Abstract
Purpose:
Sterile infiltrates following laser refractive surgery is an uncommon complication. This study was undertaken to analyze the visual outcomes of sterile infiltrates following photorefractive keratectomy (PRK).
Methods:
This retrospective study included 14 eyes that developed sterile infiltrates following PRK out of a total of 6280 eyes that underwent PRK between 2014 and 2017. Medical records of these patients, including patient demographics, characteristics of the infiltrate, presenting visual acuity, and treatment outcomes were recorded and analyzed.
Results:
The incidence of sterile corneal infiltrates post-PRK in our study was 0.22% (14/6280). The mean age of the patients was 27.42 ± 4.87 years. The uncorrected visual acuity (UCVA) at presentation was 0.49 ± 0.13 log MAR units. The mean size of the infiltrate was 3.22 ± 2.85 mm2. All cases were successfully managed medically with topical steroids. The mean UCVA and best-corrected visual acuity (BCVA) at the last follow-up visit were 0.08 ± 0.08 and 0.05 ± 0.07 log MAR units, respectively. The mean time taken for resolution of the infiltrate was 8.91 ± 4.57 days.
Conclusion:
Sterile infiltrates following PRK can be effectively treated with aggressive topical steroids. The outcome is generally favorable and does not require surgical intervention if treatment is instituted early.
Keywords: Photorefractive keratectomy, sterile corneal infiltrate, visual outcomes
Photorefractive keratectomy (PRK) is the oldest excimer laser-based technique used to alter the refractive power of the cornea.[1] The technique involves the removal of the central corneal epithelium, following which excimer laser ablation is done. The epithelium typically heals within a few days.[2,3,4] PRK for myopia is an effective procedure, which has not lost its popularity, among refractive surgeons, despite the advent of laser in-situ keratomileusis (LASIK) and small incision lenticule extraction (SMILE). It avoids the production of a corneal flap and hence eliminates potential flap-related complications, thereby yielding safer and more predictable results.[5,6,7] Though a safe surgery, numerous complications have been reported following PRK including corneal haze, overcorrection, under-correction, scarring, regression, and corneal infiltrates.[8,9,10] Corneal infiltrates following PRK can either be sterile or infective and as such, this is an uncommon complication, with few reports in the literature.[11,12,13,14,15]
The purpose of this study is to analyze the visual outcomes following successful treatment of sterile corneal infiltrates after PRK with topical steroids.
Methods
The study was approved by the Institutional Review Board and followed the tenets of the Declaration of Helsinki. Records of all 6280 patients who underwent PRK for myopia and myopic astigmatism between 2014 and 2017 were reviewed retrospectively and 14 cases with sterile corneal infiltrate were included in the study.
Surgical technique
All surgeries were performed under topical anesthesia. Using 0.5% proparacaine hydrochloride on the cornea using a PRK well, the corneal epithelium was loosened and removed using a hockey stick knife. Thereafter, ablation was performed using the excimer laser. All cases had an ablation zone of 6.5 mm. Topical 0.02% mitomycin C was applied with help of Merocel Sponge after ablation followed by a thorough wash. After the surgery, a bandage contact lens (BCL) was placed.
Postoperative regimen
The postoperative topical regimen included topical 0.5% moxifloxacin (Vigamox; Alcon Laboratories, Inc., Fort Worth, TX), 0.1% nepafenac ophthalmic suspension (Nevanac; Alcon Laboratories, Inc., Fort Worth, TX) four times a day for both eyes in each eye and preservative-free artificial tears. Topical steroids were started after 3 days of surgery following BCL removal.
The patients who presented with infiltrate in the cornea after PRK without or with pain, redness, watering, and diminution of vision after the surgery were included in the study. The infiltrate was mostly peripheral, single or multiple, circumferentially oriented, crescent-shaped, with a late epithelial breakdown and a clear zone of demarcation was present between the infiltrate and the limbus. The BCL was removed and sent for culture on chocolate agar. Corneal scraping was obtained in all cases from the site of the infiltrate and subjected to standard microbiological investigation. This included Gram's stain, 10% KOH with 0.1% calcofluor white (KOH + CFW) wet mount, and culture on blood agar, chocolate agar, brain heart infusion broth, thioglycollate broth, Sabouraud dextrose agar, and potato dextrose agar. A presumptive diagnosis of sterile infiltrate was made clinically complemented with no detection of any organism on smear examination. The diagnosis was confirmed after no significant growth was found on any culture medium until 2 weeks. The same culture media were observed for any growth weekly thereafter until the end of 1-month duration. The following data were retrieved: age, sex, the time interval between surgery and presentation, visual acuity at presentation and at last visit, location and size of the infiltrate, and time taken for complete resolution. The infiltrate was defined as central if any part of it involved the central 3 mm area of the cornea.
