Abstract
Purpose:
The aim of this study was to evaluate the safety, efficacy, and complications of V4c Toric implantable collamer Lens (TICL) implantation for myopic astigmatism in the south Indian population.
Methods:
In this retrospective observational case series, a total of 109 eyes of 67 patients who underwent V4c TICL implantation (ICL, V4C Staar Surgical, Nidau, Switzerland) between January 2012 and August 2019 were studied with a minimum follow-up period of 6 months (mean 24 months). The main outcome measures were objective and subjective refraction, uncorrected distance visual acuity, corrected distance visual acuity (CDVA), safety, predictability, adverse events, and postoperative complications.
Results:
At 6 months, mean manifest refractive spherical equivalent (SE) decreased from - 10.90 ± 3.7D preoperatively to - 0.02 ± 0.13D postoperatively (P < 0.001) and mean cylinder decreased from - 2.3 ± 1.3 D preoperatively to - 0.04 ± 0.2 D postoperatively (P < 0.001). Postoperatively, SE within ± 0.5 D and ± 1.0 D of attempted correction were achieved in 96.3 (105 eyes) and 100% (109 eyes), respectively. Manifest refractive cylinder within ± 0.5 D and ± 1.0 D of attempted correction were achieved in 97.2 (106 eyes) and 100% (109 eyes), respectively. Sixty-two percent (68 eyes) showed no change in CDVA postoperatively, and no eye had lost lines of CDVA. The safety index was 1.12, and the efficacy index was 1.10. Complications were seen in two eyes (1.8%) due to high postoperative vault requiring secondary surgical interventions.
Conclusion:
V4c TICL is a highly effective, safe, and predictable option in treating myopic astigmatism with excellent improvement in vision and spectacle independence.
Keywords: Endothelial cell density, rotational stability, toric implantable collamer lens, V4c TICL
Laser-assisted in-situ keratomileusis (LASIK) is the most widely performed procedure for correction of refractive errors, where excimer laser is used to reshape the cornea. According to a recent study, prevalence of myopia in India is 27.7% in adults.[1] LASIK improves vision by correcting myopia, hyperopia, and astigmatism. However, there are limitations when used to correct high refractive cylinder.[2] The presence of ocular residual astigmatism,[3] the disagreement between refractive and topographic astigmatism axis,[4] and the influence of internal optical astigmatism[5] may influence the outcome of LASIK for myopic astigmatism.
Phakic intraocular lenses (PIOLs) is a precise, a reproducible, and a popular procedure used to correct high ametropias in patients, contraindicated for LASIK.[6,7] The Visian Implantable Collamer Lens (ToricICL, Staar Surgical) is a posterior chamber pIOL approved by the U.S. Food and Drug Administration (FDA) for myopic astigmatism.[8] They are commercially available since 2002. ICL effectively corrects, moderate to high myopia and astigmatism.[6,8] They are commonly associated with astigmatism.[9] The Toric ICL (TICL) improves distance vision by reducing myopic astigmatism. They are intended to correct myopic astigmatism from -3D up to -20D with cylinder from 1D up to 4D at spectacle plane.[10] The challenges involved in the procedure are the accurate placement, rotational stability, residual astigmatism, and complications. Literature shows promising results for Toric Implantable Collamer Lens (ICL).[6,7] The purpose of our study is to evaluate the safety efficacy and complications of TICL V4c implantation for myopic astigmatism in south Indian population.
Methods
A retrospective, observational study of 109 eyes of 67 patie with Toric ICLV4 implantation (TICL) for myopic astigmatism, (-3D to -19D SE, Cylinder-1D to -5.5D) was done between January 2012 and August 2019 at a tertiary eye care center in South India. The study was conducted following the recommendations of the Declaration of Helsinki. Inclusion criteria included: Patients with realistic expectations, stable refraction for at least 1 year, endothelial count of >2,500 cells/mm2, anterior chamber depth (ACD) >2.8 mm, myopic astigmatism not suitable for corneal laser surgery, and age 18–40 years. The eyes with the previous history of ocular surgeries, ocular diseases such as iritis, cataract, glaucoma, and posterior segment pathology, and patients with systemic diseases were excluded. All patients underwent routine preoperative slit-lamp bio microscopic examination. The measurements before and after surgery include uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), manifest refraction (SE), keratometry, and axial length measurement from IOLMaster 700 (Carl Zeiss Meditec AG, Jena, Germany), scanning-slit topography (OrbscanIIz, Bausch and Lomb) to measure white to white diameter (WTW), central corneal thickness, and ACD, from anterior segment optical coherence tomography (AS-OCT, Visante Carl Zeiss Meditec, Dublin, California, USA), horizontal WTW, ACD, angle to angle measurement and corneal thickness, horizontal WTW measurement from the digital caliper, and a peripheral retinal examination. The IOP and endothelial cell density (ECD) were done using a Goldmann applanation tonometry and noncontact specular microscope (Topcon Corporation, Tokyo). The data entry was done and mailed to the manufacturer of VisianICL. The TICL diameters were based on the horizontal WTW distance and ACD and the TICL power from the modified vertex formula as per manufacturer's recommendations, to achieve emmetropia without glasses.
