Abstract
The Panel on Food additives and Flavourings of the EFSA was requested to update Flavouring Group Evaluation 13 using the Procedure as outlined in Commission Regulation (EC) No 1565/2000, to include an evaluation of the flavouring substances 2‐ethyl‐5‐methylfuran [FL‐no: 13.125] and 2‐octylfuran [FL‐no: 13.162]. FGE.13 revision 3 (FGE.13Rev3) deals with 26 flavourings substances of which 24 have been already evaluated to be of no safety concern. For [FL‐no: 13.125] and [FL‐no: 13.162], a concern for genotoxicity was raised in FGE.13Rev1. This concern could be ruled out based on new genotoxicity data on supporting substances in FGE.67Rev3. Subsequently, [FL‐no: 13.125 and 13.162] were evaluated, through a stepwise approach that integrates intake from current uses, toxicological threshold of concern (TTC), and available data on metabolism and toxicity, along the B‐side of the Procedure, making use of a BMDL of 8.51 mg/kg body weight (bw) per day. The Panel derived this BMDL from an oral subchronic toxicity study with the supporting substance 2‐pentylfuran [FL‐no: 13.059]. Using this BMDL, for [FL‐no: 13.125 and 13.162], adequate margins of safety were calculated based on the MSDI approach. The Panel concluded that the 26 candidate substances in FGE.13Rev3 do not give rise to safety concerns at their levels of dietary intake, when estimated on the basis of the MSDI approach. Adequate specifications for the materials of commerce have been provided for all 26 substances. Data on uses and use levels are needed for [FL‐no: 13.130]. For 21 flavouring substances [FL‐no: 13.011, 13.102, 13.108, 13.113, 13.114, 13.122, 13.125, 13.127, 13.129, 13.132, 13.133, 13.135, 13.136, 13.139, 13.141, 13.143, 13.146, 13.149, 13.162, 13.178 and 13.185], the mTAMDI intake estimates are above the TTC for their structural class and more reliable data on uses and use levels are required to finalise their evaluation.
Keywords: Furfuryl, Furan, Flavourings, sulfur‐substituted, disulfide, trisulfide, thioester, FGE.13
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
The use of flavourings is regulated under Regulation (EC) No 1334/2008 of the European Parliament and Council of 16 December 20081 on flavourings and certain food ingredients with flavouring properties for use in and on foods. On the basis of Article 9(a) of this Regulation, an evaluation and approval are required for flavouring substances.
The Union list of flavourings and source materials was established by Commission Implementing Regulation (EC) No 872/2012.2 The list contains flavouring substances for which the scientific evaluation should be completed in accordance with Commission Regulation (EC) No 1565/2000.3
1. FGE.67Rev1
On 6 July 2011, the EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) adopted an opinion on Flavouring Group Evaluation 67, Revision 1 (FGE.67Rev.1): Consideration of 40 furan‐substituted aliphatic hydrocarbons, alcohols, aldehydes, ketones, carboxylic acids and related esters, sulfides, disulfides and ethers evaluated by JECFA at the 65th meeting (JECFA, 2006b) and re‐evaluated at the 69th meeting (JECFA, 2009c).4
In its opinion, the Panel concluded that for the substances [FL‐no: 13.059, 13.069, 13.103, 13.106 and 13.148] additional toxicity/genotoxicity data are required.
2. FGE.13Rev2
On 6 July 2011, the EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) adopted an opinion on Flavouring Group Evaluation 13, Revision 2 (FGE.13Rev2): Furfuryl and furan derivatives with and without additional side‐chain substituents and heteroatoms from chemical group 14.5
In its opinion the Panel stated that it has reservations for the substances [FL‐no: 13.125 and 13.162] which could not be evaluated through the procedure due to concern of genotoxicity in vitro. For these two substances additional data are required.
On 29 April 2014, the European Flavour Association (EFFA) submitted additional data on 2‐pentylfuran [FL‐no: 13.059] from FGE.67, which is relevant to the safety assessment of this group of 7 alkylfurans.
1.1.2. Terms of Reference as provided by the requestor
The European Commission requests the European Food Safety Authority (EFSA) to evaluate this new information and, depending on the outcome, proceed to the full evaluation of these flavouring substances in accordance with Commission Regulation (EC) No 1565/2000.
1.2. Interpretation of the Terms of Reference
In FGE.67Rev1, the CEF Panel agreed with JECFA that the substances [FL‐no: 13.045, 13.054, 13.059, 13.066, 13.069, 13.070, 13.083, 13.101, 13.103, 13.105, 13.106, 13.138, 13.148, 13.163] cannot be evaluated through the Procedure, based on concerns with respect to genotoxicity. These substances are structurally related to [FL‐no: 13.125 and 13.162] evaluated in FGE.13Rev2 by the CEF Panel, who identified the same concern for genotoxicity as for the structurally related alkylfurans in FGE.67Rev2. Therefore, for all substances indicated here for FGE.67Rev2 and FGE.13Rev2, additional data were required.
Industry has submitted data on the representative substances 2‐pentylfuran [FL‐no: 13.059] and 2‐acetylfuran [FL‐no: 13.054]. Data on 2‐pentylfuran [FL‐no: 13.059] are also applicable for the candidate substances [FL‐no: 13.125 and 13.162] in FGE.13Rev3 since these are structurally related.
Based on new toxicity data on supporting substances in FGE.67Rev3, the European Commission requests EFSA to carry out a safety assessment on the substances 2‐ethyl‐5‐methylfuran [FL‐no: 13.125] and 2‐octylfuran [FL‐no: 13.162] in accordance with Commission Regulation (EC) No 1565/20003. The rest of the substances covered by the current mandate [FL‐no: 13.045, 13.054, 13.059, 13.066, 13.069, 13.070, 13.083, 13.101, 13.103, 13.105, 13.106, 13.138, 13.148 and 13.163] will be considered in FGE.67Rev3.
1.3. History of the evaluation of the substances in FGE.13
The flavouring group evaluation 13 (EFSA, 2005) included 18 flavouring substances from chemical group 14 (Annex I of Commission Regulation (EC) No 1565/20003). All the candidate substances are furan derivatives and can be divided into two subgroups, depending on the absence/presence of sulfur‐containing substituents.
The nine candidate substances in subgroup 1 are furfuryl alcohol derivatives such as esters of furfuryl alcohol [FL‐no: 13.127, 13.129, 13.132, and 13.133] or furanacrylic acid [FL‐no: 13.011], furoic acid [FL‐no: 13.136] and its esters [FL‐no: 13.102 and 13.122] and 5‐hydroxymethylfurfuraldehyde [FL‐no: 13.139].
The nine candidate substances of subgroup 2 are all sulfur‐containing furan derivatives. The sulfur is present in the molecule as a free thiol group [FL‐no: 13.108 and 13.149], as thioethers [FL‐no: 13.114, 13.145 and 13.124], as disulfides [FL‐no: 13.113, 13.144 and 13.178] or as trisulfide [FL‐no: 13.146].
The 18 candidate substances are closely structurally related to 47 flavouring substances (supporting substances) evaluated at the 55th and 59th JECFA meetings (JECFA, 2001a,b, 2002, 2003) in the groups of ‘Furfuryl alcohol and related substances’ and ‘sulfur substituted Furan derivatives’.
The AFC Panel considered that except for the flavouring substance 5‐hydroxymethylfurfuraldehyde [FL‐no: 13.139], the in vitro and in vivo data available did not give rise to concern with respect to genotoxicity of the remaining eight flavouring substances included in subgroup 1. Accordingly, the AFC Panel applied the Procedure (B‐side) to eight substances and indicated that the Procedure cannot be applied to [FL‐no: 13.139], pending submission of in vivo genotoxicity data.
Considering that the seven candidate substances of subgroup 1 (non‐sulfur‐containing) are metabolised to yield furfural and furoic acid, the toxicity of the esters of furfuryl alcohol [FL‐no: 13.127, 13.129, 13.132 and 13.133], furoic acid [FL‐no: 13.102 and 13.122] and furanacrylic acid [FL‐no: 13.011] is expected to be similar to that of the structurally related supporting substance furfural [FL‐no: 13.018] and of the candidate substance 2‐furoic acid [FL‐no: 13.136], which is the major metabolite of furfural. For furfural [FL‐no: 13.018], the AFC Panel (EFSA, 2004b) established an acceptable daily intake (ADI) value of 0.5 mg/kg bw based on an NOAEL (no observed adverse effect level) for hepatotoxicity in a 90‐day toxicity study in rats of 54 mg/kg bw per day to which a safety factor of 100 was applied. The estimated daily per capita intakes based on the MSDI approach of candidate substances in subgroup 1 were more than 500‐fold below the ADI value.
Since no toxicity data were available on the nine sulfur‐containing candidate substances in subgroup 2, the relevant NOAEL values were derived from structurally related supporting substances.
The AFC Panel concluded at step B4 of the Procedure that 17 candidate substances included in FGE.13 pose no safety concern when they are used as flavouring substances at the estimated levels of intake based on the MSDI approach.
The AFC Panel considered that for 10 of the 17 flavouring substances taken through the Procedure, the intakes, estimated on the basis of the mTAMDI, exceeded the relevant threshold for their structural class, to which the flavouring substance was assigned. Therefore, for these 10 substances, more reliable exposure data were required.
The AFC Panel requested information on geometrical isomerism/chirality for the substances [FL‐no: 13.011, 13.127 and 13.129].
The first revision of FGE.13, FGE.13Rev1, included the assessment of seven additional candidate substances [FL‐no: 13.125, 13.135, 13.141, 13.143, 13.162, 13.185 and 13.199]. Therefore, 25 substances were evaluated in FGE.13Rev1 (EFSA CEF Panel, 2010).
The evaluation of the flavouring substance 2,5‐dimethyl‐3‐(methyldithio)furan [FL‐no: 13.113] was revised because a supporting substance [FL‐no: 13.055] was identified with better structural similarity to [FL‐no: 13.113] than the one that was used in FGE.13. This new supporting substance would provide a better basis for the assessment of [FL‐no: 13.113] and with its NOAEL of 5 mg/kg bw per day, an adequate margin of safety was calculated for [FL‐no: 13.113].
For 12 flavouring substances already considered in FGE.13, the classification according to Cramer et al., 1978 was revised. These revisions were necessary to create consistency with the evaluations in FGEs 65 and 67. For the substances involved, the final conclusions were not changed.
In addition, the CEF Panel noted that the substance [FL‐no: 13.178] is synonymous with [FL‐no: 13.192],6 which was evaluated by the JECFA (JECFA no 1542) at its 69th meeting (JECFA, 2009a). For this substance in the JECFA evaluation, an MSDI for Europe of 0.24 μg per capita per day was given. This figure, which is higher and more recent than the exposure estimate in FGE.13 (0.0012 μg per capita per day), was used in FGE.13Rev1.
Based on data on the genotoxic activity of 5‐hydroxymethylfurfural [FL‐no: 13.139] there was sufficient evidence for a genotoxic potential in vitro, which could not be ruled out, due to a lack of genotoxicity data in vivo. Therefore, [FL‐no: 13.139] was not evaluated through the Procedure. For the remaining substances in subgroup Ia, the data available did not indicate a concern for genotoxicity and these were evaluated through the Procedure.
For the two candidate substances in subgroup Ib [FL‐no: 13.125 and 13.162], metabolism studies on closely related substances indicated a potential for DNA binding of metabolites. In addition, in several in vitro studies with structurally related substances, indications for genotoxic activity were obtained. These data precluded the evaluation of [FL‐no: 13.125 and 13.162], through the Procedure.
There is an absence of data on genotoxicity for 14 sulfur‐containing candidate substances included in main group II, or on the related supporting substances. Nevertheless, this did not preclude the evaluation of these substances through the Procedure.
No toxicity data were available on the sulfur‐containing furan‐derived candidate substances included in main group II. However, results from toxicity studies on 14 structurally related supporting substances and one related substance have been reported. Many of the available studies were performed either with a single dose level or multiple dose levels that produced no effects; the doses producing no adverse effects ranged from 0.45 to 10 mg/kg per day, and based on this information for each of the 14 substances in group II, an adequate margin of safety (MOS) was calculated at step B4 of the Procedure. For one substance [FL‐no: 13.114], the evaluation at step B4 was better described in FGE.13Rev1, since this had not been made explicit in FGE.13.
For 18 of the candidate substances, evaluated using the Procedure, the mTAMDI was above the threshold of concern; therefore, the CEF Panel indicated that more reliable intake data were needed.
The CEF Panel had reservations for [FL‐no: 13.185 and 13.199] (missing data on stereoisomerism) and for [FL‐no: 13.125, 13.139 and 13.162] which could not be evaluated through the Procedure due to concern of genotoxicity in vitro. For these five substances, additional data were required. For the remaining 20 substances [FL‐no: 13.011, 13.102, 13.108, 13.113, 13.114, 13.122, 13.124, 13.127, 13.129 13.132, 13.133, 13.135, 13.136, 13.141, 13.143, 13.144, 13.145, 13.146, 13.149 and 13.178], the CEF Panel concluded that they would be of ‘No safety concern at estimated levels of intake as flavouring substances’ based on the MSDI approach.
In FGE.13Rev2, two flavouring substances were added: furfuryl butyrate [FL‐no: 13.130] and 2‐methyl‐5‐propionylfuran [FL‐no: 13.155]. Therefore, 27 substances were considered in FGE.13Rev2.
For furfuryl butyrate [FL‐no: 13.130], JECFA did evaluate the specifications, but did not perform a safety evaluation. No toxicity or metabolism data and no use levels data were submitted for this substance.
In FGE.13Rev2, the genotoxicity concern for 5‐hydroxymethylfurfural [FL‐no: 13.139] was ruled out, and based on new toxicity data, the substance was evaluated via the Procedure to be of no safety concern.
New information from industry on the stereoisomeric composition of two candidate substances [FL‐no: 13.185 and 13.199] were also included in FGE.13Rev2.
In FGE.13Rev1, the substance [FL‐no: 13.135] (1‐(2‐furfurylthio)propanone) was incorrectly allocated to a subgroup of thioesters and evaluated as the thioester S‐furfuryl‐propanethioate. In FGE.13Rev2, the candidate substance [FL‐no: 13.135] was allocated to and re‐evaluated in subgroup IIa, consisting of sulfides. Since there were no further thioester candidate substances left in this FGE, the respective subgroup was deleted.
One α,β‐unsaturated ketone 2‐methyl‐5‐propionylfuran [FL‐no: 13.155] and seven supporting substances (including 2‐acetylfuran [FL‐no: 13.054]) from FGE.19 subgroup 4.5) were added in a new subgroup (Ib) of FGE.13Rev2. In the course of the assessment, the CEF Panel concluded that the α, β‐unsaturated structure in conjugation with an aromatic ring system, which is present in these eight substances as well as in acetophenone, is not considered a structural alert for genotoxicity; therefore, subgroup 4.5 was not included in the updated list of FGE.19 substances (EFSA, 2008). Nevertheless, the experimental genotoxicity data indicate that the supporting substance 2‐acetylfuran [FL‐no: 13.054] may give rise to DNA damage, which may result in chromosomal aberrations rather than gene mutations. The formation of DNA‐reactive metabolites may be anticipated (EFSA CEF Panel, 2011). The available genotoxicity data are sufficiently strong to raise a concern, which would preclude the evaluation of the substance [FL‐no: 13.155] through the Procedure.
Extensive ring opening with formation of intermediates reactive towards DNA has been reported for 2‐alkyl‐substituted furans in subgroup Ic that also includes the newly added candidate substances [FL‐no: 13.125 and 13.162]. In addition, these compounds may be metabolised to ketones (as for [FL‐no: 13.155] in subgroup Ib), for which genotoxicity may be anticipated.
The CEF Panel had reservations for three substances [FL‐no: 13.125, 13.155 and 13.162] which could not be evaluated through the Procedure due to concern for genotoxicity in vitro. For these three substances, additional data were required.
Since the last revision of FGE.13 (FGE.13Rev2), industry has withdrawn their support to 2‐methyl‐5‐propionylfuran [FL‐no: 13.155] (DG SANCO, 2012), and therefore, this substance will not be further considered in this FGE. Because the withdrawal came before publication of the Union List2, this substance was not included in this list. However, since it was included in the ‘Register’ (Commission Decision 1999/217/EC), for the sake of completeness, it is still mentioned in this revision 3 of FGE.13.
For 24 candidate substances [FL‐no: 13.011, 13.102, 13.108, 13.113, 13.114, 13.122, 13.124, 13.127, 13.129, 13.130, 13.132, 13.133, 13.135, 13.136, 13.139, 13.141, 13.143, 13.144, 13.145, 13.146, 13.149, 13.178, 13.185 and 13.199], the CEF Panel concluded, in FGE.13Rev2, that they would be of ‘No safety concern at estimated levels of intake as flavouring substances’ based on the MSDI approach.
For 19 of the 24 substances evaluated through the Procedure [FL‐no: 13.011, 13.102, 13.108, 13.113, 13.114, 13.122, 13.127, 13.129, 13.132, 13.133, 13.135, 13.136, 13.139, 13.141, 13.143, 13.146, 13.149, 13.178 and 13.185], mTAMDI values were above the threshold of concern for the respective Cramer class. For substance [FL‐no: 13.130], no use levels were provided.
| FGE | Adopted | Link | Substances |
|---|---|---|---|
| FGE.13 | 27.4.2005 | https://www.efsa.europa.eu/en/efsajournal/pub/215 | 18 |
| FGE.13Rev1 | 26.11.2009 | http://www.efsa.europa.eu/en/efsajournal/pub/1403 | 25 |
| FGE.13Rev2 | 6.7.2011 | https://www.efsa.europa.eu/en/efsajournal/pub/2313 | 27 |
| FGE.13Rev3 | 15.12.2020 | https://www.efsa.europa.eu/en/efsajournal/pub/6386 | 26 |
The present revision of FGE.13, FGE.13Rev3 concerns the evaluation of two alkylfurans, namely 2‐ethyl‐5‐methylfuran [FL‐no: 13.125] and 2‐octylfuran [FL‐no: 13.162] based on new genotoxicity and toxicity data submitted on a supporting substance, 2‐pentylfuran [FL‐no: 13.059], from FGE.67Rev3.
The present revision of FGE.13 (FGE.13Rev3) deals with 26 flavourings substances of which 24 have been already evaluated to be of no safety concern in the previous revisions of FGE.13. A summary of the history of the evaluation of the substances in FGE.13 is presented in Figure 1.
Figure 1.
Summary of the history of evaluation of the substances in FGE.13
1.4. Presentation of the substances in FGE.13Rev3
All candidate substances in FGE.13Rev3 are furan derivatives and can be divided into two main groups (I and II), depending on the absence/presence of sulfur‐containing substituents. Within these two main groups, a further differentiation in subgroups is introduced, depending on the nature of the ring substituents and the number and position of the sulfur‐containing substituents. The subgrouping of the candidate substances is shown below. The candidate substances are structurally related to 53 flavouring substances (supporting substances) evaluated by the JECFA at their 55th, 59th, 65th, 69th, 76th and 86th meetings (JECFA, 2001a,b, 2002, 2003, 2006b, 2009a, 2012, 2019) and by EFSA (EFSA, 2004b).
Only group Ic, which includes the substances evaluated in FGE.13Rev3 is described. No descriptions are given for groups Ia, Ib, IIa, IIb, IIc and IId which can be found in FGE.13Rev2 (EFSA CEF Panel, 2011).
Main group I. Non‐sulfur‐containing Furan Derivatives
Subgroup Ic: Alkyl‐substituted furans
The two candidate substances in subgroup Ic are alkyl substituted furans [FL‐no: 13.125 and 13.162] without any further functional groups. These two candidate substances are closely related to four supporting substances [FL‐no: 13.059, 13.069, 13.106, 13.148] evaluated at the 69th, 76th and 86th JECFA meeting (JECFA, 2009a, 2012, 2019) in a group of ‘Furan‐substituted substances’. These four supporting substances have been considered by EFSA in FGE.67Rev3 (EFSA FAF Panel, 2021). Previously, for this subgroup also [FL‐no: 13.029, 13.030, 13.092 and 13.103] were identified as supporting substances for the two candidates in subgroup Ic in FGE.13Rev2. However, these substances are no longer supported by industry (see FGE.67Rev3). Therefore, they have been deleted from subgroup IV of FGE.67Rev3. The substances [FL‐no: 13.029, 13.030, 13.092] were already deleted from the Union List.7
The candidate substances considered in FGE.13Rev3 and the supporting substances for each subgroup are reported in Table 1. In the last column of Table 1, the status of the evaluation by EFSA of the supporting substances and of individual members of FGE.13 is presented, based on the evaluation in FGE.13Rev2, i.e. before consideration of the information received by EFSA that leads to the present revision 3 of this FGE. In the meantime some substances have been withdrawn by industry for use as flavourings substances. This is also taken into account in the table.
Table 1.
FGE.13Rev3 – candidate and supporting substances divided into subgroups of related chemical structures. Substances listed in bold are the candidate substances in this FGE. The supporting substances from the 55th, 59th, 65th, 69th, 76th and 86th JECFA meetings and EFSA (EFSA, 2004b) are in normal type face
| FL‐no JECFA‐no | EU Register name | Structural formula | EFSA status according to FGE.13Rev2 |
|---|---|---|---|
| Main Group I: non‐sulfur‐containing furan derivatives | |||
| Subgroup Ia Structurally Related to Furfuryl alcohol | |||
| 13.011 | Ethyl furfuracrylate |

|
FGE.13 Rev1 – no safety concern |
| 13.102 | Butyl 2‐furoate |
|
FGE.13 – no safety concern |
| 13.122 | Ethyl 2‐furoate |

|
FGE.13 – no safety concern |
| 13.127 | Furfuryl 2‐methylbutyrate |
|
FGE.13 Rev1 – no safety concern |
| 13.129 | Furfuryl but‐2-enoate |