Statistical analysis
All data were entered into Microsoft Excel (2016) and analyzed using R version 16.14. Continuous variables were analyzed using the Kruskal Wallis test. Before and after comparison within the same group was analyzed using the Wilcoxon signed-rank test. A P value of less than 0.05 was considered significant.[16,17]
Results
A total of 14 eyes of 12 patients were included in the study. The incidence of sterile corneal infiltrates post-PRK in our study was 0.22% (14/6280). The mean age of the patients was 27.42 ± 4.87 years. Nine patients (75%) were females and three patients (25%) were males. The mean follow-up period was 64.86 ± 60.36 days. All patients presented with corneal infiltrates within 1 week of surgery. The mean interval between surgery and presentation was 3 ± 1.62 days. The mean uncorrected visual acuity (UCVA) at presentation was 0.49 ± 0.13 logMAR units. The mean pinhole-corrected visual acuity (PHVA) was 0.19 ± 0.13 logMAR units [Table 1]. The infiltrate was located centrally in 1 eye and peripherally in 13 eyes. The mean size of the infiltrate was 3.22 ± 2.85 mm2 [Table 2].
Table 1.
Demography and baseline parameters
| Parameter | Value |
|---|---|
| Age (mean±SD) (years) | 27.42±4.87 |
| Sex | |
| Female n (%) | 9 (75%) |
| Male n (%) | 3 (25%) |
| Time of presentation following surgery (mean±SD) (days) | 3±1.62 |
| UCVA at presentation (mean±SD) (logMAR) | 0.49±0.13 |
| PHVA at presentation (mean±SD) (logMAR) | 0.19±0.13 |
| Blepharitis n (%) | |
| Yes | 6 (42.9) |
| No | 8 (51.7) |
SD=Standard deviation, UCVA=Uncorrected visual acuity, PHVA=Pinhole visual acuity
Table 2.
Characteristics of infiltrate
| Parameter | Value |
|---|---|
| Location of infiltrate | |
| Peripheral n (%) | 13 (92.86%) |
| Central n (%) | 1 (7.14%) |
| Size of infiltrate (mean±SD) (mm) | 2.07±2.65 |
The treatment prescribed included topical 0.1% fluorometholone eye drops (FML; Allergan, Irvine, CA, USA) in five eyes, 1% prednisolone acetate ophthalmic suspension (Pred Forte; Allergan, Inc., Irvine, CA, USA) in five eyes, and 0.5% loteprednol etabonate ophthalmic suspension (Lotepred; Sun Pharma, India) in four eyes. Topical steroids were given every 2 h in initial days and tapered on weekly basis once infiltrate start resolving and scarring is evident. Excluding one patient who did not come for follow-up, resolution of the infiltrate was seen in all eyes, with a residual scar. Eight eyes had macular grade corneal scar and six patients had nebular grade scars on the resolution of the infiltrate. The central sterile infiltrate in one patient was resolved with macular grade scarring with vision 20/30p but the patient was comfortable with binocular vision. The mean time taken for resolution of the infiltrate was 8.91 ± 4.57 days. The mean UCVA and best-corrected visual acuity (BCVA) at the last follow-up visit were 0.08 ± 0.08 and 0.05 ± 0.07 logMAR units, respectively [Table 3]. There was no significant difference in the outcome parameters such as time for resolution, UCVA, BCVA, and improvement in UCVA between eyes treated with topical fluorometholone, prednisolone, and loteprednol eye drops [Table 4].
Table 3.
Outcome parameters
| Parameter | Value |
|---|---|
| Follow-up period (mean±SD) (days) | 64.86±60.36 |
| Time taken for resolution (mean±SD) (days) | 8.91±4.57 |
| UCVA at last visit (mean±SD) (logMAR) | 0.08±0.08 |
| BCVA at last visit (mean±SD) (logMAR) | 0.05±0.07 |
| Scar | |
| Yes n (%) | 7 (50) |
| No n (%) | 7 (50) |
UCVA=Uncorrected visual acuity, BCVA=Best-corrected visual acuity
Table 4.