Statistical analysis
All the statistical analysis was performed by STATA 14.0 (Texas, USA). The Shapiro–Wilk test was used to confirm the normality of the data. Continuous variables were described as mean ± SD (standard deviation) or median with interquartile range, and categorical variables were expressed as proportions. The vision was measured in Snellen equivalent and converted to logarithm of the minimal angle of resolution (log MAR) for statistical analysis. The mean of ECD, IOP were compared preoperatively, and postoperatively by paired t-test. The mean of manifest spherical equivalent, manifest cylinder, and log MAR visual acuity was compared by Wilcoxon signed-rank test. P value < 0.05 is considered as statistically significant.
Surgical technique and postoperative management
Two experienced surgeons did the Visian V4c model TICL (STAAR Surgical, Nidau, Switzerland) implantation in all patients. Thirty minutes preoperatively, pupils were dilated with tropicamide 1% and phenylephrine combination eye drop. Limbal axis marking was done using a reference marker at 3, 6, and 9'0 clock position on the limbus, before the surgery in sitting position in the slit-lamp. TICL was loaded a few minutes before the procedure. Under topical anesthesia, a 3.5 mm temporal clear corneal incision made along with two paracentesis, 1 mm width at six and 12'0 clock position. The anterior chamber (AC) was filled with hydroxypropyl methylcellulose (HPMC) 2%. The loaded ICL was then injected carefully from the injector, by keeping the nozzle of the cartridge just inside the wound and allowed to unfold. The footplates were tucked under the iris with the Vukich manipulator (Rhein Medical, Inc., Staar Surgical Co.), on all four sides and positioned along the desired axis with reference to the preoperative limbal markings with the Vukich manipulator by gentle rotation over the haptic-optic junction; remaining HPMC was washed out thoroughly, and the corneal wound hydrated after AC formation. The fellow eye was operated in the same manner within 1 or 2 days for bilateral implantations. Postoperatively, patients received Gatifloxacin and dexamethasone eye drops four times a day for 1 week and then topical nonsteroidal anti-inflammatory drugs for 2 weeks. Follow-up examinations were scheduled at 1 day, 1 week, 1 month, 2 months, 6 months, and 1 year after surgery and yearly. The assessment was on preoperative and postoperative UDVA, CDVA values (efficacy and safety indexes), achieved and expected postoperative outcomes (Predictability), endothelial cell loss (ECL), and IOP variations, rotational stability, postoperative vault between the crystalline lens and ICL measured perpendicular to the lens apex. Slit-lamp anterior segment photography was used to assess the rotational stability by serial documentation of the linear axis mark on the TICL [Fig. 1]. The independent investigator at every visit did axis marking. The rotation is defined as the difference in the intended axis and the achieved axis at the final follow-up. The assessment depends on the investigator's judgment.
Figure 1.
Slit-lamp photographic documentation of linear axis mark on the TICLV4c model
Results
One hundred nine eyes of 67 patients who underwent Toric ICLV4c (TICL) implantation between January 2012 and August 2019, with a mean follow-up of 24 months, were included in our study. Forty-one eyes (38%), 23 eyes (21%), 11 eyes (10%), and 34 eyes (31%) were followed up for 6 months, up to 1 year, up to 2 years, and above 2 years, respectively. We considered six-monthly results of the eyes since all the patients completed the follow-up, uniformly. The mean age of the patients was 23.72 ± 3.23 years (range 18–33 years). Thirty-five patients were males (52.2%), and 32 patients (47.8%) were females. The demographic data are shown in Table 1.
Table 1.
