Abstract
Vaccines against COVID-19 (and its emerging variants) are an essential global intervention to control the current pandemic situation. Vaccines often cause adverse events; however, the vast majority of adverse events following immunization (AEFI) are a consequence of the vaccine stimulating a protective immune response, and not allergic in etiology. Anaphylaxis as an AEFI is uncommon, occurring at a rate of less than 1 per million doses for most vaccines. However, within the first days of initiating mass vaccination with the Pfizer-BioNTech COVID-19 vaccine BNT162b2, there were reports of anaphylaxis from the United Kingdom and United States. More recent data imply an incidence of anaphylaxis closer to 1:200,000 doses with respect to the Pfizer-BioNTech vaccine.
In this position paper, we discuss the background to reactions to the current COVID-19 vaccines and relevant steps to mitigate against the risk of anaphylaxis as an AEFI. We propose a global surveillance strategy led by allergists in order to understand the potential risk and generate data to inform evidence-based guidance, and thus provide reassurance to public health bodies and members of the public.
Keywords: Adverse event following immunization, Anaphylaxis, COVID-19, Polyethylene glycol, Vaccine
Introduction
Vaccines against COVID-19 (and its emerging variants) are an essential global intervention to control the current pandemic situation. Vaccines often cause adverse events; however, the vast majority of adverse events following immunization (AEFI) are due to the protective immune response induced by the vaccine, and not due to an allergic reaction.
Anaphylaxis as an AEFI is uncommon, occurring at a rate of less than 1 per million doses for most vaccines.1, 2, 3, 4, 5 However, within the first days of initiating mass vaccination with the Pfizer-BioNTech COVID-19 vaccine BNT162b2, there were reports of 2 and 6 anaphylaxis events in the United Kingdom and United States, respectively.6,7 Further surveillance data reported for the United States suggest a rate closer to 1:200,000 doses for the Pfizer-BioNTech vaccine and 1:360,000 for the Moderna vaccine.8 The vaccines against COVID-19 are new and some (for example, the mRNA vaccines) have a novel mechanism of action; thus the risk of allergic reactions may be greater than for conventional vaccines. Moreover, it is not surprising that anaphylaxis has not been reported in the clinical trials to-date, given the very low incidence and the exclusion of individuals with a history of hypersensitivity reactions in most studies.
In this paper, we discuss the background to reactions to the current COVID-19 vaccines and relevant steps to mitigate against the risk of anaphylaxis as an AEFI. We propose a global surveillance strategy led by allergists in order to understand the potential risk and generate data to inform evidence-based guidance.
Potential causes of allergic reactions to COVID-19 vaccines
Allergic reactions to vaccines are generally due to adjuvants and other excipients/components in the vaccine such as preservatives and antibiotics, rather than to the active ingredient itself. Vaccines may also contain small amounts of protein present due to the production process (eg, embryonic cells).3
In the United Kingdom, there was an initial concern that patients with atopic disease might be more at risk of allergic reactions. This is because the 2 recipients in the United Kingdom who were reported to experience anaphylaxis had a history of epinephrine auto-injector carriage due to prior anaphylaxis. The UK regulator issued an advisory statement which listed prior anaphylaxis to a vaccine, medicine or food as a contra-indication.7 The US Food and Drug Administration (FDA) did not make the same stipulation, nor did the US Centers for Disease Control and Prevention (CDC), although both the UK regulator and CDC recommended that all vaccine recipients (regardless of atopic history) should be observed for 15 minutes following vaccination, and that facilities (and trained staff) must be available in all vaccination centers.9 However, subsequent surveillance data provided further reassurance and the UK regulator lifted the contra-indication 3 weeks later.10