Comparison between fluorometholone, prednisolone, and loteprednol groups
| Parameter (Mean±SD) | Fluorometholone | Prednisolone | Loteprednol | P |
|---|---|---|---|---|
| Age | 30.2±6.02 | 24.6±1.52 | 26.5±3.32 | 0.18 |
| Day of presentation after surgery | 3.4±1.14 | 1.8±1.1 | 4±2 | 0.084 |
| UCVA at presentation | 0.42±0.13 | 0.5±0.16 | 0.43±0.13 | 0.628 |
| PHVA at presentation | 0.2±0.12 | 0.18±0.15 | 0.18±0.1 | 0.962 |
| UCVA at last visit | 0.05±0.06 | 0.14±0.09 | 0.05±0.06 | 0.173 |
| BCVA at last visit | 0.05±0.06 | 0.08±0.08 | 0.05±0.06 | 0.809 |
| Time for resolution (days) | 6.75±1.5 | 12±5.57 | 8.75±5.56 | 0.303 |
| UCVA improvement (%) | 91.67±11.79 | 72.48±20.17 | 87.5±15.96 | 0.261 |
UCVA=Uncorrected visual acuity, PHVA=Pinhole visual acuity, BCVA=Best-corrected visual acuity
Discussion
Numerous theories have been proposed regarding the pathogenesis of sterile corneal infiltrate after PRK, chief amongst those being the use of non-steroidal anti-inflammatory drugs postoperatively (NSAIDs). Teal et al. were the first to report the development of sterile infiltrates in patients following PRK, and they attributed it to the use of NSAIDs with or without a contact lens instead of the then conventional post-operative practice of bandage occlusion.[10] These drugs inhibit the cyclooxygenase pathway in the metabolism of arachidonic acid, thereby increasing the production of leukotrienes and hydroxyeicosatetraenoic acid from the lipoxygenase-mediated alternate pathway. These molecules are potent chemoattractants which result in the accumulation of inflammatory cells causing infiltrates.[18,19] However, the development of such infiltrates in patients following LASIK, as well as in patients who did not receive NSAIDs following PRK, does not support this theory.[13,20]
In 1996, Teichmann et al. hypothesized that corneal infiltrates occurred as a result of the response of the circulating antibodies to the corneal heat-shock proteins (HSPs), produced after excimer laser use. This hypothesis was strengthened by reports of sterile infiltrates occurring after LASIK, PRK, phototherapeutic keratectomy (PTK), and laser-assisted subepithelial keratectomy (LASEK).[10,13,18,21,22,23] However, the absence of corneal infiltrates in a vast majority of patients who undergo excimer laser ablation, coupled with the development of sterile infiltrates in two brothers after PRK, as reported by Al-Muammar et al. may indicate a genetic predisposition in certain individuals who are more prone to develop antibodies against these HSPs.[12]
Other theories include an immunological reaction to increased meibomian gland secretions and bacterial toxins into the conjunctival cul-de-sac, released as a result of lid margin manipulation, compromise in ocular flora due to this immunological reaction, and contact lens-induced hypoxia.[20,24] Female preponderance in our study supports the theory of immunological reaction as it is more common in females. We could not assess the role played by blepharitis in the pathogenesis of this entity, though six eyes had blepharitis before the surgery. While the importance of blepharitis still remains to be proven, contact lens-induced hypoxia, as a possible cause can be ruled out owing to the development of corneal infiltration even without the use of contact lens.[13,20]
In our study, the infiltrate was located peripherally in most of the eyes (13 eyes—92.86%). Angunawela et al. postulated that the infiltrates are a result of an immunological reaction against staphylococcal antigens, which tend to get deposited in high concentrations, within areas of tear pooling, such as the periphery of the cornea in contact lens wearers.[25] However, though this may explain the predominant peripheral location of these infiltrates, it does not explain their occurrence in noncontact lens wearers.[13,20]
The infiltrates resolved in all eyes, leaving a residual scar [Fig. 1]. Most of the scars were peripheral except one which involved a small part of the central pupillary area. All the infiltrates involved the anterior one-third of the cornea. In no patient, did the corneal opacity require surgical intervention. Teal et al. found that the resolution of sterile corneal infiltrate in 17 cases has generally left the patient with permanent corneal scarring and a one to a two-line reduction in visual acuity regardless of treatment.[10] Mohammed A Al-Amry reported a case of the clinical presentation and management of a 47-year-old male myope who underwent PRK and developed bilateral sterile corneal infiltrates at 1 day postoperatively. The patient was successfully treated with aggressive topical antibiotic and topical steroid therapy. The final BCVA was 20/25 with faint corneal scarring.[11]
Figure 1.
