Abstract
Phototherapeutic keratectomy (PTK) involves treating anterior corneal lesions by superficial corneal ablation using an excimer laser (193 nm). Some of the commonly treated conditions include recurrent corneal erosions (RCE), corneal dystrophies, spheroidal degeneration, keratoconus, and corneal scars. We discuss various techniques of PTK including large area PTK, focal PTK, and multifocal PTK and alternatives to PTK. Masking agents like hyaluronate, methylcellulose, and dextran are recommended to help achieve a better outcome when ablating irregular corneal surfaces. Antifibrotic agents like mitomycin C reduce the chances of recurrence of the disease, apart from minimizing the postoperative scarring. Some of the complications include induced hyperopia and irregular astigmatism, haze, recurrence, and corneal thinning. However, earlier postoperative recovery, possibility of a repeat procedure, and ability to control the depth of ablation make PTK a promising, minimally invasive alternative to keratoplasty in cases with anterior corneal pathologies.
Keywords: Corneal dystrophy, corneal scar, excimer laser, phototherapeutic keratectomy, Phototherapeutic keratectomy
The dominant clinical interest in technology and outcomes of excimer laser surgery has primarily been for the precision and accuracy of the superficial corneal photoablation, leaving the underlying stroma clear and undisturbed. It was introduced by Munnerlyn et al. for corneal reshaping and correcting refractive errors, with the procedure being termed as photorefractive keratectomy (PRK).[1] The use of the laser was further explored in the treatment of corneal pathologies, which was termed as phototherapeutic keratectomy (PTK). This procedure received United States Food and Drug Administration (FDA) approval in 1995 when both Visx Inc. (Santa Clara, CA) and Summit Technology, Inc. (Waltham, MA) excimer lasers were approved for the treatment of corneal pathologies in the anterior one-third of the cornea. The FDA guidelines required at least 250 microns of residual bed thickness after the PTK.[2] It has been suggested that the excimer laser PTK is best suited for the corneal pathologies in the anterior 10-20% of the stroma.[3] As such, it has been successfully used in the treatment of recurrent corneal erosions (RCE),[4,5] corneal dystrophies,[6] superficial corneal opacities and corneal degenerations.[7,8]
PTK offers a less invasive and extraocular alternative to avoid or delay the need for lamellar or penetrating keratoplasties.[7,9] Earlier recovery and possibility of repeat procedures are some of the other advantages. However, recurrence of the disease, hyperopic shift, post-operative discomfort, and corneal haze are some of the known complications.[9] In this review, we discuss the indications, techniques, adjunctive treatments, and management of complications of PTK.
Principle
The term excimer is derived from “excited dimer” which are molecules bound at high energy state. These highly unstable molecules emit high energy ultraviolet (UV) radiation when they decay to the ground state. The high energy UV light thus released can be used to etch certain materials with precision. Srinivasan and Leigh described the process of ablative photodecomposition where irradiated polymers break into smaller molecules released into the surrounding atmosphere.[10]
Typically, lasers with longer wavelengths, when used to vaporize tissues, are known to cause adjacent tissue damage. However, far-ultraviolet lasers with shorter wavelengths (200-150 nm) cause sharply defined photoablation with minimal to no thermal damage to the adjacent tissue. Argon-fluoride (ArF) is a dimer that emits far UV light (193 nm) and can be used for controlled ablation of the cornea preserving the adjacent tissue.[11] Each pulse of the excimer laser is known to ablate about 0.25 μ of corneal tissue. This extreme precision is the basis for the use of excimer laser as a surgical tool.
Pre-Operative Considerations
Preoperative decision making includes determining whether the candidate is likely to benefit from PTK in the first place, and if so, careful and meticulous planning of the procedure needs to be done. The efficacy of PTK varies with patients' symptoms, nature-depth-pattern of the corneal lesions, location of the corneal lesions, planning of the procedure, and refractive status of the eye.
History taking is crucial to understand the visual disturbance that the patient is experiencing. It must be ascertained whether the symptoms can be attributed to corneal pathology and whether PTK might help alleviate those symptoms. Previous ocular history must be taken into account as well. Recurrence of latent herpetic infection has been reported after excimer laser treatments.[12,13] Corneal neurotrophicity and post-operative healing problems must be anticipated in patients with tumors of the central nervous system, cranial nerve palsies, or herpetic keratitis, due to corneal neuropathy. Severe dry eye, ocular surface disease, and eyelid malfunction can also delay epithelial healing.[3,9] Systemic diseases including diabetes mellitus and collagen vascular diseases are considered relative contraindications to PTK.[9,14]
A detailed slit-lamp examination is of utmost importance in determining the size and the extent of the corneal lesion and its distance from the visual axis. For example, an elevated lesion in the periphery, away from the visual axis, causing local tear breakdown, might benefit with a focal PTK smoothening procedure. On the other hand, similar lesions in the central cornea affecting the optical zone might need a large area PTK with the use of masking agents. Particular attention is to be paid to the level of the lesion and its depth. PTK is most effective in lesions in the anterior 10–20% of the cornea.
The refractive status of the eye also must be taken into account, especially in unilateral cases. PTK is known to cause a post-operative hyperopic shift and could lead to anisometropia.[15] In cases of corneal opacity and irregularity, a rigid gas permeable (RGP) lens could help determine the effect of the opacity versus corneal irregularity on the visual acuity.[3] Finally, the status of the limbal stem cells is important for post-operative epithelial healing.
Once it is established that the patient will likely benefit from PTK, certain pre-operative investigations might help in planning the procedure.
a. Pachymetry
The depth of the corneal lesion and the expected residual corneal thickness can be judged using optical or ultrasound pachymetry.[16] It is recommended that only superficial opacities that are within the anterior 100 μ of the cornea, be treated. Patients having deeper lesions are not ideal candidates for PTK.[14] In cases where the cornea is thin, minimal ablation is done to avoid the worsening of irregular astigmatism.[17] Although the FDA guidelines require a residual stromal thickness of 250 μ after PTK,[2] authors have suggested aiming for a residual corneal thickness of 350-400 μ to avoid the risk of ectasia.[15]
b. Corneal Topography
Topography helps in diagnosing corneal ectasias and also assessing the status of the corneal surface. Obvious irregularities are noted on topography alone while subtle Irregularities might require epithelial mapping. A decision can then be made regarding the removal of epithelium during PTK. In post-refractive surgery cases, decentred ablation zones and central islands can be demarcated using topography.[18] Use of intra-operative topography after epithelial removal to customize the PTK has also been described.[19]
c. Optical Coherence Tomography
Anterior segment optical coherence tomography (AS-OCT) allows accurate localization of the lesions and assessment of the lesion depth for planning the size and depth of ablation. AS-OCT-based PTK simulation algorithms have been described to predict the postoperative refractive outcomes.[20,21] AS-OCT has also been used in cases of recurrent corneal erosions (RCE) to measure central corneal thickness and epithelial thickness before and after PTK and assess the wound healing response.[22] Jung et al. have described the use of AS-OCT in predicting the depth of PTK required in the planning for effective removal of diffuse haze in patients with granular corneal dystrophy (GCD) type 2.[23]
Surgical Techniques
a. Standard guidelines
Typically, PTK is performed under topical anesthesia. Planning of the spot size and ablation depth is done appropriately before the start of the procedure. In general, each pulse of a broad beam excimer laser ablates 0.25 μ of the normal corneal tissue, and 50 pulses of the excimer laser induce about 1D of hyperopic shift.[15] Ayres and Rapuano[3] also suggested using the Munnerlyn formula to estimate the refractive error induced after PTK:
Dioptre change = 3 (ablation depth in microns/ablation diameter in mm)2
This formula implies more refractive error change when ablation depth is deeper.[24] Also, the change in refractive error is inversely proportional to the diameter of optic zone (OZ). For example, in cases of diffuse corneal opacities or corneal stromal dystrophies, a larger OZ (5-6.5 mm) is chosen, whereas, in cases where focal smoothening of a Salzmann's lesion or keratoconus nodule is attempted, a smaller OZ, enough to cover the elevated lesion is programmed.[17]
Many laser platforms have a PTK mode, but they each have different features. On the Visx laser (Johnson and Johnson Vision, Santa Ana, CA), the circular ablation spot can be changed from 2.0-6.5 mm diameter. In the slit mode, a rectangular ablation spot can be programmed from 0.6 to 6.5 mm in length and width, at any axis. When an ablation spot less than 2.0 mm is desired, the slit mode is used and programmed to a small square spot. The default repetition rate is 10 Hz, but many surgeons use 5-6 Hz as that makes it easier to observe the effect of the laser treatment intraoperatively. More recent excimer platforms such as the WaveLight EX 500 (Alcon, Fort Worth, TX) have faster repetition rates and may result in a myopic shift in refraction due to increased compensatory peripheral ablation.
Once the procedure is planned, epithelium, if irregular, is debrided using either a blade or 20% alcohol. This is followed by using a masking agent (discussed later) to fill the valleys and isolate the elevated areas for smoother ablation. In cases where the epithelium is smooth but the underlying stroma might be irregular, a transepithelial PTK can be performed in which case the epithelium itself acts as a natural masking agent.[14] Use of masking agent additionally can help limit the post-operative induced hyperopia.[24]
b. Use of Masking agents
Excimer laser PTK is used to smoothen the irregular corneal surface due to its ability to ablate corneal tissue with submicron precision. However, photoablation of an irregular surface leads to repetition of the same irregular contour with each pulse resulting eventually in reproducing the same shape in the deeper layers [Fig. 1]. This leads to unsatisfactory smoothening of the cornea. A masking agent or a modulator is a fluid that is used to fill the valleys and cover the deeper tissues while the protruding irregular peaks are exposed and ablated preferentially. The ideal characteristics of a masking agent include that it should be fluid, biocompatible, have ablation characteristics similar to that of cornea, and be able to mold itself to the desired shape and adhere to anterior surface of cornea.[25] Kornmehl et al. compared the different masking agents highlighting the importance of viscosity of these fluids. A fluid that is excessively viscous, might not level off as desired and cover both peaks and valleys resulting in inadequate smoothening. On the other hand, a fluid with very low viscosity, might run off not masking the irregular areas and may necessitate multiple applications. Therefore, fluids with moderate viscosity (between saline and 1% carboxymethylcellulose) are ideal candidates.[26] Sodium hyaluronate,[27] methylcellulose[28] and 0.1% dextran[26] are some of the masking agents used.
