Two recent studies published in this journal focused on SARS-CoV-2 infection among hospital workers (HWs), the first one reported the prevalence of SARS-CoV-2 carriage among HWs and the second, the clinical presentation of symptomatic HWs in order to identify new cases as early as possible and to stop nosocomial transmission1 , 2. The objective of the present study was to estimate within the hospital, the risk of in-hospital HWs infection following a high-risk exposure to SARS-CoV-2-infected subject without personal protective equipment.
We conducted the CoV-CONTACT study, a prospective cohort which included HWs, hereafter referred to as “contacts” with an high risk exposure to an SARS-CoV-2-infected person (either a patient or a colleague) hereafter referred to as “index”, in the 1000 bed Bichat Claude Bernard University Hospital (Paris, France) between March, 3rd 2020 and April, 27th 20203. Exposure was considered to be at high-risk of SARS-CoV-2 transmission if it occurred i) face-to-face, within one meter and without protective surgical or FFP2/N95 mask, and ii) during a discussion or while the index had an episode of coughing or sneezing, and iii) in the 72 h prior to, or following the virological diagnosis, or during the symptomatic period of the index.
Following exposure and upon written informed consent, daily symptoms were self-reported for 30 days; nasopharyngeal swabs for SARS-CoV-2 RT-PCR were performed at inclusion and at days 3, 5, 7 and 12; SARS-CoV-2 IgG serology (LuLISA N and EuroIMMUN4 , 5) was assessed at inclusion and at day 30. Confirmed infection was defined by positive RT-PCR or seroconversion, and possible infection by one general and one specific symptom for two consecutive days. SARS-CoV-2 seroconversion was defined as the apparition of a positive SARS-CoV-2 serology at the D30 visit, or as an at least two-fold increase of the LuLISA signal or EuroIMMUN ratio between inclusion and day 30. The primary endpoint was confirmed or possible SARS-CoV-2 infection, hereafter referred to as “SARS-CoV-2 infection”.
The 146 analysed contacts were exposed to 42 COVID-19 index. No contacts worked in a front-line COVID-19 unit (Table 1 ). Exposure to patient decreased from 67.4% (56/83) before March, 18th (the date of the widespread use of masks in the hospital) to 15.9% (10/63) after March, 18th.
Table 1.
Characteristics of the 146 contacts with high-risk exposure to SARS-CoV-2 included in the CoV-CONTACT cohort, according to the infection status at D30.
| Variable | All contacts (N = 146) | Contacts with SARS-CoV-2 infection (N = 63) | Contacts with no SARS-CoV-2 infection (N = 83) | OR [95%CI] | p-value | aOR [95%CI] | p-value |
|---|---|---|---|---|---|---|---|
| Contact characteristics | |||||||
| Age (year) | 35 [29;46] (N = 146) | 35 [28.5;45.5] (N = 63) | 35 [30;47] (N = 83) | 0.99 [0.96;1.02] | 0.46 | ||
| Male gender | 35/146 (24%) | 11/63 (17.5%) | 24/83 (28.9%) | 0.52 [0.23;1.14] | 0.11 | ||
| HW functions | |||||||
| Medical doctor / Resident / Midwife | 49/146 (33.6%) | 14/63 (22.2%) | 35/83 (42.2%) | 1 (ref) | – | 1 (ref) | – |
| Registered nurse / Certified nurse assistant /Physiotherapists / Hospital Students | 74/146 (50.7%) | 36/63 (57.1%) | 38/83 (45.8%) | 2.37 [1.11;5.22] | 0.028 | 1.76 [0.78;4.03] |
