Abstract
Phakic intraocular lenses (pIOLs) are a common solution for the surgical correction of high myopia and myopia in thin corneas. Global trends result in increasing rates of patients with high myopia which will result in increased rates of pIOL implantation. Three types of lenses can be distinguished: anterior chamber angle-supported, anterior chamber iris-fixated, and posterior chamber phakic IOLs. The efficacy of phakic intraocular lenses is generally very good, but pIOLs have undergone many changes over the years to improve the safety profile and decrease pIOL-related complications such as endothelial cell loss, corneal decompensation and cataract formation. This article describes the efficacy and safety profiles of the most recent pIOLs, as well as suggests gaps of knowledge that are deserve additional research to optimize the results of pIOLs.
Keywords: Phakic Intraocular Lens, High Myopia, Refractive Surgery
Prevalence of high myopia has increased over the past years, resulting in a so-called epidemic of myopia that is especially prevalent in East and Southeast Asia.[1,2] In these regions, myopia is seen in up to 90% of adolescents, a vast increase when compared to an incidence of maximum 30% in adolescents 60 years ago.[1,2,3] Next to genetic factors, environmental factors are the main reason for this increased prevalence of myopia. This is mainly caused by intensive education resulting in children both spending more time indoor and doing near work.[1,2,3,4,5] If children and adolescents are required to perform near work, their eyes require constant accommodation to focus incoming light onto, rather than behind the retina. This combination of accommodation and lack of natural light falling on the retina causes the eye to grow, and the axial length to increase to create a situation where constant accommodation is no longer required during near work. However, this longer axial length also results in a distant image that is focused in front of the retina, introducing (high) myopia. Studies have shown that the onset, and progression of myopia can be reduced significantly by increasing the time a child spends in daylight. A vast amount of research focuses on stopping the myopic progression in the (very) young child in order to prevent increasing incidence of high myopia, defined as -5 to -6 D or higher.[2,6] Prevention of high myopia is important since it is associated with potentially blinding complications such as myopic macular degeneration, retinal detachment, staphyloma formation, or macular retinoschizis.[1,3,5,7] Current treatments are based on using cycloplegic drops, orthokeratology (nighttime contact lenses) to temporarily change the shape of the cornea, or multifocal contact lenses.[4,5,8,9] Results seem promising in halting axial elongation, but long-term data and mechanisms have yet to be determined.[4,5,8,9] Furthermore, contact lens wear introduces a risk of corneal infection, which can result in corneal scarring requiring corneal transplantation.[10,11]
In a myopic patient non-surgical refractive correction can be obtained using contact lenses or glasses. It should be noted that refractive correction of high myopia will result in thicker contact lenses and glasses. Contact lenses in these patients are expensive, might cause problems with contact lens fitting and introduce the risk of contact lens-related infections.[10,11] Glasses are the best option to safeguard corneal health, but are aesthetically not preferred due to a misrepresentation of the contour of the face, and cause visual distortion in the peripheral visual field.
Long-term treatment of stable myopia can be obtained by three different types of surgery: laser refractive surgery, phakic intraocular lens (pIOL) implantation, and lens surgery for refractive purposes (refractive lens exchange [RLE]) [Fig. 1]. Both laser refractive surgery and refractive lens exchange are permanent, whereas refractive surgery using pIOL implantation is a reversible procedure.[12,13] For safety reasons, laser refractive surgery is performed in cases up to -8 D of myopia, depending on corneal thickness, thus excluding a large group of high myopes from treatment.[13] The relative risk of developing a retinal detachment increases four times after cataract surgery, with patients under 50 years old and patients with a long axial length reported as especially at risk for developing a retinal detachment.[14] Taking into account that cataract surgery also removes all accommodative capacity, refractive lens exchange is rarely performed in non-presbyopic patients. As a result, pIOL implantation is regularly performed as a treatment for non-presbyopic high myopes.[13,15,16]
Figure 1.
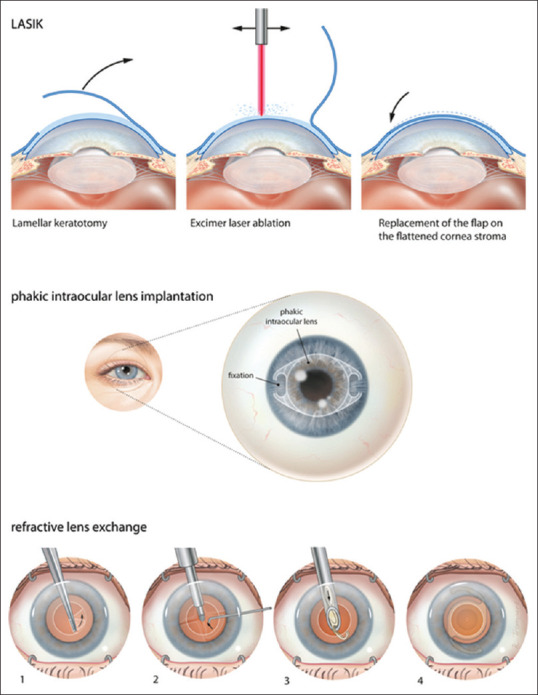
Surgical correction of refractive errors using laser refractive correction (top), phakic intraocular lens implantation (center), refractive lens exchange (bottom)
History and Development of Phakic Intraocular Lenses
Phakic IOLs can be divided into (1) anterior chamber angle supported pIOLs that function with haptics positioned in the angle where the iris and cornea meet; (2) anterior chamber iris-fixated pIOLs that use small “lobster claws” to enclavate iris-tissue and position the pIOL in front of the pupil; and (3) posterior chamber pIOLs that use plate haptics to support and position the lens in the posterior chamber [Fig. 2].[15,17]
Figure 2.
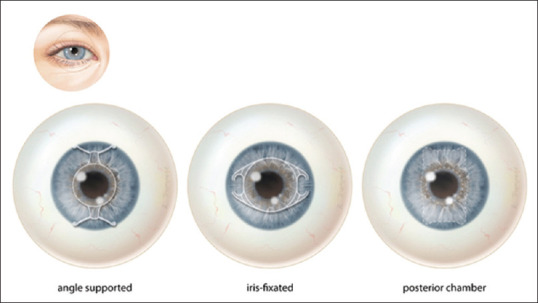
Three different phakic intraocular lenses: angle supported (left), iris-fixated (center), posterior chamber (right)
Implantation of pIOLs for the correction of myopia started in 1953 when Strampelli implanted the first rigid angle-supported pIOL. Although refractive results were promising, the high rate of corneal complications, and excessive pressure on the iris root causing inflammation and pupil malformation, resulted in withdrawal of the pIOL from the market. From the 1970s until the early 2010s multiple new angle-supported pIOLs were available, using different materials and different optic designs to try and cause less corneal complications and induce less pressure on the iris-root. Despite rendering good visual and refractive results, the complication rate of angle-supported pIOLs resulted in the withdrawal of all angle-supported pIOLs from the market [Tables 1a, 2a and 3a].[15,16,18]
Table 1a.
Overview of visual and refractive results after implantation with an anterior chamber angle supported phakic intraocular lens
| Nr. eyes [nr. pt] | Follow-up (mo) | MRSE | % within target | UDVA | CDVA | Indices | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| preop | postop | +/- 0.5D | +/- 1.0D | 20/20 (%) | 20/40 (%) | 20/20 (%) | 20/40 (%) | Efficacy | Safety | |||
| Artisan Myopia (Toric), Ophtec | ||||||||||||
| Kohnen, T., 2009[19] | 190 [190] | 24 | -10.38 | -0.23 | 72.7 | 95.7 | 57.8 | 99.4 | 85.7 | 100 | 1.04 | 1.25 |
| Knorz. M.C., 2011[20] | 360 [NR] | 36 | -10.41 | -0.24 | 78.9 | 91.3 | 46.2 | 97.1 | 80.8 | 100 | 0.92 | 1.21 |
| Mastropasqua, L., 2012[21] | 36 [NR] | 12 | NR | NR | 67 | 100 | 56 | 100 | NR | NR | 0.81 | NR |
| Yang, R.B., 2012[22] | 25 [13] | 12 | -12.08 | -0.23 | 84 | 100 | 60 | 100 | 92 | 100 | 1.04 | 1.13 |
| Kermani, O., 2013[23] | 50 [28] | 3 | -9.71 | -0.35 | 84 | NR | 69 | NR | NR | NR | NR | NR |
| Taneri, S., 2014[24] | 15 [15] | 12 | -11.25 | -0.46 | NR | NR | NR | NR | NR | NR | NR | NR |
| Aerts, A.A., 2015[25] | 79 [NR] | 24 | -10.27 | -0.17 | 91 | 95 | 73 | 100 | 85 | NR | 1.00 | 1.11 |
| Gimbel, H.V., 2015[26] | 51 [NR] | 36 | -9.26 | -0.33 | 78.4 | 92.2 | 77.6 | 98 | NR | NR | 0.93 | 1.07 |
| Kohnen, T., 2016[18] | 415 [360] | 60 | -10.41 | -0.34 | 70 | 89.8 | NR | 94.7 | 91.3 | 100 | NR | NR |
| Alio, J.L., 2017[27] | 25 [NR] | 60 | -12.27 | -0.59 | 48 | 76 | 20 | 88 | 68 | NR | 0.81 | 1.14 |
| Al-Qahtani, S., 2018[28] | 36 [21] | 36 | -10.5 | NR | NR | NR | NR | NR | 41.7 | NR | NR | NR |
Mo = Months; MRSE = Manifest refractive spherical equivalent; D = Diopter; UDVA = Uncorrected distance visual acuity; CDVA = Corrected distance visual acuity; NR = Not reported
Table 2a.
Overview of studies reporting on endothelial cell health after anterior chamber angle supported phakic intraocular lens implantation
| Nr. Eyes [Nr. pt] | Follow-up (mo) | Preop ACD (mm) | ECD (cells/mm²) | Total EC loss (%) | ||
|---|---|---|---|---|---|---|
| Preop | Postop | |||||
| AcrySof Cachet, Alcon | ||||||
| Kohnen, T., 2009[19] | 190 [190] | 12 | NR | NR | NR | -4.8 |
| Mastropasqua, L., 2012[21] | 36 [NR] | 12 | NR | NR | NR | -4.0† |
| Yang, R.B., 2012[22] | 25 [13] | 12 | NR | 2767 | 2764 | NR |
| Kermani, O., 2013[23] | 50 [28] | 3 | NR | 2650 | 2500 | NR† |
| Taneri, S., 2014[24] | 15 [15] | 12 | NR | 2676 | 2825 | NR† |
| Aerts, A.A., 2015[25] | 79 [NR] | 24 | 3.64* | 2817 | NR | -3.8[1] |
| Gimbel, H.V., 2015[26] | 51 [NR] | 36 | 3.87* | 2945 | 2768 | -3.1† |
| Kohnen, T., 2016[18] | 415 [326] | 60 | 3.73* | NR | NR | -8.9 |
| Alio, J.L., 2017[27] | 25 [NR] | 60 | NR | 2849 | 2513 | -13.7 |
| Al-Qahtani, S., 2018[28] | 36 [21] | 6 | NR | 3017 | 2775 | -7.2† |
Mo = Months; ACD = Anterior chamber depth; ECD = Endothelial cell density; EC = Endothelial cell; NR = Not reported. *as measured from the corneal epithelium, around 0.5 mm consists of corneal thickness, †measurement not repeatable based on materials and methods section, 1chronic loss from 6 months to endpoint
Table 3a.
Reported complications in eyes implanted with anterior chamber angle supported phakic intraocular lenses
| Nr. Eyes[Nr. pt] | Follow-up (mo) | Exchange (%) | Explants (%) | Complication | |||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Cataract | EC loss | Retinal | Position Related | Other | ||||
| AcrySof Cachet, Alcon | |||||||||
| Kohnen, T., 2009[19] | 190 [190] | 12 | 0.53 | 0.53 | NR | NR | NR | 32.6% ≥15° rotation,1.05% pIOL related synechiae. 0.53% explanted due to upside-down implantation | NR |
| Knorz. M.C., 2011[20] | 104 [NR] | 36 | 0.56 | 1.39 | NR | NR | NR | NR | NR |
| Gimbel, H.V., 2015[26] | 51 [NR] | 36 | NR | 1.68 | NR | 1.68 | NR | 1.68% anterior synechiae | NR |
| Kohnen, T., 2016[18] | 415 [326] | 60 | NR | 6.33 | 1.27 | 2.53 | NR | 4.9% anterior synechiae, 0.39% upside down implantation | 4.1% surgical intervention, 3.1% cataract formation, 2.5% raised IOP requiring treatment |
| Alio, J.L., 2017[27] | 25 [NR] | 60 | NR | 2 | NR | 1 | NR | 1% iris-cyst development requiring explantation | 10% additional laser refractive correction |
Mo = Months; ACD = Anterior chamber depth; ECD = Endothelial cell density; EC = Endothelial cell; NR = Not reported; pIOL = Phakic intraocular lens; IOP = Intraocular pressure
Table 1b.
