Abstract
Recent advances in the diagnosis and treatment of ectatic corneal disease have mandated a more modern staging system. The new Belin ABCD keratoconus staging system incorporates anterior and posterior curvature centered on the thinnest point of the cornea, thinnest pachymetry values and distance visual acuity in grades from 0-4. By including posterior curvature and thickness measurements based on the thinnest point, as opposed to apical, the new staging system better reflects anatomical changes seen in keratoconus and other ectatic diseases.
Keywords: ABCD, classification, ectasia, keratoconus
Keratoconus (KC) was first described in 1854 as a chronic, non-inflammatory ectatic disorder of the cornea, characterized by steepening, apical thinning and scarring leading to visual distortion.[1,2,3] Initially annual incidence rates of 2 per 100,000 and prevalence 54.5 per 100,000 were reported.[4,5,6] Different geographic regions have reported greatly differing rates. However, with modern imaging these earlier incidence and prevalence rates have been shown to be significantly underestimated.
The advent of refractive surgery has underscored the need to identify early or subclinical cases of keratoconus, as these cases are more likely to present as post-refractive surgery ectasia. This has highlighted the deficiencies in the older classification/staging systems which simply relied on anterior curvature and apical thickness readings.
Amsler- Krumeich (AK) is the most widely used, albeit outdated, system for grading of keratoconus. The system was proposed by Marc Amsler in 1947 and was based on keratometry and optical pachymetry and as such measured only central anterior curvature and apical thickness. While it was useful when rigid gas permeable lenses and penetrating keratoplasty were the only modalities of treatment,[2,3,7,8,9] the AK system has limited clinical usefulness with modern imaging which allows us to diagnose the disease at a much earlier stage than recognized with the this classification system. Newer treatments, such as corneal collagen cross-linking benefits from earlier disease identification.
Despite its shortcomings, the diagnosis of keratoconus using placido-based corneal topography is still widely used. The major drawbacks of the placido-based imaging are; that it is limited to measuring only the anterior corneal surface, completely ignoring any posterior corneal pathology, has limited corneal coverage, and has a high false-positive rate. Tomographic devices such as rotating Scheimpflug devices or anterior segment ocular computerized tomography (OCT) are capable of imaging the entire anterior segment, including both anterior and posterior corneal surfaces. Scheimpflug devices are also capable of near limbus to limbus coverage.
Moderate to advanced keratoconus is easy to diagnose clinically due to the characteristic topography, distinctive clinical signs but the challenge lies in the diagnosis of early keratoconus with near-normal best spectacle-corrected visual acuity and no easily identified clinical features. This identification of early or subclinical keratoconus is especially important in screening candidates for refractive corneal surgeries, since keratorefractive surgical procedures may cause unpredictable refractive outcomes and lead to the development of post-refractive ectasia in susceptible individuals after surgery.
Other staging or classification systems for ectatic disease have been proposed, but the vast majority ignore changes on the posterior cornea and rely on apical readings and have limitations similar to AK system. Refractive screening revealed that early or subclinical disease could not only be diagnosed prior to visual loss but that its incidence was much higher than previously known. The second landmark event was the development of CXL. Rather than treating a significant irreversible visual loss, CXL offered the opportunity to pre-emptively slow or arrest disease progression, and, if offered earlier enough, could prevent consequent vision loss.[10] Refractive surgery, CXL and the availability of newer tomographic imaging further revealed the limitations of the older imaging and classifications systems, highlighting the need for a new classification system.
The Belin ABCD classification/staging system was introduced on the Oculus Pentacam (Oculus GmbH, Wetzlar, Germany) in response to both the shortcomings of the AK system and, in part, in response to the needs outlined in the Global Consensus on Keratoconus and Ectatic Disease.[11] While initially released as a new comprehensive staging or classification system, the ABCD parameters have also subsequently been utilized in a separate progression display (Belin ABCD Progression Display). Prior progression parameters were all based on the anterior corneal surface, which meant that loss of visual acuity had to be present and further loss was required to determine progressive disease. This is counterintuitive to how we normally practice medicine, where disease (or sequalae) prevention is preferable to managing disease complications.
