Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) belongs to the family coronaviridae. It is spherical and possesses proteins called spikes, which can clamp onto the human cells. Once in close interaction with the human cells, these viruses undergo structural change and can fuse with the cell membrane. The virus enters the host and starts the process of translation and transcription in the cells and uncoated genome, respectively. Due to the rapid transmittable nature of the virus, extant actions should be taken. The fatty acids administrated orally, or intravenously, could help us gear things up in providing resistance and preventing infection. Hence, the multiplication of the virus could be hindered by arachidonic acid, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). In that context, the current review highlights the role of these unsaturated fatty acids and their derivatives such as lipoxins and resolvins in the inactivation of the enveloped coronavirus disease 2019 (COVID-19).
Keywords: Coronavirus 2, Severe acute respiratory syndrome coronavirus, Middle East respiratory syndrome, Biolipids, Angiotensin-converting enzyme 2 receptor, Docosahexaenoic acid, Eicosapentaenoic acid
Introduction
The current pandemic, as declared by the World Health Organization (WHO), has taken a toll on humankind. The reason behind this is the COVID-19, which emerged in December 2019 [1]. Recently, the existence of a total of six types of human coronavirus (HCoVs) has been reported. The virus types comprise HCoV-NL63 and HCoV-229E, and both reside in the genus alphacoronavirus (Alpha-CoV). Next up is HCoV-OC43, HCoV-HKU1, severe acute respiratory syndrome coronavirus (SARS-CoV), and Middle East respiratory syndrome coronavirus (MERS-CoV) [2]. All these viruses fit in the genus betacoronavirus. The epicenter for the same is Wuhan, China, and the virus comes out to be profoundly contagious and has shown zoonotic transmission [3]. The current scenario is substandard, and more than 100 countries have now reported laboratory-confirmed cases of COVID-19 [4]. The virus spreads very effectively into an alternate host that was not previously exposed or susceptible; therefore, one needs to be updated regularly and be prepared to control extreme virus proliferation. Table 1 shows the timeline of the emergence of viral infection in recent times.
Table 1.
Outbreak of the virus in recent past
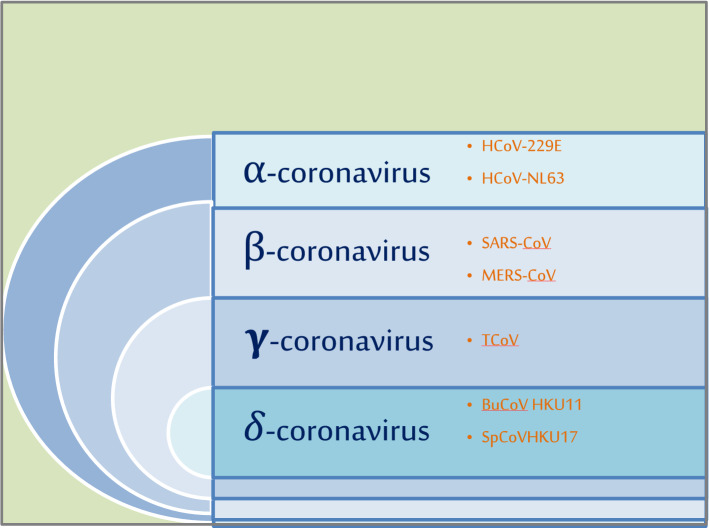
Once the virus enters the host cell, the multiplication starts and progeny viruses are produced immediately [9]. The different genera of coronavirus with examples are shown in Fig. 1. Attachment of the viral particle to the host membrane is aided by proteins called spikes to protrude out of its membrane. These spikes on the outer surface give the virus its name—coronavirus, i.e., crownlike. On similar lines, SARS and MERS outbreaks were also reported a few years back and it has been found out that the novel coronavirus that emerged in 2019 is likely to have the same genome as that of SARS-CoV [10]. Recent studies have shown that these spikes bind to some receptors on the human cell surface known as angiotensin-converting enzyme-2 (ACE2) receptors [11]. Interestingly, the same ACE2 was also the cellular receptors for the SARS-CoV. This ACE2 is the first homolog of ACE and is seen to counterpose the activity of ACE by modulating the angiotensin system. Many contemporary studies elucidate a substantial and prophylactic role of ACE2 in the renal system, cardiovascular system, and most importantly respiratory system [12]. A TEM image of the coronavirus isolated from a patient, which was later cultivated in the laboratory, shows the spikes of the virus emerging out from the outer surface which is presented in Fig. 2.
