Abstract
Purpose
COVID-19 associated hearing loss is still an ongoing matter of debate. No original studies exist on audiological effects of SARS-CoV-2 infection in hospitalized patients. The main objective was to determine whether SARS-CoV-2 may affect auditory function in clinically ill COVID-19 patients.
Materials and methods
COVID-19 patients with moderate-severe disease and without prior history of hearing abnormalities were enrolled from a tertiary referral center, and matched with controls. Participants performed an audiometric evaluation, and thresholds were compared.
Results
120 ears from 60 patients were enrolled. Patients with COVID-19 showed worse mean auditory thresholds starting from 1000 Hz through higher frequencies, when compared to controls (1000 Hz: 18.52 ± 5.49 dB HL in controls vs 25.36 ± 6.79 dB HL in COVID-19, p < 0.001; 2000Hz: 17.50 ± 5.57 dB HL in controls vs 21.96 ± 7.05 dB HL in COVID-19, p = 0.010; 3000Hz: 17.97 ± 8.07 dB HL in controls vs 25 ± 9.38 dB HL in COVID-19, p = 0.003; 4000 Hz: 20.16 ± 10.12 dB HL in controls vs 29.55 ± 11.26 dB HL in COVID-19, p = 0.001; 8000 Hz: 31.09 ± 12.75 dB HL in controls vs 40.71 ± 19.40 dB HL in COVID-19, p = 0.030; Pure Tone Average: 20.42 ± 4.29 dB HL in controls vs 24.85 ± 5.62 dB HL in COVID-19, p = 0.001). Statistical significance persisted after adjusting for confounders such as age, gender and various comorbidities (p < 0.05).
Conclusions
SARS-CoV-2 may affect hearing in COVID-19 patients with moderate-severe disease. Results are in line with the previous suggested effects of COVID-19 on auditory system. This study is expected to encourage further research on this topic.
Keywords: SARS-CoV-2, COVID-19, Coronavirus, Audiometry, Hearing
1. Introduction
Coronavirus disease 2019 (COVID-19) pandemic is an enormous public health challenge implying an outrageous number of infections and casualties worldwide. A lot is still unknown about the possible short-term and long-term effects of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection on the human physiology (Wiersinga et al., 2020). Reported symptoms such as fever, fatigue, dry cough or dyspnea may reflect the respiratory affection (Berlin et al., 2020; Carfì et al., 2020; Gandhi et al., 2020). Nevertheless, there are various case reports relating to the involvement of cranial nerves by the SARS-CoV-2, as it seems to be a reasonable neurotropic entity (Pezzini and Padovani, 2020; Yachou et al., 2020). The reported cranial mononeuropathies range from the prevalent anosmia (Vaira et al., 2020) to rarer isolated facial palsy (Lima et al., 2020) or ophthalmoparesis (Dinkin et al., 2020), but there are also descriptions of concomitant/multiple cranial neuropathies on the same patient (Gutiérrez-Ortiz et al., 2020). The vestibulocochlear system seems to be no exception, with reports concerning vestibular neuritis, disequilibrium, tinnitus and sudden hearing loss (Koumpa et al., 2020; Malayala and Raza, 2020; Viola et al., 2020).
It is well established that several viral infections may lead to hearing loss through different mechanisms, such as direct damage to the inner ear structures or indirect lesion by eliciting inflammatory responses (Cohen et al., 2014). In the particular case of SARS-CoV-2, it is still unclear whether the auditory system may be a target, although the virus has already been isolated within middle ear and mastoid tissue (Frazier et al., 2020). Interestingly, a study by Mustafa showed poorer pure-tone thresholds in high-frequencies as well as worse Transient Evoked Otoacoustic Emissions (TEOAE) amplitudes in asymptomatic COVID-19 individuals (Mustafa, 2020). To date, no studies are found regarding audiological evaluation of symptomatic or hospitalized COVID-19 patients. Thus, the objective of this study was to contribute to the fulfillment of that gap in scientific evidence: describing the hearing function of hospitalized patients with documented moderate-severe COVID-19 illness (test group), while comparing them to controls. Additionally, hypothesized predictors for hearing function on COVID-19 patients were explored. This study ultimately aims to address the impact SARS-CoV-2 may have on the hearing function of clinically ill patients.