The ABCD System of Classification
The new ABCD system utilizes 4 parameters:
Parameter “A”: Anterior Radius of Curvature in the 3.0 mm zone centered on the thinnest location of the cornea.
Parameter “B”: Posterior Radius of Curvature in the 3.0 mm zone centered on the thinnest location of the cornea
Parameter “C”: Thinnest pachymetry in μm
Parameter “D”: 'Distance Best Corrected Visual Acuity'. This parameter is not machine generated but must be entered by the user [Table 1].[8,9,12]
Table 1.
The New ABCD system for classification of keratoconus
| ABCD Criteria | A ARC (3 mm Zone) |
B PRC (3 mm Zone) |
C Thinnest Pach µm |
D BDVA |
Scarring |
|---|---|---|---|---|---|
| Stage 0 | >7.25 mm (<46.5 D) | >5.90 mm (<57.25 D) | >490 µm | =20/20 (=1.0) | - |
| Stage I | >7.05 mm (<48.0 D) | >5.70 mm (<59.25 D) | >450 µm | <20/20 (<1.0) | -, +, ++ |
| Stage II | >6.35 mm (<53.0 D) | >5.15 mm (<65.5 D) | >400 µm | <20/40 (<0.5) | -, +, ++ |
| Stage III | >6.15 mm (<55.0 D) | >4.95 mm (<68.5 D) | >300 µm | <20/100 (<0.2) | -, +, ++ |
| Stage IV | <6.15 mm (>55.0 D) | <4.95 mm (>68.5 D) | = 300 µm | <20/400 (<0.05) | -, +, ++ |
The Belin ABCD classification/staging is currently part of the Topometric/Keratoconus display. The four ABCD parameters are displayed both graphically, with the actual radius of curvature and pachymetry values, and in a classification in 5 steps ranging from zero to five. [Fig. 1].
Figure 1.
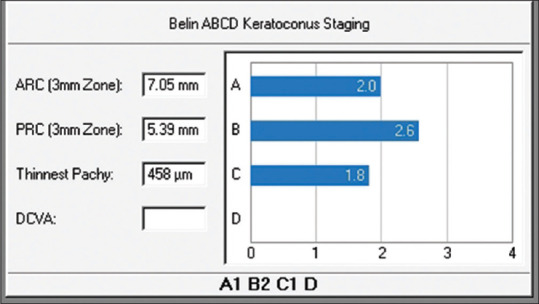
The four ABCD parameters displayed graphically
The advantages of the ABCD classification are that it describes each corneal layer independently, with its measurement centered on the thinnest point, which typically corresponds to the apex of the cone, and utilizes thinnest pachymetry as opposed to a central apical reading. In keratoconic eyes, differences between apical and thinnest point pachymetry readings can often exceed 100 microns.
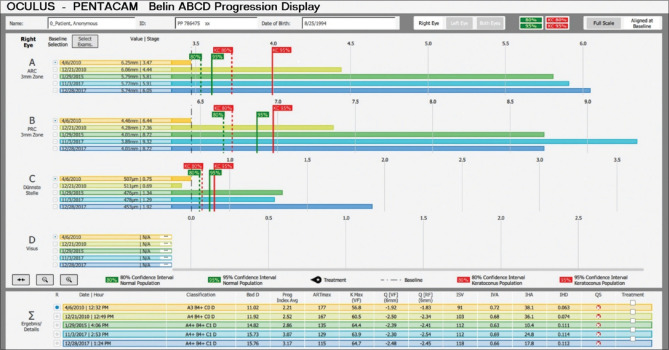
The Belin ABCD Progression Display was a further development of the ABCD staging parameters, allowing up to 8 exams to be displayed over time and examined for progressive change. Each ABCD parameter is individually displayed and one-sided confidence intervals (both 80% and 95%) for change are displayed both against a normal and keratoconic population. The normal based gates (shown in green) are suggested for young patients with early disease, while the keratoconic gates (shown in red) are meant for the more established cases [Fig. 2].
Figure 2.
