Abstract
Photorefractive keratectomy (PRK) eye surgery is widely used for patients at risk for corneal ectasia to maintain an aspheric corneal shape. Wavefront-guided (WFG) ablation profile was designed to reduce pre-existing higher-order aberrations (HOA). We aimed to compare the corneal aberrations and visual outcomes between WFG and Wavefront Optimized (WFO) PRK in patients with myopia. Eight randomized clinical trials were included. We searched PubMed, Scopus, Web of Science and CENTRAL at March 2020, and updated the search in September 2020 using relevant keywords, The data were extracted and pooled as Mean Difference (MD) with a 95% Confidence Interval (CI), using Review Manager software (version 5.4). Pooled results showed no significance between Uncorrected Distance Visual Acuity (UDVA) and Corrected Distance Visual Acuity (CDVA) between both groups underwent WFG and WFO PPR after three months follow up (MD = - 0.03; 95% CI: [-0.06, 0.00]; P = 0.07), (MD = - 0.02; 95% CI: [-0.04, 0.01]; P = 0.22) respectively. Although, no significant difference between mean manifest cylinder after three and 12 months follow up, but the total MD for mean manifest cylinder difference was significantly lower with the WFG treatment method (MD = - 0.12, (95% CI: [0.23:-0.01], P = 0.03). This shows a slight advantage of the WFG over the WFO method. The visual performance showed similarity and excellent refractive outcomes in both WFO and WFG PRK. No significant statistical differences between the two approaches. On further comparison, there was a slight advantage of the WFG over the WFO method.
Keywords: Higher-order aberration, meta-analysis, myopia, wavefront-optimized photorefractive keratectomy, wavefront-guided photorefractive keratectomy
Patients undergoing refractive surgery aim to achieve the best possible level of spectacle free vision.[1] Although laser in situ keratomileusis (LASIK) is the most commonly selected refractive procedure for rapid postoperative recovery, PRK is still used for patients at risk for Corneal ectatic disorders and to avoid potential flap complications intraoperatively and in the future.[1,2,3] However, UDVA can be improved by Myopic laser refractive surgery by producing an oblate cornea; visual quality is not enough post-surgery.[4] In myopic Laser refractive surgery, because the laser beam enters the periphery, some parts are reflected, and the circular beam becomes elliptical resulting in a decrease in the effectiveness of the laser energy.[4] Under-ablation of the peripheral corneal can be induced by these factors increasing HOA, especially spherical aberration.[5]
To overcome the problem of myopic laser refractive surgery, WFO ablation was designed to maintain an aspheric corneal shape by applying extra laser pulses to the peripheral cornea to diminish induction of spherical aberration.[6] Another procedure, WFG ablation profile was designed to reduce pre-existing HOA.[7] A few published studies compared WFG treatments with WFO treatments in patients undergoing LASIK[8] and some reports[9,10] show an improvement in the quality of vision after WFG treatment, but others[11,12] showed no difference between the two profiles.[8] WFO laser ablation profiles are designed to deliver additional treatment to the peripheral cornea in an attempt to preserve the naturally prolate shape of the cornea and minimize induction of higher-order aberrations while protecting the present aberrations of the eye.[13] WFG treatments attempt to correct both lower- and higher-order aberrations requiring preoperative measurement of the eye's aberrations using a Wavefront aberrometer.[13]
In this systematic review and meta-analysis, we aimed to compare the visual outcomes and corneal aberrations between WFO and WFG PRK and evaluate their effect on myopia for patient-perceived quality of vision to determine whether one treatment profile leads to more optimal vision than the other.
Methods
We followed the PRISMA statement guidelines[13] during the preparation of this systematic review and meta-analysis and performed all steps according to the Cochrane handbook of systematic reviews of intervention.[14] The PRISMA flow diagram for studies selected in the search process and eligibility assessment are summarized in Fig. 1.
Figure 1.
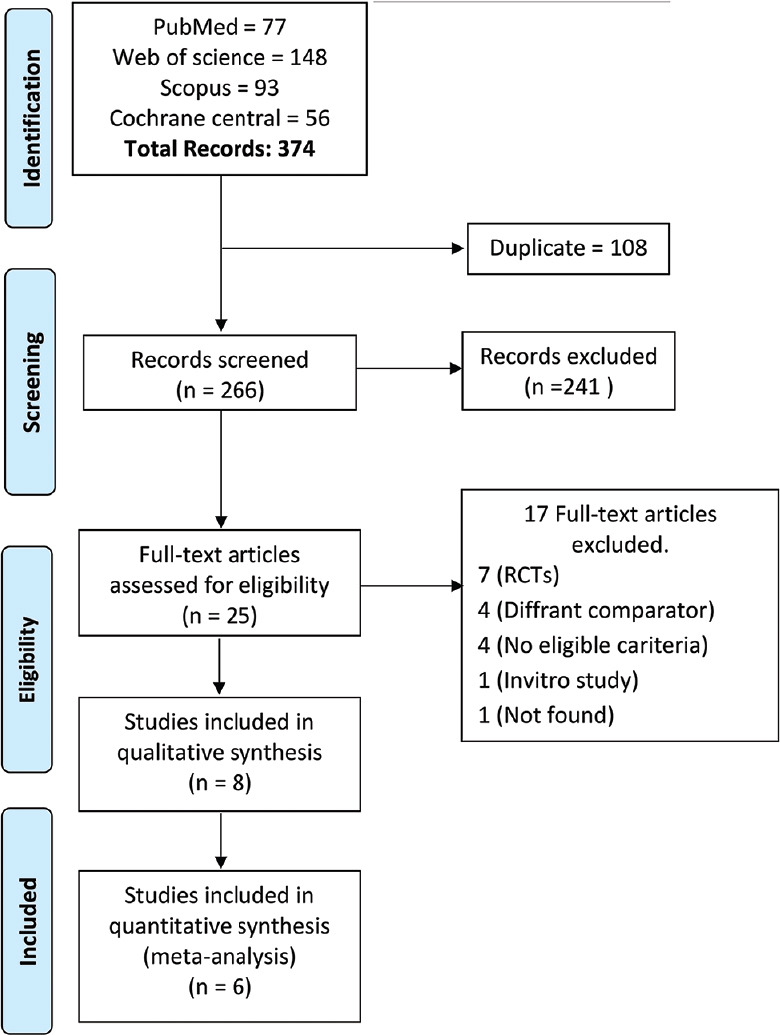
The PRISMA flow diagram for included studies.
