Abstract
Dry eye disease (DED) is a condition that is fast reaching epidemic proportions around the world. Dry eye post-refractive surgery is the leading cause of iatrogenically induced DED. The wide variety of presentations and the disparity between signs and symptoms in many patients make this a very challenging aspect of our clinical practice. There has been a paradigm shift in the way we approach and treat this condition. The International Dry eye workshop has added new knowledge and focus to our management of dry eye. A wide range of newer diagnostic modalities are available for the diagnosis of DED. Dry eye is one of the most common side effects of refractive surgery and can have a bearing the patient's perception of surgical outcomes as well. A thorough understanding of the possible underlying etiopathologies of this disease and the difference in etiopathogenesis of postrefractive dry eye is essential for optimal outcomes. It is important to approach each case in a unique fashion and customize the therapy to the patient presentation. This review article compiles all these aspects of management of dry eye in general, and postrefractive surgery dry eye in particular; from the ones commonly practiced in the clinic to the newer modalities of therapy with insights into the disease from a more practical point of view.
Keywords: Algorithm, dry eye, management, post refractive
Dry eye disease (DED) is one of the leading causes of ocular morbidity in the world with increasing number of people suffering from dry eye-related complaints.[1] It is known to have a variety of presenting symptoms and signs with underlying ocular surface pathologies, hence it's diagnosis and management can be challenging for the treating ophthalmologist.[2] DED can also be iatrogenic, secondary to medical and surgical interventions like systemic or topical medications, contact lens usage, and postsurgery including refractive surgery.[3] Refractive surgery has gained immense popularity world-wide, with more than 16 million procedures performed globally,[4,5] and dry eye is one of the most commonly reported side effect of these procedures.[6,7] Dry eye postrefractive surgery can also impact the postoperative quality of vision and quality of life[4] and therefore needs particular attention and care.[8,9]
The 2017 International Dry Eye Workshop II (DEWS II) report also defined dry eye as a multifactorial disease and highlighted the importance of ocular surface inflammation, neurosensory abnormalities and loss of homeostasis in this condition.[10] An important addition in this report TFOS DEWS II is the concept of neuropathic pain and its proposed management.[3] Dry eye after refractive surgery can be due to the general underlying problems like tear insufficiency and aqueous deficiency, meibomian gland dysfunction, or secondary to transection of corneal nerves and other specific anatomical and physiological ocular changes during the procedure.[11,12]
The identification of the underlying etiopathogenesis is integral to the management of DED in general and helps us customize primary treatment strategies. This review article aims at highlighting the methodological evaluation of DED especially related to refractive surgery and outlines a protocol based customized management.
Etiopathogenesis of Post-Refractive Surgery DED
The DED seen post-refractive surgery has multiple proposed mechanisms.
Denervation of the cornea leading to decreased corneal sensitivity
The flap creation and excimer photoablation in laser in situ keratomileusis (LASIK) and the femtosecond photo-disruption of stroma with manual extraction of the intrastromal lenticule in small incision lenticule extraction (SMILE) results in the temporary partial denervation of the cornea.[11] Photorefractive keratectomy (PRK) involves excimer photoablation of the cornea without flap creation and hence there can be some damage to the subbasal plexus of corneal nerves but less to the deeper stromal nerves than that seen in LASIK.[13] Due to the damage to corneal nerves secondary to these procedures, there is a decrease in corneal sensitivity and reduction in reflex tear secretion. This is one of the main mechanisms for DED post-refractive surgery.[11,14] The vertical side cut of the cornea in SMILE is shorter than that in a LASIK flap, thus there may be less disruption of the normal corneal nerve anatomy [Fig. 1a and b]. This has been evaluated in a number of studies with some reporting less corneal nerve damage and dry eye in SMILE than LASIK[15] and no significant difference in others.[16,17] Moreover there was no significant difference in corneal reinnervation and sensitivity between SMILE and LASIK at 6 months postoperatively.[18] Even in PRK there have been differing results with some studies claiming less reduction in tear secretion, corneal sensitivity and decreased DED in PRK compared to LASIK.[13,19,20] which was not proved in other studies.[21,22] The location of the hinge in LASIK, is another important point which is said to have a bearing on the dry eye after refractive surgery. Feng YF et al. in their metanalysis stated that a horizontal hinge led to less corneal sensory disturbance and dry eye as compared to a vertical location in early postoperative period, however, there was no statistically significant difference between the two groups at the end of 6 months post-surgery.[23]
Figure 1.