Belin ABCD progression display- The normal based gates (shown in green) are suggested for young patients with early disease, while the keratoconic gates (shown in red) are meant for the more established cases
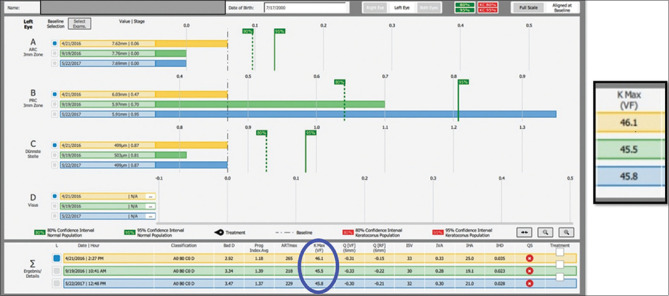
The clinical utility of the Belin ABCD Progression Display can be seen in Fig. 3, where highly significant change (progression) can be seen on the posterior corneal surface (”B” parameter) in spite of a stable anterior surface (”A” parameter) and a stable Kmax (circled in blue). The ABCD Progression Display allows the clinician a method of monitoring disease and the ability to diagnose progressive disease at a much earlier time frame than was previously possible with earlier systems limited to the anterior corneal surface, with the hope that earlier intervention can prevent visual loss, not just limiting loss after it has already occurred.
Figure 3.
Belin ABCD Progression Display showing highly significant change (progression) on the posterior corneal surface (”B” parameter) in spite of a stable anterior surface (”A” parameter) and a stable Kmax (circled in blue)
Keratoconus monitoring with the help of ABCD is proving to be an irreplaceable tool in the surgeon's armamentarium. Treatment recommendations, such as corneal cross-linking is based largely on progressive ectasia. The Global Consensus on Keratoconus and Ectatic Diseases (2015),[11] highlighted the need for better progression determinants and concluded that documented progression should require a consistent change in at least two of the following parameters:
Thinning of the cornea
Steepening of the anterior corneal curvature
Steepening of the posterior corneal curvature
An increase in the rate at which corneal thickness is changing from the periphery of the cornea to the thinnest point.
Repeatability of Pentacam
Repeatablility is the agreement in measurements taken by a single instrument and under the same conditions. The thinnest corneal thickness (TCT) and steepest keratometry values are especially important in the diagnosis of primary ectasia.[13] Hence good repeatability of these keratometric readings is important for the optimal management of keratoconus, identifying and assessing progression.[14,15]
Among the scheimpflug devices, Pentacam, Galilei, and Sirius have shown accuracy on repeated measurement of mean keratometry (Km), thinnest corneal thickness (TCT), anterior chamber depth (ACD), and mean posterior keratometry pKm.[16] Repeatability of the above-mentioned parameters is better on the Pentacam and Sirius than on Galilei. Bias in the agreement of pKm and ACD measurements is observed with all the three devices.[16] A wide 95% limits of agreement (LoA) amongst the three devices was reported in the study by Shetty et al., concludes that the three devices should not be used interchangeably for the detection of progression in keratoconus.[16]
A study by Meyer et al. assessing the repeatability and agreement of Orbscan II, Pentacam HR and Galilei tomography systems in corneas with keratoconus concluded that the Keratometric and pachymetric measurements obtained by Galilei, Pentacam, and Orbscan II were varied and independent of each other. The Orbscan II showed the least repeatability as compared to Pentacam HR and Galilei. Hence use of the Orbscan II, Pentacam HR and Galilei interchangeably may lead to inaccuracy in measurements.[17]
Hence Pentacam has shown to have the highest repeatability, and ABCD classification in pentacam has proven to be a great tool in assessing and monitoring keratoconus, but the drawback does exist that this new classification tool is limited to the pentacam and hence the availability of this device is a prerequisite to utilize this classification.