Literature search strategy
We searched PubMed, SCOPUS, Web of Science and Cochrane CENTRAL through at March 2020, and updated the search in September 2020 for relevant clinical trials comparing optimized and guided photorefractive keratotomy. We used the following search strategy: ((Wavefront-guided photorefractive keratectomy OR Wavefront-optimized photorefractive keratectomy) AND “Myopia”).
Eligibility criteria and study selection
We included studies that followed these criteria: (1) population are patients undergoing photorefractive keratotomy, (2) intervention and comparator are Wavefront-guided versus Wave-front-optimized photorefractive keratotomy operations, (3) study design: we included randomized clinical trials with no restrictions for languages. Besides, the references of included trials and relevant reviews were screened to ensure high-quality searching. We excluded animal trials, conference abstracts, non-randomized trials, and studies without relevant outcomes.
Two authors independently screened the title and abstract of all expected included studies following by full-text screening and then manually searching the references of the finally included papers eligible to meta-analysis.
Data extraction
Two independent authors performed the extraction step, and any disclosure among authors was resolved through discussion and referred to the study senior. We extracted data in a formatted data extraction excel sheet including (1) Summary as NCT, study design, population, Duration of treatment, and intervention [shown in Table 1]. (2) Baseline characteristics such as age, sex, mitomycin C use, Trefoil, and spherical aberration [shown in Table 2]. (3) Outcomes as UDVA, CDVA, mean manifest sphere, mean manifest spherical, and spherical equivalent.
Table 1.
Data summary
| Study ID | NCT | Study Design | Population | Duration of treatment | Intervention | Comparison |
|---|---|---|---|---|---|---|
| Toy et al. 2016 | - | Prospective, Randomize, Fellow eye-controlled clinical trial | 71 patients | 12 months follow up | WFO-PRK using the WaveLight Allegretto Eye-Q 400-Hz (Alcon) | WFG-PRK using the VISX CustomVue Star S4 IR |
| Sia et al. 2015 | - | Prospective randomized open-label study | 108 patients | (1,3,6,12) months follow up | Wavefront-guided | Wavefront-optimized treatment |
| Ryan et al. 2017 | 1097525 | prospective, randomized clinical trial | 56 patients | (6,12) months follow up | Wavefront-guided (WFG) | Wavefront-optimized (WFO) photorefractive keratectomy (PRK) |
| Moshirfar et al. 2011 | - | Prospective, single-masked, randomized, fellow-eye study | 23 patients | Three months follow up | WaveLight Allegretto system (Wavefront-optimized group), which utilizes the WaveLight® Allegretto 400 Hz Wave® Eye-Q Laser | VISX CustomVueTM STAR S4 IRTM Excimer Laser with Active Track |
| Maurer et al. 2014 | - | prospective randomized study | 27 patients | (1,3,6) months follow up | WFG PRK, WFG LASIK | WFO PRK and WFG LASIK |
| Lee et al. 2016 | 1135719 | prospective randomized study | 68 patients | 12 months follow up | Wavefront-guided PRK (VISX CustomVue Star S4 IR excimer laser system | Wavefront-optimized PRK (WaveLight Allegretto Wave EyeQ 400 Hz excimer laser system; Alcon Laboratories, Inc., Fort Worth, TX) |
| He et al. 2015 | 1135719 | Prospective, randomized, fellow-eye controlled clinical trial | 71 patients | (1,3,6,12) months follow up | WFG PRK (Visx CustomVue Star S4IR excimer laser system; Abbott Medical Optics) | WFO PRK (WaveLight Allegretto Wave Eye-Q 400 Hz excimer laser system; Alcon Surgical) |
| He et al. 2014 | 1135719 | Prospective, randomized, fellow eye controlled study | 71 patients | (1,3,6,12) months follow up | WFG PRK treatment by the VISX CustomVue Star S4 IR excimer laser system | WFO PRK treatment by the WaveLight Allegretto Wave Eye-Q 400 Hz excimer laser system |
Table 2.