Overview of visual and refractive results after implantation with an anterior chamber iris-fixated phakic intraocular lens
| Nr. eyes [nr. pt] | Follow-up (mo) | MRSE | % within target | UDVA | CDVA | Indices | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| preop | postop | +/- 0.5D | +/- 1.0D | 20/20 (%) | 20/40 (%) | 20/20 (%) | 20/40 (%) | Efficacy | Safety | |||
| Artisan Myopia (Toric), Ophtec | ||||||||||||
| Budo, C., 2000[31] | 249 [NR] | 36 | -12.95 | NR | 57.1 | 78.8 | 33.7 | 76.8 | 61.5 | 93.9 | 1.03 | 1.31 |
| Dick, H. B., 2003[32] | 48 [NR] | 6 | -8.90 | -0.5 | 83.3 | 100 | 85.4 | NR | NR | NR | NR | NR |
| Asano-Kato, N, 2005[33] | 11 [NR] | 24 | -12.8 | -0.71 | NR | NR | NR | NR | NR | NR | NR | NR |
| Bartels, M., 2006[34] | 20 [14] | 24 | -11.39 | -0.44 | 70 | 95 | NR | NR | NR | NR | NR | NR |
| Coullet, J., 2006[35] | 31 [31] | 12 | -10.3 | -1.01 | NR | 58 | NR | 52 | NR | NR | 0.60 | 1.13 |
| Tahzib, N.G., 2006[36] | 120 [60] | 12 | -12.09 | -0.60 | 62.4 | 81.5 | 25.8 | 68.3 | NR | NR | NR | NR |
| Tehrani, M., 2006[36] | 28 [NR] | 36 | -9.84 | NR | NR | 70 | NR | 58 | NR | NR | 1.04 | 1.12 |
| Gierek-Ciaciura, S., 2007[37] | 20 [12] | 12 | -15.73 | NR | 65 | 95 | NR | 80 | NR | NR | 1.71 | NR |
| Moshirfar, M., 2007[38] | 38 [NR] | 24 | -12.20 | -0.5 | 55 | 84 | NR | 84 | NR | NR | NR | NR |
| Tahzib, N.G., 2007[39] | 89 [49] | 72 | -10.36 | -0.71 | 50.5 | 65.1 | NR | 78.7 | 50.6 | 96.6 | 0.83 | 1.10 |
| Tahzib, N.G., 2007[39] | 89 [49] | 120 | -10.36 | -0.70 | 43.8 | 68.8 | NR | 82.0 | 52.8 | 93.3 | 0.80 | 1.10 |
| Guell, J. L., 2008[40] | 95 [NR] | 12 | -19.8 | -0.71 | NR | NR | NR | NR | NR | NR | 1.16 | 1.40 |
| Guell, J. L., 2008[40] | 169 [NR] | 12 | -11.27 | -0.49 | NR | NR | NR | NR | NR | NR | 0.95 | 1.17 |
| Silva, R.A., 2008[41] | 19 [NR] | 60 | -12.30 | 0.97 | 73.7 | 94.7 | 73.7 | 94.7 | 94.7 | NR | NR | NR |
| Stulting, R.D., 2008[42] | 386 [232] | 36 | -12.3 | NR | NR | NR | 31.1 | 83.9 | NR | NR | NR | NR |
| Tahzib, N.G., 2008[43] | 22 [13] | 12 | -9.90 | -0.21 | NR | NR | NR | NR | 77 | NR | NR | NR |
| Qasem, Q., 2010[44] | 151 [84] | 12 | -11.2 | NR | 41.7 | 64.9 | 48.3 | 86.8 | NR | NR | NR | NR |
| Hassaballa, M.A., 2011[45] | 42 [NR] | 12 | -12.89 | -0.86 | NR | 56.52 | NR | NR | NR | NR | 0.95 | 1.02 |
| Titiyal, J.S., 2012[45] | 28 [NR] | 60 | -14.23 | -0.63 | NR | NR | 21.4 | 64.3 | 50 | 92.9 | NR | NR |
| Yuan, X., 2012[47] | 84 [43] | 60 | -14.17 | NR | NR | NR | 39.3 | 100 | 65.5 | 100 | NR | NR |
| Jonker, S.M., 2019[48] | 250 [NR] | 60 | -12.58 | -0.81 | 50 | 71 | 22 | 82 | 61 | 96 | 0.83 | 1.22 |
| Jonker, S.M., 2019[48] | 136 [NR] | 120 | -12.58 | -0.72 | 31 | 52 | 44 | 96 | 46 | 95 | 0.77 | 1.24 |
| Artisan Hyperopia (Toric), Ophtec | ||||||||||||
| Dick, H. B., 2003[32] | 22 [NR] | 6 | 3.25 | -0.24 | 50 | 100 | 95.5 | NR | NR | NR | NR | NR |
| Tehrani, M., 2006[49] | 12 [NR] | 36 | 3.43 | NR | NR | 70 | NR | 73 | NR | NR | 1.14 | 1.04 |
| Guell, J. L., 2008[40] | 39 [NR] | 12 | 4.92 | -0.81 | NR | NR | NR | NR | NR | NR | 0.79 | 0.98 |
| Qasem, Q., 2010[44] | 14 [7] | 12 | 7.1 | NR | 57.1 | 85.7 | 35.7 | 92.9 | NR | NR | NR | NR |
| Artisan, Ophtec (Mixed Groups) | ||||||||||||
| Guell, J. L., 2003[50] | 27 [16] | 12 | -11.78 | -0.58 | 62.9 | 96.2 | NR | 63 | NR | NR | NR | 1.4 |
| Guell, J. L., 2008[40] | 84 [NR] | 12 | -6.82 | -0.77 | NR | NR | NR | NR | NR | NR | 0.96 | 1.26 |
| Qasem, Q., 2010[44] | 20 [11] | 12 | -9.05 | NR | 55 | 90 | 20 | 80 | NR | NR | NR | NR |
| Artiflex Myopia (Toric), Ophtec | ||||||||||||
| Coullet, J., 2006[35] | 31 [31] | 12 | -9.5 | -0.58 | NR | 83.9 | NR | 77 | NR | NR | 0.79 | 1.12 |
| Tahzib, N.G., 2008[43] | 27 [14] | 12 | -9.95 | -0.23 | NR | NR | NR | NR | 100 | NR | NR | NR |
| Dick, H. B., 2009[51] | 290 [191] | 24 | -7.33 | -0.15 | 75.2 | 94.3 | NR | 97.2 | NR | 100 | 1.00 | 1.09 |
| Doors, M., 2012[52] | 115 [73] | 6 | -7.53 | NR | 81.4 | 99 | 65.7 | 99 | 83.3 | 100 | 1.04 | 1.13 |
| Munoz, G., 2012[53] | 42 [25] | 12 | NR | NR | 66.7 | 92.9 | 38.1 | 100 | 71.4 | 100 | 0.97 | 1.11 |
| Ozerturk, Y., 2012[54] | 50 [NR] | 24 | -11.85 | -1.04 | NR | 88 | 24 | 84 | 18 | 82 | 0.79 | 1.12 |
| Ruckhofer, J., 2012[55] | 42 [24] | 6 | -7.52 | NR | NR | NR | 90 | 100 | 90 | 100 | 1.07 | 1.14 |
| Ghoreishi, M., 2020[56] | 41 [41] | 12 | -9.44 | -0.29 | 83 | 100 | NR | 100 | NR | NR | 1.08 | 1.20 |
Mo = Months; MRSE = Manifest refractive spherical equivalent; D = Diopter; UDVA = Uncorrected distance visual acuity; CDVA = Corrected distance visual acuity; NR = Not reported
Table 2b.
Overview of studies reporting on endothelial cell health after anterior chamber iris-fixated phakic intraocular lens implantation
| Nr. Eyes [Nr. pt] | Follow-up (mo) | Preop ACD (mm) | ECD (cells/mm²) | Total EC loss (%) | ||
|---|---|---|---|---|---|---|
| Preop | Postop | |||||
| Artisan Myopia, Ophtec | ||||||
| Budo, C., 2000[31] | 249 [NR] | 36 | 3.38‡ | 2876 | 2607 | NR† |
| Pop, M., 2004[57] | 293 [NR] | 24 | NR | 2631 | 2577 | -0.8† |
| Asano-Kato, N., 2005[33] | 11 [NR] | 24 | NR | 2831 | 2750 | NR† |
| Coullet, J., 2006[35] | 31 [31] | 12 | NR | 2638 | 2473 | -9.4† |
| Benedetti, S., 2007[58] | 49 [30] | 60 | NR | 2616 | 2379 | -9† |
| Gierek-Ciaciura, S, 2007[37] | 20 [12] | 12 | 3.39‡ | 2651 | NR | -6.8† |
| Moshirfar, M., 2007[38] | 38 [NR] | 24 | NR | 2713 | 2534 | -6.5† |
| Tahzib, N.G., 2007[39] | 89 [49] | 60 | 3.30‡ | 2817 | 2734 | -3.3† |
| Tahzib, N.G., 2007[39] | 89 [49] | 120 | 3.30‡ | 2817 | 2800 | -8.9† |
| Guell, J. L., 2008[40] | 89 [NR] | 60 | NR | 2836 | 2514 | -11.3† |
| Guell, J. L., 2008[40] | 165 [NR] | 60 | NR | 2755 | 2454 | -10.9† |
| Silva, R.A., 2008[41] | 19 [NR] | 60 | 3.87* | 2481 | 2156 | -14.1† |
| Stulting, R.D., 2008[42] | 386 [NR] | 36 | NR | NR | NR | -4.8† |
| Yamaguchi, T., 2008[59] | 20 [11] | 12 | 3.66* | 3139 | 3023 | NR† |
| Jonker, S.M., 2018[60] | 193 [NR] | 60 | 3.68* | 2670 | 2588 | -4.1 |
| Jonker, S.M., 2018[60] | 127 [NR] | 120 | 3.68* | 2670 | 2302 | -11.5 |
| Eldanasoury, A., 2019[61] | 90 [57] | 144 | 3.45* | 2645 | 1751 | -26.7 |
| alvis, V., 2019[62] | 67 [45] | 114 | NR | NR | NR | -18.5 |
| Shaaban, Y., 2020[63] | 20 [NR] | 36 | NR | 2707 | 2279 | -15.7† |
| Artisan Hyperopia (Toric), Ophtec | ||||||
| Guell, J. L., 2008[40] | 34 [NR] | 48 | NR | 2735 | 2560 | -6.4† |
| Galvis, V., 2019[62] | 10 [5] | 108 | NR | NR | NR | -10.5 |
| Artisan Toric, Ophtec (Mixed Groups) | ||||||
| Dick, H. B., 2003[32] | 70 [NR] | 6 | 3.47* | 2983 | 2849 | -4.5† |
| Guell, J. L., 2003[50] | 27 [16] | 12 | NR | 2649 | 2726 | 2.9† |
| Guell, J. L., 2008[40] | 67 [NR] | 36 | NR | 2632 | 2537 | -3.6† |
| Jonker, S.M., 2018[60] | 40 [NR] | 60 | 3.49* | 2695 | 2270 | -11.9 |
| Jonker, S.M., 2018[60] | 20 [NR] | 120 | 3.49* | 2695 | 2009 | -18.5 |
| Artiflex Myopia (Toric), Ophtec | ||||||
| Coullet, J., 2006[35] | 31 [31] | 12 | NR | 2654 | 2405 | -9.0† |
| Dick, H. B., 2009[51] | 290 [191] | 24 | 3.65* | 2634 | 2605 | -1.07† |
| Doors, M., 2012[52] | 115 [73] | 6 | 3.65* | 2805 | 2676 | -4.3† |
| Munoz, G., 2012[53] | 42 [25] | 12 | 3.51‡ | 2801 | 2538 | NR† |
| Ozerturk, Y., 2012[54] | 50 [NR] | 24 | 3.41‡ | 3023 | 2797 | -7.4† |
| Ruckhofer, J., 2012[55] | 42 [24] | 6 | NR | 2646 | 2627 | -0.7† |
| Jonker, S.M., 2018[64] | 137 [NR] | 60 | 3.27* | 2739 | 2480 | -10.5[1] |
| Jonker, S.M., 2018[64] | 63 [NR] | 60 | 3.24* | 2769 | 2488 | -10.2[1] |
| Shaaban, Y., 2020[63] | 20 [NR] | 36 | NR | 2899 | 2173 | -25.0† |
| Ghoreishi, M., 2020[56] | 41 [41] | 12 | NR | 2777 | 2721 | NR‡ |
Mo = Months; ACD = Anterior chamber depth; ECD = Endothelial cell density; EC = Endothelial cell; NR = Not reported. *as measured from the corneal epithelium, around 0.5 mm consists of corneal thickness, †measurement not repeatable based on materials and methods section, ‡measurement method not reported, 1chronic loss from 6 months to endpoint
Table 3b.