Figure 1.
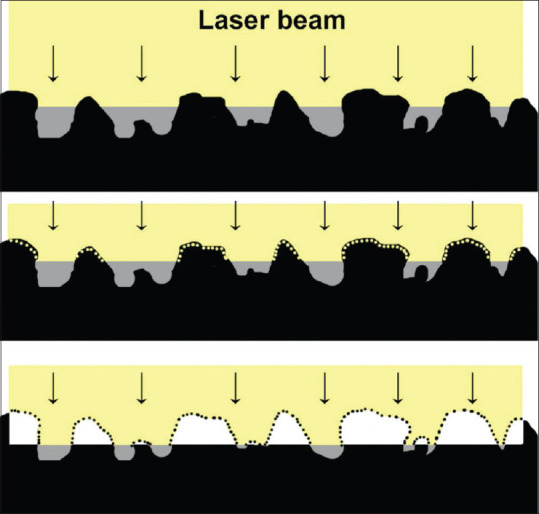
Schematic to show the use of masking agent to ablate irregular corneal surface. The masking fluid fills the valleys and cover the deeper tissues while the protruding irregular peaks are exposed and ablated preferentially
Collagen gels have also been used as modulators owing to their ablation properties being similar to the cornea.[25,29] The photo-ablatable lenticular modulator (PALM) technique was described by Katsanevaki et al., where collagen gel is applied on the irregular corneal surface, in a liquid form, at a temperature of 49°C so that it can be moulded into a lenticule using a RGP lens. They found this collagen mold to be effective as a masking agent whilst having no adverse effect on epithelial healing.[29] Bio mask is another collagen-derived masking agent which has been shown to be effective in achieving good topographic results.[30] Other than achieving an excellent smoothening of the corneal surface, the use of masking agents also helps limit the post-PTK hyperopic shift.[24]
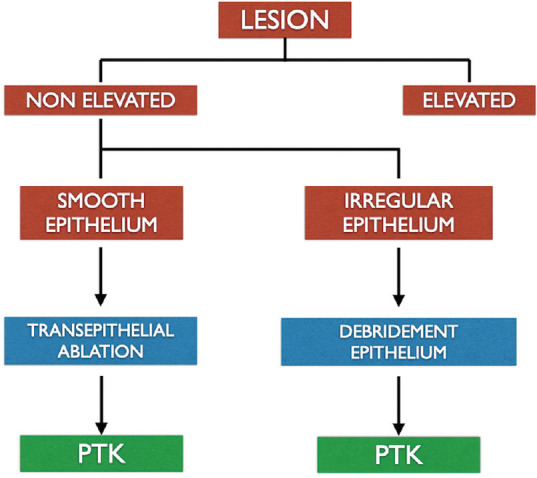
c. Transepithelial PTK
When the epithelium is smooth with an underlying irregular stromal surface, it can be left in place to act as a natural masking agent [Fig. 2]. Additional masking agent may or may not be required. When the epithelium is ablated in PTK, it appears as a blue fluorescence which disappears when the stroma is reached.[31] The masking fluids like methylcellulose might lead to formation of bubbles when exposed to excimer laser.[26] Transepithelial PTK has been described in the management of flap complications after laser in-situ keratomileusis (LASIK),[32,33] persistent epithelial defects,[34] recurrent corneal erosions,[35] corneal ectasias[36,37] and anterior stromal scarring.[38] A comparison between mechanical epithelial debridement and transepithelial excimer laser ablation revealed no significant difference in the epithelial healing post-operatively.[39]
Figure 2.
Technique of large area PTK. Large spot size used to ablate the central cornea. Peripheral small spots used to create a transition zone
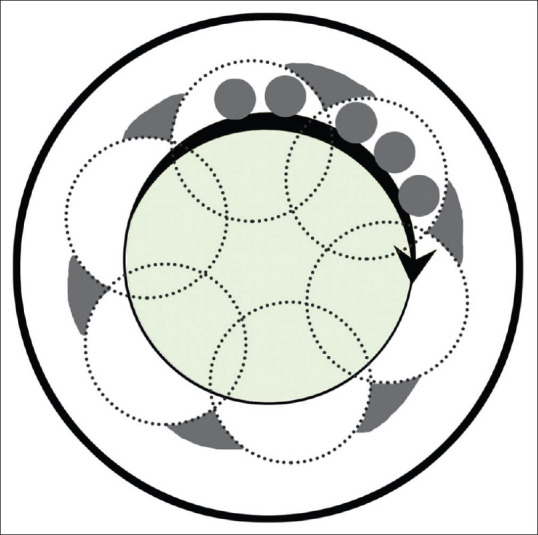
d. Large area PTK
In this technique, specific to certain lasers such as the Visx laser, a large spot size (5-6.5 mm) is chosen. A large ablation zone helps in minimizing the post-operative glare and refractive effect over the optical zone.[40] It is used for diffuse corneal opacities, scars, dystrophies, superficial keratopathies and irregularities. Once the masking agent is applied to the irregular corneal surface, the patient is asked to gaze at the fixation light and laser treatment is started. The patient's head can be rotated such that the laser beam is applied in an annular fashion. This method allows blending of the ablation zones and avoids creation of sharp edges causing topographic distortion.[41] Applying more treatment to steeper areas helps reduce irregular astigmatism.[42] Stark et al. advocated the use of additional laser of spot size 2.0 mm applied at the circumference around the ablation zone to reduce the induced hyperopia.[43] [Fig. 3].
Figure 3.
Technique of focal smoothening PTK. A spot size of 1-2 mm diameter is used and laser is fired on the nodule. The top of the elevated lesion is ablated more as compared to the surrounding area
Alternatively, a large (7.0-7.5 mm) optic zone diameter is chosen and centered over the pupil to ablate the epithelium and stroma.[2,44] The transition zone must be taken into account as in most cases, the laser spots could ablate tissue in additional 2 mm of the originally planned ablation diameter. Care must be taken to avoid damaging the limbus.[44] Additionally, the corneal periphery should not be thinned excessively, as that makes subsequent keratoplasty less successful. Only some lasers have such large ablation zones for PTK.
Rapuano described the “shoot and check” technique in which the cornea is examined at the slit-lamp after initial laser ablation and if more treatment is needed, the patient is repositioned under the laser and additional PTK is performed until satisfactory results are obtained.[2]
e. Focal smoothening
This technique is used to treat focal, elevated lesions with a small-diameter ablation zone (up to 2 mm).[44] It is used to shave off proud areas such as Salzmann's lesions or keratoconus nodules in cases where the opacities aren't able to be easily removed with a lamellar keratectomy.[45,46] The amount of elevation is measured using pachymeter, AS-OCT or estimated at the slit-lamp. The number of pulses are approximately calculated as Number of pulses = elevation in microns × 4 (since each pulse removes 0.25 μ of corneal tissue).[17] Epithelium is debrided over the elevated area, leaving behind the epithelium in surrounding normal tissue to act as a natural masking agent. If desired, additional masking agents can also be used to avoid corneal thinning. Using a spot size of 0.6-2.0 mm diameter, depending on the lesion, the laser is fired on the nodular area [Fig. 4]. The top of the elevated lesion is ablated more as compared to the surrounding area so that it is reduced down to the level of the surrounding cornea. Slit-lamp evaluation is done and laser treatment is repeated until the lesion is leveled off. Care must be taken to avoid over-treatment and creating a crater in the cornea.[2,41] However, when it comes to choosing between having an inadequately shaved lesion above the corneal surface versus having a slightly overtreated lesion, the latter is preferable as the corneal indentation can be filled with epithelium.[14] Once the lesion is focally treated, a large diameter ablation zone can be used to create the smoothest possible surface taking care not to overtreat the cornea.[2] A customized technique of PTK has been described for focal smoothening of Salzmann's nodular degeneration, in which the depth of the lesion is measured using OCT, and the horizontal and vertical dimensions of the lesion are extrapolated from Scheimpflug imaging using a Java-based image processing program. Once all the measurements are obtained, the area to be ablated is customized and treated transepithelially using a single pass.[47]
Figure 4.