Baseline and demographic characteristics of the study participants
| Characteristic | Mean±SD | Range |
|---|---|---|
| Age, years | 23.72±3.23 | 18-33 |
| Male gender, n (%) | 35 (52.2) | - |
| Laterality, n (%) | ||
| Unilateral | 25 (37.3) | - |
| Bilateral | 42 (62.7) | |
| Mean MRSE (D) | -10.9±3.7 | -19.25 to -3.50 |
| Manifest cylinder (D) | -2.33±1.3 | -5.5 to -1.0 |
| WTW, mm | 11.49±0.4 | 10.8 to 12.5 |
| ACD, mm | 3.19±0.2 | 2.8 to 3.9 |
| IOP, mm Hg | 15.29±2.87 | 9 to 24 |
| CCT, µm | 503.2±34.9 | 412 to 584 |
| ECD, cells/mm2 | 2998±313 | 2200 to 3800 |
SD=Standard deviation; D=Dioptre; MRSE=Manifest refractive spherical equivalent; WTW=Horizontal white-to-white diameter; ACD=Anterior chamber depth; IOP=Intraocular pressure; CCT=Central corneal thickness; ECD=Endothelial cell density
Preoperative mean manifest refractive spherical equivalent (MRSE) improved from -10.90 ± 3.7 diopters (D) to -0.02 ± 0.13 D postoperatively is statistically significant (P < 0.001). The mean manifest refractive cylinder decreased from -2.33 ± 1.3 D (range to -5.5 to -1 D) at baseline to -0.04 ± 0.13 D postoperatively (98.3% decrease in astigmatism, P < 0.001). Efficacy and safety parameters are shown in Table 2. The mean pre- and postoperative ECD is shown in Table 2. The rate of ECL at six months was 4%, which is statistically significant (P < 0.001). The mean postoperative vault with anterior segment optical coherence tomography (AS-OCTVisante Carl Zeiss Meditec) was 0.64 ± 0.2 mm at six months.
Table 2.
Efficacy and safety parameters after toric ICL implantation
| Baseline | Postoperative (6 months) | P | |
|---|---|---|---|
| LogMAR UDVA | |||
| Mean±SD | 1.34±0.20 | 0.08±0.13 | <0.001b |
| Median (IQR) | 1.30 (1.18, 1.48) | 0 (0, 0.18) | |
| LogMAR CDVA | |||
| Mean±SD | 0.13±0.14 | 0.06±0.11 | <0.001b |
| Median (IQR) | 0.18 (0, 0.18) | 0 (0, 0.18) | |
| MRSE (D) | |||
| Mean±SD | -10.9±3.7 | -0.02±0.13 | <0.001b |
| Median (IQR) | -10.7 (-12.9, -7.7) | 0 (0, 0) | |
| Cylinder (D) | |||
| Mean±SD | -2.33±1.3 | -0.04±0.13 | <0.001b |
| Median (IQR) | -2.0 (-3.0, -1.5) | 0 (0, 0) | |
| IOP (mm Hg) | |||
| Mean±SD | 15.29±2.87 | 15.93±2.74 | 0.029a |
| Range | 9 to 24 | 8-24 | |
| ECD (cells/mm2) | |||
| Mean±SD | 2998±313 | 2900±321 | <0.001a |
| Range | 2200-3800 | 2085-3661 |
Log MAR=Logarithm of the minimal angle of resolution; SD=Standard deviation; IQR=Interquartile range; UDVA=Uncorrected distance visual acuity; CDVA=Corrected distance visual acuity; IOP=Intraocular pressure; ECD=Endothelial cell density, aPaired t-test, bWilcoxon sign rank test
Safety outcomes
At 6 months, mean CDVA (log MAR) improved from 0.13 ± 0.14 (range 0–0.48) preoperatively to 0.06 ± 0.11 (range 0–0.48) postoperatively (P < 0.001). The proportion of eyes with a CDVA of 6/6 increased from 52.2% (57 eyes) preoperatively to more than 73.3% (80 eyes) postoperatively, and CDVA of 6/9 increased from 80.7% (88 eyes) preoperatively to more than 93.6% (102 eyes) postoperatively [Fig. 2]. At 6 months, 68 eyes (62.3%) showed no change in CDVA postoperatively. Thirty-six eyes (33.3%) gained one line, four eyes (3.6%) gained two lines, and one eye (0.9%) gained three lines. No eye had lost lines of CDVA [Fig. 3]. The safety index (postoperative CDVA divided by preoperative CDVA) was 1.12.
Figure 2.
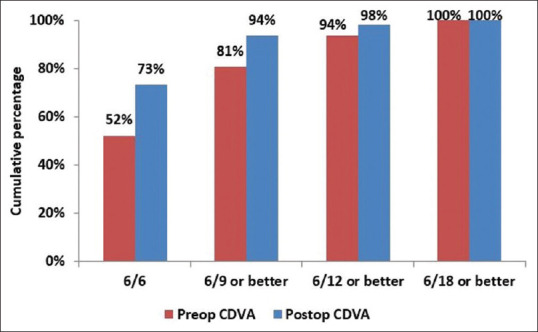
Comparison of preoperative CDVA with postoperative CDVA
Figure 3.