Polyethylene glycols
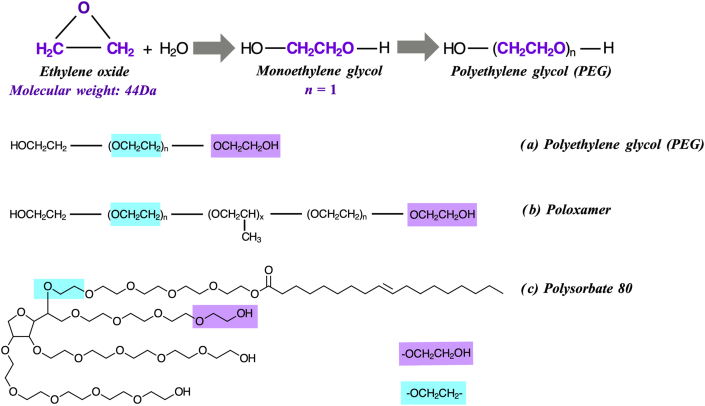
The Pfizer-BioNTech vaccine contains 2 novel lipid nanoparticles, 1 of which is “pegylated” (Polyethylene glycol, molecular weight 2000 Da, abbreviated to PEG2000). The Moderna mRNA vaccine also includes a different pegylated lipid (also a PEG2000) (Table 1). Polyethylene glycols, also known as macrogols, are a group of polyether compounds which are widely used in medicinal, cosmetic and household products (including creams and lotions, shampoo, hair dye, and dental hygiene products). They are formed via the polymerization of ethylene oxide, resulting in PEG polymers of variable chain length and thus molecular weight (Fig. 1).11 PEGs are also used as food additives, as well as in a variety of industrial processes.12 With respect to the COVID-19 vaccines, the inclusion of a pegylated nanoparticle encapsulating the mRNA impairs enzymatic degradation of the mRNA, increases its water-solubility, and thus bioavailability of the lipid nanoparticle.
Table 1.
Vaccines against SARS-Cov-2 that are currently approved as of 18 January 2021.
| Vaccine & manufacturer | Legal status (as of Jan 2021) | Vaccine type | Excipients | Hypersensitivity data to date |
|---|---|---|---|---|
| CoronaVac (Sinovac, China) | EUA for use in China (essential workers and high-risk groups), TurkeyPending: Indonesia | Inactivated vaccine (formalin with alum adjuvant) | Aluminum hydroxide, disodium hydrogen phosphate, sodium dihydrogen phosphate, sodium chloride | No anaphylaxis events reported during Phase 3 trials (33,620 participants) |
| Convidicea Ad5-nCoV (CanSino Biologics, Beijing Inst. Biotech., NPO Petrovax) | EUA in China (limited to military use only)Pending: Mexico | Recombinant adenovirus type 5 vector against spike RBD protein | N/A | No anaphylaxis events reported during Phase 3 trials (40,000 participants) |
| BBIBP-CorV (Sinopharm, Beijing Institute & Wuhan Inst. of Biological Products) | Full authorization for use in China, EUA in Bahrain, Egypt, UAE. | Inactivated SARS-CoV-2 (vero cells) + aluminum hydroxide adjuvant | Aluminum hydroxide, disodium hydrogen phosphate, sodium dihydrogen phosphate, sodium chloride, sodium hydroxide, sodium bicarbonate, M199 | No anaphylaxis events reported during Phase 3 trials (48,000 participants) |
| Pfizer-BioNTech BNT162b2 | EUA in Argentina, Bahrain, Canada, Chile, Costa Rica, Ecuador, EU, Israel, Jordan, Kuwait, Mexico, Oman, Panama, Saudi Arabia, Singapore, Switzerland, UK, USA, WHO. Pending:Australia, India, Japan | mRNA-based vaccine (encoding the viral spike (S) glycoprotein) | (4-hydroxybutyl) azanediyl)bis (hexane-6,1-diyl)bis(2-hexyldecanoate)] (ALC-0315), 2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide (ALC-0159),1,2-Distearoyl-sn-glycero-3-phosphocholine cholesterol, potassium chloride, potassium dihydrogen phosphate, sodium chloride, disodium hydrogen phosphate dihydrate, sucrose, water for injection | No anaphylaxis events attributed to vaccine reported in clinical trials (~22,000 participants randomized to active dosing).Approx. incidence of anaphylaxis 1:100,000 with routine use |
| Moderna mRNA-1273 | EUA in Canada, EU, Israel, Switzerland, UK, USA | mRNA-based vaccine (encoding the pre-fusion stabilized spike (S) glycoprotein) | Lipids (SM-102, 1,2-dimyristoyl-rac-glycero3-methoxypolyethylene glycol-2000 [PEG2000-DMG], cholesterol, and 1,2-distearoyl-snglycero-3-phosphocholine [DSPC]), tromethamine, tromethamine hydrochloride, acetic acid, sodium acetate, and sucrose. | No acute anaphylaxis reactions reported in clinical trials (~15,000 participants randomized to active dosing) |
| ChAdOx1 (Oxford/AstraZeneca; Covishield in India) | EUA in Argentina, Dominican Republic, El Salvador, EU, India, Mexico, Morocco, UK. Pending: Australia, Canada | Replication-deficient viral vector vaccine (adenovirus from chimpanzees) | L-Histidine, L-Histidine hydrochloride monohydrate, Magnesium chloride hexahydrate, polysorbate 80, Ethanol, Sucrose, Sodium chloride, Disodium edetate dihydrate, Water for injection | No anaphylaxis events reported in clinical trials (~12,000 participants randomized to active dosing) |
