Abstract
BACKGROUND
Histological changes after direct-acting antivirals (DAAs) therapy in hepatitis C virus (HCV) patients has not been elucidated. Whether the predominantly progressive, indeterminate and predominately regressive (P-I-R) score, evaluating fibrosis activity in hepatitis B virus patients has predictive value in HCV patients has not been investigated.
AIM
To identify histological changes after DAAs therapy and to evaluate the predictive value of the P-I-R score in HCV patients.
METHODS
Chronic HCV patients with paired liver biopsy specimens before and after DAAs treatment were included. Sustained virologic response (SVR) was defined as an undetectable serum HCV RNA level at 24 wk after treatment cessation. The Ishak system and P-I-R score were assessed. Inflammation improvement and fibrosis regression were defined as a ≥ 2-points decrease in the histology activity index (HAI) score and a ≥ 1-point decrease in the Ishak fibrosis score, respectively. Fibrosis progression was defined as a ≥ 1-point increase in the Ishak fibrosis score. Histologic improvement was defined as a ≥ 2-points decrease in the HAI score without worsening of the Ishak fibrosis score after DAAs therapy. The P-I-R score was also assessed. “absolutely reversing or advancing” was defined as the same directionality implied by both change in the Ishak score and posttreatment P-I-R score; and “probably reversing or advancing” was defined as only one parameter showing directionality.
RESULTS
Thirty-eight chronic HCV patients with paired liver biopsy specimens before and after DAAs treatment were included. The mean age of these patients was 40.9 ± 14.6 years and there were 53% (20/38) males. Thirty-four percent (13/38) of patients were cirrhotic. Eighty-two percent (31/38) of patients achieved inflammation improvement. The median HAI score decreased significantly after SVR (pretreatment 7.0 vs posttreatment 2.0, Z = -5.146, P = 0.000). Thirty-seven percent (14/38) of patients achieved fibrosis improvement. The median Ishak score decreased significantly after SVR (pretreatment 4.0 vs posttreatment 3.0, Z = -2.354, P = 0.019). Eighty-two percent (31/38) of patients showed histological improvement. The P-I-R score was evaluated in 61% (23/38) of patients. The progressive group showed lower platelet (P = 0.024) and higher HAI scores (P = 0.070) before treatment. In patients with stable Ishak stage after treatment: Progressive injury was seen in 22% (4/18) of patients, 33% (6/18) were classified as indeterminate and regressive changes were seen in 44% (8/18) of patients who were judged as probably reversing by the Ishak and P-I-R systems.
CONCLUSION
Significant improvement of necroinflammation and partial remission of fibrosis in HCV patients occurred shortly after DAAs therapy. The P-I-R score has potential in predicting fibrosis in HCV patients.
Keywords: Hepatitis C virus; Direct-acting antiviral agents; Necroinflammation; Fibrosis; Predominantly progressive, indeterminate and predominately regressive score; Histopathology
Core Tip: In this retrospective analysis of paired liver biopsy specimens from hepatitis C virus (HCV) patients who achieved sustained virologic response after direct-acting antivirals (DAAs) therapy, clinical and histologic features including the Ishak system and predominantly progressive, indeterminate and predominately regressive (P-I-R) scores were analyzed. Significant improvement of necroinflammation and partial remission of fibrosis in HCV patients occurred shortly after DAAs therapy. The P-I-R score has potential in predicting fibrosis in HCV patients.
INTRODUCTION
Direct-acting antivirals (DAAs) have resulted in extraordinary progress in the treatment of hepatitis C virus (HCV) infection. The potential for liver fibrosis of HCV patients to regress following successful treatment has been acknowledged for many years, particularly after eradication of HCV in most patients[1,2]. However, due to the low rate of sustained virologic response (SVR) following the interferon-ribavirin (PR) regimen, and limited indications to perform a liver biopsy after virus eradication, liver biopsy findings after successful HCV eradication have not been elucidated. Previous studies indicated the benefit of HCV eradication by PR therapy[3,4]. As DAAs have only been used in clinical practice for several years, histopathological improvement after successful DAAs therapy has only been described in a few studies and these findings were unclear[5].
Recently, a new classification, consisting of three categories of fibrosis quality: the predominantly progressive, indeterminate and predominately regressive (P-I-R) score was used to evaluate the quality of fibrosis activity based on the balance between progressive and regressive liver disease in patients with hepatitis B virus (HBV) infection, which was called the Beijing classification[6-8]. This classification was predictive of histological outcomes by uniquely incorporating histopathologic features of regressive vs progressive fibrosis, i.e., the quality of fibrosis, not only its extent and it performed well in HBV patients[6]. However, whether the Beijing classification has predictive value in HCV patients, especially after SVR, has not been investigated.
We performed this retrospective analysis of paired liver biopsy specimens of HCV patients achieving SVR after DAAs therapy. Paired liver biopsies before and after SVR with clinical and histologic features including the Ishak system and P-I-R scores were measured and analyzed.
MATERIALS AND METHODS
Study population
Chronic HCV patients with paired liver biopsy specimens before and after successful treatment with DAAs in our hospital were included in this study between January 2015 and December 2016. SVR was defined as undetectable serum HCV RNA at 24 wk after the end of treatment (EOT). Patients with other chronic liver disease (e.g., hepatitis B, hemochromatosis etc.), unqualified liver samples or incomplete clinical or laboratory data were excluded. We collected all the patients’ sociodemographic, clinical and histological information from medical records in our hospital.
