Abstract
The recent SARS-CoV-2 pandemic poses one of the greatest challenges to modern medicine. Therefore, identification of new therapeutic strategies seems essential either based on novel vaccines or drugs or simply repurposing existing drugs. Notably, due to their known safety profile, repurposing of existing drugs is the fastest and highly efficient approach to bring a therapeutic to a clinic for any new indication. One such drug that has been used extensively for decades is chloroquine (CQ, with its derivatives) either for malaria, lupus and rheumatoid arthritis. Accumulating body of evidence from experimental pharmacology suggests that CQ and related analogues also activate certain pathways that can potentially be exploited for therapeutic gain. For example, in the airways, this has opened an attractive avenue for developing novel bitter taste ligands as a new class of bronchodilators for asthma. While CQ and its derivatives have been proposed as a therapy in COVID-19, it remains to be seen whether it really work in the clinic? To this end, our perspective aims to provide a timely yet brief insights on the existing literature on CQ and the controversies surrounding its use in COVID-19. Further, we also highlight some of cell-based mechanism(s) that CQ and its derivatives affect in mediating variety of physiological responses in the cell. We believe, data emanating from the clinical studies and continual understanding of the fundamental mechanisms may potentially help in designing effective therapeutic strategies that meets both efficacy and safety criteria for COVID-19.
Keywords: Chloroquine, Bitter taste receptor, SARS-CoV2, COVID-19
1. Introduction
The coronavirus disease-2019 (COVID-19) pandemic has infected over 105 million people resulting in over 2.3 million deaths worldwide (Source: Johns Hopkins University (Dong et al., 2020)). The continual infection rate and death has turned into a global catastrophe and there is an intense search for effective drug therapy (Gates, 2020). Despite unproven clinical effectiveness and safety in COVID-19 patients, chloroquine (CQ) and hydroxychloroquine (HCQ) entered the spotlight as a potential candidate for treating this new viral disease. Limited clinical data suggest that CQ (and HCQ) is beneficial in allergic asthma and may provide further relief in steroid-resistant conditions (Charous, 1990; 1991; Charous et al., 1998; Goldstein, 1983). As lung cells are the primary target cell type(s) involved in the pathogenesis of COVID-19 infection, key molecular insights can be obtained from the published literature on CQ in the pulmonary system. Preliminary findings from observational clinical studies during COVID-19 pandemic have been recently published. In here, we sought to provide timely yet brief insights on the existing literature on CQ and the controversies surrounding its use in COVID-19. Further, we also highlight some of cell-based mechanism(s) by which CQ and its derivatives affect variety of physiological responses in the airway cells.
1.1. Discovery of chloroquine and its potential role in viral infections
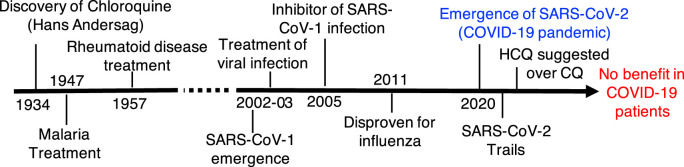
Hans Andersag's discovery of CQ occurred in 1934 but was deemed too toxic for human use. Clinical use of CQ remained unexplored and unstudied for many years, until the therapeutic value of CQ in the fight against malaria was acknowledged and clinically unveiled in 1947 (Solomon and Lee, 2009) (Fig. 1 ). CQ was originally used as a prophylactic antimalarial drug, new purpose for CQ was found as an anti-arthritis drug (Homewood et al., 1972). CQ and its derivative hydroxychloroquine (HCQ) are now used in clinical trials aimed at suppressing tumors through autophagy inhibition (Chude and Amaravadi, 2017; Maycotte et al., 2012). Interest in the potential antiviral properties of CQ appears to stem from the time during the emergence of severe acute respiratory syndrome (SARS) and accompanying discovery of the first strain of SARS coronavirus (SARS-CoV-1) in the early 2000s (Vincent et al., 2005a). CQ has been highlighted for its potential therapeutic benefit in the treatment of HIV-1/AIDS and SARS (Savarino et al., 2003; Savarino et al., 2006).
Fig. 1.
Timeline of chloroquine discovery and clinical use.