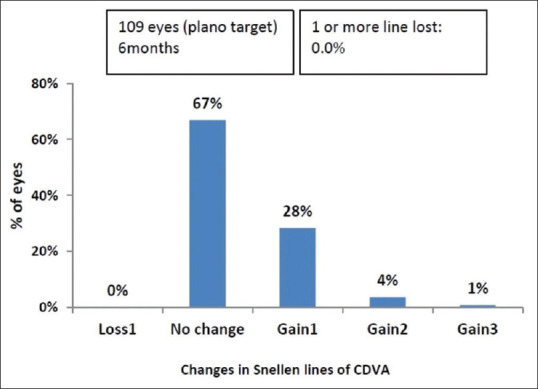
Change in Snellen lines of CDVA
Efficacy outcomes
At 6 months, preoperative mean UDVA (log MAR) improved from 1.34 ± 0.20 (range 1–1.78) to 0.08 ± 0.13 (range 0–0.48) postoperatively (P < 0.001) and the improvement in postoperative UDVA of 6/6 or better was seen in 70.6% (77 eyes) compared with preoperative CDVA (57 eyes). The efficacy index (postoperative UDVA divided by preoperative CDVA) was 1.10.
Predictability of manifest refraction
Spherical equivalent (SE): At six months, manifest SE within ± 0.5 D and ± 1.0 D of attempted correction was achieved in 96.3 (105 eyes) and 100% (109 eyes), respectively [Fig. 4]. A scatterplot of the attempted versus the achieved correction (SE) is shown in Fig. 5. Astigmatism: At six months, manifest refractive cylinder within ± 0.5 D and ± 1.0 D of attempted correction achieved in 97.2 (106 eyes) and 100% (109 eyes), respectively [Fig. 6].
Figure 4.
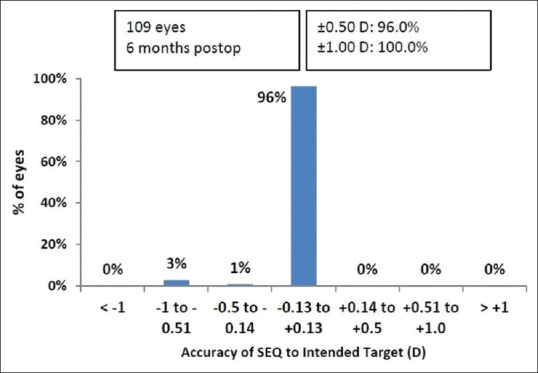
Spherical equivalent refraction accuracy
Figure 5.
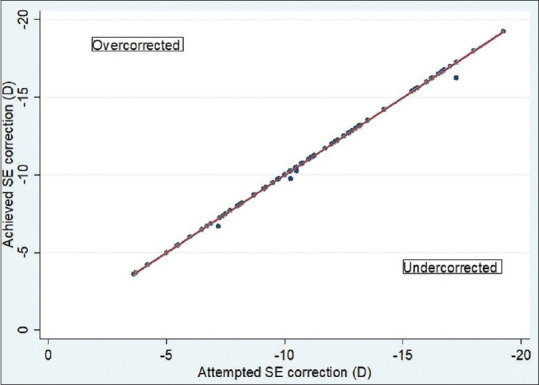
Scatter plot showing attempted versus achieved correction (spherical equivalent)
Figure 6.
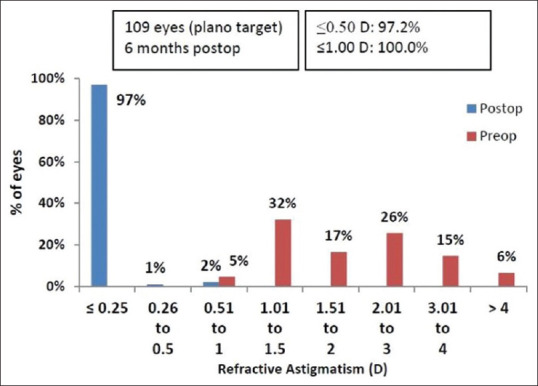
Refractive astigmatism accuracy
Stability
All the eyes showed a stable TICL position postoperatively, and none of them required realignment.
Secondary surgeries/adverse events
Two eyes (1.8%) had a high postoperative vault, requiring surgery. The average vault of these two eyes was 1.57 mm. Two eyes had ICL explanted within a mean period of 16 days. In one eye, ICL exchanged with a 0.5 mm smaller diameter size. In the second eye, ICL was explanted due to thick iris. The clear lens extraction with -1D intraocular implantation was done to avoid iris damage and pigment release with written willingness from the patient. The ECD dropped from 3341 to 2945 at 2 years follow-up. Both patients performed well and had a good vision at the final visit.