Histological evaluation
All available formalin-fixed, paraffin-embedded pretreatment and posttreatment liver biopsy specimens from patients were collected. Pretreatment was defined as 6 mo before DAAs therapy and posttreatment was defined as any time after EOT. The biopsy specimens were then reviewed by two independent experienced hepatopathologists, masked to the clinical information of these patients.
Necroinflammation activity and fibrosis stage were assessed by the Ishak modified histology activity index (HAI) grading and Ishak staging system, respectively[9]. Cirrhosis was defined as an Ishak fibrosis score ≥ 5. Inflammation improvement and fibrosis regression were defined as ≥ a 2-points decrease in the HAI score and a ≥ 1-point decrease in the Ishak fibrosis score, respectively. Fibrosis progression was defined as a ≥ 1-point increase in the Ishak fibrosis score. Histologic improvement was defined as a ≥ 2-points decrease in the HAI score without worsening of the Ishak fibrosis score after DAAs therapy.
The Beijing classification was also assessed, which was applied for liver biopsy specimens with at least focal fibrous septa formation or higher stages of scarring (Ishak staging ≥ F3). The classification consisted of three categories of fibrosis quality: Predominantly progressive, indeterminate and predominately regressive[6]. “Absolutely reversing or advancing” was defined as the same directionality implied by both change in Ishak score and posttreatment P-I-R score; and “probably reversing or advancing” was defined as only one parameter showing directionality.
Statistical analysis
Descriptive statistics were used in the analysis of patients’ sociodemographic characteristics, clinical information, histological information and P-I-R scores. Categorical variables (gender, HCV genotype, treatment group, HAI and Ishak score) were measured as percentages while continuous variables were expressed as mean ± SD (age, albumin, platelet and HCV RNA) or median (interquartile range) [alanine aminotransferase (ALT), total bilirubin (TB), international normalized ratio (INR) and liver stiffness measurement (LSM)] as appropriate. Pretreatment and posttreatment HAI grading and Ishak staging were compared using the Wilcoxon sign rank test. One-Way ANOVA (age, albumin, platelet and HCV RNA) and Kruskal-Wallis H test (ALT, TB, INR and LSM) were used to compare continuous variables and the Mantel-Haenszel chi-square test (gender, HAI and Ishak score) was used to compare categorical variables by groups of pretreatment or posttreatment P-I-R score, respectively. All statistical tests were considered significant at a P level less than 0.05. Statistical analysis was performed using SPSS 25.0 (SPSS Inc.; Chicago, IL, United States).
RESULTS
We identified 40 patients with paired liver biopsy specimens and 95% (38/40) of them were included in the analysis with available paired clinical and histological data before and after treatment. Pretreatment biopsy specimens were obtained an average of 3 mo before DAAs therapy (range: 1-6 mo) and posttreatment biopsy specimens were obtained an average of 6 mo after EOT (range: 5-7 mo).
Patient demographics, clinical and laboratory characteristics
The mean age of the patients was 40.9 ± 14.6 years. Fifty-three percent (20/38) of patients were male. Of these, the pretreatment HAI score was judged to be ≥ 10 in 29% (11/38) of patients. Thirty-four percent (13/38) of patients were cirrhotic (Ishak ≥ 5) (Table 1).
Table 1.
Clinical and histological characteristic of hepatitis C virus patients before treatment (n = 38)
| Parameters | |
| Age (yr) | 40.9 ± 14.6 |
| Males, n (%) | 20 (53) |
| ALT (U/L) | 54 (30) |
| TBil (μmol/L) | 11.5 (6.7) |
| Albumin (g/L) | 45.7 ± 3.5 |
| Platelets (× 103 μL) | 173.6 ± 63.6 |
| INR | 1.00 (0.10) |
| HCV RNA (log IU/L) | 6.24 ± 0.79 |
| LSM, kPa | 6.6 (3.6) |
| HCV genotype | |
| 1 | 23 (60) |
| 2 | 3 (8) |
| 3 | 12 (32) |
| Treatment, n (%) | |
| SOF + RBV | 17 (45) |
| DCV + ASV | 14 (37) |
| GZR + EBR | 7 (18) |
| HAI score, n (%) | |
| 0-3 | 6 (16) |
| 4-6 | 12 (32) |
| 7-9 | 9 (24) |
| ≥ 10 | 11 (29) |
| Ishak score, n (%) | |
| F0-2 | 8 (21) |
| F3-4 | 17 (45) |
| F5-6 | 13 (34) |
ALT: Alanine aminotransferase; TBil: Total bilirubin; INR: International normalized ratio; HCV: Hepatitis C virus; LSM: Liver stiffness measurement; SOF + RBV: Sofosbuvir + ribavirin; DCV + ASV: Daclatasvir + asunaprevir; GZR + EBR: Grazoprevir + elbasvir; HAI: Histology activity index.
Necroinflammation grading
Eighty-two percent (31/38) of patients achieved improvement of HAI ≥ 2. The proportion with mild or no necroinflammation (HAI range: 0-3) increased from pretreatment 16% (6/38) to posttreatment 68% (26/38). The median HAI score decreased significantly after SVR (pretreatment 7.0 vs posttreatment 2.0, Z = -5.146, P = 0.000).