0.18 |
| Non-caregiver HWs | 23/146 (15.8%) | 13/63 (20.6%) | 10/83 (12%) | 3.25 [1.17;9.36] | 0.025 | 4.06 [1.42;12.18] | 0.010 |
| Coexisting conditions | |||||||
| Obesity (BMI > 30 Kg/m²) | 27/146 (18.5%) | 13/63 (20.6%) | 14/83 (16.9%) | 1.28 [0.55;2.98] | 0.56 | ||
| Tobacco use | 36/146 (24.7%) | 17/63 (27%) | 19/83 (22.9%) | 1.24 [0.58;2.66] | 0.57 | ||
| Cardiopathy | 8/146 (5.5%) | 5/63 (7.9%) | 3/83 (3.6%) | 2.3 [0.54;11.57] | 0.27 | ||
| Chronic respiratory disease | 21/146 (14.4%) | 7/63 (11.1%) | 14/83 (16.9%) | 0.62 [0.22;1.59] | 0.33 | ||
| Chronic kidney disease | 2/146 (1.4%) | 2/63 (3.2%) | 0/83 (0%) | NE | 0.99 | ||
| Diabete | 1/146 (0.7%) | 0/63 (0%) | 1/83 (1.2%) | NE | 0.99 | ||
| Immusuppressive therapy | 7/146 (4.8%) | 4/63 (6.3%) | 3/83 (3.6%) | 1.81 [0.38;9.47] | 0.45 | ||
| Current pregnancy | 1/111 (0.9%) | 0/52 (0%) | 1/59 (1.7%) | NE | 0.99 | ||
| Type of exposition | |||||||
| Contact with > 1 index | 26/146 (17.8%) | 13/63 (20.6%) | 13/83 (15.7%) | 1.4 [0.59 ;3.3] | 0.44 | ||
| Types of index subject | |||||||
| Contacts with infected HW(s) only | 80/146 (54.8%) | 27/63 (42.9%) | 53/83 (63.9%) | 1 (ref) | – | 1 (ref) | – |
| Contacts with infected patient | 66/146 (45.2%) | 36/63 (57.1%) | 30/83 (36.1%) | 2.36 [1.21;4.65] | 0.01 | 2.62 [1.24;5.71] | 0.013 |
| Maximal SARS-CoV-2 viral load in the index subject | 9.3 [7.5;10.8] (N = 145) | 10 [7.6;10.8] (N = 62) | 8.7 [7.5;10.8] (N = 83) | 1.1 [0.93;1.31] | 0.25 | ||
| Cumulated length of exposure > 30 min | 98/143 (68.5%) | 38/61 (62.3%) | 60/82 (73.2%) | 0.61 [0.3;1.23] | 0.17 | ||
| Exposure to infected patient (N = 66) | |||||||
| Care during an aerosol-generating procedure | 6/66 (9.1%) | 3/36 (8.3%) | 3/30 (10%) | 0.82 [0.14;4.73] | 0.81 | ||
| Care without aerosol-generating procedure | 55/66 (83.3%) | 30/36 (83.3%) | 25/30 (83.3%) | 1 [0.26;3.7] | 1 | ||
| Presence in the patient's room during an aerosol-generating procedure | 22/66 (33.3%) | 13/36 (36.1%) | 9/30 (30%) | 1.32 [0.47;3.8] | 0.6 | ||
| Other type of contact | 12/66 (18.2%) | 10/36 (27.8%) | 2/30 (6.7%) | 5.38 [1.27;37.23] | 0.04 | ||
| Exposure to a SARS-CoV-2-infected HCW (N = 92) | |||||||
| Face-to-Face discussion | 86/92 (93.5%) | 31/34 (91.2%) | 55/58 (94.8%) | 0.56 [0.1;3.2] | 0.5 | ||
| Participation in a joint meeting | 25/92 (27.2%) | 9/34 (26.5%) | 16/58 (27.6%) | 0.95 [0.35;2.43] | 0.91 | ||
| Lunch sharing | 20/92 (21.7%) | 6/34 (17.6%) | 14/58 (24.1%) | 0.67 [0.22;1.89] | 0.47 | ||
| Other type of contact | 9/92 (9.8%) | 3/34 (8.8%) | 6/58 (10.3%) | 0.84 [0.17;3.42] | 0.81 |
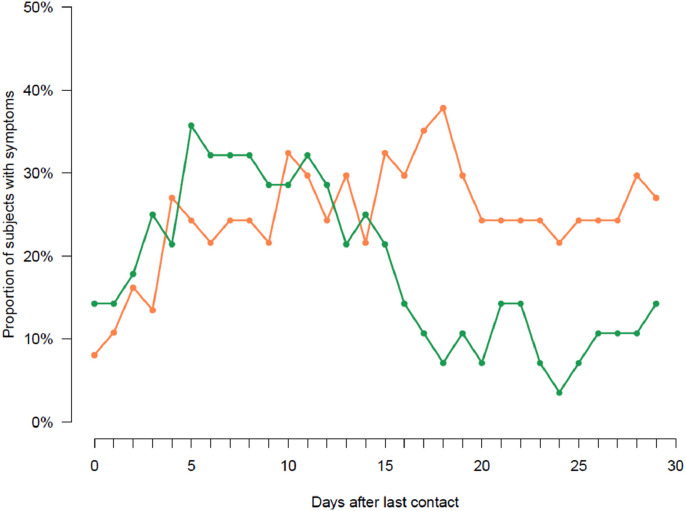
Overall, 24 /146 contact subjects (16.4%, 95%CI [11.0%−23.7%]) had at least one SARS-CoV-2-positive nasopharyngeal swab; 16/146 contact subjects (10.9%) had positive serology at inclusion which did not respond to the seroconversion definition, revealing a pre-existing infection and 31 additional contact subjects (21.2%, 95%CI [15.1%−28.9%]) exhibited a seroconversion at D30. Based on self-administered questionnaires, 59/146 contact subjects (40.4%, 95%CI [32.5%−48.9%]) met the definition of a clinical infection Fig. 1 . Seven out of 24 subjects with positive SARS-CoV-2 nasopharyngeal RT-PCR had a positive RT-PCR before the symptoms