Numerous studies have shown significant reduction in viral load with CQ treatment both in vitro and in vivo (Joshi et al., 2004; Pardridge et al., 1998; Savarino et al., 2003; Sperber et al., 1993; Tsai et al., 1990). In cellular models, CQ/HCQ have anti-SARS-CoV-2 activity with EC50 values in lower to sub mM range (Yao et al., 2020). Mechanistically, it is postulated that CQ affects the early stage of SARS-CoV replication in vitro, with the interference in glycosylation of the SARS-CoV binding site of angiotensin converting enzyme 2 (ACE2, an entry receptor for SARS-CoV) (Keyaerts et al., 2004; Vincent et al., 2005a). These studies also implied that CQ may inhibit the biosynthesis of sialic acid (Kwiek et al., 2004; Savarino et al., 2006) and given the presence of sialic acid moieties in both HIV and ACE2, the antiviral effectiveness of CQ may also involve inhibition of sialic acid biosynthesis (Savarino et al., 2006). Budding of the SARS-CoV-1 virus was identified to occur in the Golgi complex, where the envelopment with spike glycoproteins adorned with ACE2 occurs (Li et al., 2003; Ng et al., 2003). When cells are treated with CQ, its protonated form accumulates in lysosomes and Golgi. More specifically, viral infection is blocked by CQ through an increase in pH, thereby preventing virus fusion and disrupting Golgi-mediated glycosylation of spike glycoproteins and ACE2 (Vincent et al., 2005b).
1.2. Chloroquine in COVID-19: current clinical evidence and a cautionary note
Therapeutic administration of CQ has been demonstrated to effectively improve survival rate in mice infected with live influenza A H5N1 virus, which is particularly lethal in humans (Wang and Jiang, 2009; Yan et al., 2013). CQ has shown to be effective in acute lung injury induced by the H5N1 virus in this model (Yan et al., 2013). However, randomized, double-blind, placebo controlled clinical trial testing the potential efficacy of CQ as a prophylactic agent for influenza virus did not provide a favorable outcome (Paton et al., 2011). These results suggest that the preclinical efficacy of CQ did not translate into a desired clinical outcome against H5N1 virus.
The world has succumbed to the 2020 pandemic with a novel coronavirus, SARS-CoV-2, identified as the responsible pathogen (Habibzadeh et al., 2020; Moradian et al., 2020; Zhou et al., 2020b). Preliminary studies tested several FDA approved drugs such as ribavirin, penciclovir, nitazoxanide, nafamostat, CQ and two broad-spectrum antiviral drugs remdesivir (GS-5734) and favipiravir (T-705) against a clinical isolate of SARS-CoV-2. Remdesivir (Half maximal effective concentration (EC50) = 0.77 μM; selectivity index (SI) > 129.87) and CQ (EC50 = 1.13 μM, SI > 88.50) potently blocked virus infection at low-micromolar concentration and showed high SI, while other drugs showed anti-viral activity at high μM concentrations (Wang et al., 2020a). While the in vitro finding has been validated in a clinical trial with Remdesivir where it has shown to marginally reduce the hospital stay for COVID-19 patients (Wang et al., 2020b), evidence with CQ has not been encouraging to date.
At the initial epicenter of the pandemic in Wuhan, chloroquine phosphate tablet, 500 mg twice daily, was recommended for the treatment of mild, moderate and severe cases of COVID-19 after expert consensus and discussion (2020). The call for the use of hydroxychloroquine (HCQ), instead of CQ, quickly followed (Liu et al., 2020; Zhou et al., 2020a). HCQ has a lower level of tissue accumulation, fewer side-effects and is globally more available than CQ (Schrezenmeier and Dorner, 2020). HCQ is a less toxic aminoquinoline than CQ with a strong immunomodulatory effect (Barnard et al., 2006; McChesney, 1983; Sahraei et al., 2020). The right concentration of HCQ is likely to be as effective as CQ in inhibiting SARS-CoV-2 infection but scientific evidence is currently lacking (Gao et al., 2020). An open label non-randomized clinical trial showed that HCQ treatment alone significantly reduced viral load in COVID-19 patients, and HCQ effect was further reinforced by azithromycin (Gautret et al., 2020). The sample size of 42 patients used here was particularly small, and the groups of this study were not randomized. Further, another small observational study by Chen et al. on 62 patients found that HCQ significantly shortened time to clinical recovery by 2.2 days and promoted the resolution of pneumonia (Chen et al., 2020). In a high impact observational study on >1400 patients Geleris et al. found no benefit with HCQ in lowering or reducing risk of intubation or death in COVID-19 patients who were admitted to the hospital (Geleris et al., 2020). One of the main limitations of this study was the selection of patients for HCQ arm, HCQ-treated patients were severely ill at baseline than those who did not receive HCQ. The authors of the study suggested to conduct a randomized clinical trial to fully establish the clinical efficacy of CQ and HCQ in COVID-19. New clinical trial results emerged during past months clearly demonstrated that CQ/HCQ alone and in combination with other agents such as azithromycin did not show any benefit in COVID-19 (Abd-Elsalam et al., 2020; Cavalcanti et al., 2020; Group et al., 2020). Currently, NIH has halted all clinical trials on CQ/HCQ in COVID-19 which again creates a major challenge to get highly reliable data from double blind randomized clinical trials with these drugs in future. Consequent to this, the FDA strongly advised against the use of CQ of HCQ as treatment for COVID-19. This avenue of treatment is for now terminated for safer and more efficacious drugs such as statins, anti-helminthic, anti-retro viral agents. And with the approval of two mRNA-based vaccines and many others in various stages of clinical development chances are less that CQ/HCQ will be retested robustly for COVID-19 using double blind placebo controlled randomized control trials.