2. Materials and Methods
The study was conducted during November and December 2020, at the peak of Portugal’s COVID-19 second wave. A total of 60 patients and 120 ears were enrolled. Twenty-eight patients (56 ears) hospitalized in the infectious diseases’ COVID-19 Unit at Centro Hospitalar Universitário do Porto were eligible for the test group. Patients enrolled at the COVID-19 unit were either on isolated rooms or alone in a wider shared room. Eligibility criteria were as follows: 1) SARS-CoV-2 diagnostic confirmation by nasal swab and Polymerase chain reaction method (PCR); 2) hospitalization for moderate-severe SARS-CoV-2 disease; 3) clinical stability that would allow collaboration in the realization of the audiogram; 4) age < 75 years. Patients with respiratory distress signs, cognitive impairment, metabolic-infectious encephalopathy or previous reported hearing loss were excluded.
A parallel investigation using the very same method was performed at a primary care center (Unidade de Saúde Familiar Garcia de Orta, Porto) to recruit thirty-two individuals (64 ears) from the general population attending medical appointments (control sample). The audiogram was performed with the patient alone in a selected calm doctor’s office away from the waiting room. The eligibility criteria for the control group were as follows: 1) absence of known previous positivity for SARS-CoV-2 PCR, antibody or antigenic tests; 2) absence of clinical signs suggesting COVID-19 during the pandemic (anosmia, ageusia, fever, dyspnea, cough, myalgias, headache); 3) clinical stability that would allow collaboration in the realization of the audiogram; 4) age < 75 years. Patients with cognitive impairment or previously reported hearing loss were excluded.
All patients were evaluated through a simplified audiogram method using an ®Apple device running an audiogram application (app). Various papers have confirmed that these apps, when administrated in a controlled setting, can provide results that are comparable to those of conventional audiometry and can be used as a valid screening tool (Bright and Pallawela, 2016a; Masalski et al., 2018; Saliba et al., 2017; Whitton et al., 2016). There are various audiogram apps for iOS and Google-Android systems; a pre-study selection was performed testing multiple apps to fit the purposes - reasonable acuity, user-friendly interface and duration of the exam. The 2.1.5 version of “hearing test-Audiometry, Tone” from ©IT4You was chosen using the “Advanced hearing test” mode. The algorithm for audiometry used by its developer has proven to be useful in Petralex® hearing aid application technology using various types of headphones (Roe, 2018; Vashkevich et al., 2017). Although the application was designed for self-assessment, an adaptation was made to reduce either bias and device contamination, such that the patient would be in contact solely with the on-ear headphones and make a hand signal when hearing any sound; the investigator was the one operating the app to check for the audiometric thresholds. ®Sony WH-CH510 supra-aural headphones were chosen due to wireless bluetooth mode (facilitating transport and disinfection), light weight, long battery autonomy, echo cancellation and noise suppression specifications. Pure-tone auditory thresholds at 125 Hz, 250 Hz, 500 Hz, 1000 Hz, 2000 Hz, 3000 Hz, 4000 Hz and 8000 Hz were registered. An effort was made to minimize environmental noise at the Hospital and Primary care settings in order to maximize the exam acuity. In both scenarios, ambient noise level was checked prior to the examination by using ®NIOSH Sound Level Meter application by Centers for Disease Control and Prevention (CDC) since it showed high accuracy (Brown et al., 2020). Audiogram was performed when instantaneous levels were ≤60 dB(A).
Verbal consent was preferred due to the risk of contamination by droplets if paper was used. The audiogram was only performed after careful explanation about the characteristics, non-invasiveness and aim of the study. The study design complies with the Declaration of Helsinki ethical standards and was approved by the Scientific Investigation Department, Bioethical Committee and Administrative Board of Centro Hospitalar Universitário do Porto.
Statistical analysis was performed using SPSS (IBM SPSS Statistics 26). In the descriptive analysis, categorical variables are presented as percentages, and continuous variables as means and standard deviations, or medians and interquartile range for variables with skewed distributions. Normal distribution was checked using both skewness and kurtosis and Kolmogorov-Smirnov tests. The bivariate associations were analyzed using either independent t-test (parametric analysis) or Mann-Whitney test (non-parametric analysis) depending on the tests for normality, Pearson Chi-square/Fisher’s tests (95% confidence intervals) for categorical variables and Spearman’s test for continuous variables. In order to adjust for potential confounders linear regression models and ANOVA were performed to increase statistical validity. All reported p values are two-tailed, with a p value ≤ 0.05 indicating statistical significance.