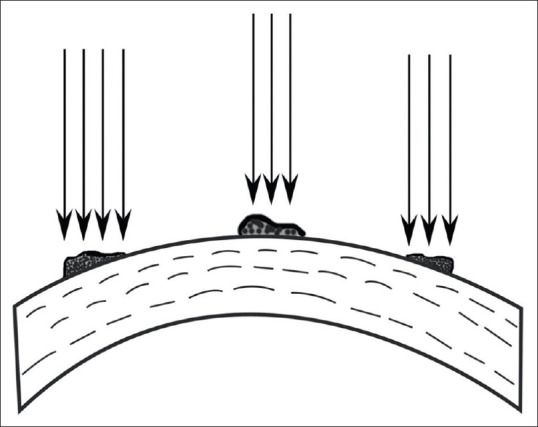
Flowchart showing algorithm for choosing between PTK following epithelial removal versus transepithelial PTK
f. Multifocal PTK
In cases of persistent epithelial defects (PED), the thickened edges are known to prevent normal epithelial healing. Kim et al. used excimer laser to smoothen out the elevated, rough edges of PED thereby allowing epithelial cell migration and promotion of healing.[48] They performed focal ablation treatments of small diameter (1 mm) in the area around the epithelial defect until a smooth corneal surface was obtained. They restricted the ablation depth to 45 μ to prevent damage to the Bowman's membrane. Some of the advantages of this technique were less risk of perforation, less refractive change and rapid re-epithelialization. In their study, 13 out of 15 eyes were completely epithelialized within one week.
g. Use of Mitomycin C
Laser-induced trauma in the area of Bowman's membrane and stroma can lead to uncontrolled proliferation of stromal keratocytes and laying down of collagen, hyaluronic acid and proteoglycans. This process of subepithelial fibrosis is clinically detectable as haze or scarring and can induce irregular astigmatism.[49] Mitomycin C (MMC) is an antimetabolite that has been used to prevent and to treat subepithelial fibrosis.[50,51] Use of 0.02% MMC for up to 2 minutes has been advocated as an adjunct to PTK to reduce haze and scarring. When being used to treat corneal surface irregularity and scarring following previous refractive surgery, the spherical error is reduced by ½ to 2/3rd considering the effect of MMC on wound healing. In cases where there is no scarring and the corneal surface is fairly regular, then the spherical error is reduced by 15-20%.[32]
In addition, MMC has the potential to prevent recurrences in anterior corneal pathologies.[52,53,54] PTK along with MMC is also useful in treating epithelial ingrowth after LASIK.[55]
Indications
a. Recurrent corneal erosions
RCE is a commonly seen condition that develops following trauma or epithelial basement membrane dystrophies (EBMD), or can be idiopathic.[56] The underlying pathology is a weakening of the adhesions between the epithelial cells and basement membrane. In case of a traumatic RCE, there is a split between the basement membrane and the Bowman's membrane which leads to poor cell adhesion. The hemidesmosomes are not impaired.[57] Whereas, in RCE related to EBMD, the hemidesmosomes seem to be affected resulting in redundant layers of basement membrane with debris of desquamating cells underneath, which affect the epithelial adhesions.[58] The initial line of management in a case of RCE includes lubricants and bandage contact lens. In refractory cases, epithelial debridement, anterior stromal puncture, diamond burr polishing, alcohol delamination and PTK have been tried.[59]
The loose and unstable corneal epithelium is removed by debridement under topical anesthesia.[6] The underlying abnormal basement membrane and debris are stripped carefully until a smooth Bowman's membrane is reached. Following this, a large area PTK is done with ablation depth of 5-7 μ.[3,60] Since the depth of stromal ablation is minimal, use of MMC is not required.[15] Excessive depth of ablation is known to cause post-operative haze, increased hyperopic shift and irregular astigmatism.[4,61]
The removal of abnormal epithelium leads to formation of new basement membrane and regeneration of basal epithelial cells. In addition, the ablation of Bowman's membrane facilitates direct contact between the epithelial cells and stromal keratocytes and stimulates the formation of new hemidesmosomes and anchoring fibrils.[60] This process takes around 2 weeks to complete and overall improves the epithelial adhesion and reduces the chances of erosions.[61] It has been shown by studies that PTK works better in cases of traumatic RCE as compared to that of non-traumatic etiology.[5,62] Transepithelial PTK has also been tried in case of RCE with similar rates of recurrence as compared to conventional PTK, the advantage being removal of only superficial epithelial cells and lesser induction of hyperopia and induced astigmatism.[63,64]
b. Corneal dystrophies
Apart from EBMD causing recurrent erosions, PTK has been used in Bowman's membrane dystrophies such as Thiel–Behnke dystrophy[65] and Reis-Bücklers dystrophy.[66]
PTK is also effective in clearing the deposits in stromal dystrophies. It has been found to be a less invasive treatment option in naïve corneas as well as in grafts with recurrent dystrophy.[67]
In lattice dystrophy, the amyloid deposits tend to be more in the anterior cornea and hence, can be treated by superficial ablation.3 Studies have shown visual improvement and reduced rate of RCE in these patients following PTK.[68,69] Granular dystrophy is characterized by bread-crumb like deposits in corneal stroma involving the central and paracentral cornea. The deposits are often more confluent in the superficial stroma and ablative treatment of these lesions is sufficient to improve vision in these patients [Fig. 5].[3] A diffuse limbus to limbus haze is seen in macular corneal dystrophy. Hafner et al. found moderate improvement in the best-corrected visual acuity (BCVA) of patients with macular dystrophy undergoing PTK [Fig. 6]. However, more than 50% of the patients eventually required penetrating keratoplasty due to a high rate of recurrence (90%).[70] One of the most important complications of PTK in corneal dystrophies is the recurrence of disease (discussed later).
Figure 5.
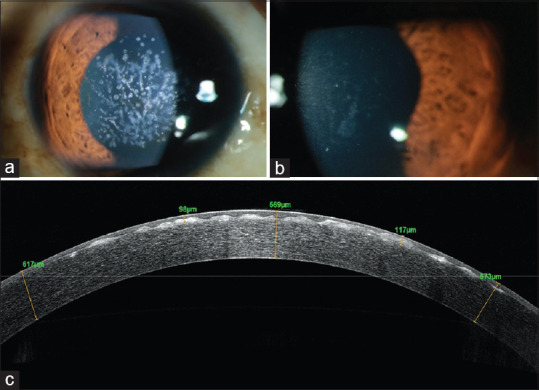
A 25-year-old female presented with decrease in vision and recurrent pricking sensation in the right eye, (a) Diffuse slit-lamp photograph of the right eye showing opacities in the anterior corneal surface suggestive of Granular Corneal Dystrophy with minimal amount of diffuse corneal haze, (a) Diffuse slit-lamp photograph of the right eye showing decrease in density of opacities on the anterior corneal surface after PTK. (c) anterior segment optical coherence tomography (OCT) showing anterior stromal scarring, epithelial irregularity and elevated anterior surface with deposits at the level of the Bowmans layer
Figure 6.
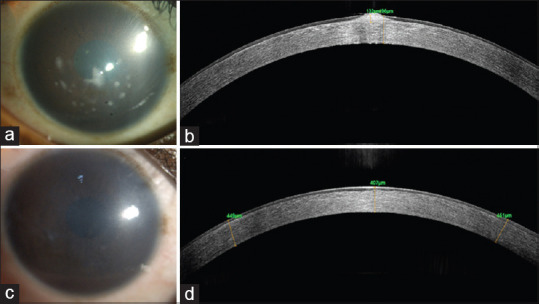
A 18-year-old male presented with photophobia and decrease in vision. (a) Diffuse slit-lamp photograph right cornea showing a diffuse corneal haze with elevated nodular deposits in the central cornea. (b) Corresponding AS-OCT of the right eye cornea showing focally elevated lesions. (c) Diffuse slit-lamp photograph of the right cornea after PTK demonstrating removal of the nodular deposits. (d) Corresponding post-PTK AS-OCT of the same area showing a slight depression that has been smoothened by epithelium
c. Corneal degenerations
(i) Climatic Droplet Keratopathy
Climatic droplet keratopathy (CDK) also known as spheroidal degeneration, is characterized by superficial subepithelial opacities and formation of golden-yellow spherules in superficial cornea in the advanced stages. It can be “smooth” or “irregular” type of CDK. PTK has been used successfully in treating CDK. A study of 75 eyes of CDK treated using PTK, found that smooth CDK was more likely to have clearer cornea than irregular CDK. Irregular CDK was also found to be associated with delayed epithelial healing.[71]
In cases with coexistent cataract, it is advisable to perform PTK before planning the cataract surgery. PTK helps regularise the cornea thus providing appropriate biometry and also improving visibility during cataract surgery.[72]
(ii) Salzmann's nodular degeneration
Salzmann's nodules cause decreased vision by inducing irregular astigmatism and encroaching directly into the visual axis. PTK has been established as an effective treatment modality to shave the nodules and regularise the cornea.[18,73] A manual keratectomy can be done to debulk the nodular lesions and then PTK along with masking agents used to smoothen the underlying irregular surface. Alternatively, the epithelium over the nodule is removed and small spot sizes used for focal smoothening followed by a larger spot size to obtain a regular surface.[3] Use of MMC may improve the quality of vision and reduce the recurrence rate.[74,75]
(iii) Band shaped keratopathy
Band shaped keratopathy (BSK) is a corneal degenerative disorder where calcific deposits cover the interpalpebral area of the cornea forming a gray-white band that is denser in the center and thins out in the periphery. When the deposits are at the level of Bowman's membrane with a smooth epithelium and little involvement of underlying stroma, it is termed as smooth BSK. On the other hand, rough BSK is a more severe form where thick calcific plaques make the epithelium irregular and deposits involve the underlying anterior stroma.[76] Superficial excimer laser ablation removes the calcific deposits and yields a clearer cornea with significant improvement in vision and symptoms.[77] In smooth BSK, the epithelium can be left intact to act as a masking agent. While in rough BSK, it is advisable to debride the epithelium and remove the thick calcific plaques manually prior to using masking agents and carrying out the photoablation. Ablation of the calcific deposits appears to fluoresce brighter than the stromal tissue and this acts as an indicator for the surgeon to stop ablating. O'Brart et al. reported success with the use of PTK in both forms of BSK with up to 95% patients showing symptomatic improvement.[76] Im et al. combined PTK with ethylenediaminetetraacetic acid chelation and amniotic membrane transplantation. Around 27% patients had visual improvement and all patients had symptomatic improvement. In 11 months of follow up, none of their patients had recurrences.[78]
d. Corneal scars
Similar to other anterior corneal pathologies, PTK is useful in removing visually disturbing scars in the superficial cornea (<100 μ). Transepithelial PTK with minimal ablation is preferred to avoid perturbation of the postoperative corneal topography and avoid significant corneal thinning.[17] The approach is to debulk the scar rather than removing it completely. Residual irregular astigmatism can be taken care of by RGP contact lenses post-operatively. A study by Dogru et al. showed that PTK could treat scars up to mid-stromal level and visual gain resulted not only from corneal regularisation and debulking the scar, but also from improved tear film stability.[79]