Baseline characteristics.
| Study ID | Age | Gender | Mean manifest cylinder (D) | Mean manifest sphere (D) | Spherical equivalent (diopters) | UDVA (Mean logMAR) | CDVA (Mean logMAR) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WFG PRK M (SD)=n | WFO PRK M (SD)=n | WFG PRK Female/Total | WFO PRK Female/Total | WFG PRK M (SD) | WFO PRK M (SD) | WFG PRK M (SD) | WFO PRK M (SD) | WFG PRK M (SD) | WFO PRK M (SD) | WFG PRK M (SD)=n | WFO PRK M (SD)=n | WFG PRK Female/ Total | WFO PRK Female/ Total | |
| Toy et al. 2017 | 40 (28.15) =71 patients | 47/71 | 0.79 (2.4) | 0.68 (2.2) | … | -4.7 (8.4) | 4.5 (8.52) | … | … | |||||
| Sia et al. 2015 | 30.4 (6.6) = 108 patients | 30.4 (6.6) =108 patients | 11/55 | 13/53 | -0.76 (0.61) | -0.63 (0.52) | 3.21 (1.72) | -3.12 (1.46) | -3.6 (1.74) | -3.43 (1.52) | 1 (0.47) | 1.1 | -0.1 (0.05) | -0.11 |
| Ryan et al. 2017 | 30.4 (6.2) =54 eyes | 30.4 (6.2) =54 eyes | 4/27 | 5/27 | -0.75 (0.61) | -0.6 (0.51) | -3.29 (1.52) | 3.23 (1.2) | -3.67 (1.50) | -3.53 (1.21) | 1.05 (0.4) | 1.11 | -0.1 (0.03) | -0.11 |
| Moshirfar et al. 2011 | 31.4 (5.80) =23 patients | 5/23 | 0.47 (0.35) | 0.48 (0.29) | -3.49 (3.58) | -3.58 (1.76) | … | … | -0.06 | -0.07 | ||||
| Maurer et al. 2014 | 29.6 (5.7) =14 patients | 29.6 (5.7) =14 patients | … | … | … | … | … | … | ||||||
| Lee et al. 2016 | = 68 patients | … | … | … | -4.55 (1.86) | -0.71 (0.56) | … | … | ||||||
| He et al., 2015 | 40 (28.15) =71 patients | 47/71 | 0.79 (2.4) | 0.68 (2.2) | -5.1 (2.25) | -4.92 (2.3) | -4.66 (2.29) | -4.55 (2.31) | 1.43 (0.61) | 1.43 (0.62) | ||||
| He et al.,2014 | 40 (28.15) =71 patients | 47/71 | 0.79 (2.4) | 0.68 (2.2) | -5.1 (2.25) | -4.92 (2.3 | -4.66 (2.29) | -4.55 (2.31) | … | … | ||||
Quality assessment
We used Cochrane's risk of bias tool to perform the quality assessment; the tool is found in chapter 8.5 of the Cochrane handbook of systematic reviews of interventions 5.1.0.[15] This ROB assessment tool included the following domains of biases: selection, performance, detection, attrition, reporting biases, and other potential sources of bias. Author judgments fall into three categories: low, unclear, or high risk of bias for each domain. We used the quality assessment table provided in (part 2, Chapter 8.5) the same book.[15] We could not assess publication bias because of the small number of included studies, less than ten studies.[16]
Data synthesis
We used the mean difference (MD) to perform analysis of continuous outcomes, and risk ratio (RR) to analyze dichotomous outcomes. The analysis was performed using (Review Manager Software, version 5.4) under a fixed-effect model in all outcome data. Statistical heterogeneity between studies was assessed by observation of the graphs and measured by Chi-square test and I-squared (I2) test for the degree of the heterogeneity. We conducted subgroup analysis according to postoperative follow-up of UDVA to stratify the surgical efficacy on UDVA.
Results
Literature search
The initial search resulted in 374 articles from 4 databases: 77 articles from PubMed, 56 articles from Cochrane, 93 articles from Scopus, and 138 articles from Web of Science. Of these 374 articles. We excluded 108 articles due to duplication. 266 articles underwent title, and abstract screening, and 241 were excluded because they did not follow our PICO criteria. The remaining 25 articles underwent full-text screening. A total of eight papers were finally included for the final qualitative analysis and six articles for quantitative analysis.
Quality assessment
Based on the Cochrane risk of bias tool that is found in chapter 8.5 of the Cochrane handbook of systematic reviews of interventions 5.1.0,[15] none of the eight studies had the selection, attrition, or reporting bias. 2 studies had performance bias as the participants and personnel were not fully blinded, and 1 study had a different type of bias. (Risk of bias summary shown in Fig. 2).
Figure 2.