3. Results
3.1. Study population
3.1.1. COVID-19 test group
All patients had registered radiological signs of pneumonia before performing the audiogram, with only 3 patients (10,7%) showing a PaO2/FiO2 ≥ 300 at Hospital admission. While performing audiometry, 75% of patients had oxygen delivery by nasal cannula, and 25% were without supplemental oxygen. Relevant descriptive results are shown in Table 1. Note that male gender predominated, with hypertension, obesity, dyslipidemia and diabetes being the most frequent associated comorbidities in this subpopulation of COVID-19 (Table 1). Dexamethasone and remdesivir were offered to most individuals prior to the audiogram (Table 1).
Table 1.
Descriptive analysis of COVID-19 patients (test group).
| Continuous variables | Mean ( Standard deviation) |
Categorical variables | Frequency (%) |
|---|---|---|---|
| Age (years) a | Gender(male) | ||
| PO2/FiO2 admission a | Obstructive sleep apnea | ||
| Sedimentation rate (mm; 1st hour)a | Autoimune disease | ||
| Fibrinogen (mg/dl; clauss)a | Diabetes Mellitus | ||
| D-Dimers (ng/mL)a | Hypertension | ||
| Activated Partial thromboplastin time (seconds) a | Dyslipidemia | ||
| Prothrombin time (seconds) a | Chronic Pulmonary disease | ||
| Ferritin (ng/mL) a | Asthma | ||
| Reactive C protein (mg/L) a | Smoking | ||
| Procalcitonin (ng/mL)a | Rhinosinusitis | ||
| HbA1C (%) | Obesity | ||
| Symptoms-to-audiogram (days) | Previous chemotherapy | ||
| PO2/FIO2 audiogramb | Dexamethasone treatment d | ||
| Length-of-stay (days) c | Remdesivir treatmentd |
- Measured at hospital admission.
- same day or most recent value from the audiogram day.
- from admission to hospital discharge.
- at the time of audiogram performance; Note: All values shown are valid percent and exclude missing values from the equation.
3.2. Primary care control group
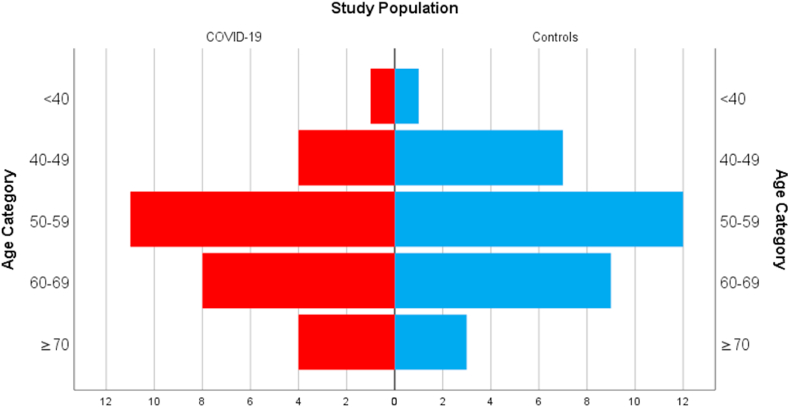
A quality analysis was performed to check for differences between test and control groups. No significant differences were found concerning gender, age or concurrent comorbidities in the primary care and the COVID-19 samples (p > 0,05) as shown in Table 2. The age distribution across both groups is shown in Fig. 1.
Table 2.
Descriptive analysis of Primary care patients (control group) and comparison with COVID-19 sample (test group).
| Variable | Control group Primary care (mean + standard deviation for age) |
Test group COVID-19 Unit (mean + standard deviation for age) |
p value (1) (for differences between groups) |
|---|---|---|---|
| Gender (male) | % | ||
| Age (years) | |||
| Obstructive sleep apnea | % | ||
| Autoimune disease | % | ||
| Diabetes Mellitus | |||
| Hypertension | |||
| Dyslipidemia | % | ||
| Chronic Pulmonary disease | % | ||
| Asthma | |||
| Smoking | |||
| Rhinosinusitis | |||
| Obesity | |||
| Previous chemotherapy |
1- Independent t-test used for comparing age between groups and Chi-Square/Fisher’s test for the other variables. Note: All values shown are valid percent and exclude missing values from the equation.
Fig. 1.
Number of patients across age categories for control and test populations.
3.3. Audiometric analysis: COVID-19 vs controls
In order to evaluate the main hypothesis of the study a bivariate analysis was first used comparing dB threshold values for each frequency in both groups. In the Independent t-test there were significant statistical higher values for auditory thresholds in COVID-19 patients beginning at 1000 Hz when compared to controls (Table 3). At 125 Hz an inverse relation existed with COVID-19 patients showing better outcome.
Table 3.