Fig. 1.
Schematic representation of different genera of the coronavirus with suitable examples
Fig. 2.
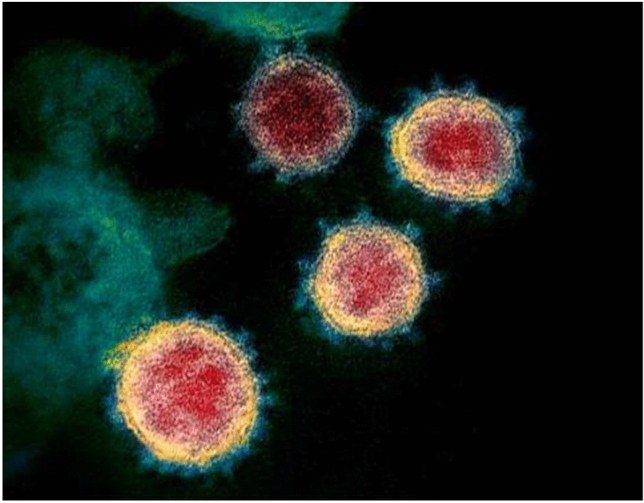
TEM image showing the COVID-19 virus, isolated from a patient, cultured in a laboratory. The image clearly shows the spikes emerging out from the viral surface [29]
Genetic constitution of SARS-CoV-2
COVID-19 is composed of a positive-strand RNA viruses (+ssRNA viruses) accomplices of a nucleoprotein [13]. This occurs within an outer covering called capsid enclosing the matrix proteins. The virus is seen to possess a minimum of six open reading frames (ORFs) in its genome. ORFs 10 and 11 play a role in the encoding of the four principle structural proteins on one-third of the genome near the 3′-terminal site. It has been discovered that the gene of ORF 8 shows potentiality as an objective for the novel CoV. The reason behind it is that ORF 8 is entirely peculiar to the novel COVID-19 only. It shows no cross-reactions to other existing kinds of coronaviruses [14]. Hence, for any kind of advances in pathogenesis or resistance, it becomes imperative to understand the phenotypic structure and genetic constitution of the same.
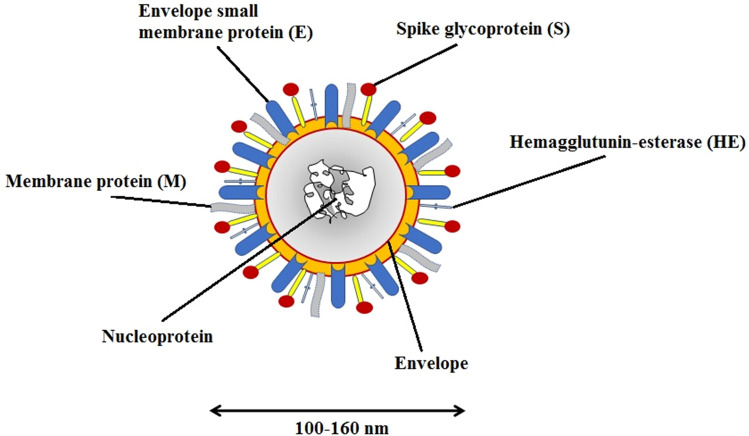
Studies have sighted clearly that this virus is a spherical or pleomorphic ‘enveloped particle’ [15]. The envelope consists of many projections (composed of the carbohydrates affixed to the polypeptide chains) which are in the shape of a club or arched. Some of the coronaviruses also comprise a ‘hemagglutinin esterase’ protein along with these glycoproteins [16].