| Covaxin (BBV152)(Bharat Biotech, India) | EUA in India | Inactivated vaccine | N/A | No events report in Phase 1 studies (n = 300) |
| Sputnik V (Gamaleya Research Inst) | Russia, Palestine | Non-replicating, two-component vector (adenovirus) against spike (S) glycoprotein | Tris (hydroxymethyl) aminomethane, sodium chloride, sucrose, magnesium chloride hexahydrate, Sodium EDTA, polysorbate 80, ethanol, water for injection | No events report in Phase 1/2 studies (n = 76) |
| EpiVacCorona (Federal Budgetary Research Institution State Research Ctr, Russia) | Regulatory approval granted in Russia on basis of Phase 1/2 studies | Peptide vaccine with alum adjuvant | Aluminum hydroxide, potassium dihydrogen phosphate, potassium chloride, sodium hydrogen phosphate dodecahydrate, sodium chloride, water for injection | Unknown |
EUA, emergency use authorization
Fig. 1.
Polymerization and molecular structure of polyethylene glycol (PEG) and PEG derivatives. Two chemical moieties are common to both PEGs and the PEG derivatives poloxamer and polysorbate; thus cross-reactivity is, in theory, possible. Reproduced from 11 with permission
PEGs were previously considered to be biologically inert, a feature which has contributed to their widespread use. They form the active ingredient in laxatives and bowel preparations, and are also used as pill binders, in the surface coatings of tablets and as a stabilizer in liquids for injection, lubricant and ultrasound gels, ointments, suppositories, depot injections, bone cements, and organ preservatives.11 PEGylated drugs are becoming more common as they prolong the circulation time of systemic drugs, by impeding metabolism or shielding the drug from immune-degradation. However, there are an increasing number of case reports of immediate-type allergy to PEGs in the literature.11,13,14
PEG allergy is very uncommon, despite its widespread use in household products, medicines, and even foods. The majority of reported reactions to PEG in the literature are due to high molecular weight PEGs,11 whereas the lower-molecular weight PEGs found in many household products seem to be less common as a cause of allergic hypersensitivity reactions. The PEG-induced reactions reported in the literature appear to be at the more severe end of the spectrum, with most cases describing multiple episodes of anaphylaxis, often requiring multiple doses of epinephrine.11 Skin testing for the investigation of PEG allergy has also been reported to induce anaphylaxis.13
The mechanism(s) which underly PEG allergy are unclear. IgE antibodies to PEG have been detected in some patients with a history of PEG-induced anaphylaxis.15 PEGs have also been shown to induce complement activation, at least in vitro,16 and may result in complement activation-related pseudo-allergy (CARPA). However, human data relating to complement activation as a mechanism for acute allergic reactions to PEG are inconclusive.11,17
The hitherto very low prevalence of PEG allergy would seem inconsistent with the number of initial cases of anaphylaxis reported to the Pfizer-BioNTech vaccine. This raises the possibility that other triggers, aside from PEG, might be contributory. mRNA-based vaccines are novel vaccines which have not been in widespread use before, so it is important to keep an open mind as to potential triggers for the reported reactions. For example, it is possible that the double stranded RNA within the vaccine is itself immunogenic. Although PEG-sensitization has not been evaluated in healthcare workers, it is possible that healthcare workers may have a higher rate of PEG-sensitization compared to the general population, thus the prioritization of healthcare workers for vaccination might have contributed to the higher-than-expected rate of anaphylaxis to the Pfizer-BioNTech vaccine.