|
FGE.13 Rev1 – no safety concern |
| 13.130 | Furfuryl butyrate |

|
FGE.13 Rev2 – no safety concern |
| 13.132 | Furfuryl hexanoate |

|
FGE.13 – no safety concern |
| 13.133 | Furfuryl isobutyrate |

|
FGE.13 – no safety concern |
| 13.136 | 2‐Furoic acid |
|
FGE.13 – no safety concern |
| 13.139 | 5‐Hydroxymethylfurfuraldehyde |
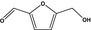
|
FGE.13 Rev2 – no safety concern |
|
13.001 745 |
5‐Methylfurfuralb |
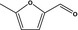
|
FGE.66 Rev1 – no safety concern |
|
13.002 746 |
Methyl 2‐furoateb |
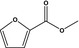
|
FGE.66 Rev1 – no safety concern |
|
13.003 747 |
Propyl 2‐furoateb |
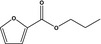
|
FGE.66 Rev1 – no safety concern |
|
13.018 450 |
Furfuralb |
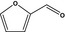
|
FGE.66 Rev1 – no safety concern |
|
13.019 451 |
Furfuryl alcoholb |
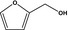
|
FGE.66 Rev1 – no safety concern |
|
13.057 743 |
Furfuryl isovalerateb |
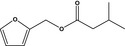
|
FGE.66 Rev1 – no safety concern |
|
13.062 740 |
Furfuryl propionateb |
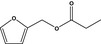
|
FGE.66 Rev1 – no safety concern |
|
13.068 741 |
Furfuryl valerateb |
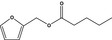
|
FGE.66 Rev1 – no safety concern |
| 13.126 | Furfuryl diethyl acetalb |
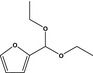
|
Supporting substance from the Register and evaluated by the AFC Panel (EFSA, 2004b). Substance not in the Union List |
|
13.128 739 |
Furfuryl acetateb |
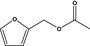
|
FGE.66 Rev1 – no safety concern |
|
13.005 749 |
Hexyl 2‐furoateb |
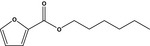
|
FGE.66 Rev1 – no safety concern |
|
13.025 748 |
Pentyl 2‐furoateb |
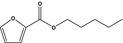
|
FGE.66 Rev1 – no safety concern |
|
13.038 752 |
2‐Phenyl‐3‐carbethoxyfuranb |
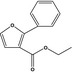
|
FGE.66 Rev1 – no safety concern |
|
13.067 742 |
Furfuryl octanoateb |
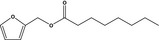
|
FGE.66 Rev1 – no safety concern |
|
13.073 750 |
Octyl 2‐furoateb |
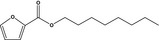
|
FGE.66 Rev1 – no safety concern |
| Subgroup Ib Alkoyl‐substituted furans | |||
| 13.155 | 2‐Methyl‐5-propionylfuran |
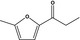
|
Not supported as candidate substance in this FGE (DG SANCO, 2012) and not included in the Union List |
|
13.054 1503 |
2‐Acetylfuranb |

|
To be evaluated in FGE.67Rev3 |
|
13.066 1506 |
3‐Acetyl‐2,5‐dimethylfuranb |
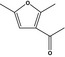
|
No longer supportedd |
|
13.070 1512 |
2‐Hexanoylfuranb |
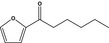
|
To be evaluated in FGE.67Rev3 |
|
13.083 1504 |
2‐Acetyl‐5‐methylfuranb |

|
To be evaluated in FGE.67Rev3 |
|
13.101 1505 |
2‐Acetyl‐3,5‐dimethylfuranb |

|
To be evaluated in FGE.67Rev3 |
|
13.105 1507 |
2‐Butyrylfuranb |
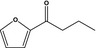
|
To be evaluated in FGE.67Rev3 |
|
13.163 1509 |
2‐Pentanoylfuranb |
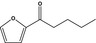
|
To be evaluated in FGE.67Rev3 |
| Subgroup Ic Alkyl‐substituted furans | |||
| 13.125 | 2‐Ethyl‐5-methylfuran |
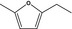
|
To be evaluated in FGE.13 Rev3 |
| 13.162 | 2‐Octylfuran |
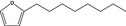
|
To be evaluated in FGE.13 Rev3 |
|
13.029 1488 |
2,5‐Dimethylfuranb |

|
Deleted from ULa |
|
13.030 1487 |
2‐Methylfuranb |

|
Deleted from ULa |
|
13.092 1489 |
2‐Ethylfuranb |
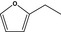
|
Deleted from ULa |
|
13.059 1491 |
2‐Pentylfuranb |
|
To be evaluated in FGE.67Rev3 |
|
13.069 1492 |
2‐Heptylfuranb |

|
To be evaluated in FGE.67Rev3 |
|
13.103 1490 |
2‐Butylfuranb |
|
No longer supportedc |
|
13.106 1493 |
2‐Decylfuranb |
|
To be evaluated in FGE.67Rev3 |
|
13.148 1494 |
3‐Methyl‐2(3‐methylbut‐2‐enyl)furanb |
|
To be evaluated in FGE.67Rev3 |
| Main Group II: Sulfur‐substituted Furan Derivatives | |||
| Subgroup IIa Sulfides | |||
| 13.114 | 2,5‐Dimethyl‐3-(methylthio)furan |
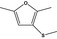
|
FGE.13 – no safety concern |
| 13.124 | Ethyl furfuryl sulfide |
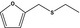
|
FGE.13 – no safety concern |
| 13.135 | 1‐(2-furfurylthio)‐propanone |
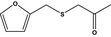
|
FGE.13 Rev1 – no safety concern |
| 13.141 | Methyl (2‐furfurylthio)acetate |
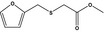
|
FGE.13 Rev1 – no safety concern |
| 13.143 | Methyl 3‐(furfurylthio)propionate |

|
FGE.13 Rev1 – no safety concern |
| 13.145 | Methyl 5‐methylfurfuryl sulfide |
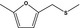
|
FGE.13 – no safety concern |
| 13.199 | 3‐[(2-methyl‐3-furyl)thio]‐butanal |
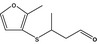
|
FGE.13 Rev2 – no safety concern |
|
13.053 1076 |
Methyl furfuryl sulfideb |

|
FGE.65 Rev1 – no safety concern |
|
13.056 1080 |
Difurfuryl sulfideb |
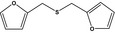
|
FGE.65 Rev1 – no safety concern |
|
13.065 1062 |
2‐Methyl‐5‐(methylthio)furanb |
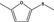
|
FGE.65 Rev1 – no safety concern |
|
13.152 1061 |
2‐Methyl‐3‐(methylthio)furanb |
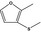
|
FGE.65 Rev1 – no safety concern |
|
13.196 1084 |
4‐(Furfurylthio) pentan‐2‐oneb |
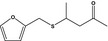
|
FGE.65 Rev1 – no safety concern |
|
13.032 1077 |
Furfuryl isopropyl sulfideb |
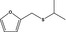
|
FGE.65 Rev1 – no safety concern |
|
13.075 1086 |
2,6‐Dimethyl‐3‐((2‐methyl‐3‐furyl)thio)heptan‐4‐oneb |
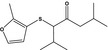
|
FGE.65 Rev1 – no safety concern |
|
13.077 1085 |
3‐((2‐Methyl‐3‐furyl)thio)heptan‐4‐oneb |
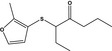
|
FGE.65 Rev1 – no safety concern |
|
13.078 1087 |
4‐((2‐Methyl‐3‐furyl)thio)nonan‐5‐oneb |

|
FGE.65 Rev1 – no safety concern |
|
13.093 1088 |
Ethyl 3‐(2‐furfurylthio)propionateb |
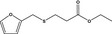
|
FGE.65 Rev1 – no safety concern |
|
13.151 1082 |
2‐Methyl‐3,5 and 6‐(furfurylthio)pyrazineb |
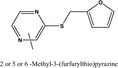
|
FGE.65 Rev1 – no safety concern |
| Subgroup IIb Thiols | |||
| 13.149 | 5‐Methyl‐2-furanmethanethiol |

|
FGE.13 – no safety concern |
| 13.108 | 4,5‐Dihydro‐3-mercapto‐2-methylfuran |
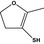
|
FGE.13 – no safety concern |
|
13.026 1072 |
2‐Furanmethanethiolb |
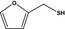
|
FGE.65 Rev1 – no safety concern |
|
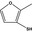
13.055 1060 |
2‐Methylfuran‐3‐thiolb |
|
FGE.65 Rev1 – no safety concern |
|
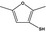
13.071 1063 |
2,5‐Dimethylfuran‐3‐thiolb |
|
FGE.65 Rev1 – no safety concern |
|
13.160 1090 |
2‐Methyltetrahydrofuran‐3‐thiolb |

|
FGE.65 Rev1 – no safety concern |
|
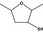
13.193 1091 |
2,5‐Dimethyltetrahydro‐3‐furanthiolb |
|
FGE.65 Rev1 – no safety concern |
| Subgroup IIc Disulfides | |||
| 13.113 | 2,5‐Dimethyl‐3-(methyldithio)furan |
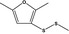
|
FGE.13Rev1 – no safety concern |
| 13.144 | Methyl 5‐methylfurfuryl disulfide |
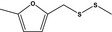
|
FGE.13 – no safety concern |
| 13.178 | 3‐(Furfuryldithio)‐2-methylfuran |
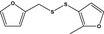
|
FGE.13 – no safety concern |
| 13.185 | 2‐Furfuryl 3‐oxo-2‐butyl disulfide |
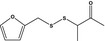
|
FGE.13 Rev2 – no safety concern |
|
13.016 1066 |
bis‐(2‐Methyl‐3‐furyl) disulfideb |
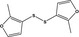
|
FGE.65 Rev1 – no safety concern |
|
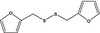
13.050 1081 |
Difurfuryl disulfideb |
|
FGE.65 Rev1 – no safety concern |
|
13.064 1078 |
Methyl furfuryl disulfideb |

|
FGE.65 Rev1 – no safety concern |
|
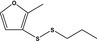
13.082 1065 |
Propyl 2‐methyl‐3‐furyl disulfideb |
|
FGE.65 Rev1 – no safety concern |
|
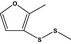
13.079 1064 |
Methyl 2‐methyl‐3‐furyl disulfideb |
|
FGE.65 Rev1 – no safety concern |
|
13.197 1079 |
Furfuryl propyldisulfideb |
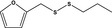
|
FGE.65 Rev1 – no safety concern |
|
13.015 1067 |
bis‐(2,5‐Dimethyl‐3‐furyl) disulfideb |
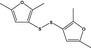
|
FGE.65 Rev1 – no safety concern |
| Subgroup IId Polysulfides | |||
| 13.146 | Methyl furfuryl trisulfide |
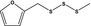
|
FGE.13 – no safety concern |
|
13.017 1068 |
bis‐(2‐Methyl‐3‐furyl) tetrasulfideb |
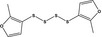
|
FGE.65 Rev1 – no safety concern |
Commission Regulation (EU) No 246/2014 of 13 March 2014 amending Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council as regards removal from the Union list of certain flavouring substances. OJ L74, 14.3.2014, p. 58–60.
Supporting substances for each subgroup.
Letter from DG‐SANTE to EFSA (DG SANTE, 2020a).
Letter from DG‐SANTE to EFSA (DG SANTE, 2020b).
For the sake of completeness, the information on identity of all substances is maintained in various tables of this FGE. Information on specifications is only maintained for the substances which are currently in the Union List (see Appendix B). For substances that are no longer in the Union List, FGE.13Rev2 can be consulted.
A summary of the safety evaluation of the flavouring substances in FGE.13 and further revisions is presented in Appendix D, Table D.1.
Table D.1.
Summary of Safety Evaluation Applying the Procedure for substances in FGE.13Rev3 (based on intakes calculated by the MSDI approach)
| FL‐no | EU Union List chemical name | Structural formula | MSDIa (μg/capita per day) | Classb Evaluation procedure pathc Outcome on the named compound and on the material of commerce | EFSA Comments |
|---|---|---|---|---|---|
| Main Group I: non‐sulfur‐containing furan derivatives | |||||
| Subgroup Ia Structurally related to furfuryl alcohol | |||||
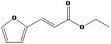
| 13.011 | (E)‐Ethyl furfuracrylate |
|
0.12 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13Rev1 The CAS no. should be changed to 53282‐12‐5 Also evaluated by JECFA as no. 2103 |
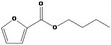
| 13.102 | Butyl 2‐furoate |
|
0.12 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13 |
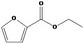
| 13.122 | Ethyl 2‐furoate |
|
0.39 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13 |
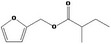
| 13.127 | Furfuryl 2‐methylbutyrate |
|
0.73 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13Rev1 |
| 13.129 | Furfuryl but‐2(E)‐enoate |
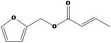
|
0.11 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13Rev1 The CAS no. should be changed to 136678‐63‐2 in the Union list |
|
13.130 759 |
Furfuryl butyrate |
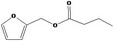
|
0.24 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13Rev2 Also evaluated by JECFA as no. 759 |
| 13.132 | Furfuryl hexanoate |
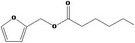
|
0.58 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13 |
| 13.133 | Furfuryl isobutyrate |
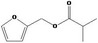
|
0.89 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13 |
| 13.136 | 2‐Furoic acid |
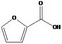
|
0.013 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13 |
| 13.139 | 5‐Hydroxymethylfurfuraldehyde |

|
0.39 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13Rev2 |
| Subgroup Ib Alkoyl‐substituted furans | |||||
| 13.155 | 2‐Methyl‐5‐propionylfuran |
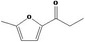
|
0.011 |
Class II No evaluation |
No longer supported by Industry (DG SANCO, 2012) |
| Subgroup Ic Alkyl‐substituted furans | |||||
| 13.125 | 2‐Ethyl‐5‐methylfuran |
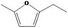
|
0.06 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13Rev3 |
| 13.162 | 2‐Octylfuran |
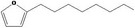
|
0.12 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13Rev3 |
| Main Group II: Sulfur‐substituted furan derivatives | |||||
| Subgroup IIa Sulfides | |||||
| 13.114 | 2,5‐Dimethyl‐3‐(methylthio)furan |
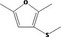
|
0.0024 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13 |
| 13.124 | Ethyl furfuryl sulfide |
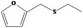
|
0.18 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13 |
| 13.135 | 1‐(2‐Furfurylthio)propanone |
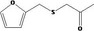
|
0.61 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13Rev1 Also evaluated by JECFA as no. 2096 |
| 13.141 | Methyl (2‐furfurylthio)acetate |
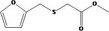
|
0.011 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13Rev1 |
| 13.143 | Methyl 3‐(furfurylthio)propionate |
|
0.011 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
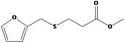
Concluded in FGE.13Rev1 |
| 13.145 | Methyl 5‐methylfurfuryl sulfide |

|
0.0024 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13 |
| 13.199 | 3‐[(2‐Methyl‐3‐furyl)thio]‐butanal |
|
1.2 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
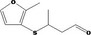
Concluded in FGE.13Rev2 Also evaluated by JECFA as no. 2095 |
| Subgroup IIb Thiols | |||||
| 13.149 | 5‐Methyl‐2‐furanmethanethiol |
|
0.37 |
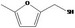
Class III B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13 |
| 13.108 | 4,5‐Dihydro‐3‐mercapto‐2‐methylfuran |
|
37 |
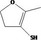
Class III B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13 Also evaluated by JECFA as no. 2097 |
| Subgroup IIc Disulfides | |||||
| 13.113 | 2,5‐Dimethyl‐3‐(methyldithio)furan |

|
0.0012 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13Rev1 |
| 13.144 | Methyl 5‐methylfurfuryl disulfide |
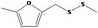
|
0.0024 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13 |
| 13.178 | 3‐[(2‐Furfuryl)dithio]‐2‐methyl‐furan |
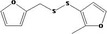
|
0.24 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13 The chemical name should be changed to 3‐[(2‐furanylmethyl)dithio]‐2‐methylfuran Also evaluated by JECFA as no. 1524 |
| 13.185 | 3‐[(2‐Furfuryl)dithio]‐2‐butanone |
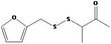
|
0.011 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13Rev2 The chemical name should be changed to 3‐[(2‐furanylmethyl)dithio]‐2‐butanone |
| Subgroup IId Polysulfides | |||||
| 13.146 | Methyl furfuryl trisulfide |
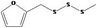
|
0.0024 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists No safety concern based on intakes calculated by the MSDI approach |
Concluded in FGE.13 |
EU MSDI: Amount added to food as flavour in (kg/year) × 10E9/(0.1 × population in Europe (= 375 × 10E6) × 0.6 × 365) = μg/capita per day.
Thresholds of concern: Class I = 1,800 μg/person per day, Class II = 540 μg/person per day, Class III = 90 μg/person per day.
Procedure path A substances can be predicted to be metabolised to innocuous products. Procedure path B substances cannot.
The names and structures of the supporting substances for the candidate substances [FL‐no: 13.125 and 13.162] considered in FGE.13Rev3 (from FGE.67Rev3) are listed in Appendix E, Table E.1, together with their evaluation status (JECFA, 2019).
Table E.1.
Summary of safety evaluations performed by JECFA and EFSA conclusions on these supporting flavouring substances from FGE.67Rev3
| JECFA conclusions | EFSA conclusions | |||
|---|---|---|---|---|
| FL‐no JECFA‐no | EU Union List chemical name | Structural formula | Classa Evaluation procedure pathb Outcome on the named compound based on the MSDI/SPETc approach | Procedural path if different from JECFA, Conclusion based on the MSDId approach on the named compound and on the material of commerce |
|
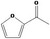
13.054 1503 |
2‐Acetylfuran |
|
Class III 4: Intake above threshold 5: Adequate NOAEL (25 mg/kg bw per day) exists |
Class III B3: Intake below threshold B4: Adequate NOAELe (22.6 mg/kg bw per day) exists No safety concern concluded in FGE.67Rev3 |
|
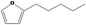
13.059 1491 |
2‐Pentylfuran |
|
Class III 4: Intake above threshold 5: Adequate NOAEL (30 mg/kg bw per day) exists |
Class III B3: Intake below threshold B4: Adequate BMDLe (8.51 mg/kg bw per day) exists No safety concern concluded in FGE.67Rev3 |
|
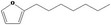
13.069 1492 |
2‐Heptylfuran |
|
Class III 4: Intake below threshold |
Class III B3: Intake below threshold B4: Adequate BMDL (8.51 mg/kg bw per day) exists No safety concern concluded in FGE.67Rev3 |
|
13.106 1493 |
2‐Decylfuran |