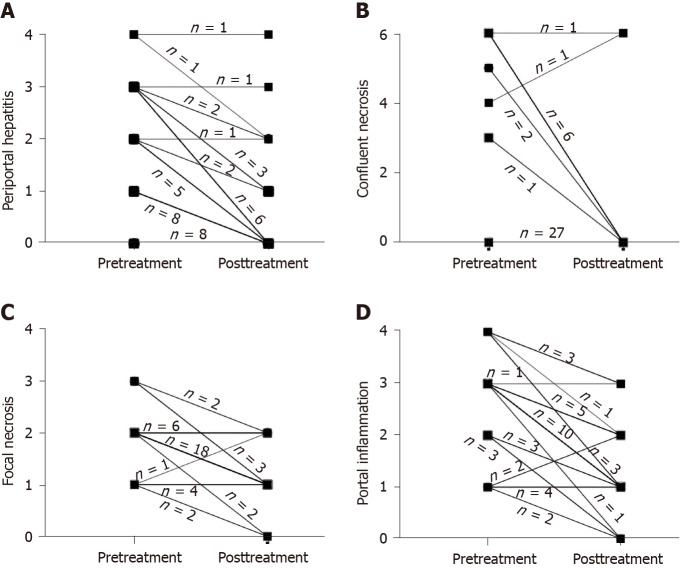
Compared to the proportion of pretreatment specimens, the proportion of absent or minimal/focal necroinflammation in individual components of the HAI score significantly decreased after SVR (periportal or periseptal interface hepatitis: 45% and 84%; confluent necrosis: 71% and 95%; focal lytic necrosis: 18% and 76%; portal inflammation: 21% and 68%). The proportion of patients with grade 3 or greater periportal hepatitis decreased from 37% (14/38) before SVR to 5% (2/38) after SVR (Figure 1A). The proportion of patients with grade 3 or greater confluent necrosis decreased from 29% (11/38) to 5% (2/38) after SVR (Figure 1B). Respectively, 71% (27/38) and 26% (10/38) of patients had a decrease or no change in focal lytic necrosis and focal inflammation score before and after SVR (Figure 1C). Eighty-two percent (31/38) of patients had decreased portal inflammation scores after SVR; and 47% (18/38) of patients had a decrease of 2-points or greater. The proportion of patients with grade 3 or greater portal inflammation decreased from 63% (24/38) to 11% (4/38) after SVR (Figure 1D). The median necroinflammation scores decreased significantly after SVR (periportal hepatitis: Pretreatment 2.0 vs posttreatment 0.0, Z = -4.620, P = 0.000; confluent necrosis: pretreatment 0.0 vs posttreatment 0.0, Z = -2.767, P = 0.006; focal lytic necrosis: pretreatment 2.0 vs posttreatment 1.0, Z = -4.670, P = 0.000; portal inflammation: pretreatment 3.0 vs posttreatment 1.0, Z = -4.826, P = 0.000).
Figure 1.
Changes in liver inflammation between pretreatment and posttreatment liver biopsies in individual patients. A: Change in periportal or periseptal interface hepatitis; B: Change in confluent necrosis; C: Change in focal lytic necrosis; D: Change in portal inflammation.
Ishak staging
Thirty-seven percent (14/38) of patients achieved fibrosis improvement and fibrosis stage in 61% (23/38) patients did not change. Overall, regression of liver fibrosis was documented in 34% (13/38) of patients, including one patient who regressed from F5 to F4. Five percent (2/38) of patients had progression of fibrosis: One progressed from F0 to F3, and the other from F5 to F6. The median Ishak score decreased significantly after SVR (pretreatment 4.0 vs posttreatment 3.0, Z = -2.354, P = 0.019).
Histological improvement as shown by the Ishak system
Eighty-two percent (31/38) of patients reached the criterion of histological improvement. Specifically, 100% (3/3), 75% (3/4), 80% (4/5) and 92% (11/12) of patients achieved histological improvement in those with a pretreatment Ishak fibrosis score of 1, 2, 3 and 4, respectively, as did 77% (10/13) of cirrhotic patients.
P-I-R classification of liver fibrosis quality
The P-I-R classification was used to evaluate 23 paired liver biopsies of patients with a pretreatment Ishak score ≥ 3. The progressive group showed lower platelet (P = 0.024) and higher HAI scores (P = 0.070), followed, in turn, by the indeterminate and regressive groups before treatment. Although patients in the regressive group were younger and had less severe necroinflammation than those in the progressive and indeterminate groups, the differences were not significant after treatment (Table 2).
Table 2.