Reported complications in eyes implanted with anterior chamber iris-fixated phakic intraocular lenses
| Nr. Eyes [Nr. pt] | Follow-up (mo) | Exchange (%) | Explants (%) | Complication | |||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Cataract | EC loss | Retinal | Position Related | Other | ||||
| Artisan Myopia (Toric), Ophtec | |||||||||
| Budo, C., 2000[31] | 249 [NR] | 36 | NR | 2.8 | 1.2 | 0.4 | 0.8% Retinal Detachment | 10% uncentered pIOL, 0.8% pupillary block | 2.4% age related cataract, 1.6% hyphema, 0.8% persistent corneal edema |
| Asano-Kato, N., 2005[33] | 11 [NR] | 24 | NR | NR | NR | NR | NR | 4.5% pigment deposition on pIOL | |
| Bartels, M., 2006[34] | 20 [14] | 24 | NR | NR | NR | NR | 1.85% Retinal Detachment | NR | 1.85% cataract |
| Benedetti, S., 2007[58] | 49 [30] | 60 | NR | NR | NR | NR | NR | 16.8% iris atrophy, 8.2% pigment deposition on pIOL | NR |
| Moshirfar, M., 2007[38] | 38 [NR] | 24 | 1.18 | NR | NR | NR | NR | 2.4% pupil deformation | 4.2% pIOL repositioning, 1.18% surgery related cataract |
| Tahzib, N.G., 2007[39] | 89 [49] | 120 | NR | 2.25 | 2.25 | NR | NR | NR | 1.12% additional laser refractive correction |
| Guell, J. L., 2008[40] | 89 [NR] | 60 | NR | 1.25 | 0.5 | 0.75 | 0.25% Macular Hemorrhage, 0.25% Retinal Detachment | NR | 15.1% additional laser refractive correction |
| Guell, J. L., 2008[40] | 165 [NR] | 60 | NR | NR | NR | NR | NR | NR | 9,8% additional laser refractive correction |
| Silva, R.A., 2008[41] | 19 [NR] | 60 | NR | 7.6 | 3.8 | NR | NR | 3.8% explanted due to glare/halo’s | NR |
| Stulting, R.D., 2008[42] | 386 [232] | 36 | 1.02 | 1.1 | 0.25 | NR | 0.51% Retinal Detachment | 2.54% refixations | NR |
| Qasem, Q., 2010[44] | 151 [84] | 12 | NR | NR | NR | NR | 2.16% Myopic Degeneration, 1.08% Retinal Detachment | 4.32% refixation | 17.9% additional laser refractive correction |
| Titiyal, J.S., 2012[46] | 28 [NR] | 60 | NR | 1.17 | NR | 1.17 | 1.17% Retinal Detachment, 1.17% Perifoveal Subretinal Hemorrhage, 1.17% Peripheral Retinal Tear | 57.41% refixation, 29.4% iris atrophy | NR |
| Moshirfar, M., 2014[65] | 213 [NR] | 65 | NR | 2.76 | 2.3 | 0.92 | NR | NR | NR |
| Chebli, S., 2018[66] | 113 [60] | 65 | NR | NR | NR | 0.9 | NR | NR | NR |
| Jonker, S.M., 2019[48] | 460 [250] | 120 | 1.09 | 17.39 | 11.09 | 5.88 | 1.09% retinal detachment, 1.74% myopic macular degeneration, 0.43% retinoschisis, 0.22% macular hole, 0.22% central serous chorioretinopathy | 1.09% refixations | 3.26% additional laser refractive correction |
| Eldanasoury, A., 2019[61] | 90 [57] | 144 | NR | 33 | 7 | 27 | NR | NR | 11% corneal edema and DSAEK |
| Galvis, V., 2019[62] | 67 [45] | 114 | NR | 4.5 | 3 | 1.5 | NR | 1.5% refixation after trauma | 29.9% over 25% total EC loss |
| Artisan Hyperopia, Ophtec | |||||||||
| Guell, J. L., 2008[40] | 28 [NR] | 60 | 4.88 | NR | NR | NR | NR | NR | 20.5% additional laser refractive correction |
| Qasem, Q., 2010[44] | 14 [7] | 12 | NR | NR | NR | NR | 2.16% Myopic Degeneration, 1.08% Retinal Detachment | 4.32% refixation | 28.6% additional laser refractive correction |
| Artiflex Myopia (Toric), Ophtec | |||||||||
| Dick, H. B., 2009[51] | 290 [191] | 24 | 0.34 | NR | NR | NR | NR | 4.8% pigment deposition on pIOL, 1.4% giant cel deposition on pIOL, 1.4% repositioning | NR |
| Doors, M., 2012[52] | 115 [73] | 6 | NR | 1.74 | NR | NR | NR | 14.8% pigment deposition on pIOL, 12.2% giant cel deposition on pIOL | 4.38% posterior synechiae |
| Munoz, G., 2012[53] | 42 [25] | 12 | NR | NR | NR | NR | NR | 16.7% pigment deposition on pIOL, 2.4% repositioning | NR |
| Ozerturk, Y., 2012[54] | 50 [NR] | 24 | NR | NR | NR | NR | 1.2% Choroidal Neovascularisation | 21.7% depositions on pIOL | NR |
| Visser, N., 2012[67] | 35 [20] | 12 | NR | NR | NR | NR | NR | 11.43% depositions on pIOL | NR |
| Galvis, V., 2019[62] | 10 [5] | 108 | NR | NR | NR | NR | NR | NR | 20% over 25% total EC loss |
| Ghoreishi, M., 2020[56] | 41 [41] | 12 | NR | NR | NR | NR | NR | 2.44% reposition due to misalignment | NR |
| Artisan/Artiflex (Toric), Ophtec (Mixed Groups) | |||||||||
| Tehrani, M., 2006[49] | 30 [NR] | 36 | NR | NR | NR | NR | NR | 17.5% pigment deposition on pIOL | NR |
| Guell, J. L., 2008[40] | 67 [NR] | 36 | 1.19 | NR | NR | NR | NR | NR | 5.95% additional laser refractive correction |
| Saxena, R., 2008[68] | 318 [NR] | mean 35 | 1.57 | 1.26 | 1.26 | NR | 0.31% Retinal Detachment | 0.31% pupillary block | NR |
| Qasem, Q., 2010[44] | 20 [11] | 12 | NR | NR | NR | NR | 2.16% Myopic Degeneration, 1.08% Retinal Detachment | 4.32% refixation | 15% additional laser refractive correction |
Mo = Months; ACD = Anterior chamber depth; ECD = Endothelial cell density; EC = Endothelial cell; NR = Not reported; pIOL = Phakic intraocular lens; IOP = Intraocular pressure
First designed in 1953 by the Dutch ophthalmologist Cornelis Binkhorst, iris-fixated IOLs were originally created for implantation after crystalline lens removal. The Binkhorst IOL, using a paper-clip design to stabilize the lens, was associated with lens instability resulting in lens luxation, corneal complications and chronic inflammation causing retinal edema and problems with intraocular pressure.[29] In 1978 the iris-fixated IOL design was updated by a second Dutch ophthalmologist, Jan Worst. He discovered that fixation of midperipheral iris tissue with small “lobster-claws” provided good stabilization of the IOL, without inducing the complications associated with chronic inflammation in the paper-clip design. Over time the Worst IOL was also implemented as a pIOL for the correction of refractive errors. Current iris-fixated pIOLs are still largely based on the Worst lens, with different materials and designs for the correction of myopia, hyperopia and astigmatism, producing excellent visual and refractive results, and low rates of complications [Tables 1a, 2a and 3a].[15,16,30]
Posterior pIOLs were launched in the 1980s, driven by the complications associated with angle-supported pIOLs. By positioning the pIOL further back and away from the cornea, it was hypothesized that the risk of corneal complications would diminish. The specific downside of this design is its proximity to the crystalline lens, and zonular fibers. Early designs of posterior pIOLs frequently touched the anterior part of the crystalline lens causing a significant anterior subcapsular cataract, whereas friction between the pIOL and iris caused pigmentary dispersion and inflammation resulting in raised intraocular pressure. Modern posterior pIOLs have evolved to use plate haptics to support them in the sulcus between the iris base and ciliary muscle, and apertures in the optic to facilitate aqueous humor flow, providing good visual and refractive results [Table 1c]. They rely on correct sizing to prevent both iris chafing or pupillary block glaucoma caused by an oversized plate pushing the iris forward, and cataract formation caused by an undersized plate resulting in insufficient support and touch between the pIOL and crystalline lens [Table 3c]. Although the altered aqueous humor flow in posterior pIOLs with apertures reduces the rate of cataract formation after implantation, correct sizing remains the main determinant in the prevention of complications. Unfortunately, there is no perfect way yet to measure the sulcus, introducing a higher risk of sizing problems.
Table 1c.
Overview of visual and refractive results after implantation with a posterior chamber phakic intraocular lens
| Nr. eyes [nr. pt] | Follow-up (mo) | MRSE | % within target | UDVA | CDVA | Indices | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| preop | postop | +/- 0.5D | +/- 1.0D | 20/20 (%) | 20/40 (%) | 20/20 (%) | 20/40 (%) | Efficacy | Safety | |||
| PRL Myopia, CIBA Vision/Zeiss Meditec | ||||||||||||
| Hoyos, J. E., 2002[69] | 17 [NR] | 12 | -18.46 | -0.22 | 53 | 82 | NR | NR | NR | NR | NR | NR |
| Pallikaris, I. G., 2004[70] | 34 [19] | 17 | -14.70 | -14.70 | 44 | 79 | NR | NR | NR | NR | NR | NR |
| Koivula, A., 2005[71] | 14 [NR] | 12 | -9.19 | -0.31 | 75 | 100 | NR | NR | NR | NR | NR | NR |
| Donoso, R., 2006[72] | 53 [39] | 8 | -17.27 | -0.23 | NR | 71.2 | NR | 60 | NR | 88.2 | 1 | 1.4 |
| Jongsareejit, A., 2006[73] | 50 [31] | 12 | NR | -0.23 | NR | NR | 44 | 82 | 54 | 84 | NR | NR |
| Verde, C. M., 2007[74] | 90 [NR] | 12 | -11.9 | 0.04 | 68 | 80 | NR | NR | NR | NR | 0.98 | 1.22 |
| Portaliou, D. M., 2011[75] | 34 [NR] | 72 | -14.080 | -0.45 | 67.6 | 91.2 | NR | NR | NR | NR | NR | NR |
| Perez-Cambrodi, R. J., 2011[76] | 35 [20] | 57 | -10.25 | -0.11 | 94.28 | 97.14 | NR | 97 | NR | 97 | 1..16 | 1.26 |
| Torun, N., 2013[77] | 53 [29] | 86 | -12.14 | -0.32 | 66.0 | 79.2 | NR | NR | NR | NR | 0.9 | 1.21 |
| PRL Hyperopia, CIBA Vision/Zeiss Meditec | ||||||||||||
| Hoyos, J. E., 2002[69] | 14 [NR] | 12 | 7.77 | -0.38 | 50 | 79 | NR | NR | NR | NR | NR | NR |
| Koivula, A., 2005[71] | 6 [NR] | 12 | 6.13 | -0.60 | 67 | 100 | NR | NR | NR | NR | NR | NR |
| Gil-Cazorla, R., 2008[78] | 16 [9] | 12 | 5.65 | 0.07 | 93.75 | 100 | NR | NR | NR | NR | 0.9 | 0.8 |
| Koivula, A., 2009[79] | 40 [25]] | 12 | 5.90 | -0.46 | 87.5 | 100 | NR | NR | NR | NR | 0.7 | 0.89 |
| PRL, CIBA Vision/Zeiss Meditec (Mixed Groups) | ||||||||||||
| Koivula, A., 2005[71] | 20 [NR] | 12 | NR | NR | 75 | 100 | 50 | 90 | NR | NR | 0.89 | 1.12 |
| ICL V4(b Toric), STAAR Surgica | ||||||||||||
| Kamiya, K., 2013[80] | 50 [28] | 36 | -9.47 | -0.22 | 82 | 98 | 86 | 98 | NR | NR | 0.94 | 1.16 |
| Sari, E.S., 2013[81] | 34 [20] | 36 | NR | NR | 52.9 | 82.4 | NR | NR | NR | NR | NR | NR |
| Alfonso, J.F., 2014[82] | 35 [20] | 12 | -7.03 | -0.18 | 97.1 | 100 | NR | NR | 77 | 100 | NR | 1.08 |
| Igarashi, A., 2014[83] | 41 [41] | 96 | -10.19 | -0.44 | 68.3 | 85.4 | 73.2 | 87.8 | NR | NR | 0.83 | 1.13 |
| Guber, I., 2016[105] | 90 [NR] | 120 | -11.4 | NR | 48.6 | 65.7 | NR | NR | NR | NR | 0.76 | 1.25 |
| Shimizu, K., 2016[84] | 26 [26] | 60 | -7.51 | -0.19 | 92 | 100 | 100 | 100 | NR | NR | NR | NR |
| ICL V4c (Toric), STAAR Surgical | ||||||||||||
| Shimizu, K., 2012[85] | 20 [20] | 6 | -7.36 | 0.01 | 100 | 100 | 100 | 100 | NR | NR | 1.03 | 1.13 |
| Shimizu, K., 2016[84] | 26 [26] | 60 | -7.54 | -0.15 | 88 | 96 | 85 | 100 | NR | NR | NR | NR |
| Kamiya, K., 2017[86] | 57 [57] | 12 | -4.29 | NR | 93 | 98 | 91 | NR | NR | NR | NR | NR |
| Kamiya, K., 2017[87] | 294 [294] | 12 | -10.31 | NR | 94 | 99 | 97 | NR | NR | NR | NR | NR |
| Pjano, M.A., 2017[88] | 28 [16] | 12 | -9.52 | -0.21 | NR | NR | 67.9 | 92.9 | NR | NR | 1.2 | 1.25 |
| Kamiya, K., 2018[9] | 365 [201] | 12 | -8.66 | NR | 90 | 98 | 94 | NR | NR | NR | NR | NR |
| Miao, H., 2018[90] | 67 [38] | 3 | -12.44 | -0.30 | 72 | 95 | 94 | 100 | NR | NR | 1.14 | 1.33 |
| Takahashi, M., 2018[91] | 42 [21] | 6 | -7.37 | -0.61 | 100 | 100 | 74 | 95 | NR | NR | NR | NR |
| Alfonso, J. F., 2019[92] | 141 [83] | 60 | -9.20 | -0.44 | 67.4 | 90.1 | 45.4 | 88.7 | 86.5 | 98.6 | 0.87 | 1.09 |
| Ghoreishi, M., 2019[93] | 41 [41] | 12 | -9.85 | -0.33 | 80 | 95 | NR | 97 | NR | NR | 1.24 | 1.40 |
| Jadidid, K., 2019[94] | 14 [14] | 36 | -8.15 | -1.02 | NR | NR | NR | NR | NR | NR | 0.75 | 1.08 |
| Qin, Q., 2019[95] | 30 [15] | 3 | -13.87 | 0.05 | NR | NR | NR | NR | NR | NR | 1.01 | 1.01 |
| Igarashi, A., 2019[96] | 44 [23] | 3 | -6.23 | NR | 93.2 | 100 | NR | NR | NR | NR | 0.91 | 1.13 |
| Niu, L., 2019[97] | 51 [31] | 12 | -14.03 | -0.67 | 69 | 92 | 69 | 100 | NR | NR | 1.14 | 1.33 |
| Nakamura, T., 2019[98] | 65 [NR] | 60 | -9.97 | NR | 81.5 | 100 | NR | NR | NR | NR | 0.77 | 0.94 |
| Nakamura, T., 2019[98] | 70 [38] | 120 | -9.97 | NR | 71.4 | 87.1 | NR | 92.9 | NR | NR | 0.66 | 0.88 |
| Yu, Z., 2020[99] | 38 [19] | 3 | -10.77 | -0.86 | 87 | 97 | NR | NR | NR | NR | 1.15 | 1.37 |
| ICL, STAAR Surgical (Mixed Groups) | ||||||||||||
| Hassaballa, M.A., 2011[45] | 26 [NR] | 12 | -12.44 | -0.63 | NR | 53.84 | NR | NR | NR | NR | 0.95 | 1.18 |
| Moya, T., 2015[100] | 110 [NR] | 144 | -16.90 | -1.77 | NR | 34.3 | NR | NR | NR | NR | 0.65 | 1.22 |
| IPCL (Toric), CareGroup IOL | ||||||||||||
| Vasavada, V., 2018[101] | 30 [16] | 36 | -16.50 | -0.89 | 45 | 69 | 46 | NR | NR | NR | 1.02 | 0.64 |
| Sachdev, G., 2019[102] | 134 [NR] | 12 | -10.31 | NR | 88 | 95.8 | 51 | 96 | NR | NR | NR | NR |
| EYECRYL, Biotech | ||||||||||||
| Yasa, D., 2018[103] | 58 [29] | 12 | -13.41 | -0.44 | 62 | 93 | NR | NR | NR | NR | 1.2 | 1.39 |
| Yasa, D., 2020[104] | 43 [23] | 6 | -10.3 | -0.36 | 70 | 91 | NR | NR | 16 | 88 | 1.25 | 1.41 |
Mo = Months; MRSE = Manifest refractive spherical equivalent; D = Diopter; UDVA = Uncorrected distance visual acuity; CDVA = Corrected distance visual acuity; NR = Not reported
Table 3c.
