Abstract
Acute Kidney Injury (AKI) is a frequent complication in critically ill patients with Coronavirus disease 2019 (COVID-19), and it has been associated with worse clinical outcomes, especially when Kidney Replacement Therapy (KRT) is required. A condition of hypercoagulability has been frequently reported in COVID-19 patients, and this very fact may complicate KRT management. Sustained Low Efficiency Dialysis (SLED) is a hybrid dialysis modality increasingly used in critically ill patients since it allows to maintain acceptable hemodynamic stability and to overcome the increased clotting risk of the extracorporeal circuit, especially when Regional Citrate Anticoagulation (RCA) protocols are applied. Notably, given the mainly diffusive mechanism of solute transport, SLED is associated with lower stress on both hemofilter and blood cells as compared to convective KRT modalities. Finally, RCA, as compared with heparin-based protocols, does not further increase the already high hemorrhagic risk of patients with AKI. Based on these premises, we performed a pilot study on the clinical management of critically ill patients with COVID-19 associated AKI who underwent SLED with a simplified RCA protocol. Low circuit clotting rates were observed, as well as adequate KRT duration was achieved in most cases, without any relevant metabolic complication nor worsening of hemodynamic status.
Keywords: COVID-19, Acute kidney injury, Sustained low-efficiency dialysis, Regional citrate anticoagulation
1. Introduction
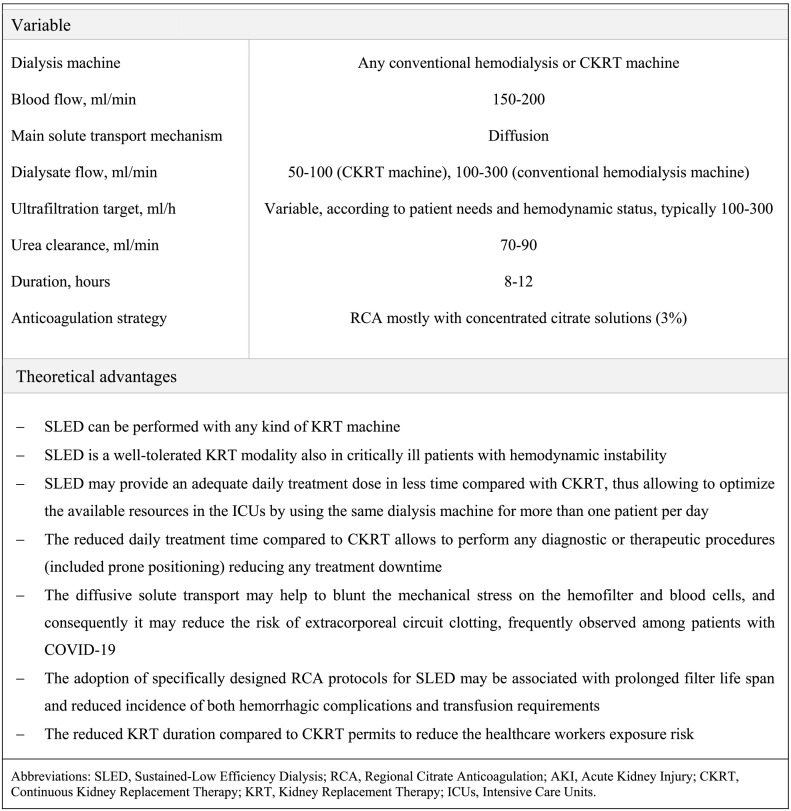
In the course of the severe acute respiratory syndrome associated with Coronavirus disease 2019 (COVID-19) the percentage of patients with Acute Kidney Injury (AKI) requiring Kidney Replacement Therapy (KRT) varies significantly among different studies, ranging from 0% to 35% according to the number of critically ill patients included, geographic location, racial and ethnic groups considered, prevalence of comorbidities and local institutional procedures [1,2]. The pathophysiological mechanism of this specific form of AKI remains still unclear. Indeed, while in most cases the etiology seems to be directly related to sepsis and multiorgan dysfunction, specific alterations associated with the viral infection have not been completely excluded [1,3]. Remarkably, a hypercoagulable state attributed to systemic inflammation, platelet disfunction and microvascular alterations is often reported, and it has been associated with a high incidence of thrombotic complications [4,5]. In this complex clinical scenario, continuous KRT (CKRT) have been frequently adopted in the Intensive Care Unit (ICU) patients with hemodynamic instability [1]. However, a high rate of extracorporeal circuit clotting has been often described, leading to frequent treatment downtime, increased transfusion needs and waste of health resources [[4], [5], [6], [7]]. Sustained Low Efficiency Dialysis (SLED) is a modality of Prolonged Intermittent KRT (PIKRT) with a typical 8–12 h treatment time, which is increasingly used in ICU patients, since it combines the most favorable characteristics of both CKRT and conventional Intermittent Hemodialysis (IHD) [8] (Fig. 1 ). PIKRT can be performed either with CKRT or IHD machines, providing adequate daily treatment dose in less time compared with CKRT, thus optimizing the often limited available resources in the overcrowded clinical context of SARS-CoV-2 in the ICUs [1]. Moreover, the diffusive solute transport characteristic of this KRT technique may help reduce the mechanical stress on the hemofilter, and consequently the risk of extracorporeal circuit clotting [9]. Given the prolonged duration of SLED, an anticoagulation strategy is commonly recommended. The most recent guidelines on AKI suggest the use of Regional Citrate Anticoagulation (RCA) in all patients without specific contraindications for citrate, irrespective of the bleeding risk [10,11]. Indeed, several clinical studies have extensively demonstrated the advantages of RCA over systemic heparin anticoagulation protocols in terms of prolonged filter life span and reduced incidence of both hemorrhagic complications and transfusion requirements [12]. In particular, the percentage of premature treatment interruption secondary to circuit clotting is significantly higher in patients on CKRT with heparin-based protocols compared to those based on RCA protocols [13].