Conclusion
ABCD classification has superseded other classification systems and has proved to be of great value in assessing the course of various ectatic diseases and monitoring the treatment. By the inclusion of both anterior and the posterior surfaces and the pachymetry at the thinnest point rather than the apex, it has guided the clinician in treating in a more specific and personalized way. Effective application of the analysis of the ABCD classification in the surgical setup will help in refining surgical outcomes post cross-linking as well as post-refractive surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Nottingham J. London: J Churchill; 1854. Practical Observations on Conical Cornea: And On The Short Sight, and Other Fefects of Vision Connected with It. [Google Scholar]
- 2.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 3.Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthal. 1984;28:293–322. doi: 10.1016/0039-6257(84)90094-8. [DOI] [PubMed] [Google Scholar]
- 4.Gorskova EN, Sevost'ianov EN. [Epidemiology of keratoconus in the Urals] Vestn Oftalmol. 1998;114:38–40. [PubMed] [Google Scholar]
- 5.Jonas JB, Nangia V, Matin A, Kulkarni M, Bhojwani K. Preva- lence and associations of keratoconus in rural Maharashtra in central India: The central India eye and medical study. Am J Ophthalmol. 2009;148:760–5. doi: 10.1016/j.ajo.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101:267–73. doi: 10.1016/0002-9394(86)90817-2. [DOI] [PubMed] [Google Scholar]
- 7.Amsler M. Kératocõne classique et kératocône fruste; arguments unitaires. Ophthalmologica. 1946;111:96–101. doi: 10.1159/000300309. [DOI] [PubMed] [Google Scholar]
- 8.Belin MW, Duncan JK. Keratoconus: The ABCD grading system. Klin Monbl Augenheilkd. 2016;233:701–7. doi: 10.1055/s-0042-100626. [DOI] [PubMed] [Google Scholar]
- 9.Grisevic S, Gilevska F, Biscevic A, Ahmedbegovic-Pjano M, Bohac M, Pidro A. Keratoconus progression classification one year after performed crosslinking method based on ABCD keratoconus grading system. Acta Inform Med. 2020;28:18–23. doi: 10.5455/aim.2020.28.18-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamiya K, Ishii R, Shimizu K, Igarashi A. Evaluation of corneal elevation, pachymetry and keratometry in keratoconic eyes with respect to the stage of Amsler-Krumeich classification. Br J Ophthalmol. 2014;98:459–63. doi: 10.1136/bjophthalmol-2013-304132. [DOI] [PubMed] [Google Scholar]
- 11.Gomes JA, Tan D, Rapuano CJ, Belin MW, Ambrósio R, Jr, Guell JL, et al. Group of Panelists for the Global Delphi panel of Keratoconus and Ectatic diseases. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34:359–69. doi: 10.1097/ICO.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 12.Smolek MK, Klyce SD. Current keratoconus detection methods compared with a neural network approach. Invest Ophthalmol Vis Sci. 1997;38:2290–9. [PubMed] [Google Scholar]
- 13.Li Y, Meisler DM, Tang M, Lu ATH, Thakrar V, Reiser BJ, et al. Keratoconus diagnosis with optical coherence tomography pachymetry mapping. Ophthalmology. 2008;115:2159–66. doi: 10.1016/j.ophtha.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kymionis GD, Kontadakis GA, Kounis GA, Portaliou DM, Karavitaki AE, Magarakis M, et al. Simultaneous topography-guided PRK followed by corneal collagen cross- linking for keratoconus. J Refract Surg. 2009;25(suppl):807–11. doi: 10.3928/1081597X-20090813-09. [DOI] [PubMed] [Google Scholar]
- 15.Choi JA, Kim MS. Progression of keratoconus by longitudinal assessment with corneal topography. Invest Ophthalmol Vis Sci. 2012;53:927–35. doi: 10.1167/iovs.11-8118. [DOI] [PubMed] [Google Scholar]
- 16.Shetty R, Arora V, Jayadev C, Nuijts RM, Kumar M, Puttaiah NK, et al. Repeatability and agreement of three Scheimpflug-based imaging systems for measuring anterior segment parameters in keratoconus. Invest Ophthalmol Vis Sci. 2014;55:5263–8. doi: 10.1167/iovs.14-15055. [DOI] [PubMed] [Google Scholar]
- 17.Meyer JJ, Gokul A, Vellara HR, Prime Z, McGhee CNJ. Repeatability and agreement of Orbscan II, Pentacam HR and Galilei tomography systems in corneas with keratoconus. Am J Ophthalmol. 2017;175:122–8. doi: 10.1016/j.ajo.2016.12.003. [DOI] [PubMed] [Google Scholar]