Reported complications in eyes implanted with posterior chamber phakic intraocular lenses
| Nr. Eyes [Nr. pt] | Follow-up (mo) | Exchange (%) | Explants (%) | Complication | |||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Cataract | EC loss | Retinal | Position Related | Other | ||||
| PRL Myopia, CIBA Vision/Zeiss Meditec | |||||||||
| oyos, J. E., 2002[69] | 17 [NR] | 12 | 18 | NR | NR | NR | NR | 6% cortical lens opacity | NR |
| Pallikaris, I. G., 2004[70] | 34 [19] | 17 | NR | NR | NR | NR | NR | 2.9% anterior opacification due to lens touch | 28.5% glare and halo’s, 8.8% damaged anterior capsule during iridectomy creation, 5.9% glaucoma requiring surgery |
| Donoso, R., 2006[72] | 53 [39] | 8 | NR | 3.8 | NR | NR | 1.9% retinal detachment | 3.8% lens subluxation through zonules | NR |
| Jongsareejit, A., 2006[73] | 50 [31] | 12 | NR | 2 | 2 | NR | 2% macular hemorrhage | 2% pupillary block glaucoma | NR |
| Portaliou, D. M., 2011[75] | 34 [NR] | 72 | NR | NR | NR | NR | NR | 8.8% damaged anterior capsule, pigment dispersion, 2.9% lens decentration | NR |
| Perez-Cambrodi, R. J., 2011[76] | 35 [20] | 57 | NR | NR | NR | NR | 2.86% retinal detachment | 5.71% lens decentration, 2.86% cortical opcifications | NR |
| Torun, N., 2013[77] | 53 [29] | 86 | NR | 9.3 | 7.5 | NR | 3.8% retinal detachment | 11.3% asymptomatic anterior cataract, 9.4% slight lens decentration, 1.8% lens decentration requiring explantation | NR |
| PRL Hyperopia, CIBA Vision/Zeiss Meditec | |||||||||
| Hoyos, J. E., 2002[69] | 14 [NR] | 12 | NR | NR | NR | NR | NR | 14% pupillary block glaucoma, 7% pigmentary dispersion | 28% glare and halo’s |
| Gil-Cazorla, R., 2008[78] | 16 [9] | 12 | NR | NR | NR | NR | NR | NR | 50% additional laser refractive correction to correct astigmatism |
| PRL, CIBA Vision/Zeiss Meditec (Mixed Groups) | |||||||||
| Koivula, A., 2005[71] | 20 [NR] | 12 | NR | NR | NR | NR | NR | 10% pupillary block glaucoma | NR |
| ICL V3/V4, STAAR Surgical | |||||||||
| Moya, T., 2015[100] | 110 [NR] | 144 | 1.38 | 11.8 | 7.6 | NR | 3.47% Retinal Detachment | 13.88% significant opacities, 0.69% subluxation needing exchange, 0.69% incorrect sizing needing exchange | NR |
| ICL V4(b Toric), STAAR Surgical | |||||||||
| Kamiya, K., 2013[80] | 50 [25] | 36 | NR | NR | NR | NR | NR | 12% ≥10° rotation requiring repositioning, 8% asymptomatic anterior catatarct | NR |
| Sari, E.S., 2013[81] | 34 [20] | 36 | NR | NR | NR | NR | NR | 8.8% repositioning, 5.8% asymptomatic anterior catatarct | NR |
| Igarashi, A., 2014[83] | 41 [41] | 96 | NR | NR | 4.9 | NR | NR | 9.8% asymptomatic anterior cataract | 9.8% asymptomatic nuclear cataract |
| Guber, I., 2016[105] | 90 [NR] | 120 | NR | NR | 18.3 | NR | 1.5% Choroidal Neovascularisation, 0.8% Macular Hole, 0.8% Central Pigment Atrophy | 54.8% lens opacities, 12% increased IOP requiring treatment | NR |
| Shimizu, K., 2016[84] | 26 [26] | 60 | NR | NR | NR | NR | NR | 3.1% anterior cataract, 3.1% ≥30° rotation | 3.1% additional laser refractive correction, |
| ICL V4(c Toric), STAAR Surgical | |||||||||
| Kamiya, K., 2017[87] | 294 [294] | 12 | 0.7 | NR | NR | NR | NR | 0.3% ≥30° rotation, 0,3% iritis | 0.3% additional laser refractive correction |
| Kamiya, K., 2018[89] | 365 [201] | 12 | 0.6 | NR | NR | NR | NR | 0.6% ≥30° rotation | NR |
| Takahashi, M., 2018[91] | 42 [21] | 6 | NR | NR | NR | NR | NR | 19% glare and halo in the early postoperative period | NR |
| Reháková, T., 2019[106] | 63 [32] | 24 | NR | 3.1 | NR | NR | NR | 3.1% explanted due to decentration and acute glaucoma | NR |
| Choi, J., 2019[107] | 110 [60] | 120 | 1.9 | 5.5 | 5.5 | NR | 0.9% rhegmatogenous retinal detachment | 19.1% lens opacities (9.1% anterior subcapsular opacities), 1.8% exchange due to acute glaucoma, 0.9% pigment dispersion requiring trabeculectomy | NR |
| Niu, L., 2019[97] | 51 [31] | 12 | NR | NR | NR | NR | NR | 7.8% with a small vault due to incorrect haptic placement in the sulcus | NR |
| Nakamura, T., 2019[98] | 114 [61] | 120 | NR | NR | 3.5 | NR | NR | 10.5% anterior subcapsular opacities | NR |
| IPCL (Toric), CareGroup IOL | |||||||||
| Vasavada, V., 2018[101] | 30 [16] | 36 | NR | NR | NR | NR | NR | 3.3% clinically significant vault reduction over 3 years, 3.3% anterior subcapsular cataract with good VA untill 3y postop | NR |
| Sachdev, G., 2019[102] | 134 [NR] | 12 | NR | NR | 0.7 | NR | NR | 2.9% anterior subcapsular cataract, 0.7% pupillary block glaucoma, 4.47% ≥5° rotation requiring repositioning | 0.7% hypopion/TASS |
Mo = Months; ACD = Anterior chamber depth; ECD = Endothelial cell density; EC = Endothelial cell; NR = Not reported; pIOL = Phakic intraocular lens; IOP = Intraocular pressure
One posterior pIOL type that was taken of the market did not require measurement of the sulcus. This lens relied on the natural flow of intraocular fluid to keep the pIOL from touching the crystalline lens. However, its instability increased the rate of cataract formation, the rate of pIOL luxation behind the crystalline lens in the vitreous, and made it unsuitable for the correction of astigmatism.[15,16,108]
Outcomes and Complications of Phakic Intraocular Lenses
Large numbers of studies have shown that all three types of pIOLs render excellent results with regard to uncorrected visual acuity, distance corrected visual acuity, and residual refractive error [Tables 1a-1c]. Other outcome measures reported after pIOL implantation are mainly related to the safety of the pIOL in the eye and report complications associated with the cornea, crystalline lens, or retina.[16]
The cornea is essential in refracting light – functioning as a positive lens – and its clarity is of vital importance to procure a clear image on the retina. Corneal clarity is maintained by the innermost cells of the cornea, called the endothelial cells. Endothelial cells function as an active pump that transports water from the corneal stroma, essential because excessive intracorneal water causes corneal edema and compromises its clarity. Although the number of endothelial cells decreases with advancing age, it remains sufficiently high to retain adequate pump function.[12,109,110,111] Any intraocular surgery causes a surgery-related, acute peak in endothelial cell loss that varies per person as well as per type of surgery. Additionally, the intraocular presence of a pIOL increases chronic endothelial cell loss. Therefore, endothelial cell density should be checked with regular intervals, both to monitor individual safety as well as safety of the specific pIOL in general.[112] On a patient-level these measurements are performed to prevent corneal transplantation due to endothelial cell loss and corneal decompensation. On a population level, regular measurements and standardized reporting can detect trends to remove high-risk pIOLs from the market. Recent evidence have led to withdrawal of angle-supported pIOLs because of increased levels of endothelial cell loss [Tables 2a-2c].[15,16,18,112,113]
Table 2c.
Overview of studies reporting on endothelial cell health after posterior chamber phakic intraocular lens implantation
| Nr. Eyes [Nr. pt] | Follow-up (mo) | Preop ACD (mm) | ECD (cells/mm²) | Total EC loss (%) | ||
|---|---|---|---|---|---|---|
| Preop | Postop | |||||
| PRL Myopia, CIBA Vision/Zeiss Meditec | ||||||
| Koivula, A., 2005[71] | 14 [NR] | 12 | NR | 2989 | 2771 | -7.4† |
| Torun, N., 2013[77] | 53 [29] | 86 | NR | 2581 | NR | -6.4 |
| PRL Hyperopia, CIBA Vision/Zeiss Meditec | ||||||
| Koivula, A., 2005[71] | 6 [NR] | 12 | NR | 3198 | 3031 | -6.2† |
| Koivula, A., 2009[79] | 40 [25] | 12 | NR | NR | NR | -3.8† |
| PRL, CIBA Vision/Zeiss Meditec (Mixed Groups) | ||||||
| Koivula, A., 2005[71] | 20 [NR] | 12 | NR | NR | NR | -7.1† |
| ICL V4(b Toric), STAAR Surgical | ||||||
| Kamiya, K., 2013[80] | 50 [28] | 36 | 3.23* | 2753 | 2682 | NR† |
| Sari, E.S., 2013[81] | 34 [20] | 36 | 3.22* | 3307 | 3102 | -7.8† |
| Alfonso, J.F., 2014[82] | 35 [20] | 12 | NR | 2755 | 2634 | NR† |
| Igarashi, A., 2014[83] | 41 [41] | 96 | 3.24* | 2819 | 2626 | -6.2† |
| Shimizu, K., 2016[84] | 26 [26] | 60 | 3.11‡ | 2750 | 2711 | -1.2† |
| Goukon, H.. 2017[114] | 25 [25] | 24 | 3.16‡ | 2829 | 2798 | -1.1† |
| ICL V4(c Toric), STAAR Surgical | ||||||
| Shimizu, K., 2012[85] | 20 [20] | 6 | 3.13* | 2798 | 2720 | -2.8† |
| Shimizu, K., 2016[84] | 26 [26] | 60 | 3.13‡ | 2803 | 2799 | -0.5† |
| Goukon, H., 2017[114] | 34 [34] | 24 | 3.14‡ | 2816 | 2806 | -0.3† |
| Kamiya, K., 2017[87] | 57 [57] | 12 | 3.08* | NR | NR | -0.1† |
| Kamiya, K., 2017[87] | 294 [294] | 12 | 3.14* | NR | NR | -0.1† |
| Pjano, M.A., 2017[88] | 28 [16] | 12 | 3.48* | 2656 | 2512 | -5.5† |
| Alfonso, J. F., 2019[92] | 141 [83] | 60 | 3.16 | 2657 | 2645 | -0.4† |
| Reháková, T., 2019[106] | 63 [32] | 24 | NR | 3271 | 2803 | -13.5† |
| Choi, J., 2019[107] | 71 [NR] | 120 | NR | 2889 | 2749 | NR† |
| Niu, L., 2019[97] | 51 [31] | 12 | 2.74 | 3235 | 2964 | -8.4† |
| Nakamura, T., 2019[98] | 70 [38] | 120 | 3.19 | 2739 | 2581 | -5.3† |
| Ghoreishi, M., 2020[56] | 41 [41] | 12 | NR | 2723 | 2672 | NR‡ |
| IPCL (Toric), CareGroup IOL | ||||||
| Vasavada, V.,2018[101] | 30 [16] | 36 | 3.28* | 3036 | 2655 | -9.7† |
| Sachdev, G., 2019[102] | 134 [NR] | 12 | 3.21* | 2755 | NR | -2.0† |
| 17 [10] | 24 | 3.44 | 2552 | 2299 | -9.9 | |
| EYECRYL, Biotech | ||||||
| Yasa, D., 2018[103] | 58 [29] | 12 | NR | 2713 | 2608 | -3.9† |
| Yasa, D., 2020[104] | 43 [23] | 6 | 3.23 | 2719 | 2779 | NR† |
Mo = Months; ACD = Anterior chamber depth; ECD = Endothelial cell density; EC = Endothelial cell; NR = Not reported. *as measured from the corneal epithelium, around 0.5 mm consists of corneal thickness, †measurement not repeatable based on materials and methods section, ‡measurement method not reported
Although high myopes are known to develop cataract at a younger age, pIOLs may also lead to accelerated cataract formation, causing loss of accommodation and decreased visual acuity, requiring pIOL explantation combined with cataract surgery [Tables 3a-3c].[12,16] Cataract formation after uneventful pIOL implantation is thought to be associated with inflammation in all pIOL types, but patients with posterior chamber pIOLs are especially at risk for cataract formation due to the design of the pIOL [Tables 3a-3c].[12,16,116] As highlighted previously, the position of the posterior pIOL in proximity to the crystalline lens, combined with known preoperative sizing difficulties, frequently induces contact between the pIOL and crystalline lens. As a result of this friction, these patients are at a high risk to develop anterior subcapsular cataract, which can require cataract surgery at a much younger age, whilst adhesions between the crystalline lens and its surrounding capsule increase the difficulty of the surgery. Fortunately, recent modifications with additional apertures in the optic have presumably changed the aqeous humor flow and resulted in a decreased rate of cataract formation [Table 3c].[117]
High myopia is associated with retinal complications because the retina is stretched out and becomes thinner, increasing the risk of developing weak spots in the retina. Ultimately these weak spots can result in complications such as myopic macular degeneration, retinal detachment, macular retinoschizis or choroidal neovascularization [Tables 3a-3c].[6] Even though these complications can occur regardless of intraocular surgery, it is known that cataract surgery significantly increases the risk of developing a retinal detachment, especially when surgery is performed in younger patients.[118,119] The causative mechanism might be that cataract surgery requires the removal of the crystalline lens, changing the dynamics of the posterior part of the eye, whereas in pIOL implantation changes are only applied in the anterior segment of the eye, which is unlikely to result in a similar risk.