Polysorbate 80 and PEG
Some of the current non-mRNA-based COVID vaccines contain polysorbate 80. However, in contrast to PEG, polysorbate 80 is an excipient in many existing vaccines (eg, DTaP and its analogues, HepB, HPV, pneumococcal conjugate vaccine, influenza vaccines, zoster) and many medicines; it is also in widespread use as an emulsifier in foods (E433). At least 70% of injectable biological agents and monoclonal antibody treatments contain polysorbates, usually polysorbate 80.17
Polysorbates are derived from PEGs, but tend to be of lower molecular weights (eg, polysorbate 80 has a molecular weight of 1310 Da), and thus may be much less likely to trigger an allergic reaction. Polysorbates have been reported to induce anaphylaxis-like reactions in animal models (typically via an IgE-independent pathway),18 but there are very few cases of clinical reactivity in humans in the literature.18, 19, 20 This is very surprising, given the widespread use of polysorbates in many pharmaceuticals and in particular, vaccines. IgE-sensitization (without clinical reactivity) is considered to be more common.11,13,14 This may also explain why patients with PEG-allergy tend to tolerate polysorbates in food.11 Whether patients with allergy to polysorbates react to higher molecular weight PEGs is unclear. The CDC currently advises against administering either the Pfizer-BioNTech or AstraZeneca vaccines in individuals with allergy to polysorbates.9
There are 2 reported cases in the literature of patients with immediate-type allergy to PEG who had cross-reactivity to polysorbate 80 at skin testing.19 However, in our experience, most patients with PEG allergy tolerate polysorbate 80, including in vaccines. Of note, the AstraZeneca ChAdOx1 vaccine contains polysorbate 80 at a concentration of <100 μg per dose. This is equivalent to the amount of polysorbate 80 found in most injected influenza vaccines, and less than half the amount in influenza vaccines targeted to those over age 65 (eg, Fluad).17 Given the very widespread use of these vaccines on an annual basis, it is reassuring to note that influenza vaccines have not associated with a higher rate of hypersensitivity reactions (akin to that observed for the PEGylated Pfizer-BioNTech COVID-19 vaccine). This is the basis for current guidance from Public Health England in the United Kingdom, that individuals with PEG allergy can receive the AstraZeneca ChAdOx1 vaccine,21 although it would seem prudent to keep these patients for at least 30 min observation post vaccination.
Relevance of allergy in patient selection for vaccination
Healthcare workers must follow local authorizations and policy in terms of indications and contra-indications for vaccines against COVID-19. The schema in Table 2 provides an outline for precautions which may be prudent dependent on patient history. The anaphylaxis reactions reported to date almost always occurred within 15–30 min of vaccination. There is little information on the risk of vaccine-associated reactions in patients with mast cell disease (such as mastocytosis) nor those who meet published criteria for mast cell activation syndrome (MCAS);22 in the absence of any data, we recommend that such patients can be vaccinated, but may benefit from extended observation of at least 30 min.23
Table 2.