It has been noted that the viral membrane is composed of a total of three to four proteins as shown in Fig. 3. ‘M protein’ is present in ample amount, where M stands for membrane glycoprotein which passes over the bilayered viral membrane three times, vacating a small NH2-terminal domain outside the virus and a long-COOH terminal domain, i.e., the cytoplasmic domain inside it [17]. The other proteins like the envelope protein (E), spike protein (S), nucleocapsid (N) as well as membrane protein (M) are encoded by the 3′-terminal site of this genome. This particular genome also encodes for the eight accessory proteins [18]. Peplomers are constituted by some S proteins as a type 1 glycoprotein membrane [16].
Fig. 3.
Structure of enveloped coronavirus
The chief initialization for neutralizing antiserum/antibodies is done by the S protein itself. The intracellular formation of virus particles is determined completely by M protein without any requirement of the S protein. The length of the spike in the virus is important to be recognized as it is probable to play a salient role in the pathogenesis as well as the treatment of the same [19]. Lately, sequence analysis of nCoV uncovered that it comprehends a classical genome structure of CoV and permeates with the clump of viruses of genus betacoronavirus that encircle the following viruses: bat-SARS-like (SL)-ZC45, bat-SL ZXC21, SARS-CoV, and MERS-CoV. Established on the phylogenetic tree of CoVs, it is ruled out that 2019-nCoV is more sharply related to bat-SL-CoVZC45 and bat-SL-CoVZXC21 and not so closely related to SARS-CoV [20].
ACE-2 as therapeutic target in COVID-19
Our skin, lymph node, the brain, nasopharynx, the small intestines, the kidneys, stomach, colon, thymus, bone marrow, oral and nasal mucosa, spleen, liver, and blood vessels are the chief organs explicating ACE2. These could be the objective of the SARS-CoV-2 virus [21]. ‘Angiotensinogen’ as a pivotal substratum of the rennin–angiotensin system (RAS) is primarily produced by the liver and is then sliced up to produce Ang1 with the assistance of rennin (proangiotensin). Talking about the pulmonary circulation, AngI is seen to get readily stimulated to AngII by ‘angiotensin-converting enzyme’ (ACE) [22]. In this progression, ACE operates as a peptidyl-dipeptidase and further unfolds the decapeptide AngI to an eight-amino-acid peptide called AngII. AngII is one of the eminently recognized vasopressors [23].
The binding of SARS-CoV-2 virus and ACE 2 receptor has only increased the infectivity level, leading to the outbreak of COVID-19 [24]. A remarkable number of known compounds with combative consequences of the SARS-CoV-2 virus for bandaging with the ACE2 receptors could be a fresh territory in research to undertake and clutch the problem with SARS-CoV-2. Henceforth, ACE2 is seen as a flicker of hope for the unwelcomed scenario [25].
Role of bioactive lipids in inactivation of enveloped viruses
FA and envelope inactivation
Long-chain fatty acids (LC-PUFAs) such as AA, EPA, and DHA play a significant role in curtail of inflammatory activity. They are very essential for human health; however, a more investigation is required about their role in the control of viral infection [26]. Naked viruses show no effect on UFAs, but inactivation of some enveloped viruses is associated with the action of FA on them [27]. Some electro-micrographs have shown that the association of FA with the enveloped viruses disintegrated the envelope. In a study, non-saturated monoglycerides, as well as alcohols about a chain length of 16–18C were endowed to be extremely potent inactivators of enveloped viruses, namely herpes simplex virus type II [28]. Hence, some relation between the FA and viral inactivation could be established.