onset; the first positive nasopharyngeal RT-PCR was observed as early as six days before symptoms onset. At day 30, 63/146 contacts (43.2%, 95%CI [35.1%−51.6%]) had SARS-CoV-2 infection (confirmed in 35 (23.9%, 95%CI [17.5%; 31.9%]), and possible in 28 (19.2%, 95%CI [13.3%; 26.7%])). In the multivariable analysis, the variables associated with SARS-CoV-2 infection were being a non-caregiver HW (aOR = 4.1, 95%CI [1.4; 12.2], p = 0.010) and being exposed to a SARS-CoV-2-infected patient (aOR = 2.6, 95%CI [1.2; 5.7], p = 0.013) rather to an infected colleague (Table 1).
Fig. 1.
Proportions of symptomatic contact subjects among the 146 contacts of the CoV-CONTACT cohort. The orange curve corresponds to contacts subjects with confirmed SARS-CoV-2 infection (i.e., virologically- or immunologically-proven, n = 35). The green curve corresponds to contacts subjects with possible SARS-CoV-2 infection (i.e., clinically-suspected without viro-immunological confirmation, n = 28).
Following universal masking for HWs on March, 18th in our hospital, high-risk exposure to SARS-CoV-2-positive patients dropped by 4 and high-risk exposure to SARS-CoV-2-positive colleagues became predominant, making colleagues-to-colleagues transmission a potentially major route of infection6. Of note, none of the exposures between a HW and a SARS-CoV-2 infected patient occurred in the front-line services where the mask was worn by all caregivers from the beginning of the epidemic. These exposures occurred, prior to universal masking, in second-line services in which patients had not been previously identified as COVID-19. The profession of the contact subjects was associated with infection, but we did not find any association with the type of activities of the HWs.
The 10.9% rate of HWs with SARS-Cov-2 antibodies at inclusion revealing a pre-existing infection while they were not working in front-line services, is close to the seroprevalence of 8.8% reported in the Paris area in the general population during this period7 , 8. In addition to these HWs already infected at inclusion, 31 others (21.2% of the total population) seroconverted at day 30.
We cannot state with certainty that contacts meeting the definition of confirmed infection acquired their infection as a result of the exposure leading to their inclusion in the study. There are several arguments in favor of the link between exposure and infection: the RT-PCR positivity within 12 days after contact, the chronology of symptom onset after contact, and the seroconversion rate observed within the 30 days following the exposure, which is much higher than that observed in the community between March and May 20207 , 8. In addition, the subjects included were counseled to strictly adhere to protective measures to avoid any chain of transmission during the D0-D30 period, limiting the risk of further exposure.
All together, the rate of transmission observed in HWs after high-risk exposure, which could be as large as 43%, and close to a recent report9, strengthens the conclusion that universal masking of HW, both during contacts with patients and colleagues, and at all times, as soon as the epidemic has been identified, is essential to prevent HWs infection and maintain hospital capacities during outbreaks10.