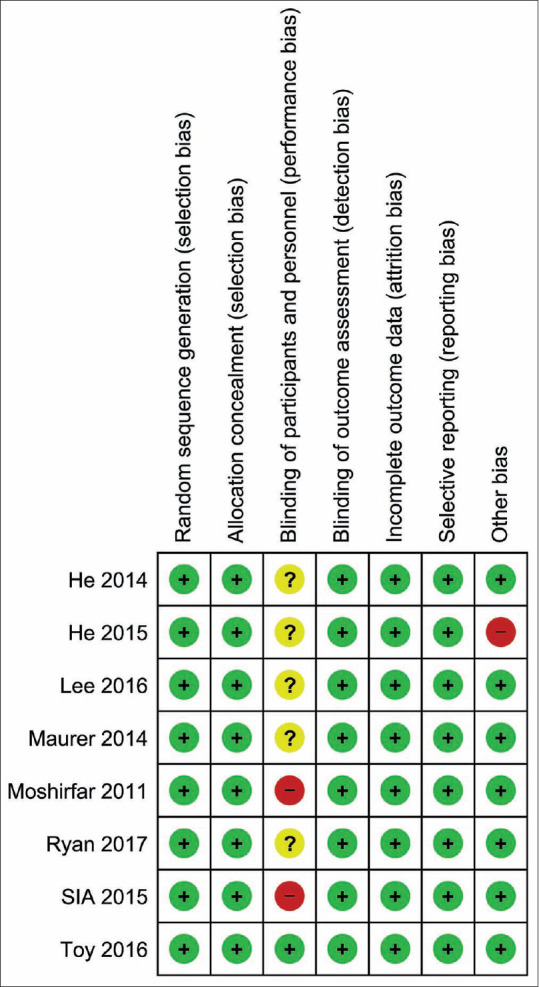
Risk of Bias assessment summary of the included studies
Outcomes
1. UDVA
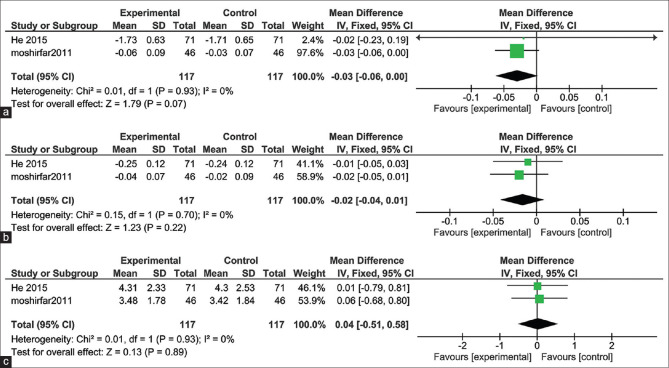
After 3 months follow up, the pooled studies showed no significant difference in UDVA of 20/20 in the eyes underwent WFG and WFO PRK (MD = -0.03; 95% CI: [-0.06, 0.00]; P = 0.07). Pooled results were homogenous (I2 = 0%, P = 0.93)[8,17] Fig. 3a.
Figure 3.
(a) Uncorrected Distance Visual Acuity (UDVA) after 3 months follow-up. (b) Corrected Distance Visual Acuity (CDVA) after 3 months follow-up. (c) Spherical Equivalent after 3 months follow-up
2. CDVA
The pooled studies showed no significant difference in CDVA of 20/20 in the eyes underwent WFG and WFO PRK after 3 months follow up (MD = -0.02; 95% CI: [-0.04, 0.01]; P = 0.22). Pooled results were homogenous (I2 = 0%, P = 0.70)[8,17] Fig. 3b.
3. Spherical Equivalent
The pooled studies showed no significant difference in the spherical equivalent of 20/20 in the eyes underwent WFG and WFO PRK after 3 months follow up (MD = 0.04; 95% CI: [-0.51, 0.58]; P = 0.89). Pooled results were homogenous (I2 = 0%, P = 0.93)[8,17] Fig. 3c.
4. Follow up assessment of UDVA
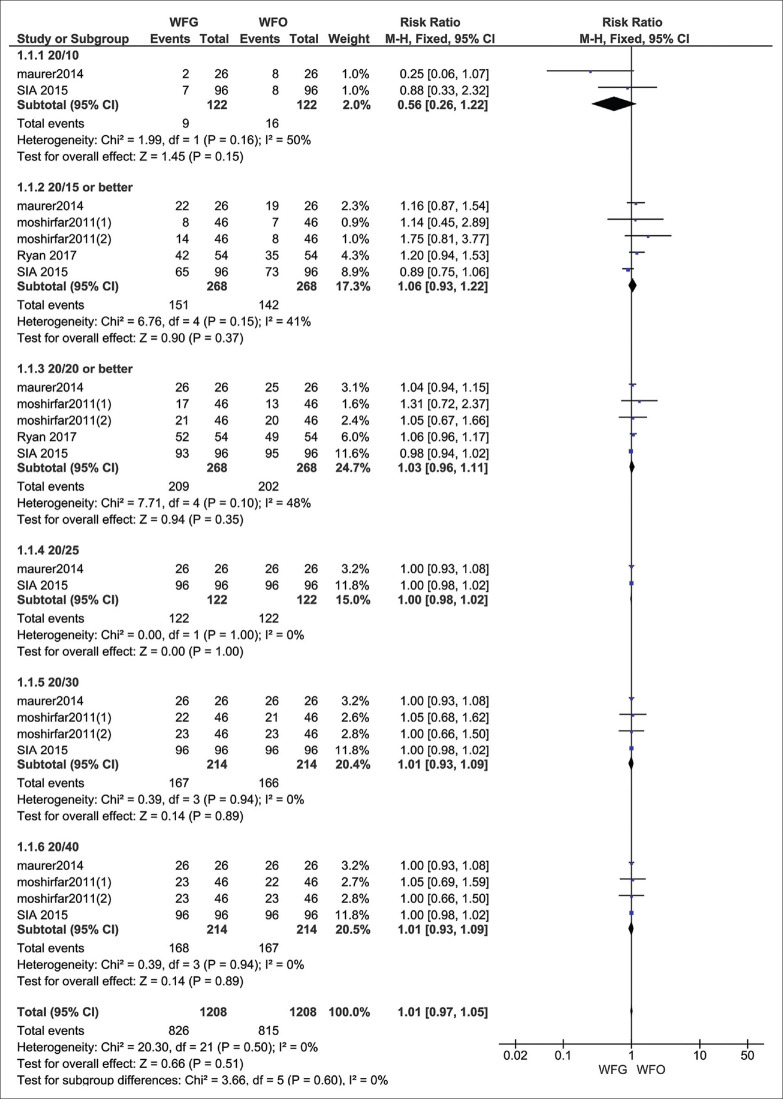
The pooled studies showed no significant difference in UDVA of 20/10 in the eyes underwent WFG and WFO PRK after 6, 12 months follow-up (RR = 0.56, 95% CI: [0.26, 1.22], P = 0.15), Pooled results were homogenous (I2 = 50%, P = 0.16).[18,19] UDVA of 20/15 in the eyes underwent WFG and WFO PRK after 1, 3, 6, 12 months follow-up (RR = 1.06, 95% CI: [0,93 1.22], P = 0.37), Pooled results were homogenous (I2 = 41%, P = 0.15).[17,18,19,20] UDVA of 20/20 in the eyes underwent WFG and WFO PRK after 1, 3, 6, 12 follow-up (RR = 1.03, 95% CI: [0.96, 1.11], P = 0.35), Pooled results were homogenous (I2 = 48%, P = 0.10).