Clinical and pathological parameters of hepatitis C virus patients by pretreatment and posttreatment predominantly progressive, indeterminate and predominately regressive score (n = 23)
| Parameters | Progressive | Indeterminate | Regressive | P value |
| Pretreatment, n (%) | 15 (65) | 7 (30) | 1 (4) | |
| Age (yr) | 50.3 ± 12.3 | 43.9 ± 12.5 | 27.0 | 0.154 |
| Males, n (%) | 6 (40) | 3 (43) | 0 (0) | 0.709 |
| ALT (U/L) | 66 (57) | 48 (38) | 50 (0) | 0.469 |
| TBil (μmol/L) | 14.0 (8.0) | 12.0 (6.0) | 9.0 (0.0) | 0.642 |
| Albumin (g/L) | 44.0 ± 3.9 | 47.0 ± 3.1 | 45.0 | 0.233 |
| Platelets (× 103 μL) | 129.3 ± 60.2 | 188.0 ± 51.5 | 268.0 | 0.024 |
| INR | 1.04 (0.16) | 1.00 (0.2) | 1.00 (0.00) | 0.307 |
| HCV RNA (log IU/L) | 6.01 ± 0.66 | 6.46 ± 0.90 | 6.60 | 0.375 |
| LSM, kPa | 8.8 (14.8) | 6.6 (2.9) | 6.6 (0.0) | 0.331 |
| HAI score, n (%) | 0.070 | |||
| 0-3 | 0 (1) | 1 (14) | 0 (0) | |
| 4-6 | 1 (7) | 2 (29) | 1 (100) | |
| 7-9 | 4 (27) | 3 (43) | 0 (0) | |
| ≥ 10 | 10 (67) | 1 (14) | 0 (0) | |
| Ishak score, n (%) | 0.072 | |||
| 3-4 | 4 (27) | 5 (71) | 1 (100) | |
| 5-6 | 11 (73) | 2 (29) | 0 (0) | |
| Posttreatment, n (%) | 5 (22) | 9 (39) | 9 (39) | |
| Age (yr) | 50.4 ± 12.0 | 47.8±14.1 | 45.4 ± 13.4 | 0.802 |
| Males, n (%) | 1 (20) | 3 (33) | 5 (56) | 0.384 |
| ALT (U/L) | 25 (21) | 17 (8) | 15 (15.5) | 0.340 |
| TBil (μmol/L) | 19 (10) | 10 (6) | 14 (9) | 0.149 |
| Albumin (g/L) | 44.0 ± 4.2 | 44.9 ± 1.9 | 46.0 ± 1.8 | 0.353 |
| Platelets (× 103 μL) | 156.4 ± 63.3 | 184.4 ± 80.3 | 151.1 ± 65.8 | 0.552 |
| INR | 1.13 (0.15) | 1.00 (0.14) | 1.09 (0.15) | 0.052 |
| LSM, kPa | 11.5 (22.8) | 7.8 (7.4) | 6.8 (5.2) | 0.683 |
| HAI score, n (%) | 0.338 | |||
| 0-3 | 1 (20) | 4 (44) | 6 (67) | |
| 4-6 | 2 (40) | 4 (44) | 3 (33) | |
| 7-9 | 1 (20) | 0 (0) | 0 (0) | |
| ≥ 10 | 1 (20) | 1 (11) | 0 (0) | |
| Ishak score, n (%) | 0.339 | |||
| 3-4 | 2 (40) | 6 (67) | 6 (67) | |
| 5-6 | 3 (60) | 3 (33) | 3 (33) |
ALT: Alanine aminotransferase; TBil: Total bilirubin; INR: International normalized ratio; HCV: Hepatitis C virus; LSM: Liver stiffness measurement; HAI: Histology activity index.
Posttreatment P-I-R fibrosis quality vs changes in Ishak fibrosis stage between pretreatment and posttreatment
A comparison of posttreatment P-I-R fibrosis quality with changes in Ishak fibrosis stage between pretreatment and posttreatment is shown in Table 3. In 4 of 23 (17%) patients whose Ishak score decreased by 1 stage, none showed progressive injury after treatment, with 25% (1/4) and 75% (3/4) showing regressive and indeterminate, respectively. In those with stable Ishak stages, progressive injury after treatment was seen in 22% (4/18) of patients, 33% (6/18) were classified as indeterminate and regressive changes were seen in 44% (8/18) of patients who were judged as probably reversing by the Ishak and P-I-R systems. Only one patient with an increased Ishak stage in the posttreatment biopsy also showed progressive changes.
Table 3.
Posttreatment predominantly progressive, indeterminate and predominately regressive score vs changes in Ishak stage to evaluate disease progression or regression (n = 23)
| Ishak (pre-post) |
Posttreatment P-I-R score (n = 23) |
||
| Progressive (n = 5) | Indeterminate (n = 9) | Regressive (n = 9) | |
| Increase (n = 1) | Absolutely advancing 100% (1 of 1) | 0 | 0 |
| Stable (n = 18) | Probably advancing 22% (4 of 18) | 33% (6 of 18) | Probably reversing 44% (8 of 18) |
| Decrease (n = 4) | 0 | 75% (3 of 4) | Absolutely reversing 33% (1 of 3) |
P-I-R: Predominantly progressive, indeterminate and predominately regressive.