Discussion
ICL V4c model has a 360 um central hole, which eliminates the need for peripheral iridotomies, improves aqueous circulation through the hole, and reduces pupillary block and cataract formation.[11,12] The erroneous preoperative evaluation and incorrect sizing of the ICL leads to complications. The high cost and glaucoma are significant challenges in the Indian population.[13] Literature reveals promising results on the refractive and visual outcome following TICL for myopic astigmatism.[6,7,14] Our study shows favorable results with the TICLV4c model for myopic astigmatism too in south Indian context. Lee et al.[15] were the first to investigate the safety, efficacy, and rotational stability of the V4c model. The safety index was 1.38; the efficacy index was 1.35 at 12 months, 60% had a refractive cylinder of 0.25D or less at 6 months, and 100% had UDVA of 20/20 at 6 months. The U.S. FDA has conducted a clinical trial of TICL and demonstrated its efficacy and predictability in 210 eyes for a range of SE between 2.38 and 19.5 D myopia.[16] Preoperative mean MRSE of -9.36 D had decreased to 0.05 D postoperatively. The mean manifest refractive cylinder decreased from 1.93 D ± 0.84 at baseline to 0.51 D ± 0.48, with a 73.6% decrease in astigmatism. Pothireddy et al.[7] retrospectively studied outcomes of TICLs in the Indian eyes with a mean age of 24.5 years, similar to our study. The mean refractive cylinder decreased from more than 2.00 D preoperatively to less than 0.50 D postoperatively, and 84.5% of eyes were within 1.00 D of the target refraction. In our study, at 6 months, preoperative mean MRSE improved from -10.90 ± 3.7 D to -0.02 ± 0.13 D in the postoperative period, and mean manifest refractive cylinder decreased from -2.33 ± 1.3 D to -0.04 ± 0.13 D postoperatively, a 98.3% decrease in astigmatism was noted. Our patients' mean age was 23.72 ± 3.23 years, a much younger group compared to other studies. At 6 months, 96.3% of eyes were within ± 0.5D, and 100% were within ± 1 D of the intended SE correction. Likewise, 97.2% of eyes were within ± 0.5D, and 100% were within ± 1 D of the intended astigmatic correction. The safety index of 1.12 indicates the significant improvement of CDVA from preoperative to 6 months postoperatively and efficacy index of 1.1, indicates a significant difference between the UDVA and CDVA preoperatively, which was comparable with the previous studies.[6,11,15,16]
An ideal ICL vault is between 500 and 700 μm.[17] The complications such as cataract occur when the vault is <250 μm and glaucoma with vault >750 μm.[18,19] Shimizu et al.[20] compared the efficacy of conventional ICL to hole ICL and found clinically significant cataract in one eye (3%) implanted with conventional ICL. IPCL (V1) model cause clinically significant cataract when compared to other ICL models.[21] The mean postoperative vault of our patients was 0.64 ± 0.2 mm at 6 months. None of our patients developed cataracts after 2 years of follow up. We attribute it to the proper ICL sizing, presence of a central hole, and the young age group of our patients.
Guber et al.[22] compared the efficacy and safety of V4 and V4cTICLs after 10 years of follow-up and showed no change in IOP in both ICLs. Gonzalez-Lopez et al.[23] reported an increase in IOP in 5 out of 100 eyes after V4cICL. They attributed it to obstruction of TMW or central hole, by residual viscosurgical devices and miosis. In our study, mean preoperative and postoperative IOP was 15.29 ± 2.9 and 15.94 ± 2.7 mm Hg, respectively, statistically not significant (P < 0.029). Postoperative IOP was normal. Higueras-Esteban et al.[24] studied IOP measurements in V4b and V4c ICLs and found no difference between them. The evaluation of the rotational stability of TICL is pivotal to achieve high efficacy for the correction of astigmatism. Very few studies assessed the rotational stability of TICLs through various methods. Lee et al.[15] measured it, with OPD scan III (Nidek Co., Ltd), the absolute degree of rotation at 6 months was 3.87* +/- 3.07*, comparable to the study by Hyun et al.[11] who studied the rotational stability with digital anterior segment photography (DASP) 7, 11. In total, 79.2% of eyes with V4c TICL and 70.8% of eyes with V4 TICL had rotational stability < 5 degrees until the last follow-up. The V4c TICL is stored in a balanced salt solution, do not change its size after implantation resulting in better stability in contrast to V4 TICL, stored in sodium chloride (NaCl), and hence, enlarges by 1.05 times in the eye within 2–3 days, therefore vulnerable to rotation before it fully enlarges. Many authors[25,26] have studied the rotational stability using DASP. We assessed the rotational stability with serial slit-lamp photographic documentation and found no change in our patients' axis. Hashemian et al.[27] had to do rerotation in 1 eye of V4c (2.2%) and three V4b groups (7.5%). Garcia-De la Rosa et al.[19] did repositioning of V4cTICL in one eye.
