Abstract
Background
Moderate and severe COVID-19 patients typically present with pneumonia. In this study we aimed to detect the occurrence of pulmonary residuals as a late sequela of COVID-19 and to identify it's predictors among moderate and severe cases.
Methods
This observational prospective study involved 85 COVID-19 patients confirmed by real time polymerase chain reaction (RT-PCR) nasopharyngeal swab, patients were recruited in the period of 1 st of June to 1 st of July. Demographic and clinical data were obtained for each patient. Chest imaging was performed initially and after 3 weeks to detect post COVID pulmonary residuals.
Results
The study population included 74 (87.1%) moderate and 11 (12.9%) severe patients. Patients with older age, male gender, high BMI and initial chest CT of consolidation/mixed consolidation and ground glass opacities (GGOs) had more frequent post COVID-19 pulmonary residuals (P 0.003, 0.026, 0.031, 0.035) respectively. There was a statistically significant difference between patients who showed complete resolution and patients who developed pulmonary residuals regarding the lymphocyte count, serum CRP and ferritin levels (P 0.0001). After logistic regression, male gender, high BMI, initial chest CT of consolidation/mixed consolidation and GGOs, lymphocytopenia, high serum CRP and ferritin levels were the predictors of pulmonary residuals. While the age wasn't statistically significant.
Conclusion
38.5% of moderate and severe COVID-19 patients tend to have pulmonary residuals. Independent predictors of pulmonary residuals as a sequela of COVID-19 are male gender, high BMI, initial chest CT of consolidation and mixed consolidation/GGOs, lymphocytopenia, high serum CRP and ferritin levels.
Keywords: Consolidation, Ground glass opacities, Post Covid-19, Pulmonary fibrosis
1. Introduction
At the end of the year 2019, the world witnessed the emergence of the COVID-19 (coronavirus disease) outbreak caused by severe acute respiratory syndrome coronavirus 2 (SARS-COV-2).1 It all started in Wuhan city, China. The disease crossed China's borders to several other countries and was declared as a pandemic.2
Rapid disease progression as well as diversity of the disease's clinical presentation that ranges from mild flu like illness to fatal forms of the disease targeting mainly the lungs,3 made it crucial to develop stratagems to identify early predictors of COVID-19. This assisted in prognosticating the disease course, helped in triaging patients along with implementing an efficient management approach to every one thus improving survival as well as conserving limited resources.4
Residual pulmonary imaging abnormalities and breathing difficulties were reported in patients recovered from previous coronavirus diseases.5 , 6
Various mechanisms of lung injury in COVID-19 have been described, with both viral and immune-mediated mechanisms being involved.7 COVID-19 lung injury is followed by acute inflammation and an attempt at pulmonary tissue repair. This result in the restoration of normal pulmonary architecture or it may proceed to lung fibrosis with architectural distortion and irreversible lung dysfunction.8
Persistent pulmonary lesions secondary to SARS-COV-2 infection was one of the disease outcomes that required research attention. Early identification of those patients and treatment not only hinders disease progression to progressive fibrosis, but might also advocates near complete resolution.
The aim of this study was to identify the occurrence of persistent pulmonary lesions as one of the sequelae of COVID-19 and to detect clinical, laboratory and radiological predictors that could help in the early recognition of those who are likely to had pulmonary residuals.
2. Methods
2.1. Study design and study population
This observational prospective study was performed on COVID-19 patients who tested positive by the real time polymerase chain reaction (RT-PCR) of nasopharyngeal sample in the period from first of June to first of July 2020. Patients were enrolled from two quarantine-hospitals in Cairo government.
According to the clinical management of COVID-19 as declared by the World Health Organization (WHO), the diagnosis and classification of severity of COVID-19 disease were established.9 Mild COVID-19: patients with COVID-19 infection without clinical signs of pneumonia or hypoxia. Moderate COVID-19: patients with clinical signs of pneumonia (fever, cough, dyspnea, tachypnea) but no signs of severe pneumonia and oxygen saturation ≥ 90% on room air. Severe COVID-19: patients with clinical signs of pneumonia and one of the following: respiratory rate >30 breaths/min; severe respiratory distress; or oxygen saturation <90% on room air.
