Abstract
Rationale:
There is a robust relationship between anxiety disorders, including post traumatic stress disorder (PTSD) and substance abuse. In fact, 30–50% of people seeking treatment for substance abuse have a comorbid diagnosis for PTSD. Heroin use is at epic proportions in the United States and is commonly used by people with co-occurring PTSD symptoms and substance use disorder.
Objectives:
Here we combined animal assays of acute restraint stress and contingent heroin self-administration (SA) to study co-morbidity between stress disorders and opioid use disorder, and identify shifts in anxiety-like behaviors following stress and/or heroin in response to a stress-conditioned cue. Our objective for this approach was to determine the long-term impact of acute restraint stress and heroin self-administration on stress reactivity and basic reward processes.
Methods:
We used 2-h acute restraint stress paired with an odor stimulus to condition a stress cue (CS) for testing of subsequent stress reactivity in a burying task, and reinstatement and extinction to heroin seeking. Rats were also tested for social place preference for measures of social reward and anxiety-like behaviors.
Results:
Stress rats exhibited multiple levels of disrupted behavior including enhanced acquisition of heroin intake and reinstatement in response to the stress CS, as well as delayed extinction in response to the stress CS. All rats developed a social place preference, but stress rats spent more time in nose-to-nose contact with the unfamiliar rat while heroin rats spent time exploring the chamber. In the burying task, stress shortened latencies to bury the CS, and increased burying and immobility in male and female rats relative to sham counterparts.
Conclusions:
Acute restraint stress results in anxiety-like behaviors and a stress associated cue is sufficient to reinstate extinguished heroin seeking. This project has the potential to elucidate the complex relationship between stress/anxiety disorders, including some PTSD-like characteristics, and the onset, maintenance, and relapse to heroin seeking.
Keywords: PTSD, opioids, anxiogenic phenotype, relapse, addiction, stress, anxiety
Introduction
Individuals with stress or anxiety disorders are particularly vulnerable to opioid abuse (Conway et al. 2006), and a diagnosis of post-traumatic stress disorder (PTSD) substantially increases the probability of developing substance use disorders (SUDs). For example, veterans diagnosed with PTSD have a 30–50% comorbidity with SUDs (Seal et al. 2012); and, 30–60% of women seeking treatment for SUDs have a comorbid diagnosis for PTSD induced by sexual or physical assault (Najavits et al. 1997) (Cohen and Hien 2006). Moreover, severity of stress exposure correlates with greater substance use (Ouimette et al. 1996), and stress is a risk factor in triggering relapse (Sinha 2001). PTSD is an anxiety disorder caused by exposure to a traumatic event, followed by an inability to extinguish memory of that trauma (McCauley et al. 2012). Symptoms can occur within 3 months or may not occur until years later. According to the Diagnostic and Statistical Manual (DSM-5) of the American Psychiatric Association, PTSD occurs when marked physiological reactions to internal or external cues that symbolize or resemble an aspect of the traumatic event re-occur resulting in intense psychological distress and physiological reactivity. Triggering events that resemble aspects of the traumatic event, including smell, induce physical sensations or memories of the trauma (Friedman et al. 2011) often leading to avoidance efforts. Even though the link between stress and SUDs is evident, effective treatments for comorbid PTSD and SUDs are not available.
Opioid addiction is at epic proportions in the United States likely due to increased prescription opioid distribution (Clark and Schumacher 2017). Each day more than 90 people in the United States die from opioid overdose (Scholl et al. 2018). Abuse of prescription opioids alone costs approximately 78.5 billion dollars per year in the US, representing a significant economic burden (Florence et al. 2016). The rise of opioid-related deaths and the economic burden it presents has created a public health crisis and warrants research to better understand and combat the abuse. Numerous studies have established overlaps in brain region involvement in anxiety and stress related disorders, including post-traumatic stress disorder (PTSD), and generalized substance use disorders (SUD) (Maren et al. 2013; Woodward et al. 2006). Recently, combined animal models of acute restraint stress with contingent drug self-administration revealed an overlap in neuroplasticity of glutamatergic synaptic function in nucleus accumbens core (NAcore) and potentiated acquisition of cocaine and alcohol self-administration (SA) (Garcia-Keller et al. 2016; Garcia-Keller et al. 2019). Specifically, stress rats showed decreased expression and function of the main glutamate transporter (GLT-1), and potentiated excitatory synapses on medium spiny neurons in the NAcore 3 weeks after the stress exposure (Garcia-Keller et al. 2016; Garcia-Keller et al. 2013). These neuroadaptations resemble the changes induced by self-administered drugs, including cocaine, heroin, nicotine, and alcohol (Scofield et al. 2016), demonstrating overlapping neurocircuitries and mechanisms (Woodward et al. 2006). Combined, these findings contribute to the understanding of the clinical studies showing that patients with comorbid PTSD/SUDs have greater drug use severity and poorer treatment outcomes than patients diagnosed with either PTSD or SUDs alone (Boden et al. 2012; Clark et al. 2001; DiMaggio et al. 2009; Ouimette et al. 1996).
Given the potentiated acquisition of cocaine SA and the common neuroplasticity with drugs of abuse, it is possible that acute stress is both a precursor to addiction as well as a precipitator of relapse, rendering those individuals with PTSD and/or anxiety related disorders at an elevated risk of developing an addiction and relapsing after protracted abstinence (Sinha 2001). To test this hypothesis, we used an established model of conditioned stress for the study of PTSD-like symptoms (Garcia-Keller et al. 2019) with heroin seeking and anxiety-like behaviors. Support for this model comes from the premise that people with PTSD avoid exposure to stress inducing stimuli or contexts, therefore relapse of PTSD symptoms or drug seeking are most likely to occur in the presence of an environmental or a contextual stimulus paired with the original stressor (Berardi et al. 2012; Liberzon and Abelson 2016). As such, conditioned stressors are posited to be useful models for co-morbid stress and substance use disorder (Berardi et al. 2012; Liberzon and Abelson 2016). Recently, Garcia-Keller (2019) and colleagues, demonstrated that a conditioned stressor reinstated cocaine and alcohol seeking. Acute restraint stress was paired with a neutral odor to condition a Pavlovian association between the experience of the stress and an olfactory stimulus to emulate heightened reactivity to stress associated cues found in PTSD type disorders.
Here, we combined acute restraint stress with heroin SA and evaluated whether acute restraint stress would potentiate acquisition of heroin taking and if exposure to the stress stimulus induced heroin seeking during abstinence and extinction (Experiment 1). We also characterized the effects of acute stress and heroin SA on approach/avoidance behaviors. Avoidance behaviors are part of the diagnostic criteria for stress disorders and are quantifiable in rodent models. Such tasks exploit the use of environmental stimuli that may be perceived as rewarding or threatening (Lezak et al. 2017). We tested the hypothesis that acute stress modifies approach/avoidance behaviors through social reward place conditioning (Experiment 2) and defensive burying of the CS (Experiment 3).
2. Materials & Methods:
Figure 1 depicts the experimental timeline and animal distribution for the Experiments 1, 2 and 3. In brief, all rats underwent restraint stress or were sham exposed in the presence of a conditioning odor cue (lemon or sandalwood oil scent). Three weeks later, rats were given access to contingent heroin or saline SA followed by a three-week abstinence period. Social interaction and defensive burying occurred on abstinence day 7.
Figure 1.