1.3. Early clinical evidence supporting chloroquine in airways disease
Early clinical studies from the 1980s and 1990s demonstrated that HCQ is a steroid-sparing agent due to its anti-inflammatory effect making it a potentially good candidate for the treatment of severe asthma (Charous, 1990). Interest in CQ in asthma treatment slowed and lack of further clinical validation halted the progression of these early observations. In 2010, a seminal work identified bitter taste receptors as a target for providing effective bronchodilation and a future anti-asthma therapy, these studies highlighted the effectiveness of CQ as a bitter ligand (Deshpande et al., 2010). Further, we have used CQ as an agonist of type II taste receptors (T2Rs) for studying airway smooth muscle functions including the involvement of T2R agonists in autophagy-related signaling in mitigating airway remodeling in experimental models of asthma (Pan et al., 2017). Whilst we and others may have found CQ to be a useful drug to study the mechanisms involved in promoting airway smooth muscle relaxation (Deshpande et al., 2010), it may provide additional beneficial effects particularly as an immunomodulator (McAlinden et al., 2019; Nayak et al., 2019a; Nayak et al., 2019b; Sharma et al., 2017). In the airways, specialized epithelial cells known as solitary chemosensory cells (SCCs) have been previously identified as epithelial chemosensors in the sinonasal mucosa that express T2Rs and respond to “bitter” irritants to promote local neurogenic inflammation (Saunders et al., 2014) and drive release of antimicrobial peptides (Lee et al., 2017; Lee et al., 2014). Similarly, in the upper airways SCCs are known to expand protective inflammatory signaling in response to viral infections (Kohanski et al., 2018; Patel et al., 2018). From the experience of H1N1 influenza, it was discovered that differentiation and proliferation of SCCs occur in the distal lung after influenza virus-induced injury while these cells are entirely absent in uninjured lungs (Kohanski et al., 2018; Patel et al., 2018). These observations may have direct implication in the treatment of COVID-19 as beneficial effect remains to be established in experimental models or in randomized clinical trials.
CQ binds to the bitter taste receptors (expressed in SCCs and airway smooth muscle cells) namely TAS2R3, TAS2R7, TAS2R10 and TAS2R39 (Manson et al., 2014; Meyerhof et al., 2010). Stimulation of airway smooth muscle cells with CQ leads to airway relaxation (Deshpande et al., 2010; Pulkkinen et al., 2012). CQ has been tested in a prophylactic and treatment model of allergic airways disease (murine asthma). CQ mitigated airway inflammation, remodeling, mucus secretion and airway hyperresponsiveness, some of the cardinal features of allergen-induced asthma in mice (Sharma et al., 2017). CQ has been shown have an anti-mitogenic effect on airway smooth muscle, inhibiting the growth of human airway smooth muscle cells by activating T2Rs (Sharma et al., 2016) and more recently it has been shown that CQ can also target mitochondrial-signaling to reduce airway smooth muscle cell growth (Pan et al., 2017). These in vitro observations further confirmed the in vivo findings where CQ was found to be effective in targeting airway smooth muscle remodeling (McAlinden et al., 2019). CQ is also known to affect macroautophagy (autophagy), a fundamental physiological process that occurs in all eukaryotic cells (Mizushima and Levine, 2010). Autophagy is also referred to as cell's inner recycling mechanism, with a key role in maintaining cellular homeostasis. In an experimental model of allergic asthma in mice, CQ provided therapeutic benefit by affecting autophagy and by reducing TGFβ1-dependent profibrotic signalling (Halwani et al., 2011; McAlinden et al., 2019).
1.4. Chloroquine targets multiple signaling pathways
Studies using CQ to modulate autophagy in various experimental models both in vitro and in vivo has shown to be effective so far. CQ has a lower selectivity profile which may not provide an effective treatment for COVID-19. The main mechanism by which CQ inhibits autophagy is by preventing the fusion of the autophagosomes with lysosomes (Mauthe et al., 2018). Independent of autophagy pathways, CQ can also induce a severe disorganization of the Golgi complex (Kellokumpu et al., 2002), which in turn could drive disruption of autophagolysosomes (Mauthe et al., 2018). CQ is used in an experimental setting to assess role of autophagy in multiple cell-types. CQ can also affect intracellular trafficking via autophagy pathway that potentially affect ACE2 cytosolic membrane exposure and interaction with SARS-CoV-2 thereby inhibiting entry of virus in the cell (Shojaei et al., 2020). On the other hand, evidence suggests that CQ can inhibit cytokine secretion and therefore inhibit SARS-CoV-2 induced cytokine storm via inhibition of intracellular trafficking and regulation of unfolded protein response (Sureda et al., 2020; Zeki et al., 2014). More detailed investigation is needed to establish a clear relevance of autophagy in COVID-19-induced lung pathology to further modulators of autophagy pathway as therapeutic agents (Drozdzal et al., 2020).