|
Class III 4: Intake above threshold 5: Adequate NOAEL (30 mg/kg bw per day) exists |
Class III B3: Intake below threshold B4: Adequate BMDL (8.51 mg/kg bw per day) exists No safety concern concluded in FGE.67Rev3 |
|
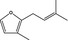
13.148 1494 |
3‐Methyl‐2(3‐methylbut‐2‐enyl)furan |
|
Class III 4: Intake above threshold 5: Adequate NOAEL (45 mg/kg bw per day) exists |
Class III B3: Intake below threshold B4: Adequate BMDL (8.51 mg/kg bw per day) exists No safety concern concluded in FGE.67Rev3 |
Thresholds of concern: Class I = 1,800 μg/person per day, Class II = 540 μg/person per day, Class III = 90 μg/person per day.
WHO technical Report Series 1014. Evaluation of certain food additives. Eighty‐sixth report of the Joint FAO/WHO Expert Committee on Food Additives.
The highest intake estimate based on either the MSDI or SPET approach will be used in the comparison to the TTC.
EU MSDI: Amount added to food as flavouring in (kg/year) × 10E9/(0.1 × population in Europe (= 375 × 10E6) × 0.6 × 365) = μg/capita per day.
NOAEL or BMDL derived by EFSA see Section 3.4.
2. Data and methodologies
2.1. Data
The applicant did not provide new toxicity data on 2‐ethyl‐5‐methylfuran [FL‐no: 13.125] and on 2‐octylfuran [FL‐no: 13.162]. Genotoxicity and toxicity data have been provided for the supporting substance 2‐pentylfuran [FL‐no: 13.059] and for 2‐acetylfuran [FL‐no: 13.054], evaluated in FGE.67Rev3.
Additional information was provided by the applicant during the assessment process in response to requests from EFSA sent on 1/4/2015, 18/12/2017, 29/11/2018, 29/4/2020, 15/7/2020 (see Documentation provided to EFSA n. 6, 26, 27, 30, 31, 32, 3, 4, 21, 23). Moreover, industry provided updated poundage and use levels data (Documentation provided to EFSA n.22).
The new available data considered in the present revision of FGE.13 are summarised in Table 2.
Table 2.
Data evaluated in FGE.13Rev3 and FGE.67Rev3
| FL‐no | Chemical name | Data provided for the current revision 3 of FGE.13 | Appendix (Table nr) and relevant section of the opinion | Documentation provided to EFSA/Reference |
|---|---|---|---|---|
| 13.125 | 2‐Ethyl‐5‐methylfuran | Use levels, poundage data | Appendix C (Tables C.2 and C.6); Section 3.2 | EFFA (2017, 2020c) |
| 13.162 | 2‐Octylfuran | Use levels, poundage data | Appendix C (Tables C.2 and C.6); Section 3.2 | EFFA (2017, 2020c) |
| 13.059 | 2‐Pentylfurana |
Genotoxicity and toxicity data Use levels poundage data |
Appendix H (Tables H.1, H.2), Appendix I (Table I.1); Sections 3.3.2 and 3.3.3 | New York Medical College (2012), Covance (2014), Charles River (2020b), Gulf South Research Institute (1971a,b), Product Safety Labs (2016, 2017), EFFA (2020a,b) |
| 13.054 | 2‐Acetylfurana |
Genotoxicity and toxicity data Use levels poundage data |
Appendix H (Tables H.1, H.2), Appendix I (Table I.1); Sections 3.3.2 and 3.3.3 | Covance (2016), Charles River (2020a), Bio‐Research Laboratories (1985), EFFA (2020a,b) |
Data on the supporting substance 2‐pentylfuran and 2‐acetylfuran are evaluated in FGE.67Rev3.
In addition, the following references were used:
-
–
JECFA monograph and report of the 65th meeting (JECFA, 2006a,b), JECFA monograph and report of the 69th meeting (JECFA, 2009a,b), 76th JECFA report (JECFA, 2012) and 86th JECFA report (JECFA, 2019).
-
–
EFSA scientific opinion on FGE.67Rev2 (EFSA CEF Panel, 2015a).
-
–
EFSA scientific opinion on FGE.67Rev3 (EFSA FAF Panel, 2021).
-
–
EFSA scientific opinion on FGE.13Rev2 (EFSA CEF Panel, 2011).
2.2. Methodologies
This opinion was elaborated following the principles described in the EFSA Guidance on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing guidance documents from the EFSA Scientific Committee. The assessment strategy applied for the evaluation programme of flavouring substances, as laid down in Commission Regulation (EC) No 1565/2000, is based on the Opinion on a Programme for the Evaluation of Flavouring substances of the Scientific Committee on Food (SCF, 2017).
2.2.1. Procedure for the safety evaluation of flavouring substances
The approach for safety evaluation of chemically defined flavouring substances as referred to in Commission Regulation (EC) No 1565/2000, named the ‘Procedure’, is described in Appendix A.
2.2.2. Approach used for the calculation of exposure
The approach used for calculation of the intake of the flavouring substances is described in Appendix A (point ‘a) Intake’) and in Appendix C (Section C.2 ‘mTAMDI calculation’).
3. Assessment
The 24 flavouring substances already evaluated in the previous revisions of FGE.13 and substance [FL‐no: 13.155], which is no longer supported as a candidate substance, will not be further discussed. Thus, in FGE.13Rev3, only two substances [FL‐no: 13.125 and 13.162] will be evaluated. Nevertheless, for the sake of completeness, the information for all 27 substances is maintained in the various tables of this FGE.
3.1. Specifications
Purity criteria for the 26 candidate substances (NB: [FL‐no: 13.155] is no longer a candidate substance) have been provided by the flavouring industry (JECFA, 2001c; EFFA, 2003, 2004b; Flavour Industry, 2009).
Judged against the requirements in Annex II of Commission Regulation (EC) No 1565/20003, the information is adequate for all 26 candidate substances. However, the Panel noted that:
the chemical name of [FL‐no: 13.178] should be changed from 3‐[(2-furfuryl)dithio]‐2-methyl‐furan to 3‐[(2-furanylmethyl)dithio]‐2-methylfuran;
the chemical name of [FL‐no: 13.185] should be changed from 3‐[(2-furfuryl)dithio]‐2-butanone to 3‐[(2-furanylmethyl)dithio]‐2-butanone;
the CAS no. of [FL‐no: 13.011] should be changed from 623‐20-1 to 53282‐12-5 and the CAS no. of [FL‐no: 13.129] should be changed from 59020‐84-7 to 136678‐63-2.
No new information on specifications has been provided for the substances in FGE.13Rev3 since the previous revision of the FGE. The available specifications including minimum assay values are presented in table format in Appendix B.
Stereoisomers
It is recognised that geometrical and optical isomers of substances may have different properties. Their flavour may be different; they may have different chemical properties resulting in possible variation of their absorption, distribution, metabolism, elimination and toxicity. Thus, information must be provided on the configuration of the flavouring substance, i.e. whether it is one of the geometrical/optical isomers, or a defined mixture of stereoisomers. The available specifications of purity will be considered in order to determine whether the safety evaluation carried out for candidate substances for which stereoisomers may exist can be applied to the material of commerce. Flavouring substances with different configurations should have individual chemical names and codes (CAS number, FLAVIS number etc.).
Three of the 26 candidate substances possess a chiral centre [FL‐no: 13.127, 13.185 and 13.199]. For all three substances, the industry has informed that the commercial substance is the racemate (EFFA, 2010).
Two of the 26 candidate substances can exist as geometrical isomers [FL‐no: 13.011 and 13.129], and in both cases, industry has informed that the commercial substance is the (E)‐isomer (see Appendix B).
3.2. Intake data
3.2.1. Natural occurrence in food
For the two candidate substances that are evaluated in FGE.13Rev3, a search in VCF online database (VCF (2020) showed for [FL‐no: 13.125] natural occurrence in e.g. barley, beef, coffee, hazelnut, fish, shrimps and soybean. Quantitative data were available for hazelnut (0.115–2.412 mg/kg) and for shrimps (0.011 mg/kg). For [FL‐no: 13.162] natural occurrence was identified qualitatively in beef, coriander seed, hazelnut, olive, potato, walnut and whey protein. In chicken, trace amounts were reported. For the remaining substances in this group of flavouring substances, information on natural occurrence in food has been presented in FGE.13Rev2.
3.2.2. Estimated daily per capita intake (MSDI approach)
The intake estimation is based on the maximised survey‐derived daily intake (MSDI (SCF, 1999)) approach. The data underlying this approach are obtained from surveys on annual production volumes in Europe. These surveys were initially conducted in 1995 by the International Organization of the Flavour Industry (IOFI), in which flavour manufacturers reported the total amount of each flavouring substance incorporated into food sold in the EU during the previous year (IOFI, 1995). The intake approach does not consider the possible natural occurrence in food.
Average per capita intake (MSDI) is estimated on the assumption that the amount added to food is consumed by 10% of the EU population8 (Eurostat, 1998). This is derived for candidate substances from estimates of annual volume of production provided by Industry and incorporates a correction factor of 0.6 to allow for incomplete reporting (60%) in the industry surveys (SCF, 1999).
The total annual volume of production of the 26 candidate substances for use as flavouring substances in Europe has been reported to be approximately 360 kg (EFFA, 2003, 2004a, 2020c; Flavour Industry, 2009, 2010) and for 42 supporting substances approximately 7800 kg (IOFI, 1995; EFFA, 2004a, 2009, 2011, 2020b).
On the basis of the annual volumes of production reported for the 26 candidate substances, the daily per capita intakes for each of these flavourings have been estimated (Appendix C, Table C.6). More than 85% of the total annual volume of production for the candidate substances is accounted by one candidate substance, 4,5‐dihydro‐3‐mercapto‐2‐methylfuran [FL‐no: 13.108]. The estimated daily per capita intake of this candidate substance from use as a flavouring substance is 37 μg, and below 1.2 μg for each of the remaining candidate substances (Table C.6).
Table C.6.
Estimated intakes based on the MSDI approach and the mTAMDI approach
| FL‐no | EU Register name | MSDI (μg/capita per day) | mTAMDI (μg/person per day) | Structural class | TTC (μg/person per day) |
|---|---|---|---|---|---|
| 13.122 | Ethyl 2‐furoate | 0.39 | 3,900 | Class II | 540 |
| 13.130 | Furfuryl butyrate | 0.24 | – | Class II | 540 |
| 13.136 | 2‐Furoic acid | 0.013 | 1,400 | Class II | 540 |
| 13.139 | 5‐Hydroxymethylfurfuraldehyde | 0.39 | 1,600 | Class II | 540 |
| 13.145 | Methyl 5‐methylfurfuryl sulfide | 0.0024 | 160 | Class II | 540 |
| 13.125 | 2‐Ethyl‐5‐methylfuran | 0.06 | 540 | Class III | 90 |
| 13.162 | 2‐Octylfuran | 0.12 | 540 | Class III | 90 |
| 13.011 | (E)‐Ethyl furfuracrylate | 0.12 | 3,900 | Class III | 90 |
| 13.102 | Butyl 2‐furoate | 0.12 | 3,900 | Class III | 90 |
| 13.108 | 4,5‐Dihydro‐3‐mercapto‐2‐methylfuran | 37 | 160 | Class III | 90 |
| 13.113 | 2,5‐Dimethyl‐3‐(methyldithio)furan | 0.0012 | 160 | Class III | 90 |
| 13.114 | 2,5‐Dimethyl‐3‐(methylthio)furan | 0.0024 | 160 | Class III | 90 |
| 13.124 | Ethyl furfuryl sulfide | 0.18 | 78 | Class III | 90 |
| 13.127 | Furfuryl 2‐methylbutyrate | 0.73 | 3,900 | Class III | 90 |
| 13.129 | Furfuryl but‐2(E)‐enoate | 0.11 | 3,900 | Class III | 90 |
| 13.132 | Furfuryl hexanoate | 0.58 | 3,900 | Class III | 90 |
| 13.133 | Furfuryl isobutyrate | 0.89 | 3,900 | Class III | 90 |
| 13.135 | 1‐(2‐Furfurylthio)propanone | 0.61 | 780 | Class III | 90 |
| 13.141 | Methyl (2‐furfurylthio)acetate | 0.011 | 400 | Class III | 90 |
| 13.143 | Methyl 3‐(furfurylthio)propionate | 0.011 | 420 | Class III | 90 |
| 13.144 | Methyl 5‐methylfurfuryl disulfide | 0.0024 | 78 | Class III | 90 |
| 13.146 | Methyl furfuryl trisulfide | 0.0024 | 160 | Class III | 90 |
| 13.149 | 5‐Methyl‐2‐furanmethanethiol | 0.37 | 160 | Class III | 90 |
| 13.178 | 3‐[(2‐Furanylmethyl)dithio]‐2‐methyl‐furan | 0.24 | 160 | Class III | 90 |
| 13.185 | 3‐[(2‐Furanylmethyl)dithio]‐2‐butanone | 0.011 | 420 | Class III | 90 |
| 13.199 | 3‐[(2‐Methyl‐3‐furyl)thio]‐butanal | 1.2 | 49 | Class III | 90 |
New information on production figures has been provided. Poundage data for [FL‐no: 13.125 and 13.162] are 0.5 kg and 1 kg, respectively (EFFA, 2020c).
3.2.3. Intake Estimated on the Basis of the Modified TAMDI (mTAMDI)
The method for calculation of modified theoretical added maximum daily intake (mTAMDI) values is based on the approach used by SCF up to 1995 (SCF, 1995).
The assumption is that a person may consume a certain amount of flavourable foods and beverages per day.
Updated use levels for the two candidate substances [FL‐no: 13.125 and 13.162] have been provided (EFFA, 2017). No use levels are available for [FL‐no: 13.130].
The detailed information on use levels and the comparison of the MSDI and mTAMDI intake estimations are reported in Appendix C (Tables C.2, C.6) for 25 candidate flavouring substances in FGE.13Rev3. In the case where different use levels were reported for different food categories the highest reported normal use level was used for the calculation of mTAMDI.
Table C.2.
Normal and maximum use levels (mg/kg) for the 26 candidate substances in FGE.13Rev3
| FL‐no | Food categories | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal use levels (mg/kg)a , b Maximum use levels (mg/kg) | |||||||||||||||||||
| 01.0 | 02.0 | 03.0 | 04.1 | 04.2 | 05.0 | 05.3 | 06.0 | 07.0 | 08.0 | 09.0 | 10.0 | 11.0 | 12.0 | 13.0 | 14.1 | 14.2 | 15.0 | 16.0 | |
| 13.011 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
|
| 13.102 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
|
| 13.108 |
0.4 2 |
0.2 1 |
0.4 2 |
0.3 1.5 |
– – |
0.4 2 |
0.2 1 |
0.4 2 |
0.1 0.4 |
0.1 0.4 |
– – |
– – |
0.2 1 |
0.4 2 |
0.2 1 |
0.4 2 |
1 5 |
0.2 1 |
|
| 13.113 |
0.4 2 |
0.2 1 |
0.4 2 |
0.3 1.5 |
– – |
0.4 2 |
0.2 1 |
0.4 2 |
0.1 0.4 |
0.1 0.4 |
– – |
– – |
0.2 1 |
0.4 2 |
0.2 1 |
0.4 2 |
1 5 |
0.2 1 |
|
| 13.114 |
0.4 2 |
0.2 1 |
0.4 2 |
0.3 1.5 |
– – |
0.4 2 |
0.2 1 |
0.4 2 |
0.1 0.4 |
0.1 0.4 |
– – |
– – |
0.2 1 |
0.4 2 |
0.2 1 |
0.4 2 |
1 5 |
0.2 1 |
|
| 13.122 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
|
| 13.124 |
0.2 1 |
0.1 0.5 |
0.2 1 |
0.2 1 |
– – |
0.2 1 |
0.1 0.5 |
0.2 1 |
0.1 0.2 |
0.1 0.2 |
– – |
– – |
0.1 0.5 |
0.2 1 |
0.1 0.3 |
0.2 1 |
0.4 2 |
0.1 0.5 |
|
| 13.125 |
1.7 3.85 |
0.2 0.38 |
0.11 0.68 |
– – |
– – |
2 6.34 |
0.65 2 |
1.4 4 |
2.38 8.8 |
1 2.66 |
– – |
– – |
– – |
0.47 2 |
– – |
0.47 1.03 |
0.2 1 |
– – |
0.2 0.2 |
| 13.127 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
|
| 13.129 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
|
| 13.130 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 13.132 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
|
| 13.133 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
|
| 13.135 |
0 0 |
0 0 |
0 0 |
0 0 |
– – |
2 7 |
0 0 |
1.5 6 |
1.1 6 |
0 0 |
– – |
– – |
0 0 |
0 0 |
1.5 7 |
0.5 2 |
1.5 7 |
0 0 |
|
| 13.136 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
5 25 |
2 10 |
– – |
1 5 |
1 5 |
– – |
– – |
2 10 |
3 15 |
2 10 |
5 25 |
5 25 |
2 10 |
|
| 13.139 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
4 20 |
2 10 |
5 25 |
1 5 |
1 5 |
– – |
– – |
2 10 |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
|
| 13.141 |
0.5 2.5 |
0.2 1 |
0.5 2.5 |
0.4 2 |
– – |
1 5 |
0.2 1 |
2 10 |
0.2 1 |
0.2 1 |
– – |
– – |
0.3 1.5 |
0.5 2.5 |
0.2 1 |
1 5 |
1 5 |
0.4 2 |
|
| 13.143 |
0.5 2.5 |
0.2 1 |
0.5 2.5 |
0.4 2 |
– – |
1 5 |
0.2 1 |
2 10 |
0.2 1 |
0.2 1 |
– – |
– – |
0.3 1.5 |
0.5 2.5 |
0.2 1 |
1 5 |
2 10 |
0.4 2 |
|
| 13.144 |
0.2 1 |
0.1 0.5 |
0.2 1 |
0.2 1 |
– – |
0.2 1 |
0.1 0.5 |
0.2 1 |
0.1 0.2 |
0.1 0.2 |
– – |
– – |
0.1 0.5 |
0.2 1 |
0.1 0.3 |
0.2 1 |
0.4 2 |
0.1 0.5 |
|
| 13.145 |
0.4 2 |
0.2 1 |
0.4 2 |
0.3 1.5 |
– – |
0.4 2 |
0.2 1 |
0.4 2 |
0.1 0.4 |
0.1 0.4 |
– – |
– – |
0.2 1 |
0.4 2 |
0.2 1 |
0.4 2 |
1 5 |
0.2 1 |
|
| 13.146 |
0.4 2 |
0.2 1 |
0.4 2 |
0.3 1.5 |
– – |
0.4 2 |
0.2 1 |
0.4 2 |
0.1 0.4 |
0.1 0.4 |
– – |
– – |
0.2 1 |
0.4 2 |
0.2 1 |
0.4 2 |
1 5 |
0.2 1 |
|
| 13.149 |
0.4 2 |
0.2 1 |
0.4 2 |
0.3 1.5 |
– – |
0.4 2 |
0.2 1 |
0.4 2 |
0.1 0.4 |
0.1 0.4 |
– – |
– – |
0.2 1 |
0.4 2 |
0.2 1 |
0.4 2 |
1 5 |
0.2 1 |
|
| 13.162 |
1.7 3.85 |
0.2 0.38 |
0.11 0.68 |
– – |
– – |
2 6.34 |
0.65 2 |
1.4 4 |
2.38 8.8 |
1 2.66 |
– – |
– – |
– – |
0.47 2 |
– – |
0.47 1.03 |
0.2 1 |
– – |
0.2 0.2 |
| 13.178 |
0.4 2 |
0.2 1 |
0.4 2 |
0.3 1.5 |
– – |
0.4 2 |
0.2 1 |
0.4 2 |
0.1 0.4 |
0.1 0.4 |
– – |
– – |
0.2 1 |
0.4 2 |
0.2 1 |
0.4 2 |
1 5 |
0.2 1 |
|
| 13.185 |
0.5 2.5 |
0.2 1 |
0.5 2.5 |
0.4 2 |
– – |
1 5 |
0.2 1 |
2 10 |
0.2 1 |
0.2 1 |
– – |
– – |
0.3 1.5 |
0.5 2.5 |
0.2 1 |
1 5 |
2 10 |
0.4 2 |
|
| 13.199 |
0.005 0.01 |
0.05 0.1 |
0.001 0.003 |
0.001 0.003 |
0.001 0.003 |
0.005 0.01 |
0.005 0.01 |
0.05 0.1 |
0.05 0.2 |
0.05 0.1 |
– – |
– – |
0.05 0.1 |
0.005 0.01 |
0.002 0.005 |
0.005 0.01 |
2 10 |
0.005 0.01 |
|
‘Normal use’ is defined as the average of reported usages and ‘maximum use’ is defined as the 95th percentile of reported usages (EFFA, 2002).
‘Normal and maximum use levels’ provided by industry for 25 of the 26 candidate substances in the present flavouring group (EFFA, 2003, 2004b, 2007, 2017; Flavour Industry, 2009, 2010).
According to the Flavour Industry, the normal use levels for these 25 candidate substances are in the range of 0.001–20 mg/kg food, and the maximum use levels are in the range of 0.003–100 mg/kg food (EFFA, 2003, 2004b, 2017; Flavour Industry, 2009).
The mTAMDI values for the four candidate substances from structural class II (see Appendix C) range from 160 to 3,900 μg/person per day. For 21 candidate substances from structural class III the mTAMDIs range from 49 to 3,900 μg/person per day.
For detailed information on use levels and intake estimations based on the mTAMDI approach, see Appendix C.
3.2.4. Considerations of combined intakes from use as flavouring substances
Because of structural similarities of candidate and supporting substances, it can be anticipated that many of the flavourings are metabolised through the same metabolic pathways and that the metabolites may affect the same target organs (see Section 3.3.1). Further, in case of combined exposure to structurally related flavourings, the pathways could be overloaded. Therefore, combined intake should be considered. As flavouring substances not included in this Flavouring Group Evaluation may also be metabolised through the same pathways, the combined intake estimates presented here are only preliminary. Currently, the combined intake estimates are only based on MSDI exposure estimates, although it is recognised that this may lead to underestimation of exposure. After completion of all FGEs, this issue should be readdressed. The combined exposure is calculated for each subgroup considering also the supporting substances. In the case of subgroup Ic, the combined exposure will take into account exposures to [FL‐no: 13.125 and 13.162], and to the supporting substances from FGE.67Rev3 [FL‐no: 13.059, 13.069, 13.106, 13.148].
The total estimated combined daily per capita intake of structurally related flavourings is estimated by summing the MSDI for individual candidate substances evaluated through the Procedure. The 26 candidate substances are structurally related to 42 supporting substances evaluated by the JECFA at their 55th, 59th, 69th and 86th meetings (JECFA, 2001a,b, 2002, 2003, 2009b, 2019) or by EFSA (EFSA, 2004b). This number of 42 does not include flavouring substances which are no longer supported by industry or have been deleted from the Union List (see Table 1).
For the present evaluation, the combined intake will be estimated for each subgroup as defined in Section 1.4. The combined intake is estimated for candidate substances together with their supporting substances. In cases where the subgroups include substances belonging to different structural classes according the Cramer classification, the combined intake will be estimated for each structural class, separately. For example, in the case of subgroup Ic, the combined exposure will take into account exposures to candidate substances [FL‐no: 13.125 and 13.162] and to the supporting substances from FGE.67Rev3 [FL‐no: 13.059, 13.069, 13.106, 13.148], all from structural class III. Each combined intake estimate will be compared to the threshold of concern value for the relevant structural class. In the table below, the combined intake is given for each subgroup and structural class within the subgroups for both candidate and supporting substances.
Table 3.
Total combined intake estimates (based on MSDI) for the different subgroups in FGE.13Rev3. The combined intake for candidate and supporting substances in each structural class is also presented
| Combined intake based on the MSDI approach (μg/capita per day) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Subgroup Ia | Subgroup Ic | Subgroup IIa | Subgroup IIba | Subgroup IIca | Subgroup IId | |||
| SC II | SC III | SC III | SC II | SC III | SC III | SC III | SC III | |
| Candidate substances | 1.0 | 2.6 | 0.18 | 0.0024 | 2.0 | 37 | 0.25 | 0.0024 |
| No of substances | 4 | 6 | 2 | 1 | 6 | 2 | 4 | 1 |
| Supporting substances | 850 | 2.6 | 3.9 | 2.2 | 6.9 | 55 | 0.89 | 0.97 |
| No of substancesa | 10 | 5 | 4 | 2 | 8 | 3 | 4 | 1 |
| Total | 850 | 5.2 | 4.1 | 2.2 | 8.9 | 92 | 1.1 | 0.97 |
The total number of supporting substances included in the combined exposure estimates adds up to 37 rather than 42. This is because in subgroup IIb and subgroup IIc there are no structural class II candidate substances whereas there are two and three, respectively, among the supporting substances. Therefore, these five supporting substances have not been included in the combined intake calculations.
On the basis of the reported annual production volumes in Europe (EFFA, 2003, 2004b, 2017, 2020b,c), the combined estimated daily per capita intake as flavourings is below the threshold of concern for the structural class for all subgroups and structural classes except for subgroup Ia (structural class II) and subgroup IIb (structural class III), where the total combined intakes for candidate and supporting substances are 850 and 92 μg/capita per day, respectively. These estimates exceed the threshold for structural class II substances of 540 μg/capita per day, and for structural class III substances of 90 μg/capita per day. However, for subgroup Ia, more than 50% of the total combined daily per capita intake of 850 μg comes from furfural for which, together with the furfural component of furfural diethyl acetal, an ADI of 0.5 mg/kg bw per day has been established by EFSA (EFSA, 2004b). For subgroup IIb, the total combined intake for the class III substances of 92 μg/capita per day also exceeds the threshold for the structural class of 90 μg/person per day. One of the substances in the group of supporting substances from structural class III, 2‐methyltetrahydrofuran‐3‐thiol [FL‐no: 13.160], also accounts for more than 50% of the combined MSDI for this group. In a 90‐day toxicity study by Kappeler (2014), a NOAEL of 5 mg/kg bw per day for male and female Crl:CD (SD) rats, could be established for 2‐methyltetrahydrofuran‐3‐thiol in FGE.65Rev1 (EFSA CEF Panel, 2015b). The MSDI of 92 μg/capita per day correspond to 0.0015 mg/kg bw per day, which provides a margin of safety of more than 3,000 for this subgroup.
The combined estimated daily per capita intake of the substances in subgroup Ic [FL‐no: 13.125 and 13.162] plus those for the four supporting substances (from FGE.67Rev3) assigned to structural class III is 4.1 μg, which does not exceed the threshold of concern of 90 μg/person per day for substances belonging to structural class III.
The background figures for the different exposure estimates are given in Appendix C.
3.3. Biological and toxicological data
3.3.1. Absorption, Distribution, Metabolism and Elimination (ADME)
The candidate substances in FGE.13Rev3 are furan derivatives which can be divided into subgroups based on their chemical structure (see Table 4). In FGE.13Rev3 only information on subgroup Ic is reported. Information on all other subgroups can be retrieved in FGE.13Rev2 (EFSA CEF Panel, 2011).
Table 4.
Candidate substances divided into subgroups of related chemical structures
| FL‐no | EU Register name | Structural formula | Structural class |
|---|---|---|---|
| Main Group I: non‐sulfur‐containing furan derivatives | |||
| Subgroup Ia Structurally related to furfuryl alcohol | |||
| 13.011 | Ethyl furfuracrylate |
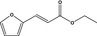
|
III |
| 13.102 | Butyl 2‐furoate |
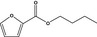
|
III |
| 13.122 | Ethyl 2‐furoate |