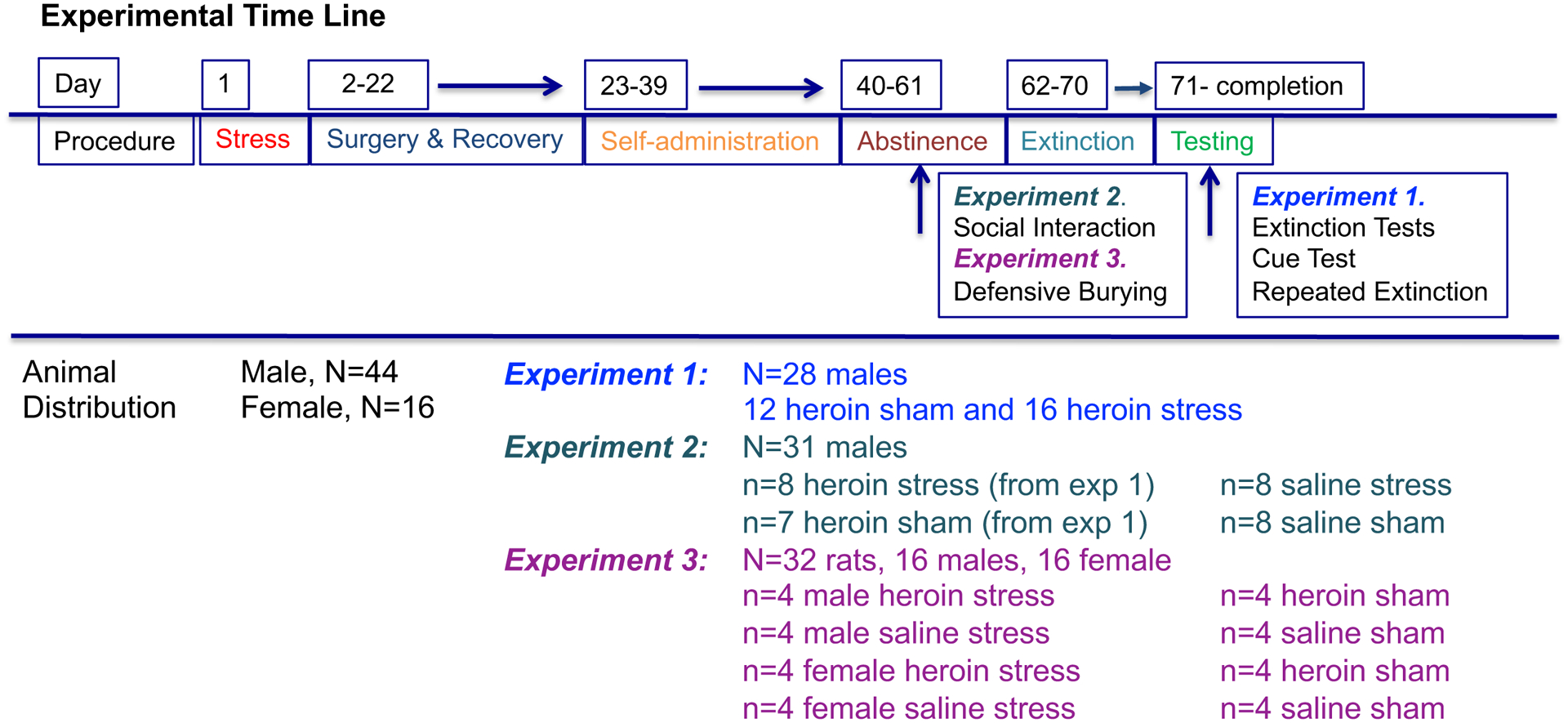
Experimental timeline and animal distribution for the experiments. All rats went through acute restraint stress or sham conditioning, surgery, recovery and self-administration of either heroin or saline. In experiment 1, rats tested for CS reactivity, CS and cued reinstatement. A subset went onto daily extinction of the CS and heroin cue combined (i.e., repeated extinction). In experiment 2, during abstinence, rats that went through heroin or saline self-administration were tested for social interaction. (Heroin rats were a subset of rats from experiment 1). In experiment 3, male and female rats underwent a defensive burying task using the CS as the threatening stimulus.
2.1. Subjects
A total of 43 male (Experiments 1 and 2) and 16 male and 16 female (Experiment 3), age matched, Sprague-Dawley rats (Envigo, Indianapolis, IN, USA) were used to complete all experiments. All rats were pair-housed on a normal 12:12 light-dark cycle in a temperature and humidity-controlled vivarium. Rats were given 5 days to acclimate after arrival before the beginning of the experiments. Rat chow (Envigo, Indianapolis, IN, USA) and water were available ad libitum throughout the study. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina and were in accordance with the “Guide for the Care and Use of Laboratory Rats” of the Institute of Laboratory Animal Resources on Life Sciences, National Research Council.
2.2. Acute Restraint Stress and Scent Exposure
Rats were randomly assigned into sham or stress groups. The stress rats were inserted into flat bottom restrainers (PLAS Labs, Thomas Scientific, Swedensboro, NJ, USA) that restricted movement and did not allow for escape for a total of 2 hours during the beginning of the dark cycle. Along with restraint, 3 drops of an odor fluid (stress conditioned stimulus, “stress CS”) were placed in the cage to elicit an association between the odor and the physiological discomfort of restraint. The scents used were lemon (LM, dōTERRA Intl., West Pleasant Grove, UT, USA) and sandalwood (SW, Wyndmere Naturals, Minneapolis, MN, USA). Each scent was placed in a petri dish proximal to the restrainer for the duration of the restraint stress. Rats in the sham group received exposure to the odor without the stress experience; thus exposure to this odor is termed a “sham CS” throughout. Each partner in a pair of rats (sham or stress) were exposed to the same scent and stress/sham condition as their cage mate. Weights were obtained just prior to, the day following the stress, and every week thereafter through protocol completion. Rats were given 14 days after sham or stress treatment before surgery.
2.3. Surgery
Rats were anesthetized with IP injections of ketamine (66 mg/kg; Vedco Inc, St. Joseph, MO, USA), xylazine (1.3 mg/kg; Lloyd Laboratories, Shenandoah, IA, USA), and equithesin (0.5 ml/kg; sodium pentobarbital 4 mg/kg, chloral hydrate 17 mg/kg, 21.3 mg/kg magnesium sulfate heptahydrate dissolved in 44% propylene glycol, 10% ethanol solution). Ketorolac (2.0 mg/kg, IP; Sigma Chemical, St. Louis, MO, USA) and cefazolin (0.2 g/kg, Patterson Veterinary, Saint Paul, MN, USA) were given before surgery as an analgesic and antibiotic, respectively. Catheters (constructed with Silastic tubing, Dow Corning Corporation, Midland, MI, USA) were inserted 4 cm into the right jugular vein and secured with silk sutures. The opposite end of the tubing ran subcutaneously and exited through a small incision on the back below the shoulder blades where an external port was exposed.
2.4. Heroin Self-Administration
Seven days after surgery (21 days after stress or sham conditioning), rats began SA of either heroin or saline. Heroin (Research Triangle Institute Intl., Research Triangle Park, NC, USA) was diluted in saline. SA procedures were conducted for 3 hours during the rat’s light cycle in SA chambers (30 × 20 × 20 cm) that were enclosed in sound reducing compartments with a ventilation fan (Med Associates, Fairfax, VT, USA) and connected to a computerized data collection program (MED PC, Med Associates). Each chamber had two retractable levers with a white stimulus light above each lever, house light, and tone generator. The infusion tubing was enclosed in steel spring leashes (Plastics One Inc., Roanoke, VA, USA) that connected to the external infusion port and a weighted swivel apparatus (Instech, Plymouth Meeting, PA, USA). The swivel was suspended above the box to allow the rat unrestricted movement throughout the chamber. Heroin SA was conducted 5 days per week.
We used an increased fixed ratio schedule or a descending dose regimen to determine if the protocol in which rats administer heroin would impact acquisition of heroin SA. Specifically, in Experiment 1 and 2 the dose 40 μg/infusion was held constant throughout, but the response requirement to obtain the drug increased such that for the first 5 days rats were on a fixed ratio (FR) 1 schedule of reinforcement, followed by an FR3 for 3 days, and then concluding the experiment on an FR5. In Experiment 3 rats began a descending dose schedule of 2 days on high dose (100 μg/infusion), 2 days on mid dose (50 μg/infusion), and a minimum of 12 days on low dose (25 μg/infusion) (Shen et al. 2014). The house light remained on throughout the sessions and pressing the active lever resulted in a heroin infusion and a 5 second cue (illumination of the white stimulus light over the active lever and activation of tone generator; 78 dB, 4.5 kHz), followed by a 20s time out length between. During the time-out period, responses on the active and inactive levers were recorded, but no stimulus nor drug were presented. Saline SA rats were used for control purposes. Specifically, in Experiments 2 and 3 control rats underwent sham or stress conditioning, catheter implantation surgery, recovery and saline SA. All handling and experimenter protocols occurred under the same conditions however lever pressing resulted in an infusion of saline (50 μl volume) rather than heroin.