Descriptive analysis of Audiometric Thresholds: Primary care (control group) vs COVID-19 patients (test group).
| Pure-tone audiometric thresholds (Hz)1 |
Control group Primary care (mean + standard deviation dB) |
Test group COVID-19 Unit (mean + standard deviation dB) |
p value (1) (for differences between groups) |
|---|---|---|---|
| 125 | |||
| 250 | |||
| 500 | |||
| 1000 | |||
| 2000 | |||
| 3000 | |||
| 4000 | |||
| 8000 | |||
| PTA2 |
1- Left + right ear mean was calculated for each patient for analysis. 2- Pure tone average calculated with left + right ear mean values of 500, 1000 and 2000 Hz. Independent t-test used for comparing subgroups. Statistically significant p values are highlighted in bold.
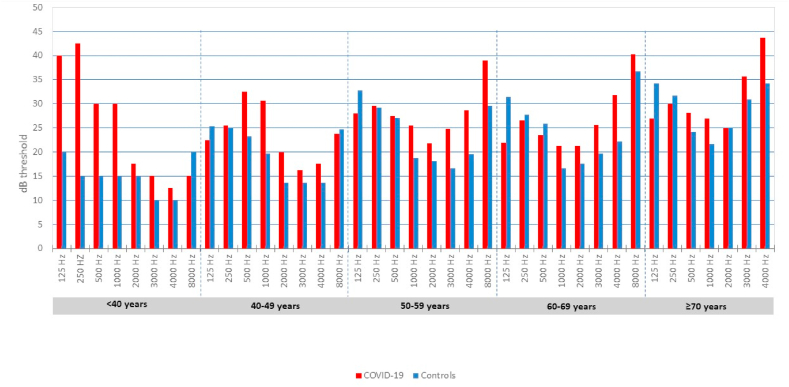
To confirm this trend, age categories were created and the Independent t-test was run inside those categories for finer comparison (see Fig. 2), at the cost of having a limited sample size for each category (Fig. 1). In the [40–50] years category COVID-19 patients showed higher thresholds in the 500 Hz (23,21 ± 4.73 dB HL in controls vs 32.50 ± 8.90 dB HL in COVID-19, p = 0.047), 1000 Hz (19.64 ± 4.43 dB HL in controls vs 30.62 ± 2.39 dB HL in COVID-19, p < 0.001) and pure tone average (PTA) (18.81 ± 3.66 dB HL in controls vs 27.71 ± 4.58 dB in COVID-19, p = 0.020). In the [50–60] years category COVID-19 patients showed higher thresholds in the 125 Hz (32.71 ± 3.10 dB HL in controls vs 27.95 ± 4.98 dB HL in COVID-19, p = 0.015), 1000 Hz (18.75 ± 5.17 dB HL in controls vs 25.45 ± 4.85 dB HL in COVID-19, p = 0.004), 3000 Hz (16.67 ± 4.44 dB HL in controls vs 24.77 ± 8.62 dB HL in COVID-19, p = 0.009) and 4000 Hz (19.79 ± 7.03 dB HL in controls vs 28.64 ± 8.61 dB HL in COVID-19, p = 0.0015). In the [60–70] and ≥70 years group no significant differences were registered although there was a trend towards significance in higher frequencies. Fig. 2 illustrates the pure-tone threshold distribution across age categories for both COVID-19 and control groups.
Fig. 2.
Pure-tone audiometric thresholds across multiple age groups: COVID-19 vs controls.
A linear regression was performed for multivariate analysis considering age, gender and the multiple comorbidities as potential confounders. The outcome was defined as the pure-tone threshold mean in each of the various frequencies. Pure-tone thresholds were significantly worse in COVID-19 patients beginning at 1000 Hz even after confoundment adjustment methods (Table 4). There was also a significant difference between the two groups concerning pure tone average (PTA) with COVID-19 patients showing worse outcomes. At 125 Hz an inverse relation existed with COVID-19 patients showing better outcome.
Table 4.
Results from linear regression model with all the comorbidities, age and gender as potential confounders taken in count.
| COVID-19 as an independent variable predicting Pure-Tone threshold | β1 | SE β2 | Beta 3 | t4 | P value |
|---|---|---|---|---|---|
| 125 Hz | |||||
| 250 Hz | |||||
| 500 Hz | |||||
| 1000 Hz | |||||
| 2000 Hz | |||||
| 3000 Hz | |||||
| 4000 Hz | |||||
| 8000 Hz | |||||
| PTA5 |
1) β stands for unstandardized regression coefficient; 2) Standard error for unstandardized regression coefficient; 3) Standardized Regression coefficient; 4) ; 5) Pure tone average calculated with left + right ear mean values of 500, 1000 and 2000 Hz. Statistically significant p values are highlighted in bold.