Mechanism of fatty acids against viral infection
In the time of pandemic with rising cases day by day, it has been found out that building up of our innate immunity blocks progression of COVID-19 at early stages, while omega-3 polyunsaturated fatty acids (n-3 PUFAs) show immunomodulation effects [29]. It is known that omega-3 fatty acids exert anti-viral effects by inhibiting influenza virus replication and the use of omega-3 fatty acids might upgrade oxygenation in COVID-19 patients especially [30]. This might happen due to the counterintuitive increase in oxidative stress and inflammation due to increased susceptibility of cellular membranes to damage. n-3 PUFAs also interfere with virus entry and replication. n-3 PUFAs may modulate the membrane rafts where ACE2 and transmembrane protease serine 2 (TMPRSS2) are mainly expressed and lipid raft modulation may be an option to reduce ACE2-mediated virus infection [31]. As an important part of the membrane phospholipids, n-3 PUFAs can modulate the properties of the membrane. Some of the properties which are regulated are the membrane fluidity and protein complex assembly in lipid rafts. Domains, both raft and non-raft, may also modulate the expression, stability, and enzymatic activities of ACE2 and TMPRSS2. The number and size of these rafts also influence these activities. Among omega-3 long-chain PUFAs, DHA is described to directly regulate the formation of lipid rafts [32].
The fatty acids play a significant role in viral infection. The more the number of carbon chains, the maximum will be the activity of fatty acids against viral infection [33]. Several theories are elucidating the mechanism of fatty acids against viral infection. Previously, the Rous sarcoma virus can be successfully inactivated by a 1% solution of sodium oleate leading to the disappearance of viral infection. A fraction of UFAs from pig pancreatic tissue strongly inhibits chicken sarcoma virus. Treatment of 10 µg/mL of oleic acid inactivates the viral envelope, while with 50 µg/mL concentration completely disrupts any possible viral particles. Mouse mammary viral particles can be inactivated by cream from human milk which finally degrades viral envelope [33]. Electron micrograph study revealed that 1 mg/mL of linoleic completely disrupts viral envelope showing potent anti-viral activity [34]. The exact mechanism of viral inactivation by fatty acid is unknown; however, the fatty acids cause disintegration of bilayer lipid envelope with high efficacy.
Antimicrobial role of the FA
The SARS, SARS-CoV-2, and MERS are all enfolded with an envelope shape similar genome [35]. It has been discovered that some UFAs including AA might hold the capacity to disintegrate their envelopes. Hence, we can say that these polyunsaturated fatty acids (PUFAs) hold a decisive position here. Occasionally, the virus contagiousness process includes internalization, particle assembly, and lastly the virus liberation. AA, EPA, and DHA have certain metabolites that show both anti- and pro-inflammatory properties. These bioactive compounds intervene in the cellular mechanism by promoting phagocytosis, thus getting hold of possible antigens, and generate immunocytes. These metabolites are resourcefulness for reducing microbial load too [11]. Some metabolites such as lipoxin A4, resolvins, protectins, and maresins function as endogenous antimicrobial molecules, and so their appropriate use may aid in decreasing the morbidity and mortality due to SARS-CoV-2, SARS, and MERS. It has been seen that short-chain and long-chain saturated fatty acids do not possess anti-viral activities even at their highest tested amounts, whereas medium-chain saturated and long-chain UFAs show high anti-viral activity against the enveloped viral particles [36]. High anti-viral actions were shown by the monoglycerides of these FAs. This has a direct effect on the viral envelope, causing its leakage. At much higher concentrations, these were seen to completely disintegrate the envelope, as well as the viral particle, including its plasma membrane, and ultimately result in cell lysis. Some studies have shown that free fatty acids (FFAs) induce endoplasmic reticulum (ER) stress and block anti-viral activity (in the case of interferon-alpha against hepatitis C virus). This mechanism involves the accumulation of fat intracellularly in the hepatitis C virus (HCV) cell culture that is later seen to induce ER stress and also defect in the JAK-STAT signaling pathway which ultimately deflates the anti-viral response [37].