Schema for contra-indications and precautions when considering vaccination for COVID-19. Adapted from 9
 |
Management of anaphylaxis
The anaphylaxis episodes to the Pfizer-BioNTech vaccine reported to date have responded to treatment with epinephrine (adrenaline), administered according to International guidelines.24 Note, however, that at least for the cases reported in the United Kingdom, more than 1 dose of epinephrine was required. Staff in all vaccination settings should be trained in the management of anaphylaxis, and have the necessary equipment available. Further guidance relating to the management of anaphylaxis in the vaccination setting can be found in Appendix A to this article.
A blood sample for evaluation of mast cell tryptase is often of critical importance for accurate diagnosis of vaccine-associated anaphylaxis, particularly in adults, as the history and even symptomology can be ambiguous. The blood sample should be taken 0.5–2 h after a reaction,24 along with a further convalescent/baseline sample (taken at least 24 h after complete resolution of symptoms).
In the context of treating an acute episode of anaphylaxis to a PEG-containing vaccine, it is worth noting that some medicines (such as oral antihistamines) contain PEG.13 In general, liquid antihistamines tend not to contain PEG, but clinicians are advised to check their local formulary for specific information.
Choice of vaccine after anaphylaxis to a first vaccine dose
Individuals who develop a systemic allergic reaction to a vaccine should not receive a second dose of that vaccine, nor a vaccine with similar excipients. For example, patients who react with systemic symptoms of the Pfizer BioNTech vaccine should not be given a second dose of this vaccine, nor should they receive a dose of the Moderna vaccine which is also a mRNA-based vaccine which contains PEG. Although data are not currently available as to protection offered using a combination of different vaccines, current UK advice is that the AstraZeneca vaccine can be used as an alternative (if not otherwise contraindicated, for example, in a patient with a proven allergy to polysorbate 80).21
Advice from the British Society for Allergy and Clinical Immunology (BSACI) (and published by Public Health England) advises that individuals who develop just a localized urticarial skin reaction (without systemic symptoms) to the first dose of a COVID-19 vaccine should receive the second dose using the same vaccine, in a setting with full resuscitation facilities (eg, a hospital); a 30 min observation period is advised.21
Diagnostic testing
Various algorithms have been proposed for the investigation of vaccine allergy and PEG-associated anaphylaxis.13,17 “The investigation of potential PEG-allergy is complicated, and skin testing (both skin prick testing and intradermal testing) has been associated with systemic reactions, including anaphylaxis;13,17,25 evaluation should therefore only be performed by those with the relevant expertise and in the appropriate setting. We advise clinicians to consider obtaining venous access prior to skin testing with PEGs, in patients with a history of possible anaphylaxis to PEG. Whilst the concentration of PEG-2000 in the Pfizer-BioNTech vaccine is very small, it is sufficient to elicit a positive intradermal response in PEG-allergic patients. However, the vaccine is also able to elicit a delayed intradermal response in vaccinated individuals without PEG allergy (S Farooque & P Turner, personal communication).
Surveillance
The speed and necessity with which vaccines against COVID-19 are being rolled-out requires additional surveillance in order for the community to be able to provide reassurance to the vast majority of individuals and identify potential risk factors for those very few who do experience allergic reactions to the vaccines. This is particularly important in the current climate of vaccine hesitancy. Furthermore, there is a concern as to whether the first dose of vaccine might cause IgE-sensitization, and thus increase the risk of anaphylaxis to a subsequent booster dose of vaccine. In addition, the second dose of the Pfizer vaccine causes a higher rate of AEFI;26 this is not uncommon for non-live vaccines, and is generally thought to be due to a priming effect of the first dose. Whether this impacts on the risk of allergic reactions to the new COVID-19 vaccines is unclear.