(a) Diagrammatic representation of the normal corneal nerve anatomy with distribution at various levels. (b) Diagrammatic representation of differences in nerve transection in LASIK v/s SMILE
These changes to the corneal nerves can be studied in the eye by in vivo confocal microscopy (IVCM). This technique can be used to demonstrate the transection of corneal nerves post-refractive surgery and serial imaging can also demonstrate the slow regeneration of nerve fibers, which usually takes 3–6 months.[14] However, in some patients the nerve fiber regeneration can be significantly lower even 1-year post-surgery.[24] Tests using the Cochet-Bonnet esthesiometer to check corneal sensitivity show that corneal sensation recovers to pre-operative levels by around 6 months post-surgery. However, some patients do experience the symptoms for a longer duration.[13]
Reduced blink rate
The decreased corneal sensitivity may affect the corneal-blink reflex-lacrimal gland pathway resulting in a reduced blink rate and decreased reflex aqueous tear secretion.[11] Since blinking contributes to the expression of meibum from the meibomian glands (MG) in the eye lids, the lipid layer secretion is also dependent on the blink. Reduction in the blink rate and an incomplete blink may result in subsequent reduction in the MG lipid secretion and evaporative DED.[24]
Corneal curvature change associated tear film instability
Changes in corneal curvature may alter the friction encountered between the cornea and lid, leading to an unstable tear film and dry eye.[25]
Mucin associated tear film instability
The pressure exerted due to the suction applied during the refractive surgery can damage conjunctival goblet cells resulting in reduced mucin secretion.[26] In addition, the corneal nerve disruption alters the membrane-associated mucin expression on the epithelium, leading to an unstable tear film.[27]
Pain without stain
Postrefractive dry eye can also be due to neuropathic corneal pain (NCP) which is also termed as corneal allodynia, corneal neuralgia, corneal neuropathy or keratoneuralgia.[28] This has now been included in the TFOS DEWS II report as a separate condition needing special care.[3] The symptoms of NCP and DED can overlap with patients complaining of pain, discomfort, burning, irritation and grittiness in the eyes.[1] It can be triggered by an ocular surgery or infection in some cases or be related to non-ocular causes, neurological, or psychiatric conditions.[29] Corneal refractive surgery like LASIK, SMILE or PRK can be a cause for neuropathic pain. One classical feature is the disproportionately increased symptoms as compared to signs.
Various possible etiopathogenesis of post-refractive DED have been summarized in Fig. 2. In addition, patients may also suffer from aqueous deficiency and evaporative dry eye not related to the refractive surgery. A routine screening for DED is important before planning refractive surgery on a patient as these patients can have a significant worsening of the disease postoperatively if undiagnosed and not managed adequately. Patients are screened for DED as per normal protocol DED in the out-patient department. There are no additional special tests for checking dry eye before refractive surgery.
Figure 2.
Flow chart of the probable pathophysiology of post-refractive surgery DED
Preoperative Evaluation for DED in Patients Undergoing Refractive Surgery
History and slit-lamp examination
A detailed history preoperatively is essential and should include history of contact lens intolerance, allergy, medication use, previous chemical injury, long-term use of antiglaucoma medications and associated systemic diseases like rheumatoid arthritis, Sjogren's syndrome, rosacea, systemic lupus erythematosus.[30] If suspecting underlying systemic disease, a timely referral to an immunologist/rheumatologist will help in the management of the systemic condition and better management of the DED. Overlapping demographics of patients opting for a refractive surgery and those suffering from the above-mentioned conditions emphasizes the need for careful history taking and evaluation.[31,32]
Patient questionnaires
Validated questionnaires like the Ocular Surface Disease Index (OSDI), Dry Eye Questionnaire (DEQ-5) and Impact of Dry Eye on Everyday Living (IDEEL) are useful to preoperatively pick up symptomatology suggestive of dry eye.[33,34]
Examination – ocular surface assessment
Evaluation of the face, eyelids including meibomian glands, blink patterns, tear film, conjunctiva and cornea are essential in DED. Meibomian gland dysfunction (MGD) is graded using different classifications and scales.[35]
Routine tests for Dry eye evaluation including tear meniscus height,[36] schirmers test with and without anesthesia,[37,38] tear break time[21] and ocular surface staining using fluorescein, rose Bengal or lissamine green form are an important part of the preoperative evaluation,[39] and the same tests must be repeated postoperatively if patient develops symptoms suggestive of dry eye. Care should be taken to not touch the strip to the ocular surface to avoid false staining while instilling the dye. It is important to look for a characteristic pattern of corneal staining termed LASIK induced neuroepitheliopathy (LINE) seen in postrefractive surgery patients if they are symptomatic [Fig. 3].[40]
Figure 3.