Treatment protocol followed the National Institute of Health treatment guidelines.10 Accordingly, steroids were used in treatment protocol of moderate cases requiring supplemental oxygen and in severe cases who requiring mechanical ventilation.
Any adult patient suffering from moderate and severe COVID-19 disease was included in this study. While mild cases according to WHO classification and pediatric age group patients were excluded.
2.2. Patients' sampling
All patients with moderate and severe COVID-19 infection who were recruited in the period from first of June to first of July 2020 and committed to the follow up visit, who fulfilled the eligibility criteria and accept to participate in the study were included (85 patients).
2.3. Data collection
A set of demographic and clinical data were obtained for each patient (age, gender, body mass index (BMI), smoking history and comorbid conditions).
Each patient was subjected to a checklist of COVID-19 symptoms with yes/no response including the constitutional symptoms as fever, fatigue and bony aches as well as respiratory symptoms as cough, dyspnea and sore throat in addition to headache, anosmia and diarrhea.
Laboratory assessment as complete blood picture, urea, creatinine, liver transaminases, serum C-reactive protein (CRP), D-dimer and ferritin were collected within 24 hours of symptom onset and oxygen saturation was recorded.
Analysis of initial chest CT findings is performed according to “Glossary of Terms for Thoracic Imaging” established by the Fleischner Society in 2008.11 CT findings including predominant pattern, distribution of lesions and number of affected lobes were recorded. Predominant pattern includes pure ground glass opacities (GGOs) – GGOs with linear opacities - pure consolidation – mixed consolidation and GGOs).
2.4. Outcome
Follow up of patient's symptoms and oxygen saturation were recorded together with follow up chest CT was performed to assess the disease prognosis after 3 weeks of symptom onset and was compared to the initial chest CT. As notable radiographic progression occurred within the first 4 weeks followed by stabilization of these shadows.12
2.5. Statistical analysis
The data were analyzed using IBM SPSS (Statistical Package of Social Science) version 23. Normality distribution of the data was tested by using Shapiro–Wilk test. Clinical characteristics, and laboratory data of the patients were presented by using the median and inter quartile range (IQR) for not normally distributed numerical data and by using frequency and percentage for qualitative data. The difference between group I (included patients whose follow up chest CT improved after 3 weeks of symptom onset), and group II (whose follow up chest CT showed persistent pulmonary lesions) regarding the clinical characteristics of the patient, laboratory findings and chest CT findings were assessed through chi-squared test for categorical variables and through Mann–Whitney for quantitative variables. Binary logistic regression analysis was done to identify predictors of post COVID-19 persistent pulmonary lesions after being adjusted for their potential mutual confounding effect. The results are presented as odds ratios (OR) and 95% confidence intervals (CIs). P-value ≤ 0.05 was considered statistically significant. All tests were two-tailed.
2.6. Ethical considerations
The aim and nature of the study was explained for patient's relatives before inclusion. An informed written consent was obtained from relatives of participants before enrollment. The study design conformed to the requirements of Revised Helsinki Declaration of biomedical ethics.
3. Results
3.1. Demographics and clinical characteristics of the study population
The study population included 85 patients who had moderate and severe COVID-19 infection, they were 74 (87.1%) moderate and 11 (12.9%) severe according to WHO classification, 56.5% were males and 43.5% were females with median age of 52 and interquartile range (IQR) (37.5–59.5) years. Other clinical characteristics of the study population were summarized in Table (1) . The patients were divided into two groups according to their follow up chest CT after 3 weeks. Group I (n = 51) included patients whose follow up chest CT improved either complete resolution or residual linear opacities. While group II (n = 32) whose follow up chest CT showed persistent pulmonary lesions to COVID-19 infection. 2 patients of the study population had severe COVID-19 disease and died of respiratory failure within first week of illness.
Table 1.