Fig. 1.
Typical setting and theoretical advantages of SLED with RCA in the specifical clinical context of critically ill patients with COVID-19 associated AKI.
In this report we present preliminary data from a pilot study of critically ill patients with SARS CoV-2 related AKI who underwent 49 SLED sessions with a simplified RCA protocol. This approach allowed both to overcome the increased clotting risk observed in this peculiar clinical setting, and to deliver the prescribed dialysis dose.
2. Case series
One-hundred thirty-five consecutive patients with COVID-19 were admitted to the General ICU of the Parma University Hospital between February 23 and May 5, 2020. AKI, defined according to the most recent guidelines [14], was detected in 43 (31,9%) patients. Five (11.6%) of these patients were scheduled to be started on KRT; however, one patient died six hours before the effective start of KRT. The demographic and clinical characteristics at ICU admission of patients underwent KRT are reported in Table 1 . All patients were mechanically ventilated and required prone positioning. The coagulation profile was within the normal range in all patients (Table 1); all of them were given subcutaneous low molecular weight heparin as prophylaxis. In particular, given the evidence of reduced kidney function at ICU admission, 3 of 4 patients received a reduced dose of enoxaparin 4.000 UI once daily and the other a full dose of 6.000 UI. KRT was started after an average time from ICU admission of 5 days. The main indication for starting KRT was progressive fluid overload associated with stage 3 oliguric AKI. Each patient underwent repeated SLED sessions, for a total of 49 treatments. The extracorporeal treatment was performed either by using the Prismax System (Baxter) with polyacrylonitrile AN69 filters (ST 150, 1.5 m2, Baxter) or, alternatively, the AK200S Ultra type 1 machine (Gambro, Medolla, Italy) with polysulfone filters (F8HPS, 1.8 m2, Fresenius, Italy), on the basis of machine availability at the moment. Double lumen 12 Fr dialysis catheters were used. The prescribed SLED duration was 8–12 h with a prescribed net fluid removal per hour of 100–300 mL/h, according to patient's needs and hemodynamic status. The concentrated citrate solution (ACD-A; citrate anion 112.9 mmol/L or 3%, of which 0.8% from citric acid and 2.2% from trisodium citrate, Fresenius Italy) was infused in the extracorporeal circulation before the filter at in proportion to the blood flow rate (Qb, 150–200 mL/min –QACD-A approximately 250–300 mL/h), aiming at a target 2.5–3 mmol/L citrate concentration in the hemofilter. When the CKRT dialysis machine was used, a calcium and bicarbonate containing solution (Baxter Prismasol 4: Ca2+ 1.75 mmol/L, HCO3 − 32 mmol/L, Na+ 140 mmol/L, Cl− 113.5 mmol/L, K+ 4 mmol/L, Mg2+ 0.5 mmol/L; Lactate 3 mmol/L; Glucose 6.1 mmol/L) was selected as dialysis fluid and set at 100 mL/min. Alternatively, when the conventional hemodialysis machine was used, the on-line generated ultrapure dialysate with a Ca++ concentration of 1.25 mmol/L was run at 300 mL/min in a countercurrent direction. Calcium gluconate (10% solution, calcium 0.24 mmol/mL) was infused in a central venous line to maintain the systemic ionized calcium (s-Ca2+) concentration within the normal range (0.90–1.20 mmol/L). In particular, the infusion was started at 5 mL/h whenever s-Ca2+ fell below 0.9 mmol/L. The intradialytic variables related to RCA are reported in Table 2 . Electrolytes and acid-base parameters were maintained within the normal range without any relevant episode of hypo-hypercalcemia and metabolic acidosis/alkalosis. Calcium supplementation at fixed rate 1.13 mmol/h was needed in 13/49 treatments. Calcium ratio was steadily maintained below the threshold of 2.5. Clinical monitoring data during SLED treatments are reported in Table 2. The ultrafiltration target was achieved in most sessions, with only a mild decrease in Systolic Blood Pressure (SBP). Vasopressor amines were being infused before SLED start in 23 sessions, and infusion was initiated in the course of 2 sessions, in both cases at the second hour of treatment. We did not observe any statistically significant difference in the vasopressors dose in course of SLED sessions; no SLED session was interrupted for severe hemodynamic instability. No KRT-related bleeding complication was observed. Three out of 49 (6.1%) SLED sessions were prematurely interrupted for circuit clotting (Table 1); a complete restitution the extracorporeal circuit blood to the patient was achieved in 2 of the 3 cases. Relevant modifications of operative SLED parameters in the course of treatment were not required, and the extracorporeal circuit pressures remained within the safety limits. In all of the four patients SLED was discontinued following recovery of an adequate urine output.