The integral procedure of the antimicrobial activity of FAs entailing AA too may incorporate their capacity to induce not only the leakage but also the bacteriolysis of membranes of the cell, even incorporating the degradation of a viral proteinaceous envelope, and numerous other biological effects, which are metabolous [11]. All this is not only delimited to inhibition of respiratory activity; many other roles like consequences on the passage of amino acids and uncoupling of ‘oxidative phosphorylation’ have been analyzed as well [38]. Studies were also conducted to check the effect of fatty acids on certain microbes. The results indicated that the inhibitory functioning of a non-metabolized fatty acid on E. coli is built upon the biological metabolous acts and also on the stability of the outer membrane [39]. This background familiarity points out that PUFAs as well as their metabolites were designated as the ‘bioactive lipids’ suggestive of a meaningful role. Other studies were done on the microbes such as Staphylococcus aureus and coagulase-negative Staphylococci; group A Streptococci existent on the human being’s skin surface displayed that these are comprehensively inefficient or fruitless in a way to root any infection by the skin surface lipids; chiefly, UFA Streptococcus was seen to expire within 5 min after they were exposed to OA (oleic acid) [40]. The reason behind it was the change in the stability of their outer sheath on exposure to the acid [39].
Influence of DHA and EPA
‘Saturated fatty acids’ with either long or short chains implied almost no or a very little anti-viral effect at the most immense concentration vis-a-vis medium-chained saturated and UFA with long chains. The latter were all eminently effective against tackling the enveloped viruses, even though the extent of fatty acid is mandatory for maximal viral inactivation heterogeneous by a huge factor of 20 times [41]. Monoglycerides of these FAs implied a high anti-viral action, in a few occurrences at an amount that is ten times reduced than that of the free FAs. Anti-viral fatty acids were endowed to affect the ‘viral envelope,’ causing leaking out and, at larger concentrations, an entire degradation of envelope and the viral particles [36]. These also facilitated dissolution of the sheaths, i.e., plasma membrane of tissue culture cells giving rise to lysis and death of the cell.
Studies have also shown that linoleic acid (LNA) and its derivatives EPA and DHA are omega-3 fatty acids which are generally recognized for their profitable ramifications on our well-being and status of health. Studies also depicted that DHA and EPA have disparate effects on the behavior of leukocytes such as ‘phagocytosis, chemotactic response, and cytokine production’ [42].
Even so, the efficacy of these fatty acids and their metabolites on microorganism’s sheaths and their antioxidant characteristics are shown to obstruct the development of microbes. This proves that in this way the human welfare can be achieved. Immune cells in humans are ordinarily plenteous in arachidonic acid; however, this, along with EPA and DHA contents, could be revised via their oral administration [43]. Hence, omega-3 fatty acids could be studied and considered a substitution or adjunctive remedial agents. This all is only because of their antimicrobial and immunomodulatory functions. It is anyways known that a continuing nutritional supplementation of omega-3 fatty acids is more favorable [44]. It is seen to lower swellings and the severity of illness and enhance the survival rates, analogously to omega-6 fatty acids [34].
Cholesterol 25-hydroxylase (CH25H) and non-enveloped viruses
‘Interferon-stimulating gene supposedly gives rise to a reticulum-associated sheath protein called CH25H, and it is capable of hindering duplication of viruses [23]. This study was conducted on the encephalomyocarditis virus (EMCV), a non-enveloped ssRNA virus. But the repercussions of the same on EMCV are not precisely explicated [45].
This specific viral species spreads the infection to many mammals, giving rise to many diseases like inflammation of the brain: encephalitis, other neurological illness, diabetic problems, reproduction-related disorders, problems in the heart, etc. [46].
To combatant the emerging viral infections, approaches like using fatty acids have unfurled. In the last few years, FA relevance as ‘drug delivery adjuvants’ is realized and biolipid is yet another approach showing promising results. The quest for molecules that can block the virus coupling to the human cell requires knowledge of binding modes of viral peptides and human receptors. Hence, blocking the attachment site of the virus in the upper respiratory tract is a preventive measure against the viral particle. The aftermath reveals that FAs, taken orally or intravenously, lead to rejuvenation from contagious illnesses and might prevent these infections or provide resistance [8, 47–49].