As CQ and HCQ are being approved as “Off Label” drugs for COVID-19, in many jurisdictions, randomized controlled clinical trials must be carried out to prove whether these drugs are actually effective against COVID-19. Additionally, research effort must be directed to test inhaled formulations of CQ and HCQ as this approach will deliver drug locally into the lungs which is the target for COVID-19 entry. This approach may be advantageous in reducing the actual dose of CQ and HCQ which at higher doses do have serious adverse effects (Ciszowski et al., 2005; Weniger, 1979).
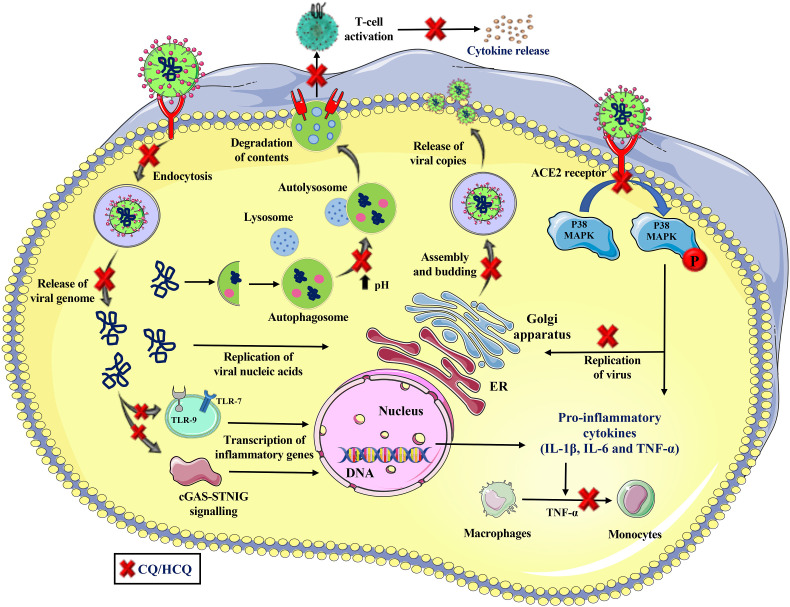
Mechanisms by which CQ and HCQ can activate beneficial immune signaling during viral infection must be explored. Inhaled CQ is effective in reducing inflammation in both bronchoalveolar lavage (BAL) and in lung tissue in murine models of asthma. Further, evidence supports immunomodulatory function of CQ (Ben-Zvi et al., 2012), and like many other bitter ligands can activate SCCs that can help expand immune response in the lung during COVID-19 (Lee et al., 2017; Lee et al., 2014). We have shed some light on the potential mechanisms by which CQ and its derivatives may provide beneficial effects during lung infection. We believe that drugs which target lysosomes or the Golgi complex and disrupt the glycosylation and assembly of S proteins may also prove beneficial in treating SARS-CoV-2 associated infections and lung injury in humans (Dwek et al., 2002). Approaches targeting ER α-glucosidases I and II (involved in processing glycoproteins) and the ceramide-specific glucosyltransferase (involved in glucosphingholipid synthesis) could be a potential strategy for treating viral infections, without compromising the host cell (Dwek et al., 2002; Watanabe et al., 2019). Potential mechanisms by which CQ and HCQ may work in viral infections including SARS-CoV-2 induced COVID-19 are summarized in the schematic (Fig. 2 ).
Fig. 2.
A Multitude of mechanisms by which CQ/HCQ may work in COVID-19. CQ/HCQ may prevent the entry of virus (either by blocking endocytosis or inhibition of the N-glycosylation of the cell surface viral receptor ACE2 or the viral spike (S) proteins or inhibition of the synthesis of cell membrane sialic acids) and release of viral genome leading to reduction in transcription of inflammatory genes and induction of pro-inflammatory cytokines. CQ/HCQ can also modulate innate and adaptive immune cell activation, cytokine response and inflammation by various mechanisms that leads to reduction in cellular inflammation. CQ/HCQ also affects viral replication, assembly and budding of viral proteins leading to reduction in viral copies in the host cells.