Clinical photograph of LASIK induced neurotrophic epitheliopathy staining (LINE)
Advanced Diagnostic Modalities can be Added to the Evaluation Preoperatively if Suspecting Dry Eye
Interferometry
Tear film interferometry is one of the newer modalities of dry eye assessment. It measures the nature, lipid layer thickness (LLT) and lipid layer breakup using interference patterns. The LipiView (TearScience Inc., Morrisville, NC, USA) and IDRA (SBI Sistemi Inc, Strada Torino, Italy) are some of the commercially available interferometers.[41]
Meibography can be used to assess the morphology of the meibomian glands and loss as this can be a contributor to the dry eye.[42]
Confocal microscopy
In vivo confocal microscopy (IVCM) is a non-invasive, high-resolution imaging tool that images the cornea at the different levels and delineates cellular changes. It is useful in following up nerve and inflammation-related changes seen in the cornea postrefractive surgery and in DED. The number and density of sub-basal and stromal nerve cells is deranged in DED related conditions.[43] Bead-like formation, micro-neuromas, tortuosity and irregular branching and increased corneal dendritic cell density [cDCD] are also seen on IVCM in patients of DED [Fig. 4a].[43,44] Changes in keratocyte count, stromal changes and nerve density have been shown to occur after refractive surgery.[45,46] IVCM can also demonstrate the corneal nerve-related changes postrefractive surgery, and nerve regeneration on serial follow up [Fig. 4b]. In a subset of patients who have NCP, in vivo confocal microscopy (IVCM) demonstrated decreased corneal nerves and the presence of microneuromas indicative of the swelling of the injured nerve terminal.[47,48] These findings could aid in diagnosis and planning management.
Figure 4.
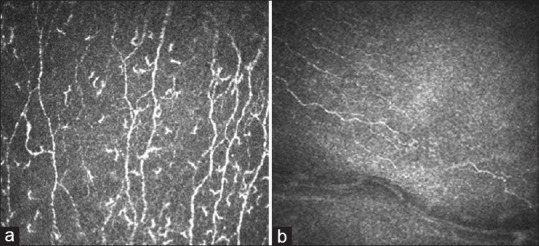
(a) Beading of nerves , increased tortuosity of corneal nerves (arrow), increased dendritic cells (star) seen on IVCM in patients of DED. (b) Early nerve regeneration post LASIK seen on IVCM
Inflammatory biomarkers
One of the important underlying factors in the pathogenesis of DED is ocular surface inflammation.[49] Identification and quantification of the inflammatory markers in DED can help the clinicians to customize DED management especially if it is detected in a pre-refractive surgery patient as the associated inflammation can have an impact on healing and outcomes postrefractive surgery.[49,50,51] Matrix metalloproteinase 9 (MMP9) is one of the commonly studied inflammatory markers and can now be measured using an outpatient point of care diagnostic test called the InflammaDry (Rapid Pathogen Screening, Quidel Corporation).[33] However, an easy diagnostic kit which could measure a larger range of inflammatory factors known to be associated with this condition could give much more information and help customize treatment.[49]
Management of dry eye related to refractive surgery
The TFOS DEWS II has given very comprehensive recommendations for management of DED. It elaborates on treatment aspects ranging from education of the patient regarding environmental modifications and disease prognostication to treatment options available for different types of DED. It also talks about the newer thermal and light-based therapies for MGD and role of anti-inflammatory agents, autologous serum and oral secretagogues across disease severity.[52] The postrefractive surgery DED management remains the same, except that postoperatively the topical anti-inflammatory and immunomodulatory medications like cyclosporine may need to be continued for longer duration (up to 4 months) depending on certain preoperative characteristics.[53]
Post-operative topical medications to treat and prevent dry eye
Tear substitutes
Tear substitutes help in lubricating the surface and also relieve patient symptomatology protecting the surface against desiccation and promoting tear retention.[52,54] A large number of topical formulations are available containing carboxymethyl cellulose (CMC), hydroxypropyl methylcellulose (HPMC), sodium hyaluronate, polyvinyl alcohol, polyvinylpyrrolidone and polyethylene glycol. Sodium Hyaluronate (HA) in addition has been shown to bind well to ocular surface and has potential wound healing properties.[55] Lubricating eye drops are selected based on the mechanism of action, composition, viscosity, osmolarity/osmolality, pH and preservative agents.