Acknowledgments
CoVCONTACT study group
Principal investigator: Duval Xavier
Steering Committee: Burdet Charles, Duval Xavier, Lina Bruno, Tubiana Sarah, Van Der Werf Sylvie
CoV-CONTACT Clinical Centers: Abad Fanny, Abry Dominique, Alavoine Loubna, Allain Jean-Sébastien, Amiel-Taieb Karline, Audoin Pierre, Augustin Shana, Ayala Sandrine, Bansard Hélène, Bertholon Fréderique,Boissel Nolwenn, Botelho-Nevers Elisabeth, Bouiller Kévin, Bourgeon Marilou, Boutrou Mathilde, Brick Lysiane, Bruneau Léa, Caumes Eric, Chabouis Agnès, Chan Thien Eric, Chirouze Catherine, Coignard Bruno, Costa Yolande, Costenoble Virginie, Cour Sylvie, Cracowski Claire, Cracowski Jean Luc, Deplanque Dominique, Dequand Stéphane, Desille-Dugast Mireille, Desmarets Maxime, Detoc Maelle, Dewitte Marie, Djossou Felix, Ecobichon Jean-Luc, Elrezzi Elise, Faurous William, Fortuna Viviane, Fouchard Julie, Gantier Emilie, Gautier Céline, Gerardin Patrick, Gerset Sandrine, Gilbert Marie, Gissot Valérie, Guillemin Francis, Hartard Cédric, Hazevis Béatrice, Hocquet Didier, Hodaj Enkelejda, Ilic-Habensus Emila, Jeudy A, Jeulin Helene, Kane Maty,Kasprzyk Emmanuelle, Kikoine John, Laine Fabrice, Laviolle Bruno, Lebeaux David, Leclercq Anne, Ledru Eric, Lefevre Benjamin, Legoas Carole, Legrand Amélie, Legrand Karine, Lehacaut Jonathan, Lehur Claire, Lemouche Dalila, Lepiller Quentin, Lepuil Sévérine, Letienne Estelle, Lucarelli Aude, Lucet Jean-Christophe, Madeline Isabelle, Maillot Adrien, Malapate Catherine, Malvy Denis, Mandic Milica, Marty-Quinternet Solène, Meghadecha Mohamed, Mergeay-Fabre Mayka, Mespoulhe Pauline, Meunier Alexandre, Migaud Maria-Claire, Motiejunaite Justina, Nathalie Gay, Nguyen Duc, Oubbea Soumaya, Pagadoy Maïder, Paris Adeline, Paris Christophe, Payet Christine, Peiffer-Smadja Nathan, Perez Lucas, Perreau Pauline, Pierrez Nathalie, Pistone Thierry, Postolache Andreea, Rasoamanana Patrick, Reminiac Cécile, Rexah Jade, Roche-Gouanvic Elise, Rousseau Alexandra, Schoemaecker Betty, Simon Sandrine, Soler Catherine, Somers Stéphanie, Sow Khaly, Tardy Bernard, Terzian Zaven, Thy Michael, Tournier Anne, Tyrode Sandrine, Vauchy Charline, Verdon Renaud, Vernet Pauline, Vignali Valérie, Waucquier Nawal
Coordination and statistical analyses: Burdet Charles, Do Thi Thu Huong, Laouénan Cédric, Mentre France, Pauline Manchon, Tubiana Sarah, Dechanet Aline, Letrou Sophie, Quintin Caroline, Frezouls Wahiba
Virological lab: Le Hingrat Quentin, Houhou Nadhira, Damond Florence, Descamps Dianes, Charpentier Charlotte, Visseaux Benoit, Vabret Astrid, Lina Bruno, Bouscambert Maud, Van Der Werf Sylvie, Behillil Sylvie, Gaillanne Laurence, Benmalek Nabil, Attia Mikael, Barbet Marion, Demeret Caroline, Rose Thierry, Petres Stéphane, Escriou Nicolas, Barbet Marion, Petres Stéphane, Escriou Nicolas, Goyard Sophie
Biological center: Kafif Ouifiya, Piquard Valentine, Tubiana Sarah
Partners: RECOVER, REACTING, Santé Publique France (Coignard Bruno, Mailles Alexandra), Agences régionales de santé (Simondon Anne, Dreyere Marion, Morel Bruno, Vesval Thiphaine)
Sponsor: Inserm
Amat Karine, Ammour Douae, Aqourras Khadija, Couffin-Cadiergues Sandrine, Delmas Christelle, Desan Vristi, Doute Jean Michel, Esperou Hélène, Hendou Samia, Kouakam Christelle, Le Meut Guillaume, Lemestre Soizic, Leturque Nicolas, Marcoul Emmanuelle, Nguefang Solange, Roufai Layidé
Genetic: Laurent Abel, Sophie Caillat-ZucmanClinicalTrial.