ECL in our study was 4% at 6 months (P < 0.001), which was comparable to other studies.[28,29] Edelhauser et al.[30] reported a cumulative ECL between 8.4 and 8.9% over 3 years and between 8.4 and 9.5% over 4 years. The authors implicated surgical trauma in the initial ECL due to prolonged corneal endothelial remodeling. A similar observation was confirmed by few studies where the percentage of hexagonal cells (polymorphism) and the coefficient of variation (polymegathism) achieved stability over a period, with the loss rate no longer clinically significant.[31,32]
In Al Sabaani et al.[33] in a cohort of 787 eyes, explantation was done in 30 eyes (3.8%) due to incorrect sizing of ICL, cataract, high residual astigmatism, retinal detachment, and intolerable glare. Brar et al.[34] reported an explantation rate of 1.98% (19 eyes) in a large retrospective case series of 957 eyes, including 536 TICL implantations. The reasons were cataract in eight eyes (42.1%), high vault in six eyes (31.6%), and frequent rotation in five eyes (26.3%). Kaur et al.[35] while evaluating the causes for explantation of ICL in his study reported that chipped haptic of ICL during insertion was one reason, other notable reasons were shallow vault with recurrent uveitis and acute postoperative endophthalmitis. In our study, ICL was explanted in two eyes (1.8%) due to the high vault (average 1.57 mm), within a mean duration of 16 days. In one eye, ICL exchanged with a 0.5 mm smaller diameter size. In the second eye, the clear lens extraction with foldable IOL implantation was carried out at 8 months. Both patients have performed well and had a good vision at the final visit. Even though several complications remain, benefits supersede the risks. It is a retrospective study with a short follow-up, although the study period was 7 years. We recommend a prospective study with larger sample size, longer follow-up to evaluate ECD, and cataract formation.
Conclusion
To conclude, V4cTICL had improved the quality of life and vision in all our patients, achieving a 98.3% decrease in astigmatism, and 33.3% gained one line more than the preoperative CDVA. ECD loss was 4%, which is comparable to many other previous studies. The procedure was 100% predictable, safer, has a useful efficacy index and good rotational stability. Both eyes with high vault were effectively managed, with the added advantage of easy removal and exchange of ICLs. Considering that benefits outweigh the risks, V4TICL is a safe and effective procedure, particularly in high myopia and astigmatism levels. The V4c model with a central hole offers additional safety omitting laser peripheral iridotomies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sheeladevi S, Seelam B, Nukella PB, Borah RR, Ali R, Keay L. Prevalence of refractive errors, uncorrected refractive error, and presbyopia in adults in India: A systematic review. Indian J Ophthalmol. 2019;67:583–92. doi: 10.4103/ijo.IJO_1235_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keir NJ, Simpson T, Jones LW, Fonn D. Wavefront-guided LASIK for myopia: Effect on visual acuity, contrast sensitivity, and higher order aberrations. J Refract Surg. 2009;25:524–33. doi: 10.3928/1081597X-20090512-06. [DOI] [PubMed] [Google Scholar]
- 3.Teus MA, Arruabarrena C, Hernández-Verdejo JL, Cañones R, Mikropoulos DG. Ocular residual astigmatism's effect on high myopic astigmatism LASIK surgery. Eye (Lond) 2014;28:1014–9. doi: 10.1038/eye.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bragheeth MA, Dua HS. Effect of refractive and topographic astigmatic axis on LASIK correction of myopic astigmatism. J Refract Surg. 2005;21:269–75. doi: 10.3928/1081-597X-20050501-10. [DOI] [PubMed] [Google Scholar]
- 5.Qian Y-S, Huang J, Liu R, Chu R-Y, Xu Y, Zhou X-T, et al. Influence of internal optical astigmatism on the correction of myopic astigmatism by LASIK. J Refract Surg. 2011;27:863–8. doi: 10.3928/1081597X-20110629-01. [DOI] [PubMed] [Google Scholar]
- 6.Alfonso JF, Lisa C, Alfonso-Bartolozzi B, Pérez-Vives C, Montés-Micó R. Collagen copolymer toricphakic intraocular lens for myopic astigmatism: One-year follow-up. J Cataract Refract Surg. 2014;40:1155–62. doi: 10.1016/j.jcrs.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 7.Pothireddy R, Reddy KP, Senthil S, Rao HL. Posterior chamber toricphakic intraocular lenses for myopic astigmatism: First experience in India. J Cataract Refract Surg. 2012;38:1583–9. doi: 10.1016/j.jcrs.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 8.Sanders DR, Doney K, Poco M ICL in treatment of Myopia study group. United States food and drug administration clinical trial of the Implantable collamer lens (ICL) for moderate to high myopia: Three-year follow-up. Ophthalmology. 2004;111:1683–92. doi: 10.1016/j.ophtha.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 9.Heidary G, Ying G-S, Maguire MG, Young TL. The impact of sphere on cylinder type and severity in a high myopia cohort. Invest Ophthalmol Vis Sci. 2004;45:2747. [Google Scholar]
- 10.Staar Surgical [Internet] cited 2020 May 7. Available from: https://staar.com/news/2018/staar-surgical-announcesapproval-by-the-fda-of-the-visian-toric-icl-for-the-correction-ofmyopia-with-astigmatism .