|
II |
| 13.127 | Furfuryl 2‐methylbutyrate |
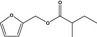
|
III |
| 13.129 | Furfuryl but‐2‐enoate |

|
III |
| 13.130 | Furfuryl butyrate |
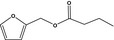
|
II |
| 13.132 | Furfuryl hexanoate |
|
III |
| 13.133 | Furfuryl isobutyrate |
|
III |
| 13.136 | 2‐Furoic acid |

|
II |
| 13.139 | 5‐Hydroxymethylfurfuraldehyde |
|
II |
| Subgroup Ib Alkoyl‐substituted furans | |||
| 13.155a | 2‐Methyl‐5‐propionylfuran |
|
II |
| Subgroup Ic Alkyl‐substituted furans | |||
| 13.125 | 2‐Ethyl‐5‐methylfuran |
|
IIIb |
| 13.162 | 2‐Octylfuran |
|
IIIb |
| Main Group II: Sulfur‐substituted furan derivatives | |||
| Subgroup IIa Sulfides | |||
| 13.114 | 2,5‐Dimethyl‐3‐(methylthio)furan |
|
III |
| 13.124 | Ethyl furfuryl sulfide |
|
III |
| 13.135 | 1‐(2‐Furfurylthio)propanone |
|
III |
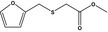
| 13.141 | Methyl (2‐furfurylthio)acetate |
|
III |
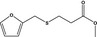
| 13.143 | Methyl 3‐(furfurylthio)propionate |
|
III |
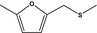
| 13.145 | Methyl 5‐methylfurfuryl sulfide |
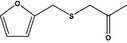
|
II |
| 13.199 | 3‐[(2‐methyl‐3‐furyl)thio]‐butanal |
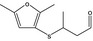
|
III |
| Subgroup IIb Thiols | |||
| 13.149 | 5‐Methyl‐2‐furanmethanethiol |
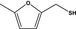
|
III |
| 13.108 | 4,5‐Dihydro‐3‐mercapto‐2‐methylfuran |
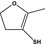
|
III |
| Subgroup IIc Disulfides | |||
| 13.113 | 2,5‐Dimethyl‐3‐(methyldithio)furan |

|
III |
| 13.144 | Methyl 5‐methylfurfuryl disulfide |
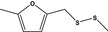
|
III |
| 13.178 | 3‐(Furfuryldithio)‐2‐methylfuran |
|
III |
| 13.185 | 2‐Furfuryl 3‐oxo‐2‐butyl disulfide |
|
III |
| Subgroup IId Polysulfides | |||
| 13.146 | Methyl furfuryl trisulfide |