[17,18,19,20] UDVA of 20/25 in the eyes underwent WFG and WFO PRK after 6,12 months follow-up (RR = 1.00, 95% CI: [0.98, 1.02], P = 1.00), Pooled results were homogenous (I2 = 0%, P = 1.00).[18,19] UDVA of 20/30 in the eyes underwent WFG and WFO PRK after 1, 3, 6, 12 months follow-up (RR = 1.01, 95% CI: [0.93, 1.09], P = 0.89), Pooled results were homogenous (I2 = 0%, P = 0.94).[17,18,19] UDVA of 20/40 in the eyes underwent WFG and WFO PRK after 1, 3, 6, 12 follow-up (RR = 1.01, 95% CI: [0.93, 1.09], P = 0.89), Pooled results were homogenous (I2 = 0%, P = 0.94).[17,18,19] UDVA was generally almost the same in both groups (RR = 1.01, 95% CI: [0.97:1.05], P = 0.51) The pooled results were homogenous (I2 = 0%, P = 0.60) Fig. 4.
Figure 4.
Follow up assessment of UDVA
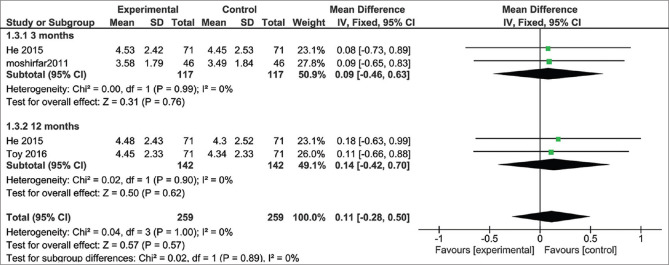
5. Mean Manifest sphere
The pooled studies showed no significant difference in the mean manifest sphere after 3 months follow up (MD = 0.09%; 95% CI: [-0.46, 0.63], P = 0.76), and after 12 months (MD = 0.14; 95% CI: [-0.46, 0.70], P = 0.62).[8,17,21] Pooled results were homogenous at 3 and 6 months (I2 = 0%, P = 0.99), (I2 = 0%, P = 0.90) respectively. The total mean difference is 0.11 D for WFG vs WFO (95% CI: [0.28:0.5], P = 0.57), The pooled results were homogenous (I2 = 0%, P = 0.89) Fig. 5.
Figure 5.
Mean Manifest sphere after 3 and 12 months follow-up
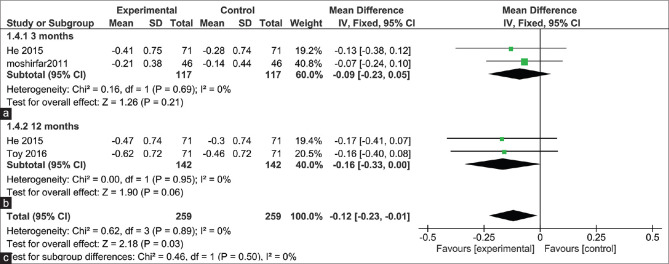
6. Mean manifest cylinder
The pooled studies showed no significant difference in the mean manifest cylinder after 3 months follow up (MD = -0.09, 95% CI: [-0.23, 0.05], and after 12 months (MD = -0.16, 95% CI: [-0.33, 0.00], Pooled results were homogenous at 3 and 6 months (I2 = 0%, P = 0.89) but the total mean difference for mean manifest cylinder difference was significantly lower with the WFG treatment method being (MD = -0.12, (95% CI: [0.23:-0.01], P = 0.03), The pooled results were homogenous (I2 = 0%, P = 0.50) [Fig. 6].[8,18,22]
Figure 6.