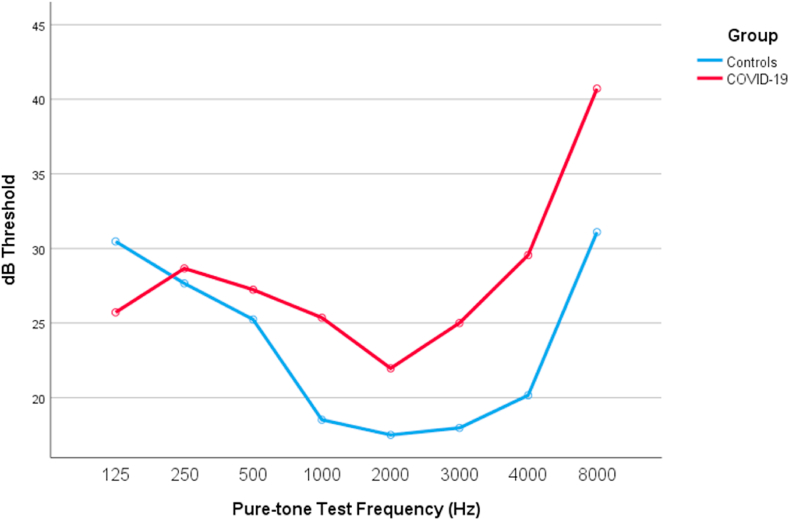
A repeated measures ANOVA comparison using the Bonferroni correction confirmed that patients with COVID-19 had significantly higher auditory thresholds starting from 1000 Hz through higher frequencies, when compared to the control group (see Fig. 3). In fact, the auditory affection in COVID-19 patients directly increased with frequency. From this model results, COVID-19 explains at least 12.3% of the audiological variance registered between test and control groups (F (1,58) = 8.477, p = 0.005). In order to definitely confirm that age (presbycusis) did not interfered with this model, an additional between-subjects factorial ANOVA was performed to account for age categories. Results sustained that COVID-19 had deleterious effects on hearing even when age was considered (F (1,50) = 7.836, p = 0.007). No synergic interaction effect was noted between COVID-19 and age on hearing loss (F (1,50) = 0.360, p = 0.836).
Fig. 3.
Pure-tone audiometric thresholds means for both test and control groups.
3.4. Predictors of hearing loss in COVID-19
After meeting the primary objective of the study in the previous section, a wide analysis was made in order to search for predictors of worse auditory thresholds in this population of COVID-19 patients. Fibrinogen levels at admission were related to worse auditory thresholds at 4000 Hz (p = 0.015), and so did D-dimers at admission (p = 0.017) after performing the Spearman’s test. Importantly, no impact was found on auditory thresholds in the various frequencies for patients treated with remdesivir compared to patients not receiving that treatment (p > 0.05 in the T-test for the all frequencies). The same was true for patients receiving or not receiving dexamethasone (p > 0.05). No associations were found between PO2/FiO2 at admission, PO2/Fi02 at audiogram, sedimentation rate, activated partial thromboplastin time, prothrombin time, reactive C protein, procalcitonin, HbA1C, symptoms-to-audiogram time or final length of hospital stay and auditory thresholds (p > 0.05).
4. Discussion
There is a lack of discussion on the relationship between COVID-19 and hearing, despite the plethora of literature on COVID-19. Hearing loss and tinnitus are symptoms that have been seen in patients with COVID-19 (Degen et al., 2020; Koumpa et al., 2020; Sriwijitalai and Wiwanitkit, 2020; Viola et al., 2020). However, affection of hearing by COVID-19 may not be limited to a clinically perceived loss, as recently suggested by Mustafa (Mustafa, 2020). Many COVID-19 patients present with remarkable disconnect between profound hypoxemia yet without proportional signs of respiratory distress i.e. “happy” hypoxemia (Dhont et al., 2020). Will in fact COVID-19 also be able to elicit an “happy hypoacusis” subclinical hearing loss? Despite the need for large scale studies, it seems reasonable to consider the possibility of a relationship between COVID-19 and hearing loss. SARS-CoV-2 is believed to bind to the ACE-2 receptor which was recently seen to be expressed in epithelial cells of the middle ear, as well as the stria vascularis and spiral ganglion in mice (Uranaka et al., 2020). Furthermore, SARS-CoV-2 infection causes an inflammatory response and an increase in cytokines known to be potentially harmful to cochlear structures (Koumpa et al., 2020; Tan et al., 2013; Vallamkondu et al., 2020). Both a direct entry into the cochlea or secondary inflammation are possible mechanisms that could occur in the setting of SARS-CoV-2 infection (Koumpa et al., 2020). Other potential mechanism already documented in some viruses is the ability to affect brainstem, especially considering SARS-CoV-2 neurotropic character (Machado et al., 2020; Mustafa, 2020). Because COVID-19 is mainly a respiratory tract disease, its potential to affect Eustachian tube function and middle ear mucosa leading to conductive hearing loss should also be considered.