Discussion
As reported in Tables 1a-2a-2c, and 3a-3c previous publications show large variations in the duration of follow-up, and reported outcome measures. This is especially true when reporting safety outcomes as endothelial cell loss, complications, and number and reason of pIOL explantations Tables 2a-2c, and 3a-3c.
Cataract formation in patients with pIOLs can be caused by (A) aging, (B) crystalline lens damage, or possibly by (C) insufficient aqueous humor circulation.[16,105,120,121]
-
A)
With advancing age the crystalline lens becomes sclerotic and loses flexibility, resulting in decreased accommodative capacity and requirement of reading glasses (presbyopia).[4,109,122] Age-related structural changes in the fibers and proteins of the crystalline lens result in refractive changes, increased light scatter and a decreased lens clarity (cataract)[122]
-
B)
Crystalline lens trauma due to forceful irrigation, complicated pIOL implantation, or intermittent touch of an incorrectly sized posterior chamber pIOL have all been described in previous papers.[16,105,123] Sizing of posterior chamber pIOLs is especially difficult because there is no clear correlation between anterior segment measurements and sulcus diameter. Insufficient sizing induces higher risks of excessively small or large vaults, causing crystalline lens touch or angle closure, respectively – risks that are decreased in the presence of a peripheral iridectomy in anterior chamber or central hole in posterior chamber pIOLs. Numerous conference meetings and scientific papers report on the optimization of sizing of posterior chamber pIOLs, but further research will hopefully result in finding the 'gold standard' for preoperative sizing[124,125]
-
C)
Aqueous humor circulation is responsible for the distribution of nutrients towards the surface of the crystalline lens.[122] Computer simulations using iris-fixated and posterior chamber pIOLs have shown that altered aqueous humor flow is unlikely to cause cataract formation in iris-fixated pIOLs and posterior chamber pIOLs with a central hole.[120] The models in these studies focus on the assessment of shear stress on the surface of the crystalline lens and corneal endothelium. Importantly, they show an increased shear stress on the surface of the crystalline lens in posterior chamber pIOLs with a small vault.[120] It is currently unclear if daily eye movements (saccades) affect aqueous humor flow and nutrient distribution. New simulations need to be performed in order to assess whether flow is altered and if this might influence cataract formation or EC loss.
Endothelial cell loss is the second most common complication related to pIOL implantation. Three hypotheses exist, attributing EC loss to either (i) the proximity of the pIOL to the corneal endothelium, (ii) the pIOL-related change in aqueous humor flow, or (iii) the chronic subclinical inflammatory response to the pIOL.[48,60,64]
-
(i)
A shorter distance between the anterior chamber pIOL and the corneal endothelium is associated with an increased EC loss.[25,48,60] Posterior chamber pIOLs are designed to be positioned further away from the endothelium and are therefore assumed to cause less EC loss. This theory cannot be definitely confirmed due to incomplete or absent reporting on EC loss in recent research on posterior chamber pIOLs. A 2018 paper by our group casts additional doubt upon this theory, because it does not identify a smaller preoperative anterior chamber depth as a significant risk-factor for EC loss in eyes with foldable iris-fixated pIOLs.[64] Adequately designed studies are needed to prove the validity of this hypothesis and assess if there is additional benefit to posterior chamber pIOLs regarding EC loss
-
(ii)
Alterations in aqueous humor flow have been assessed in iris-fixated and posterior chamber pIOLs using computer simulation, and have shown a significant change in aqueous humor flow that is unlikely to result in increased EC loss. As mentioned previously, none of these studies have implemented the daily movements (saccades) of the eye in their estimations.[120] New simulations are required to assess whether these movements result in significant changes in aqueous humor flow that could explain EC loss, and to assess whether design changes are required in pIOLs currently available for implantation
-
(iii)
Research regarding the possibility of subclinical inflammation has been met with technical difficulties in the past.[126] Few methods are available to assess minimal inflammation - for example, in eyes not presenting with either cell, flare or the Tyndall Effect during slit-lamp evaluation. Laser Flare Meters have been used in the past to assess inflammation, and the first study using a Laser Flare Cell Meter to assess inflammation in patients with a pIOL was published in the early nineties.[127] Studies that were performed in the years following showed increased flare in eyes with angle-supported and iris-fixated pIOLs, as compared to healthy patients without these lenses, and showed highly variable inflammation in eyes with iris-fixated P IOLs.[116] However, high inter- and intra-observer variability, low repeatability, and the time-consuming nature of these measurements have prohibited the implementation of the Laser Flare Cell Meter in the clinical care pathway.
Regardless of the cause of EC loss, EC monitoring is essential whenever a pIOL is implanted. In 2006, the AFSSAPS published a guideline reporting an ECD of 1500 cells/mm2 or less as a reason for pIOL explantation, after the Vivarte angle-supported pIOL had to be taken of the market.[128] After consulting with large numbers of specialists from the field, the AAO published a second, more extensive guideline in 2018.[112] It describes the importance of correct ECD measurements and provides specific endpoints when reporting ECD (i.e., the proportion of eyes with ≥25% EC loss after 3 years). In addition, it also refers to clause D.4.2 of the ANSI standard Z80.13 Phakic Intraocular Lenses standard for recommendations on how to perform ECD measurements. Studies should report the mean of three acceptable measurements of the central cornea, identifying at least 100 cells per frame, and use the center-to-center method with the same non-contact specular microscope throughout the study.[112] Identifying 100 cells per image can be challenging: non-contact specular microscopes are capable of capturing 120 to 170 cells per image, depending on ECD and the quality of the image. Contact specular microscopes on the other hand can capture 700 to 3000 cells per image, depending on the skill of the technician.[129] Contact specular microscopy however is time-consuming and invasive, as well as a skill that requires a certain level of training and upkeep, making it more difficult to implement in a busy practice. Another option to increase the number of analyzed cells is to use the corner method instead of the center-to center-method.[130] A 2010 study confirmed that the corner method is likely to benefit representation of ECD and morphological characteristics in transplanted corneas, but did not find clinically relevant differences between these measurement methods in healthy corneas.[130] The corner method takes up significantly more time in the clinic and probably only has additional value in studies on transplanted or diseased corneal endothelium, or in studies focusing on morphometric data. Selection of 100 contiguous cells in a non-contact specular microscopic image is challenging, even in healthy corneas. For this reason, most studies reporting ECD data select 50 contiguous cells and report the mean of three ECD measurements to provide reliable results. The ANSI standard might press researchers to select cells that would not qualify as clearly identifiable, possibly resulting in misrepresentation of ECD and EC morphology. It is important to acknowledge that neither the AFSSAPS, nor the AAO criteria present the researcher with cut-off values as to what proportion of eyes is considered 'safe' at a predefined time point.[112,128] The observed rates of EC loss clearly indicate the need for higher preoperative ECD in each age group, in order to provide a safe number of ≥1500 cells/mm2, when cataract surgery becomes necessary.[60,64] Additional risk-factors for increased EC loss differed between rigid (PMMA) and foldable (silicone) iris-fixated pIOLs, prompting the need for research on different intraocular materials (i.e., differences in intraocular inflammation) as a cause for increased EC loss.[60,64]
Phakic IOL implantation yields excellent visual and refractive results. An analysis of the preoperative characteristics of patients implanted with pIOLs identify (high) myopia as the main reason for surgery, followed by (high) astigmatism or (high) hyperopia Tables 1a-1c. Multiple studies assessing patient satisfaction, spectacle independence and occurrence of bothersome side-effects have indeed reported excellent outcomes, and few bothersome side effects (glare, halo's) after implantation with different types of pIOLs.[36,131] Several questionnaires are available for the evaluation of refractive errors, with three questionnaires (i.e., Quality of Life Impact of Refractive Correction [QIRC][visual symptoms], Quality of Vision [QoV][quality of life], Near Activity Visual Questionnaire [NAVQ][activity limitations]) showing slightly superior results in the assessment of refractive surgery.[132]
Decreased visual acuity over time has been reported with pIOLs, and can be attributed to refractive changes or occurrence of complications. Recent publications by our group report significant changes in refractive error over time in a mainly myopic population.[48] Age-related myopisation due to cataract formation did not entirely account for these changes. Subgroup analyses imply a significant increase in axial length over time in a highly myopic – but small – patient population. Increasing axial length is known to occur in the growing, adolescent eye, but is assumed to stop at around the age of 21. These hypotheses are based on older epidemiological studies that have used cross-sectional analyses to report axial length, resulting in data implying a decrease in axial length with age.[133,134,135] The data presented in the abovementioned papers suggest that axial length keeps increasing after reaching adulthood. New studies are necessary to determine if this is indeed the case, and if so, if it only occurs in (high) myopes.[48]
It is hard to confirm an association between progressive cataract formation and myopisation based on research since there are no guidelines defining how to describe the rate and progression of cataract formation in patients with pIOLs. Three options can be identified to gather information on cataract formation in a study population.
-
(I)
Cataract formation results in altered refraction and visual acuity, and standardization of refractive and visual results are applied by a number of journals. The arrival of standardized six- and nine graphs has resulted in standardized outcome measures in the vast majority of newly published papers on refractive surgery.[136,137] Via these criteria it can be computed how many eyes show a significant decrease in visual acuity or change in refractive error. To conclude if these changes are indeed related to cataract formation, ophthalmologists are dependent on the authors to provide an adequate and sufficient explanation of the cause of these changes
-
(II)
Structural evaluation of cataract formation using grading tools requires the investigator to visually quantify the amount of cataract. Unfortunately, this divides patients into categories rather than assigning a numerical value that can be used to assess cataract progression (i.e., for the categories two versus four, the amount of cataract does not increase two-fold in the second category).[138,139,140,141,142,143] Although some Scheimpflug and optical coherence tomography devices can objectively measure lens characteristics, this is not fully automated yet and further studies are needed to optimize clinical use[144,145]
-
(III)
Survival analyses provide a definitive cut-off measure, working with a binary outcome measure (explantation yes/no). However, they do not provide insights into the progression of cataract formation and implementation is difficult in studies reporting short-term follow-up or small numbers of patients. For future reference, long-term studies with large numbers of patients are the preferred option, reporting refractive changes and their causes, as well as survival analyses and the total number of explantations due to cataract formation. Survival analyses should be attempted in small studies, but they should always be supplemented with the mean time and total number of explantations in order to provide sufficient standardization over time.
Conclusion
Phakic IOL implantation in highly myopic patients is associated with excellent visual and refractive outcomes shortly after surgery. Due to age-related and morphometric changes, visual and refractive outcomes might change over time. The exact mechanisms causing this are yet unknown and require additional research to formulate definitive recommendations. Until then, surgeons implanting pIOLs in highly myopic patients should inform their patients of the possibility that their refractive error might change slightly over time. As shown by the large variability in the reported data, there are still large variations in the assessment and description of complications occurring in patients with pIOLs. Further standardization of outcomes is required to make sure that complications are reported reliably and results can be compared. Specific attention should be paid towards reporting EC loss, cataract formation, including developing methods to reliably assess cataract progression over time, and patient-reported outcomes using validated questionnaires.
Financial support and sponsorship
Nil.
Conflicts of interest
Soraya M.R. Jonker, Tos T.J.M. Berendschot, Annick E. Ronden, Isabelle E.Y. Saelens: none. Noël J.C. Bauer: Alcon (C, L, S), Bausch & Lomb (C, L), Ophtec (S). Rudy M.M.A. Nuijts: Abbott (S), Alcon (C, L, S), Asico (C), Bausch & Lomb (S), Carl-Zeiss (S), HumanOptics (S), Ophtec (S), TheaPharma (S, C).