Table 1.
Demographic and clinical characteristics at ICU admission, and SLED parameters.
| Variable | Pt. 1 | Pt. 2 | Pt. 3 | Pt. 4 |
|---|---|---|---|---|
| Age, yr | 49 | 63 | 54 | 47 |
| Male | Y | Y | Y | Y |
| Comorbidities, | ||||
| Arterial hypertension | N | Y | Y | N |
| Diabetes mellitus | Y | Y | Y | N |
| CKD | N | N | N | N |
| APACHE II score | 36 | 31 | 22 | 30 |
| SOFA score | 16 | 14 | 12 | 16 |
| Invasive mechanical ventilation | Y | Y | Y | Y |
| Serum creatinine, mg/dL | 2 | 0.6 | 4.2 | 1.5 |
| BUN, mg/dl | 24 | 18 | 80 | 26 |
| Platelet count, x 103/mL | 118 | 284 | 248 | 448 |
| INR | 1.33 | 1.34 | 1.28 | 1.32 |
| aPTT ratio | 1.02 | 1.11 | 1.22 | 1.02 |
| ICU stay, days | 33 | 40 | 48 | 75 |
| Duration of mechanical ventilation, days | 30 | 38 | 48 | 70 |
| Death in the ICU | N | N | N | N |
| Death during hospital stay | N | N | N | N |
| Prescribed SLED sessions, n/patient | 23 | 16 | 2 | 8 |
| SLED duration, hours (median, IQ range) | 12 (9.5–12.5) | 8 (8–12) | 10 (9–11) | 10 (8.7–12) |
| Causes of SLED interruption | ||||
| Programmed end of treatment (%) | 18 (78.3) | 14 (87.5) | 2 (100) | 6 (75) |
| Circuit clotting (%) | 2 (8.7) | 1 (6.2) | - | - |
| CVC malfunctioning (%) | 2 (8.7) | 1 (6.2) | - | 1 (12.5) |
| Other clinical reasons (%) | 1 (4.3) | - | - | 1 (12.5) |
| Duration of KRT, days | 29 | 27 | 4 | 8 |
ICU, Intensive Care Unit; SLED, Sustained Low-Efficiency Dialysis; Pt, Patient; CKD, Chronic Kidney Disease; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; BUN, Blood Urea Nitrogen; CVC, Central Venous Catheter; KRT, Kidney Replacement Therapy. Y, Yes; N, No.
Table 2.
Intradialytic clinical monitoring and intradialytic variables related to Regional Citrate Anticoagulation.