The primary objective of evaluating hearing function of COVID-19 hospitalized patients by matching them with general population was met. Our results point to a pure-tone loss beginning at 1000 Hz and extending through 2000, 3000, 4000 and 8000 Hz when compared to controls. The study conducted by Mustafa in asymptomatic COVID-19 patients had significant differences beginning only at 4000 and extending to 6000 and 8000 Hz (Mustafa, 2020). Hence, our study is partly in line with the asymptomatic COVID-19 counterpart by supporting the idea that COVID-19 infection can have deleterious effects on cochlear hair cell functions(Mustafa, 2020). The difference however is that in COVID-19 hospitalized patients significance was also found further beyond between 1000 and 8000 Hz. The latter may be due to the fact that: 1) viral load is greater in symptomatic patients – when direct damage is considered as a causal mechanism to cochlear system structures; 2) Immune system activation is greater in our study’s population – eliciting moderate-severe disease and concomitant greater attack to inner ear structures; 3) Our sample size is slightly larger and this difference reflects a pre-existent statistical tendency.
After exploring the disparity between test and control groups, an effort was made to look for marker predictors of poor auditory outcomes in the COVID-19 hospitalized group. A relationship was found between D-Dimer and fibrinogen values and Pure-Tone thresholds at 4000 Hz. Microangiopathic events were already reported in COVID-19 (Kirschenbaum et al., 2020). In theory, a vascular event could contribute to cochlear dysfunction in these patients, especially in the case of sudden hearing loss in symptomatic patients (Kirschenbaum et al., 2020). Nevertheless, our findings relate to an isolated frequency, making us favor a direct viral/inflammatory/brainstem modulation explanation for the findings. Literature also refers to remdesivir as a potential ototoxic drug. No significant effect of remdesivir treatment was observed in auditory thresholds in this study (Ciorba et al., 2020).
The tonal audiogram is a commonly used tool to measure a subject’s self-perception of sound, being an instrument for evaluation of the cochlear system. The routinely used material to perform the examination would imply a soundproof cabin and clinically calibrated headphones. One can understand that patient transferring for audiology facilities could endanger feasibility due to potential exposure of other professionals to SARS-CoV-2, costs of disinfection and the need for additional organizational circuits. The creation of this simplified method allowed the testing of isolated patients and made possible an otherwise arduous design. These kind of assessments are not expected to replace clinical audiometry; however, they may prove useful in difficult scenarios (Bright and Pallawela, 2016b; Masalski et al., 2018; Saliba et al., 2017; Wang et al., 2014). Measuring of bone conduction thresholds and tympanograms were not performed due to technical limitations, making difficult to assure that cochlear or neural hearing loss explain the results. Further studies are needed contemplating alternative methods, a larger sample size or comparing different COVID-19 subpopulations.
5. Conclusions
To our Knowledge this is the first original study to address the auditory function of hospitalized COVID-19 patients. Our findings suggest that SARS-CoV-2 may elicit unnoted hearing loss in symptomatic COVID-19 patients with moderate-severe disease, irrespectively of age. Importantly, it is in line with the previous suggested effect of COVID-19 on auditory function. Anecdotal reports of sudden hearing loss exist, but a more silent way of hearing impingement may ensue. The relationship between COVID-19 and hearing loss as well as the mechanism involved require further research.
CRediT authorship contribution statement
Francisco Alves de Sousa: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data curation, Writing - original draft. Rodrigo Pinto Costa: Investigation, Data curation, Resources, Writing - review & editing. Sandra Xará: Writing - review & editing, Supervision. Ana Nóbrega Pinto: Writing - review & editing, Supervision, Project administration. Cecília Almeida e Sousa: Supervision, Project administration.
Declaration of competing interest
This is an independent study. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Authors have no competing interests to declare.