References
- 1.WHO. World Health Organization Brien Holden Vision Institute; 2017. The Impact of Myopia and High Myopia. [Google Scholar]
- 2.Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036–42. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Morgan IG, He M, Rose KA. Epidemic of pathologic myopia: What can laboratory studies and epidemiology tell us? Retina. 2017;37:989–97. doi: 10.1097/IAE.0000000000001272. [DOI] [PubMed] [Google Scholar]
- 4.Azar DT, Azar NF, Brodie SE, Hoffer KJ, Korn TS, Mauger TF, et al. Clinical Optics United States of America: American Academy of Ophthalmology. 2017 [Google Scholar]
- 5.Huang J, Wen D, Wang Q, McAlinden C, Flitcroft I, Chen H, et al. Efficacy comparison of 16 interventions for myopia control in children: A network meta-analysis. Ophthalmology. 2016;123:697–708. doi: 10.1016/j.ophtha.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Tideman JW, Snabel MC, Tedja MS, van Rijn GA, Wong KT, Kuijpers RW, et al. Association of axial length with risk of uncorrectable visual impairment for europeans with myopia. JAMA Ophthalmol. 2016;134:1355–63. doi: 10.1001/jamaophthalmol.2016.4009. [DOI] [PubMed] [Google Scholar]
- 7.Tideman JW, Polling JR, Voortman T, Jaddoe VW, Uitterlinden AG, Hofman A, et al. Low serum vitamin D is associated with axial length and risk of myopia in young children. Eur J Epidemiol. 2016;31:491–9. doi: 10.1007/s10654-016-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Q, Liu Y, Tighe S, Zhu Y, Su X, Lu F, et al. Retardation of myopia progression by multifocal soft contact lenses. Int J Med Sci. 2019;16:198–202. doi: 10.7150/ijms.30118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y, Xu F, Zhang T, Liu M, Wang D, Chen Y, et al. Orthokeratology to control myopia progression: A meta-analysis. PLoS One. 2015;10:e0124535. doi: 10.1371/journal.pone.0124535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu HY, Chu HS, Wang IJ, Chen WL, Hu FR. Microbial keratitis in Taiwan: A 20-Year update. Am J Ophthalmol. 2019;205:74–81. doi: 10.1016/j.ajo.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Kam KW, Yung W, Li GKH, Chen LJ, Young AL. Infectious keratitis and orthokeratology lens use: A systematic review. Infection. 2017;45:727–35. doi: 10.1007/s15010-017-1023-2. [DOI] [PubMed] [Google Scholar]
- 12.Bowling B. China: Elsevier; 2016. Kanski's Clinical Ophthalmology. [Google Scholar]
- 13.Aalders-Deenstra V, Bartels M, Beerthuizen J, Aalders-Deenstra V, Bartels M, Beerthuizen J, et al. Consensus Refractie Chirurgie. 3 ed. Nederlands Gezelschap voor Refractie Chirurgie (NGRC); 2006. [Google Scholar]
- 14.Bjerrum SS, Mikkelsen KL, La Cour M. Risk of pseudophakic retinal detachment in 202,226 patients using the fellow nonoperated eye as reference. Ophthalmology. 2013;120:2573–9. doi: 10.1016/j.ophtha.2013.07.045. [DOI] [PubMed] [Google Scholar]
- 15.Guell JL, Morral M, Kook D, Kohnen T. Phakic intraocular lenses part 1: Historical overview, current models, selection criteria, and surgical techniques. J Cataract Refract Surg. 2010;36:1976–93. doi: 10.1016/j.jcrs.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Kohnen T, Kook D, Morral M, Guell JL. Phakic intraocular lenses: Part 2: Results and complications. J Cataract Refract Surg. 2010;36:2168–94. doi: 10.1016/j.jcrs.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Bowes Hamill M, Berdy GJ, Davidson RS, Majmudar PA, Randleman JB, Shamie N, et al. Refractive Surgery United States of America: American Academy of Ophthalmology. 2017 [Google Scholar]
- 18.Kohnen T, Maxwell WA, Holland S. Correction of moderate to high myopia with a foldable, angle-supported phakic intraocular lens: Results from a 5-year open-label trial. Ophthalmology. 2016;123:1027–35. doi: 10.1016/j.ophtha.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 19.Kohnen T, Knorz MC, Cochener B, Gerl RH, Arné JL, Colin J, et al. AcrySof phakic angle-supported intraocular lens for the correction of moderate-to-high myopia: One-year results of a multicenter European study. Ophthalmology. 2009;116:1314–21. doi: 10.1016/j.ophtha.2009.01.041. 21e1-3. [DOI] [PubMed] [Google Scholar]
- 20.Knorz MC, Lane SS, Holland SP. Angle-supported phakic intraocular lens for correction of moderate to high myopia: Three-year interim results in international multicenter studies. J Cataract Refract Surg. 2011;37:469–80. doi: 10.1016/j.jcrs.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 21.Mastropasqua L, Toto L, Vecchiarino L, Doronzo E, Mastropasqua R, Di Nicola M. AcrySof cachet phakic intraocular lens in myopic patients: Visual performance, wavefront error, and lens position. J Refract Surg. 2012;28:267–74. doi: 10.3928/1081597X-20120222-01. [DOI] [PubMed] [Google Scholar]
- 22.Yang RB, Zhao SZ. AcrySof phakic angle-supported intraocular lens for the correction of high to extremely high myopia: One-year follow-up results. Int J Ophthalmol. 2012;5:360–5. doi: 10.3980/j.issn.2222-3959.2012.03.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kermani O, Oberheide U, Gerten G. Rotation stability of the cachet angle-supported phakic intraocular lens. J Refract Surg. 2013;29:390–4. doi: 10.3928/1081597X-20130515-03. [DOI] [PubMed] [Google Scholar]
- 24.Taneri S, Oehler S, Heinz C. Inflammatory response in the anterior chamber after implantation of an angle-supported lens in phakic myopic eyes. J Ophthalmol. 2014;2014:923691. doi: 10.1155/2014/923691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aerts AA, Jonker SM, Wielders LH, Berendschot TT, Doors M, De Brabander J, et al. Phakic intraocular lens: Two-year results and comparison of endothelial cell loss with iris-fixated intraocular lenses. J Cataract Refract Surg. 2015;41:2258–65. doi: 10.1016/j.jcrs.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 26.Gimbel HV, Norton NR, Amritanand A. Angle-supported phakic intraocular lenses for the correction of myopia: Three-year follow-up. J Cataract Refract Surg. 2015;41:2179–89. doi: 10.1016/j.jcrs.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Alio JL, Plaza-Puche AB, Cavas F, Yébana Rubio P, Sala E. An angle-supported foldable phakic intraocular lens for correction of myopia: A five-year follow-up. Arch Soc Esp Oftalmol. 2017;92:4–11. doi: 10.1016/j.oftal.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Al-Qahtani S, Al-Afraj K, Al-Jindan M, Al-Beshri AS, Khandekar R. Short and long-term outcomes of angle supported phakic intraocular lens implantation in high myopic eyes. Int J Ophthalmol. 2018;11:888–90. doi: 10.18240/ijo.2018.05.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cornelius D, Binkhorst MD. At the forefront of the IOL revolution. J Cataract Refract Surg. 1997;23:306–7. doi: 10.1016/s0886-3350(97)80170-8. [DOI] [PubMed] [Google Scholar]
- 30.Alpar JJ. Professor Jan Gerben Frans Worst. J Cataract Refract Surg. 2016;42:809. doi: 10.1016/j.jcrs.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 31.Budo C, Hessloehl JC, Izak M, Luyten GP, Menezo JL, Sener BA, et al. Multicenter study of the Artisan phakic intraocular lens. J Cataract Refract Surg. 2000;26:1163–71. doi: 10.1016/s0886-3350(00)00545-9. [DOI] [PubMed] [Google Scholar]
- 32.Dick HB, Alio J, Bianchetti M, Christiaans BJ, El-Danasoury MA, Güell JL, et al. Toric phakic intraocular lens: European multicenter study. Ophthalmology. 2003;110:150–62. doi: 10.1016/s0161-6420(02)01447-1. [DOI] [PubMed] [Google Scholar]
- 33.Asano-Kato N, Toda I, Hori-Komai Y, Sakai C, Fukumoto T, Arai H. Experience with the Artisan phakic intraocular lens in Asian eyes. J Cataract Refract Surg. 2005;31:910–5. doi: 10.1016/j.jcrs.2004.08.057. [DOI] [PubMed] [Google Scholar]
- 34.Bartels MC, Saxena R, van den Berg TJ, van Rij G, Mulder PG, Luyten GP, et al. The influence of incision-induced astigmatism and axial lens position on the correction of myopic astigmatism with the Artisan toric phakic intraocular lens. Ophthalmology. 2006;113:1110–7. doi: 10.1016/j.ophtha.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Coullet J, Guell JL, Fournie P, Grandjean H, Gaytan J, Arné JL, et al. Iris-supported phakic lenses (rigid vs foldable version) for treating moderately high myopia: Randomized paired eye comparison. Am J Ophthalmol. 2006;142:909–16. doi: 10.1016/j.ajo.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 36.Tahzib NG, Bootsma SJ, Eggink FA, Nuijts RM. Functional outcome and patient satisfaction after Artisan phakic intraocular lens implantation for the correction of myopia. Am J Ophthalmol. 2006;142:31–9. doi: 10.1016/j.ajo.2006.01.088. [DOI] [PubMed] [Google Scholar]
- 37.Gierek-Ciaciura S, Gierek-Lapinska A, Ochalik K, Mrukwa-Kominek E. Correction of high myopia with different phakic anterior chamber intraocular lenses: ICARE angle-supported lens and Verisyse iris-claw lens. Graefes Arch Clin Exp Ophthalmol. 2007;245:1–7. doi: 10.1007/s00417-006-0374-7. [DOI] [PubMed] [Google Scholar]
- 38.Moshirfar M, Holz HA, Davis DK. Two-year follow-up of the Artisan/Verisyse iris-supported phakic intraocular lens for the correction of high myopia. J Cataract Refract Surg. 2007;33:1392–7. doi: 10.1016/j.jcrs.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Tahzib NG, Nuijts RM, Wu WY, Budo CJ. Long-term study of Artisan phakic intraocular lens implantation for the correction of moderate to high myopia: Ten-year follow-up results. Ophthalmology. 2007;114:1133–42. doi: 10.1016/j.ophtha.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 40.Guell JL, Morral M, Gris O, Gaytan J, Sisquella M, Manero F. Five-year follow-up of 399 phakic Artisan-Verisyse implantation for myopia, hyperopia, and/or astigmatism. Ophthalmology. 2008;115:1002–12. doi: 10.1016/j.ophtha.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 41.Silva RA, Jain A, Manche EE. Prospective long-term evaluation of the efficacy, safety, and stability of the phakic intraocular lens for high myopia. Arch Ophthalmol. 2008;126:775–81. doi: 10.1001/archopht.126.6.775. [DOI] [PubMed] [Google Scholar]
- 42.Stulting RD, John ME, Maloney RK, Assil KK, Arrowsmith PN, Thompson VM, et al. Three-year results of Artisan/Verisyse phakic intraocular lens implantation. Results of the United States Food And Drug Administration clinical trial. Ophthalmology. 2008;115:464–72. doi: 10.1016/j.ophtha.2007.08.039. e1. [DOI] [PubMed] [Google Scholar]
- 43.Tahzib NG, MacRae SM, Yoon G, Berendschot TT, Eggink FA, Hendrikse F, et al. Higher-order aberrations after implantation of iris-fixated rigid or foldable phakic intraocular lenses. J Cataract Refract Surg. 2008;34:1913–20. doi: 10.1016/j.jcrs.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 44.Qasem Q, Kirwan C, O'Keefe M. 5-year prospective follow-up of Artisan phakic intraocular lenses for the correction of myopia, hyperopia and astigmatism. Ophthalmologica. 2010;224:283–90. doi: 10.1159/000299179. [DOI] [PubMed] [Google Scholar]
- 45.Hassaballa MA, Macky TA. Phakic intraocular lenses outcomes and complications: Artisan vs Visian ICL. Eye (Lond) 2011;25:1365–70. doi: 10.1038/eye.2011.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Titiyal JS, Sharma N, Mannan R, Pruthi A, Vajpayee RB. Iris-fixated intraocular lens implantation to correct moderate to high myopia in Asian-Indian eyes: Five-year results. J Cataract Refract Surg. 2012;38:1446–52. doi: 10.1016/j.jcrs.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 47.Yuan X, Ping HZ, Hong WC, Yin D, Ting Z. Five-year follow-up after anterior iris-fixated intraocular lens implantation in phakic eyes to correct high myopia. Eye (Lond) 2012;26:321–6. doi: 10.1038/eye.2011.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jonker SMR, Berendschot T, Ronden AE, Saelens IEY, Bauer NJC, Nuijts RMMA. Long-term changes in visual outcomes and ocular morphometrics after myopic and toric phakic intraocular lens implantation: Five- and 10-year results. J Cataract Refract Surg. 2019;45:1470–9. doi: 10.1016/j.jcrs.2019.05.044. [DOI] [PubMed] [Google Scholar]
- 49.Tehrani M, Dick HB. Iris-fixated toric phakic intraocular lens: Three-year follow-up. J Cataract Refract Surg. 2006;32:1301–6. doi: 10.1016/j.jcrs.2006.02.058. [DOI] [PubMed] [Google Scholar]
- 50.Guell JL, Vazquez M, Malecaze F, Manero F, Gris O, Velasco F, et al. Artisan toric phakic intraocular lens for the correction of high astigmatism. Am J Ophthalmol. 2003;136:442–7. doi: 10.1016/s0002-9394(03)00295-2. [DOI] [PubMed] [Google Scholar]
- 51.Dick HB, Budo C, Malecaze F, Güell JL, Marinho AA, Nuijts RM, et al. Foldable Artiflex phakic intraocular lens for the correction of myopia: Two-year follow-up results of a prospective European multicenter study. Ophthalmology. 2009;116:671–7. doi: 10.1016/j.ophtha.2008.12.059. [DOI] [PubMed] [Google Scholar]