Preservative in tears can increase inflammation and hence preservative-free medications are preferred, especially when the drops have to be instilled more than 4 times a day over long periods of time.[54,56] Benzalkonium chloride and chlorobutanol preservatives in excess can damage the corneal epithelium resulting in symptoms mimicking the DED itself. Newer preservatives like purite (sodium chlorite) sodium perborate, are shown to cause less harm to the ocular surface.[57,58] Thus, preservative-free eye drops should be recommended to patients who require more frequent instillation of eye drops in order to prevent the preservative related damage to corneal and conjunctival surface health.
Anti-inflammatory treatments
Inflammation is a known underlying feature of various types of DED and is further increased post-refractive surgery. Use of anti-inflammatory medications like topical steroids and cyclosporine may have an important role in post-operative care. This helps in reducing and breaking the inflammatory cascade which is key to alleviating symptoms and treating the disease.[10,52]
Topical steroids - Steroids act on the inflammatory cascade, by blocking cyclooxygenase. They also have local immuno-modulatory activity by inhibiting certain transcription factors.[59] Topical corticosteroid use decreases ocular irritation and inflammation.[52] Since they are a part of the normal post-operative regime in refractive surgery, they are key to reducing the incidence of dry eye symptoms in these patients in the early post-operative period and can be used for 4-6 weeks in tapering doses.[52] The strength of steroid used would depend on the severity of inflammation in the eye. Patients prescribed topical corticosteroids for dry eye should be monitored for adverse effects like raised intraocular pressure and cataract.[52]
Topical Cyclosporine A (CsA) - Topical administration of Cyclosporine A (CsA) has an important role in treatment of DED post-refractive surgery.] It acts by preventing activation of T-cells and production of inflammatory cytokines.[60] It has also shown some effect on increasing tear secretion and improve both the symptoms and signs of DED in several studies.[60,61] Since the pathophysiology of dry eye post-refractive surgery is slightly different from regular dry eye, it is important to control the inflammation which may improve patient symptoms even without signs. Topical cyclosporine is usually prescribed for a minimum duration of 3 months because the onset of action is slower than steroids, and can also be started 1 month preoperatively if patient is having dry eye preoperatively.[53,62]
Treatment of meibomian gland dysfunction
If the patient has chronic MGD preoperatively, it is advisable to treat this before planning refractive surgery to avoid postoperative complications. The goal of all treatments for MGD is to improve the flow of meibomian gland secretions and reduce inflammation thus leading to normal tear film stability. This can be done by conventional methods like lid hygiene, warm compress, gland expression, oral and topical medications or newer procedural therapies like the thermal pulsation system[63,64] and Intense pulsed light[65,66] if the patient is nonresponsive or faster resolution is required. These procedures have shown good effect even in postrefractive dry eye, but care must be taken not to perform thermal pulsation in early postoperative period to avoid flap related issues.
Essential fatty acids - Usage of oral Omega-3 fatty acids like eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA] and plant-based sources 5-aminolevulinate [ALA] have been shown to improve symptoms and decrease inflammation and ocular surface staining in some studies,[67] but not in others.[68]
Role of Vitamin D in DED- A significant association has been found between DED and vitamin D and a protective role of Vitamin D has also been postulated.[69] It has an important role in wound healing and an association has also been found with the corneal dendritic cell density and inflammatory factors in tears,[70,71] It can be used as adjuvant therapy for patients with DED non-responsive to conventional therapy.
Fig. 5 summarizes the algorithmic approach to diagnosing and managing DED related to refractive surgery. In addition to the features specific to postrefractive dry eye, it is important not to miss easily diagnosable causes for DED preoperatively. As discussed in the TFOS DEWS II report, since DED can have a lot of overlap between aqueous and evaporative disease, it is useful to plan therapy based on the severity of disease as well as the symptoms and signs.[52] This has to be done both preoperatively and postoperatively if patient is symptomatic.
Figure 5.