Concluding remarks and prospects for future research
To emerge, combatant approaches like using fatty acids have unfurled in the last few years and the role of fatty acids as ‘drug delivery adjuvants’ is realized. Biolipid is yet another approach showing promising results. The quest for molecules that can block the virus coupling to the human cell requires foreknowledge of binding modes of viral peptides and human receptors. Hence, blocking the attachment site of the virus in the upper respiratory tract is a preventive measure against the viral particle. The aftermath reveals that FAs, taken orally or intravenously, led to rejuvenation from contagious illnesses and might prevent these infections or provide resistance.
Acknowledgements
This work was supported by the Department of Biotechnology (DBT), New Delhi, India, under Project Grant No. BT/PR/15650/AAQ/3/815/2016.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Bleibtreu A, Bertine M, Bertin C, Houhou-Fidouh N, Visseaux B. Focus on middle east respiratory syndrome coronavirus (MERS-CoV) Med Mal Infect. 2020;50(3):243–251. doi: 10.1016/j.medmal.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babcock GJ, Esshaki DJ, Thomas WD, Jr, Ambrosino DM. Amino Acids 270 to 510 of the severe acute respiratory syndrome coronavirus spike protein are required for interaction with receptor. J Virol. 2004;78(9):4552–4560. doi: 10.1128/JVI.78.9.4552-4560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee A, Mossman K, Miller M. Bat influenza viruses: making a double agent of MHC class II. Trends Microbiol. 2020;28(9):703–706. doi: 10.1016/j.tim.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belur LR, Michael W. Incorporation of palmitic acid or oleic acid into macrophage membrane lipids exerts differential effects on the function of normal mouse peritoneal macrophages. Biochim Biophysca Acta Lipids Lipid Metab. 1984;792(2):141–148. doi: 10.1016/0005-2760(84)90215-7. [DOI] [PubMed] [Google Scholar]
- 5.Bordi L, Piralla A, Lalle E, Giardina F, Colavita F, Tallarita M, Sberna G, Novazzi F, Meschi S, Castilletti C, Brisci A, Minnucci G, Tettamanzvi V, Baldanti F, Capobianchi MR. Rapid and sensitive detection of SARS-CoV-2 RNA using the Simplexa™ COVID-19 direct assay. J Clin Virol. 2020;128:104416. doi: 10.1016/j.jcv.2020.104416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imai Y, Kuba K, Ohto-Nakanishi T, Penninger JM. Angiotensin-converting enzyme 2 (ACE2) in disease pathogenesis. Circ J. 2020;74(3):405–410. doi: 10.1253/circj.CJ-10-0045. [DOI] [PubMed] [Google Scholar]
- 7.Carocci M, Bakkli-Kasimi L. The encephalomyocarditis virus. Virulence. 2012;3(4):351–367. doi: 10.4161/viru.20573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimitrov DS. The secret life of ACE2 as a receptor for the SARS virus. Cell. 2003;115(6):652–653. doi: 10.1016/S0092-8674(03)00976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Speert DP, Wannamaker LW, Clawson CC. Bactericidal effect of oleic acid on group a streptococci: mechanism of action. Infect Immun. 1979;26(3):1202–1210. doi: 10.1128/iai.26.3.1202-1210.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calder PC. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2007;77(5–66):327–335. doi: 10.1016/j.plefa.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Fay JP, Farias R. Inhibitory action of a non-metabolizable fatty acid on the growth of Escherichia coli: role of metabolism and outer membrane integrity. J Bacteriol. 1977;132(3):790–795. doi: 10.1128/jb.132.3.790-795.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest. 2003;111(9):1265–1273. doi: 10.1172/JCI18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorjão R, Azevedo-Martins AK, Rodrigues HG, Abdulkader F, Arcisio-Miranda M, Procopio J, Curi R. Comparative effects of DHA and EPA on cell function. Pharmacol Ther. 2009;122(1):56–64. doi: 10.1016/j.pharmthera.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Gunduz F, Aboulnasr FM, Chandra PK, Hazari S, Poat B, Baker DP, Balart LA, Dash S. Free fatty acids induce ER stress and block antiviral activity of interferon alpha against hepatitis C virus in cell culture. Virol J. 2012;3(9):1–12. doi: 10.1186/1743-422X-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinojosa CA, Gonzalev-Juarbe N, Rahman MM, Fernandes G, Orihuela CJ, Restrepo MI. Omega-3 fatty acids in contrast to omega-6 protect against pneumococcal pneumonia. Microb Pathog. 