- 52.Doors M, Budo CJ, Christiaans BJ, Luger M, Marinho AA, Dick HB, et al. Artiflex Toric foldable phakic intraocular lens: Short-term results of a prospective European multicenter study. Am J Ophthalmol. 2012;154:730–9e2. doi: 10.1016/j.ajo.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 53.Munoz G, Cardoner A, Albarran-Diego C, Ferrer-Blasco T, Belda-Salmerón L. Iris-fixated toric phakic intraocular lens for myopic astigmatism. J Cataract Refract Surg. 2012;38:1166–75. doi: 10.1016/j.jcrs.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 54.Ozerturk Y, Kubaloglu A, Sari ES, Koytak A, Capkin M, Akçay L, et al. Foldable iris-fixated phakic intraocular lens implantation for the correction of myopia: Two years of follow-up. Indian J Ophthalmol. 2012;60:23–8. doi: 10.4103/0301-4738.91340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruckhofer J, Seyeddain O, Dexl AK, Grabner G, Stoiber J. Correction of myopic astigmatism with a foldable iris-claw toric phakic intraocular lens: Short-term follow-up. J Cataract Refract Surg. 2012;38:582–8. doi: 10.1016/j.jcrs.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 56.Ghoreishi M, Kashfi A, Peyman M, Mohammadinia M. Comparison of toric implantable collamer lens and toric artiflex phakic IOLs in terms of visual outcome, a paired contralateral eye study. Am J Ophthalmol. 2020;219:186–94. doi: 10.1016/j.ajo.2020.06.021. [DOI] [PubMed] [Google Scholar]
- 57.Pop M, Payette Y. Initial results of endothelial cell counts after Artisan lens for phakic eyes: An evaluation of the United States Food and Drug Administration Ophtec Study. Ophthalmology. 2004;111:309–17. doi: 10.1016/j.ophtha.2003.05.025. [DOI] [PubMed] [Google Scholar]
- 58.Benedetti S, Casamenti V, Benedetti M. Long-term endothelial changes in phakic eyes after Artisan intraocular lens implantation to correct myopia: Five-year study. J Cataract Refract Surg. 2007;33:784–90. doi: 10.1016/j.jcrs.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 59.Yamaguchi T, Negishi K, Yuki K, Saiki M, Nishimura R, Kawaguchi N, et al. Alterations in the anterior chamber angle after implantation of iris-fixated phakic intraocular lenses. J Cataract Refract Surg. 2008;34:1300–5. doi: 10.1016/j.jcrs.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 60.Jonker SM, Berendschot T, Ronden AE, Saelens IE, Bauer NJ, Nuijts RM. Long-term endothelial cell loss in patients with artisan myopia and artisan toric phakic intraocular lenses: 5- and 10-year results. Ophthalmology. 2018;125:486–94. doi: 10.1016/j.ophtha.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 61.Eldanasoury AM, Roozbahani M, Tolees S, Arana C. Long-term effect of anterior chamber depth on endothelial cell density in patients with iris-fixated phakic intraocular lenses. J Refract Surg. 2019;35:493–500. doi: 10.3928/1081597X-20190708-01. [DOI] [PubMed] [Google Scholar]
- 62.Galvis V, Villamil JF, Acuña MF, Camacho PA, Merayo-Lloves J, Tello A, et al. Long-term endothelial cell loss with the iris-claw intraocular phakic lenses (Artisan®) Graefes Arch Clin Exp Ophthalmol. 2019;257:2775–87. doi: 10.1007/s00417-019-04506-9. [DOI] [PubMed] [Google Scholar]
- 63.Shaaban YM, Badran TAF. Three-year effect of phakic intraocular lenses on the corneal endothelial cell density. Clin Ophthalmol. 2020;14:149–55. doi: 10.2147/OPTH.S236041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jonker SMR, Berendschot T, Ronden AE, Saelens IEY, Bauer NJ, Nuijts RM. Five-year endothelial cell loss after implantation with artiflex myopia and artiflex toric phakic intraocular lenses. Am J Ophthalmol. 2018;194:110–9. doi: 10.1016/j.ajo.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 65.Moshirfar M, Imbornoni LM, Ostler EM, Muthappan V. Incidence rate and occurrence of visually significant cataract formation and corneal decompensation after implantation of Verisyse/Artisan phakic intraocular lens. Clin Ophthalmol. 2014;8:711–6. doi: 10.2147/OPTH.S59878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chebli S, Rabilloud M, Burillon C, Kocaba V. Corneal endothelial tolerance after iris-fixated phakic intraocular lens implantation: A model to predict endothelial cell survival. Cornea. 2018;37:591–5. doi: 10.1097/ICO.0000000000001527. [DOI] [PubMed] [Google Scholar]
- 67.Visser N, Berendschot TT, Bauer NJ, Nuijts RM. Vector analysis of corneal and refractive astigmatism changes following toric pseudophakic and toric phakic IOL implantation. Invest Ophthalmol Vis Sci. 2012;53:1865–73. doi: 10.1167/iovs.11-8868. [DOI] [PubMed] [Google Scholar]
- 68.Saxena R, Boekhoorn SS, Mulder PG, Noordzij B, van Rij G, Luyten GP. Long-term follow-up of endothelial cell change after Artisan phakic intraocular lens implantation. Ophthalmology. 2008;115:608–13e1. doi: 10.1016/j.ophtha.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 69.Hoyos JE, Dementiev DD, Cigales M, Hoyos-Chacón J, Hoffer KJ. Phakic refractive lens experience in Spain. J Cataract Refract Surg. 2002;28:1939–46. doi: 10.1016/s0886-3350(02)01439-6. [DOI] [PubMed] [Google Scholar]
- 70.Pallikaris IG, Kalyvianaki MI, Kymionis GD, Panagopoulou SI. Phakic refractive lens implantation in high myopic patients: One-year results. J Cataract Refract Surg. 2004;30:1190–7. doi: 10.1016/j.jcrs.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 71.Koivula A, Petrelius A, Zetterstrom C. Clinical outcomes of phakic refractive lens in myopic and hyperopic eyes: 1-year results. J Cataract Refract Surg. 2005;31:1145–52. doi: 10.1016/j.jcrs.2004.11.059. [DOI] [PubMed] [Google Scholar]
- 72.Donoso R, Castillo P. Correction of high myopia with the PRL phakic intraocular lens. J Cataract Refract Surg. 2006;32:1296–300. doi: 10.1016/j.jcrs.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 73.Jongsareejit A. Clinical results with the medennium phakic refractive lens for the correction of high myopia. J Refract Surg. 2006;22:890–7. doi: 10.3928/1081-597X-20061101-09. [DOI] [PubMed] [Google Scholar]
- 74.Verde CM, Teus MA, Arranz-Marquez E, Cazorla RG. Medennium posterior chamber phakic refractive lens to correct high myopia. J Refract Surg. 2007;23:900–4. doi: 10.3928/1081-597X-20071101-06. [DOI] [PubMed] [Google Scholar]
- 75.Portaliou DM, Kymionis GD, Panagopoulou SI, Kalyvianaki MI, Grentzelos MA, Pallikaris IG. Long-term results of phakic refractive lens implantation in eyes with high myopia. J Refract Surg. 2011;27:787–91. doi: 10.3928/1081597X-20110628-01. [DOI] [PubMed] [Google Scholar]
- 76.Perez-Cambrodi RJ, Pinero DP, Madrid-Costa D, Blanes-Mompó FJ, Ferrer-Blasco T, Cerviño A. Medium-term visual, refractive, and intraocular stability after implantation of a posterior chamber phakic intraocular lens to correct moderate to high myopia. J Cataract Refract Surg. 2011;37:1791–8. doi: 10.1016/j.jcrs.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 77.Torun N, Bertelmann E, Klamann MK, Maier AK, Liekfeld A, Gonnermann J. Posterior chamber phakic intraocular lens to correct myopia: Long-term follow-up. J Cataract Refract Surg. 2013;39:1023–8. doi: 10.1016/j.jcrs.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 78.Gil-Cazorla R, Teus MA, Arranz-Marquez E, Marina-Verde C. Phakic refractive lens (Medennium) for correction of +4.00 to +6.00 diopters: 1-year follow-up. J Refract Surg. 2008;24:350–4. doi: 10.3928/1081597X-20080401-06. [DOI] [PubMed] [Google Scholar]
- 79.Koivula A, Zetterstrom C. Phakic intraocular lens for the correction of hyperopia. J Cataract Refract Surg. 2009;35:248–55. doi: 10.1016/j.jcrs.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 80.Kamiya K, Shimizu K, Kobashi H, Igarashi A, Komatsu M. Three-year follow-up of posterior chamber toric phakic intraocular lens implantation for moderate to high myopic astigmatism. PLoS One. 2013;8:e56453. doi: 10.1371/journal.pone.0056453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sari ES, Pinero DP, Kubaloglu A, Evcili PS, Koytak A, Kutlutürk I, et al. Toric implantable collamer lens for moderate to high myopic astigmatism: 3-year follow-up. Graefes Arch Clin Exp Ophthalmol. 2013;251:1413–22. doi: 10.1007/s00417-012-2172-8. [DOI] [PubMed] [Google Scholar]
- 82.Alfonso JF, Lisa C, Alfonso-Bartolozzi B, Pérez-Vives C, Montés-Micó R. Collagen copolymer toric phakic intraocular lens for myopic astigmatism: One-year follow-up. J Cataract Refract Surg. 2014;40:1155–62. doi: 10.1016/j.jcrs.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 83.Igarashi A, Shimizu K, Kamiya K. Eight-year follow-up of posterior chamber phakic intraocular lens implantation for moderate to high myopia. Am J Ophthalmol. 2014;157:532–9e1. doi: 10.1016/j.ajo.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 84.Shimizu K, Kamiya K, Igarashi A, Kobashi H. Long-term comparison of posterior chamber phakic intraocular lens with and without a central hole (Hole ICL and Conventional ICL) implantation for moderate to high myopia and myopic astigmatism: Consort-compliant article. Medicine (Baltimore) 2016;95:e3270. doi: 10.1097/MD.0000000000003270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shimizu K, Kamiya K, Igarashi A, Shiratani T. Early clinical outcomes of implantation of posterior chamber phakic intraocular lens with a central hole (Hole ICL) for moderate to high myopia. Br J Ophthalmol. 2012;96:409–12. doi: 10.1136/bjophthalmol-2011-300148. [DOI] [PubMed] [Google Scholar]
- 86.Kamiya K, Takahashi M, Takahashi N, Shoji N, Shimizu K. Monovision by implantation of posterior chamber phakic intraocular lens with a central hole (Hole ICL) for early presbyopia. Sci Rep. 2017;7:11302. doi: 10.1038/s41598-017-11539-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kamiya K, Shimizu K, Igarashi A, Kitazawa Y, Kojima T, Nakamura T, et al. Posterior chamber phakic intraocular lens implantation: Comparative, multicentre study in 351 eyes with low-to-moderate or high myopia. Br J Ophthalmol. 2018;102:177–81. doi: 10.1136/bjophthalmol-2017-310164. [DOI] [PubMed] [Google Scholar]
- 88.Pjano MA, Pidro A, Biscevic A, Grisevic S, Pandzic B, Cerovic V. Refractive outcomes of posterior chamber phakic intraocular lens implantation for correction of myopia and myopic astigmatism. Med Arch. 2017;71:93–6. doi: 10.5455/medarh.2017.71.93-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kamiya K, Shimizu K, Igarashi A, Kitazawa Y, Kojima T, Nakamura T, et al. Posterior chamber phakic intraocular lens implantation in eyes with an anterior chamber depth of less than 3 mm: A multicenter study. Sci Rep. 2018;8:13322. doi: 10.1038/s41598-018-31782-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miao H, Chen X, Tian M, Chen Y, Wang X, Zhou X. Refractive outcomes and optical quality after implantation of posterior chamber phakic implantable collamer lens with a central hole (ICL V4c) BMC Ophthalmol. 2018;18:141. doi: 10.1186/s12886-018-0805-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Takahashi M, Kamiya K, Shoji N, Kato S, Igarashi A, Shimizu K. Intentional undercorrection by implantation of posterior chamber phakic intraocular lens with a central hole (Hole ICL) for early presbyopia. Biomed Res Int. 2018;2018:6158520. doi: 10.1155/2018/6158520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alfonso JF, Fernandez-Vega-Cueto L, Alfonso-Bartolozzi B, Montés-Micó R, Fernández-Vega L. Five-year follow-up of correction of myopia: Posterior chamber phakic intraocular lens with a central port design. J Refract Surg. 2019;35:169–76. doi: 10.3928/1081597X-20190118-01. [DOI] [PubMed] [Google Scholar]
- 93.Ghoreishi M, Abdi-Shahshahani M, Peyman A, Pourazizi M. A model for predicting sulcus-to-sulcus diameter in posterior chamber phakic intraocular lens candidates: Correlation between ocular biometric parameters. Int Ophthalmol. 2019;39:661–6. doi: 10.1007/s10792-018-0859-5. [DOI] [PubMed] [Google Scholar]
- 94.Jadidi K, Mosavi SA, Nejat F, Aghamolaei H, Daryabari SH, Torabi H, et al. Use of low-vault posterior chamber collagen copolymer phakic intraocular lenses for the correction of myopia: A 3-year follow-up. Graefes Arch Clin Exp Ophthalmol. 2019;257:1555–60. doi: 10.1007/s00417-019-04336-9. [DOI] [PubMed] [Google Scholar]