In summary, although CQ or HCQ have been promoted as potential anti-COVID-19 drugs, the evidence for their clinical effectiveness is insufficient in COVID-19. The USFDA has revoked Emergency Use Authorization (EUA) for CQ and HCQ as the known risks outweighs potential benefits with their use. CQ and its derivatives affect a multitude of mechanisms in the lung therefore further research is warranted to identify structurally similar drugs that are safe in viral associated diseases including COVID-19.
Acknowledgements
DAD is supported by the National Institutes of Health (NIH) grants HL137030 and HL146645.
References
- Abd-Elsalam S., Esmail E.S., Khalaf M., Abdo E.F., Medhat M.A., Abd El Ghafar M.S., Ahmed O.A., Soliman S., Serangawy G.N., Alboraie M. Hydroxychloroquine in the treatment of COVID-19: a multicenter randomized controlled study. Am. J. Trop. Med. Hyg. 2020;103:1635–1639. doi: 10.4269/ajtmh.20-0873. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Barnard D.L., Day C.W., Bailey K., Heiner M., Montgomery R., Lauridsen L., Chan P.K., Sidwell R.W. Evaluation of immunomodulators, interferons and known in vitro SARS-coV inhibitors for inhibition of SARS-coV replication in BALB/c mice. Antivir. Chem. Chemother. 2006;17:275–284. doi: 10.1177/095632020601700505. [DOI] [PubMed] [Google Scholar]
- Ben-Zvi I., Kivity S., Langevitz P., Shoenfeld Y. Hydroxychloroquine: from malaria to autoimmunity. Clin. Rev. Allergy Immunol. 2012;42:145–153. doi: 10.1007/s12016-010-8243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcanti, A.B., Zampieri, F.G., Rosa, R.G., Azevedo, L.C.P., Veiga, V.C., Avezum, A., Damiani, L.P., Marcadenti, A., Kawano-Dourado, L., Lisboa, T., Junqueira, D.L.M., de Barros, E.S.P.G.M., Tramujas, L., Abreu-Silva, E.O., Laranjeira, L.N., Soares, A.T., Echenique, L.S., Pereira, A.J., Freitas, F.G.R., Gebara, O.C.E., Dantas, V.C.S., Furtado, R.H.M., Milan, E.P., Golin, N.A., Cardoso, F.F., Maia, I.S., Hoffmann Filho, C.R., Kormann, A.P.M., Amazonas, R.B., Bocchi de Oliveira, M.F., Serpa-Neto, A., Falavigna, M., Lopes, R.D., Machado, F.R., Berwanger, O., Coalition covid-19 Brazil, I.I., 2020. Hydroxychloroquine with or without azithromycin in mild-to-moderate covid-19. N. Engl. J. Med. 383, 2041-2052. [DOI] [PMC free article] [PubMed]
- Charous B.L. Open study of hydroxychloroquine in the treatment of severe symptomatic or corticosteroid-dependent asthma. Ann. Allergy. 1990;65:53–58. [PubMed] [Google Scholar]
- Charous B.L. Effectiveness of long-term treatment of severe asthma with hydroxychloroquine (HCQ) Ann. N. Y. Acad. Sci. 1991;629:432–433. doi: 10.1111/j.1749-6632.1991.tb38008.x. [DOI] [PubMed] [Google Scholar]
- Charous B.L., Halpern E.F., Steven G.C. Hydroxychloroquine improves airflow and lowers circulating IgE levels in subjects with moderate symptomatic asthma. J. Allergy Clin. Immunol. 1998;102:198–203. doi: 10.1016/s0091-6749(98)70086-7. [DOI] [PubMed] [Google Scholar]
- Chen Z., Hu J., Zhang Z., Jiang S., Han S., Yan D., Zhuang R., Hu B., Zhang Z. medRxiv; 2020. Efficacy of hydroxychloroquine in patients with COVID-19:results of a randomized clinical trial. [Google Scholar]
- Chude C.I., Amaravadi R.K. Targeting autophagy in cancer: update on clinical trials and novel inhibitors. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18061279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciszowski K., Winnik L., Groszek B., Klys M., Kolodziej J. [Acute chloroquine intoxication--rare, but always serious: case reports and literature review] Przegl. Lek. 2005;62:501–507. [PubMed] [Google Scholar]
- Deshpande D.A., Wang W.C., McIlmoyle E.L., Robinett K.S., Schillinger R.M., An S.S., Sham J.S., Liggett S.B. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat. Med. 2010;16:1299–1304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drozdzal S., Rosik J., Lechowicz K., Machaj F., Kotfis K., Ghavami S., Los M.J. FDA approved drugs with pharmacotherapeutic potential for SARS-CoV-2 (COVID-19) therapy. Drug Resist. Updates. 2020;53:100719. doi: 10.1016/j.drup.2020.100719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwek R.A., Butters T.D., Platt F.M., Zitzmann N. Targeting glycosylation as a therapeutic approach. Nat. Rev. Drug Discov. 2002;1:65–75. doi: 10.1038/nrd708. [DOI] [PubMed] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- Gates B. Responding to covid-19 - a once-in-a-century pandemic? N. Engl. J. Med. 2020;382:1677–1679. doi: 10.1056/NEJMp2003762. [DOI] [PubMed] [Google Scholar]
- Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honore S., Colson P., Chabriere E., La Scola B., Rolain J.M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G., Labella A., Manson D., Kubin C., Barr R.G., Sobieszczyk M.E., Schluger N.W. Observational study of hydroxychloroquine in hospitalized patients with covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J.A. Hydroxychloroquine for asthma. Am. Rev. Respir. Dis. 1983;128:1100–1101. doi: 10.1164/arrd.1983.128.6.1100. [DOI] [PubMed] [Google Scholar]
- Group R.C., Horby P., Mafham M., Linsell L., Bell J.L., Staplin N., Emberson J.R., Wiselka M., Ustianowski A., Elmahi E., Prudon B., Whitehouse T., Felton T., Williams J., Faccenda J., Underwood J., Baillie J.K., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Lim W.S., Montgomery A., Rowan K., Tarning J., Watson J.A., White N.J., Juszczak E., Haynes R., Landray M.J. Effect of hydroxychloroquine in hospitalized patients with covid-19. N. Engl. J. Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibzadeh P., Mofatteh M., Ghavami S., Roozbeh J. The potential effectiveness of acetazolamide in the prevention of acute kidney injury in COVID-19: a hypothesis. Eur. J. Pharmacol. 2020;888:173487. doi: 10.1016/j.ejphar.2020.173487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halwani R., Al-Muhsen S., Al-Jahdali H., Hamid Q. Role of transforming growth factor-beta in airway remodeling in asthma. Am. J. Respir. Cell Mol. Biol. 2011;44:127–133. doi: 10.1165/rcmb.2010-0027TR. [DOI] [PubMed] [Google Scholar]
- Homewood C.A., Warhurst D.C., Peters W., Baggaley V.C. Lysosomes, pH and the anti-malarial action of chloroquine. Nature. 1972;235:50–52. doi: 10.1038/235050a0. [DOI] [PubMed] [Google Scholar]
- Joshi S.R., Butala N., Patwardhan M.R., Daver N.G., Kelkar D. Low cost anti-retroviral options: chloroquine based ARV regimen combined with hydroxyurea and lamivudine: a new economical triple therapy. J. Assoc. Phys. India. 2004;52:597–598. [PubMed] [Google Scholar]
- Kellokumpu S., Sormunen R., Kellokumpu I. Abnormal glycosylation and altered Golgi structure in colorectal cancer: dependence on intra-Golgi pH. FEBS Lett. 2002;516:217–224. doi: 10.1016/s0014-5793(02)02535-8. [DOI] [PubMed] [Google Scholar]
- Keyaerts E., Vijgen L., Maes P., Neyts J., Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem. Biophys. Res. Commun. 2004;323:264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohanski M.A., Workman A.D., Patel N.N., Hung L.Y., Shtraks J.P., Chen B., Blasetti M., Doghramji L., Kennedy D.W., Adappa N.D., Palmer J.N., Herbert D.R., Cohen N.A. Solitary chemosensory cells are a primary epithelial source of IL-25 in patients with chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2018;142:460–469. doi: 10.1016/j.jaci.2018.03.019. e467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiek J.J., Haystead T.A., Rudolph J. Kinetic mechanism of quinone oxidoreductase 2 and its inhibition by the antimalarial quinolines. Biochemistry. 2004;43:4538–4547. doi: 10.1021/bi035923w. [DOI] [PubMed] [Google Scholar]
- Lee R.J., Hariri B.M., McMahon D.B., Chen B., Doghramji L., Adappa N.D., Palmer J.N., Kennedy D.W., Jiang P., Margolskee R.F., Cohen N.A. Bacterial d-amino acids suppress sinonasal innate immunity through sweet taste receptors in solitary chemosensory cells. Sci. Signal. 2017;10 doi: 10.1126/scisignal.aam7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R.J., Kofonow J.M., Rosen P.L., Siebert A.P., Chen B., Doghramji L., Xiong G., Adappa N.D., Palmer J.N., Kennedy D.W., Kreindler J.L., Margolskee R.F., Cohen N.A. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J. Clin. Invest. 2014;124:1393–1405. doi: 10.1172/JCI72094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson M.L., Safholm J., Al-Ameri M., Bergman P., Orre A.C., Sward K., James A., Dahlen S.E., Adner M. Bitter taste receptor agonists mediate relaxation of human and rodent vascular smooth muscle. Eur. J. Pharmacol. 2014;740:302–311. doi: 10.1016/j.ejphar.