Whilst many countries have their own surveillance system, the incidence of anaphylaxis to vaccines is so small that we propose a global strategy. Therefore, World Allergy Organization (WAO) in conjunction with Imperial College London and the Network for Online Registration of Anaphylaxis (NORA) have established a system for healthcare professionals to register potential cases of anaphylaxis and allergic reactions to the different COVID-19 vaccines. We encourage our colleagues around the world to provide de-identified information which will then be assessed against the validated criteria published by the Brighton Collaboration with respect to Anaphylaxis as an AEFI,27 as summarized in Appendix B. These data will allow us, as a community, to monitor the occurrence of allergic reactions to the different COVID-19 vaccines in use. We intend to make this information public via the WAO and NORA websites.
REPORT CASES OF ALLERGIC REACTIONS TO COVID VACCINES AT: Bit.ly/wao-covid.
Summary
In the current climate where widespread uptake of vaccines against COVID-19 is a key global intervention to control the pandemic, it is essential that vaccination proceeds safely and with as few barriers as possible. Both the public and healthcare workers need reassurance that the vaccines are safe. We hope guidance such as this document, and the collection of anonymized patient data relating to possible allergic reactions to the vaccines, will play a constructive role in achieving this aim.
Abbreviations
AEFI, adverse event following immunization; CARPA, complement activation-related pseudo-allergy; CDC, US Centers for Disease Control and Prevention; COVID-19, Coronavirus Disease 2019; FDA, US Food & Drug Administration; MCAS, mast cell activation syndrome; mRNA, messenger RNA; NORA, Network for Online Registration of Anaphylaxis; PEG, Polyethylene glycol; WAO, World Allergy Organization
Funding
None.
Consent for publication
All authors provided input into the manuscript, reviewed the final draft and provided consent for publication.
Ethics approval
Not applicable.
Author contributions
Drs Turner and Worm wrote the first draft of the manuscript, which was then reviewed, amended, and approved by all co-authors.
Availability of data and materials
Not applicable.
Declaration of competing interest
Dr. Ansotegui reports personal fees from Mundipharma, Roxall, Sanofi, MSD, Faes Farma, Hikma, UCB, Astra Zeneca, Stallergenes, Abbott, and Bial, outside the submitted work.
Dr. Cardona reports personal fees from ALK, Allergy Therapeutics, LETI, Thermofisher, Merck, Astrazeneca, and GSK, outside the submitted work. Former chair of the WAO Anaphylaxis Committee. Member of the working group of the EAACI anaphylaxis guidelines. Chair of the SLAAI anaphylaxis Committee.
Dr. Campbell is a part-time employee of DBV Technologies and reports grants from National Health and Medical Research Council of Australia and personal fees from Allergenis, Westmead Fertility Centre, and Financial Markets Foundation for Children.
Dr. Ebisawa reports personal fees from Mylan, outside the submitted work.
Dr. El-Gamal has nothing to disclose.
Dr. Fineman has nothing to disclose.
Dr. Geller has nothing to disclose.
Dr. Gonzalez-Estrada has nothing to disclose.
Dr. Greenberger reports personal fees from Wolters Kluwer book, Wolters Kluwer Uptodate, and Allergy Therapeutics, outside the submitted work; and Expert testimony: Legal on anaphylaxis.
Dr. Leung has nothing to disclose.
Dr. Levin has nothing to disclose.
Dr Muraro reports grants and personal fees from Aimmune and personal fees from DVB, Mylan, ALK and Nestle outside the submitted work.
Dr. Sanchez Borges has nothing to disclose.
Dr. Senna has nothing to disclose.
Dr. Sheikh has nothing to disclose.
Dr. Tanno has nothing to disclose.
Dr. Thong has nothing to disclose.