2020;141:103979. doi: 10.1016/j.micpath.2020.103979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Husson M-O, Ley D, Portal C, Gottrand M, Hueso T, Desseyn J-L, Gottrand F. Modulation of host defence against bacterial and viral infections by omega-3 polyunsaturated fatty acids. J Infect. 2016;73(6):523–535. doi: 10.1016/j.jinf.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Kakhki RK, Kakhki MK, Neshani A. COVID-19 target: a specific target for novel coronavirus detection. Gene Rep. 2020;20:100740. doi: 10.1016/j.genrep.2020.100740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katdare A, Thakkar S, Dhepale S, Khunt D, Misra M. Fatty acids as essential adjuvants to treat various ailments and their role in drug delivery: a review. Nutrition. 2019;65:138–157. doi: 10.1016/j.nut.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar P, Lee J-H, Beyenal H, Lee J. Fatty acids as antibiofilm and antivirulence agents. Trends Microbiol. 2020;28(9):763–768. doi: 10.1016/j.tim.2020.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Li S, Li L, Zhu H, Shi M, Fan H, Gao YN, Wang XW, Jiang P, Bai J. Cholesterol 25-hydroxylase inhibits encephalomyocarditis virus replication through enzyme activity-dependent and independent mechanisms. Vet Microbiol. 2020;245:108658. doi: 10.1016/j.vetmic.2020.108658. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Liu S, Zhang S, Ju Q, Zhang S, Yang Y, Wang H. Current treatment approaches for COVID-19 and the clinical value of transfusion-related technologies. Transfus Apher Sci. 2020;59(5):102839. doi: 10.1016/j.transci.2020.102839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi L, Mao C, Xu Z, Zhang L. Angiotensin-converting enzymes and drug discovery in cardiovascular diseases. Drug Discov Today. 2010;15(9–10):332–341. doi: 10.1016/j.drudis.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moletta L, Pierobon ES, Capovilla G, Costantini M, Salvador R, Merigliano S, Valmasoni M. International guidelines and recommendations for surgery during Covid-19 pandemic: a systematic review. Int J Surg. 2020;79:180–188. doi: 10.1016/j.ijsu.2020.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mousavizadeh L, Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis. J Microbiol Immunol Infect. 2020. (In Press Corrected Proof). [DOI] [PMC free article] [PubMed]
- 28.Petrosillo N, Viceconte G, Ergonul O, Ippolito G, Petersen E. COVID-19, SARS and MERS: Are they closely related? Clin Microbiol Infect. 2020;26(6):729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang JP-C, Pariante CM, Su K-P. Omega-3 fatty acids in the psychological and physiological resilience against COVID-19. Prostaglandins Leukot Essent Fatty Acids. 2020;161:102177. doi: 10.1016/j.plefa.2020.102177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shakoor H, Feehan J, Al Dhaheri AS, Ali HI, Platat C, Ismail LC, Apostolopoulos V, Stojanovska L. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas. 2021;143:1–9. doi: 10.1016/j.maturitas.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weill P, Plissonneau C, Legrand P, Rioux V, Thibault R. May omega-3 fatty acid dietary supplementation help reduce severe complications in Covid-19 patients? Biochimie. 2020;179:275–280. doi: 10.1016/j.biochi.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wassall SR, Leng X, Canner SW, Pennington ER, Kinnun JJ, Cavazos AT, Dadoo S, Johnson D, Heberle FA, Katsaras J, Shaikh S. Docosahexaenoic acid regulates the formation of lipid rafts: a unified view from experiment and simulation. Biochim Biophys Acta Biomembr. 2018;1860(10):1985–1993. doi: 10.1016/j.bbamem.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergsson G, Hilmarsson H, Thormar H. Antibacterial, antiviral and antifungal activities of lipids. In: Thormar H, editors. Lipid and essential oils as antmicrobial agents. Chapter 3. Chichester, UK: Wiley; 2010. p. 47–80.