2.5. Abstinence, Extinction, and CS Reinstatement Testing
Following SA, rats underwent 3 weeks of abstinence during which they were weighed and handled on a daily basis but were not placed back into the SA context (see next section for behavioral tests that occurred during abstinence). Rats then underwent 3-hour daily extinction sessions for a minimum of 8 days, where responses on both the active and inactive levers were recorded, but no stimulus or drug were presented. On extinction day 1, a “CS reactivity test” was conducted by placing a dish containing the sham/stress CS into the SA chamber. Extinction criterion was less than 25 active lever presses for the final two days of extinction consecutively. After meeting extinction criterion, rats then underwent two reinstatement tests. During the “CS Reinstatement Tests”, an odor dish was placed within the SA apparatus. The dish contained the sham/stress CS or was empty. Tests were counterbalanced with a minimum of 2 days on extinction procedures between each test with rats required to have <25 active lever presses for each day consecutively. After the CS reinstatement test rats received an additional cue test in which active lever pressing resulted in presentation of the light+tone heroin associated cue. A subset of rats (7 stress and 7 sham) underwent a final extinction phase, in which the sham/stress CS was repeatedly placed in the chamber over the course of 10 days and a lever press resulted in presentation of the heroin cues. During tests, presses on the active and inactive lever were recorded but no drug was given.
2.6. Behavioral Testing
2.6.1. Social interaction and social reward place preference.
Social interaction tasks assess approach/avoidance behaviors in response to an unfamiliar rat. The ratio of time spent with and without the social partner can be taken as an index of anxiety-like behavior (Lezak et al. 2017). These tasks also assess social reward as an index of basic reward processing. In Experiment 2, a subset of rats (8 stress and 7 sham) that went through heroin self-administration were used to determine whether prior restraint stress and heroin experience has an impact on social interaction and social reward place conditioning. Control rats were either stress or sham and went through simultaneous saline SA. This task occurred in a round open field (125 cm diameter) and behavior was recoded with Ethovision tracking software in 10 min sessions. On day 1 rats were placed in the open field and given 10 mins to explore the environment, this is considered a pre-interaction measure. White tape was used to mark a 11 × 7 inch square on the floor of the apparatus to indicate the area where the caged rats would go on subsequent sessions. The amount of time spent in this area was recorded. On days 2, 3, and 4 an unfamiliar rat was placed in a wire cage [11 × 7 × 7, (l × w × h)] on one side of the open field. During these sessions, we evaluated the amount of time rats spent near the caged rat, entrances into the zone, the number of nose touches, and bouts of cage climbing for a 10 min session. On day 5, rats were returned to the empty open field for a post-interaction assessment and time spent in the area that previously held the conspecific (denoted by the white tape) was recorded.
2.6.2. Defensive Burying.
Defensive burying measures approach/avoidance behaviors through exploiting an innate response in rodents to bury threatening objects (Pinel and Treit 1978). Often shock-prods are used as an aversive stimulus to initiate the burying behavior in control rats but unconditioned stimuli (e.g., predator odors) or conditioned stimuli, rather than shocks, also elicit burying behavior (Pinel and Treit 1978). Here, we used the sham/stress CS to test cue reactivity during a defensive burying task. The CS was the dish containing the odor previously paired with restraint stress or sham treatment. In Experiment 3, the defensive burying test was conducted in a standard home cage (18.5 × 10 × 8.5) 8 days after heroin exposure ceased. Bedding was placed in one half of the cage opposite to a dish containing the sham/stress CS. Each rat was placed facing away from the CS on the bedding side of the cage. Behavior was digitally recorded for 10 min and manually scored by an experimenter blind to the experimental conditions. The home cage was cleaned with isopropyl between experiments and fresh bedding was used for each animal. A repertoire of behaviors was quantified including: 1) ambulation, 2) rearing, 3) immobility, 4) burying, 5) grooming, and 6) exploring the stimulus based on the criteria defined in De Boer and Koolhaas (De Boer and Koolhaas 2003).
2.7. Data Analysis
Analysis of variance (ANOVA) were used to analyze the heroin SA, extinction, cue reactivity data, and social interaction data. The independent variables were stress condition (sham and stress), drug SA (saline and heroin), and sex (male and female). Differences in days to acquire were determined with Log-rank Mantel-Cox test for rats in Experiment 1 and 2 (see Figure 1). Groups are denoted throughout as sham/saline, stress/saline, sham/heroin, and stress/heroin. Lever presses were the primary dependent measures during heroin maintenance and extinction. Lever presses during extinction and reinstatement tests were analyzed with a 2 × 2 × 3 mixed variable ANOVA with stress (sham and stress) and sex as the between subject variables and test (ext, CS, empty dish, and cue) as the within subject variable. The saline SA groups were not included in the test day analysis due to low levels of responding. Drug intake in mg/kg was another dependent variable during heroin maintenance. During social interaction “% time spent” with the unfamiliar rat was calculated between time spent inside and outside the zone [time in zone/(time in zone + time outside zone).
To assess differential defensive burying behavior across conditions, a generalized linear mixed effects model was developed. Data are clustered on rat (across behavior using a compound symmetric structure) and assuming a log-linear distribution (negative binomial). Time spent (seconds) in each behavior (ambulation, rearing, immobility, burying, grooming, and exploring the stimulus) during the defensive burying task was the main dependent variable in the defensive burying repertoire. The behavior specific main effects of stress group, drug group, and sex as well as drug effects stratified by stress condition are assessed using group level means. Pairwise comparisons beyond the a priori hypothesis were conducted only in instances of significant statistical interactions (p<0.05). To control for multiplicity in the defensive burying analysis, the false discovery rate (Benjamini and Hochberg 1995) was applied to all a priori tests noted above. Comparison specific significance was set (p<.007) to control for an experiment wise false discovery rate of less than 1%. Linear regression data are reported as slope coefficients and associated standard errors. Statistical analyses and graphs were generated using SAS version 9.4 (SAS institute, Cary NC, USA) and GraphPad Prism 8.2 software. The significance level was α=0.05 unless otherwise noted.
3. Results
3.1. Experiment One: Acute restraint stress exposure potentiated heroin intake and stress cue reactivity
3.1.1. Acute restraint stress exposure potentiated heroin intake.
Exposure to restraint stress increased heroin self-administration as indicated by the number of days it took to reach SA criterion, defined as earning a minimum of 10 infusions during the 3-hour session (Figure 2A), compared to sham animals (Log-rank Mantel-Cox test Chi2=3.9, p=0.048).
Figure 2. Acute stress reduced days to acquire heroin self-administration and increased stress reactivity following extinction.