Acknowledgments
We would like to express deepest gratitude to the clinical body of the Infectious Diseases Department from Centro Hospitalar Universitário do Porto, so as to chief nurse officer Teresa Cruz and her team for the unwavering support of this work during difficult times fighting against the COVID-19 pandemic.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
Contributor Information
Francisco Alves de Sousa, Email: franciscoalvesousa@gmail.com, u13387@chporto.min-saude.pt.
Rodrigo Pinto Costa, Email: rodrigo.costa92@gmail.com.
Sandra Xará, Email: xarazita@yahoo.com.
Ana Nóbrega Pinto, Email: ananobregapinto@gmail.com.
Cecília Almeida e Sousa, Email: director.orl@hgsa.min-saude.pt.
References
- Berlin D.A., Gulick R.M., Martinez F.J. Severe covid-19. N. Engl. J. Med. 2020;383(25):2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- Bright T., Pallawela D. Validated smartphone-based apps for ear and hearing assessments: a review. JMIR Rehabil. Assist. Technol. 2016;3:e13. doi: 10.2196/rehab.6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright T., Pallawela D. Validated smartphone-based apps for ear and hearing assessments: a review. JMIR Rehabil. Assist. Technol. 2016;3:e13. doi: 10.2196/rehab.6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P., Biggs T., Crossley E., Singh T. The accuracy of iPhone applications to monitor environmental noise levels. Laryngoscope. 2020;131 doi: 10.1002/lary.28590. [DOI] [PubMed] [Google Scholar]
- Carfì A., Bernabei R., Landi F., Group for the G.A.C.-19 P.-A.C.S. Persistent symptoms in patients after acute COVID-19. J. Am. Med. Assoc. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciorba A., Corazzi V., Skarżyński P.H., Skarżyńska M.B., Bianchini C., Pelucchi S., Hatzopoulos S. Don’t forget ototoxicity during the SARS-CoV-2 (Covid-19) pandemic! Int. J. Immunopathol. Pharmacol. 2020;34:2. doi: 10.1177/2058738420941754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B.E., Durstenfeld A., Roehm P.C. Viral causes of hearing loss: a review for hearing health professionals. Trends Hear. 2014;18:1–17. doi: 10.1177/2331216514541361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen C., Lenarz T., Willenborg K. Acute profound sensorineural hearing loss after COVID-19 pneumonia. Mayo Clin. Proc. 2020;95:1801–1803. doi: 10.1016/j.mayocp.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhont S., Derom E., Van Braeckel E., Depuydt P., Lambrecht B.N. The pathophysiology of “happy” hypoxemia in COVID-19. Respir. Res. 2020;21:1–9. doi: 10.1186/s12931-020-01462-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkin M., Gao V., Kahan J., Bobker S., Simonetto M., Wechsler P., Harpe J., Greer C., Mints G., Salama G., Tsiouris A.J., Leifer D. COVID-19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology. 2020;95:221–223. doi: 10.1212/WNL.0000000000009700. [DOI] [PubMed] [Google Scholar]
- Frazier K.M., Hooper J.E., Mostafa H.H., Stewart C.M. SARS-CoV-2 virus isolated from the mastoid and middle ear: implications for COVID-19 precautions during ear surgery. JAMA Otolaryngol. Neck Surg. 2020;146:964–966. doi: 10.1001/jamaoto.2020.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi R.T., Lynch J.B., del Rio C. Mild or moderate covid-19. N. Engl. J. Med. 2020;383:1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Ortiz C., Méndez-Guerrero A., Rodrigo-Rey S., San Pedro-Murillo E., Bermejo-Guerrero L., Gordo-Mañas R., de Aragón-Gómez F., Benito-León J. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95:e601–e605. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- Kirschenbaum D., Frontzek K., Steiger P., Dietler S., Abela I.A., Lutterotti A., Stippich C., Globas C., Varga Z. Large and small cerebral vessel involvement. 2020. 3719-3722. [DOI] [PMC free article] [PubMed]
- Koumpa F.S., Forde C.T., Manjaly J.G. Sudden irreversible hearing loss post COVID-19. BMJ Case Rep. 2020;13:13–15. doi: 10.1136/bcr-2020-238419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima M.A., Silva M.T.T., Soares C.N., Coutinho R., Oliveira H.S., Afonso L., Espíndola O., Leite A.C., Araujo A. Peripheral facial nerve palsy associated with COVID-19. J. Neurovirol. 2020;26:941–944. doi: 10.1007/s13365-020-00912-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado C., DeFina P., Chinchilla M., Machado, Yanín, Machado, Yazmina Brainstem dysfunction in SARS-COV-2 infection can be a potential cause of respiratory distress. Neurol. 2020;68:989–993. doi: 10.4103/0028-3886.299165. [DOI] [PubMed] [Google Scholar]