Dr. Turner reports grants from UK Medical Research Council, NIHR/Imperial BRC, UK Food Standards Agency, End Allergies Together, Jon Moulton Charity Trust; personal fees and non-financial support from Aimmune Therapeutics, DBV Technologies and Allergenis, personal fees and other from ILSI Europe and UK Food Standards Agency, outside the submitted work; current Chairperson of the WAO Anaphylaxis Committee, and joint-chair of the Anaphylaxis Working group of the UK Resuscitation Council.
Dr. Worm reports other from Allergopharma GmbH & Co. KG, other from ALK-Abelló Arzneimittel GmbH, other from Mylan Germany GmbH, other from Leo Pharma GmbH, other from Sanofi-Aventis Deutschland GmbH, other from Regeneron Pharmaceuticals, other from DBV Technologies S.A, other from Stallergenes GmbH, other from HAL Allergie GmbH, other from Bencard Allergie GmbH, other from Aimmune Therapeutics UK Limited, other from Actelion Pharmaceuticals Deutschland GmbH, other from Novartis AG, other from Biotest AG, other from AbbVie Deutschland GmbH & Co. KG, other from Lilly Deutschland GmbH, outside the submitted work.
Acknowledgments
We thank Drs Sophie Farooque and Alessia Baseggio Conrado (London) for reviewing a draft of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2021.100517.
Appendix A and Appendix B. Supplementary data
The following is the supplementary data to this article:
References
- 1.McNeil M.M., Weintraub E.S., Duffy J. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137(3):868–878. doi: 10.1016/j.jaci.2015.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su J.R., Moro P.L., Ng C.S., Lewis P.W., Said M.A., Cano M.V. Anaphylaxis after vaccination reported to the vaccine adverse event reporting system, 1990-2016. J Allergy Clin Immunol. 2019 Apr;143(4):1465–1473. doi: 10.1016/j.jaci.2018.12.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dreskin S.C., Halsey N.A., Kelso J.M. International Consensus (ICON): allergic reactions to vaccines. World Allergy Organ J. 2016 Sep 16;9(1):32. doi: 10.1186/s40413-016-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nilsson L., Brockow K., Alm J. Vaccination and allergy: EAACI position paper, practical aspects. Pediatr Allergy Immunol. 2017 Nov;28(7):628–640. doi: 10.1111/pai.12762. [DOI] [PubMed] [Google Scholar]
- 5.Erlewyn-Lajeunesse M., Hunt L.P., Heath P.T., Finn A. Anaphylaxis as an adverse event following immunisation in the UK and Ireland. Arch Dis Child. 2012;97(6):487–490. doi: 10.1136/archdischild-2011-301163. [DOI] [PubMed] [Google Scholar]
- 6.Clark T. Presentation to ACIP COVID-19 vaccines work group December 19 2020 meeting. 2020. Anaphylaxis following m-RNA COVID-19 vaccine receipt.https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-12/slides-12-19/05-COVID-CLARK.pdf Available at. [Google Scholar]
- 7.Raine J. Medicines and Healthcare products Regulatory Agency (MHRA); 9 December 2020. Confirmation of Guidance to Vaccination Centres on Managing Allergic Reactions Following COVID-19 Vaccination with the Pfizer/BioNTech Vaccine.https://www.gov.uk/government/news/confirmation-of-guidance-to-vaccination-centres-on-managing-allergic-reactions-following-covid-19-vaccination-with-the-pfizer-biontech-vaccine press release. Available at. [Google Scholar]
- 8.COVID-19 vaccine safety update. 27 January 2021., 2021. [Accessed 6 Feb 2021]
- 9.Interim Clinical Considerations for Use of mRNA COVID-19 Vaccines Currently Authorized in the United States - Appendix B. United States Centers for Disease Control and Prevention; Atlanta, GA: 2020. cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html (Reviewed December 20, 2020). Available at. [Google Scholar]
- 10.Medicines and Healthcare Products Regulatory Agency (MHRA) Oxford University/AstraZeneca COVID-19 vaccine approved; 30 December 2020. https://www.gov.uk/government/news/oxford-universityastrazeneca-covid-19-vaccine-approved press release. Available at. [Google Scholar]