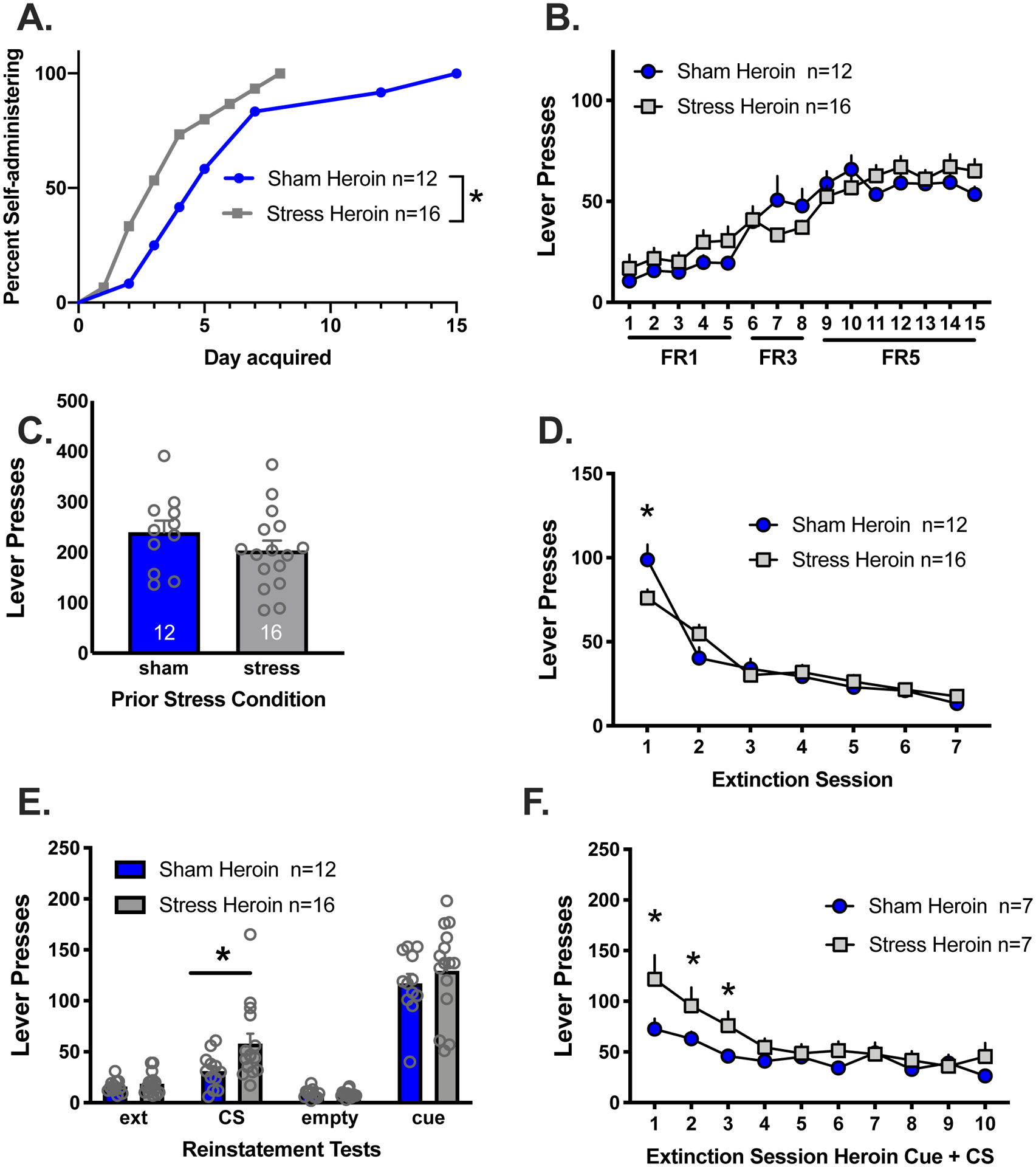
A) Stress rats acquired the lever press response for heroin in fewer days than sham rats. B) Active lever presses in stress and sham rats self-administering heroin did not differ between stress groups. C) There were no differences between sham and stress rats on the first day of extinction with the stress/sham CS. Sham and stress rats all extinguished lever press responding over the 8 days of extinction. Sham rats had increased responding on extinction day 1 relative to stress rats in response to the sham CS. E) Stress rats lever pressed more in response to the stress CS relative to sham rats. Both groups reinstated to heroin conditioned cues. F) Stress rats responded more than sham rats in response to daily extinction with the heroin conditioned cue combined with the stress/sham CS
* Indicates significant difference between stress and sham groups, p<0.05.
All the stress rats were at performance criteria by day 8 of heroin SA whereas the entire group of sham rats did not reach criteria until day 15. Active lever pressing increased relative to the change in FR schedule (Figure 2B) [main effect of day, F(14, 336)=38.19, p<0.0001]. There was also an interaction between stress group and day [F(14,336)=2.24, p<.005], however post hoc comparisons did not reach statistical significance. There were no differences in heroin intake between groups (data not shown).
3.1.2. A history of acute restraint stress increased stress CS reinstatement and delayed extinction of heroin conditioned cues.
At the end of the abstinence period rats were placed back into the SA chamber for a stress reactivity test in which the stress/sham CS was present. Responding was similar in both groups (Fig 2C). Both groups extinguished responding over the 7 days (Fig 2D) [main effect of day, F(6,156)=85.44, p<0.001]. There was a significant interaction between stress group and extinction day [F(6,156)=4.64, p<0.0002] with sham rats responding more than stress groups on day 1 (Holm-Sidak’s, p<0.05). During the sham/stress CS reinstatement test, stress rats reinstated to a greater extent than sham rats (Fig 2E), [t(26)=2.73, p<0.007] but both groups reinstated equally in response to heroin conditioned cues. Importantly, responding to the sham CS did not differ during the CS test and extinction or the “empty dish” test session (Holm-Sidak’s, p>0.05). In stress rats, lever responding significantly increased in response to the stress CS relative to extinction and the “empty dish” test (Holm-Sidak’s, p<0.05). During the final extinction phase the CS was present in the environment and lever pressing resulted in heroin cues. Responding was elevated on the first 3 days of this extinction phase in rats with a history of restraint stress. Lever pressing extinguished over days in both groups [Fig 2F, F(9,108)=14.64, p<0.001] and there was an interaction between stress group and day [F(9,108)=2.44, p<0.02]. Planned comparisons between groups showed that stress rats responded more on days 1–3 relative to sham (p’s<0.05).
3.2. A history of acute restraint stress exposure and heroin SA modified social interaction but not social reward.
Sham and Stress rats spent more time in an area where they previously experienced the opportunity for social interaction regardless of whether they self-administered saline or heroin [Figure 3A, main effect of test day, F(1,54)=57.77, p<0.001]. Activity within the chamber was also higher post social interaction regardless of stress or heroin condition [Figure 3B, main effect of test day, F(1,54)=12.59, p<0.0008]. We also evaluated behavior during the conditioning sessions including: % time spent with the unfamiliar rat, nose touches, climbs onto the cage, and activity within the chamber. Using a traditional marker of social interaction [time in zone/(time in zone + time outside zone), there were no differences between groups in the % of time spent with the unfamiliar rat (Fig 3C). However, the number of nose touches (Fig 3D) were higher in stress rats indicated by a main effect of stress group [F(1,27)=9.04, p<0.006]. Cage climbs (Fig 3E) were increased in saline rats relative to heroin indicated by a main effect of drug group [F(1,27)=17.65, p<0.003]. Heroin rats were more active (Fig 3F) during the session relative to saline rats indicated by a main effect of drug group [F(1,27)=15.08, p<0.006].
Figure 3. Acute stress and heroin self-administration do not interfere with social reward place conditioning but result in different patterns of social behavior.
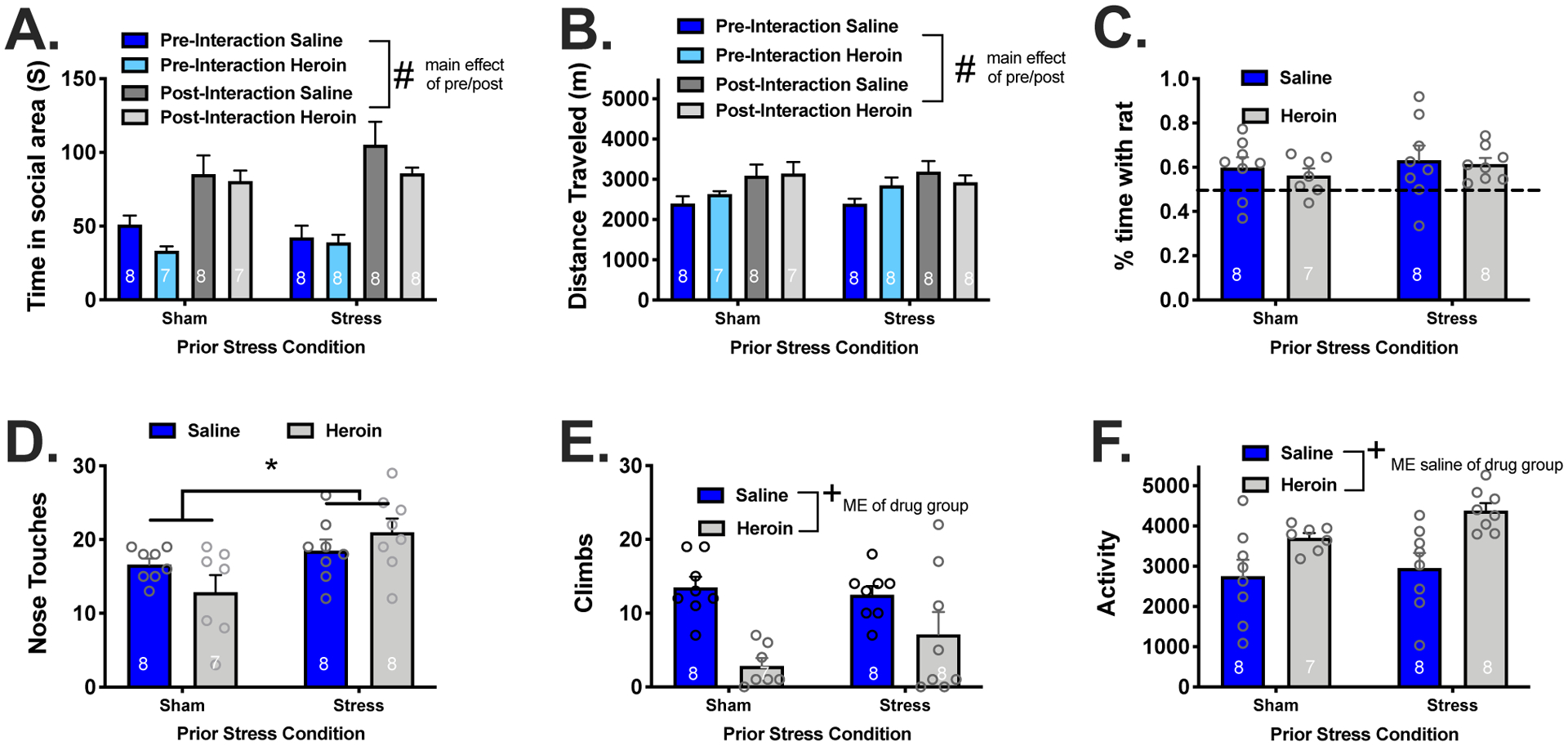
(A) All groups conditioned a preference for the area of the apparatus in which social interaction was available. (B) There were no group differences in the percent of time spent interacting with the rat. (C) Stress rats spent more time in nose-to-nose contact with the caged rat. (D) Heroin rats climbed on top of the cage less than their saline counterparts and (E) had increased locomotor activity during the conditioning sessions.