The etiology of the scar is also important. Scars caused by chemical burns often require removal of fibrovascular pannus by manual dissection. It has been suggested that in the presence of accompanying limbal stem cell deficiency, PTK could have advantages over keratoplasty by being a tissue-sparing procedure and inducing minimal surgical trauma.[80] Scars secondary to herpetic keratitis can be treated with PTK when there is no active inflammation.[80] After the procedure, patients must be closely monitored for a possible reactivation of the herpetic infection; a short course of prophylactic oral antivirals is often used after PTK in these eyes.[12,13]
e. Bullous keratopathy
Bullous keratopathy involves corneal endothelial decompensation causing stromal hydration and subsequent epithelial edema. The swollen epithelium loses the adhesions with the underlying basement membrane. Epithelial edematous cysts can combine together and form bullae which can later rupture and cause pain. PTK has been found to be effective in symptomatic bullous keratopathy. The excimer laser ablation improves epithelial cell adhesions to the basement membrane and reduces formation of bullae.[81] It is also hypothesized that the reduced corneal thickness helps reduce the osmotic load and the endothelium can better maintain the dehydrated state of stroma. After epithelial debridement, a large area PTK with 7-8 mm spot size with around 1 mm of blend zone is advisable.[82] Maini et al. studied the effect of depth of ablation on symptom alleviation in bullous keratopathy. They observed that deeper ablations (25% of the corneal thickness) have better results as compared to superficial ablations (25 μ). Deeper photoablation possibly destroys the subepithelial nerve plexus more effectively as compared to the superficial PTK. This induced neurotrophicity reduced the symptoms in patients with persistent epithelial defects. Though there is a theoretical risk of infective keratitis, the authors did not report any such case in their study.[82] Apart from pain relief, visual gain with a more stable tear film has also been reported in these patients.[83]
f. Keratitis
PTK has been effectively tried in infective as well as non-infective keratitis. Excimer laser ablation sterilizes the infected corneal surface by ablation of the tissue and killing the microbes, not by affecting the viability of the organisms.[84] PTK not only helps by eradicating the microbes, but also regularises the cornea and improves vision. In general, it is more effective in superficial infections refractory to conventional medical therapy. In case of deeper infections, PTK alone is not effective. However, it helps by reducing the microbial load in the tissue, improving drug penetration potentially improving the recovery time.[85,86] The risk of perforation must be kept in mind when performing ablative treatments for deeper infections.[84]
Studies have demonstrated the benefits of PTK in treating keratomycosis that is refractory to medical management. Li et al. reported that up to 83% of patients in their cohort improved after PTK.[86] A study by Lin et al. concluded that patients having fungal keratitis undergoing PTK had a faster recovery time than those who were on antifungals alone.[85] Both the studies reported rapid re-epithelialization and visual gain after PTK. However, the benefits of this procedure must be balanced with the risks of potential aerosol spread of infective material in the laser room.
Apart from fungal keratitis, excimer laser ablation has been successfully used in treating infections caused by Staphylococcus,[87] Mycobacteria,[88] Pseudomonas[89] and early Acanthamoeba keratitis.[90] Apart from infective ulcers, PTK is useful in shield ulcers[91] and post-burn ulcers.[92]
g. Keratoconus
Conventional crosslinking (CXL) to halt progression in keratoconus required epithelial debridement for adequate riboflavin penetration into the stroma. Transepithelial PTK can replace the manual epithelial debridement before CXL is done.[93] It is known that the epithelium is significantly thinner at the apex of the cone and is surrounded by an annular ring of thickened epithelium.[94] Transepithelial PTK removes the epithelium and part of anterior stroma at the apex of the cone in these cases. By using the epithelium as a masking agent, it helps regularise the corneal surface. The Cretan protocol involves transepithelial ablation in a diameter of 6.5-7 mm zone and depth of 50 μ, followed by enlarging the de-epithelialized zone by mechanical debridement to 9 mm. CXL is then carried out.[95] A study of 23 patients treated using this protocol showed a significant improvement in corneal astigmatism and visual acuity (uncorrected and best-corrected).[36] Although there is a risk of corneal thinning and progression of ectasia, studies have not reported complications post-operatively.[95] Grentzelos et al. described Cretan protocol plus where transepithelial PTK and conventional PRK was followed by CXL. They reported significant flattening of keratometry and improvement of visual acuity at one year follow-up.[96]
PTK is also used in management of keratoconus nodules developed in patients with chronic contact lens use. Similar to Salzmann's nodules, the keratoconus nodules can be manually debulked prior to using PTK to achieve corneal surface regularisation [Fig. 7].[3]
Figure 7.
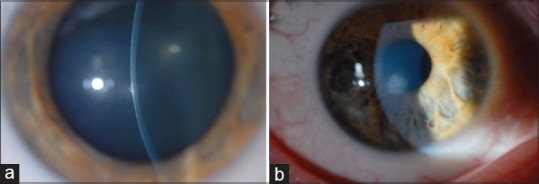
A 28-year-old male patient diagnosed with keratoconus and using contact lenses for 2 years presented to the clinic with intolerance to CL and decrease in vision. (a) Slit-lamp photograph of the right eye of the patient showing central elevated keratoconus nodule in the cornea, (b) Diffuse slit-lamp photograph of the right eye of the patient after PTK showing smooth anterior surface and decrease in density of the corneal scarring
h. Refractive surgery complications
A number of complications of refractive surgeries not amenable to medical treatment are successfully managed by PTK. Visually significant subepithelial fibrosis may develop after several years following radial keratotomy (RK) affecting the visual acuity. This could be treated by superficial debridement and followed by PTK [Fig. 8].[97,98]
Figure 8.
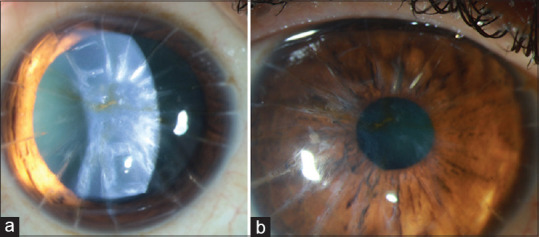
A 53-year-old patient with history of radial keratotomy (RK) in 1999 presented with decrease in vision for 7 years in the left eye. (a) Diffuse slit-lamp photograph of the left eye showing central anterior stromal scarring in the visual axis and RK incisions. (b) Diffuse slit-lamp photograph of the left eye showing minimal anterior stromal scarring in the visual axis after PTK
PRK is known to be associated with corneal haze. Sometimes, post-PRK haze fails to resolve spontaneously and may be addressed by PTK with MMC [Fig. 9].[15] PTK has also been tried in the treatment of flap folds. Following LASIK, a malposition in the flap may cause folds to form which, if left untreated for a long time, have a tendency to recur. PTK helps by reducing the thickness of the Bowman's membrane and reducing its memory, thereby preventing recurrence of these folds.[99] Other flap complications where PTK is useful include buttonholing of flap or irregular flap. An early treatment with transepithelial PTK with MMC deep enough to ablate the interface completely is advocated to prevent scarring and epithelial ingrowth.[15]
Figure 9.
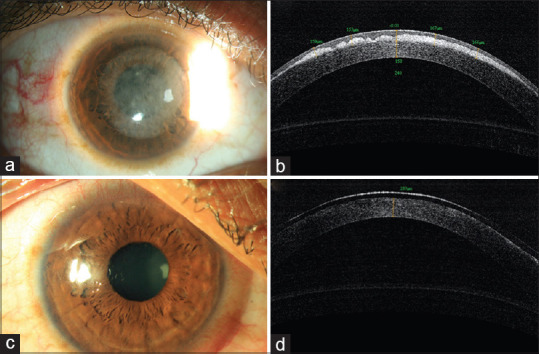
A 25 year old male was seen 5 months following PRK in both eyes with complaints of glare and drop in vision. (a) Diffuse slit-lamp photography in sclerotic scatter demonstrating diffuse cornea haze in the central 6.5 mm are of the cornea. (b) Corresponding AS-OCT of the same eye shows dense haze restricted to the sub epithelial zone and a saw toothed appearance, the anterior surface of which has been smoothened by epithelium. (c) Diffuse slit-lamp photograph 1 day following PTK for removal of the haze showing a clear cornea. (d) Corresponding AS-OCT of the cornea 1-day post PTK with clear anterior stroma. A bandage contact lens is also seen
Central islands and decentred ablation zones can be corrected using PTK to achieve a smooth surface. Correction of the contour in case of decentred ablation might need topography-guided PTK to prevent worsening.[15,100] Photoablation in the management of epithelial ingrowth[54] and diffuse lamellar keratitis (DLK)[101] has also been described.
Post-operative Complications
Recurrence of disease
As mentioned earlier, certain conditions can recur after PTK. The Bowman's layer and stromal dystrophies almost always recur, although some more quickly than others, even after a keratoplasty. Thiel–Behnke and Reis–Bücklers corneal dystrophies recur the fastest, within a few years. Granular and lattice corneal dystrophies tend to recur over 3–6 years, whereas macular and Schnyder corneal dystrophies often take decades to recur. Fortunately, when these dystrophies recur, the opacities are often in the anterior stroma, making them quite amenable to repeat treatment with PTK.[6] Recurrent erosions can also recur after PTK, and repeat PTK may be successful. When Salzmann's nodules recur after PTK, they too can be managed with repeat PTK, but the results tend not to be as good as initial treatments. MMC should be considered in eyes with Salzmann's nodular degeneration, especially if recurrent.[51]
Refractive error induced by PTK
Induced hyperopia due to central corneal flattening is the most commonly seen refractive error after PTK.[43] Ablation of peripheral corneal lesions could induce irregular astigmatism or even myopia.[3] Munnerlyn's formula[1] has been used by authors to estimate the amount of refractive error induced.[6] Use of masking agents, transition zone and using large spot size for treatment are some of the measures used to reduce the induced refractive error.[6]
Corneal haze and opacities
Deeper stromal ablations during PTK may lead to post-operative scarring and haze. The haze caused by photoablation is reticular in appearance and restricted to the ablation zone. This must be differentiated from residual scar that is more deep seated and may extend beyond the area treated by PTK.[9] When deep ablations are necessary, MMC 0.02% (for 30-120 seconds) should be considered to reduce the chance of visually significant corneal haze.