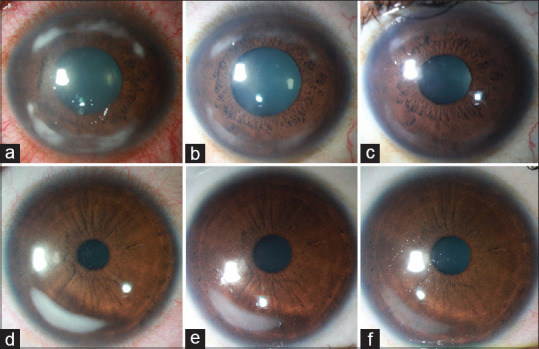
Diffuse illumination slit lamp serial photographs of two patients. (a) Crescentic peripheral corneal infiltrates with severe conjunctival congestion at presentation. (b) Subsidence of congestion with a reduction in the size of the infiltrates 3 days after starting topical steroids. (c) Resolution of the infiltrate with scarring 7 days after initiation of treatment. (d) Single foci of infiltrate with mild congestion at the presentation of another patient. (e) Improvement 3 days after the starting of intensive topical steroids. (f) Resolution of infiltrate with residual peripheral scarring
Abdulrahman Al-Muammar reported bilateral peripheral corneal infiltrate developed on the third postoperative day in two brothers who underwent bilateral photorefractive keratectomy. The patients were treated with antibiotics and low concentration steroids until the negative culture was reported 48 h later when intensive topical steroids were started. The infiltrate resolved by day 10 with a residual subepithelial peripheral corneal haze that was apparent 8 months after surgery with unaided visual acuity of 20/20 in both the eyes.[12] Tova Lifshitz et al. reported five eyes of peripheral sterile corneal infiltrates after refractive surgery. All cases improved after several days of topical steroid and antibiotic treatment and systemic steroid. Final visual acuity was 20/25 or better in all cases.[13] Mounir et al. reported the occurrence of sterile infiltrates in three eyes of two patients following simultaneous PRK and collagen cross-linking. One eye received a lower potency steroid as compared to the other eyes, resulting in a large central scar, which necessitated penetrating keratoplasty.[14]
Rao et al. reported painless inferior subepithelial infiltrates away from the site of ablation in both eyes after excimer laser PRK for myopia in a 26-year-old man. Clinical characteristics of the corneal infiltrates resembled staphylococcal-immune infiltrates. The condition responded to treatment with topical diluted steroids and antibiotics. There was no residual corneal scarring. The infiltrates resolved with UCVA of 20/30 in the right eye and 20/20 in the left eye.[20] Mohammad-Ali Javadi and Sepehr Feizi reported a case of severe sterile keratitis and corneal scar after collagen crosslinking in keratoconic eye necessitating corneal transplantation (deep anterior lamellar keratoplasty) after which vision improved from 20/400 to BCVA of 20/30.[26]
The mean uncorrected visual acuity at presentation was 0.49 ± 0.13 logMAR units which improved to 0.08 ± 0.08 at the last visit. Though we had an overall favorable visual outcome, the visual recovery in such cases is variable, due to the presence of a residual scar in many cases.[11,12,13,14,15] The BCVA at the last visit was 0.05 ± 0.07 logMAR. In the absence of the data on pre-treatment BCVA, we could not analyze the difference between the BCVA at the presentation of infiltrate and at the last follow-up visit after resolution.
In conclusion, the development of sterile infiltrates following PRK, though not common, is not an exceedingly rare phenomenon. It is important to differentiate this entity from post-refractive infectious keratitis, as the management for these two conditions is very contrasting. The peripheral location of these infiltrates, usually between first to the fourth postoperative day, anterior stromal involvement, and diffuse inflammation, without an identifiable focus of infection, aids in the diagnosis. Corneal scraping is advisable for ruling out infectious etiology. However, in scenarios where scraping is not possible, more frequent follow-up visits with a high degree of suspicion for infective keratitis is advocated. Early aggressive treatment with steroids results in a good outcome, limiting the development of a residual scar.