|
III |
Substance not supported (DG SANCO, 2012) and not included in the Union List.
Determined with OECD Toolbox (version 4.3.1 available online https://www.oecd.org/chemicalsafety/risk-assessment/oecd-qsar-toolbox.htm).
3.3.1.1. Main Group I
Subgroup Ic
The candidate substances in subgroup Ic [FL‐no: 13.125 and 13.162] are substituted furans which in contrast to the candidate substances in subgroup Ia do not bear any functional (carbonyl) groups in the side chain. Based on the limited data available, also for these substances absorption from the GI‐tract may be anticipated, similar to the subgroup Ia substances. Mono‐alkyl furans, such as the candidate substance 2‐octylfuran [FL‐no: 13.162], may be subject to oxidation (possibly epoxidation of the unsubstituted double bond in the furan ring) followed by ring opening and rearrangement to keto‐aldehydes. For several 2‐alkyl‐substituted furans reactivity of their metabolites towards proteins and DNA has been demonstrated, resulting in toxicity to liver and kidneys. Oxidation of the C1′‐carbon of the alkyl substituent may result in the formation of an α,β‐unsaturated ketone with the carbonyl group connected to the aromatic double bonds in the furan ring, similar to acetophenone. A study on the metabolism of 2,5‐dimethylfuran demonstrated that this substance is subject to ring‐opening resulting in the formation of a reactive intermediate, probably hex‐3‐ene‐2,5‐dione, which showed reactivity towards free protein‐thiol and amino groups.
Concerning the potential neurotoxicity of the unsaturated gamma diketone hex‐3‐ene‐2,5‐dione, the Panel concluded in FGE.67Rev3 (EFSA FAF Panel, 2021) that there is no solid ground to raise a concern for potential formation of neurotoxic protein adducts.
The ring‐opening products of alkylfurans are reactive towards DNA and proteins and the two candidate substances in subgroup Ic are examples of these alkylfurans. Therefore, it is concluded that the candidate substances included in main group I cannot be predicted to be metabolised only to innocuous compounds.
A detailed description of the toxicokinetic features of alkylfurans, among which [FL‐no: 13.125 and 13.162] is reported in Appendix F.
3.3.2. Genotoxicity studies
Genotoxicity studies were available only on some of the candidate substances included in main group I or on their related supporting substances. In FGE.13Rev3, only data related to the substances in subgroup Ic are reported. Information on other substances are available in FGE.13Rev2 (EFSA CEF Panel, 2011).
No data are available on the genotoxic properties of the two candidate substances [FL‐no: 13.125 and 13.162] in subgroup Ic.
Several studies were found with 2‐methylfuran [FL‐no: 13.030]9 and 2,5‐dimethylfuran [FL‐no: 13.029].9 Negative results were obtained in a limited bacterial reverse gene mutation test with S. Typhimurium (TA97 and TA100 strains only, no data on cytotoxicity, no duplicate trial (Shinohara et al., 1986)). However, a concentration‐related positive response with limited validity (e.g. no clear data on cytotoxicity; no clear description of scoring criteria) was obtained with both substances in a chromosome aberration test in Chinese hamster ovary cells with and without metabolic activation in the presence or absence of metabolic activation (Stich et al., 1981). Both substances also gave a positive response in a rec‐assay for bacterial DNA‐repair (Shinohara et al., 1986), but the predictive value of this test system is considered to be limited.
For 2‐alkyl‐ and 2,5‐dialkyl‐substituted furans, formation of reactive ring opening products cannot be excluded (see Section 3.3.1 and Appendix F). These reactive intermediates can bind covalently to DNA, which might result in genotoxic events. In an alternative metabolic pathway, these flavouring substances may also be converted to ketones which are structurally related to the substances in former subgroup Ib, and for these substances, a concern for genotoxicity has been identified (based on previously reported data on the supporting substance 2‐acetylfuran [FL‐no: 13.054] from FGE.67). Therefore, owing to the anticipated metabolism of the two candidate substances in subgroup Ic into possible genotoxic metabolites a concern for genotoxicity was raised. However, newly submitted genotoxicity data are available for the supporting substances 2‐acetylfuran [FL‐no: 13.054] and 2‐pentylfuran [FL‐no: 13.059] evaluated in FGE.67Rev3 (EFSA FAF Panel, 2021). The summary of that evaluation is reported below, for detailed description of the evaluation of the genotoxicity studies on [FL‐no: 13.054, 13.059], see FGE.67Rev3 (EFSA FAF Panel, 2021).
3.3.2.1. Genotoxicity evaluation of the supporting substance 2‐pentylfuran [FL‐no: 13.059] from FGE.67Rev3
For 2‐pentylfuran [FL‐no: 13.059], no in vitro data were available to the Panel. Industry submitted an in vivo comet assay in mice (New York Medical College, 2012). The Panel considered that this study was not sufficiently reliable to conclude on the genotoxicity of 2‐pentylfuran in mice.
2‐Pentylfuran was tested in vivo in a combined comet assay and micronucleus assay in rats. Results from the comet assay in liver were negative, suggesting that 2‐pentylfuran did not induce gene mutations or clastogenic effects in the tissue in which the potential for activation to genotoxic metabolites is expected. In the same study, 2‐pentylfuran did not increase micronucleated cell frequency in bone marrow. However, the Panel noted that no decrease in the percentage of polychromatic erythrocytes was observed, and hence, exposure of the bone marrow to 2‐pentylfuran could not be confirmed. No information was available from blood analyses to show systemic exposure. Moreover, the clinical signs of toxicity observed in this study or in an additional 90‐day repeated dose toxicity study in rats are not sufficient to demonstrate the systemic exposure to the tested substance.
Based on the observations above, the Panel considered that the available in vivo micronucleus assay is not adequate to rule out potential chromosomal damage induced by 2‐pentylfuran [FL‐no: 13.059].
To resolve the above concerns, the Panel requested to test 2‐pentylfuran [FL‐no: 13.059] in an in vitro micronucleus assay in human peripheral blood lymphocytes with fluorescence in situ hybridisation (FISH) analysis. In this in vitro test, 2‐pentylfuran did not induce micronuclei (MN), indicating that the testing substance does not induce chromosomal damage. Based on the available data, the Panel concluded in FGE.67Rev3 that for 2‐pentylfuran [FL‐no: 13.059], the concern for genotoxicity is ruled out.
3.3.2.2. Genotoxicity evaluation of the supporting substance 2‐acetylfuran [FL‐no: 13.054] from FGE.67Rev3
From studies considered in previous versions of this FGE, a concern for genotoxicity was raised for 2‐acetylfuran (see above). The studies showed limitations and the CEF Panel decided that additional information was necessary for its evaluation. Following the request of the Panel, an in vivo combined gene mutation and micronucleus assay in transgenic mice was submitted. 2‐Acetylfuran did not increase the mutation frequency in liver and duodenum, indicating that the testing substance does not induce gene mutations. In the same study, 2‐acetylfuran did not increase the percentage of micronucleated cells in peripheral blood. However, the Panel noted that in the micronucleus arm of the study, no positive control was included. Moreover, no decrease in the percentage of reticulocytes was observed, and hence, systemic exposure to 2‐acetylfuran could not be confirmed. The clinical signs of toxicity observed in this study and in a 28/90‐day repeated dose toxicity study in rats (Bio‐Research Laboratories, 1985) were not sufficient to demonstrate the systemic exposure to the tested substance in mice. The use of toxicity data from a different species as evidence of systemic exposure is not recommended by EFSA Scientific Committee (2017a).
Based on the observations above, the Panel considered that for 2‐acetylfuran, there is no concern for gene mutations. However, the available in vivo micronucleus assay was not adequate to rule out potential chromosomal damage induced by 2‐acetylfuran [FL‐no: 13.054]. Therefore, the Panel requested to test 2‐acetylfuran in an in vitro micronucleus assay in human peripheral blood lymphocytes with FISH analysis. This study showed that 2‐acetylfuran did not induce MN, indicating that the substance does not induce chromosomal damage and is not aneugenic. Based on the available data, the Panel concluded in FGE.67Rev3 that for 2‐acetylfuran [FL‐no: 13.054], the concern for genotoxicity is ruled out.
The Panel considered that the genotoxicity data on the supporting substances 2‐acetylfuran [FL‐no: 13.054] and 2‐pentylfuran [FL‐no: 13.059] allow to rule out the genotoxicity concern for these two supporting substances. Therefore, the structurally related candidate substances [FL‐no: 13.125 and 13.162] can be evaluated through the procedure.
3.3.3. Toxicological data
No toxicity data have been provided for [FL‐no: 13.125 and 13.162]. However, toxicity studies are available for the supporting substance 2‐pentylfuran [FL‐no: 13.059] and for 2‐acetylfuran [FL‐no: 13.054] evaluated in FGE.67Rev3. Summary results of the toxicity studies described below are reported Appendix I, Table I.1.
Table I.1.
Summary of toxicity data evaluated in FGE.67Rev3
| Chemical name [FL‐no] | Species; Sex No/group | Route | Doses (mg/kg bw per day) | Duration (days) | NOAEL or BMDL (mg/kg bw per day) | Reference | Comments |
|---|---|---|---|---|---|---|---|
| 2‐acetylfuran [13.054] | Rat; M & F; 32 rats for control and lowest dose; 12 rats for the mid dose; 10 rats for the highest dose | Diet | 0, 5, 25, 100 (nominal) | 28 | Bio‐Research Laboratories (1985) | ||
| 0, 5, 25 (nominal, equal to 22.6 in males and 27 in females) | 90 | 22.6 | Study conducted according to OECD TG 408 and GLP | ||||
| 2‐pentylfuran [13.059] | Mice; M & F; 5 animals/group | Gavage | 0, 800, 1,000, 1,260, 1,600, 2,000 | Single dose acute toxicity study | LD50 about 1,200 mg/kg | Gulf South Research Institute (1971a) | Only summary available |
| Rats; M & F; 23 animals/group | Diet | 0, 25.6 | 90 | – | Gulf South Research Institute (1971b) | ||
| Rat; M & F 5/sex/dose level | Diet | 0, 100, 250 and 500 | 14 | – | Product Safety Labs (2016) | ||
| Rat; M & F 10/sex/dose level | Oral gavage | 0, 30, 100 and 150 | 90 | 8.51 | Product Safety Labs (2017) | Study conducted according to OECD TG 408 and GLP. Value 8.51 is BMDL22. The study authors proposed a NOAEL of 30 mg/kg bw per day |
3.3.3.1. 2‐Pentylfuran [FL‐no: 13.059] – toxicity studies
2‐Pentylfuran was tested in a single dose oral acute toxicity study in Swiss Webster mice at these doses: 0, 800, 1,000, 1,260, 1,600, 2,000 mg/kg bw (Gulf South Research Institute, 1971a, only data summary available). The LD50 derived was 1,185 mg/kg bw for males and 1,220 mg/kg bw for females.
2‐Pentylfuran was tested in a 90‐day toxicity study in Sprague Dawley rats (Gulf South Research Institute, 1971b), administered in the diet at 25.6 mg/kg per day. Average liver weights of males and liver and kidney weights of female were statistically significant higher than control. The study authors considered that this result was due to a low average organ weight in control and not to a real enlargement of tissues in treated animals. Histological lesions were observed in lungs, which were associated to a virus infection. No other histological findings were observed.
The Panel considered to base the toxicological evaluation on a more recent 90‐day toxicity study (Product Safety Labs, 2017) where three dose levels were tested and which is compliant with OECD TG 408 and good laboratory practice (GLP) principles.
2‐Pentylfuran was tested in a 90‐day toxicity study in CRL Sprague‐Dawley® CD® IGS rats (10/sex per dose group). 2‐Pentylfuran was formulated in corn oil and administered at dose levels of 0, 30, 100 and 150 mg/kg bw per day (Product Safety Labs, 2017).
All animals survived until the end of the study.
Statistical significant changes were observed in several haematology parameters and clinical chemistry parameters, which correlated with histopathology findings in spleen and liver.
The main effects of 2‐pentylfuran in the rat are haematological effects, in particular on the red blood cells and, related to this, effects on reticulocyte counts and on spleen. The test substance appears to increase the turn‐over of erythrocytes.
The main effect in the liver was centrilobular hepatocellular hypertrophy, which was accompanied by an increase in relative and absolute liver weights. Some indication for liver toxicity was obtained from the increase in plasma sorbitol dehydrogenase, but other clinical chemistry parameters and histopathology did not support this.
The results for all haematological, clinical chemistry, urinalysis parameters and the data on body and liver weight changes were subjected to dose‐response modelling, using the EFSA PROAST web tool,10 in line with the EFSA Scientific Committee guidance document (EFSA Scientific Committee, 2017b). Instead of using the default value of 5% for the BMR, the Panel employed endpoint‐specific benchmark responses (BMR), based on the theory developed by Slob (2017). This theory takes better account of the natural variability in the measured parameters than the default BMRs. This results in biologically more plausible BMRs and subsequently more plausible BMDLs.
The BMDL for mean corpuscular volume was the lowest BMDL identified among the haematological parameters (14 and 25.4 mg/kg bw per day for males and females, respectively), based on a BMR of 2.6%.
Among the clinical chemistry data, the BMDL of plasma total bilirubin (which amounted to 8.51 or 18.3 mg/kg bw per day for males and females, respectively) was chosen based on a BMR of 22%.
For the decrease in body weights and the increases in absolute and relative liver weights BMDLs could be estimated. The lowest reliable BMDL for these parameters were 52.6 and 29.9 mg/kg bw per day for increased absolute liver weight in males and females, respectively, based on a BMR of 15%.
The Panel concluded that for the evaluation of 2‐pentylfuran and the structurally related flavouring substances, the BMDL of 8.51 mg/kg bw per day in males can be used as the reference point, as all other reliable BMDLs were higher than this BMDL for bilirubin. More details and graphical representation are reported in FGE.67Rev3 (EFSA FAF Panel, 2021).
3.3.3.2. 2‐Acetylfuran [FL‐no: 13.054] – Repeated‐dose toxicity studies
2‐Acetylfuran was tested in Sprague‐Dawley CD rats in a combined 28‐day and 90‐day toxicity study (Bio‐Research Laboratories, 1985). The study was conducted in compliance with the United States Food and Drug Administration's Good Laboratory Practice Regulations.
Groups of male and female Sprague‐Dawley rats were exposed through the diet to 0, 5, 25 or 100 mg 2‐acetylfuran/kg bw per day (nominal dosing).
Results after 28 days of exposure
In the animals of the 100 mg/kg group, a statistically significant decrease in body weight was observed in males and females at week 3 (–15 or –35%, respectively). In these animals also a decreased feed consumption (–15% in the males and –17% in the females) was observed. At week 4, the animals were sacrificed together with half of the 5 mg/kg bw and control group animals. In the 100 mg/kg male animals, glucose and alkaline phosphatase in serum were significantly decreased and blood urea nitrogen (BUN) was significantly increased. For glucose and alkaline phosphatase in the females, a similar change was observed as in the males, but statistical significance was not reached. In females, BUN was not affected. A significant increase in relative liver weight was observed in males and females dosed at 100 mg/kg per day. In males also an increase of relative gonad (testis + epididymis) weight was observed. Other parameters in haematology, urinalysis, clinical chemistry or organ weights and histopathology were not affected. No adverse effects were observed in the animals from the 5 mg/kg group.
Results after 90 days of exposure
At study termination, the body weights of the males and females in the 25 mg/kg bw dose group were statistically significantly lower (by 10%) than those of the control animals. The decreases in body weight were rather limited and connected to a reduced feed consumption (–4% in the males and –15% in the females). Since no findings attributable to treatment were noted in the clinical, haematological and histopathological examinations or in the clinical chemistry and urinalysis parameters, the Panel identified an NOAEL of 25 mg/kg bw per day (nominal value) from this study, which is the highest dose tested up to 90‐day of exposure. This NOAEL is equal to 22.6 mg/kg bw per day in males and 27 mg/kg bw per day in females.
3.4. Application of the Procedure for the safety evaluation of flavouring substances
The application of the Procedure is based on intakes estimated on the basis of the MSDI approach. Where the mTAMDI approach indicates that the intake of a flavouring substance might exceed its corresponding threshold of concern, the Panel requires more precise data on use and use levels. For comparison of the intake estimations based on the MSDI approach and the mTAMDI approach, see Section 3.2 and Appendix C.
Based on new experimental data on genotoxicity on the structurally related substances 2‐acetylfuran and 2‐pentylfuran [FL‐no:13.059] (FGE.67Rev3), which rule out the concerns for genotoxicity for [FL‐no: 13.125 and 13.13.162], the Procedure for the safety evaluation of flavouring substances as outlined in Appendix A has been applied to the two candidate substances. The stepwise evaluations are summarised in Appendix A.
Step 1
The two candidate substances [FL‐no: 13.125 and 13.162] are allocated to structural class III according to the decision tree approach by Cramer et al. (1978), see Table 4.
Step 2
Taking into account the metabolic pathways described in Section 3.3.1, the two candidate substances are not predicted to be metabolised only to innocuous products. Therefore, the evaluation of 2‐ethyl‐5‐methylfuran [FL‐no: 13.125] and 2‐octylfuran [FL‐no: 13.162] proceeds via the B‐side of the evaluation scheme.
Step B3
The two candidate substances [FL‐no: 13.125] and [FL‐no: 13.162], which have been assigned to structural class III, have estimated European daily per capita intakes (MSDI) of 0.06 and 0.12 μg, respectively (Appendix C). These intakes are below the threshold of concern of 90 μg/person per day for structural class III. Therefore, the safety evaluation proceeds to step B4 for both candidate substances.
Step B4
Based on the MSDI exposure estimates for the two candidate substances and the BMDL of 8.51 mg/kg bw per day for increased plasma total bilirubin, observed in a 90‐day oral toxicity study with 2‐pentylfuran, adequate margins of safety of 8.5 × 106 and 4.3 × 106 can be calculated for [FL‐no: 13.125] and [FL‐no: 13.162], respectively. It is concluded that the use of these two substances as flavourings in food does not raise a safety concern when based on the MSDI approach.
3.5. Comparison of the intake estimations based on the mTAMDI approach with the structural class thresholds
For three candidate substances [FL‐no: 13.122, 13.136 and 13.139] in structural class II, evaluated through the Procedure previously, the mTAMDI range from 1,400 to 3,900 μg/person per day, which is above the threshold of concern for this Cramer class. For another previously evaluated substance [FL‐no: 13.145], the mTAMDI is 160 μg/person per day, which is below its class threshold of 540 μg/person per day (class II). For the structural class II substance [FL‐no: 13.130] no use levels were provided and no mTAMDI can be calculated.
The estimated mTAMDI exposure estimates for 18 [FL‐no: 13.011, 13.102, 13.108, 13.113, 13.114, 13.125, 13.127, 13.129, 13.132, 13.133, 13.135, 13.141, 13.143, 13.146, 13.149, 13.162, 13.178 and 13.185] substances assigned to structural class III, range from 160 to 3,900 μg/person per day and are above the threshold of concern for their structural class (90 μg/person per day). For the remaining three substances from structural class III [FL no: 13.124, 13.144 and 13.199], the mTAMDIs range from 49 to 78 μg/person per day, which is below the threshold of concern.
Thus, for four structural class II substances and for 18 structural class III substances, further information is required. This would include more reliable intake data and then, if required, additional toxicological data. For comparison of the MSDI and mTAMDI values, see Appendix C.
4. Discussion
The present revision of FGE.13, FGE.13Rev3, concerns the evaluation, in total, of 26 candidate flavouring substances. New data included are additional toxicity and genotoxicity studies for the supporting substances 2‐acetylfuran [FL‐no: 13.054] and 2‐pentylfuran [FL‐no: 13.059].
Three of the 26 flavouring substances possess a chiral centre [FL‐no: 13.127, 13.185 and 13.199] industry has informed that the commercial substances are racemates in all three cases. Two of the 26 substances can exist as geometrical isomers [FL‐no: 13.011 and 13.129] and in both cases industry has informed that the commercial substances are the (E)‐isomers.
Five candidate substances are classified into structural class II [FL‐no: 13.122, 13.130, 13.136, 13.139 and 13.145] and 21 candidate substances are classified into structural class III [FL‐no: 13.011, 13.102, 13.108, 13.113, 13.114, 13.124, 13.125, 13.127, 13.129, 13.132, 13.133, 13.135, 13.141, 13.143, 13.144, 13.146, 13.149, 13.162, 13.178, 13.185 and 13.199].
According to the default MSDI approach, the 26 flavouring substances in this group have intakes in Europe from 0.0012 to 37 μg/capita per day, which are below the thresholds of concern for both structural class II (540 μg/person per day) and structural class III (90 μg/person per day) substances.
On the basis of the reported annual production volumes in Europe (MSDI approach), the combined estimated daily per capita intake as flavourings is below the threshold of concern for the structural class for all subgroups and structural classes except for subgroup Ia, structural class II and subgroup IIb where the total combined intake for candidate and supporting substances is 850 and 92 μg/capita per day, respectively, which exceeds the threshold for structural class II substances of 540 μg/person per day and for structural class III substances of 90 μg/person per day. However, for subgroup Ia, 50% of the combined daily per capita intake of 850 μg results from furfural for which, together with the furfural component of furfural diethyl acetal, an ADI of 0.5 mg/kg bw per day has been established by EFSA (EFSA, 2004b). For subgroup IIb, the total combined intake for the class III substances of 92 μg/capita per day also exceeds the threshold for the structural class of 90 μg/person per day. One of the substances in the group of supporting substances from structural class III, 2‐methyltetrahydrofuran‐3‐thiol [FL‐no: 13.160], also accounts for more than 50% of the combined MSDI for this group. In a 90‐day toxicity study by Kappeler (2014), a NOAEL of 5 mg/kg bw per day for male and female Crl:CD (SD) rats could be established for 2‐methyltetrahydrofuran‐3‐thiol in FGE.65Rev1 (EFSA CEF Panel, 2015b). The MSDI of 92 μg/capita per day corresponds to 0.0015 mg/kg bw per day, which can provide a margin of safety of more than 3,000.
In previous versions of this FGE, no concerns for genotoxicity were raised for the candidate substances in subgroups Ia and IIa‐d.
However, for the candidate substance in group Ib, in vitro and in vivo genotoxicity data were available for one supporting substance (2‐acetylfuran [FL‐no: 13.054]). For subgroup Ic, data on in vitro genotoxicity were provided for two supporting substances [FL‐no: 13.029, 13.030]9 (Appendix G). For one of these, also data on in vivo genotoxicity were provided. In addition, data for a supporting substance [FL‐no: 13.054] for subgroup Ib contribute to the evaluation of the genotoxic potential of substances in subgroup Ic due to structural similarity of metabolites of the Ic candidates with the substances in subgroup Ib.
For the supporting substance in subgroup Ib (2‐acetylfuran) and for a supporting substance in subgroup Ic (2‐pentylfuran), data were submitted that ruled out the concern for genotoxicity for the remaining substances in subgroup Ic ([FL‐no: 13.125 and 13.162]). The remaining substance in subgroup Ib [FL‐no:13.155] is no longer supported (DG SANCO, 2012).
Based on information on absorption, distribution, metabolism and excretion on candidate and supporting substances, it cannot be concluded that any of the 26 candidate substances in this FGE is metabolised only to innocuous metabolites. In FGE13Rev2, the CEF Panel concluded at step B4 of the Procedure that 24 candidate substances [FL‐no: 13.011, 13.102, 13.108, 13.113, 13.114, 13.122, 13.124, 13.127, 13.129, 13.130, 13.132, 13.133, 13.135, 13.136, 13.139, 13.141, 13.143, 13.144, 13.145, 13.146, 13.149, 13.178, 13.185 and 13.199] do not pose a safety concern when used as flavouring substances at the estimated levels of intake based on the MSDI approach.
In this revision of FGE.13 (Revision 3), the FAF Panel calculated margins of safety of 8.5 × 106 and 4.3 × 106 for the two substances in subgroup Ic [FL‐no: 13.125 and 13.162], respectively, at step B4 of the Procedure, by comparison of their MSDI intakes estimates with the BMDL of 8.51 mg/kg bw per day for the supporting substance 2‐pentylfuran. This BMDL was calculated from the data on increased plasma total bilirubin obtained from a 90‐day oral toxicity study with this substance based on a BMR of 22% change. From these margins of safety, the FAF Panel concluded that the candidate flavouring substances [FL‐no: 13.125 and 13.162] do not raise a safety concern when used as flavouring substances at the estimated levels of intake based on the MSDI approach.