ME, main effect
# Indicates a significant difference between pre and post test, p<0.05.
* Indicates a significant difference between stress and sham rats, p<0.05.
+ Indicates a significant difference between heroin and saline rats, p<0.05.
3.3. Experiment Three: The effects of acute restraint stress and heroin self-administration on defensive burying in male and female rats.
3.3.1. A history of acute restraint stress increased stress CS reactivity in a defensive burying task.
We modified the SA procedure to a descending dose protocol in order to assess male and female rats for cue reactivity in a defensive burying task. As expected, there were no significant differences in active lever responding between male or female, sham or stress groups during heroin or saline SA (Figure 4A, data collapsed across sex). Females (mean±SEM, 29.38±2.65) took more heroin than males (mean±SEM, 21.83±1.42) when adjusted for mg/kg body weight throughout the SA phase [t(42)=2.63, p<0.012]. This finding is consistent with our reports of cocaine and methamphetamine (Bernheim et al. 2017; Leong et al. 2017; Weber et al. 2018), in which females also took more drug than males when measured in mg/kg body weight but did not differ on the behavioral output (lever presses) to receive the drug..
Figure 4. Acute restraint stress increased latency to bury and % time burying the CS and increased time immobile during the session.
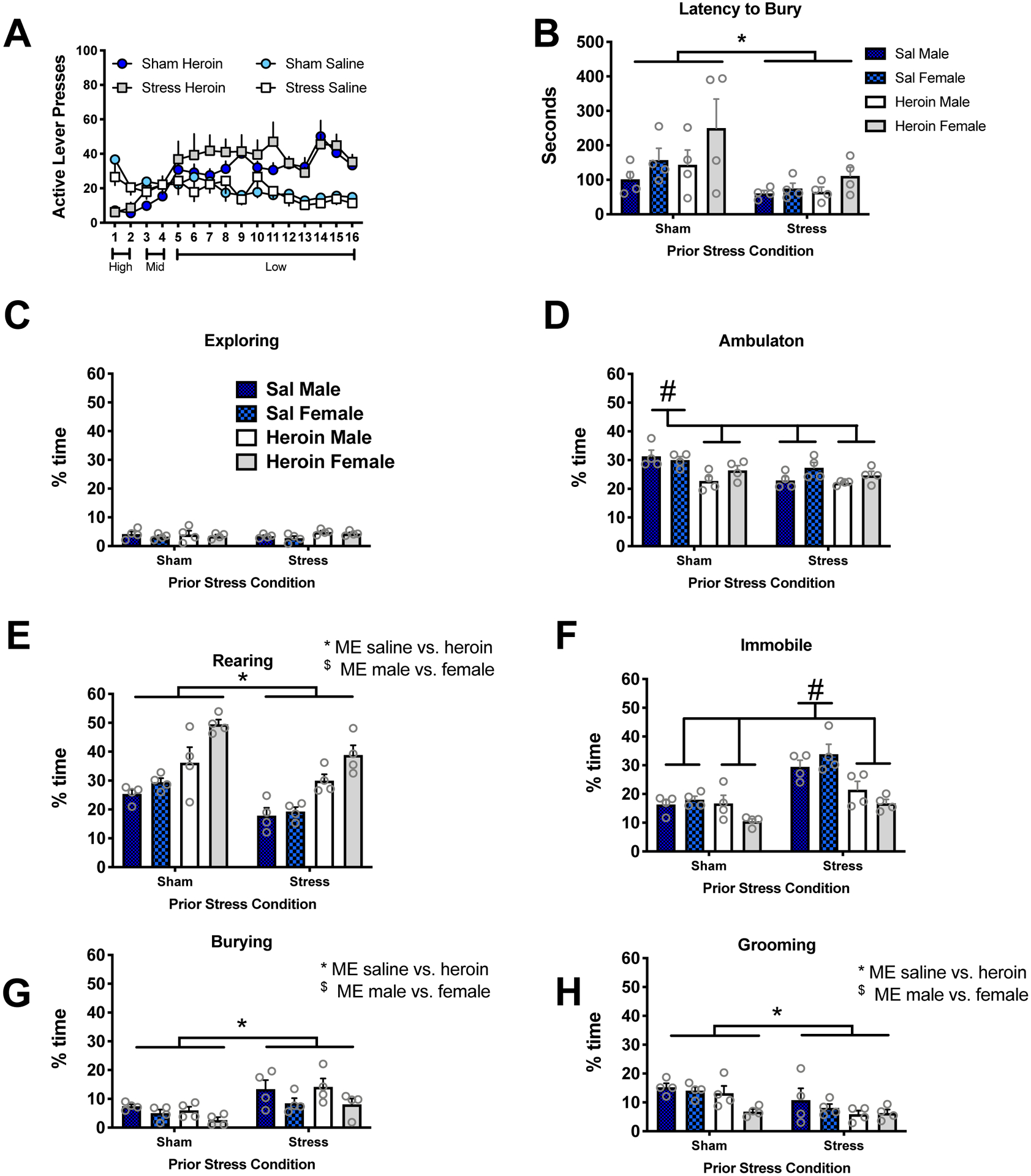
(A) Lever presses did not differ between stress groups or sexes. Data is collapsed across sex. B) Latency to the first burying bout was faster in stress rats. C-H) The complete behavioral repertoire evaluated in a defensive burying task in response the stress conditioned stimulus for sham and heroin rats including exploring (C), ambulation (D), rearing (E), immobility (F), burying (G), and grooming (H). Significant behavior differences were noted between animals exposed to sham as compared to acute stress for all behaviors except exploration of the CS (p<.09). Specifically, sham conditioned animals spent more time engaged in ambulation, grooming, and rearing with less time engaged in burying the CS or sitting immobile. Significant behavioral differences were also identified between saline and heroin SA groups with saline rats spending more time engaged in ambulation, grooming, and immobility and less time rearing relative to the heroin SA rats. Male rats also spent more time engaged in burying the CS and less time in ambulation and rearing relative to females. Significant effects on each of the behaviors for Sham vs Stress, Saline vs Heroin, and Males vs. Females are indicated in Table 1 along with confidence intervals and risk ratios.
ME, main effect
# Indicates a significant difference with all other groups, p<0.007
* Indicates significant difference between stress and sham groups, p<0.007.
+ Indicates significant difference between saline and heroin groups, p<0.007.
$ Indicates significant difference between male and female groups, p<0.007.