Mean manifest cylinder after 3 and 12 months follow-up
A total of 96 patients out of 164 patients in the WFG group had Mitomycin C applied to their eyes.[18] Comparing the change in HOAs from baseline between groups, the mean increase in coma was 0.2 microns in the WFG eyes (0.12 SD) and 0.21 microns in the WFO eyes (0.12 SD). Changes in other types of HOAs, such as trefoil, and spherical aberration were 0.16 microns (0.09 SD) for WFG and 0.17 microns (0.11 SD) for WFO group in trefoil aberration and 0.1 microns (0.12 SD) for WFG and 0.11 microns (0.14 SD) for WFO group in spherical aberration.[8]
Discussion
We established a systematic review and meta-analysis to compare the visual outcomes and corneal aberrations between WFG and WFO PRK in patients with myopia. The data from our analysis estimated that there is no significant difference between WFG and WFO in UDVA, CDVA, mean manifest sphere and spherical equivalent but the total of MD of manifest cylinder after three and 12 months follow-up was significantly lower with the WFG ablation profile. To our knowledge, we have comprehensively reported the outcomes following WFG and WFO PRK covering up to 12 months after surgery.
The wavefront-based technique has better clinical results and visual performance when compared to conventional therapy.[1,10,22] WFO and WFG photoablation techniques also reduce the postoperative occurrence of HOAs comparing to other conventional therapies.[23]
He et al. found no significant induction of HOAs after WFO vs WFG therapies. Still, WFG had a significant advantage over WFO in postoperative UDVA, residual refractive errors, and contrast sensitivity.[8] Moshifar et al. investigated the three months outcomes following WFG and WFO ablation profile in PRK and found that both platforms produced equivalent refractive safety, predictability, and change in spherical aberration; but WFG PRK yielded slightly better results in contrast sensitivity.[24] Sia et al. demonstrated that both techniques have comparable excellent refractive efficacy, safety, predictability, and stability profiles.[28]
Yu et al. observed 100 patients randomized to WFG treatment or WFO LASIK treatment in both eyes with questionnaires which showed no difference between the two groups in terms of patient satisfaction.[25] Patients were observed for six months and questioned about the level of glare, halos, light sensitivity, fluctuations of vision, ghost images, and starbursts they experienced. They again found no differences between the two treatment groups except perhaps an increase in ghost images in the WFO group at six months. Similar to previous studies, symptoms increased from baseline at the first month but returned to preoperative levels from three months onward. This reversal in the symptoms was most likely due to epithelial remodelling.[26]
Although WFG treatment has the advantage of precisely individualized correction of higher-order aberrations at the time of surgery, small gains in visual acuity, predictability, and HOAs compared to WFO in laser-assisted LASIK, both methods of PRK have been shown to result in comparable visual results.[8] It is important to be able to predict the change in corneal curvature induced for a given amount of refractive correction on both treatment modalities for preoperative planning and future intraocular lens selection. Regarding future cataract surgery, after myopic laser photoablation, corneal power calculations are typically overestimated, causing the selection of underpowered intraocular lenses, and subsequently, the hyperopic surprise may occur.[10,22,27,28,29,30,31,32,33,34]
WFG and WFO treatment profiles differ such that while WFG was developed to consider pre-existing HOAs, WFO was designed to consider HOAs induced by conventional treatment, particularly spherical aberration.[18] Yet again, WFO treatment may be preferred if it has overall equivalent outcomes to WFG treatment because WFO can be more easily performed without needing to analyze aberrometry data.[8] Several studies have compared the quality of vision after WFG and WFO treatments in patients undergoing LASIK.[10,12,35,36,37] Some have shown no difference between the two profiles.[36,37] However, other studies suggested improved results with WFG treatment.[34,37]
Quality of evidence
We included six Randomized Clinical Trials (RCTs) to the quantitative analysis constituting a strong level of evidence. All steps were performed according to the Cochrane handbook and PRISMA checklist.[14,15] By measuring the quality level, the included studies are ranged from low, moderate to high quality.
The main strength and limitation of our study
This is the most comprehensive report to date on outcomes following WFG and WFO PRK covering up to 12 months postoperative refractive outcomes. All outcomes were homogenous under the fixed-effect model. It is important to note that our review was limited by studies reporting UDVA at different times following the procedure, some studies reported UDVA at three months, others at 6, while others at 12, and some studies had strict enough follow up to report it on all those time periods. Also, we have included a limited number of trials.