For three candidate substances [FL‐no: 13.122, 13.136 and 13.139] in structural class II, the mTAMDI intake estimates are above the threshold of concern for this Cramer class. For one structural class II substance [FL‐no: 13.145], the mTAMDI is below the class II threshold and for another substance [FL‐no: 13.130], no use levels were provided, so no mTAMDI can be calculated for this substance.
The mTAMDI intake estimates for 18 flavouring substances [FL‐no: 13.011, 13.102, 13.108, 13.113, 13.114, 13.125, 13.127, 13.129, 13.132, 13.133, 13.135, 13.141, 13.143, 13.146, 13.149, 13.162, 13.178 and 13.185], all assigned to structural class III are above the threshold of concern for their class. For the remaining three substances from structural class III [FL‐no: 13.124, 13.144 and 13.199], the mTAMDIs are below the threshold of concern.
5. Conclusions
For all 26 candidate substances [FL‐no: 13.011, 13.102, 13.108, 13.113, 13.114, 13.122, 13.124, 13.125, 13.127, 13.129, 13.130, 13.132, 13.133, 13.135, 13.136, 13.139, 13.141, 13.143, 13.144, 13.145, 13.146, 13.149, 13.162, 13.178, 13.185 and 13.199], the Panel concluded that they would be of ‘No safety concern at estimated levels of intake as flavouring substances’ when evaluated based on the MSDI approach.
For four substances [FL‐no: 13.124, 13.144, 13.145 and 13.199], there is no concern when the exposure was estimated based on the mTAMDI approach.
For 21 substances, more detailed information on uses and normal and maximum use levels are needed to refine the mTAMDI estimates in order to finalise their evaluation. Upon submission of such data, additional data on toxicity may become necessary.
For one substance [FL‐no: 13.130], use levels are needed to calculate the mTAMDI estimate.
Adequate specifications including complete purity criteria and identity for the materials of commerce have been provided for all 26 flavouring substances evaluated through the Procedure.
6. Recommendation
The FAF Panel recommends the European Commission to consider:
to request updated and detailed information on uses and use levels for the following 22 flavouring substances:
[FL‐no: 13.011, 13.102, 13.108, 13.113, 13.114, 13.122, 13.125, 13.127, 13.129, 13.130, 13.132, 13.133, 13.135, 13.136, 13.139, 13.141, 13.143, 13.146, 13.149, 13.162, 13.178 and 13.185];
to change the chemical name of [FL‐no: 13.178] from 3‐[(2-furfuryl)dithio]‐2-methyl‐furan to 3‐[(2-furanylmethyl)dithio]‐2-methylfuran in the Union list (Appendix B – Table B.1);
to change the chemical name of [FL‐no: 13.185] from 3‐[(2-furfuryl)dithio]‐2-butanone to 3‐[(2-furanylmethyl)dithio]‐2-butanone in the Union list (Appendix B – Table B.1);
to change the CAS no. of [FL‐no: 13.011] from 623‐20-1 to 53282‐12-5 and the CAS no. of [FL‐no: 13.129] from 59020‐84-7 to 136678‐63-2 in the Union list (Appendix B – Table B.1).
Table B.1.
Summary table on specifications data for flavouring substances in FGE.13Rev3 (for chemical structures, see Appendix D) that are included in the Union List. Substance [FL‐no: 13.155], which is no longer supported by industry and not included in the EU Union list is referenced in the previous version, FGE.13Rev2 (EFSA CEF Panel, 2011)
| Information included in the EU Union list Regulation (EC) No 1334/2008 as amended | Most recent available specifications dataa | EFSA comments | |||||
|---|---|---|---|---|---|---|---|
| FL‐no. FEMA no. CoE no. CAS no. | Chemical name | Purity of the named compound | Phys. form Mol. formula Mol. weight | Solubility(c) Solubility in ethanol(d) | Boiling point, °C(e) Melting point, °C ID test Assay minimum (isomers distribution/secondary components) | Refrac. Index(f) Spec. gravity(g) | |
|
13.011 – 545 623‐20‐1 |
(E)‐Ethyl furfuracrylate | (b) |
Liquid C9H10O3 166.18 |
Practically insoluble or insoluble Freely soluble |
229 14 MS 95% |
1.544–1.550 1.092–1.098 |
JECFA no. 2103 CAS no. does not specify (Z) or (E) isomer. CAS no. in Union List should be changed to 53282‐12‐5 |
|
13.102 – – 583‐33‐5 |
Butyl 2‐furoate | (b) |
Liquid C9H12O3 168.19 |
Practically insoluble or insoluble Freely soluble |
233 n.a. MS 95% |
1.469–1.475 1.052–1.058 |
|
|
13.108 4683 – 26486‐13‐5 |
4,5‐Dihydro‐3‐mercapto‐2‐methylfuran | (b) |
Liquid C5H8OS 116.18 |
Slightly soluble Freely soluble |
160 n.a. MS 95% |
1.497–1.503 1.047–1.053 |
JECFA no. 2097 |
|
13.113 – – 61197‐06‐6 |
2,5‐Dimethyl‐3‐(methyldithio)furan | (b) |
Solid C7H10OS2 174.28 |
Practically insoluble or insoluble Freely soluble |
284 45 MS 95% |
n.a. n.a. |
|
|
13.114 – – 63359‐63‐7 |
2,5‐Dimethyl‐3‐(methylthio)furan | (b) |
Liquid C7H10OS 142.22 |
Practically insoluble or insoluble Freely soluble |
63 (13 hPa) n.a. MS 95% |
1.503–1.509 1.042–1.048 |
|
|
13.122 – 10588 614‐99‐3 |
Ethyl 2‐furoate | (b) |
Solid C7H8O3 140.14 |
Practically insoluble or insoluble Freely soluble |
196 36 MS 99% |
n.a. n.a. |
|
|
13.124 – – 2024‐70‐6 |
Ethyl furfuryl sulfide | (b) |
Liquid C7H10OS 142.22 |
Slightly soluble Freely soluble |
73 (13 hPa) n.a. MS 95% |
1.509–1.515 1.047–1.053 |
|
|
13.125 – 10942 1703‐52‐2 |
2‐Ethyl‐5‐methylfuran | (b) |
Liquid C7H10O 110.16 |
Practically insoluble or insoluble Freely soluble |
118 n.a. MS 95% |
1.443–1.449 0.890–0.896 |
|
|
13.127 – 10643 13678‐61‐0 |
Furfuryl 2‐methylbutyrate | (b) |
Liquid C10H14O3 182.22 |
Practically insoluble or insoluble Freely soluble |
263 n.a. MS 95% (racemate) |
1.455–1.461 1.009–1.015 |
|
|
13.129 – – 59020‐84‐7 |
Furfuryl but‐2(E) ‐enoate | (b) |
Liquid C9H10O3 166.17 |
Practically insoluble or insoluble Freely soluble |
245 n.a. NMR 95% |
1.491–1.497 1.034–1.040 |
CAS no. does not specify (Z) or (E) isomer. CAS no. in the Union List should be changed to 136678‐63‐2 |
|
13.130 – 638 623‐21‐2 |
Furfuryl butyrate | (b) |
Liquid C9H12O3 168.19 |
Insoluble Miscible |
212 n.a. IR 99 |
1.457–1.462 1.051–1.057 |
JECFA no. 759 |
|
13.132 – – 39252‐02‐3 |
Furfuryl hexanoate | (b) |
Liquid C11H16O3 196.25 |
Practically insoluble or insoluble Freely soluble |
224 n.a. MS 98% |
1.452–1.458 1.003–1.013 |
|
|
13.133 – 10641 6270‐55‐9 |
Furfuryl isobutyrate | (b) |
Liquid C9H12O3 168.19 |
Practically insoluble or insoluble Freely soluble |
85 (20 hPa) n.a. MS 95% |
1.497–1.503 1.028–1.034 |
|
|
13.135 4676 – 58066‐86‐7 |
1‐(2‐Furfurylthio)propanone | (b) |
Liquid C8H10O2S 170.04 |
Insoluble Soluble |
240.4 (1.2Torr) n.a. IR NMR 95% |
1.525–1.531 1.146–1.154 |
JECFA no. 2096 |
|
13.136 – 10098 88‐14‐2 |
2‐Furoic acid | (b) |
Solid C5H4O3 112.08 |
Slightly soluble Freely soluble |
231 132 MS 95% |
n.a. n.a. |
|
|
13.139 – 11112 67‐47‐0 |
5‐Hydroxymethylfurfuraldehyde | (b) |
Solid C6H6O3 126.11 |
Slightly soluble Freely soluble |
154 (16 hPa) 34 MS 95% |
n.a. n.a. |
|
|
13.141 – – 108499‐33‐8 |
Methyl (2‐furfurylthio)acetate | (b) |
Liquid C8H10O3S 186.23 |
Practically insoluble or insoluble Freely soluble |
287 n.a. MS 95% |
1.510–1.520 1.195–1.205 |
|
|
13.143 – – 94278‐26‐9 |
Methyl 3‐(furfurylthio)propionate | (b) |
Liquid C9H12O3S 200.25 |
Practically insoluble or insoluble Freely soluble |
310 n.a. MS 95% |
1.509–1.519 1.160–1.170 |
|
|
13.144 – – 78818‐78‐7 |
Methyl 5‐methylfurfuryl disulfide | (b) |
Solid C7H10OS2 174.28 |
Practically insoluble or insoluble Freely soluble |
279 32 NMR 95% |
n.a. n.a. |
|
|
13.145 – 11522 13679‐60‐2 |
Methyl 5‐methylfurfuryl sulfide | (b) |
Liquid C7H10OS 142.22 |
Slightly soluble Freely soluble |
84 (20 hPa) n.a. NMR 95% |
1.509–1.515 1.048–1.054 |
|
|
13.146 – – 66169‐00‐4 |
Methyl furfuryl trisulfide | (b) |
Solid C6H8OS3 192.32 |
Practically insoluble or insoluble Freely soluble |
320 43 NMR 95% |
n.a. n.a. |
|
|
13.149 – – 59303‐05‐8 |
5‐Methyl‐2‐furanmethanethiol | (b) |
Liquid C6H8OS 128.19 |
Slightly soluble Freely soluble |
62 (17 hPa) n.a. MS 95% |
1.523–1.529 1.041–1.047 |
|
|
13.162 – 10965 4179‐38‐8 |
2‐Octylfuran | (b) |
Liquid C12H20O 180.29 |
Practically insoluble or insoluble Freely soluble |
103 (16 hPa) n.a. MS 95% |
1.313–1.319 0.892–0.898 |
|
|
13.178 4119 – 109537‐55‐5 |
3‐[(2‐Furfuryl)dithio]‐2‐ methyl‐furan | (b) |
Solid C10H10O2S2 226.32 |
Practically insoluble or insoluble Freely soluble |
398 122 NMR 95% |
n.a. n.a. |
JECFA no. 1524 The chemical name in the Union List should be changed to 3‐[(2‐furanylmethyl)dithio]‐2‐methylfuran |
|
13.185 – – 159113‐17‐4 |
3‐[(2‐Furfuryl)dithio]‐2‐ butanone | (b) |
Solid C9H12O2S2 216.32 |
Practically insoluble or insoluble Freely soluble |
374 77 NMR 95% (racemate, EFFA 2010) |
n.a. n.a. |
The chemical name in the Union List should be changed to 3‐[(2‐furanylmethyl)dithio]‐2‐butanone |
|
13.199 4501 – 915971‐43‐6 |
3‐[(2‐Methyl‐3‐furyl)thio]‐butanal | (b) |
Liquid C9H12O2S 184.26 |
Practically insoluble Soluble |
n.a. Decomposition at 198°C IR NMR MS 98% (racemate, EFFA, 2010) |
1.5122–1.5222 1.101–1.121 |
JECFA no. 2095 |
Documentation provided to EFSA (EFFA, 2003, 2004b, Flavour Industry, 2009, 2010) and JECFA (2001c).
At least 95% unless otherwise specified.
Solubility in water, if not otherwise stated.
Solubility in 95% ethanol, if not otherwise stated.
At 1,013.25 hPa, if not otherwise stated.
At 20°C, if not otherwise stated.
At 25°C, if not otherwise stated.
7. Documentation as provided to EFSA
Asquith JC, 1989. Bacterial reverse mutation assay ST 15C 89. Firmenich SA. Toxicol study no. M/AMES/18216. September 1989. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Bio‐Research Laboratories, 1985. A combined 28‐day and 90‐day toxicity study of four test articles [2‐furyl methyl ketone, benzophenone, 3‐(2-furyl)acrolein and isobutyl 3-(2‐furyl)propionate] administered orally (in the diet) to the albino rat. Bio‐Research Laboratories LTD, project no. 81238. Unpublished report submitted by EFFA to DG SANTE.
Charles River, 2020a. 2‐Acetylfuran, in vitro micronucleus assay in cultured human peripheral blood lymphocytes. Charles River Laboratories, Study no. 00968014. 15 January 2020. Unpublished report submitted by EFFA to EFSA.
Charles River, 2020b. 2‐Pentylfuran, in vitro micronucleus assay in cultured human peripheral blood lymphocytes. Charles River Laboratories, Study no. 00968015. 12 March 2020. Unpublished report submitted by EFFA to EFSA.
Covance, 2014. 2‐Pentylfuran: Combined bone marrow micronucleus test and Comet assay in the liver of treated rats. Covance Laboratories Ltd. Study no. 8297732. 11 July 2014. Unpublished final report submitted by EFFA to DG SANTE.
Covance, 2016. Acetyl furan: Transgenic gene mutation assay in Muta(™) mice. Covance Laboratories Ltd. Study no. 8332627. August 16, 2016. Unpublished report submitted by EFFA to EFSA.
DG SANCO (Directorate General for Health and Consumer Affairs), 2012. Information from DG SANCO 07/02 2012, concerning a list of 100 non‐supported Register substances and a list of 30 Register substances for which no data have been submitted or which appears as duplicates in the Register.
DG SANTE (Directorate General for Health and Food Safety), 2020a. Letter from European Commission DG‐SANTE (Ares (2020) 3647124) informing of the withdrawal from the Union List of the flavouring substance 2‐butylfuran [FL‐no: 13.103].
DG SANTE (Directorate General for Health and Food Safety), 2020b. Letter from European Commission DG‐SANTE (Ares (2020) 3942736) informing of the withdrawal from the Union List of the flavouring substance 3‐acetyl‐2,5-dimethylfuran [FL‐no: 13.066].
Durward R, 2007a. Furyl methyl ketone: unscheduled DNA synthesis (UDS) assay liver in vitro. Safepharm Laboratories Ltd. Project no. 1834/0005. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Durward R, 2007b. Furyl methyl ketone: in vivo liver unscheduled DNA synthesis (UDS) assay. Safepharm Laboratories Ltd. Project no. 1834/0004. Unpublished report submitted by EFFA to FLAVIS Secretariat.
EFFA (European Flavour Association), 2002. Letter from EFFA to Dr. Joern Gry, Danish Veterinary and Food Administration. Dated 31 October 2002. Re.: Second group of questions. FLAVIS/8.26.
EFFA (European Flavour Association), 2003. Submission 2003‐3. Flavoring group evaluation of 19 flavoring substances (candidate chemicals) of the chemical group 14 (Annex I of 1565/2000/EC) structurally related to furfuryl alcohol and related substances [JECFA/WHO FAS 46/55] and sulfur substituted furan derivates [JECFA/WHO FAS 50/59] used as flavoring substances. 31 January, 2003. Unpublished report submitted by EFFA to FLAVIS Secretariat. FLAVIS/8.21.
EFFA (European Flavour Association), 2004a. Intake ‐ Collection and collation of usage data for flavouring substances. Letter from Dan Dils, EFFA to Torben Hallas‐Møller, EFSA. May 31, 2004.
EFFA (European Flavour Association), 2004b. Submission 2003‐3 Addendum. Supplement of eight flavouring substances (candidate chemicals) to the flavouring group evaluation of chemical group 14 (Annex I of 1565/2000/EC) structurally related to furfuryl alcohol and related substances [JECFA/WHO FAS 46/55] and sulfur substituted furan derivatives [JECFA/WHO FAS 50/59] used as flavouring substances 3 June 2004. Unpublished report submitted by EFFA to FLAVIS Secretariat. FLAVIS/8.68.
EFFA (European Flavour Association), 2007. E‐mail from Jan Demyttenaere, EFFA to Flavis Secretariat, National Food Institute, Technical University of Denmark. Dated 8 February 2007. RE: FLAVIS submissions ‐ use levels for Category 14.2 ‐ Alcoholic beverages FLAVIS/8.70.
EFFA (European Flavour Association), 2009. Poundage data on selected substances in FGE.65 and FGE.67. Private communication from EFFA to the FLAVIS secretariat. 21 October 2009. FLAVIS/8.111.
EFFA (European Flavour Association), 2010. EFFA Letters to EFSA on clarification of specifications and isomerism for which data were requested in published FGEs.
EFFA (European Flavour Association), 2011. Poundage data on selected substances in FGE.66 and FGE.67. Private communication from EFFA to the FLAVIS secretariat. 27 May 2011. FLAVIS/8.111.
EFFA (European Flavour Association), 2017. Poundage data and use levels on flavouring substances under evaluation by EFSA. Supplementary data provided by EFFA following a request by the European Commission.
EFFA (European Flavour Association), 2020a. Addendum of Additional Data Relevant to the Flavouring Group Evaluation of the Chemical Group 14 (Annex I of 1565/2000/EC) Consideration of 28 furan‐substituted compounds evaluated by JECFA at the 55th, 65th, and 69th meetings (JECFA, 2001, 2006, 2009) and by EFSA in FGE.67Rev2 (2015). Addendum to FGE.67Rev2. Prepared by: International Organization of the Flavor Industry, 15/3/2020.
EFFA (European Flavour Association), 2020b. EFFA Submission of updated poundages and use levels on furans of FGE.67. Data submitted by EFFA to EFSA on voluntary basis on 5 May 2020.
EFFA (European Flavour Association), 2020c. EFFA Submission of updated poundages and use levels on furans of FGE.13 & FGE.67. Data submitted by EFFA to EFSA on 5 May 2020.
Flavour Industry, 2009. Unpublished information submitted by Flavour Industry to FLAVIS Secretariat. A‐13rev1 [FL‐no: 13.199].
Flavour Industry, 2010. Unpublished information submitted by Flavour Industry to DG SANCO and forwarded to EFSA. A‐10Rev3 and A‐13Rev2 [FL‐no: 10.170 and 13.135].
Gulf South Research Institute, 1971a. Acute toxicological evaluations of chemicals with mice – 2‐pentyl furan. GSRI project no. NC‐398. 2 February, 1971. Unpublished report submitted by EFFA to EFSA.
Gulf South Research Institute, 1971b. Subacute toxicity evaluation of 2‐pentyl furan with rats. GSRI project no. NC‐403. 4 January, 1971. Unpublished report submitted by EFFA to EFSA.
Kappeler KV, 2014. A 90‐Day Oral (Gavage) Toxicity Study of 2‐Methyltetrahydrofuran‐3-thiol in Rats. WIL Research. Study no. WIL‐968011. 21 August 2014. Unpublished report.
IOFI (International Organization of the Flavour Industry), 1995. European inquiry on volume of use. IOFI, International Organization of the Flavor Industry, 1995.
New York Medical College, 2012. Comparison of Furan and 2‐Pentylfuran Genotoxicity Measured by in vivo COMET Assays in Mouse Liver Study No. NYMC‐CSL 10‐72, 3 January 2012. Chemical Safety Laboratory Department of Pathology, New York Medical College, Valhalla, NY. Private communication to the International Organization of the Flavor Industry (IOFI), Brussels, Belgium. Unpublished report submitted by EFFA to EFSA.
Product Safety Labs, 2016. 2‐Pentylfuran: A 14‐day dietary toxicity/palatability study in rats. Product Safety Labs. Study no. 42347. May 12, 2016. Unpublished report submitted by EFFA to EFSA.
Product Safety Labs, 2017. 2‐Pentylfuran: A 90‐day oral gavage study in rats. Product Safety Labs. Study no. 42348. January 19, 2017. Unpublished report submitted by EFFA to EFSA.
Abbreviations
- ADI
Acceptable Daily Intake
- AFC
Food Additives, Flavourings, Processing Aids and Materials in Contact with Food
- BMD
Benchmark Dose
- BMDL
Benchmark Dose lower boundary of confidence interval (95% single sided)
- BUN
Blood Urea Nitrogen
- bw
Body weight
- CAS
Chemical Abstract Service
- CEF
Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CHO
Chinese hamster ovary (cells)
- CoE
Council of Europe
- DNA
Deoxyribonucleic acid
- EC
European Commission
- EFFA
European Flavour Association
- EFSA
The European Food Safety Authority
- EU
European Union
- FAO
Food and Agriculture Organization of the United Nations
- FEMA
Flavor and Extract Manufacturers Association
- FGE
Flavouring Group Evaluation
- FISH
Fluorescence In Situ Hybridization
- FLAVIS (FL)
Flavour Information System (database)
- GI
Gastrointestinal
- GLP
Good Laboratory Practice
- GPT
Glutamic Pyruvic Transaminase
- GSH
Glutathione
- ID
Identity
- IP
Intra Peritoneal
- IOFI
International Organization of the Flavour Industry
- IR
Infrared spectroscopy
- JECFA
The Joint FAO/WHO Expert Committee on Food Additives
- LD50
Lethal Dose, 50%; Median lethal dose
- MN
Micronuclei
- MS
Mass spectrometry
- MSDI
Maximised Survey‐derived Daily Intake
- mTAMDI
Modified Theoretical Added Maximum Daily Intake
- No
Number
- NOAEL
No Observed Adverse Effect Level
- NOEL
No Observed Effect Level
- OECD
Organisation for Economic Co‐operation and Development
- SCF
Scientific Committee on Food
- SMART
Somatic Mutation and Recombination Test
- TAMDI
Theoretical Added Maximum Daily Intake
- UDS
Unscheduled DNA Synthesis
Appendix A – Procedure of the safety evaluation
1.
The approach for a safety evaluation of chemically defined flavouring substances as referred to in Commission Regulation (EC) No 1565/20003, named the ‘Procedure’, is shown in schematic form in Figure A.1. The Procedure is based on the Opinion of the Scientific Committee on Food expressed on 2 December 1999 (SCF, 1999), which is derived from the evaluation Procedure developed by the Joint FAO/WHO Expert Committee on Food Additives at its 44th, 46th and 49th meetings (JECFA, 1995, 1996, 1997, 1999).11
Figure A.1.
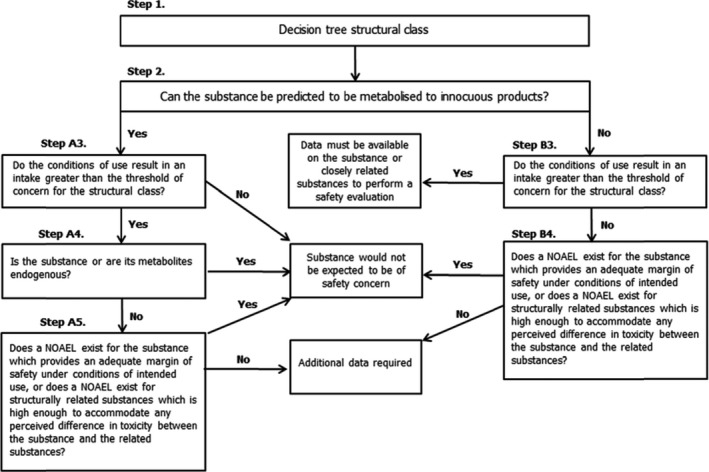
Procedure for the safety evaluation of chemically defined flavouring substances
The Procedure is a stepwise approach that integrates information on intake from current uses, structure–activity relationships, metabolism and, when needed, toxicity. One of the key elements in the Procedure is the subdivision of flavourings into three structural classes (I, II, III) for which toxicological thresholds of concern (TTCs) (human exposure thresholds) have been specified. Exposures below these TTCs are not considered to present a safety concern.
Class I contains flavourings that have simple chemical structures and efficient modes of metabolism, which would suggest a low order of oral toxicity. Class II contains flavourings that have structural features that are less innocuous, but are not suggestive of toxicity. Class III comprises flavourings that have structural features that permit no strong initial presumption of safety, or may even suggest significant toxicity (Cramer et al., 1978). The TTCs for these structural classes of 1,800, 540 or 90 μg/person per day, respectively, are derived from a large database containing data on subchronic and chronic animal studies (JECFA, 1996).
In step 1 of the Procedure, the flavourings are assigned to one of the structural classes. The further steps address the following questions:
Can the flavourings be predicted to be metabolised to innocuous products12 (step 2)?
Do their exposures exceed the TTC for the structural class (steps A3 and B3)?
Are the flavourings or their metabolites endogenous13 (step A4)?
Does a NOAEL exist on the flavourings or on structurally related substances (steps A5 and B4)?
In addition to the data provided for the flavouring substances to be evaluated (candidate substances), toxicological background information available for compounds structurally related to the candidate substances is considered (supporting substances), in order to assure that these data are consistent with the results obtained after application of the Procedure.
The Procedure is not to be applied to flavourings with existing unresolved problems of toxicity. Therefore, the right is reserved to use alternative approaches if data on specific flavourings warranted such actions.
The following issues are of special importance:
a) Intake
Annual production volumes of the flavouring substances as surveyed by the Industry can be used to calculate the ‘Maximised Survey‐derived Daily Intake’ (MSDI) by assuming that the production figure only represents 60% of the use in food due to underreporting and that 10% of the total EU population are consumers (SCF, 1999).
However, the Panel noted that due to year‐to‐year variability in production volumes, to uncertainties in the underreporting correction factor and to uncertainties in the percentage of consumers, the reliability of intake estimates on the basis of the MSDI approach is difficult to assess.
The Panel also noted that in contrast to the generally low per capita intake figures estimated on the basis of this MSDI‐approach, in some cases the regular consumption of products flavoured at use levels reported by the Flavour Industry in the submissions would result in much higher intakes. In such cases, the human exposure thresholds below which exposures are not considered to present a safety concern might be exceeded.
Considering that the MSDI model may underestimate the intake of flavouring substances by certain groups of consumers, the SCF recommended also taking into account the results of other intake assessments (SCF, 1999).
One of the alternatives is the ‘Theoretical Added Maximum Daily Intake’ (TAMDI) approach which is calculated on the basis of standard portions and upper use levels (SCF, 1995) for flavourable beverages and foods in general, with exceptional levels for particular foods. This method is regarded as a conservative estimate of the actual intake in most consumers because it is based on the assumption that the consumer regularly eats and drinks several food products containing the same flavouring substance at the upper use level.
One option to modify the TAMDI approach is to base the calculation on normal rather than upper use levels of the flavouring substances. This modified approach is less conservative (e.g. it may underestimate the intake of consumers being loyal to products flavoured at the maximum use levels reported). However, it is considered as a suitable tool to screen and prioritise the flavouring substances according to the need for refined intake data (EFSA, 2004a).
The method for the modified TAMDI (mTAMDI) calculations is described in Appendix C.
To gather information on the occurrence and levels of a flavouring substance in natural sources, the Triskelion database is used (available at the following link https://www.vcf-online.nl/VcfHome.cfm/).
b) Genotoxicity
As reflected in the opinion of SCF (1999), the Panel has in its evaluation focused on a possible genotoxic potential of the flavouring substances or of structurally related substances. Generally, substances for which the Panel has concluded that there is an indication of genotoxic potential in vitro will not be evaluated using the EFSA Procedure until further genotoxicity data are provided. Substances for which a genotoxic potential in vivo has been concluded, will not be evaluated through the Procedure.
Appendix B – Specifications for substances in FGE.13Rev3
1.
Appendix C – Exposure estimates
C.1. Normal and maximum use levels
For each of the 18 Food categories (Table C.1) in which the candidate substances are used, Flavour Industry reports a ‘normal use level’ and a ‘maximum use level’. According to the industry, the ‘normal use’ is defined as the average of reported usages and ‘maximum use’ is defined as the 95th percentile of reported usages (EFFA, 2002). The normal and maximum use levels in different food categories have been extrapolated from figures derived from 12 model flavouring substances (EFFA, 2004a).
Table C.1.
Food categories according to Commission Regulation (EC) No 1565/20003 (Annex III)
| Food category | Description |
|---|---|
| 01.0 | Dairy products, excluding products of category 02.0 |
| 02.0 | Fats and oils, and fat emulsions (type water‐in‐oil) |
| 03.0 | Edible ices, including sherbet and sorbet |
| 04.1 | Processed fruit |
| 04.2 | Processed vegetables (incl. mushrooms & fungi, roots & tubers, pulses and legumes), and nuts & seeds |
| 05.0 | Confectionery |
| 06.0 | Cereals and cereal products, incl. flours & starches from roots & tubers, pulses & legumes, excluding bakery |
| 07.0 | Bakery wares |
| 08.0 | Meat and meat products, including poultry and game |
| 09.0 | Fish and fish products, including molluscs, crustaceans and echinoderms |
| 10.0 | Eggs and egg products |
| 11.0 | Sweeteners, including honey |