- 11.Wenande E., Garvey L.H. Immediate-type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy. 2016;46(7):907–922. doi: 10.1111/cea.12760. [DOI] [PubMed] [Google Scholar]
- 12.Fruijtier-Polloth C. Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology. 2005;214:1–38. doi: 10.1016/j.tox.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Sellaturay P., Nasser S., Ewan P. Polyethylene glycol-induced systemic allergic reactions (anaphylaxis) J Allergy Clin Immunol Pract. 2020 Oct 1;S2213–2198(20) doi: 10.1016/j.jaip.2020.09.029. 31007-2. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 14.Bruusgaard-Mouritsen M., Johansen J., Garvey L. Allergy to polyethylene glycol has significant impact on daily life. Author. July 06, 2020 doi: 10.22541/au.159402782.23497540. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Z.H., Stone C.A., Jr., Jakubovic B. Anti-PEG IgE in anaphylaxis associated with polyethylene glycol. J Allergy Clin Immunol Pract. 2020 Nov 17;S2213–2198(20) doi: 10.1016/j.jaip.2020.11.011. 31231-9. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamad I., Hunter A.C., Szebeni J., Moghimi S.M. Poly(ethylene glycol)s generate complement activation products in human serum through increased alternative pathway turnover and a MASP-2-dependent process. Mol Immunol. 2008;46(2):225–232. doi: 10.1016/j.molimm.2008.08.276. [DOI] [PubMed] [Google Scholar]
- 17.Banerji A., Wickner P.G., Saff R., Khan D.A., Phillips E., Blumenthal K.G. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and approach. J Allergy Clin Immunol Pract. 2020 doi: 10.1016/j.jaip.2020.12.047. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maggio E. Polysorbates, biotherapeutics and anaphylaxis: a review. Bioprocess Int. 2017 https://bioprocessintl.com/manufacturing/formulation/polysorbates-biotherapeutics-and-anaphylaxis-a-review/ Available at. [Google Scholar]
- 19.Stone C.A., Jr., Liu Y., Relling M.V. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7(5):1533–1540.e8. doi: 10.1016/j.jaip.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badiu I., Geuna M., Heffler E., Rolla G. Hypersensitivity reaction to human papillomavirus vaccine due to polysorbate 80. BMJ Case Rep. 2012 May 8:2012. doi: 10.1136/bcr.02.2012.5797. bcr0220125797. PMID: 22605841; PMCID: PMC3351639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Public Health England COVID-19: the green book. 2021. assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/948757/Greenbook_chapter_14a_v4.pdf (chapter 14)a. Available at.
- 22.Valent P., Akin C., Bonadonna P. Proposed diagnostic algorithm for patients with suspected mast cell activation syndrome. J Allergy Clin Immunol Pract. 2019;7(4):1125–1133.e1. doi: 10.1016/j.jaip.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zanoni G., Zanotti R., Schena D., Sabbadini C., Opri R., Bonadonna P. Vaccination management in children and adults with mastocytosis. Clin Exp Allergy. 2017;47(4):593–596. doi: 10.1111/cea.12882. [DOI] [PubMed] [Google Scholar]
- 24.Cardona V., Ansotegui I.J., Ebisawa M. World allergy organization anaphylaxis guidance 2020. World Allergy Organ J. 2020;13(10):100472. doi: 10.1016/j.waojou.2020.100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wylon K., Dölle S., Worm M. Polyethylene glycol as a cause of anaphylaxis. Allergy Asthma Clin Immunol. 2016;12:67. doi: 10.1186/s13223-016-0172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polack F.P., Thomas S.J., Kitchin N. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ju Rüggeberg, Gold M.S., Bayas J.M., Brighton Collaboration Anaphylaxis Working Group Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5675–5684. doi: 10.1016/j.vaccine.2007.02.064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.