Algorithmic approach to evaluation and management of DED pre and post refractive surgery with emphasis on subdivision by type and grade of disease
Conclusion
The management of DED is evolving and the treatment of post refractive dry eye needs a comprehensive understanding of the possible pathomechanisms. In this article we detail the different aspects of postrefractive dry eye and present an algorithmic approach for it's management. A combination of detailed history, simple tests and newer advances in understanding will help optimize outcomes in these patients. Due to the varied pathophysiology in the postrefractive DED, this algorithm may be further customized for optimal results. A systematic approach to the disease with a balance of tests and diagnostics as discussed, followed by appropriate medications for adequate duration will give the best outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Şimşek C, Doǧru M, Kojima T, Tsubota K. Current management and treatment of dry eye disease. Turk J Ophthalmol. 2018;48:309–13. doi: 10.4274/tjo.69320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeev MS, Miller DD, Latkany R. Diagnosis of dry eye disease and emerging technologies. Clin Ophtalmol. 2014;8:581–90. doi: 10.2147/OPTH.S45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomes JAP, Azar DT, Baudouin C, Efron N, Hirayama M, Horwath-Winter J, et al. TFOS DEWS II iatrogenic report. Ocul Surf. 2017;15:511–38. doi: 10.1016/j.jtos.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Solomon KD, Fernandez de Castro LE, Sandoval HP, Groat B, Neff KD, Ying MS, et al. LASIK world literature review: Quality of life and patient satisfaction. Ophthalmology. 2009;116:691–701. doi: 10.1016/j.ophtha.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 5.Statista. Number of LASIK surgeries in the United States from 1996 to 2020 Statista. 2019. [Last accessed on 2019 Dec 08]. Available from: https://wwwstatistacom/statistics/271478/number-of-lasik-surgeries-in-the-us/
- 6.Solomon R, Donnenfeld ED, Perry HD. The effects of LASIK on the ocular surface. Ocul Surf. 2004;2:34–44. doi: 10.1016/s1542-0124(12)70022-8. [DOI] [PubMed] [Google Scholar]
- 7.Ambrósio R, Tervo T, Wilson SE. LASIK-associated dry eye and neurotrophic epitheliopathy: Pathophysiology and strategies for pre- vention and treatment. J Refract Surg. 2008;24:396–407. doi: 10.3928/1081597X-20080401-14. [DOI] [PubMed] [Google Scholar]
- 8.Ang RT, Dartt DA, Tsubota K. Dry eye after refractive surgery. Curr Opin Ophthalmol. 2001;12:318–22. doi: 10.1097/00055735-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Nariani A, Gupta P. Dry eye and refractive surgery outcomes. Curr Ophthalmol Rep. 2016;4:8–14. [Google Scholar]
- 10.Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–83. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Kobashi H, Kamiya K, Shimizu K. Dry eye after small incision lenticule extraction and femtosecond laser-assisted LASIK: Meta-analysis. Cornea. 2017;36:85–91. doi: 10.1097/ICO.0000000000000999. [DOI] [PubMed] [Google Scholar]
- 12.D'Souza S, Petznick A, Tong L, et al. Comparative analysis of two femtosecond LASIK platforms using iTRAQ quantitative proteomics. Invest Ophthalmol Vis Sci. 2014;55:3396–402. doi: 10.1167/iovs.14-14113. [DOI] [PubMed] [Google Scholar]
- 13.Bower KS, Sia RK, Ryan DS, Mines MJ, Dartt DA. Chronic dry eye in photorefractive keratectomy and laser in situ keratomileusis: Manifestations, incidence, and predictive factors. J Cataract Refract Surg. 2015;41:2624–34. doi: 10.1016/j.jcrs.2015.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee B, Mclaren J, Eric J, Hodge D, Bourne W. Reinnervation in the cornea afterLASIK. Invest Ophthalmol Vis Sci. 2002;43:3660–4. [PubMed] [Google Scholar]
- 15.Cai WT, Liu QY, Ren CD, Wei QQ, Liu JL, Wang QY, et al. Dry eye and corneal sensitivity after small incision lenticule extraction and femtosecond laser assisted in situ keratomileusis: A meta-analysis. Int J Ophthalmol. 2017;18(10):632–8. doi: 10.18240/ijo.2017.04.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang C, Ding H, He M, Liu L, Liu L, Li G, et al. Comparison of early changes in ocular surface and inflammatory mediators between femto- second lenticule extraction and small-incision lenticule extraction. PLoS One. 2016;11:e0149503. doi: 10.1371/journal.pone.0149503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y, Yang Y. Dry eye after small incision lenticule extraction and LASIK for myopia. J Refract Surg. 2014;30:186–90. doi: 10.3928/1081597X-20140219-02. [DOI] [PubMed] [Google Scholar]