- 34.Thormar H, Isaacs CE, Brown HR, Barshatzky MR, Pessolano T. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob Agents Chemother. 1987;31(1):27–31. doi: 10.1128/AAC.31.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das UN. Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: a review. J Adv Res. 2018;11:57–66. doi: 10.1016/j.jare.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olena A. Researchers find 380 amino acid substitutions between 2019-nCoV and severe acute respiratory syndrome (SARS)-related coronaviruses. https://www.the-scientist.com/news-opinion/scientists-compare-novel-coronavirus-to-sars-and-mers-viruses-67088. 2020.
- 37.Coronavirus disease 2019 (COVID-2019) situation report-48. World Health Organization.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200308-sitrep-48-covid-19.pdf?sfvrsn=16f7ccef_4. 2020.
- 38.Roshanravan N, Ghaffari S, Hedayati M. Angiotensin converting enzyme-2 as therapeutic target in COVID-19. Diabetes Metab Syndr. 2020;14(4):637–639. doi: 10.1016/j.dsx.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saghazadeh A, Rezaei N. Towards treatment planning of COVID-19: rationale and hypothesis for the use of multiple immunosuppressive agents: anti-antibodies, immunoglobulins, and corticosteroids. Int Immunopharmocol. 2020;84:106560. doi: 10.1016/j.intimp.2020.106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sands JA, Landin P, Auperin D, Reinhardt A. Enveloped virus inactivation by fatty acid derivatives. Antimicrob Agents Chemother. 1979;15(1):134–136. doi: 10.1128/AAC.15.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santos PCJL, Krieger JE, Pereira AC. Renin-angiotensin system, hypertension, and chronic kidney disease. J Pharmacol Sci. 2012;120(2):77–88. doi: 10.1254/jphs.12R03CR. [DOI] [PubMed] [Google Scholar]
- 42.Anand KB, Karade S, Sen S, Gupta RM. SARS-CoV-2: Camazotz’s curse. Med J Armed Forces India. 2020;76(2):136–141. doi: 10.1016/j.mjafi.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Struck A-W, Axmann M, Pfefferle S, Drosten C, Meyer B. A hexapeptide of the receptor-binding domain of SARS corona virus spike protein blocks viral entry into host cells via the human receptor ACE2. Antiviral Res. 2012;94(3):288–296. doi: 10.1016/j.antiviral.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swanson D, Block R, Mousa SA. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr. 2012;3(1):1–7. doi: 10.3945/an.111.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vansteenkiste K, Limbergen TV, Decaluwé R, Tignon M, Cay B, Maes D. Clinical problems due to encephalomyocarditis virus infections in two pig herds. Porc Health Manag. 2016;2(1):19. doi: 10.1186/s40813-016-0036-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villanueva RA, Rouille Y, Dubuisson J. Interactions between virus proteins and host cell membranes during the viral life cycle. Int Rev Cytol. 2005;245:171–244. doi: 10.1016/S0074-7696(05)45006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P, Meng J, Zhu Z, Zhang Z, Wang J, Sheng J, Quan L, Xia Z, Tan W, Cheng G, Jiang T. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27(3):325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie M, Chen Q. Insight into 2019 novel coronavirus—an updated interim review and lessons from SARS-CoV and MERS-CoV. Int J Infect Dis. 2020;94:119–124. doi: 10.1016/j.ijid.2020.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Y. Unveiling the origin and transmission of 2019-nCoV. Trends Microbiol. 2020;28(4):239–240. doi: 10.1016/j.tim.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]