Keratectasia
Post-PTK keratectasia is not unknown. Vinciguerra et al. reported iatrogenic keratectasia when PTK was done in thin corneas such as in post-refractive surgery cases. They suggested a wide ablation diameter to avoid focal stress and subsequent corneal weakening.[102] Ectasia has also been reported in a cornea of normal thickness with band keratopathy.[103]
Infective keratitis after PTK
Reactivation of herpetic keratitis following PTK is well-documented.[12,104] Corneal trauma induced by photoablation and use of topical steroids post-PTK in the presence of viral shedding in the tear fluids are the predisposing factors.[12] Bacterial keratitis following PTK for spheroidal degeneration[105] and RCE[7] have been reported.
Corneal Transplantation and PTK
There are few studies evaluating the outcomes of grafts in corneas that have previously undergone a PTK. A study comparing the outcomes of penetrating keratoplasty in eyes which have undergone PTK versus naïve corneas revealed no significant difference in keratometric outcomes, complications and graft survival between the two groups. However, they did not evaluate the rate of recurrence of disease in the graft.[106] Another study evaluated the outcomes of deep anterior lamellar keratoplasty (DALK) in eyes that have undergone PTK for GCD. They concluded that previous PTK was a risk factor for recurrence of disease.[107]
PTK has been used successfully to treat recurrence of dystrophies in a graft.[54,65] However, chances of graft rejection due to inflammation must be borne in mind.[9] PTK following endothelial keratoplasties has not shown any adverse effects on the graft survival.[108]
Alternatives to PTK
Although PTK is effective in treating the anterior corneal pathologies, it is limited by the depth of the lesion, and post-operative induced irregular astigmatism, hyperopia and scarring. In cases of deeper lesions, anterior lamellar keratoplasties provide a viable option. Superficial anterior lamellar keratoplasty (SALK) is used for lesions restricted to the anterior 30-50% of the corneal stroma.[109] The corneal tissue can be dissected either using microkeratome[109] or femtosecond laser.[109] The decision regarding the surgical procedure of choice depends on the type of lesion, depth of the lesion, and corneal topography [Fig. 10]. Microkeratome-assisted anterior lamellar keratoplasty (ALK) can be successfully used when the lesions are limited to anterior 250 μ. There is a possibility of donor tissue mismatch in terms of size, shape and thickness. Interface irregularity leading to visually significant haze is common.[109] On the other hand, femtosecond laser-assisted anterior lamellar keratoplasty (FALK) leads to a smoother interface and better visual outcome. The depth of laser dissection can be customized based on the depth of the lesion. Owing to better visual results, ease of technique and better donor-to-recipient fit, FALK may be a favorable option in corneas with regular topography. However, femtosecond laser penetration is affected in dense scar tissue and may prove to be ineffective in such cases.[110] In cases where the corneal topography is irregular and depth of corneal scars is varied, hemi-automated lamellar keratoplasty (HALK) is useful, where the recipient cornea undergoes a manual dissection and the donor cornea is prepared using a microkeratome.[111]
Figure 10.
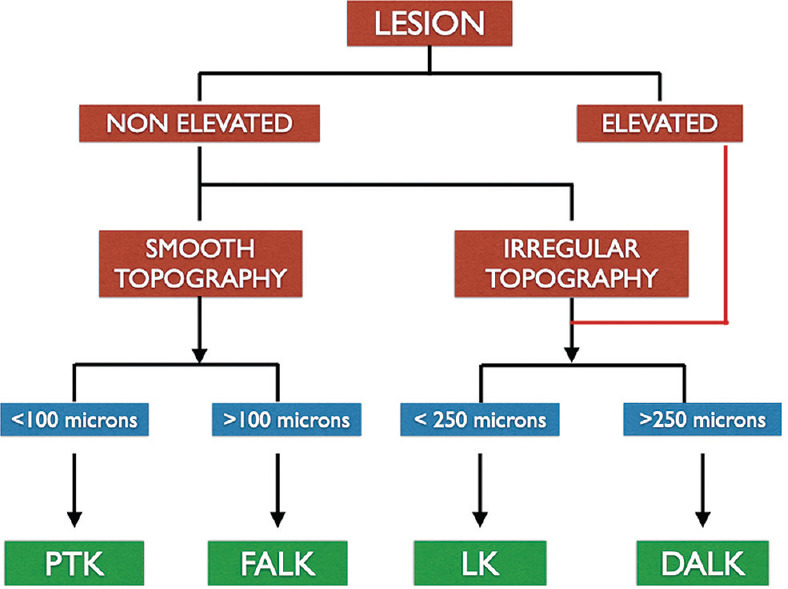
Flowchart highlighting the algorithm for selecting the appropriate procedure for a lesion in the anterior stroma as an alternative to PTK. (PTK – Phototherapeutic Keratectomy, FALK – Femtosecond Assisted Anterior Lamellar Keratoplasty, LK – Lamellar Keratoplasty, DALK – Deep Anterior Lamellar Keratoplasty)
Conclusion
In summary, PTK is an effective method to manage anterior corneal pathologies and offers advantages including repeatability, faster visual recovery and being minimally invasive. Attention to preoperative evaluation and accurate measurement of depth of lesion, corneal thickness and topography, may lead to improved outcomes. Risk of haze, hyperopic shifts and recurrence of disease are the important complications that might need to be addressed in the postoperative period.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Munnerlyn CR, Koons SJ, Marshall J. Photorefractive keratectomy: A technique for laser refractive surgery. J Cataract Refract Surg. 1988;14:46–52. doi: 10.1016/s0886-3350(88)80063-4. [DOI] [PubMed] [Google Scholar]
- 2.Rapuano CJ. Excimer laser phototherapeutic keratectomy. Int Ophthalmol Clin. 1996;36:127–36. doi: 10.1097/00004397-199603640-00017. [DOI] [PubMed] [Google Scholar]
- 3.Ayres BD, Rapuano CJ. Excimer laser phototherapeutic keratectomy. Ocul Surf. 2006;4:196–206. doi: 10.1016/s1542-0124(12)70166-0. [DOI] [PubMed] [Google Scholar]
- 4.Cavanaugh TB, Lind DM, Cutarelli PE, Mack RJ, Durrie DS, Hassanein KM, et al. Phototherapeutic keratectomy for recurrent erosion syndrome in anterior basement membrane dystrophy. Ophthalmology. 1999;106:971–6. doi: 10.1016/S0161-6420(99)00540-0. [DOI] [PubMed] [Google Scholar]
- 5.Jain S, Austin DJ. Phototherapeutic keratectomy for treatment of recurrent corneal erosion. J Cataract Refract Surg. 1999;25:1610–4. doi: 10.1016/s0886-3350(99)00262-x. [DOI] [PubMed] [Google Scholar]
- 6.Rapuano CJ. Excimer laser phototherapeutic keratectomy in eyes with anterior corneal dystrophies: Preoperative and postoperative ultrasound biomicroscopic examination and short-term clinical outcomes with and without an antihyperopia treatment. Trans Am Ophthalmol Soc. 2003;101:371–99. [PMC free article] [PubMed] [Google Scholar]
- 7.Fagerholm P. Phototherapeutic keratectomy: 12 years of experience. Acta Ophthalmol Scand. 2003;81:19–32. doi: 10.1034/j.1600-0420.2003.00015.x. [DOI] [PubMed] [Google Scholar]
- 8.Das S, Langenbucher A, Pogorelov P, Link B, Seitz B. Long-term outcome of excimer laser phototherapeutic keratectomy for treatment of Salzmann's nodular degeneration. J Cataract Refract Surg. 2005;31:1386–91. doi: 10.1016/j.jcrs.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 9.Nagpal R, Maharana PK, Roop P, Murthy SI, Rapuano CJ, Titiyal JS, et al. Phototherapeutic keratectomy. Surv Ophthalmol. 2020;65:79–108. doi: 10.1016/j.survophthal.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan R. Kinetics of the ablative photodecomposition of organic polymers in the far ultraviolet (193 nm) Journal of Vacuum Science & Technology B: Microelectronics Processing and Phenomena. 1983;1(1):923–6. [Google Scholar]
- 11.Trokel SL, Srinivasan R, Braren B. Excimer laser surgery of the cornea. Am J Ophthalmol. 1983;96:710–5. doi: 10.1016/s0002-9394(14)71911-7. [DOI] [PubMed] [Google Scholar]
- 12.Deai T, Fukuda M, Tomoda Y, Higaki S, Hayashi K, Shimomura Y. Excimer laser photokeratectomy reactivates latent herpes simplex virus. Jpn J Ophthalmol. 2004;48:570–2. doi: 10.1007/s10384-004-0112-9. [DOI] [PubMed] [Google Scholar]
- 13.Vrabec MP, Durrie DS, Chase DS. Recurrence of herpes simplex after excimer laser keratectomy. Am J Ophthalmol. 1992;15(114):96–7. doi: 10.1016/s0002-9394(14)77418-5. [DOI] [PubMed] [Google Scholar]
- 14.Rapuano CJ. Phototherapeutic keratectomy: Who are the best candidates and how do you treat them? Curr Opin Ophthalmol. 2010;21:280–2. doi: 10.1097/ICU.0b013e32833a8e0d. [DOI] [PubMed] [Google Scholar]
- 15.Wilson SE, Marino GK, Medeiros CS, Santhiago MR. Phototherapeutic keratectomy: Science and art. J Refract Surg. 2017;33:203–10. doi: 10.3928/1081597X-20161123-01. [DOI] [PubMed] [Google Scholar]
- 16.Reinstein DZ, Aslanides IM, Silverman RH, Asbell PA, Coleman DJ. High-frequency ultrasound corneal pachymetry in the assessment of corneal scars for therapeutic planning. CLAO J. 1994;20:198–203. doi: 10.1097/00140068-199407000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Hersh PS, Burnstein Y, Carr J, Etwaru G, Mayers M. Excimer laser phototherapeutic keratectomy. Surgical strategies and clinical outcomes. Ophthalmology. 1996;103:1210–22. doi: 10.1016/s0161-6420(96)30520-4. [DOI] [PubMed] [Google Scholar]