Conclusion
Results of the visual performance show that eyes achieve similar and excellent refractive outcomes in both WFO and WFG PRK. There are no significant statistical differences between the two approaches. Only slightly better results, lower surgical side effects, and higher patient satisfaction was noticed in WFG treated eyes, giving it a slight advantage over the WFO method. Larger sample sizes and more studies are required to show equivalency or superiority of WFG compared to WFO effectively. We recommend further, and more extensive studies are conducted with stricter follow up and more diverse follow-up checklists to determine the best guidelines for myopia PRK treatment
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.He L, Manche EE. Prospective randomized contralateral eye evaluation of subjective quality of vision after wavefront-guided or wavefront-optimized photorefractive keratectomy. J Refract Surg. 2014;30:6–12. doi: 10.3928/1081597X-20131217-01. [DOI] [PubMed] [Google Scholar]
- 2.Nassiri N, Safi S, Amiri MA, Sheibani K, Safi H, Panahi N, et al. Visual outcome and contrast sensitivity after photorefractive keratectomy in low to moderate myopia: Wavefront-optimized versus conventional methods. J Cataract Refract Surg [Internet] 2011;37:1858–64. doi: 10.1016/j.jcrs.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 3.Moshirfar M, Hatch BB, Ollerton AJ, Sikder S, Mifflin MD. A prospective, contralateral comparison of photorefractive keratectomy (PRK) versus thin-flap LASIK: Assessment of visual function. Clin Ophthalmol. 2011;2011:451–7. doi: 10.2147/OPTH.S18967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang MJ, Hwang J, Chung SH. Comparison of corneal wavefront-optimized and wavefront-guided alcohol-assisted photorefractive keratectomy using schwind amaris 750s laser for myopia. Korean J Ophthalmol. 2020;34:210–8. doi: 10.3341/kjo.2019.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cano D, Barbero S, Marcos S. Comparison of real and computer-simulated outcomes of LASIK refractive surgery. J Opt Soc Am A. 2004;21:926. doi: 10.1364/josaa.21.000926. [DOI] [PubMed] [Google Scholar]
- 6.Biebesheimer JB, Kang TS, Huang CY, Yu F, Hamilton DR. Development of an advanced nomogram for myopic astigmatic wavefront-guided laser in situ keratomileusis (LASIK) Ophthalmic Surg Lasers Imaging. 2011;42:241–7. doi: 10.3928/15428877-20110303-01. [DOI] [PubMed] [Google Scholar]
- 7.Phusitphoykai N, Tungsiripat T, Siriboonkoom J, Vongthongsri A. Comparison of conventional versus wavefront-guided laser in situ keratomileusis in the same patient. J Refract Surg. 19(2 Suppl):S217–20. doi: 10.3928/1081-597X-20030302-08. [DOI] [PubMed] [Google Scholar]
- 8.He L, Manche EE. Contralateral eye-to-eye comparison of wavefront-guided and wavefront-optimized photorefractive keratectomy: A randomized clinical trial. JAMA Ophthalmol. 2015;133:51–9. doi: 10.1001/jamaophthalmol.2014.3876. [DOI] [PubMed] [Google Scholar]
- 9.Sáles CS, Manche EE. One-year outcomes from a prospective, randomized, eye-to-eye comparison of wavefront-guided and wavefront-optimized LASIK in myopes. Ophthalmology. 2013;120:2396–402. doi: 10.1016/j.ophtha.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Padmanabhan P, Mrochen M, Basuthkar S, Viswanathan D, Joseph R. Wavefront-guided versus wavefront-optimized laser in situ keratomileusis: Contralateral comparative study. J Cataract Refract Surg. 2008;34:389–97. doi: 10.1016/j.jcrs.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Straziota CE, Randleman BJ, Stulting DR. Visual acuity and higher-order aberrations with wavefront-guided and wavefront-optimized laser in situ keratomileusis. J Cataract Refract Surg. 2010;36:437–41. doi: 10.1016/j.jcrs.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 12.Stonecipher KG, Kezirian GM. Wavefront-optimized versus wavefront-guided LASIK for myopic astigmatism with the ALLEGRETTO WAVE: Three-month results of a prospective FDA trial. J Refract Surg. 2008;24:S424–30. doi: 10.3928/1081597X-20080401-20. [DOI] [PubMed] [Google Scholar]
- 13.Myrowitz EH, Chuck RS. A comparison of wavefront-optimized and wavefront-guided ablations. Curr Opin Ophthalmol. 2009;20:247–50. doi: 10.1097/icu.0b013e32832a2336. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PloS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green S, Higgins PT. Cochrane Handbook: Cochrane Reviews: Ch 8: Assessing risk of bias in included studies. Cochrane Handbook for: Systematic Reviews of Interventions. 2011:3–10. [Google Scholar]
- 16.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moshirfar M, Churgin DS, Betts BS, Hsu M, Sikder S, Neuffer M, et al. Prospective, randomized, fellow eye comparison of WaveLight® Allegretto Wave® Eye-Q versus VISX CustomVue ™ STAR S4 IR ™ in photorefractive keratectomy: Analysis of visual outcomes and higher-order aberrations. Clin Ophthalmol. 2011;5:1185–93. doi: 10.2147/OPTH.S24319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sia RK, Ryan DS, Stutzman RD, Pasternak JF, Eaddy JB, Logan LA, et al. Wavefront-guided versus wavefront-optimized photorefractive keratectomy: Clinical outcomes and patient satisfaction. J Cataract Refract Surg. 2015;41:2152–64. doi: 10.1016/j.jcrs.2015.10.054. [DOI] [PubMed] [Google Scholar]