- Malayala S.V., Raza A. A case of COVID-19-induced vestibular neuritis. Cureus. 2020;12 doi: 10.7759/cureus.8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masalski M., Grysiński T., Kręcicki T. Hearing tests based on biologically calibrated mobile devices: comparison with pure-tone audiometry. JMIR mHealth uHealth. 2018;6:1–12. doi: 10.2196/mhealth.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa M.W.M. Audiological profile of asymptomatic Covid-19 PCR-positive cases. Am. J. Otolaryngol. 2020;41(3) doi: 10.1016/j.amjoto.2020.102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzini A., Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat. Rev. Neurol. 2020;16:636–644. doi: 10.1038/s41582-020-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe M.C. An application of the medical research council’s guidelines for evaluating complex interventions: a usability study assessing smartphone-connected listening devices in adults with hearing loss. Educ. Rev. 2018;16:227–230. doi: 10.1080/0013191640160307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba J., Al-Reefi M., Carriere J.S., Verma N., Provencal C., Rappaport J.M. Accuracy of mobile-based audiometry in the evaluation of hearing loss in quiet and noisy environments. Otolaryngol. neck Surg. Off. J. Am. Acad. Otolaryngol. Neck Surg. 2017;156:706–711. doi: 10.1177/0194599816683663. [DOI] [PubMed] [Google Scholar]
- Sriwijitalai W., Wiwanitkit V. Hearing loss and COVID-19: a note. Am. J. Otolaryngol. 2020;41:102473. doi: 10.1016/j.amjoto.2020.102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W.J.T., Thorne P.R., Vlajkovic S.M. Noise-induced cochlear inflammation. World J. Otorhinolaryngol. 2013;3:89–99. doi: 10.5319/wjo.v3.i3.89. [DOI] [Google Scholar]
- Uranaka T., Kashio A., Ueha R., Sato T., Bing H., Ying G., Kinoshita M., Kondo K., Yamasoba T. Expression of Ace 2, Tmprss2, and furin in mouse ear tissue. 2020. bioRxiv 2020.06.23.164335. [DOI] [PubMed]
- Vaira L.A., Salzano G., Deiana G., De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020;130:1787. doi: 10.1002/lary.28692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallamkondu J., John A., Wani W.Y., Ramadevi S.P., Jella K.K., Reddy P.H., Kandimalla R. SARS-CoV-2 pathophysiology and assessment of coronaviruses in CNS diseases with a focus on therapeutic targets. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2020;1866:165889. doi: 10.1016/j.bbadis.2020.165889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashkevich M., Azarov E., Petrovsky N., Petrovsky A. Petralex: a smartphone-based real-time digital hearing aid with combined noise reduction and acoustic feedback suppression. Signal Process. - Algorithms, Archit. Arrange. Appl. Conf. Proceedings, SPA 2017-Septe. 2017:249–254. doi: 10.23919/SPA.2017.8166873. [DOI] [Google Scholar]
- Viola P., Ralli M., Pisani D., Malanga D., Sculco D., Messina L., Laria C., Aragona T., Leopardi G., Ursini F., Scarpa A., Topazio D., Cama A., Vespertini V., Quintieri F., Cosco L., Cunsolo E.M., Chiarella G. Tinnitus and equilibrium disorders in COVID-19 patients: preliminary results. Eur. Arch. oto-rhino-laryngology Off. J. Eur. Fed. Oto-Rhino-Laryngological Soc. Affil. with Ger. Soc. Oto-Rhino-Laryngology - Head Neck Surg. 2020:1–6. doi: 10.1007/s00405-020-06440-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.C., Zupancic S., Ray C., Cordero J., Demke J.C. Hearing test app useful for initial screening, original research shows. Hear. J. 2014;67:32–34. doi: 10.1097/01.HJ.0000455839.29274.d6. [DOI] [Google Scholar]
- Whitton J.P., Hancock K.E., Shannon J.M., Polley D.B. Validation of a self-administered audiometry application: an equivalence study. Laryngoscope. 2016;126:2382–2388. doi: 10.1002/lary.25988. [DOI] [PubMed] [Google Scholar]
- Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA, J. Am. Med. Assoc. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- Yachou Y., El Idrissi A., Belapasov V., Ait Benali S. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neurol. Sci. 2020;41:2657–2669. doi: 10.1007/s10072-020-04575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]