- 18.Li M, Niu L, Qin B, Zhou Z, Ni K, Le Q, et al. Confocal comparison of corneal reinnervation after small incision lenticule extraction and femtosecond laser in situ keratomileusis. PLoS One. 2013;8:e81435. doi: 10.1371/journal.pone.0081435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JB, Ryu CH, Kim J, Kim EK, Kim HB. Comparison of tear secretion and tear film instability after photorefractive keratectomy and laser in situ keratomileusis. J Cataract Refract Surg. 2000;26:1326–31. doi: 10.1016/s0886-3350(00)00566-6. [DOI] [PubMed] [Google Scholar]
- 20.Matsui H, Kumano Y, Zushi I, Yamada T, Matsui T, Nishida T. Corneal sensation after correction of myopia by photorefractive keratectomy and laser in situ keratomileusis. J Cataract Refract Surg. 2001;27:370–3. doi: 10.1016/s0886-3350(00)00756-2. [DOI] [PubMed] [Google Scholar]
- 21.Dooley I, D'Arcy F, O'Keefe M. Comparison of dry-eye disease severity after laser in situ keratomileusis and laser-assisted subepithelial keratectomy. J Cataract Refract Surg. 2012;38:1058–64. doi: 10.1016/j.jcrs.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 22.Erie JC, McLaren JW, Hodge DO, Bourne WM. Recovery of corneal subbasal nerve density after PRK and LASIK. Am J Ophthalmol. 2005;140:1059–64. doi: 10.1016/j.ajo.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 23.Feng YF, Yu JG, Wang DD, Li JH, Huang JH, Shi JL, et al. The effect of hinge location on corneal sensation and dry eye after LASIK: A systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2013;251:357–66. doi: 10.1007/s00417-012-2078-5. [DOI] [PubMed] [Google Scholar]
- 24.Battat L, Macri A, Dursun D, Pflugfelder SC. Effects of laser in situ keratomileusis on tear production, clearance, and the ocularsurface. Ophthalmology. 2001;108:1230–5. doi: 10.1016/s0161-6420(01)00623-6. [DOI] [PubMed] [Google Scholar]
- 25.Szczesna DH, Kulas Z, Kasprzak HT, Stenevi U. Examination of tear film smoothness on corneae after refractive surgeries using a non-invasive interferometric method. J Biomed Opt. 2009;14:064029. doi: 10.1117/1.3275850. [DOI] [PubMed] [Google Scholar]
- 26.Konomi K, Chen LL, Tarko RS, Scally A, Schaumberg DA, Azar D, et al. Preoperative characteristics and a potential mechanism of chronic dry eye after LASIK. Invest Ophthalmol Vis Sci. 2008;49:168–74. doi: 10.1167/iovs.07-0337. [DOI] [PubMed] [Google Scholar]
- 27.Shin SY, Lee YJ. Conjunctival changes induced by LASIK suction ring in a rabbit model. Ophthalmic Res. 2006;38:343–9. doi: 10.1159/000096229. [DOI] [PubMed] [Google Scholar]
- 28.Dieckmann G, Goyal S, Hamrah P. Neuropathic corneal pain: Approaches for management. Ophthalmology. 2017;124:S34–47. doi: 10.1016/j.ophtha.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shehadeh-Mashor R, Mimouni M, Shapira Y, Sela T, Munzer G, Kaiserman I. Risk factors for dry eye after refractive surgery. Cornea. 2019;38:1495–9. doi: 10.1097/ICO.0000000000002152. [DOI] [PubMed] [Google Scholar]
- 30.Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15:438–510. doi: 10.1016/j.jtos.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Belmonte C, Nichols JJ, Cox SM, Brock JA, Begley CG, Bereiter DA, et al. TFOS DEWS II pain and sensation report. Ocul Surf. 2017;15:404–37. doi: 10.1016/j.jtos.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stefanski AL, Tomiak C, Pleyer U, Dietrich T, Burmester GR, Dörner T. The diagnosis and treatment of Sjögren's syndrome. Dtsch Arztebl Int. 2017;114:354–61. doi: 10.3238/arztebl.2017.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15:539–74. doi: 10.1016/j.jtos.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Barber L, Khodai O, Croley T, Lievens C, Montaquila S, Ziemanski J, et al. Dry eye symptoms and impact on vision-related function across International Task Force guidelines severity levels in the United States. BMC Ophthalmol. 2018;18:260. doi: 10.1186/s12886-018-0919-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomlinson A, Bron AJ, Korb DR, Amano S, Paugh JR, Pearce EI, et al. The international workshop on meibomian gland dysfunction: Report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;52:2006–49. doi: 10.1167/iovs.10-6997f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thulasi P, Djalilian AR. Update in current diagnostics and therapeutics of dry eye disease. Ophthalmology. 2017;124:S27–33. doi: 10.1016/j.ophtha.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shapiro A, Merin S. Schirmer test and break-up time of tear film in normal subjects. Am J Ophthalmol. 1979;88:752–7. doi: 10.1016/0002-9394(79)90678-0. [DOI] [PubMed] [Google Scholar]