Animals were abstinent for 7 days and then tested for CS reactivity in a defensive burying test. Figure 4B depicts the latency (seconds) to the first burying bout. The sham group was slower to begin burying the CS [main effect of stress [F(1,24)=9.87, p<.005] relative to the stress group. There were no other main effects or significant interactions in the latency to bury. Figure 4C–H depicts the complete behavioral repertoire recorded during the defensive burying task for rats presented by the % time engaged in the behaviors including exploring (C), ambulation (D), rearing (E), immobility (F), burying (G), and grooming (H). Significant behavior differences were noted between animals exposed to sham as compared to acute stress (Table 1) for all behaviors except exploration of the CS (p<.09). Sham conditioned animals spent a greater percentage of time engaged in ambulation relative to stress animals (Fig 4D, p<.0004), as did rats that self-administered saline relative to heroin (p<.0001), and female relative to male rats (p<.006). Sham rats spent a greater percentage of time rearing relative to stress rats (Fig 4E, p<.0006); whereas saline rats engaged in rearing less than heroin rats (p<.0001). Females also exhibited greater rearing than males (p=.0006). Stress rats spent a greater percentage of time immobile during the session relative to sham conditioned rats (Fig 4F, p<.001). Stress/Saline rats spent less time immobile than sham conditioned rats and Stress/Heroin rats (p<.0001). Time spent burying (Fig 4G) was increased in stress conditioned rats relative to sham (p<.001) and higher in males relative to females (p<.0001). Time spent grooming (H) was higher in sham conditioned rats compared to stress (p<.0003) and male compared to female rats (p<.0005).
Table 1.
Summary of defensive burying data.
| Stress vs. Sham | Heroin vs. Saline | Females vs. Males | ||||
|---|---|---|---|---|---|---|
| Behavior | RR (95% CI) | Δ (95% CI) | RR (95% CI) | Δ (95% CI) | RR (95% CI) | Δ (95% CI) |
| Explore CS | 1.01 (0.80,1.27) | 0 (−16,16) | 1.23 (0.98,1.55) | 3 (−13,19) | 0.83 (0.66,1.05) | −4 (−20,12) |
| Ambulation | 0.88 (0.82,0.95) | −22 (−38,−6) | 0.86 (0.81,0.93) | −33 (−49,−17) | 1.10 (1.03,1.27) | 15 (−1,31) |
| Rearing | 0.74 (0.66,0.83) | −54 (−70,−39) | 1.69 (1.51,1.89) | 84 (68,99) | 1.22 (1.09, 1.37) | 43 (27,59) |
| Immobile | 1.62 (1.42,1.84) | 60 (44,75) | 0.68 (0.60,0.77) | −57 (−73,−41) | 0.89 (0.78,1.01) | −6 (−22,10) |
| Burying | 2.16 (1.62,2.88) | 34 (18,49) | 0.81 (0.61,1.08) | −9 (−24,7) | 0.57 (0.43,0.76) | −25 (−40,−9) |
| Grooming | 0.64 (0.51,0.82) | −27 (−43,−12) | 0.65 (0.51,0.83) | −28 (−43,−12) | 0.79 (0.62,1.00) | −15 (−31,1) |
Statistical analyses were conducted with generalized linear mixed effects model. Estimates are from model based means and standard errors. Relative risk estimates (RR) are noted as the increased probability of greater time involved in each behavior (Stress-Sham, Heroin-Saline, Females-Males) and the associated 95% CI. Deltas (Δ) are noted as difference in mean seconds performing each behavior (Stress-Sham, Heroin-Saline, Females-Males) and the associated 95% CI. Stress vs. Sham: Positive differences and risk ratio’s greater than 1.0 note that the stress group performed the task for greater time than the sham group. Heroin vs. Saline: Positive differences and risk ratio’s greater than 1.0 note that heroine rats performed the task for greater time than saline rats. Female vs. Male: Positive differences and risk ratio’s greater than 1.0 note that female rats performed the task for greater time than male rats.
Indicates p<.007 corrected
Two interaction contrasts also emerged between stress condition and drug group. Specifically, there were stress × drug group interactions on ambulation (Fig 4D, p<0.03) and the interaction approached significance on immobility (Fig 4F, p<0.055). Results indicate that the difference between saline and heroin rats in ambulation time under the sham condition (RR=0.79 (95% CI: 0.72–0.89); p<0.01) was attenuated and no longer statistically different than under the acute stress condition (RR=0.93 (95% CI: 0.85–1.12); p=0.14). Under the sham condition, heroin rats spend less time in ambulation than saline rats. Sham/saline rats spent more time in ambulation than sham/heroin, stress/heroin, and stress/saline rats. The ambulation time was not different between heroin rats and saline rats that were in the stress condition. The pattern of immobility time was in the opposite direction; results indicate that the difference between saline and heroin rats in immobility time under the acute stress condition (RR=0.60 (95% CI: 0.51–0.71); p<0.01) was slightly stronger than under the sham condition (RR=0.77 (95% CI: 0.64–93); p<0.01). Stress/saline rats spent more time immobile than the other three groups. Although saline rats, in general, spent more time immobile than heroin rats, those in the stress condition had a much greater difference than the sham condition.
4. Discussion
There is high comorbidity among stress disorders and addiction. Stress can impact multiple facets of the addiction cycle including initial acquisition and use patterns, and is a common predictor of relapse (Shaham and Stewart 1995). Individuals with stress or anxiety disorders, like PTSD, are at an elevated risk of not only developing a SUD, including opioid use disorder, but also in relapsing after cessation of drug taking (Sinha 2001). In order to better understand this phenomenon, we used a single episode of acute restraint stress and classically conditioned a neutral odor with the experience (Deslauriers et al. 2018; Garcia-Keller et al. 2019). We first observed that stress increased the rate of acquisition for heroin self-administration and that re-exposure to the stress CS was sufficient to induce heroin seeking, consistent with what was observed with different drugs of abuse, like cocaine and alcohol (Garcia-Keller et al. 2019). Further, the combined stress CS with the heroin conditioned cues delayed extinction responding relative to the sham CS with heroin conditioned cues. Re-exposure to the CS also changed the behavior repertoire in stress rats in a defensive burying task. Such that, stress rats spent more time burying and immobile relative to sham rats, indicating changes in both active and passive coping strategies. Further, rats with a history of acute restraint stress had increased nose-to-nose contact with a non-familiar rat.
Presentation of the stress CS on heroin extinction and reinstatement.
Stress/heroin rats reached SA criteria (>10 infusions/day) in fewer days than sham/heroin rats indicating that acute stress enhances the reinforcing value of heroin (Shaham and Stewart 1995) similar to what was observed with cocaine (Garcia-Keller et al. 2016) and alcohol (Garcia-Keller et al. 2019). This finding is relevant in the context that addiction is a cyclical disorder demarked by distinct phases of acquisition, maintenance, abstinence and relapse. Whereas, stress impacted the acquisition phase, lever presses during maintenance were similar between stress/heroin and sham/heroin rats.
Extinction procedures initiate a new learning contingency when lever responding no longer results in drug delivery. This change in drug contingency demarks a stressful time point in which drug craving may be enhanced, particularly on the first day (Kohtz and Aston-Jones 2017). We placed rats back into the SA environment following 21 days of abstinence with the sham/stress CS in the chamber. We predicted that a history of acute restraint stress would increase extinction responding particularly in the presence of the conditioned odor. However, a history of acute stress had no effect on lever pressing under this condition (Fig 2C). In fact, stress rats responded less on the active lever on extinction day one suggesting a restraint-induced reduction in heroin seeking that was equivalent over the rest of the extinction sessions (Fig 2D). The first extinction phase (Fig 2D) only extinguished the drug-taking context and not the drug conditioned cues or the stress CS. The second extinction phase (Fig 2F), extinguished the heroin cue (light + tone) and the sham/stress CS, and we report that a history of restraint stress delayed extinction of the heroin conditioned cue when the stress CS was also present. The difference between sham and stress rats was the nature of the conditioned odor association. Specifically, sham rats were only required to extinguish responding of the heroin conditioned cues due to their sham CS condition. In contrast, stress rats were required to extinguish responding to the heroin paired cues in the presence of the stress CS. Given that heroin seeking remained higher when the stress CS was present relative to the sham CS, we conclude that the stress CS potentiated motivated responding for heroin cues. Importantly, when the sham/stress CS was absent, both groups equally reinstated to the heroin associated cue alone, indicating that increased drug seeking is dependent on the presence of the stress CS. This pattern of results is aligned with human PTSD-like symptomology in which patients are resistant to extinguish memory of the trauma or trauma associated cues (McCauley et al. 2012) presumably due to deficits in fear extinction (Maren et al. 2013).