- 18.Rapuano CJ, Laibson PR. Excimer laser phototherapeutic keratectomy for anterior corneal pathology. CLAO J. 1994;20:253–7. doi: 10.1097/00140068-199410000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Vinciguerra P, Camesasca FI. Custom phototherapeutic keratectomy with intraoperative topography. J Refract Surg. 2004;20:S555–63. [PubMed] [Google Scholar]
- 20.Cleary C, Li Y, Tang M, Samy El Gendy NM, Huang D. Predicting transepithelial phototherapeutic keratectomy outcomes using Fourier domain optical coherence tomography. Cornea. 2014;33:280–7. doi: 10.1097/ICO.0000000000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori H, Miura M, Iwasaki T, Goto H, Sakurai Y, Watanabe Y, et al. Three-dimensional optical coherence tomography-guided phototherapeutic keratectomy for granular corneal dystrophy. Cornea. 2009;28:944–7. doi: 10.1097/ICO.0b013e31819670c2. [DOI] [PubMed] [Google Scholar]
- 22.Wirbelauer C, Scholz C, Häberle H, Laqua H, Pham DT. Corneal optical coherence tomography before and after phototherapeutic keratectomy for recurrent epithelial erosions. J Cataract Refract Surg. 2002;28:1629–35. doi: 10.1016/s0886-3350(02)01366-4. [DOI] [PubMed] [Google Scholar]
- 23.Jung SH, Han KE, Stulting RD, Sgrignoli B, Kim T, Kim EK. Phototherapeutic keratectomy in diffuse stromal haze in granular corneal dystrophy type 2. Cornea. 2013;32:296–300. doi: 10.1097/ICO.0b013e31824a2288. [DOI] [PubMed] [Google Scholar]
- 24.Dogru M, Katakami C, Yamanaka A. Refractive changes after excimer laser phototherapeutic keratectomy. J Cataract Refract Surg. 2001;27:686–92. doi: 10.1016/s0886-3350(01)00802-1. [DOI] [PubMed] [Google Scholar]
- 25.Englanoff JS, Kolahdouz-Isfahani AH, Moreira H, Cheung DT, Nimni ME, Trokel SL, et al. In situ collagen gel mold as an aid in excimer laser superficial keratectomy. Ophthalmology. 1992;99:1201–8. doi: 10.1016/s0161-6420(92)31822-6. [DOI] [PubMed] [Google Scholar]
- 26.Kornmehl EW, Steinert RF, Puliafito CA. A comparative study of masking fluids for excimer laser phototherapeutic keratectomy. Arch Ophthalmol. 1991;109:860–3. doi: 10.1001/archopht.1991.01080060124039. [DOI] [PubMed] [Google Scholar]
- 27.Alió JL, Belda JI, Shalaby AM. Correction of irregular astigmatism with excimer laser assisted by sodium hyaluronate. Ophthalmology. 2001;108:1246–60. doi: 10.1016/s0161-6420(01)00602-9. [DOI] [PubMed] [Google Scholar]
- 28.Fasano AP, Moreira H, McDonnell PJ, Sinbawy A. Excimer laser smoothing of a reproducible model of anterior corneal surface irregularity. Ophthalmology. 1991;98:1782–5. doi: 10.1016/s0161-6420(91)32050-5. [DOI] [PubMed] [Google Scholar]
- 29.Katsanevaki VJ, Ginis HS, Naoumidi II, Pallikaris IG. The PALM technique: Histological findings of masked phototherapeutic keratectomy on rabbit corneas. BMC Ophthalmol. 2003;3:4. doi: 10.1186/1471-2415-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kremer F, Aronsky M, Bowyer B, Stevens SX. Treatment of corneal surface irregularities using biomask as an adjunct to excimer laser phototherapeutic keratectomy. Cornea. 2002;21:28–32. doi: 10.1097/00003226-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Tuft S, al-Dhahir R, Dyer P, Zhu ZH. Characterization of the fluorescence spectra produced by excimer laser irradiation of the cornea. Invest Ophthalmol Vis Sci. 1990;31:1512–8. [PubMed] [Google Scholar]
- 32.Muller LT, Candal EM, Epstein RJ, Dennis RF, Majmudar PA. Transepithelial phototherapeutic keratectomy/photorefractive keratectomy with adjunctive mitomycin-C for complicated LASIK flaps. J Cataract Refract Surg. 2005;31:291–6. doi: 10.1016/j.jcrs.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 33.Ibarz Barberá M, García González M, Teus Guezala M. [Transepithelial phototherapeutic keratectomy to treat chronic laser in situ keratomileusis-flap macrostriae. A case review] Arch Soc Esp Oftalmol. 2012;87:407–10. doi: 10.1016/j.oftal.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Churashov SV, Kudryashova EV, Kulikov AN, Boiko EV, Chernysh VF, Maltsev DS. “Wet” transepithelial phototherapeutic keratectomy in the management of persistent epithelial defects in the graft. Clin Ophthalmol. 2018;12:895–901. doi: 10.2147/OPTH.S161018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kymionis GD, Grentzelos MA, Mikropoulos DG, Rallis KI. Transepithelial phototherapeutic keratectomy for recurrent corneal erosions in a patient with previous corneal collagen cross-linking. J Refract Surg. 2012;28:732–4. doi: 10.3928/1081597X-20120911-01. [DOI] [PubMed] [Google Scholar]
- 36.Kymionis GD, Grentzelos MA, Kankariya VP, Liakopoulos DA, Karavitaki AE, Portaliou DM, et al. Long-term results of combined transepithelial phototherapeutic keratectomy and corneal collagen crosslinking for keratoconus: Cretan protocol. J Cataract Refract Surg. 2014;40:1439–45. doi: 10.1016/j.jcrs.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 37.Cagil N, Sarac O, Yesilirmak N, Caglayan M, Uysal BS, Tanriverdi B. Transepithelial phototherapeutic keratectomy followed by corneal collagen crosslinking for the treatment of pellucid marginal degeneration: Long-term results. Cornea. 2019;38:980–5. doi: 10.1097/ICO.0000000000002003. [DOI] [PubMed] [Google Scholar]
- 38.Rush SW, Matulich J, Rush RB. Long-term outcomes of optical coherence tomography-guided transepithelial phototherapeutic keratectomy for the treatment of anterior corneal scarring. Br J Ophthalmol. 2014;98:1702–6. doi: 10.1136/bjophthalmol-2014-305366. [DOI] [PubMed] [Google Scholar]
- 39.Kanitkar KD, Camp J, Humble H, Shen DJ, Wang MX. Pain after epithelial removal by ethanol-assisted mechanical versus transepithelial excimer laser debridement. J Refract Surg. 2000;16:519–22. doi: 10.3928/1081-597X-20000901-06. [DOI] [PubMed] [Google Scholar]
- 40.Horgan SE, McLaughlin-Borlace L, Stevens JD, Munro PM. Phototherapeutic smoothing as an adjunct to photorefractive keratectomy in porcine corneas. J Refract Surg. 1999;15:331–3. doi: 10.3928/1081-597X-19990501-08. [DOI] [PubMed] [Google Scholar]
- 41.Hersh PS, Spinak A, Garrana R, Mayers M. Phototherapeutic keratectomy: Strategies and results in 12 eyes. Refract Corneal Surg. 1993;9(2 Suppl):S90–5. [PubMed] [Google Scholar]
- 42.Gibralter R, Trokel SL. Correction of irregular astigmatism with the excimer laser. Ophthalmology. 1994;101:1310. doi: 10.1016/s0161-6420(94)31174-2. [DOI] [PubMed] [Google Scholar]
- 43.Stark WJ, Chamon W, Kamp MT, Enger CL, Rencs EV, Gottsh JD. Clinical follow-up of 193-nm ArF excimer laser photokeratectomy. Ophthalmology. 1992;99:805–12. doi: 10.1016/s0161-6420(92)31896-2. [DOI] [PubMed] [Google Scholar]
- 44.Sekundo W, Geerling G. [Phototherapeutic keratectomy. Basic principles, techniques and indications] Ophthalmologe. 2006;103:563–9. doi: 10.1007/s00347-006-1359-y. [DOI] [PubMed] [Google Scholar]
- 45.Steinert RF, Puliafito CA. Excimer laser phototherapeutic keratectomy for a corneal nodule. Refract Corneal Surg. 1990;6:352. [PubMed] [Google Scholar]
- 46.Moodaley L, Liu C, Woodward EG, O'Brart D, Muir MK, Buckley R. Excimer laser superficial keratectomy for proud nebulae in keratoconus. Br J Ophthalmol. 1994;78:454–7. doi: 10.1136/bjo.78.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chacra LM, Arba-Mosquera S, Awwad ST. Customized ablation area PTK as a technique for Salzmann's degeneration and other focal stromal pathologies. J Refract Surg. 2020;36:340–4. doi: 10.3928/1081597X-20200226-01. [DOI] [PubMed] [Google Scholar]
- 48.Kim MS, Song SW, Kim JH, Woo HM. Multifocal phototherapeutic keratectomy for the treatment of persistent epithelial defect. J Cataract Refract Surg. 2000;26:1753–7. doi: 10.1016/s0886-3350(00)00640-4. [DOI] [PubMed] [Google Scholar]
- 49.Bechara SJ, Grossniklaus HE, Waring GO. Subepithelial fibrosis after myopic epikeratoplasty. Report of a case. Arch Ophthalmol. 1992;110:228–32. doi: 10.1001/archopht.1992.01080140084032. [DOI] [PubMed] [Google Scholar]