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Mauthe M., Orhon I., Rocchi C., Zhou X., Luhr M., Hijlkema K.J., Coppes R.P., Engedal N., Mari M., Reggiori F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy. 2018;14:1435–1455. doi: 10.1080/15548627.2018.1474314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maycotte P., Aryal S., Cummings C.T., Thorburn J., Morgan M.J., Thorburn A. Chloroquine sensitizes breast cancer cells to chemotherapy independent of autophagy. Autophagy. 2012;8:200–212. doi: 10.4161/auto.8.2.18554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlinden K.D., Deshpande D.A., Ghavami S., Xenaki D., Sohal S.S., Oliver B.G., Haghi M., Sharma P. Autophagy activation in asthma airways remodeling. Am. J. Respir. Cell Mol. Biol. 2019;60:541–553. doi: 10.1165/rcmb.2018-0169OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McChesney E.W. Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. Am. J. Med. 1983;75:11–18. doi: 10.1016/0002-9343(83)91265-2. [DOI] [PubMed] [Google Scholar]
- Meyerhof W., Batram C., Kuhn C., Brockhoff A., Chudoba E., Bufe B., Appendino G., Behrens M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses. 2010;35:157–170. doi: 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]
- Mizushima N., Levine B. Autophagy in mammalian development and differentiation. Nat. Cell Biol. 2010;12:823–830. doi: 10.1038/ncb0910-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradian N., Ochs H.D., Sedikies C., Hamblin M.R., Camargo C.A., Jr., Martinez J.A., Biamonte J.D., Abdollahi M., Torres P.J., Nieto J.J., Ogino S., Seymour J.F., Abraham A., Cauda V., Gupta S., Ramakrishna S., Sellke F.W., Sorooshian A., Wallace Hayes A., Martinez-Urbistondo M., Gupta M., Azadbakht L., Esmaillzadeh A., Kelishadi R., Esteghamati A., Emam-Djomeh Z., Majdzadeh R., Palit P., Badali H., Rao I., Saboury A.A., Jagan Mohan Rao L., Ahmadieh H., Montazeri A., Fadini G.P., Pauly D., Thomas S., Moosavi-Movahed A.A., Aghamohammadi A., Behmanesh M., Rahimi-Movaghar V., Ghavami S., Mehran R., Uddin L.Q., Von Herrath M., Mobasher B., Rezaei N. The urgent need for integrated science to fight COVID-19 pandemic and beyond. J. Transl. Med. 2020;18:205. doi: 10.1186/s12967-020-02364-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak A.P., Shah S.D., Michael J.V., Deshpande D.A. Bitter taste receptors for asthma therapeutics. Front. Physiol. 2019;10:884. doi: 10.3389/fphys.2019.00884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak A.P., Villalba D., Deshpande D.A. Bitter taste receptors: an answer to comprehensive asthma control? Curr. Allergy Asthma Rep. 2019;19:48. doi: 10.1007/s11882-019-0876-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M.L., Tan S.H., See E.E., Ooi E.E., Ling A.E. Proliferative growth of SARS coronavirus in Vero E6 cells. J. Gen. Virol. 2003;84:3291–3303. doi: 10.1099/vir.0.19505-0. [DOI] [PubMed] [Google Scholar]
- Pan S., Sharma P., Shah S.D., Deshpande D.A. Bitter taste receptor agonists alter mitochondrial function and induce autophagy in airway smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2017;313:L154–l165. doi: 10.1152/ajplung.00106.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W.M., Yang J., Diagne A. Chloroquine inhibits HIV-1 replication in human peripheral blood lymphocytes. Immunol. Lett. 1998;64:45–47. doi: 10.1016/s0165-2478(98)00096-0. [DOI] [PubMed] [Google Scholar]
- Patel N.N., Kohanski M.A., Maina I.W., Triantafillou V., Workman A.D., Tong C.C.L., Kuan E.C., Bosso J.V., Adappa N.D., Palmer J.N., Herbert D.R., Cohen N.A. Solitary chemosensory cells producing interleukin-25 and group-2 innate lymphoid cells are enriched in chronic rhinosinusitis with nasal polyps. Int. Forum Allergy Rhinol. 2018 doi: 10.1002/alr.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton N.I., Lee L., Xu Y., Ooi E.E., Cheung Y.B., Archuleta S., Wong G., Wilder-Smith A. Chloroquine for influenza prevention: a randomised, double-blind, placebo controlled trial. Lancet Infect. Dis. 2011;11:677–683. doi: 10.1016/S1473-3099(11)70065-2. [DOI] [PubMed] [Google Scholar]
- Pulkkinen V., Manson M.L., Safholm J., Adner M., Dahlen S.E. The bitter taste receptor (TAS2R) agonists denatonium and chloroquine display distinct patterns of relaxation of the Guinea pig trachea. Am. J. Physiol. Lung Cell Mol. Physiol. 2012;303:L956–L966. doi: 10.1152/ajplung.00205.2012. [DOI] [PubMed] [Google Scholar]