- 38.Li N, Deng XG, He MF. Comparison of the Schirmer I test with and without topical anesthesia for diagnosing dry eye. Int J Ophthalmol. 2012;5:478–81. doi: 10.3980/j.issn.2222-3959.2012.04.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bron AJ, Argueso P, Irkec M, Bright FV. Clinical staining of the ocular surface: Mechanisms and interpretations. Prog Retin eye Res. 2015;44:36e61. doi: 10.1016/j.preteyeres.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Ambrósio R, Jr, Tervo T, Wilson SE. LASIK-associated dry eye and neurotrophic epitheliopathy: Pathophysiology and strategies for prevention and treatment. J Refract Surg. 2008;24:396–407. doi: 10.3928/1081597X-20080401-14. [DOI] [PubMed] [Google Scholar]
- 41.Eom Y, Lee JS, Kang SY, Kim H, Song JS. Correlation between quantitative measurements of tear film lipid layer thickness and meibomian gland loss in patients with obstructive meibomian gland dysfunction and normal controls. Am J Ophthalmol. 2013;155:1104–10. doi: 10.1016/j.ajo.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 42.Arita R, Suehiro J, Haraguchi T, Shirakawa R, Tokoro H, Amano S. Objective image analysis of the meibomian gland area. Br J Ophthalmol. 2014;98:746–55. doi: 10.1136/bjophthalmol-2012-303014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khamar P, Nair AP, Shetty R, Vaidya T, Subramani M, Ponnalagu M, et al. Dysregulated tear fluid nociception- associated factors, corneal dendritic cell density, and vitamin D levels in evaporative dry eye. Invest Ophthalmol Vis Sci. 2019;60:2532–42. doi: 10.1167/iovs.19-26914. [DOI] [PubMed] [Google Scholar]
- 44.Tuominen IS, Konttinen YT, Vesaluoma MH, Moilanen JA, Helintö M, Tervo TM. Corneal innervation and morphology in primary Sjögren's syndrome. Invest Ophthalmol Vis Sci. 2003;44:2545–9. doi: 10.1167/iovs.02-1260. [DOI] [PubMed] [Google Scholar]
- 45.Moilanen JA, Vesaluoma MH, Muller LJ, Tervo TM. Long-term corneal morphology after PRK by in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2003;44:1064–9. doi: 10.1167/iovs.02-0247. [DOI] [PubMed] [Google Scholar]
- 46.Linna TU, Vesaluoma MH, Perez-Santonja JJ, Petroll MW, Alió JL, Tervo TMT. Effect of myo- pic LASIK on corneal sensitivity and morphology of subbasal nerves. IOVS. 2000;41:393–7. [PubMed] [Google Scholar]
- 47.Theophanous C, Jacobs DS, Hamrah P. Corneal neuralgia after LASIK. Optom Vis Sci. 2015;92:e233–40. doi: 10.1097/OPX.0000000000000652. [DOI] [PubMed] [Google Scholar]
- 48.Moein HR, Dieckmann G, Abbouda A, Pondelis N, Jamali N, Salem Z. In vivo confocal microscopy demonstrates the presence of microneuromas and may allow differentiation of patients with corneal neuropathic pain from dry eye disease. Invest Ophthalmol Vis Sci. 2017:58. ARVO E-Abstract2656. [Google Scholar]
- 49.D'Souza S, Tong L. Practical issues concerning tear protein assays in dry eye. Eye Vis. 2014;1:6. doi: 10.1186/s40662-014-0006-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar NR, Khamar P, Shetty R, Sharma A, Shetty N, Pahuja N, et al. Identification of novel predictive factors for post surgical corneal haze. Sci Rep. 2019;9:16980. doi: 10.1038/s41598-019-53123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shetty R, Sethu S, Chevour P, Deshpande K, Pahuja N, Nagaraja H, et al. Lower vitamin D level and distinct tear cytokine profile were observed in patients with mild dry eye signs but exaggerated symptoms. Transl Vis Sci Technol. 2016;5:16. doi: 10.1167/tvst.5.6.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones L, Downie LE, Korb D, Benitez-Del-Castillo JM. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15:575–628. doi: 10.1016/j.jtos.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 53.Shtein RM. Post-LASIK dry eye. Expert Rev Ophthalmol. 2011;6:575–82. doi: 10.1586/eop.11.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Asbell PA. Increasing importance of dry eye syndrome and the ideal artificial tear: Consensus views from a roundtable discussion. Curr Med Res Opin. 2006;22:2149–57. doi: 10.1185/030079906X132640. [DOI] [PubMed] [Google Scholar]