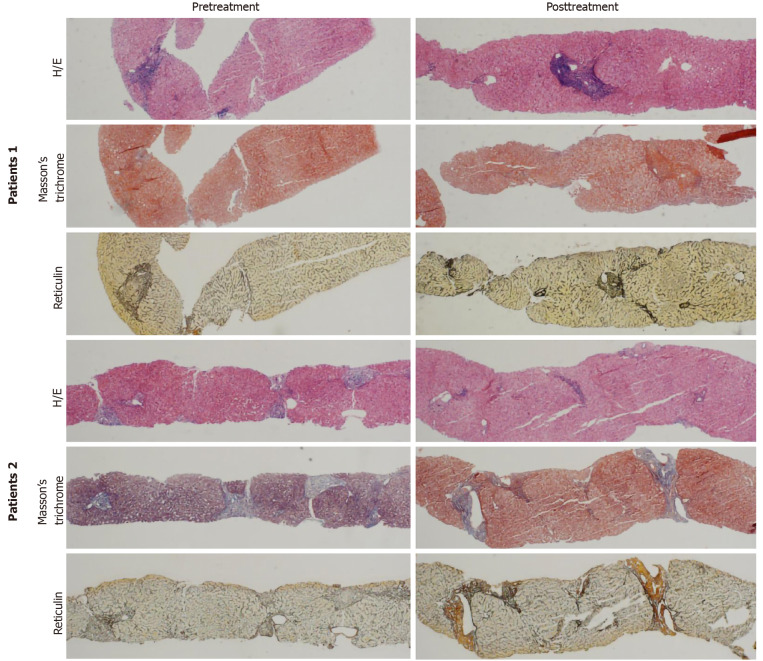
Eight “probably reversing” cases are shown in Table 4. Fibrosis staging of these patients was stable after SVR. Histology of two “probably reversing” cases before and after SVR with DAAs is shown in Figure 2.
Table 4.
The three fibrosis scoring or staging systems of the 8 “probably reversing” cases
| Case No. |
P-I-R
|
Ishak score
|
LSM (kPa)
|
|||
|
Pre
|
Post
|
Pre
|
Post
|
Pre
|
Post
|
|
| 1 | P | R | 6 | 6 | 6.8 | 6.1 |
| 2 | P | R | 6 | 6 | 7.4 | 5.6 |
| 3 | I | R | 3 | 3 | 4.9 | 4.1 |
| 4 | P | R | 6 | 6 | 24.2 | 27 |
| 5 | P | R | 5 | 5 | 6.3 | 3.6 |
| 6 | I | R | 4 | 4 | 5.8 | 5.6 |
| 7 | P | R | 6 | 6 | 14.0 | 10.7 |
| 8 | I | R | 5 | 5 | 6.9 | 7.6 |
P-I-R: Predominantly progressive, indeterminate and predominately regressive; P: Progressive; I: Indeterminate; R: Regressive; LSM: Liver stiffness measurement; Pre: Pretreatment; Post: Posttreatment.
Figure 2.
Histology of two “probably reversing” cases before and after sustained virologic response with direct-acting antivirals. Patient 1 is a 55-year-old male with genotype 1b treated with daclatasvir + asunaprevir for 24 wk: Pre-histology activity index (HAI) 14, pre-Ishak 6, pre-predominantly progressive, indeterminate and predominately regressive (P-I-R) predominantly progressive, post-HAI 4, post-Ishak 6, post-P-I-R predominantly progressive. Patient 2 is a 39-year-old female with genotype 3 treated with sofosbuvir + ribavirin for 24 wk: Pre-HAI 6, pre-Ishak 3, pre-P-I-R indeterminate, post-HAI 3, post-Ishak 3, post-P-I-R predominately regressive. Hematoxylin and eosin (10 ×); Masson’s trichrome (4 ×); reticulin (4 ×). H/E: hematoxylin and eosin.
DISCUSSION
Our study of 38 paired liver biopsy specimens from HCV patients showed the histological changes after successful antiviral therapy with DAAs. Significant improvement of necroinflammation was achieved and fibrosis staging improved or did not worsen in most patients. Eighty-two percent of patients reached the criterion of histological improvement. The analysis of P-I-R classification of 23 paired liver biopsies showed that 65% of biopsies were in the progressive groups before SVR, which reduced to only 22% after SVR. Liver biopsy findings after successful HCV eradication by DAAs therapy have not been elucidated. Our study is important due to the availability of paired histological data before and after SVR.
Previous studies comparing liver biopsies of HCV patients before and after SVR indicated improvements in inflammation and fibrosis scores after SVR[4,10]. However, a small subset of patients display persistent hepatic inflammation and/or progression to cirrhosis despite SVR[5,11,12], and regression of fibrosis varies[4,13,14].
A 5-year follow-up study on HCV patients with 49 paired pre-treatment and long-term follow-up biopsies showed that 45 (92%) patients had a decrease in the combined inflammation score after their 4th year of follow-up[4], and another study demonstrated long-term changes in liver histology following treatment of chronic HCV showed that patients with SVR had a significant decline in mean inflammation[15]. The longest hepatitis C study with paired liver samples had a median interval between the two biopsies of 93 mo: In total, 71% of patients with SVR treated with PR achieved inflammation improvement, while 23% worsened[12]. These studies reached relatively similar conclusions according to paired liver biopsies: Histological inflammation in hepatitis C patients with SVR significantly improved after long-term follow-up. However, in one study with paired liver biopsies from hepatitis C patients after successful interferon therapy, histological improvement was significant, but mild inflammation persisted in 87% of patients even after more than 4 years[16]. In another study of allograft liver biopsy specimens from patients achieving SVR after a liver transplant (LT) for HCV infection, fibrosis continued to progress in 23% of patients[5]. It should be noted that these conclusions were established based on evidence from the PR regimen. Data on the DAAs regimen are limited: A recent study on the histological findings of 25 HCV patients after successful DAAs therapy before LT, showed that 24% (6/25) of patients still had mild inflammation (HAI: 5-8), and 4% (1/25) had moderate inflammation (HAI: 9-12) after SVR. These proportions were similar to those in patients without SVR[11]. Our finding that significant improvement of necroin-flammation (improvement of HAI ≥ 2) was achieved in 82% of patients in a very short time after SVR demonstrated the rapid improvement in inflammation following successful DAAs treatment.