Gov identification number: NCT0425989
Contributor Information
Principal investigator:
Xavier Duval, Charles Burdet, Xavier Duval, Bruno Lina, Sarah Tubiana, Sylvie Van Der Werf, Fanny Abad, Dominique Abry, Loubna Alavoine, Jean-Sébastien Allain, Karline Amiel-Taieb, Pierre Audoin, Shana Augustin, Sandrine Ayala, Hélène Bansard, Fréderique Bertholon, Nolwenn Boissel, Elisabeth Botelho-Nevers, Kévin Bouiller, Marilou Bourgeon, Mathilde Boutrou, Lysiane Brick, Léa Bruneau, Eric Caumes, Agnès Chabouis, Eric Chan Thien, Catherine Chirouze, Bruno Coignard, Yolande Costa, Virginie Costenoble, Sylvie Cour, Claire Cracowski, Luc Cracowski Jean, Dominique Deplanque, Stéphane Dequand, Mireille Desille-Dugast, Maxime Desmarets, Maelle Detoc, Marie Dewitte, Felix Djossou, Jean-Luc Ecobichon, Elise Elrezzi, William Faurous, Viviane Fortuna, Julie Fouchard, Emilie Gantier, Céline Gautier, Patrick Gerardin, Sandrine Gerset, Marie Gilbert, Valérie Gissot, Francis Guillemin, Cédric Hartard, Béatrice Hazevis, Didier Hocquet, Enkelejda Hodaj, Emila Ilic-Habensus, Jeudy A, Helene Jeulin, Maty Kane, Emmanuelle Kasprzyk, John Kikoine, Fabrice Laine, Bruno Laviolle, David Lebeaux, Anne Leclercq, Eric Ledru, Benjamin Lefevre, Carole Legoas, Amélie Legrand, Karine Legrand, Jonathan Lehacaut, Claire Lehur, Dalila Lemouche, Quentin Lepiller, Sévérine Lepuil, Estelle Letienne, Aude Lucarelli, Jean-Christophe Lucet, Isabelle Madeline, Adrien Maillot, Catherine Malapate, Denis Malvy, Milica Mandic, Solène Marty-Quinternet, Mohamed Meghadecha, Mayka Mergeay-Fabre, Pauline Mespoulhe, Alexandre Meunier, Maria-Claire Migaud, Justina Motiejunaite, Gay Nathalie, Duc Nguyen, Soumaya Oubbea, Maïder Pagadoy, Adeline Paris, Christophe Paris, Christine Payet, Nathan Peiffer-Smadja, Lucas Perez, Pauline Perreau, Nathalie Pierrez, Thierry Pistone, Andreea Postolache, Patrick Rasoamanana, Cécile Reminiac, Jade Rexah, Elise Roche-Gouanvic, Alexandra Rousseau, Betty Schoemaecker, Sandrine Simon, Catherine Soler, Stéphanie Somers, Khaly Sow, Bernard Tardy, Zaven Terzian, Michael Thy, Anne Tournier, Sandrine Tyrode, Charline Vauchy, Renaud Verdon, Pauline Vernet, Valérie Vignali, Nawal Waucquier, Charles Burdet, Huong Do Thi Thu, Cédric Laouénan, France Mentre, Manchon Pauline, Sarah Tubiana, Aline Dechanet, Sophie Letrou, Caroline Quintin, Wahiba Frezouls, Quentin Le Hingrat, Nadhira Houhou, Florence Damond, Dianes Descamps, Charlotte Charpentier, Benoit Visseaux, Astrid Vabret, Bruno Lina, Maud Bouscambert, Sylvie Van Der Werf, Sylvie Behillil, Laurence Gaillanne, Nabil Benmalek, Mikael Attia, Marion Barbet, Caroline Demeret, Thierry Rose, Stéphane Petres, Nicolas Escriou, Marion Barbet, Stéphane Petres, Nicolas Escriou, Sophie Goyard, Ouifiya Kafif, Valentine Piquard, Sarah Tubiana, Bruno Coignard, Alexandra Mailles, Anne Simondon, Marion Dreyere, Bruno Morel, and Thiphaine Vesval
Sponsor: Inserm:
Karine Amat, Douae Ammour, Khadija Aqourras, Sandrine Couffin-Cadiergues, Christelle Delmas, Vristi Desan, Michel Doute Jean, Hélène Esperou, Samia Hendou, Christelle Kouakam, Guillaume Le Meut, Soizic Lemestre, Nicolas Leturque, Emmanuelle Marcoul, Solange Nguefang, Layidé Roufai, Laurent Abel, and Sophie Caillat-Zucman