| Variable | SLED Start | SLED 2 h | SLED 6 h | SLED 10 h | SLED 12 h | P | |
|---|---|---|---|---|---|---|---|
| SBP, mmHg | 130.1 (20.4) | 129.0 (24.0) | 119.9 (23.2) | 117.2 (24.4) | 118.4 (15.0) | 0.0046 | |
| DBP, mmHg | 62.2 (10.55) | 62.2 (13.98) | 61.6 (9.48) | 60.7 (11.53) | 61.1 (11.42) | NS | |
| Heart rate, bpm | 94.9 (12.4) | 96.8 (13.5) | 96.9 (14.8) | 100.2 (13.5) | 94.5 (15.2) | NS | |
| Dopamine, mcg/Kg/min | 0.73 (2.08) | 0.67 (1.98) | 0.89 (2.47) | 0.78 (2.27) | 0.71 (2.28) | NS | |
| Norepinephrine, mcg/Kg/min | 0.06 (0.11) | 0.06 (0.11) | 0.06 (0.14) | 0.05 (0.15) | 0.05 (0.15) | NS | |
| Dobutamine, mcg/Kg/min | 0.14 (0.37) | 0.14 (0.37) | 0.14 (0.37) | 0.09 (0.31) | 0.09 (0.31) | NS | |
| SpO2, % | 97.5 (2.9) | 98.0 (2.1) | 97.2 (6.6) | 98.6 (1.7) | 99.3 (0.8) | NS | |
| FiO2, % | 50.9 (20.0) | 50.3 (18.1) | 51.4 (18.6) | 50.6 (17.5) | 51.1 (15.0) | NS | |
| ACT, sec | 110.6 (10.53) | 111.8 (10.54) | 116.8 (10.87) | 110.9 (6.33) | 109.1 (14.25) | NS | |
| Ionized calcium (s-Ca2+), mmol/L | 1.16 (0.08) | 1.12 (0.07) | 1.16 (0.07) | 1.15 (0.08) | 1.16 (0.08) | NS | |
| Bicarbonate (HCO3−), mmol/L | 24.4 (2.6) | 24.7 (2.7) | 24.6 (1.8) | 25.9 (3.2) | 26.5 (1.1) | ||
Data are presented as mean (SD). Values were measured on patients' arterial line if not otherwise indicated.
Abbreviations: SLED, Sustained Low Efficiency Dialysis; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; SpO2, Peripheral Oxygen Saturation, FiO2, Fraction of Inspired Oxygen; ACT, Activated coagulation time; NS, not statistically significant.
3. Discussion
Our pilot study suggests that, in patients with COVID-19 associated AKI, SLED with RCA is a well-tolerated KRT modality which allows to deliver the prescribed dialysis dose and to maintain an adequate fluid balance even in patients with severe hemodynamic instability. According to our knowledge, the present study is the first to provide data on the use of RCA in the course of SLED in critically ill patients with COVID-19 associated AKI. Indeed, the adoption of a KRT technique mainly based on diffusion reduces the mechanical stress on both the hemofilter and blood cells, leading to a lower incidence of extracorporeal circuit clotting, particularly common in COVID-19 patients with AKI [[4], [5], [6], [7]]. Moreover, given the dramatically elevated number of patients requiring an intensive clinical management in this clinical setting, the shortening of daily treatment time characteristic of SLED as compared to CKRT, might contribute to deliver the dialysis treatment to more patients by using the same dialysis machine (Fig. 1). Our report confirms the feasibility and safety of PIKRT, recently reported in a prospective observational study performed in 136 critically ill patients with COVID-19 associated AKI [15], where 108/130 PIKRT sessions (83%) were performed with a systemic heparin-based anticoagulation protocol. The ultrafiltration target was attained in most cases; however, in this report 17 sessions (13%) based on a heparin-based protocol were complicated by premature interruption for extracorporeal circuit clotting [15]. In this context, notably, circuit clotting rates are particularly high in patients undergoing convective CKRT (i.e. Continuous Veno-Venous Hemofiltration, CVVH), despite the higher delivered dose or the use of pre-dilution modality [3,8]. Frequent premature circuit clotting was also reported in a recent study on 37 critically ill patients with SARS-CoV-2 who underwent Continuous Veno-Venous Hemodiafiltration (CVVHDF) with a heparin-coated hemodiafilter aimed at enhancing cytokine adsorption [16]. In our study, the application of a simplified RCA protocol specifically designed for the SLED modality significantly contributed to achieve the prescribed dialysis duration, without any relevant metabolic complications, even in presence of impaired liver function [12]. As a matter of fact, it is well known that RCA protocols optimized the hemofilter efficiency and circuit life span, without interfering with systemic coagulation, even in patients with very high bleeding risk [[11], [12], [13]].
In conclusion, notwithstanding the limited number of observations which require further confirmation in a study with a more numerous patient population, our pilot study suggests that SLED with RCA is a safe and feasible KRT modality in critically ill patients with COVID-19 associated AKI requiring KRT. Moreover, given the peculiar operative setting, this form of KRT may allow to reduce the otherwise increased rate of extracorporeal circuit clotting, particularly when RCA protocols are applied. Finally, by shortening treatment time, SLED could represent a rationale therapeutic alternative to CKRT, since it allows health resources optimization in the ICU.
Financial disclosure statement
None.