| 12.0 | Salts, spices, soups, sauces, salads, protein products etc. |
| 13.0 | Foodstuffs intended for particular nutritional uses |
| 14.1 | Non‐alcoholic (‘soft’) beverages, excl. dairy products |
| 14.2 | Alcoholic beverages, incl. alcohol‐free and low‐alcoholic counterparts |
| 15.0 | Ready‐to‐eat savouries |
| 16.0 | Composite foods (e.g. casseroles, meat pies, mincemeat) – foods that could not be placed in categories 01.0–15.0 |
The ‘normal and maximum use levels’ have been provided by industry for 25 of the 26 candidate substances in FGE.13Rev3 (EFFA, 2003, 2004b, 2007, 2017; Flavour Industry, 2009, 2010). For [FL‐no: 13.130], information on uses and use levels is missing (Table C.2).
C.2. mTAMDI calculation
The method for calculation of modified Theoretical Added Maximum Daily Intake (mTAMDI) values is based on the approach used by SCF up to 1995 (SCF, 1995). The assumption is that a person may consume the amount of flavourable foods and beverages listed in Table C.3. These consumption estimates are then multiplied by the reported use levels in the different food categories and summed up.
Table C.3.
Estimated amount of flavourable foods, beverages and exceptions assumed to be consumed per person per day (SCF, 1995)
| Class of product category | Intake estimate (g/day) |
|---|---|
| Beverages (non‐alcoholic) | 324.0 |
| Foods | 133.4 |
| Exception a: Candy, confectionery | 27.0 |
| Exception b: Condiments, seasonings | 20.0 |
| Exception c: Alcoholic beverages | 20.0 |
| Exception d: Soups, savouries | 20.0 |
| Exception e: Others, e.g. chewing gum | E.g. 2.0 (chewing gum) |
The mTAMDI calculations are based on the normal use levels reported by industry. The seven food categories used in the SCF TAMDI approach (SCF, 1995) correspond to the 18 food categories as outlined in Commission Regulation (EC) No 1565/20003 and reported by the flavour industry in the following way (see Table C.4):
Beverages (SCF, 1995) correspond to food category 14.1
Foods (SCF, 1995) correspond to the food categories 1, 2, 3, 4.1, 4.2, 6, 7, 8, 9, 10, 13 and/or 16
Exception a (SCF, 1995) corresponds to food categories 5 and 11
Exception b (SCF, 1995) corresponds to food category 15
Exception c (SCF, 1995) corresponds to food category 14.2
Exception d (SCF, 1995) corresponds to food category 12
Exception e (SCF, 1995) corresponds to others, e.g. chewing gum.
Table C.4.
Distribution of the 18 food categories listed in Commission Regulation (EC) No 1565/20003 into the seven SCF food categories used for TAMDI calculation (SCF, 1995)
| Food Categories according to Commission Regulation (EC) No 1565/20003 | Distribution of the seven SCF food categories | |||
|---|---|---|---|---|
| Key | Description | Food | Beverages | Exceptions |
| 01.0 | Dairy products, excluding products of category 02.0 | Food | ||
| 02.0 | Fats and oils, and fat emulsions (type water‐in‐oil) | Food | ||
| 03.0 | Edible ices, including sherbet and sorbet | Food | ||
| 04.1 | Processed fruit | Food | ||
| 04.2 | Processed vegetables (incl. mushrooms & fungi, roots & tubers, pulses and legumes), and nuts & seeds | Food | ||
| 05.0 | Confectionery | Exception a | ||
| 06.0 | Cereals and cereal products, incl. flours & starches from roots & tubers, pulses & legumes, excluding bakery | Food | ||
| 07.0 | Bakery wares | Food | ||
| 08.0 | Meat and meat products, including poultry and game | Food | ||
| 09.0 | Fish and fish products, including molluscs, crustaceans and echinoderms | Food | ||
| 10.0 | Eggs and egg products | Food | ||
| 11.0 | Sweeteners, including honey | Food | ||
| 12.0 | Salts, spices, soups, sauces, salads, protein products, etc. |
Exception a Exception d |
||
| 13.0 | Foodstuffs intended for particular nutritional uses | Food | ||
| 14.1 | Non‐alcoholic (‘soft’) beverages, excl. dairy products | Beverages | ||
| 14.2 | Alcoholic beverages, incl. alcohol‐free and low‐alcoholic counterparts | Exception c | ||
| 15.0 | Ready‐to‐eat savouries | Exception b | ||
| 16.0 | Composite foods (e.g. casseroles, meat pies, mincemeat) – foods that could not be placed in categories 01.0–15.0 | Food | ||
Table C.5.
Use of the 25 candidate substances for which use levels have been provided
| Food category | Description | Flavourings used |
|---|---|---|
| 01.0 | Dairy products, excluding products of category 2 | All 25 |
| 02.0 | Fats and oils, and fat emulsions (type water‐in‐oil) | All 25 |
| 03.0 | Edible ices, including sherbet and sorbet | All 25 |
| 04.1 | Processed fruits | All 25 except [FL‐no: 13.125 and 13.162] |
| 04.2 | Processed vegetables (incl. mushrooms & fungi, roots & tubers, pulses and legumes), and nuts & seeds | Only [FL‐no: 13.199] |
| 05.0 | Confectionery | All 25 |
| 06.0 | Cereals and cereal products, incl. flours & starches from roots & tubers, pulses & legumes, excluding bakery | All 25 |
| 07.0 | Bakery wares | All 25 except [FL‐no: 13.136] |
| 08.0 | Meat and meat products, including poultry and game | All 25 |
| 09.0 | Fish and fish products, including molluscs, crustaceans and echinoderms | All 25 except [FL‐no: 13.162 and 13.125] |
| 10.0 | Eggs and egg products | None |
| 11.0 | Sweeteners, including honey | None |
| 12.0 | Salts, spices, soups, sauces, salads, protein products etc. | All 25 |
| 13.0 | Foodstuffs intended for particular nutritional uses | All 25 except [FL‐no: 13.162 and 13.125] |
| 14.1 | Non‐alcoholic (‘soft’) beverages, excl. dairy products | All 25 |
| 14.2 | Alcoholic beverages, incl. alcohol‐free and low‐alcoholic counterparts | All 25 |
| 15.0 | Ready‐to‐eat savouries | All 25 except [FL‐no: 13.162] |
| 16.0 | Composite foods (e.g. casseroles, meat pies, mincemeat) – foods that could not be placed in categories 1–15 | All 25 |
The MSDI and mTAMDI intake estimates for flavouring substances in FGE.13Rev3 are reported in the table below.
Appendix D – Summary of safety evaluations for substances in FGE.13Rev3
1.
Appendix E – Summary of safety evaluations for supporting substances from FGE.67Rev3
1.
Appendix F – Metabolism
F.1. Introduction
The candidate substances in FGE.13Rev2 are furan derivatives which can be divided into two main groups (I and II). Ten candidate substances in subgroup Ia are furfuryl alcohol derivatives. In subgroup Ib, there was only one candidate substance, an alkoyl‐substituted furan which was not supported by Industry and not included in the Union List 4. The two candidate substances in subgroup Ic are alkyl‐substituted furans, without functional groups in the side chains.
The 14 candidate substances in main group II are furan derivatives, containing sulfur substituents as mono‐, di‐ and tri‐sulfides (subgroups IIa, IIc and IId) or free thiol groups (subgroup IIb). The candidate substance 4,5‐dihydro‐3‐mercapto‐2‐methylfuran [FL‐no: 13.108] in subgroup IIb is a non‐aromatic furan derivative.
The subgrouping has been presented in Table 1 (Section 1.4). Further details on the structural properties and the metabolism of the substances in the subgroups Ia, Ib, IIa, IIb, IIc and IId are presented in FGE.13Rev2 (EFSA CEF Panel, 2011) and are not further discussed in this revision of FGE.13.
In this Annex in FGE.13Rev3, only information on subgroup Ic is reported.
F.2. Subgroup Ic – Alkyl‐substituted furans
F.2.1. Hydrolysis of esters
Ester hydrolysis is not an issue for the substances in subgroups Ic.
F.2.2. Absorption, distribution and elimination
Alkylfuran derivatives exhibit rapid uptake, metabolism and excretion. Male Sprague‐Dawley rats administered 100 mg [14C]‐2‐methylfuran/kg bw in sesame oil via intraperitoneal injection showed radiolabelled 2‐methylfuran [FL‐no: 13.030] metabolites in the 12‐h urine (Ravindranath and Boyd, 1991). Maximal hepatic radioactivity was detected at 4 h post‐administration.
Tissue distribution of 50–200 mg [14C]‐2‐methylfuran/kg bw over 24 h showed the presence of radiolabel from greatest to least as follows: liver > kidney > lung > blood. The maximal amount of radiolabel was detected in the liver at 8 h post administration, followed by a steady decline up to 24 h (Ravindranath et al., 1986).
Based on these data, the members of this group of furan‐substituted aliphatic hydrocarbons, alcohols, aldehydes, ketones, carboxylic acids and related esters are anticipated to be rapidly absorbed, distributed through key organs involved in metabolic processes and then eliminated, primarily in the urine.
F.2.3. Biotransformation
No data were available on the candidate substances in subgroup Ic. However, some data on supporting substances have been summarised by the JECFA (JECFA, 2009a). The following text is based on this JECFA evaluation in which some modifications have been included after consultation of the original publications.
For the candidate substances in subgroup Ic, it is of relevance that alkyl‐substituted furan derivatives may undergo cytochrome P450‐mediated side‐chain oxidation to yield an alcohol functional group located at the position bonded directly to the furan ring. The resulting secondary alcohol may be excreted in the urine primarily as the glucuronic acid or sulfate conjugate, or it may be converted to the corresponding ketone, which may also be excreted in the urine. This kind of side‐chain oxidation, preferably at the C1’ position of furan, is similar to that observed with other alkyl‐substituted heterocyclic derivatives (e.g. pyridine derivatives and indoles) (Hawksworth and Scheline, 1975; Thornton‐Manning et al., 1993). It is noted that the resulting secondary furyl alcohol forms an α,β‐unsaturated carbonyl system with the double bonds in the furan ring. In addition to side‐chain oxidation, the furan ring can undergo cytochrome P450‐induced oxidation followed by opening of the ring to yield reactive 2‐enal or 2‐enedial intermediates. It is not entirely clear if in these reactions also epoxide intermediates are involved, but if so, they have to be very unstable. The 2‐enedial intermediates have been shown to form protein and DNA adducts. They also may conjugate readily with GSH, but as their GSH conjugates are unstable, this conjugation offers no protection. However, conjugation with cysteine results in a stable non‐reactive product (Ravindranath et al., 1983, 1984, 1986; Ravindranath and Boyd, 1985, 1991).
Initial in vitro experiments in rat microsomal preparations suggested that high concentrations of alkyl‐substituted furans are partly metabolised to reactive acetylacrolein‐type intermediates (Ravindranath et al., 1983, 1984). Acetylacrolein is a potent microsomal mixed‐function oxidase inhibitor that has been reported to bind covalently and irreversibly to the oxidising enzyme, thus inactivating it (Ravindranath and Boyd, 1985).
Acetylacrolein (= 4‐oxo‐pent‐2‐enal) is a potent microsomal mixed‐function oxidase inhibitor which has been reported to bind covalently and irreversibly to the oxidising enzyme, thus deactivating it (Ravindranath and Boyd, 1985). Significant protein binding (> 55 nmol/mg protein) was reported when 10 mmol/L of [2‐14C]‐methylfuran were incubated with rat hepatic microsomes in the presence of NADPH and oxygen. In the absence of oxygen or NADPH, little binding was observed (< 2 nmol/mg protein). These findings suggest that NADPH‐dependent oxidation of 2‐methylfuran is a prerequisite for protein binding. Increased protein binding (> 80 nmol/mg protein) was also reported when Sprague‐Dawley rats were pre‐treated with phenobarbital, a cytochrome P450 inducer, while decreased or no protein binding was observed in the presence of piperonyl butoxide or N‐octyl imidazole, both of which inhibit cytochrome P450. The Vmax and Km for 2‐methylfuran metabolism in phenobarbital pre‐treated rats were 0.81 μmol/2 mg microsomal protein per min and 0.463 mmol/l, respectively, and those in rats without phenobarbital pre‐treatment were 0.53 μmol/2 mg microsomal protein per min and 1.417 mmol/L, respectively. These values suggest that 2‐methylfuran undergoes cytochrome P450‐mediated oxidation to yield a reactive metabolite (i.e. acetylacrolein) which binds covalently to protein (Ravindranath and Boyd, 1985). With 3‐methylfuran, a similar ring opening product (3‐methyl‐but‐2‐enedial) has been found (Ravindranath et al., 1983, 1984).
In the same study (Ravindranath and Boyd, 1985), when acetylacrolein at 0.25 mmol/L (24.5 μg/mL) was added to the incubation mixture, microsomal metabolism of 2‐methylfuran was almost completely inhibited (covalent binding was 1.5% of the control incubation). At a concentration of 0.5 mmol acetylacrolein/L (49.1 μg/mL), no metabolism of 2‐methylfuran was detectable, suggesting that acetylacrolein inhibits cytochrome P450‐mediated oxidation, probably through direct covalent binding with the enzyme. Thus, 2‐methylfuran is a suicide substrate for this enzyme. Conjugation of the reactive metabolite with sulfhydryl trapping agents, including cysteine (10 mmol/L) and GSH (10 mmol/L), showed a marked decrease in microsomal protein binding, suggesting that sulfhydryl conjugation plays a role in the detoxication of acetylacrolein. Cysteine was the better trapping agent for the prevention of microsomal protein binding when compared with GSH, semicarbazide, lysine or N‐acetylcysteine. The authors postulated that cysteine forms a stable cyclic conjugate with α, β‐unsaturated aldehydes, whereas the ability of GSH to form stable conjugates with α, β‐unsaturated aldehydes varies (Esterbauer et al., 1975, 1976; Ravindranath and Boyd, 1985).
Other in vitro experiments support the conclusion that cytochrome P450‐mediated oxidation of 2‐methyl‐furan is directly related to its toxicity. This was studied in hepatocytes isolated from adult male Wistar rats that were untreated or treated with phenobarbitone (0.1% in drinking water for 5 days) or β‐naphthoflavone (80 mg/kg bw by intraperitoneal injection daily for 3 days). The cultured hepatocytes were incubated with 2‐methylfuran at 0, 100, 300, 600 or 1,000 μmol/L (0, 8.2, 24.6, 49.3 and 82.1 μg/mL, respectively) for 24 h. The median lethal concentrations (LC50 values) for untreated, phenobarbitone‐treated or β‐naphthoflavone‐treated hepatocytes were 794, 34 and 57 μmol/L (65.2, 2.8 and 4.7 μg/mL), respectively, indicating that enzyme induction increased the toxicity of 2‐methylfuran (Hammond and Fry, 1991).
Male Sprague‐Dawley rats (150–200 g) were administered a single dose of 50, 100, 200 or 400 mg 2‐methylfuran/kg bw in sesame oil by intraperitoneal injection and were sacrificed 24 h later. The 50 mg/kg bw group did not show any evidence of liver necrosis, but they exhibited endothelial injury, with blebbing of the endothelium into the vascular lumen of the central veins. Animals given 100, 200 or 400 mg 2‐methylfuran/kg bw showed a dose‐related increase in the severity of hepatocellular injury (e.g. eosinophilic cytoplasm, vacuolation), centrilobular necrosis and necrosis and sloughing of the bronchiolar epithelium, which, at the high dose, resulted in complete obliteration of numerous respiratory and terminal bronchioles. Dose‐related increases in serum glutamic pyruvic transaminase (GPT) were observed up to 200 mg 2‐methylfuran/kg bw; however, the levels of serum GPT in the animals given 50 mg 2‐methylfuran/kg bw were not significantly higher than those of the control rats. Free GSH levels in the liver, lungs and kidneys, investigated over a period of 0.5–36 h after administration of 100 mg 2‐methylfuran/kg bw, were initially decreased (67.5% of control in the liver and 87% of control in the kidneys at 0.5 h), but then reached or exceeded control levels within 8–24 h (137% of control in the kidneys and 130% of control in the lungs at 12 h). Tissue distribution and covalent binding studies were conducted over a period of 0.5–24 h after an intraperitoneal dose of 100 mg [14C]‐2‐methylfuran/kg bw. The radiolabelled [14C]‐2‐methylfuran covalently bound to protein was detected at the highest concentration in the liver, followed by the kidney, lung and blood. Liver and kidney DNA also showed covalent binding of 14C label. Maximal covalent binding to DNA was observed in the liver at 1 h and in the kidney at 4 h. With phenobarbital pretreatment, a twofold increase in binding in the liver was observed. Conversely, N‐octylimidazole pretreatment decreased the level of covalent binding of the 14C label to proteins and DNA in the liver, lung and kidney. Increased and decreased protein binding and hepatotoxicity measured as serum GPT levels were observed in rats pretreated with phenobarbital and N‐octylimidazole, respectively. 3‐Methylcholanthrene or piperonyl butoxide pretreatment did not affect either covalent binding or hepatotoxicity. These results provide evidence that bioactivation of 2‐methylfuran by a CYP system is a prerequisite for tissue necrosis in rats (Ravindranath et al., 1986).
In a study examining GSH and cysteine conjugation on the toxic potential of 2‐methylfuran, male Sprague‐Dawley rats were treated subcutaneously with a 900 mg/kg bw dose of buthionine sulfoximine 1.5 h prior to intraperitoneal administration of 100 mg [14C]2‐methylfuran/kg bw prepared in sesame oil. Marked decreases in covalent DNA and protein binding in the liver and reduced hepatotoxicity, as indicated by lower serum GPT levels, were observed. Buthionine sulfoximine treatment revealed a transient increase in plasma cysteine levels, concurrent with a decrease in GSH levels. However, administration of 100 mg 2‐methylfuran/kg bw 1.5 h after buthionine sulfoximine administration significantly reduced plasma cysteine levels and increased (20%) urinary elimination of 2‐methylfuran‐labelled metabolites compared with the control group ([14C]‐2‐methylfuran only). Subcutaneous pretreatment with diethylmaleate, a depletor of liver GSH, at 0.4 ml/kg bw increased binding to liver proteins and increased hepatotoxicity, as indicated by a rise in serum GPT levels compared with rats that received only 2‐methylfuran. Subcutaneous pretreatment of rats with GSH synthesis promoter L‐2‐oxothiazolidine‐4‐carboxylate at a dose of 1,000 mg/kg bw resulted in a marked increase of covalent protein binding in the liver and potentiated hepatotoxicity (increased serum GPT levels compared with rats that received only 2‐methyl‐furan). When rats were pretreated with both buthionine sulfoximine and L‐2‐oxothiazolidine‐4‐carboxylate, a marked decrease in covalent protein binding in the liver and hepatotoxicity, as indicated by a reduction in serum GPT levels, was observed. No unchanged 2‐methylfuran was observed in the urine, indicating that pretreatment did not inhibit metabolic processes (Ravindranath and Boyd, 1991). The authors proposed that buthionine sulfoximine pretreatment indirectly aids in the detoxication of 2‐methylfuran through a reduction of GSH supply and an increase in the availability of cysteine, which forms a more stable conjugate with acetylacrolein than GSH.
Adult male Swiss albino mice (10–15 per group) were administered 2‐ethylfuran (commercial grade, FL‐no: 13.092) at 200 mg/kg bw in sesame oil via intraperitoneal injection with or without phenobarbital, piperonyl butoxide or cobalt(II) chloride pretreatment. The mortality rates were 1/10, 2/10, 3/15 and 2/11 for the untreated, phenobarbital pretreatment, piperonyl butoxide pretreatment and cobalt(II) chloride pretreatment groups, respectively. 2‐Ethylfuran produced a moderate necrosis of the liver and mild to moderate necrosis of the kidneys. The kidney necrosis was described as a coagulative lesion of the proximal convoluted tubules of the outer cortex, without damage to the glomerular or medullary cells. Piperonyl butoxide and cobalt(II) chloride decreased the severity of necrosis in the liver and kidney (McMurtry and Mitchell, 1977).
In the same study, mice were injected intraperitoneally with 70 mg 2‐acetylfuran [FL‐no: 13.054] (commercial grade)/kg bw in 0.9% sodium chloride, with and without phenobarbital pretreatment, and 80 mg 2‐acetylfuran/kg bw, with and without cobalt(II) chloride pretreatment. The mortality rates were 1/12, 0/12, 0/12 and 0/12 for the 70 mg 2‐acetylfuran/kg bw, 70 mg 2‐acetylfuran/kg bw plus phenobarbital, 80 mg 2‐acetylfuran/kg bw, and 80 mg 2‐acetylfuran/kg bw plus cobalt(II) chloride treatment groups, respectively. Mice treated with 2‐acetylfuran showed no evidence of toxicity in the kidneys. Hepatic necrosis, described as midzonal‐centrilobular necrosis of the parenchymal hepatocytes, was mild in severity with cobalt(II) chloride pretreatment, showing a marked decrease in the incidence and severity of necrosis (McMurtry and Mitchell, 1977).
Ten male ICR mice were injected intraperitoneally with 2‐ethylfuran (analytical reagent grade) at 2.6 mmol/kg bw (250 mg/kg bw) in sesame oil. Histopathology of tissues collected 24 h later revealed extensive proximal tubular necrosis of the kidneys and focal hydroptic degeneration of the liver. Significant increases in the plasma urea nitrogen level (approximately five times control level) and GPT level were reported (Wiley et al., 1984).
Severe bronchiolar necrosis was reported when 2‐ethylfuran (2.6 mmol/kg bw or 250 mg/kg bw) in sesame oil was administered by intraperitoneal injection to male ICR mice. Administration of 1.56 mmol 2‐ethylfuran/kg bw (150 mg/kg bw) via intraperitoneal injection to five male ICR mice showed approximately a doubling, compared with control values, of the amount of [14C]‐thymidine incorporation into pulmonary DNA measured at 3 days after dosing, which indicates increased cell replication and lung repair (Gammal et al., 1984).
In a study of the tumour‐inhibiting properties of 2‐heptylfuran [FL‐no: 13.069], increased cytosolic glutathione S‐transferase activity was observed in tissue preparations of the liver, forestomach and small bowel mucosa isolated from 7‐week‐old female A/J mice (five mice per group) that received doses of 12, 25, 50 or 80 μmol of 2‐heptylfuran dissolved in cottonseed oil via gavage every other day for a total of three doses. A 50 μmol dose of 2‐heptylfuran showed a significant increase in acid‐soluble sulfhydryl levels, which is a good measure of GSH content in tissues, in all four tissue types (liver, small bowel mucosa, forestomach and lung) when compared with controls. At lower dose levels, the increases became inconsistent. At the highest dose level, the increase was lower than at 50 μmol probably because of the toxicity of the substance (Lam and Zheng, 1992).
In summary, 2‐alkyl‐substituted furans can be metabolised by side‐chain oxidation to initially yield the 1’‐alcohol derivative, which can be either conjugated and excreted or oxidised to the corresponding (α,β,‐unsaturated) ketone. The conversion to the ketone is anticipated to be reversible, in which case the ketones are reduced to the corresponding alcohols and excreted mainly in the urine. In a second pathway, the furan ring can be oxidised and may undergo rapid ring opening to yield reactive 2‐ene‐1,4‐dicarbonyls (e.g. acetylacrolein) possibly through an unstable epoxide intermediate. These reactive 2‐ene‐1,4‐dicarbonyls can be conjugated with available sulfhydryl trapping agents, such as GSH and cysteine, or can be covalently bound to proteins and DNA.
Much less information is available on the metabolism of dialkylfurans, of which the candidate substance [FL‐no: 13.125] is an example. For 2,5‐dimethylfuran, the formation of the reactive metabolite hex‐3‐ene‐2,5‐dione has been postulated, based on studies with dimethylfuran in microsomal incubates, using trapping agents (Wang et al., 2015). It could be demonstrated that this intermediate showed reactivity towards amino‐ or thiol‐moieties in proteins in these in vitro systems. In protein‐digests of various tissues (liver, heart, lung, kidney, serum), dimethylfuran‐derived adducts were detected after intraperitoneal administration of 2,5‐dimethylfuran to mice.
F.3. Summary
The mono‐alkyl furans from subgroup Ic, such as the candidate substance 2‐octylfuran [FL‐no: 13.162], may undergo oxidation (possibly epoxidation of the unsubstituted double bond) and rearrangement to an oxo‐aldehyde (a ring‐opening product). For several 2‐alkyl‐substituted furans reactivity of these oxo‐aldehydes towards proteins and DNA has been demonstrated, resulting in toxicity to liver and kidneys. In addition, oxidation of the C1’‐carbon of the alkyl substituent may result in the formation of an α,β‐unsaturated ketone, for which genotoxicity might be anticipated. For 2,5‐dimethylfuran, the formation of hex‐3‐ene‐2,5‐dione has been postulated.
F.4. Conclusion
For candidate substances in subgroup Ic, oxidation and opening of the furan ring, which results in the formation of reactive and toxic metabolites can be anticipated. It cannot be concluded that these substances are metabolised only into innocuous products.
Appendix G – Genotoxicity studies on supporting substances considered in FGE.13Rev2 and in FGE.67Rev2
1.
Table G.1.
In vitro and in vivo genotoxicity data for furan‐substituted substances evaluated by the JECFA at the 65th (JECFA, 2006b) and 69th meeting (JECFA, 2009a)
| Chemical name [FL‐no] JECFA‐no. | Test system | Test object | Concentration/dose and test conditions | Results | Reference |
|---|---|---|---|---|---|
| In vitro | |||||
|
2‐Methylfuran [13.030]s 1487 |
Reverse mutation | S. Typhimurium TA98 and TA100 | 0.165, 0.330, 0.495 or 0.660 μmol/plate (13.5, 27.1, 40.6 or 54.2 μg/plate)a | Negativeb | Shinohara et al. (1986) |
| Reverse mutation | S. Typhimurium TA98, TA100, TA102 and TA1535 | Up to 10,000 μg/plate | Negative(b),(c),(d) | Zeiger et al. (1992) | |
| Reverse mutation | S. Typhimurium TA97 and TA104 | Up to 10,000 μg/plate | Equivocal(b),(c),(d) | Zeiger et al. (1992) | |
| Reverse mutation | S. Typhimurium TA98, TA100 and TA102 | 11 nmol/plate to 1.1 mmol/plate (0.9–90,310 μg/plate)a | Negativeb | Aeschbacher et al. (1989) | |
| DNA damage | B. subtilis H17 (rec+) and M45 (rec−) | 0.16, 16 or 1,600 μg/disc |