- 11.Hyun J, Lim DH, Eo DR, Hwang S, Chung ES, Chung TY. A comparison of visual outcome and rotational stability of two types of toric implantable collamer lenses (TICL): V4 versus V4c. PLoS One. 2017;12:e0183335. doi: 10.1371/journal.pone.0183335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Miao H, Naidu RK, Wang X, Zhou X. Comparison of early changes in and factors affecting vault following posterior chamber phakic Implantable collamer Lens implantation without and with a central hole (ICL V4 and ICL V4c) BMC Ophthalmol. 2016;16:161. doi: 10.1186/s12886-016-0336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandey S, Sharma V. Commentary: Expanding indications of newer and economically viable phakic posterior chamber intraocular lens designs. Indian J Ophthalmol. 2019;67:1066–7. doi: 10.4103/ijo.IJO_173_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamiya K, Shimizu K, Aizawa D, Igarashi A, Komatsu M, Nakamura A. One-year follow-up of posterior chamber toricphakic intraocular lens implantation for moderate to high myopic astigmatism. Ophthalmology. 2010;117:2287–94. doi: 10.1016/j.ophtha.2010.03.054. [DOI] [PubMed] [Google Scholar]
- 15.Lee H, Kang S, Choi J, Ha B, Kim E, Seo KY, et al. Rotational stability and visual outcomes of V4c ToricPhakic intraocular lenses. J Refract Surg. 2018;34:489–96. doi: 10.3928/1081597X-20180521-01. [DOI] [PubMed] [Google Scholar]
- 16.Sanders DR, Schneider D, Martin R, Brown D, Dulaney D, Vukich J, et al. Toric implantable collamer lens for moderate to high myopic astigmatism. Ophthalmology. 2007;114:54–61. doi: 10.1016/j.ophtha.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 17.Schmidinger G, Lackner B, Pieh S, Skorpik C. Long-term changes in posterior chamber phakic intraocular collamer lens vaulting in myopic patients. Ophthalmology. 2010;117:1506–11. doi: 10.1016/j.ophtha.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Kojima T, Maeda M, Yoshida Y, Ito M, Nakamura T, Hara S, et al. Posterior chamber phakic implantable collamer lens: Changes in vault during 1 year. J Refract Surg. 2010;26:327–32. doi: 10.3928/1081597X-20090617-11. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-De la Rosa G, Olivo-Payne A, Serna-Ojeda JC, Salazar-Ramos MS, Lichtinger A, Gomez-Bastar A, et al. Anterior segment optical coherence tomography angle and vault analysis after toric and non-toric implantable collamer lens V4c implantation in patients with high myopia. Br J Ophthalmol. 2018;102:544–48. doi: 10.1136/bjophthalmol-2017-310518. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu K, Kamiya K, Igarashi A, Kobashi H. Long-term comparison of posterior chamber phakic intraocular lens with and without a central hole (Hole ICL and Conventional ICL) implantation for moderate to high myopia and myopic astigmatism: Consort-compliant article. Medicine (Baltimore) 2016;95:e3270. doi: 10.1097/MD.0000000000003270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sachdev GS, Singh S, Ramamurthy S, Rajpal N, Dandapani R. Comparative analysis of clinical outcomes between two types of posterior chamber phakic intraocular lenses for correction of myopia and myopic astigmatism. Indian J Ophthalmol. 2019;67:1061–5. doi: 10.4103/ijo.IJO_1501_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guber I, Mouvet V, Bergin C, Perritaz S, Othenin-Girard P, Majo F. Clinical outcomes and cataract formation rates in eyes 10 years after posterior phakic lens implantation for myopia. JAMA Ophthalmol. 2016;134:487–94. doi: 10.1001/jamaophthalmol.2016.0078. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Lopez F, Bilbao-Calabuig R, Mompean B, de Rojas V, Luezas J, Djodeyre MR, et al. Intraocular pressure during the early postoperative period after 100 consecutive implantations of posterior chamber phakic intraocular lenses with a central hole. J Cataract Refract Surg. 2013;39:1859–63. doi: 10.1016/j.jcrs.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 24.Higueras-Esteban A, Ortiz-Gomariz A, Gutiérrez-Ortega R, Villa-Collar C, Abad-Montes JP, Fernandes P, et al. Intraocular pressure after implantation of the Visian implantable collamer lens with centra FLOW without iridotomy. Am J Ophthalmol. 2013;156:800–5. doi: 10.1016/j.ajo.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 25.Park SC, Kwun YK, Chung ES, Ahn K, Chung TY. Postoperative astigmatism and axis stability after implantation of the STAAR toric implantable collamer Lens. J Refract Surg. 2009;25:403–9. doi: 10.3928/1081597X-20090422-01. [DOI] [PubMed] [Google Scholar]
- 26.Sheng XL, Rong WN, Jia Q, Liu YN, Zhuang WJ, Gu Q, et al. Outcomes and possible risk factors associated with axis alignment and rotational stability after implantation of the toric implantable collamer lens for high myopic astigmatism. Int J Ophthalmol. 2012;5:459–65. doi: 10.3980/j.issn.2222-3959.2012.04.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashemian SJ. Comparison of visual outcomes and complications of posterior chamber phakic intraocular lens with and without a central hole implantation for correction of high myopic astigmatism. J Eye Cataract Surg. 2018;4:56. [Google Scholar]
- 28.Kamiya K, Shimizu K, Igarashi A, Hikita F, Komatsu M. Four-year follow-up of posterior chamber phakic intraocular lens implantation for moderate to high myopia. Arch Ophthalmol. 2009;127:845–50. doi: 10.1001/archophthalmol.2009.67. [DOI] [PubMed] [Google Scholar]
- 29.Alfonso JF, Baamonde B, Fernández-Vega L, Fernandes P, González-Méijome JM, Montés-Micó R. Posterior chamber collagen copolymer phakic intraocular lenses to correct myopia: Five-year follow-up. J Cataract Refract Surg. 2011;37:873–80. doi: 10.1016/j.jcrs.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 30.Edelhauser HF, Sanders DR, Azar R, Lamielle H. Corneal endothelial assessment after ICL implantation. J Cataract Refract Surg. 2004;30:576–83. doi: 10.1016/j.jcrs.2003.09.047. [DOI] [PubMed] [Google Scholar]
- 31.Chung TY, Lee MO, Park SC, Ahn K, Chung ES. Changes in iridocorneal angle structure and trabecular pigmentation with STAAR implantable collamer lens during 2 years. J Refract Surg. 2009;25:251–8. doi: 10.3928/1081597X-20090301-03. [DOI] [PubMed] [Google Scholar]
- 32.Dejaco-Ruhswurm I, Scholz U, Pieh S, Hanselmayer G, Lackner B, Italon C, et al. Long-term endothelial changes in phakic eyes with posterior chamber intraocular lenses. J Cataract Refract Surg. 2002;28:1589–93. doi: 10.1016/s0886-3350(02)01210-5. [DOI] [PubMed] [Google Scholar]
- 33.Al Sabaani N, Behrens A, Jastanieah S, Al Malki S, Al Jindan M, Al Motowa S. Causes of phakic implantable collamer lens explantation/exchange at king Khaled eye specialist hospital. Middle East Afr J Ophthalmol. 2016;23:293–5. doi: 10.4103/0974-9233.194076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brar S, Ganesh S, Pandey R. Incidence and factors responsible for implantable collamer lens (ICL) explantation and outcomes of further management-5 year retrospective study. EC Ophthalmol. 2015;3:231–9. [Google Scholar]
- 35.Kaur M, Titiyal JS, Falera R, Sinha R, Sharma N. Indications for explant of implantable collamer lens. Eye (Lond) 2018;32:838–40. doi: 10.1038/eye.2017.307. [DOI] [PMC free article] [PubMed] [Google Scholar]