Clinical studies have clearly established that most patients demonstrated marked improvements in fibrosis following SVR after PR therapy[1,4,12]. However, in large clinical trials a minority of patients (7%-13%) maintained their level of fibrosis or even progressed to cirrhosis despite achieving SVR[5,10,17,18]. The above results are all derived from clinical studies of PR. The effect of DAAs on fibrosis in HCV patients after SVR has been unclear up to now. Noninvasive methods for assessing fibrosis have been applied[19-22]. However, histological improvement of fibrosis in HCV patients after successful DAAs treatment has not been extensively studied. We found fibrosis regression in 37% (14/38) of patients; however, one study indicated a weak effect of SVR on fibrosis regression in cirrhotic HCV patients after DAAs therapy before LT[11]. In another study of liver histopathology after LT in HCV patients after successful therapy with PR or DAAs, 23% of patients still had fibrosis progression[5]. The fact that only 34% of patients in our study were cirrhotic and were facing more complicated conditions after LT could explain the distinctions between our results and others.
The P-I-R score has been proposed to evaluate the quality of fibrosis activity based on the balance between progressive and regressive liver disease in HBV patients[6-8]. Our research first determined whether the P-I-R score had predictive value in HCV patients especially after SVR with DAAs. In 23 patients with paired liver biopsy samples, 65% of pretreatment biopsies were in the progressive groups, while the proportion decreased to 22% after SVR. Patients with a progressive form of fibrosis showed higher total HAI scores and portal inflammation than those with a regressive form of fibrosis. In 8 patients who were probably reversing, the posttreatment P-I-R scores were almost consistent with the changing tendency of noninvasive measurements of fibrosis indicating that the predictive value of P-I-R may be similar to that of noninvasive measurements. However, further assessment should be performed in future studies.
CONCLUSION
Our retrospective study of 38 paired liver biopsy specimens showed histological improvements in HCV patients with SVR after successful DAAs treatment. Histological improvement including necroinflammation and fibrosis were seen in most patients within a very short time after SVR. A preliminary examination of the application of the P-I-R score was performed in HCV patients. However, in view of the small sample size and short-term follow-up, larger and longer-term prospective studies and predictive factors for histological improvement after DAAs treatment should be performed in future studies.
ARTICLE HIGHLIGHTS
Research background
Histological changes after direct-acting antivirals (DAAs) therapy in hepatitis C virus (HCV) patients have not been elucidated. Whether the predominantly progressive, indeterminate and predominately regressive (P-I-R) score has predictive value in HCV patients has not been investigated.
Research motivation
The key issues are whether DAAs improve histopathology in HCV patients rapidly and whether the P-I-R score has predictive value in HCV patients. The results will provide important information on the histological response to DAAs therapy and the potential value of the P-I-R score in HCV patients.
Research objectives
To obtain a full understanding of the effect of DAAs on the liver, we assessed the histological changes after DAAs therapy. We also evaluated the predictive value of the P-I-R score in HCV patients.
Research methods
Chronic HCV patients with paired liver biopsy specimens before and after DAAs treatment were included. Sustained virologic response was defined as an undetectable serum HCV RNA level at 24 wk after treatment cessation. The Ishak system and P-I-R score were assessed.
Research results
Thirty-eight chronic HCV patients were included. Their mean age was 40.9 ± 14.6 years and 53% (20/38) were male. Eighty-two percent (31/38) and 37% (14/38) of patients achieved inflammation and fibrosis improvement, respectively. The P-I-R score was evaluated in 61% (23/38) of patients. In patients with stable Ishak stage after treatment: Progressive injury was seen in 22% (4/18) of patients, 33% (6/18) were classified as indeterminate and regressive changes were seen in 44% (8/18) of patients who were judged as probably reversing by the Ishak and P-I-R systems. Our results increased the knowledge on liver biopsy findings after successful HCV eradication by DAAs therapy. However, in view of the small sample size and short-term follow-up, larger and longer-term prospective studies and predictive factors for histological improvement after DAAs treatment should be performed in future studies.
Research conclusions
Significant improvement of necroinflammation and partial remission of fibrosis in HCV patients occurred shortly after DAAs therapy. The P-I-R score has potential in predicting fibrosis in HCV patients.
Research perspectives
Larger and longer-term prospective studies and predictive factors for histological improvement after DAAs treatment should be performed in future studies.
ACKNOWLEDGEMENTS
We thank Liu HX, MD for help with the statistical review.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Institutional Review Board of Peking University People’s Hospital (2019PHB036-01) and informed consent was waived.
Informed consent statement: Patients were not required to give informed consent for this study as the analysis used anonymous clinical data. The Institutional Review Board of Peking University People’s Hospital approved waiving informed consent.