Negative Positive(b),(e) |
Shinohara et al. (1986) | |
| Chromosomal aberration | CHO cells | 0–150 mmol/L (0–12,315 μg/mL)a | Positive(b),(f) | Stich et al. (1981) | |
|
2,5‐Dimethylfuran [13.029]s 1488 |
Reverse mutation | S. Typhimurium TA98 and TA100 | 0.165, 0.330, 0.495 or 0.660 μmol/plate (13.5, 27.1, 40.6 or 54.2 μg/plate)g | Negativeb | Shinohara et al. (1986) |
| Reverse mutation | S. Typhimurium TA98 and TA100 | Not specified | Negativeb | Lee et al. (1994) | |
| Reverse mutation | S. Typhimurium TA97, TA98, TA100 and TA1535 | Up to 3,333 μg/plate | Negative(b),(c),(d) | Zeiger et al. (1992) | |
| DNA damage | B. subtilis H17 (rec+) and M45 (rec−) | 190, 1,900 or 9,500 μg/disc |
Negative Positive(b),(h) |
Shinohara et al. (1986) | |
| Chromosomal aberration | Chinese hamster V79 cells | 1 mmol/L (96.13 μg/mL)g | Negative | Ochi and Ohsawa (1985) | |
| Chromosomal aberration | CHO cells | 0–20 mmol/L (0–1,923 μg/mL)g | Positive(b),(f) | Stich et al. (1981) | |
|
3‐Methyl‐2‐(3‐methylbut‐2‐enyl)‐furan [13.148] 1494 |
Reverse mutation | S. Typhimurium TA98, TA100, TA1535 and TA1537 | 3.2, 16, 80, 400 or 2,000 μg/plate | Negativeb | Asquith (1989) |
|
2‐Acetylfuran [13.054] 1503 |
Reverse mutation | S. Typhimurium TA98 and TA100 | 0.165, 0.330, 0.495 or 0.660 μmol/plate (13.5, 27.1, 40.6 or 54.2 μg/plate)i |
Negative Positive(b),(j) |
Shinohara et al. (1986) |
| DNA damage | E. coli PQ37 (SOS chromotest) | Not specified | Slightly positivei | Eder et al. (1993) | |
| DNA damage | B. subtilis H17 (rec+) and M45 (rec−) | 550, 5,500 or 55,000 μg/disc |
Negative Positive(b),(k) |
Shinohara et al. (1986) | |
| Chromosomal aberration | CHO cells | 0–112.6 mmol/L (0–13,220 μg/mL)i | Positive(b),(l),(m) | Stich et al. (1981) | |
| UDS | Human hepatocytes | 2.19, 4.38, 8.75, 17.5, 35, 70, 140 or 280 μg/mL | Negative | Durward (2007a) | |
| In vivo | |||||
|
2‐Methylfuran [13.030]s 1487 |
Chromosomal aberration | Mouse bone marrow cells and spermatocytes | 1,000, 2,000 or 4,000 mg/kg (100, 200 or 400 mg/kg bw per day)n | Negative | Subramanyam et al. (1989) |
|
2‐Acetylfuran [13.054] 1503 |
Chromosomal aberration | Mouse bone marrow | 1,000, 2,000 or 3,000 mg/L (20, 40 or 60 mg/kg bw)o | Positive(p),(q) | Sujatha et al. (1993) |
| Chromosomal aberration | Mouse spermatocytes | 1,000, 2,000 or 3,000 mg/L (20, 40 or 60 mg/kg bw)o | Negativer | Sujatha et al. (1993) | |
| SCE | Mouse bone marrow | 1,000, 2,000 or 3,000 mg/L (20, 40 or 60 mg/kg bw)o | Positive | Sujatha (2007) | |
| UDS | Rat liver | 7 or 21 mg/kg bw | Negative | Durward (2007b) | |
CHO: Chinese hamster ovary; SCE, sister chromatid exchange; UDS, unscheduled DNA synthesis.
Calculated using relative molecular mass of 2‐methylfuran = 82.1.
With and without metabolic activation.
Preincubation method.
Occasional incidences of slight to complete clearing of the background lawn at the higher concentrations.
Negative at all concentrations with metabolic activation; positive without metabolic activation.
Clastogenic activity decreased with metabolic activation (statistical significance of results was not specified).
Calculated using relative molecular mass of 2,5‐dimethylfuran = 96.13.
Positive at every concentration without metabolic activation; with metabolic activation, negative at 190 μg/disc, but positive at higher concentrations.
Calculated using relative molecular mass of 2‐furyl methyl ketone = 110.11.
Positive only in strain TA98 with an increase in the presence of metabolic activation.
Negative at 550 μg/disc; positive at 5,500 and 55,000 μg/disc (with and without metabolic activation).
Cytotoxicity was observed at 12 398 μg/mL (112.6 mmol/L) in the presence of metabolic activation.
Clastogenic activity increased with metabolic activation (statistical significance of results was not specified).
Mice received 2‐methylfuran in the diet for 5 consecutive days at 24‐h intervals.
Two experimental protocols were utilised. In one experiment, animals received single oral dose administrations of the test compound. In the other experiment, the test compound was orally administered once per day at the same concentrations as in the single‐dose study for 5 consecutive days with 24‐h intervals between doses.
No effects observed at 20 mg/kg bw dose level and only mild, but significant (p < 0.05) effects seen at higher concentrations in bone marrow cells.
Chromosomal aberrations were observed in the presence of significant mitodepression.
A single statistically significant occurrence of increased chromosomal aberrations observed 3 weeks following a single dose administration in the 60 mg/kg bw test group; statistically significant increases in polyploidy and XY univalents observed at weeks 3 and 4 at 60 mg/kg bw in multiple dose‐treated rats.
Substance deleted from the Union list. Commission Regulation (EU) No 246/2014 of 13 March 2014 amending Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council as regards removal from the Union list of certain flavouring substances. OJ L 74, 14.3.2014, p. 58–60.
Appendix H – Genotoxicity studies on supporting substances from FGE.67Rev3
1.
No genotoxicity studies are available on candidate substances from subgroup Ic. Available studies on the supporting substance 2‐pentylfuran [FL‐no: 13.059] and on 2‐acetylfuran [FL‐no: 13.054] are presented in Tables H.1 and H.2.
Table H.1.
Summary of in vitro genotoxicity data evaluated in FGE.67Rev3
| Chemical name [FL‐no] | Test system | Test object | Concentrations of substance and test conditions (μg/mL) | Result | Reference | Comments |
|---|---|---|---|---|---|---|
|
2‐acetylfuran [13.054] |
Micronucleus assay | Human peripheral blood lymphocytes | From 34.7 to 1,111(a),(b),(c) | Negative | Charles River (2020a) | Reliable without restrictions. Study performed in compliance with OECD TG 487 and GLP |
|
2‐pentylfuran [13.059] |
Micronucleus assay | Human peripheral blood lymphocytes |
From 54.5 to 100.8(a),(b) From 51.4 to 91.1c |
Negative | Charles River (2020b) | Reliable without restrictions. Study performed in compliance with OECD TG 487 and GLP |
Without S9 metabolic activation, 4 + 20 h treatment.
With S9 metabolic activation, 4 + 20 h treatment.
Without S9 metabolic activation, 24 h treatment.
Table H.2.
Summary of in vivo genotoxicity data evaluated in FGE.67Rev3
| Chemical name [FL‐no] | Test system | Test object | Route | Dose (mg/kg bw per day) | Result | Reference | Comments |
|---|---|---|---|---|---|---|---|
| 2‐acetylfuran [13.054] | Micronucleus assay (peripheral blood) | Muta® Mice, M | Gavage | 0, 15, 30 and 60 | Negative | Covance (2016) | Reliable with restrictions (no clear evidence of bone marrow exposure). Study performed in compliance with GLP and according to OECD TG 474, but positive control was not included |
| Gene mutation assay in liver and duodenum | Negative | Reliable without restrictions. Study performed in compliance with GLP and according to OECD TG 488 | |||||
|
2‐pentylfuran [13.059] |
Comet assay (liver) | B6C3F1 mice, M | Gavage | 508 |
Positive Negative in the presence of proteinase K |
New York Medical College (2012) |
Insufficient reliability The study aimed at comparing furan and 2‐pentylfuran. Liver was sampled 3h after treatment |
| 762 | Negative both with and without proteinase K | ||||||
| 508, 762 |
Negative both with and without proteinase K Re‐estimate: reduction of tail length at the highest dose, both with and without proteinase K |
||||||
|
2‐pentylfuran [13.059] |
Micronucleus assay (bone marrow) | Han Wistar rats, M | Gavage | 42.5, 85, 170a | Negative | Covance (2014) | Reliable with restrictions (no clear evidence of bone marrow exposure). Study performed in compliance with GLP and according to OECD TG 474 |
| Comet assay (liver) | Negative | Reliable without restrictions. The study was performed in compliance with recommendations of the Comet and IWGT workshop, Japanese Center for the Validation of Alternative Methods (JaCVAM) and current literature |
M: male.
Administered via gavage in three doses at times 0, 24 and 45 h with sacrifice and harvest at 48 h.
Appendix I – Toxicity studies on supporting substances from FGE.67Rev3
1.
No toxicity studies are available on candidate substances from subgroup Ic. Available studies on the supporting substance 2‐pentylfuran [FL‐no: 13.059] and on 2‐acetylfuran [FL‐no: 13.054] are presented in Table I.1.
Suggested citation: EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , Younes M, Aquilina G, Castle L, Engel K‐H, Fowler P, Frutos Fernandez MJ, Fürst P, Gundert‐Remy U, Gürtler R, Husøy T, Manco M, Moldeus P, Passamonti S, Shah R, Waalkens‐Berendsen I, Wölfle D, Wright M, Benigni R, Bolognesi C, Chipman K, Cordelli E, Degen G, Marzin D, Svendsen C, Carfì M, Vianello G and Mennes W, 2021. Scientific Opinion on Flavouring Group Evaluation 13 Revision 3 (FGE.13Rev3): furfuryl and furan derivatives with and without additional side‐chain substituents and heteroatoms from chemical group 14. EFSA Journal 2021;19(2):6386, 61 pp. 10.2903/j.efsa.2021.6386
Requestor: European Commission
Question numbers: EFSA‐Q‐2014‐00421, EFSA‐Q‐2014‐00422
Panel members: Gabriele Aquilina, Laurence Castle, Karl‐Heinz Engel, Paul Fowler, Maria Jose Frutos Fernandez, Peter Fürst, Ursula Gundert‐Remy, Rainer Gürtler, Trine Husøy, Melania Manco, Wim Mennes, Peter Moldeus, Sabina Passamonti, Romina Shah, Maged Younes, Ine Waalkens‐Berendsen, Detlef Wölfle and Matthew Wright.
Acknowledgements: The Panel wishes to thank the following hearing expert for the support provided to this scientific output: Karin Nørby.
Adopted: 15 December 2020
Notes
Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. OJ L 354, 31.12.2008, p. 34–50.
Commission implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC. OJ L 267, 2.10.2012, p. 1–161.
Commission Regulation (EC) No 1565/2000 of 18 July 2000 laying down the measures necessary for the adoption of an evaluation programme in application of Regulation (EC) No 2232/96. OJ L 180, 19.7.2000, p. 8–16.
EFSA Journal 2011;9(10):2315.
EFSA Journal 2011;9(8):2313.
The substance [FL‐no: 13.192] has not been added to the Union List, i.e. Commission Implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC.
Commission Regulation (EU) No 246/2014 of 13 March 2014 amending Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council as regards removal from the Union list of certain flavouring substances. OJ L74, 14.3.2014, p. 58–60.
EU figure 375 millions (Eurostat, 1998). This figure relates to EU population at the time for which production data are available, and is consistent (comparable) with evaluations conducted prior to the enlargement of the EU. No production data are available for the enlarged EU.
This substance has been deleted from the Union list, see Commission Implementing Regulation (EU) No 246/2014 of 13 March 2014 amending Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council as regards removal from the Union list of certain flavouring substances. OJ L 74, 14.3.2014, p. 58–60.
https://efsa.openanalytics.eu/ freely available
The FAF Panel is aware that a Revised Procedure for the Safety Evaluation of Flavouring agents has been agreed by JECFA (JECFA, 2016). The EFSA Scientific Committee has developed a modified procedure for evaluation of substances based on the TTC approach (EFSA Scientific Committee, 2019). However, these developments have no impact on the present evaluation, which should follow the requirements as set out in Commission Regulation (EC) No 1565/2000.
‘Innocuous products’: products that are known or readily predicted to be harmless to humans at the estimated intakes of the flavouring agent (JECFA, 1997).
‘Endogenous substances’: intermediary metabolites normally present in human tissues and fluids, whether free or conjugated; hormones and other substances with biochemical or physiological regulatory functions are not included (JECFA, 1997).
References
- Aeschbacher HU, Wolleb U, Loliger J, Spadone JC and Liardon R, 1989. Contribution of coffee aromaconstituents to the mutagenicity of coffee. Food and Chemical Toxicology, 27, 227–232. [DOI] [PubMed] [Google Scholar]
- Cramer GM, Ford RA and Hall RL, 1978. Estimation of toxic hazard ‐ a decision tree approach. Food and Cosmetics Toxicology, 16, 255–276. [DOI] [PubMed] [Google Scholar]
- Eder E, Scheckenbach S, Deininger C and Hoffman C, 1993. The possible role of alpha, beta‐unsaturated carbonyl compounds in mutagenesis and carcinogenesis. Toxicology Letters, 67, 87–103. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2004a. Minutes of the 7th Plenary meeting of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food, Held in Brussels on 12‐13 July 2004. Brussels, 28 September 2004. Available online: https://www.efsa.europa.eu/en/events/event/afc040712
- EFSA (European Food Safety Authority), 2004b. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with food (AFC) on a request from the Commission related to furfural and furfural diethylacetal. Question number EFSA‐Q‐2003‐236. Adopted by written procedure on 2 June 2004. EFSA Journal 2004;2(7):67, 27 pp. 10.2903/j.efsa.2004.67 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2005. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) on a request from the Commission related to Flavouring Group Evaluation 13: Furfuryl and furan derivatives with and without additional side‐chain substituents and heteroatoms from chemical group 14 (Commission Regulation (EC) No 1565/2000 of 18 July 2000). EFSA Journal 2005;3(7):215, 73 pp. 10.2903/j.efsa.2005.215 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008. Scientific Opinion. List of alpha, beta‐unsaturated aldehydes and ketones representative of FGE.19 substances for genotoxicity testing ‐ Statement of the Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). EFSA Journal 2008;17(7):910, 7 pp. 10.2903/j.efsa.2008.910 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2010. Scientific Opinion on Flavouring Group Evaluation 13, Revision 1 (FGE.13Rev1): Furfuryl and furan derivatives with and without additional side‐chain substituents and heteroatoms from chemical group 14. EFSA Journal 2010;8(4):1403, 112 pp. 10.2903/j.efsa.2010.1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2011. Scientific Opinion on Flavouring Group Evaluation 13, Revision 2 (FGE.13Rev2): Furfuryl and furan derivatives with and without additional side‐chain substituents and heteroatoms from chemical group 14. EFSA Journal 2011;9(8):2313, 126 pp. 10.2903/j.efsa.2011.2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2015a. Scientific Opinion on Flavouring Group Evaluation 67, Revision 2 (FGE.67Rev2): consideration of 28 furan‐substituted compounds evaluated by JECFA at the 55th, 65th and 69th meetings (JECFA, 2001, 2006a, 2009b). EFSA Journal 2015;13(5):4115, 107 pp. 10.2903/j.efsa.2015.4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2015b. Scientific Opinion on Flavouring Group Evaluation 65, Revision 1 (FGE.65Rev1): consideration of sulfur‐substituted furan derivatives used as flavouring agents evaluated by JECFA (59th meeting) structurally related to a subgroup of substances within the group of ‘Furfuryl and furan derivatives with and without additional side‐chain substituents and heteroatoms from chemical group 14’ evaluated by JECFA in FGE.13Rev2 (2011). EFSA Journal 2015;13(2):4024, 44 pp. 10.2903/j.efsa.2015.4024 [DOI] [Google Scholar]
- EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings), 2021. Scientific Opinion on Flavouring Group Evaluation 67, Revision 3 (FGE.67Rev3): consideration of 23 furan‐substituted compounds evaluated by JECFA at the 55th, 65th 69th and 86th meetings. EFSA Journal 2021;19(1):6362, 83 pp. 10.2903/j.efsa.2021.6362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: general principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2017a. Scientific Opinion on the clarification of some aspects related to genotoxicity assessment. EFSA Journal 2017;15(12):5113, 25 pp. 10.2903/j.efsa.2017.5113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2017b. Update: use of the benchmark dose approach in risk assessment. EFSA Journal 2017;15(1):4658, 41 pp. 10.2903/j.efsa.2017.4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2019. Guidance on the use of the Threshold of Toxicological Concern approach in food safety assessment. EFSA Journal 2019;17(6):5708, 17 pp. 10.2903/j.efsa.2019.5708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H, Zollner H and Scholz N, 1975. Reaction of glutathione with conjugated carbonyls. Zeitschrift für Naturforschung, 30c, 466–473. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Ertl A and Scholz N, 1976. The reaction of cysteine with alpha, beta‐unsaturated aldehydes. Tetrahedron, 32, 285–289. [Google Scholar]
- Eurostat , 1998. Total population. Cited in Eurostat, 2004.
- Gammal LM, Wiley RA, Traiger G, Haschek WM and Baraban S, 1984. Toxicity‐distribution relationships among 3‐alkylfurans in the mouse lung. Toxicology, 30, 177–184. [DOI] [PubMed] [Google Scholar]
- Hammond AH and Fry JR, 1991. The use of hepatocytes cultured from inducer‐treated rats in the detection of cytochrome p‐450‐mediated cytotoxicity. Toxicology in Vitro, 5, 133–137. [DOI] [PubMed] [Google Scholar]
- Hawksworth G and Scheline RR, 1975. Metabolism in the rat of some pyrazine derivatives having flavour importance in foods. Xenobiotica, 5, 389–399. [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1995. Evaluation of certain food additives and contaminants. Forty‐fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives. 14‐23 February 1995. WHO Technical Report Series, no. 859. Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1996. Toxicological evaluation of certain food additives. The forty‐fourth meeting of the Joint FAO/WHO Expert Committee on Food Additives and contaminants. WHO Food Additives Series: 35. IPCS, WHO, Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1997. Evaluation of certain food additives and contaminants. Forty‐sixth report of the Joint FAO/WHO Expert Committee on Food Additives. Geneva, 6‐15 February 1996. WHO Technical Report Series, no. 868. Geneva. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1999. Evaluation of certain food additives and contaminants. Forty‐ninth report of the Joint FAO/WHO Expert Committee on Food Additives. Rome, 17‐26 June 1997. WHO Technical Report Series, no. 884. Geneva. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2001a. Evaluation of certain food additives and contaminants. Fifty‐fifth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 901. Geneva, 6‐15 June 2000.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2001b. Safety evaluation of certain food additives and contaminants. Fifty‐fifth meeting of the Joint FAO/WHO Expert Committee on Food Additives, WHO Food Additives Series: 46. IPCS, WHO, Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2001c. Compendium of food additive specifications. Addendum 9. Joint FAO/WHO Expert Committee of Food Additives 57th session. Rome, 5‐14 June 2001. FAO Food and Nutrition paper 52 Add. 9.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2002. Evaluation of certain food additives. Fifty‐ninth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 913. Geneva, 4‐13 June 2002.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2003. Safety evaluation of certain food additives. Fifty‐ninth meeting of the Joint FAO/WHO Expert Committee on Food Additives, WHO Food Additives Series: 50. IPCS, WHO, Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2006a. Evaluation of certain food additives. Sixty‐fifth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 934. Geneva, 7‐16 June 2005.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2006b. Safety evaluation of certain food additives and contaminants. Sixty‐fifth meeting of the Joint FAO/WHO Expert Committee on Food Additives, WHO Food Additives Series: 56. IPCS, WHO, Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2009a. Safety evaluation of certain food additives and contaminants. Sixty‐ninth meeting of the Joint FAO/WHO Expert Committee on Food Additives, WHO Food Additives Series: 60. IPCS, WHO, Geneva 2009. Available online: http://whqlibdoc.who.int/publications/2009/9789241660600_eng.pdf (May 2009)
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2009b. Evaluation of certain food additives. Sixty‐ninth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 952. Rome, 17‐26 June 2008. Available online: http://whqlibdoc.who.int/trs/WHO_TRS_952_eng.pdf (May 2009).
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2012. Evaluation of certain food additives. Seventy‐sixth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 974.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2016. Evaluation of certain food additives. Eighty‐second report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 1000.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2019. Evaluation of certain food additives. Eighty‐sixth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 1014.
- Lam L and Zheng B, 1992. Inhibitory effects of 2‐n‐heptylfuran and 2‐n‐butylthiophene on benzo[a]pyrene‐induced lung and forestomach tumorigenesis in A/J mice. Nutrition and Cancer, 17, 19–26. [DOI] [PubMed] [Google Scholar]
- Lee H, Bian SS and Chen YL, 1994. Genotoxicity of 1,3‐dithiane and 1,4‐dithiane in the CHO/SCE assay and the Salmonella/microsomal test. Mutation Research, 321, 213–218. [DOI] [PubMed] [Google Scholar]
- McMurtry RJ and Mitchell JR, 1977. Renal and hepatic necrosis after metabolic activation of 2‐substituted furans and thiophenes, including furosemide and cephaloridine. Toxicology and Applied Pharmacology, 42, 285–300. [DOI] [PubMed] [Google Scholar]
- Ochi T and Ohsawa M, 1985. Participation of active oxygen species in the induction of chromosomal aberrations by cadmium chloride in cultured Chinese hamster cells. Mutation Research, 143, 137–142. [DOI] [PubMed] [Google Scholar]
- Ravindranath V and Boyd MR, 1985. Metabolic activation of 2‐methylfuran by rat microsomal systems. Toxicology and Applied Pharmacology, 78, 370–376. [DOI] [PubMed] [Google Scholar]
- Ravindranath V and Boyd MR, 1991. Effect of modulators of glutathione synthesis on the hepatotoxicity of 2‐methylfuran. Biochemical Pharmacology, 41, 1311–1318. [DOI] [PubMed] [Google Scholar]
- Ravindranath V, Burka LT and Boyd MR, 1983. Isolation and characterization of the reactive metabolites of 2‐methylfuran (2‐MF) and 3‐methylfuran (3‐MF). Pharmacologist, 25, 171. [Google Scholar]
- Ravindranath V, Burka LT and Boyd MR, 1984. Reactive metabolites from the bioactivation of toxic methylfurans. Science, 224, 884–886. [DOI] [PubMed] [Google Scholar]
- Ravindranath V, McMenamin MG, Dees JH and Boyd MR, 1986. 2‐Methylfuran toxicity in rats ‐ role of metabolic activation in vivo . Toxicology and Applied Pharmacology, 85, 78–91. [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee for Food), 1995. Scientific Committee for Food. First annual report on chemically defined flavouring substances. May 1995, 2nd draft prepared by the SCF Working Group on Flavouring Substances (Submitted by the SCF Secretariat, 17 May 1995). CS/FLAV/FL/140-Rev2. Annex 6 to Document III/5611/95, European Commission, Directorate‐General III, Industry.
- SCF (Scientific Committee on Food), 1999. Opinion on a programme for the evaluation of flavouring substances (expressed on 2 December 1999). Scientific Committee on Food. SCF/CS/FLAV/TASK/11 Final 6/12/1999. Annex I the minutes of the 119th Plenary meeting. European Commission, Health & Consumer Protection Directorate‐General.
- Shinohara K, Kim E and Omura H, 1986. Furans as the mutagens formed by amino‐carbonyl reactions In: Fujimaki M, Namiki M and Kato H (eds.). Amino‐Carbonyl Reactions in Food and Biological Systems. Elsevier, New York. [Google Scholar]
- Slob W, 2017. A general theory of effect size, and its consequences for defining the benchmark response (BMR) for continuous endpoints. Critical Reviews in Toxicology, 47, 342–351. [DOI] [PubMed] [Google Scholar]
- Stich HF, Rosin MP, Wu CH and Powrie WD, 1981. Clastogenicity of furans found in food. Cancer Letters, 13, 89–95. [DOI] [PubMed] [Google Scholar]
- Subramanyam S, Sailaja D and Rathnaprabha D, 1989. Genotoxic assay of two dietary furans by some in vivo cytogenetic parameters. Environment Molecular Mutagensis, 14, 239. [Google Scholar]
- Sujatha PS, 2007. Genotoxic evaluation of furfuryl alcohol and 2‐furyl methyl ketone by sister chromatid exchange (SCE) analysis. Journal of Health Science, 53, 124–127. [Google Scholar]
- Sujatha PS, Jayanthi A and Subramanyam S, 1993. Evaluation of the clastogenic potential of 2‐furyl methyl ketone in an in vivo mouse system. Medical Science Research, 21, 675–678. [Google Scholar]
- Thornton‐Manning JR, Nichols WK, Manning BW, Skiles GL and Yost GS, 1993. Metabolism and bioactivation of 3‐methylindole by Clara cells, alveolar macrophages and subcellular fractions from rabbit lungs. Toxicology and Applied Pharmacology, 122, 182–190. [DOI] [PubMed] [Google Scholar]
- VCF (Volatile Compounds in Food), 2020. online. Available online: https://www.vcf-online.nl/VcfHome.cfm
- Wang K, Li W, Chen J, Peng Y and Zheng J, 2015. Detection of cysteine‐ and lysine‐based protein adductions by reactive metabolites of 2,5‐dimethylfuran. Analytica Chimica Acta, 896, 93–101. [DOI] [PubMed] [Google Scholar]
- Wiley RA, Traiger GJ, Baraban S and Gammal LM, 1984. Toxicity‐distribution relationships among 3‐alkylfurans in mouse liver and kidney. Toxicology and Applied Pharmacology, 74, 1–9. [DOI] [PubMed] [Google Scholar]
- Zeiger E, Anderson B, Haworth S, Lawlor T and Mortelmans K, 1992. Salmonella mutagenicity tests: V. Results from the testing of 311 chemicals. Environmental and Molecular Mutagenesis, 19, 2–141. [DOI] [PubMed] [Google Scholar]