- Sahraei Z., Shabani M., Shokouhi S., Saffaei A. Aminoquinolines against coronavirus disease 2019 (COVID-19): chloroquine or hydroxychloroquine. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders C.J., Christensen M., Finger T.E., Tizzano M. Cholinergic neurotransmission links solitary chemosensory cells to nasal inflammation. Proc. Natl. Acad. Sci. U. S. A. 2014;111:6075–6080. doi: 10.1073/pnas.1402251111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect. Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino A., Di Trani L., Donatelli I., Cauda R., Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect. Dis. 2006;6:67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrezenmeier E., Dorner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 2020;16:155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- Sharma P., Panebra A., Pera T., Tiegs B.C., Hershfeld A., Kenyon L.C., Deshpande D.A. Antimitogenic effect of bitter taste receptor agonists on airway smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2016;310:L365–L376. doi: 10.1152/ajplung.00373.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P., Yi R., Nayak A.P., Wang N., Tang F., Knight M.J., Pan S., Oliver B., Deshpande D.A. Bitter taste receptor agonists mitigate features of allergic asthma in mice. Sci. Rep. 2017;7:46166. doi: 10.1038/srep46166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaei S., Suresh M., Klionsky D.J., Labouta H.I., Ghavami S. Autophagy and SARS-CoV-2 infection: apossible smart targeting of the autophagy pathway. Virulence. 2020;11:805–810. doi: 10.1080/21505594.2020.1780088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon V.R., Lee H. Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur. J. Pharmacol. 2009;625:220–233. doi: 10.1016/j.ejphar.2009.06.063. [DOI] [PubMed] [Google Scholar]
- Sperber K., Kalb T.H., Stecher V.J., Banerjee R., Mayer L. Inhibition of human immunodeficiency virus type 1 replication by hydroxychloroquine in T cells and monocytes. AIDS Res. Hum. Retrovir. 1993;9:91–98. doi: 10.1089/aid.1993.9.91. [DOI] [PubMed] [Google Scholar]
- Sureda A., Alizadeh J., Nabavi S.F., Berindan-Neagoe I., Cismaru C.A., Jeandet P., Los M.J., Clementi E., Nabavi S.M., Ghavami S. Endoplasmic reticulum as a potential therapeutic target for covid-19 infection management? Eur. J. Pharmacol. 2020;882:173288. doi: 10.1016/j.ejphar.2020.173288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai W.P., Nara P.L., Kung H.F., Oroszlan S. Inhibition of human immunodeficiency virus infectivity by chloroquine. AIDS Res. Hum. Retrovir. 1990;6:481–489. doi: 10.1089/aid.1990.6.481. [DOI] [PubMed] [Google Scholar]
- Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Jiang C. Avian influenza H5N1: an update on molecular pathogenesis. Sci. China Ser. C Life Sci. 2009;52:459–463. doi: 10.1007/s11427-009-0059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y., Luo G., Wang K., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., Wang Y., Ding D., Wu F., Tang X., Ye X., Ye Y., Liu B., Yang J., Yin W., Wang A., Fan G., Zhou F., Liu Z., Gu X., Xu J., Shang L., Zhang Y., Cao L., Guo T., Wan Y., Qin H., Jiang Y., Jaki T., Hayden F.G., Horby P.W., Cao B., Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:P1569–P1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Bowden T.A., Wilson I.A., Crispin M. Exploitation of glycosylation in enveloped virus pathobiology. Biochim. Biophys. Acta Gen. Subj. 2019;1863:1480–1497. doi: 10.1016/j.bbagen.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weniger H. World health Organization; Geneva: 1979. Review of Side Effects and Toxicity of Chloroquine. [Google Scholar]
- Yan Y., Zou Z., Sun Y., Li X., Xu K.F., Wei Y., Jin N., Jiang C. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res. 2013;23:300–302. doi: 10.1038/cr.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., Zhan S., Lu R., Li H., Tan W., Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki A., Ott S., Sandhu K., Ghavami S., Kenyon N. Modulating Eotaxin-3 Production and Secretion from Human Airway Epithelial Cells. American Thoracic Society Meeting. Am J Respir Crit Care Med; San Diego, CA, USA: 2014. The complex roles of endoplasmic reticulum stress and autophagy; p. A5683. [Google Scholar]
- Zhou D., Dai S.M., Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J. Antimicrob. Chemother. 2020 doi: 10.1093/jac/dkaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]