- 55.Ho WT, Chiang TH, Chang SW, Chen YH, Hu FR, Wang IJ. Enhanced corneal wound healing with hyaluronic acid and high-potassium artificial tears. Clin Exp Optom. 2013;96:536–41. doi: 10.1111/cxo.12073. [DOI] [PubMed] [Google Scholar]
- 56.Jee D, Park SH, Kim MS, Kim EC. Antioxidant and inflammatory cytokine in tears of patients with dry eye syndrome treated with preservative-free versus preserved eye drops. Invest Ophthalmol Vis Sci. 2014;55:5081–9. doi: 10.1167/iovs.14-14483. [DOI] [PubMed] [Google Scholar]
- 57.Göbbels M, Spitznas M. Influence of artificial tears on corneal epithelium in dry-eye syndrome. Graefes Arch Clin Exp Ophthalmol. 1989;227:139–41. doi: 10.1007/BF02169786. [DOI] [PubMed] [Google Scholar]
- 58.Noecker RJ, Herrygers LA, Anwaruddin R. Corneal and conjunctival changes caused by commonly used glaucoma medications. Cornea. 2004;23:490–6. doi: 10.1097/01.ico.0000116526.57227.82. [DOI] [PubMed] [Google Scholar]
- 59.Pflugfelder SC, Maskin SL, Anderson B, Chodosh J, Holland EJ, De Paiva CS, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol. 2004;138:444–57. doi: 10.1016/j.ajo.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 60.Utine CA, Stern M, Akpek EK. Clinical review: Topical ophthalmic use of cyclosporin A. Ocul Immunol Inflammation. 2010;18:352–61. doi: 10.3109/09273948.2010.498657. [DOI] [PubMed] [Google Scholar]
- 61.Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107:631–9. doi: 10.1016/s0161-6420(99)00176-1. [DOI] [PubMed] [Google Scholar]
- 62.Perry HD, Solomon R, Donnenfeld ED, Perry AR, Wittpenn JR, Greenman HE, et al. Evaluation of topical cyclosporine for the treatment of dry eye disease. Arch Ophthalmol. 2008;126:1046–50. doi: 10.1001/archopht.126.8.1046. [DOI] [PubMed] [Google Scholar]
- 63.Lane SS, DuBiner HB, Epstein RJ, Ernest PH, Greiner JV, Hardten DR, et al. A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea. 2012;31:396–404. doi: 10.1097/ICO.0b013e318239aaea. [DOI] [PubMed] [Google Scholar]
- 64.Schallhorn CS, Schallhorn JM, Hannan S, Schallhorn SC. Effectiveness of an eyelid thermal pulsation procedure to treat recalcitrant dry eye symptoms after laser vision correction. J Refract Surg. 2017;33:30–6. doi: 10.3928/1081597X-20161006-05. [DOI] [PubMed] [Google Scholar]
- 65.Vigo L, Giannaccare G, Sebastiani S, Pellegrini M, Carones F. Intense pulse light for the treatment of dry eye owing to meibomian gland dysfunction. J Vis Exp. 2019 doi: 10.3791/57811. doi: 103791/57811. [DOI] [PubMed] [Google Scholar]
- 66.Dell SJ. Intense pulsed light for evaporative dry eye disease. Clin Ophthalmol. 2017;11:1167–73. doi: 10.2147/OPTH.S139894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wojtowicz JC, Butovich I, Uchiyama E, Aronowicz J, Agee S, McCulley JP. Pilot, prospective, randomized, double-masked, placebo-controlled clinical trial of an omega-3 supplement for dry eye. Cornea. 2011;30:308–14. doi: 10.1097/ICO.0b013e3181f22e03. [DOI] [PubMed] [Google Scholar]
- 68.Rosenberg ES, Asbell PA. Essential fatty acids in the treatment of dry eye. Ocul Surf. 2010;8:18–28. doi: 10.1016/s1542-0124(12)70214-8. [DOI] [PubMed] [Google Scholar]
- 69.Askari G, Rafie N, Miraghajani M, Heidari Z, Arab A. Association between vitamin D and dry eye disease: A systematic review and meta-analysis of observational studies. Cont Lens Anterior Eye. 2020;43:418–25. doi: 10.1016/j.clae.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 70.D'souza S, Ghosh A, Pahuja N, Deshmukh R, Ahuja P, Sainani K, et al. Dysregulated tear fluid nociception-associated factors, corneal dendritic cell density, and vitamin D levels in evaporative dry eye. Invest Ophthalmol Vis Sci. 2019;60:2532–42. doi: 10.1167/iovs.19-26914. [DOI] [PubMed] [Google Scholar]
- 71.Hwang JS, Lee YP, Shin YJ. Vitamin D enhances the efficacy of topical artificial tears in patients with dry eye disease. Cornea. 2019;38:304–10. doi: 10.1097/ICO.0000000000001822. [DOI] [PubMed] [Google Scholar]