- 50.Porges Y, Ben-Haim O, Hirsh A, Levinger S. Phototherapeutic keratectomy with mitomycin C for corneal haze following photorefractive keratectomy for myopia. J Refract Surg. 2003;19:40–3. doi: 10.3928/1081-597X-20030101-08. [DOI] [PubMed] [Google Scholar]
- 51.Majmudar PA, Forstot SL, Dennis RF, Nirankari VS, Damiano RE, Brenart R, et al. Topical mitomycin-C for subepithelial fibrosis after refractive corneal surgery. Ophthalmology. 2000;107:89–94. doi: 10.1016/s0161-6420(99)00019-6. [DOI] [PubMed] [Google Scholar]
- 52.Marcon AS, Rapuano CJ. Excimer laser phototherapeutic keratectomy retreatment of anterior basement membrane dystrophy and Salzmann's nodular degeneration with topical mitomycin C. Cornea. 2002;21:828–30. doi: 10.1097/00003226-200211000-00020. [DOI] [PubMed] [Google Scholar]
- 53.Ayres BD, Hammersmith KM, Laibson PR, Rapuano CJ. Phototherapeutic keratectomy with intraoperative mitomycin C to prevent recurrent anterior corneal pathology. Am J Ophthalmol. 2006;142:490–2. doi: 10.1016/j.ajo.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 54.Miller A, Solomon R, Bloom A, Palmer C, Perry HD, Donnenfeld ED. Prevention of recurrent Reis-Bücklers dystrophy following excimer laser phototherapeutic keratectomy with topical mitomycin C. Cornea. 2004;23:732–5. doi: 10.1097/01.ico.0000127476.37175.6d. [DOI] [PubMed] [Google Scholar]
- 55.Kymionis G, Ide T, Yoo S. Flap amputation with phototherapeutic keratectomy (PTK) and adjuvant mitomycin C for severe post-LASIK epithelial ingrowth. Eur J Ophthalmol. 2009;19:301–3. doi: 10.1177/112067210901900223. [DOI] [PubMed] [Google Scholar]
- 56.Brown N, Bron A. Recurrent erosion of the cornea. Br J Ophthalmol. 1976;60:84–96. doi: 10.1136/bjo.60.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Y-T, Huang C-W, Huang F-C, Tseng S-Y, Tseng S-H. The cleavage plane of corneal epithelial adhesion complex in traumatic recurrent corneal erosion. Mol Vis. 2006;12:196–204. [PubMed] [Google Scholar]
- 58.Tripathi RC, Bron AJ. Ultrastructural study of non-traumatic recurrent corneal erosion. Br J Ophthalmol. 1972;56:73–85. doi: 10.1136/bjo.56.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maini R, Loughnan MS. Phototherapeutic keratectomy re-treatment for recurrent corneal erosion syndrome. Br J Ophthalmol. 2002;86:270–2. doi: 10.1136/bjo.86.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O'Brart DP, Muir MG, Marshall J. Phototherapeutic keratectomy for recurrent corneal erosions. Eye (Lond) 1994;8:378–83. doi: 10.1038/eye.1994.90. [DOI] [PubMed] [Google Scholar]
- 61.Das S, Seitz B. Recurrent corneal erosion syndrome. Surv Ophthalmol. 2008;53:3–15. doi: 10.1016/j.survophthal.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 62.Dedes W, Faes L, Schipper I, Bachmann LM, Thiel MA. Phototherapeutic keratectomy (PTK) for treatment of recurrent corneal erosion: Correlation between etiology and prognosis-prospective longitudinal study. Graefes Arch Clin Exp Ophthalmol. 2015;253:1745–9. doi: 10.1007/s00417-015-2990-6. [DOI] [PubMed] [Google Scholar]
- 63.Giessler S, Duncker GI. [Recurrent corneal erosion after mechanical trauma. Results of transepithelial phototherapeutic keratectomy] Ophthalmologe. 2001;98:950–4. doi: 10.1007/s003470170042. [DOI] [PubMed] [Google Scholar]
- 64.Holzer MP, Auffarth GU, Specht H, Kruse FE. Combination of transepithelial phototherapeutic keratectomy and autologous serum eyedrops for treatment of recurrent corneal erosions. J Cataract Refract Surg. 2005;31:1603–6. doi: 10.1016/j.jcrs.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 65.Amm M. [Photo-therapeutic keratectomy (PTK)--a successful treatment for Thiel-Behnke dystrophy and its recurrence] Ophthalmologe. 1999;96:489–93. doi: 10.1007/s003470050442. [DOI] [PubMed] [Google Scholar]
- 66.Rogers C, Cohen P, Lawless M. Phototherapeutic keratectomy for Reis Bucklers' corneal dystrophy. Aust N Z J Ophthalmol. 1993;21:247–50. doi: 10.1111/j.1442-9071.1993.tb00963.x. [DOI] [PubMed] [Google Scholar]
- 67.Reddy JC, Rapuano CJ, Nagra PK, Hammersmith KM. Excimer laser phototherapeutic keratectomy in eyes with corneal stromal dystrophies with and without a corneal graft. Am J Ophthalmol. 2013;155:1111–8e2. doi: 10.1016/j.ajo.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 68.Das S, Langenbucher A, Seitz B. Excimer laser phototherapeutic keratectomy for granular and lattice corneal dystrophy: A comparative study. J Refract Surg. 2005;21:727–31. doi: 10.3928/1081-597X-20051101-12. [DOI] [PubMed] [Google Scholar]
- 69.Chiambaretta F, Rozier B, Pilon F, Gérard M, Coulangeon LM, Creveaux I, et al. [Phototherapeutic keratectomy in the treatment of lattice corneal dystrophy type I] J Fr Ophtalmol. 2004;27:747–53. doi: 10.1016/s0181-5512(04)96209-2. [DOI] [PubMed] [Google Scholar]
- 70.Hafner A, Langenbucher A, Seitz B. Long-term results of phototherapeutic keratectomy with 193-nm excimer laser for macular corneal dystrophy. Am J Ophthalmol. 2005;140:392–6. doi: 10.1016/j.ajo.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 71.Badr IA, al-Rajhi A, Wagoner MD, Dunham T, Teichmann KD, Cameron JA Phototherapeutic keratectomy for climatic droplet keratopathy, KKESH excimer laser study group. King Khaled eye specialist hospital. J Refract Surg. 1996;12:114–22. doi: 10.3928/1081-597X-19960101-21. [DOI] [PubMed] [Google Scholar]
- 72.Salah T, el Maghraby A, Waring GO. Excimer laser phototherapeutic keratectomy before cataract extraction and intraocular lens implantation. Am J Ophthalmol. 1996;122:340–8. doi: 10.1016/s0002-9394(14)72060-4. [DOI] [PubMed] [Google Scholar]
- 73.Maloney RK, Thompson V, Ghiselli G, Durrie D, Waring GO, O'Connell M. A prospective multicenter trial of excimer laser phototherapeutic keratectomy for corneal vision loss. The summit phototherapeutic keratectomy study group. Am J Ophthalmol. 1996;122:149–60. doi: 10.1016/s0002-9394(14)72006-9. [DOI] [PubMed] [Google Scholar]
- 74.Reddy JC, Rapuano CJ, Felipe AF, Nagra PK, Hammersmith KM. Quality of vision after excimer laser phototherapeutic keratectomy with intraoperative mitomycin-C for Salzmann nodular degeneration. Eye Contact Lens. 2014;40:213–9. doi: 10.1097/ICL.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 75.Khaireddin R, Katz T, Baile RB, Richard G, Linke SJ. Superficial keratectomy, PTK, and mitomycin C as a combined treatment option for Salzmann's nodular degeneration: A follow-up of eight eyes. Graefes Arch Clin Exp Ophthalmol. 2011;249:1211–5. doi: 10.1007/s00417-011-1643-7. [DOI] [PubMed] [Google Scholar]
- 76.O'Brart DP, Gartry DS, Lohmann CP, Patmore AL, Kerr Muir MG, Marshall J. Treatment of band keratopathy by excimer laser phototherapeutic keratectomy: Surgical techniques and long term follow up. Br J Ophthalmol. 1993;77:702–8. doi: 10.1136/bjo.77.11.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stewart OG, Morrell AJ. Management of band keratopathy with excimer phototherapeutic keratectomy: Visual, refractive, and symptomatic outcome. Eye (Lond) 2003;17:233–7. doi: 10.1038/sj.eye.6700327. [DOI] [PubMed] [Google Scholar]
- 78.Im S-K, Lee K-H, Yoon K-C. Combined ethylenediaminetetraacetic acid chelation, phototherapeutic keratectomy and amniotic membrane transplantation for treatment of band keratopathy. Korean J Ophthalmol. 2010;24:73–7. doi: 10.3341/kjo.2010.24.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dogru M, Katakami C, Miyashita M, Hida E, Uenishi M, Tetsumoto K, et al. Visual and tear function improvement after superficial phototherapeutic keratectomy (PTK) for mid-stromal corneal scarring. Eye (Lond) 2000;14(Pt 5):779–84. doi: 10.1038/eye.2000.204. [DOI] [PubMed] [Google Scholar]
- 80.Vinciguerra P, Albè E, Rosetta P, Di Iorio E, Pellegrini G. Custom phototherapeutic keratectomy and autologous fibrin-cultured limbal stem cell autografting: A combined approach. J Refract Surg. 2008;24:323–4. doi: 10.3928/1081597X-20080401-02. [DOI] [PubMed] [Google Scholar]