Conflict-of-interest statement: Dr. Rao reports grants from National Natural Science Foundation of China (NSFC) (81870406), grants from Beijing Natural Science Foundation (7182174), grants from China National Science and Technology Major Project for Infectious Diseases Control during the 13th Five-Year Plan Period (2017ZX10202202), during the conduct of the study.
Manuscript source: Unsolicited manuscript
Peer-review started: October 28, 2020
First decision: December 3, 2020
Article in press: December 26, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Trifan A S-Editor: Chen XF L-Editor: Webster JR P-Editor: Ma YJ
Contributor Information
Rui Huang, Peking University Hepatology Institute, Beijing Key Laboratory of Hepatitis C and Immunotherapy for Liver Diseases, Beijing International Cooperation Base for Science and Technology on NAFLD Diagnosis, Peking University People’s Hospital, Beijing 100044, China.
Hui-Ying Rao, Peking University Hepatology Institute, Beijing Key Laboratory of Hepatitis C and Immunotherapy for Liver Diseases, Beijing International Cooperation Base for Science and Technology on NAFLD Diagnosis, Peking University People’s Hospital, Beijing 100044, China. raohuiying@pkuph.edu.cn.
Ming Yang, Hepatopancreatobiliary Center, Beijing Tsinghua Changgung Hospital, Tsinghua University, Beijing 102218, China.
Ying-Hui Gao, Peking University Hepatology Institute, Beijing Key Laboratory of Hepatitis C and Immunotherapy for Liver Diseases, Beijing International Cooperation Base for Science and Technology on NAFLD Diagnosis, Peking University People’s Hospital, Beijing 100044, China.
Jian Wang, Peking University Hepatology Institute, Beijing Key Laboratory of Hepatitis C and Immunotherapy for Liver Diseases, Beijing International Cooperation Base for Science and Technology on NAFLD Diagnosis, Peking University People’s Hospital, Beijing 100044, China.
Qian Jin, Peking University Hepatology Institute, Beijing Key Laboratory of Hepatitis C and Immunotherapy for Liver Diseases, Beijing International Cooperation Base for Science and Technology on NAFLD Diagnosis, Peking University People’s Hospital, Beijing 100044, China.
Dan-Li Ma, Peking University Hepatology Institute, Beijing Key Laboratory of Hepatitis C and Immunotherapy for Liver Diseases, Beijing International Cooperation Base for Science and Technology on NAFLD Diagnosis, Peking University People’s Hospital, Beijing 100044, China.
Lai Wei, Hepatopancreatobiliary Center, Beijing Tsinghua Changgung Hospital, Tsinghua University, Beijing 102218, China.
Data sharing statement
No additional data are available.
References
- 1.Pockros PJ, Hamzeh FM, Martin P, Lentz E, Zhou X, Govindarajan S, Lok AS. Histologic outcomes in hepatitis C-infected patients with varying degrees of virologic response to interferon-based treatments. Hepatology. 2010;52:1193–1200. doi: 10.1002/hep.23809. [DOI] [PubMed] [Google Scholar]
- 2.Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, Ling MH, Albrecht J. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303–1313. doi: 10.1053/gast.2002.33023. [DOI] [PubMed] [Google Scholar]
- 3.McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling MH, Cort S, Albrecht JK. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 4.George SL, Bacon BR, Brunt EM, Mihindukulasuriya KL, Hoffmann J, Di Bisceglie AM. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology. 2009;49:729–738. doi: 10.1002/hep.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitcomb E, Choi WT, Jerome KR, Cook L, Landis C, Ahn J, Te HS, Esfeh J, Hanouneh IA, Rayhill SC, Gibson W, Plesec T, Koo J, Wang HL, Hart J, Pai RK, Westerhoff M. Biopsy Specimens From Allograft Liver Contain Histologic Features of Hepatitis C Virus Infection After Virus Eradication. Clin Gastroenterol Hepatol. 2017;15:1279–1285. doi: 10.1016/j.cgh.2017.04.041. [DOI] [PubMed] [Google Scholar]
- 6.Sun Y, Zhou J, Wang L, Wu X, Chen Y, Piao H, Lu L, Jiang W, Xu Y, Feng B, Nan Y, Xie W, Chen G, Zheng H, Li H, Ding H, Liu H, Lv F, Shao C, Wang T, Ou X, Wang B, Chen S, Wee A, Theise ND, You H, Jia J. New classification of liver biopsy assessment for fibrosis in chronic hepatitis B patients before and after treatment. Hepatology. 2017;65:1438–1450. doi: 10.1002/hep.29009. [DOI] [PubMed] [Google Scholar]
- 7.Theise ND, Jia J, Sun Y, Wee A, You H. Progression and regression of fibrosis in viral hepatitis in the treatment era: the Beijing classification. Mod Pathol. 2018;31:1191–1200. doi: 10.1038/s41379-018-0048-0. [DOI] [PubMed] [Google Scholar]