The limitations of this study are its retrospective nature and inability to assess the risk factors involved in the pathogenesis of this condition. Also, the sample size for comparison of outcomes between the fluorometholone, prednisolone, and loteprednol groups was inadequate, which could, in part, explain why there was no significant difference between these groups. An objective analysis of residual scar formation was not done which could have helped in evaluating the effect of the scar in more detail. Post-PRK, corneal lesions after healing may cause a decrease in visual acuity and even higher-order aberrations (HOA) can occur. We have preoperative and postoperative data of corneal HOAs. But, curvature changes and HOAs can occur because of PRK surgery as well as because of a residual scar after the resolution of sterile corneal infiltrates after PRK, and differentiation between both was not possible as there was no control group in our study to compare. This can also be considered as one of the limitations of our study.
There is limited literature on the occurrence of sterile infiltrates after PRK, and to the best of our knowledge, this is the largest case series including such patients.[11,12,13,14]
Conclusion
Our study indicates the visual outcome of patients developing sterile infiltrate after PRK is good. As most of these patients have infiltrates in the periphery of the cornea and resolution takes place with scar formation in the periphery, the visual acuity of these patients remains good in most of the cases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Jesper Hjortdal. Surface Ablation Techniques for Myopia – A Review of the Advances Over the Past 25 Years. European Ophthalmic Review. 2017;11:31–4. [Google Scholar]
- 2.Lindstrom RL, Sher NA, Chen V, Bowers RA, Frantz JM, Brown DC, et al. Use of the 193-NM excimer laser for myopic photorefractive keratectomy in sighted eyes: A multicenter study. Trans Am Ophthalmol Soc. 1991;89:155–82. [PMC free article] [PubMed] [Google Scholar]
- 3.Seiler T, Wollensak J. Myopic photorefractive keratectomy with the excimer laser: One-year follow-up. Ophthalmology. 1991;98:1156–63. doi: 10.1016/s0161-6420(91)32157-2. [DOI] [PubMed] [Google Scholar]
- 4.Dausch D, Klein R, Schröder E. Photoablative, refractive keratectomy in treatment of myopia. A case study of 134 myopic eyes with 6-months follow-up. Fortschr Ophthalmol. 1991;88:770–6. [PubMed] [Google Scholar]
- 5.McAlinden C. Corneal refractive surgery: Past to present. Clin Exp Optom. 2012;95:386–98. doi: 10.1111/j.1444-0938.2012.00761.x. [DOI] [PubMed] [Google Scholar]
- 6.Shojaei A, Mohammad-Rabei H, Eslani M, Elahi B, Noorizadeh F. Long-term evaluation of complications and results of photorefractive keratectomy in myopia: An 8-year follow-up. Cornea. 2009;28:304–10. doi: 10.1097/ICO.0b013e3181896767. [DOI] [PubMed] [Google Scholar]
- 7.Wen D, McAlinden C, Flitcroft I, Tu R, Wang Q, Alió J, et al. Postoperative efficacy, predictability, safety, and visual quality of laser corneal refractive surgery: A network meta-analysis. Am J Ophthalmol. 2017;178:65–78. doi: 10.1016/j.ajo.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Diakonis VF, Kankariya VP, Kymionis GD, Kounis G, Kontadakis G, Gkenos E, et al. Long term followup of photorefractive keratectomy with adjuvant use of mitomycin C? J Ophthalmol. 2014;2014:821920. doi: 10.1155/2014/821920. doi: 10.1155/2014/821920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seiler T, Holschbach A, Derse M, Jean B, Genth U. Complications of myopic photorefractive keratectomy with the excimer laser. Ophthalmology. 1994;101:153–60. doi: 10.1016/s0161-6420(94)31371-6. [DOI] [PubMed] [Google Scholar]