- 81.Thomann U, Meier-Gibbons F, Schipper I. Phototherapeutic keratectomy for bullous keratopathy. Br J Ophthalmol. 1995;79:335–8. doi: 10.1136/bjo.79.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maini R, Sullivan L, Snibson GR, Taylor HR, Loughnan MS. A comparison of different depth ablations in the treatment of painful bullous keratopathy with phototherapeutic keratectomy. Br J Ophthalmol. 2001;85:912–5. doi: 10.1136/bjo.85.8.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sharma N, Prakash G, Sinha R, Tandon R, Titiyal JS, Vajpayee RB. Indications and outcomes of phototherapeutic keratectomy in the developing world. Cornea. 2008;27:44–9. doi: 10.1097/ICO.0b013e318157a111. [DOI] [PubMed] [Google Scholar]
- 84.Gottsch JD, Gilbert ML, Goodman DF, Sulewski ME, Dick JD, Stark WJ. Excimer laser ablative treatment of microbial keratitis. Ophthalmology. 1991;98:146–9. doi: 10.1016/s0161-6420(91)32323-6. [DOI] [PubMed] [Google Scholar]
- 85.Lin C-P, Chang C-W, Su C-Y. Phototherapeutic keratectomy in treating keratomycosis. Cornea. 2005;24:262–8. doi: 10.1097/01.ico.0000148313.78933.68. [DOI] [PubMed] [Google Scholar]
- 86.Li L-M, Zhao L-Q, Qu L-H, Li P. Excimer laser phototherapeutic keratectomy for the treatment of clinically presumed fungal keratitis. J Ophthalmol. 2014;2014:963287. doi: 10.1155/2014/963287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lindbohm N, Moilanen JAO, Vesaluoma MH, Tervo TMT. Acinetobacter and staphylococcus aureus ulcerative keratitis after laser in situ keratomileusis treated with antibiotics and phototherapeutic keratectomy. J Refract Surg. 2005;21:404–6. doi: 10.3928/1081-597X-20050701-19. [DOI] [PubMed] [Google Scholar]
- 88.Kymionis GD, Kankariya VP, Kontadakis GA. Combined treatment with flap amputation, phototherapeutic keratectomy, and collagen crosslinking in severe intractable post-LASIK atypical mycobacterial infection with corneal melt. J Cataract Refract Surg. 2012;38:713–5. doi: 10.1016/j.jcrs.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 89.Frucht-Pery J, Golan G, Hemo I, Zauberman H, Shapiro M. Efficacy of topical gentamicin treatment after 193-nm photorefractive keratectomy in an experimental pseudomonas keratitis model. Graefes Arch Clin Exp Ophthalmol. 1995;233:532–4. doi: 10.1007/BF00183436. [DOI] [PubMed] [Google Scholar]
- 90.Kandori M, Inoue T, Shimabukuro M, Hayashi H, Hori Y, Maeda N, et al. Four cases of acanthamoeba keratitis treated with phototherapeutic keratectomy. Cornea. 2010;29:1199–202. doi: 10.1097/ICO.0b013e3181d3d674. [DOI] [PubMed] [Google Scholar]
- 91.Cameron JA, Antonios SR, Badr IA. Excimer laser phototherapeutic keratectomy for shield ulcers and corneal plaques in vernal keratoconjunctivitis. J Refract Surg. 1995;11:31–5. doi: 10.3928/1081-597X-19950101-09. [DOI] [PubMed] [Google Scholar]
- 92.Lakimenko S, Buznyk O, Shchypun S. Treatment of post-burn persistent corneal ulcers with excimer laser phototherapeutic keratectomy. Prospective clinical trial. Klin Oczna. 2010;112:195–200. [PubMed] [Google Scholar]
- 93.Kymionis GD, Grentzelos MA, Karavitaki AE, Kounis GA, Kontadakis GA, Yoo S, et al. Transepithelial phototherapeutic keratectomy using a 213-nm solid-state laser system followed by corneal collagen cross-linking with riboflavin and UVA irradiation. J Ophthalmol. 2010;2010:146543. doi: 10.1155/2010/146543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reinstein DZ, Gobbe M, Archer TJ, Silverman RH, Coleman DJ. Epithelial, stromal, and total corneal thickness in keratoconus: Three-dimensional display with artemis very-high frequency digital ultrasound. J Refract Surg. 2010;26:259–71. doi: 10.3928/1081597X-20100218-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kymionis GD, Grentzelos MA, Kounis GA, Diakonis VF, Limnopoulou AN, Panagopoulou SI. Combined transepithelial phototherapeutic keratectomy and corneal collagen cross-linking for progressive keratoconus. Ophthalmology. 2012;119:1777–84. doi: 10.1016/j.ophtha.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 96.Grentzelos MA, Kounis GA, Diakonis VF, Siganos CS, Tsilimbaris MK, Pallikaris IG, et al. Combined transepithelial phototherapeutic keratectomy and conventional photorefractive keratectomy followed simultaneously by corneal crosslinking for keratoconus: Cretan protocol plus. J Cataract Refract Surg. 2017;43:1257–62. doi: 10.1016/j.jcrs.2017.06.047. [DOI] [PubMed] [Google Scholar]
- 97.Majmudar PA, Raviv T, Dennis RF, Epstein RJ. Subepithelial fibrosis after RK. J Cataract Refract Surg. 2000;26:1433–4. doi: 10.1016/s0886-3350(00)00678-7. [DOI] [PubMed] [Google Scholar]
- 98.Fong YC, Chuck RS, Stark WJ, McDonnell PJ. Phototherapeutic keratectomy for superficial corneal fibrosis after radial keratotomy. J Cataract Refract Surg. 2000;26:616–9. doi: 10.1016/s0886-3350(99)00363-6. [DOI] [PubMed] [Google Scholar]
- 99.Hernandez-Matamoros J, Iradier MT, Moreno E. Treating folds and striae after laser in situ keratomileusis. J Cataract Refract Surg. 2001;27:350–2. doi: 10.1016/s0886-3350(00)00791-4. [DOI] [PubMed] [Google Scholar]
- 100.Rachid MD, Yoo SH, Azar DT. Phototherapeutic keratectomy for decentration and central islands after photorefractive keratectomy. Ophthalmology. 2001;108:545–52. doi: 10.1016/s0161-6420(00)00595-9. [DOI] [PubMed] [Google Scholar]
- 101.Leu G, Hersh PS. Phototherapeutic keratectomy for the treatment of diffuse lamellar keratitis. J Cataract Refract Surg. 2002;28:1471–4. doi: 10.1016/s0886-3350(01)01311-6. [DOI] [PubMed] [Google Scholar]
- 102.Vinciguerra P, Munoz MIT, Camesasca FI, Grizzi F, Roberts C. Long-term follow-up of ultrathin corneas after surface retreatment with phototherapeutic keratectomy. J Cataract Refract Surg. 2005;31:82–7. doi: 10.1016/j.jcrs.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 103.Miyata K, Takahashi T, Tomidokoro A, Ono K, Oshika T. Iatrogenic keratectasia after phototherapeutic keratectomy. Br J Ophthalmol. 2001;85:247–8. doi: 10.1136/bjo.85.2.238j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fagerholm P, Ohman L, Orndahl M. Phototherapeutic keratectomy in herpes simplex keratitis. Clinical results in 20 patients. Acta Ophthalmol (Copenh) 1994;72:457–60. doi: 10.1111/j.1755-3768.1994.tb02796.x. [DOI] [PubMed] [Google Scholar]
- 105.al-Rajhi AA, Wagoner MD, Badr IA, al-Saif A, Mahmood M. Bacterial keratitis following phototherapeutic keratectomy. J Refract Surg. 1996;12:123–7. doi: 10.3928/1081-597X-19960101-22. [DOI] [PubMed] [Google Scholar]
- 106.Szentmáry N, Langenbucher A, Hafner A, Seitz B. Impact of phototherapeutic keratectomy on the outcome of subsequent penetrating keratoplasty in patients with stromal corneal dystrophies. Am J Ophthalmol. 2004;137:301–7. doi: 10.1016/j.ajo.2003.08.039. [DOI] [PubMed] [Google Scholar]
- 107.Salouti R, Hosseini H, Eghtedari M, Khalili MR. Deep anterior lamellar keratoplasty with melles technique for granular corneal dystrophy. Cornea. 2009;28:140–3. doi: 10.1097/ICO.0b013e3181861cdd. [DOI] [PubMed] [Google Scholar]
- 108.Lee BS, Hardten DR. Visual and subjective outcomes of phototherapeutic keratectomy after Descemet's stripping endothelial keratoplasty. Clin Ophthalmol. 2014;8:1011–5. doi: 10.2147/OPTH.S63982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Patel AK, Scorcia V, Kadyan A, Lapenna L, Ponzin D, Busin M. Microkeratome-assisted superficial anterior lamellar keratoplasty for anterior stromal corneal opacities after penetrating keratoplasty. Cornea. 2012;31:101–5. doi: 10.1097/ICO.0b013e31820c9fd1. [DOI] [PubMed] [Google Scholar]
- 110.Shousha MA, Yoo SH, Kymionis GD, Ide T, Feuer W, Karp CL, et al. Long-term results of femtosecond laser-assisted sutureless anterior lamellar keratoplasty. Ophthalmology. 2011;118:315–23. doi: 10.1016/j.ophtha.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 111.Yuen LH, Mehta JS, Shilbayeh R, Lim L, Tan DT. Hemi-automated lamellar keratoplasty (HALK) Br J Ophthalmol. 2011;95:1513–8. doi: 10.1136/bjophthalmol-2011-300195. [DOI] [PubMed] [Google Scholar]