CRediT authorship contribution statement
Di Mario Francesca: Conceptualization, Methodology, Investigation, Data curation, Writing - original draft. Regolisti Giuseppe: Methodology, Investigation, Formal analysis, Writing - review & editing. Di Maria Alessio: Investigation, Data curation. Parmigiani Alice: Investigation, Data curation. Benigno Giuseppe Daniele: Investigation, Data curation. Picetti Edoardo: Resources, Data curation. Barbagallo Maria: Resources, Data curation. Greco Paolo: Resources, Data curation. Maccari Caterina: Resources, Data curation. Fiaccadori Enrico: Supervision, Methodology, Writing - review & editing, Project administration.
Declaration of Competing Interest
Disclosure of potential conflicts of interest: the authors declare that they have no conflict of interest.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Nadim M.K., Forni L.G., Mehta R.L., et al. COVID-19-associated acute kidney injury: consensus report of the 25th acute disease quality initiative (ADQI) workgroup. Nat Rev Nephrol. 2020;16(12):747–764. doi: 10.1038/s41581-020-00356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farouk S.S., Fiaccadori E., Cravedi P., Campbell K.N. COVID-19 and the kidney: what we think we know so far and what we don’t. J Nephrol. 2020;20:1–6. doi: 10.1007/s40620-020-00789-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossi G.M., Delsante M., Pilato F.P., et al. Kidney biopsy findings in a critically ill COVID-19 patient with Dialysis-dependent acute kidney injury: a case against “SARS-CoV-2 nephropathy”. Kidney Int Rep. 2020;5(7):1100–1105. doi: 10.1016/j.ekir.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helms J., Tacquard C., Severac F., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Endres P., Rosovsky R., Zhao S., et al. Filter clotting with continuous renal replacement therapy in COVID-19. J Thromb Thrombolysis. 2020;7:1–5. doi: 10.1007/s11239-020-02301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sise M.E., Baggett M.V., Shepard J.O., Stevens J.S., Rhee E.P. Case 17-2020: a 68-year-old man with Covid-19 and acute kidney injury. N Engl J Med. 2020;382(22):2147–2156. doi: 10.1056/NEJMcpc2002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiaccadori E., Regolisti G., Cademartiri C., et al. Efficacy and safety of a citrate-based protocol for sustained low-efficiency dialysis in AKI using standard dialysis equipment. Clin J Am Soc Nephrol. 2013;8(10):1670–1678. doi: 10.2215/CJN.00510113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricci Z., Ronco C., Bachetoni A., et al. Solute removal during continuous renal replacement therapy in critically ill patients: convection versus diffusion. Crit Care. 2006;10(2):R67. doi: 10.1186/cc4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;S2:1–138. [Google Scholar]
- 11.Fiaccadori E., Pistolesi V., Mariano F., et al. Regional citrate anticoagulation for renal replacement therapies in patients with acute kidney injury: a position statement of the work group “renal replacement therapies in critically ill patients” of the Italian Society of Nephrology. J Nephrol. 2015;28(2):151–164. doi: 10.1007/s40620-014-0160-2. [DOI] [PubMed] [Google Scholar]
- 12.Morabito S., Pistolesi V., Tritapepe L., Fiaccadori E. Regional citrate anticoagulation for RRTs in critically ill patients with AKI. Clin J Am Soc Nephrol. 2014;9(12):2173–2188. doi: 10.2215/CJN.01280214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarbock A., Küllmar M., Kindgen-Milles D., et al. Effect of regional citrate anticoagulation vs systemic heparin anticoagulation during continuous kidney replacement therapy on Dialysis filter life span and mortality among critically ill patients with acute kidney injury: a randomized clinical trial. JAMA. 2020;324(16):1629–1639. doi: 10.1001/jama.2020.18618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey A.S., Eckardt K.U., Dorman N.M., et al. Nomenclature for kidney function and disease: report of a kidney disease: improving global outcomes (KDIGO) consensus conference. Kidney Int. 2020;97(6):1117–1129. doi: 10.1016/j.kint.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez-Sandoval J.C., Gaytan-Arocha J.E., Xolalpa-Chávez P., et al. Prolonged intermittent renal replacement therapy for acute kidney injury in COVID-19 patients with acute respiratory distress syndrome. Blood Purif. 2020;26:1–9. doi: 10.1159/000510996. [DOI] [PubMed] [Google Scholar]
- 16.Villa G., Romagnoli S., De Rosa S., et al. Blood purification therapy with a hemodiafilter featuring enhanced adsorptive properties for cytokine removal in patients presenting COVID-19: a pilot study. Crit. Care. 2020;24(1)):605. doi: 10.1186/s13054-020-03322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]