References
- 1.Brown C.S., Clare K., Chand M., Andrews J., Auckland C., Beshir S., et al. Snapshot PCR surveillance for SARS-CoV-2 in hospital staff in England. J Infect. Sep 2020;81(3):427–434. doi: 10.1016/j.jinf.2020.06.069. PubMed PMID:32615198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jary A., Flandre P., Chabouis A., Nguyen S., Marot S., Burrel S., et al. Clinical presentation of Covid-19 in health care workers from a French University Hospital. J Infect. 2020 Sep;81(3):e61–e63. doi: 10.1016/j.jinf.2020.06.048. PubMed PMID: 32579992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lescure F.X., Bouadma L., Nguyen D., Parisey M., Wicky P.H., Behillil S., et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. Jun 2020;20(6):697–706. doi: 10.1016/S1473-3099(20)30200-0. PubMed PMID: 32224310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anna F., Goyard S., Lalanne A., Nevo F., Gransagne M., Souque P., et al. High seroprevalence but short-lived immune response to SARS-CoV-2 infection in Paris. medRxiv. 2020 doi: 10.1101/2020102520219030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theel E.S., Harring J., Hilgart H., Granger D. Performance characteristics of four high-throughput immunoassays for detection of IgG antibodies against SARS-CoV-2. J Clin Microbiol. Jun 8 2020 doi: 10.1128/JCM.01243-20. PubMed PMID: 32513859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Contejean A., Leporrier J., Canoui E., Alby-Laurent F., Lafont E., Beaudeau L., et al. Comparing dynamics and determinants of SARS-CoV-2 transmissions among health care workers of adult and pediatric settings in central Paris. Clin Infect Dis. Jul 15 2020 doi: 10.1093/cid/ciaa977. PubMed PMID: 32663849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santé Publique France. Point épidémiologie hebdomadaire. https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/documents/bulletin-national/covid-19-point-epidemiologique-du-23-juillet-2020. Accessed July 28, 2020.
- 8.Le Vu S., Jones G., Anna F., Rose T., Richard J., Bernard-Stoecklin S., et al. Prevalence of SARS-CoV-2 antibodies in France: results from nationwide serological surveillance. medRxiv. 2020 doi: 10.1101/2020102020213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houlihan C.F., Vora N., Byrne T., Lewer D., Kelly G., Heaney J., et al. Pandemic peak SARS-CoV-2 infection and seroconversion rates in London frontline health-care workers. Lancet. Jul 25 2020;396(10246):e6–e7. doi: 10.1016/S0140-6736(20)31484-7. PubMed PMID: 32653078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houghton C., Meskell P., Delaney H., Smalle M., Glenton C., Booth A., et al. Barriers and facilitators to healthcare workers' adherence with infection prevention and control (IPC) guidelines for respiratory infectious diseases: a rapid qualitative evidence synthesis. Cochrane Database Syst Rev. Apr 21 2020;4 doi: 10.1002/14651858.CD013582. PubMed PMID: 32315451. [DOI] [PMC free article] [PubMed] [Google Scholar]