- 95.Qin Q, Wu Z, Bao L, Chen H, Yang L, He Z, et al. Evaluation of visual quality after EVO-ICL implantation for hypermyopia: An observational study. Medicine (Baltimore) 2019;98:e17677. doi: 10.1097/MD.0000000000017677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Igarashi A, Shimizu K, Kato S, Kamiya K. Predictability of the vault after posterior chamber phakic intraocular lens implantation using anterior segment optical coherence tomography. J Cataract Refract Surg. 2019;45:1099–104. doi: 10.1016/j.jcrs.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 97.Niu L, Miao H, Han T, Ding L, Wang X, Zhou X. Visual outcomes of Visian ICL implantation for high myopia in patients with shallow anterior chamber depth. BMC Ophthalmol. 2019;19:121. doi: 10.1186/s12886-019-1132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakamura T, Isogai N, Kojima T, Yoshida Y, Sugiyama Y. Posterior chamber phakic intraocular lens implantation for the correction of myopia and myopic astigmatism: A retrospective 10-year follow-up study. Am J Ophthalmol. 2019;206:1–10. doi: 10.1016/j.ajo.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 99.Yu Z, Li J, Song H. Short-time evaluation on intraocular scattering after implantable collamer lens implantation for correcting high myopia. BMC Ophthalmol. 2020;20:235. doi: 10.1186/s12886-020-01482-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moya T, Javaloy J, Montes-Mico R, Beltrán J, Muñoz G, Montalbán R. Implantable collamer lens for myopia: Assessment 12 years after implantation. J Refract Surg. 2015;31:548–56. doi: 10.3928/1081597X-20150727-05. [DOI] [PubMed] [Google Scholar]
- 101.Vasavada V, Srivastava S, Vasavada SA, Sudhalkar A, Vasavada AR, Vasavada VA. Safety and efficacy of a new phakic posterior chamber iol for correction of myopia: 3 years of follow-up. J Refract Surg. 2018;34:817–23. doi: 10.3928/1081597X-20181105-01. [DOI] [PubMed] [Google Scholar]
- 102.Sachdev G, Ramamurthy D. Long-term safety of posterior chamber implantable phakic contact lens for the correction of myopia. Clin Ophthalmol. 2019;13:137–42. doi: 10.2147/OPTH.S185304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yasa D, Urdem U, Agca A, Yildirim Y, Kepez Yildiz B, Kandemir Beşek N. Early results with a new posterior chamber phakic intraocular lens in patients with high myopia. J Ophthalmol. 2018;2018:1329874. doi: 10.1155/2018/1329874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yaşa D, Köse B, Aǧca A. Rotational stability of a new posterior chamber toric phakic intraocular lens. J Ophthalmol. 2020;2020:1624632. doi: 10.1155/2020/1624632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guber I, Mouvet V, Bergin C, Perritaz S, Othenin-Girard P, Majo F, et al. Clinical outcomes and cataract formation rates in eyes 10 years after posterior phakic lens implantation for myopia. JAMA Ophthalmol. 2016;134:487–94. doi: 10.1001/jamaophthalmol.2016.0078. [DOI] [PubMed] [Google Scholar]
- 106.Řeháková T, Veliká V, Rozsíval P, Jirásková N. Correction of myopia and myopic astigmatism by implantation of a phakic posterior chamber implantable collamer lens. Cesk Slov Oftalmol. 2019;74:147–52. doi: 10.31348/2018/1/4-4-2018. [DOI] [PubMed] [Google Scholar]
- 107.Choi JH, Lim DH, Nam SW, Yang CM, Chung ES, Chung TY. Ten-year clinical outcomes after implantation of a posterior chamber phakic intraocular lens for myopia. J Cataract Refract Surg. 2019;45:1555–61. doi: 10.1016/j.jcrs.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 108.Perez-Cambrodi RJ, Pinero DP, Ferrer-Blasco T, Cerviño A, Brautaset R. The posterior chamber phakic refractive lens (PRL): A review. Eye (Lond) 2013;27:14–21. doi: 10.1038/eye.2012.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Remington LA. St Louis, Missouri, United States of America: Elsevier Butterworth Heinemann; 2012. Clinical Anatomy and Physiology of the Visual System. [Google Scholar]
- 110.Weisenthal RW, Afshari NA, Bouchard CS, Colby KA, Rootman DS, Tu EY, et al. External Disease and Cornea. United States of America: American Academy of Ophthalmology; 2016. [Google Scholar]
- 111.Bourne WM, Nelson LR, Hodge DO. Central corneal endothelial cell changes over a ten-year period. Invest Ophthalmol Vis Sci. 1997;38:779–82. [PubMed] [Google Scholar]
- 112.MacRae S, Holladay JT, Hilmantel G, Calogero D, Masket S, Stark W, et al. Special report: American Academy of Ophthalmology Task Force recommendations for specular microscopy for phakic intraocular lenses. Ophthalmology. 2017;124:141–2. doi: 10.1016/j.ophtha.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 113.Ostovic M, Hofmann C, Klaproth OK, Kohnen T. Corneal decompensation and angle-closure glaucoma after upside-down implantation of an angle-supported anterior chamber phakic intraocular lens. J Cataract Refract Surg. 2013;39:806–9. doi: 10.1016/j.jcrs.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 114.Goukon H, Kamiya K, Shimizu K, Igarashi A. Comparison of corneal endothelial cell density and morphology after posterior chamber phakic intraocular lens implantation with and without a central hole. Br J Ophthalmol. 2017;101:1461–5. doi: 10.1136/bjophthalmol-2016-309363. [DOI] [PubMed] [Google Scholar]
- 115.Stodulka P, Slovak M, Sramka M, Polisensky J, Liska K. Posterior chamber phakic intraocular lens for the correction of presbyopia in highly myopic patients. J Cataract Refract Surg. 2020;46:40–4. doi: 10.1097/j.jcrs.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 116.Perez-Santonja JJ, Iradier MT, Benitez del Castillo JM, Serrano JM, Zato MA. Chronic subclinical inflammation in phakic eyes with intraocular lenses to correct myopia. J Cataract Refract Surg. 1996;22:183–7. doi: 10.1016/s0886-3350(96)80216-1. [DOI] [PubMed] [Google Scholar]
- 117.Packer M. The Implantable Collamer Lens with a central port: Review of the literature. Clin Ophthalmol. 2018;12:2427–38. doi: 10.2147/OPTH.S188785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McCannel CA, Atebara NH, Kim SJ, Leonard BC, Rosen RB, Sarraf D, et al. Retina and Vitreous. United States of America: American Academy of Ophthalmology; 2017. [Google Scholar]
- 119.Tuft SJ, Minassian D, Sullivan P. Risk factors for retinal detachment after cataract surgery: A case-control study. Ophthalmology. 2006;113:650–6. doi: 10.1016/j.ophtha.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 120.Fernandez-Vigo JI, Marcos AC, Agujetas R, Montanero JM, Sánchez-Guillén I, García-Feijóo J, et al. Computational simulation of aqueous humour dynamics in the presence of a posterior-chamber versus iris-fixed phakic intraocular lens. PLoS One. 2018;13:e0202128. doi: 10.1371/journal.pone.0202128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schmidinger G, Lackner B, Pieh S, Skorpik C. Long-term changes in posterior chamber phakic intraocular collamer lens vaulting in myopic patients. Ophthalmology. 2010;117:1506–11. doi: 10.1016/j.ophtha.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 122.Jick SL, Beardsley TL, Brasington CR, Buznego C, Grostern RJ, Park L, et al. Lens and Cataract. United States of America: American Academy of Ophthalmology; 2018. [Google Scholar]
- 123.Steinwender G, Varna-Tigka K, Shajari M, Kohnen T. Anterior subcapsular cataract caused by forceful irrigation during implantation of a posterior chamber phakic intraocular lens with a central hole. J Cataract Refract Surg. 2017;43:969–74. doi: 10.1016/j.jcrs.2017.03.045. [DOI] [PubMed] [Google Scholar]
- 124.Gonzalez-Lopez F, Mompean B, Bilbao-Calabuig R, Vila-Arteaga J, Beltran J, Baviera J. Dynamic assessment of light-induced vaulting changes of implantable collamer lens with central port by swept-source OCT: Pilot study. Transl Vis Sci Technol. 2018;7:4. doi: 10.1167/tvst.7.3.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee H, Kang DSY, Choi JY, Ha BJ, Kim EK, Seo KY, et al. Analysis of pre-operative factors affecting range of optimal vaulting after implantation of 12.6-mm V4c implantable collamer lens in myopic eyes. BMC Ophthalmol. 2018;18:163. doi: 10.1186/s12886-018-0835-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tahzib NG, Eggink FA, Frederik PM, Nuijts RM. Recurrent intraocular inflammation after implantation of the Artiflex phakic intraocular lens for the correction of high myopia. J Cataract Refract Surg. 2006;32:1388–91. doi: 10.1016/j.jcrs.2006.02.082. [DOI] [PubMed] [Google Scholar]
- 127.Alio JL, Hoz Fde L, Ismail MM. Subclinical inflammatory reaction induced by phakic anterior chamber lenses for the correction of high myopia. Ocul Immunol Inflamm. 1993;1:219–24. doi: 10.3109/09273949309085021. [DOI] [PubMed] [Google Scholar]
- 128.Bernard P, Fournier M. Definitive stop of marketing, product recall and follow-up of implanted patients Presbyopic intraocular lenses NEWLIFE/VIVARTE PRESBYOPIC Agence Française de Sécurité Sanitaire des Produits de Santé (AFSSAPS) 2007;2016 [Google Scholar]
- 129.McCarey BE, Edelhauser HF, Lynn MJ. Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices, and new intraocular drugs and solutions. Cornea. 2008;27:1–16. doi: 10.1097/ICO.0b013e31815892da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Patel SV, McLaren JW, Bachman LA, Bourne WM. Comparison of flex-center, center, and corner methods of corneal endothelial cell analysis. Cornea. 2010;29:1042–7. doi: 10.1097/ICO.0b013e3181cc7a60. [DOI] [PubMed] [Google Scholar]
- 131.Ieong A, Hau SC, Rubin GS, Allan BD. Quality of life in high myopia before and after implantable Collamer lens implantation. Ophthalmology. 2010;117:2295–300. doi: 10.1016/j.ophtha.2010.03.055. [DOI] [PubMed] [Google Scholar]
- 132.Kandel H, Khadka J, Lundstrom M, Goggin M, Pesudovs K. Questionnaires for measuring refractive surgery outcomes. J Refract Surg. 2017;33:416–24. doi: 10.3928/1081597X-20170310-01. [DOI] [PubMed] [Google Scholar]
- 133.Atchison DA, Markwell EL, Kasthurirangan S, Pope JM, Smith G, Swann PG. Age-related changes in optical and biometric characteristics of emmetropic eyes. J Vis. 2008;8:291–0. doi: 10.1167/8.4.29. [DOI] [PubMed] [Google Scholar]
- 134.Gudmundsdottir E, Arnarsson A, Jonasson F. Five-year refractive changes in an adult population: Reykjavik eye study. Ophthalmology. 2005;112:672–7. doi: 10.1016/j.ophtha.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 135.McBrien NA, Adams DW. A longitudinal investigation of adult-onset and adult-progression of myopia in an occupational group. Refractive and biometric findings. Invest Ophthalmol Vis Sci. 1997;38:321–33. [PubMed] [Google Scholar]
- 136.Reinstein DZ, Archer TJ, Randleman JB. JRS standard for reporting astigmatism outcomes of refractive surgery. J Refract Surg. 2014;30:654–9. doi: 10.3928/1081597X-20140903-01. [DOI] [PubMed] [Google Scholar]
- 137.Dupps WJ, Jr, Kohnen T, Mamalis N, Rosen ES, Koch DD, Obstbaum SA, et al. Standardized graphs and terms for refractive surgery results. J Cataract Refract Surg. 2011;37:1–3. doi: 10.1016/j.jcrs.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 138.Chylack LT, Jr, Leske MC, Sperduto R, Khu P, McCarthy D. Lens opacities classification system. Arch Ophthalmol. 1988;106:330–4. doi: 10.1001/archopht.1988.01060130356020. [DOI] [PubMed] [Google Scholar]
- 139.Chylack LT, Jr, Leske MC, McCarthy D, Khu P, Kashiwagi T, Sperduto R. Lens opacities classification system II (LOCS II) Arch Ophthalmol. 1989;107:991–7. doi: 10.1001/archopht.1989.01070020053028. [DOI] [PubMed] [Google Scholar]
- 140.Chylack LT, Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, et al. The lens opacities classification system III. The longitudinal study of cataract study group. Arch Ophthalmol. 1993;111:831–6. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 141.Hall AB, Thompson JR, Deane JS, Rosenthal AR. LOCS III versus the Oxford Clinical Cataract Classification and Grading System for the assessment of nuclear, cortical and posterior subcapsular cataract. Ophthalmic Epidemiol. 1997;4:179–94. doi: 10.3109/09286589709059192. [DOI] [PubMed] [Google Scholar]
- 142.Sparrow JM, Bron AJ, Brown NA, Ayliffe W, Hill AR. The oxford clinical cataract classification and grading system. Int Ophthalmol. 1986;9:207–25. doi: 10.1007/BF00137534. [DOI] [PubMed] [Google Scholar]
- 143.Chylack LT, Jr, Wolfe JK, Friend J, Khu PM, Singer DM, McCarthy D, et al. Quantitating cataract and nuclear brunescence, the Harvard and LOCS systems. Optom Vis Sci. 1993;70:886–95. doi: 10.1097/00006324-199311000-00005. [DOI] [PubMed] [Google Scholar]
- 144.Makhotkina NY, Berendschot T, van den Biggelaar F, Weik ARH, Nuijts RMMA. Comparability of subjective and objective measurements of nuclear density in cataract patients. Acta Ophthalmol. 2018;96:356–63. doi: 10.1111/aos.13694. [DOI] [PubMed] [Google Scholar]
- 145.Chen D, Li Z, Huang J, Yu L, Liu S, Zhao YE, et al. Lens nuclear opacity quantitation with long-range swept-source optical coherence tomography: Correlation to LOCS III and a Scheimpflug imaging-based grading system. Br J Ophthalmol. 2019;103:1048–53. doi: 10.1136/bjophthalmol-2018-312661. [DOI] [PubMed] [Google Scholar]