Acute restraint stress and heroin SA on anxiety-like behaviors.
Social factors can also have a profound impact on maintaining abstinence from drug use (Garmendia et al. 2008; Hostetler and Ryabinin 2014), reducing anxiety disorder symptoms (Price et al. 2018), and enhancing other treatment approaches (Cox et al. 2017). Neither acute stress nor heroin SA impacted social reward place conditioning indexed by pre/post measures of time spent in the social paired area. A caveat to this conclusion is that all rats underwent the socialization sequence so there is no place comparison relative to rats that were conditioned to an empty chamber rather than a conspecific. Regardless, rats did develop a preference to an area where they previously interacted with a conspecific, indexed as greater time spent in the “social” area post-interaction vs. pre-interaction. During the 3 consecutive days of conditioning stress and heroin rats spent the same amount of time with the conspecific as sham rats. However, stress rats had more nose-to-nose interactions than sham rats suggesting that a history of acute stress increased direct social interaction. Albeit speculative, this finding is reminiscent of PTSD patients responding positively to social supports (Price et al. 2018). A history of heroin SA also moderated social interactions because during the conditioning sessions heroin rats spent more time exploring the chamber relative to their saline counterparts. Activity during social interaction is indicative of anxiety-like behaviors (Lezak et al. 2017) suggesting that abstinence from heroin may lend itself to an anxiogenic state independent of a history of acute stress.
During abstinence we assessed the capacity of the CS to elicit defensive behaviors in rodents (Lezak et al. 2017) by exploiting an innate response in rodents to bury threatening objects (Pinel and Treit 1978). Here we used the stress/sham CS as the threatening object. Stress rats spent their time immobile or actively involved in burying the dish containing the CS. Further, the stress groups had shorter latencies to the first burying bout. This behavioral pattern provides evidence that the odor is indeed functioning as a CS due to the unique behavioral profile of the defensive burying repertoire of the stress rats relative to the sham exposed group.
In general, sham conditioned animals spent more time engaged in ambulation, grooming and rearing and less time engaged in burying the CS or remaining immobile. Significant behavioral differences were also evident between saline and heroin SA groups. Saline rats spent more time engaged in ambulation, grooming and immobile and less time rearing relative to the heroin SA rats. Sex differences revealed male rats spent more time burying and less time in ambulation and rearing relative to females. This sex difference in ambulation and rearing is not surprising given that females have greater baseline activity relative males (Leong et al. 2016; Zhou et al. 2015). Interestingly, stress/saline rats of both sexes spent the greatest amount of time immobile relative to all other groups. We predicted that a history of stress conditioning and heroin SA would potentiate responding in response to the stress CS. Rather than potentiating, heroin SA seemed to normalize immobility in stress/heroin rats. However, the defensive burying repertoire consists of multiple competing behaviors. Heroin rats spent the greatest % of time rearing, which we suggest can be classified as an escape type behavior. In all, a history of stress conditioning and heroin self-administration uniquely changes the response repertoire within the defensive burying task.
Importantly, these exacerbated responses in stress reactivity (burying and immobility) occurred 7 weeks after the initial stressful experience and did not involve exposure to the primary stressor. This detail is relevant in regard to PTSD type behaviors because often symptomatic episodes do not result from the primary stressor but from conditioned stimuli or “triggers” associated with the original stressor that evoke memories, psychological distress, or physiological responses (Fitzgerald et al. 2018; Maren et al. 2013). Coping strategies in response to CS or “triggers” influence PTSD development. Avoidant coping is linked to increased PTSD symptom development following trauma (Gil and Weinberg 2015; Hooberman et al. 2010). Coping consists of active and avoidant strategies. Active coping reflects attempts to change perceptions of the stressor or qualities of the stressor (e.g., burying). In contrast, avoidant coping involves actions and thought processes used to escape direct confrontation with the stressor (e.g., immobility) (Wu et al. 2013). Burying in a defensive burying task is an active, adaptive coping style; whereas, immobility in response to a perceived threat is regarded as a passive, maladaptive coping style (Fucich and Morilak 2018). Rodents shift from active to passive coping following a history of chronic mild stress (Fucich and Morilak 2018; Paredes and Morilak 2019). Our data did not follow a shift from active (burying) to passive (immobile) coping previously described because stress increased both of these variables relative to sham rats. This difference is most likely due to the use of the CS as a perceived threat rather than a shock probe. Our methodology renders the sham rats without a perceived threat during the task, these animals are responding to a non-associated odor; whereas stress rats are in contact with a stress CS that can “trigger” a traumatic episode. In stress rats, both burying, and immobility were increased relative to sham animals suggesting increased involvement in both active and passive coping strategies. This is particularly relevant to PTSD because passive or avoidant coping strategies can exacerbate PTSD symptomology (Mattson et al. 2018; McNeill and Galovski 2015; Thompson et al. 2018).
Conclusions
In conclusion, stress is a well-known precipitant to relapse in human and drug seeking in animal models, and our study expands current research on stress and addiction by demonstrating that a conditioned stressor can reinstate and exacerbate drug seeking under multiple reinstatement modalities. Most stress reinstatement protocols use foot shock or a pharmacological agent to induce reinstatement of drug seeking. Importantly, to include Pavlovian processes and more closely simulate stress disorders we used a previously neutral stimulus (sight, sound, smell) paired with a physiological response (stress, fear, anger) thereby producing a stress CS. We then used this CS, to test measures of cue reactivity in a defensive burying task and heroin seeking. Stress rats had multiple levels of disrupted behavior including: 1) enhanced acquisition of heroin intake, 2) enhanced reinstatement and extinction responding in the presence of the stress CS, 3) enhanced stress reactivity in a burying task resulting in an atypical maladaptive burying strategy, and 4) increased nose-to-nose contact with a non-familiar rat. Taken together, this model seems to address some of the characteristics observed in anxiety and stress related disorders, including PTSD (substance use disorder, anxiety, maladaptive coping strategies) and may be useful for future studies seeking to understand circuits recruited in this pathology and eventually help develop therapeutic approaches.
Acknowledgments
We thank Jordan L. Hopkins for technical assistance. Heroin was supplied by the National Institute on Drug Abuse.
Funding, Disclosure, Conflict of Interest The authors do not have any competing financial or any other conflicts of interests in relation to this work. Heroin was supplied by the NIDA drug supply program. The data collected for this manuscript were supported by the National Institute of Health, National Institute on Drug Abuse grants: P50 DA016511 (CMR), U54 DA016511 (CMR, PWK), T32 DA728823 (RW), and College of Charleston Honors College Summer Enrichment Grant (JSC) and R25 DA033680 (JSC). This work was conducted in a facility constructed with support from NIDA C06RR015455.