- 19.Maurer T, Deaver D, Howell C, Moyer S, Nguyen O, Mueller G, et al. Military target task performance after wavefront-guided (WFG) and wavefront-optimized (WFO) photorefractive keratectomy (PRK) Sens Technol Glob Heal Mil Med Environ Monit IV. 2014;9112:91120U. [Google Scholar]
- 20.Ryan DS, Sia RK, Stutzman RD, Pasternak JF, Howard RS, Howell CL, et al. Wavefront-guided versus wavefront-optimized photorefractive keratectomy: Visual and military task performance. Mil Med. 2017;182:e1636–44. doi: 10.7205/MILMED-D-15-00576. [DOI] [PubMed] [Google Scholar]
- 21.Toy BC, Manche EE. Vector analysis of 1-year astigmatic outcomes from a randomized fellow eye comparison of photorefractive keratectomy using 2 excimer laser platforms. Eye Contact Lens. 2018;44:S71–6. doi: 10.1097/ICL.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 22.Razmju H, Rezaei L, Nasrollahi K, Fesharaki H, Attarzadeh H, Footami FJ. IOLMaster versus manual keratometry after photorefractive keratectomy. J Ophthalmic Vis Res. 2011;6:160–5. [PMC free article] [PubMed] [Google Scholar]
- 23.Karimian F, Feizi S, Jafarinasab MR. Conventional versus custom ablation in photorefractive keratectomy: Randomized clinical trial. J Cataract Refract Surg. 2010;36:637–43. doi: 10.1016/j.jcrs.2009.10.050. [DOI] [PubMed] [Google Scholar]
- 24.Hatch BB, Moshirfar M, Ollerton AJ, Sikder S, Mifflin MD. A prospective, contralateral comparison of photorefractive keratectomy (PRK) versus thin-flap LASIK: Assessment of visual function. Clin Ophthalmol. 2011;5:451–7. doi: 10.2147/OPTH.S18967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu J, Chen H, Wang F. Patient satisfaction and visual symptoms after wavefront-guided and wavefront-optimized LASIK with the WaveLight platform. J Refract Surg. 2008;24:477–86. doi: 10.3928/1081597X-20080501-05. [DOI] [PubMed] [Google Scholar]
- 26.Abbas UL, Hersh PS, Summit PRK Study Group. Late natural history of corneal topography after excimer laser photorefractive keratectomy. Ophthalmology. 2001;108:953–9. doi: 10.1016/s0161-6420(01)00549-8. [DOI] [PubMed] [Google Scholar]
- 27.Seitz B, Langenbucher A, Nguyen NX, Kus MM, Küchle M. Underestimation of intraocular lens power for cataract surgery after myopic photorefractive keratectomy. Ophthalmology. 1999;106:693–702. doi: 10.1016/S0161-6420(99)90153-7. [DOI] [PubMed] [Google Scholar]
- 28.Rosa N, Cennamo G, Rinaldi M. Correlation between refractive and corneal topographic changes after photorefractive keratectomy for myopia. J Refract Surg. 2001;17:129–33. doi: 10.3928/1081-597X-20010301-06. [DOI] [PubMed] [Google Scholar]
- 29.Rosa N, Capasso L, Lanza M, Furgiuele D, Romano A. Reliability of the IOLMaster in measuring corneal power changes after photorefractive keratectomy. J Cataract Refract Surg. 2004;30:409–13. doi: 10.1016/S0886-3350(03)00583-2. [DOI] [PubMed] [Google Scholar]
- 30.Peter R, Hazeghi M, Job O, Wienecke L, Schipper I. Manual keratometry and videokeratography after photorefractive keratectomy. J Cataract Refract Surg. 2000;26:1748–52. doi: 10.1016/s0886-3350(00)00696-9. [DOI] [PubMed] [Google Scholar]
- 31.Odenthal MT, Eggink CA, Melles G, Pameyer JH, Geerards AJ, Beekhuis WH. Clinical and theoretical results of intraocular lens power calculation for cataract surgery after photorefractive keratectomy for myopia. Arch Ophthalmol. 2002;120:431–8. doi: 10.1001/archopht.120.4.431. [DOI] [PubMed] [Google Scholar]
- 32.McCarthy M, Gavanski GM, Paton KE, Holland SP. Intraocular lens power calculations after myopic laser refractive surgery: A comparison of methods in 173 eyes. Ophthalmology. 2011;118:940–4. doi: 10.1016/j.ophtha.2010.08.048. [DOI] [PubMed] [Google Scholar]
- 33.Chen S, Hu FR. Correlation between refractive and measured corneal power changes after myopic excimer laser photorefractive surgery. J Cataract Refract Surg. 2002;28:603–10. doi: 10.1016/s0886-3350(01)01323-2. [DOI] [PubMed] [Google Scholar]
- 34.Lee HK, Choe CM, Ma KT, Kim EK. Measurement of contrast sensitivity and glare under mesopic and photopic conditions following wavefront-guided and conventional LASIK surgery. J Refract Surg. 2006;22:647–55. doi: 10.3928/1081-597X-20060901-05. [DOI] [PubMed] [Google Scholar]
- 35.Falavarjani KG, Hashemi M, Modarres M, Sanjari MS, Darvish N, Gordiz A. Topography-guided vs wavefront-optimized surface ablation for myopia using the wavelight platform: A contralateral eye study. J Refract Surg. 2011;27:13–7. doi: 10.3928/1081597X-20100310-02. [DOI] [PubMed] [Google Scholar]
- 36.Tran DB, Shah V. Higher order aberrations comparison in fellow eyes following intraLase LASIK with wavelight allegretto and customcornea LADArvision4000 systems. J Refract Surg. 2006;22:S961–4. doi: 10.3928/1081-597X-20061101-25. [DOI] [PubMed] [Google Scholar]
- 37.Miraftab M, Seyedian MA, Hashemi H. Wavefront-guided vs wavefront-optimized LASIK: A randomized clinical trial comparing contralateral eyes. J Refract Surg. 2011;27:245–50. doi: 10.3928/1081597X-20100812-02. [DOI] [PubMed] [Google Scholar]