- 8.Wang B, Sun Y, Zhou J, Wu X, Chen S, Wu S, Liu H, Wang T, Ou X, Jia J, You H. Advanced septa size quantitation determines the evaluation of histological fibrosis outcome in chronic hepatitis B patients. Mod Pathol. 2018;31:1567–1577. doi: 10.1038/s41379-018-0059-x. [DOI] [PubMed] [Google Scholar]
- 9.Brunt EM. Grading and staging the histopathological lesions of chronic hepatitis: the Knodell histology activity index and beyond. Hepatology. 2000;31:241–246. doi: 10.1002/hep.510310136. [DOI] [PubMed] [Google Scholar]
- 10.Lee YA, Friedman SL. Reversal, maintenance or progression: what happens to the liver after a virologic cure of hepatitis C? Antiviral Res. 2014;107:23–30. doi: 10.1016/j.antiviral.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Putra J, Schiano TD, Fiel MI. Histological assessment of the liver explant in transplanted hepatitis C virus patients achieving sustained virological response with direct-acting antiviral agents. Histopathology. 2018;72:990–996. doi: 10.1111/his.13453. [DOI] [PubMed] [Google Scholar]
- 12.Chu CY, Cheng CH, Chen HL, Lin IT, Wu CH, Lee YK, Bair MJ. Long-term histological change in chronic hepatitis C patients who had received peginterferon plus ribavirin therapy with sustained virological response. J Formos Med Assoc. 2019;118:1129–1137. doi: 10.1016/j.jfma.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, Kuroki T, Nishiguchi S, Sata M, Yamada G, Fujiyama S, Yoshida H, Omata M. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132:517–524. doi: 10.7326/0003-4819-132-7-200004040-00002. [DOI] [PubMed] [Google Scholar]
- 14.Tachi Y, Hirai T, Miyata A, Ohara K, Iida T, Ishizu Y, Honda T, Kuzuya T, Hayashi K, Ishigami M, Goto H. Progressive fibrosis significantly correlates with hepatocellular carcinoma in patients with a sustained virological response. Hepatol Res. 2015;45:238–246. doi: 10.1111/hepr.12331. [DOI] [PubMed] [Google Scholar]
- 15.Shiffman ML, Sterling RK, Contos M, Hubbard S, Long A, Luketic VA, Stravitz RT, Fuchs M, Sanyal AJ. Long term changes in liver histology following treatment of chronic hepatitis C virus. Ann Hepatol. 2014;13:340–349. [PubMed] [Google Scholar]
- 16.Tsuda N, Yuki N, Mochizuki K, Nagaoka T, Yamashiro M, Omura M, Hikiji K, Kato M. Long-term clinical and virological outcomes of chronic hepatitis C after successful interferon therapy. J Med Virol. 2004;74:406–413. doi: 10.1002/jmv.20190. [DOI] [PubMed] [Google Scholar]
- 17.Poynard T, Moussalli J, Munteanu M, Thabut D, Lebray P, Rudler M, Ngo Y, Thibault V, Mkada H, Charlotte F, Bismut FI, Deckmyn O, Benhamou Y, Valantin MA, Ratziu V, Katlama C FibroFrance-GHPS group. Slow regression of liver fibrosis presumed by repeated biomarkers after virological cure in patients with chronic hepatitis C. J Hepatol. 2013;59:675–683. doi: 10.1016/j.jhep.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Maylin S, Martinot-Peignoux M, Moucari R, Boyer N, Ripault MP, Cazals-Hatem D, Giuily N, Castelnau C, Cardoso AC, Asselah T, Féray C, Nicolas-Chanoine MH, Bedossa P, Marcellin P. Eradication of hepatitis C virus in patients successfully treated for chronic hepatitis C. Gastroenterology. 2008;135:821–829. doi: 10.1053/j.gastro.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 19.Facciorusso A, Del Prete V, Turco A, Buccino RV, Nacchiero MC, Muscatiello N. Long-term liver stiffness assessment in hepatitis C virus patients undergoing antiviral therapy: Results from a 5-year cohort study. J Gastroenterol Hepatol. 2018;33:942–949. doi: 10.1111/jgh.14008. [DOI] [PubMed] [Google Scholar]
- 20.Knop V, Hoppe D, Welzel T, Vermehren J, Herrmann E, Vermehren A, Friedrich-Rust M, Sarrazin C, Zeuzem S, Welker MW. Regression of fibrosis and portal hypertension in HCV-associated cirrhosis and sustained virologic response after interferon-free antiviral therapy. J Viral Hepat. 2016;23:994–1002. doi: 10.1111/jvh.12578. [DOI] [PubMed] [Google Scholar]
- 21.Bachofner JA, Valli PV, Kröger A, Bergamin I, Künzler P, Baserga A, Braun D, Seifert B, Moncsek A, Fehr J, Semela D, Magenta L, Müllhaupt B, Terziroli Beretta-Piccoli B, Mertens JC. Direct antiviral agent treatment of chronic hepatitis C results in rapid regression of transient elastography and fibrosis markers fibrosis-4 score and aspartate aminotransferase-platelet ratio index. Liver Int. 2017;37:369–376. doi: 10.1111/liv.13256. [DOI] [PubMed] [Google Scholar]
- 22.Singh S, Facciorusso A, Loomba R, Falck-Ytter YT. Magnitude and Kinetics of Decrease in Liver Stiffness After Antiviral Therapy in Patients With Chronic Hepatitis C: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:27–38.e4. doi: 10.1016/j.cgh.2017.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.