Footnotes
Conflict of Interest: None
References
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B 57: 289–300. [Google Scholar]
- Berardi A, Trezza V, Campolongo C (2012) Modeling specific phobias and posttraumatic stress disorder in rodents: the challenge to convey both cognitive and emotional features. Rev Neurosci 23: 645–57. [DOI] [PubMed] [Google Scholar]
- Bernheim A, Leong KC, Berini C, Reichel CM (2017) Antagonism of mGlu2/3 receptors in the nucleus accumbens prevents oxytocin from reducing cued methamphetamine seeking in male and female rats. Pharmacology, biochemistry, and behavior 161: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden MT, Kimerling R, Jacobs-Lentz J, Bowman D, Weaver C, Carney D, Walser R, Trafton JA (2012) Seeking Safety treatment for male veterans with a substance use disorder and post-traumatic stress disorder symptomatology. Addiction (Abingdon, England) 107: 578–86. [DOI] [PubMed] [Google Scholar]
- Clark DJ, Schumacher MA (2017) America’s Opioid Epidemic: Supply and Demand Considerations. Anesthesia and analgesia 125: 1667–1674. [DOI] [PubMed] [Google Scholar]
- Clark HW, Masson CL, Delucchi KL, Hall SM, Sees KL (2001) Violent traumatic events and drug abuse severity. Journal of substance abuse treatment 20: 121–7. [DOI] [PubMed] [Google Scholar]
- Cohen LR, Hien DA (2006) Treatment outcomes for women with substance abuse and PTSD who have experienced complex trauma. Psychiatric services (Washington, DC) 57: 100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, Grant BF (2006) Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. The Journal of clinical psychiatry 67: 247–57. [DOI] [PubMed] [Google Scholar]
- Cox DW, Bakker AM, Naifeh JA (2017) Emotion Dysregulation and Social Support in PTSD and Depression: A Study of Trauma-Exposed Veterans. Journal of traumatic stress 30: 545–549. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Koolhaas JM (2003) Defensive burying in rodents: ethology, neurobiology and psychopharmacology. European journal of pharmacology 463: 145–61. [DOI] [PubMed] [Google Scholar]
- Deslauriers J, Toth M, Der-Avakian A, Risbrough VB (2018) Current Status of Animal Models of Posttraumatic Stress Disorder: Behavioral and Biological Phenotypes, and Future Challenges in Improving Translation. Biological psychiatry 83: 895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaggio C, Galea S, Li G (2009) Substance use and misuse in the aftermath of terrorism. A Bayesian meta-analysis. Addiction (Abingdon, England) 104: 894–904. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JM, DiGangi JA, Phan KL (2018) Functional Neuroanatomy of Emotion and Its Regulation in PTSD. Harvard review of psychiatry 26: 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florence CS, Zhou C, Luo F, Xu L (2016) The Economic Burden of Prescription Opioid Overdose, Abuse, and Dependence in the United States, 2013. Medical care 54: 901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman MJ, Resick PA, Bryant RA, Brewin CR (2011) Considering PTSD for DSM-5. Depression and anxiety 28: 750–69. [DOI] [PubMed] [Google Scholar]
- Fucich EA, Morilak DA (2018) Shock-probe Defensive Burying Test to Measure Active versus Passive Coping Style in Response to an Aversive Stimulus in Rats. Bio-protocol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Keller C, Kupchik YM, Gipson CD, Brown RM, Spencer S, Bollati F, Esparza MA, Roberts-Wolfe DJ, Heinsbroek JA, Bobadilla AC, Cancela LM, Kalivas PW (2016) Glutamatergic mechanisms of comorbidity between acute stress and cocaine self-administration. Molecular psychiatry 21: 1063–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Keller C, Martinez SA, Esparza MA, Bollati F, Kalivas PW, Cancela LM (2013) Cross-sensitization between cocaine and acute restraint stress is associated with sensitized dopamine but not glutamate release in the nucleus accumbens. The European journal of neuroscience 37: 982–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Keller C, Smiley C, Monforton C, Melton S, Kalivas PW, Gass J (2019) N-Acetylcysteine treatment during acute stress prevents stress-induced augmentation of addictive drug use and relapse. Addiction biology: e12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmendia ML, Alvarado ME, Montenegro M, Pino P (2008) [Social support as a protective factor of recurrence after drug addiction treatment]. Revista medica de Chile 136: 169–78. [PubMed] [Google Scholar]
- Gil S, Weinberg M (2015) Coping strategies and internal resources of dispositional optimism and mastery as predictors of traumatic exposure and of PTSD symptoms: A prospective study. Psychological trauma : theory, research, practice and policy 7: 405–11. [DOI] [PubMed] [Google Scholar]
- Hooberman J, Rosenfeld B, Rasmussen A, Keller A (2010) Resilience in trauma-exposed refugees: the moderating effect of coping style on resilience variables. The American journal of orthopsychiatry 80: 557–563. [DOI] [PubMed] [Google Scholar]
- Hostetler CM, Ryabinin AE (2014) Social partners prevent alcohol relapse behavior in prairie voles. Psychoneuroendocrinology 39: 152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohtz AS, Aston-Jones G (2017) Cocaine Seeking During Initial Abstinence Is Driven by Noradrenergic and Serotonergic Signaling in Hippocampus in a Sex-Dependent Manner. Neuropsychopharmacology 42: 408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong K, Freeman L, Berini C, Ghee S, See R, Reichel C (2017) Oxytocin Reduces Cocaine Cued Fos Activation in a Regionally Specific Manner. Int J Neuropsychopharmacol 20: 844–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong K, Zhou L, Ghee S, See R, Reichel C (2016) Oxytocin decreases cocaine taking, cocaine seeking, and locomotor activity in female rats. Experimental and clinical psychopharmacology 24: 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak KR, Missig G, Carlezon WA Jr. (2017) Behavioral methods to study anxiety in rodents. Dialogues in clinical neuroscience 19: 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Abelson JL (2016) Context Processing and the Neurobiology of Post-Traumatic Stress Disorder. Neuron 92: 14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I (2013) The contextual brain: implications for fear conditioning, extinction and psychopathology. Nature reviews Neuroscience 14: 417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson E, James L, Engdahl B (2018) Personality Factors and Their Impact on PTSD and Post-traumatic Growth is Mediated by Coping Style Among OIF/OEF Veterans. Military medicine. [DOI] [PubMed] [Google Scholar]
- McCauley JL, Killeen T, Gros DF, Brady KT, Back SE (2012) Posttraumatic Stress Disorder and Co-Occurring Substance Use Disorders: Advances in Assessment and Treatment. Clinical psychology : a publication of the Division of Clinical Psychology of the American Psychological Association 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill SA, Galovski TE (2015) Coping Styles Among Individuals with Severe Mental Illness and Comorbid PTSD. Community mental health journal 51: 663–73. [DOI] [PubMed] [Google Scholar]
- Najavits LM, Weiss RD, Shaw SR (1997) The link between substance abuse and posttraumatic stress disorder in women. A research review. The American journal on addictions 6: 273–83. [PubMed] [Google Scholar]
- Ouimette PC, Wolfe J, Chrestman KR (1996) Characteristics of posttraumatic stress disorder-alcohol abuse comorbidity in women. Journal of substance abuse 8: 335–46. [DOI] [PubMed] [Google Scholar]
- Paredes D, Morilak DA (2019) A Rodent Model of Exposure Therapy: The Use of Fear Extinction as a Therapeutic Intervention for PTSD. Frontiers in behavioral neuroscience 13: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinel JP, Treit D (1978) Burying as a defensive response in rats. Journal of Comparative and Physiological Psychology 92: 708–712. [Google Scholar]
- Price M, Lancaster CL, Gros DF, Legrand AC, van Stolk-Cooke K, Acierno R (2018) An Examination of Social Support and PTSD Treatment Response During Prolonged Exposure. Psychiatry 81: 258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G (2018) Drug and Opioid-Involved Overdose Deaths - United States, 2013–2017. MMWR Morbidity and mortality weekly report 67: 1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, Roberts-Wolfe D, Kalivas PW (2016) The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacol Rev 68: 816–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal KH, Shi Y, Cohen G, Cohen BE, Maguen S, Krebs EE, Neylan TC (2012) Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. Jama 307: 940–7. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J (1995) Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology 119: 334–41. [DOI] [PubMed] [Google Scholar]
- Shen HW, Scofield MD, Boger H, Hensley M, Kalivas PW (2014) Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. The Journal of neuroscience : the official journal of the Society for Neuroscience 34: 5649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R (2001) How does stress increase risk of drug abuse and relapse? Psychopharmacology 158: 343–59. [DOI] [PubMed] [Google Scholar]
- Thompson NJ, Fiorillo D, Rothbaum BO, Ressler KJ, Michopoulos V (2018) Coping strategies as mediators in relation to resilience and posttraumatic stress disorder. Journal of affective disorders 225: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber RA, Logan CN, Leong KC, Peris J, Knackstedt L, Reichel CM (2018) Regionally Specific Effects of Oxytocin on Reinstatement of Cocaine Seeking in Male and Female Rats. Int J Neuropsychopharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward SH, Kaloupek DG, Streeter CC, Kimble MO, Reiss AL, Eliez S, Wald LL, Renshaw PF, Frederick BB, Lane B, Sheikh JI, Stegman WK, Kutter CJ, Stewart LP, Prestel RS, Arsenault NJ (2006) Hippocampal volume, PTSD, and alcoholism in combat veterans. The American journal of psychiatry 163: 674–81. [DOI] [PubMed] [Google Scholar]
- Wu G, Feder A, Cohen H, Kim JJ, Calderon S, Charney DS, Mathe AA (2013) Understanding resilience. Frontiers in behavioral neuroscience 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ghee SM, See RE, Reichel CM (2015) Oxytocin differentially affects sucrose taking and seeking in male and female rats. Behavioural brain research 283: 184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]