- 10.Teal P, Breslin C, Arshinoff S, Edmison D. Corneal subepithelial infiltrates following excimer laser photorefractive keratectomy. J Cataract Refract Surg. 1995;21:516–8. doi: 10.1016/s0886-3350(13)80208-8. [DOI] [PubMed] [Google Scholar]
- 11.Al-Amry MA. Severe bilateral paralimbal sterile infiltrates after photorefractive keratectomy. Middle East Afr J Ophthalmol. 2014;21:83–5. doi: 10.4103/0974-9233.124114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Muammar A. Peripheral sterile corneal infiltrate in two brothers after photorefractive keratectomy. Saudi J Ophthalmol. 2011;25:305–8. doi: 10.1016/j.sjopt.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lifshitz T, Levy J, Mahler O, Levinger S. Peripheral sterile corneal infiltrates after refractive surgery. J Cataract Refract Surg. 2005;31:1392–5. doi: 10.1016/j.jcrs.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 14.Mounir A, Anbar M, Radwan G. Sterile corneal infiltrates after simultaneous photorefractive keratectomy and corneal crosslinking. JCRS Online Case Rep. 2017;5:46–8. [Google Scholar]
- 15.Soltani Moghadam R, Rahimi F, Fard MA, Esfandiarpour B, Medghalchi A. Bilateral ring-shaped marginal keratitis after photorefractive keratectomy: A case report. Iran J Ophthalmol. 2011;3:71–4. [Google Scholar]
- 16.Stephanie Glen. “Kruskal Wallis H Test: Definition, Examples & Assumptions” From StatisticsHowTocom: Elementary Statistics for the rest of us! https://wwwstatisticshowtocom/kruskal-wallis/
- 17.Winters R, Winters A, Amedee RG. Statistics: A brief overview. Ochsner J. 2010;10:213–6. [PMC free article] [PubMed] [Google Scholar]
- 18.Lahners WJ, Hardten DR, Lindstrom RL. Peripheral keratitis following laser in situ keratomileusis. J Refract Surg. 2003;19:671–5. doi: 10.3928/1081-597X-20031101-10. [DOI] [PubMed] [Google Scholar]
- 19.Ku EC, Lee W, Kothari HV, Scholer DW. Effect of diclofenac sodium on the arachidonic acid cascade. Am J Med. 1986 Apr 28;80(4B):18–23. doi: 10.1016/0002-9343(86)90074-4. doi: 10.1016/0002-9343(86)90074-4. PMID: 3085488. [DOI] [PubMed] [Google Scholar]
- 20.Rao SK, Fogla R, Rajagopal R, Sitalakshmi G, Padmanabhan P. Bilateral corneal infiltrates after excimer laser photorefractive keratectomy. J Cataract Refract Surg. 2000;26:456–9. doi: 10.1016/s0886-3350(99)00348-x. [DOI] [PubMed] [Google Scholar]
- 21.Teichmann KD, Cameron J, Huaman A, Rahi AH, Badr I. Wessely-type immune ring following phototherapeutic keratectomy. J Cataract Refract Surg. 1996;22:142–6. doi: 10.1016/s0886-3350(96)80284-7. [DOI] [PubMed] [Google Scholar]
- 22.Yu EYW, Rao SK, Cheng ACK, Law RWK, Leung ATS, Lam DSC. Bilateral peripheral corneal infiltrates after simultaneous myopic laser in situ keratomileusis. J Cataract Refract Surg. 2002;28:891–4. doi: 10.1016/s0886-3350(01)01095-1. [DOI] [PubMed] [Google Scholar]
- 23.Ambrósio R, Periman LM, Netto MV, Wilson SE. Bilateral marginal sterile infiltrates and diffuse lamellar keratitis after laser in situ keratomileusis. J Refract Surg. 2003;19:154–8. doi: 10.3928/1081-597X-20030301-11. [DOI] [PubMed] [Google Scholar]
- 24.Donshik PC, Suchecki JK, Ehlers WH. Peripheral corneal infiltrates associated with contact lens wear. Trans Am Ophthalmol Soc. 1995;93:49–64. [PMC free article] [PubMed] [Google Scholar]
- 25.Angunawela RI, Arnalich-Montiel F, Allan BDS. Peripheral sterile corneal infiltrates and melting after collagen crosslinking for keratoconus. J Cataract Refract Surg. 2009;35:606–7. doi: 10.1016/j.jcrs.2008.11.050. [DOI] [PubMed] [Google Scholar]
- 26.Javadi M-A, Feizi S. Sterile keratitis following collagen crosslinking. J Ophthalmic Vis Res. 2014;9:510–3. doi: 10.4103/2008-322X.150832. [DOI] [PMC free article] [PubMed] [Google Scholar]