Clinical characteristics and laboratory findings of the study population.
| All patients (n = 85) | ||
|---|---|---|
| Age [Median (IQR)] years | 52 (37.5–59.5) | |
| Gender [n (%)] | Males | 48 (56.5%) |
| Females | 37 (43.5%) | |
| BMI [Median (IQR)] kg/m2 | 29.7 (25.2–33.2) | |
| Medical comorbiditiesa[n (%)] | 29 (34.1%) | |
| Chronic lung diseaseb[n (%)] | 9 (10.6%) | |
| Smokers [n (%)] | 14 (16.5%) | |
| Laboratory data [Median (IQR)] | ||
| Hb (g/dl) | 13.2 (12–14.3) | |
| TLC (/cmm) | 5 (3.8–7.5) | |
| NLR | 2.1 (1.2–4) | |
| Absolute neutrophil count (/μL) | 3.1 (1.9–4.8) | |
| Lymphocytic count (/μL) | 1.4 (1–1.95) | |
| Lymphocytes % | 30 (19–40) | |
| Neutrophil % | 61 (50–72) | |
| Platelets (10³/cmm) | 218 (174.5–260.5) | |
| Urea (mg/dl) | 26 (20–38) | |
| Creatinine (mg/dl) | 0.8 (0.7–1) | |
| ALT (U/l) | 23 (17–35) | |
| AST (U/l) | 26 (18–40) | |
| CRP (mg/L) | 20 (6–60) | |
| D-Dimer (μg/mL) | 0.4 (0.3–0.8) | |
| Ferritin (ng/mL) | 250 (100–374) | |
| Severity of infection [n (%)] | Moderate | 74 (87.1%) |
| Severe | 11 (12.9%) | |
| Clinical symptoms [n (%)] | ||
| Anosmia | 21 (24.7%) | |
| Diarrhea | 44 (51.8%) | |
| Sore throat | 19 (22.4%) | |
| Cough | 66 (77.6%) | |
| Dyspnea | 44 (51.8%) | |
| Fever | 72 (84.7%) | |
| Bony aches | 52 (61.2%) | |
| Fatigue | 48 (56.5%) | |
| Headache | 34 (40%) | |
19 patients with controlled hypertension, 16 patients were diabetic, 10 patients with ischemic heart disease.
Chronic lung disease included 5 patients with chronic obstructive pulmonary disease, 2 patients with bronchial asthma, 1 patient with idiopathic pulmonary fibrosis and 1 patient with sarcoidosis. BMI: body mass index, IQR: interquartile range, Hb: hemoglobin, TLC: total leucocyte count, NLR: Neutrophil leucocyte ratio, ALT: alanine transaminase, AST: aspartate aminotransferase, CRP: C-reactive protein.
3.2. Demographics and clinical characteristics in relation to outcome
The median age in group I was 45 (IQR = 35–56) while in group II was 55 (IQR = 50.2–64.7) years with statistically significant difference between 2 groups (P = 0.003). Also there was statistically significant difference between the two groups in gender with more predilection to males in group II (P = 0.026).
Patients who developed persistent pulmonary lesions recorded significantly higher median BMI than patients showed complete resolution (P = 0.031).
Regarding the medical comorbidities (diabetes, hypertension, cardiovascular disease), 55.6% of the patients in group II had medical comorbidities compared to 44.4% in group I with statistically significant difference between the two groups, (P value = 0.027) (Table 2 ).
Table 2.
Clinical characteristics and laboratory findings between patients showed complete resolution and patients developed organizing pneumonia.
| Group I | Group II | |||
|---|---|---|---|---|
| Age | 45 (35–56) | 55 (50.2–64.7) | 0.003 | |
| Gender [n (%)] | Males | 24 (47%) | 23 (71.8%) | 0.026 |
| Females | 27 (53%) | 9 (28.2%) | ||
| BMI [Median (IQR)] | 28.7 (25–31.5) | 31.1 (27.4–36.7) | 0.031 | |
| Medical comorbiditiesa[n (%)] | 12 (23.5%) | 15 (46.8%) | 0.027 | |
| Chronic lung diseaseb[n (%)] | 4 (7.8%) | 5 (15.6%) | 0.26 | |
| Smokers [n (%)] | 7 (13.7%) | 7 (21.8%) | 0.33 | |
| Laboratory data [Median (IQR)] | ||||
| Hb (g/dl) | 13.2 (12–14.5) | 13.3 (12.1–14.3) | 0.81 | |
| TLC (/cmm) | 5 (4–7.4) | 4.8 (3.6–7.8) | 0.62 | |
| NLR | 1.8 (1.1–3.3) | 2.8 (1.5–6.7) | 0.008 | |
| Absolute neutrophil count (/μL) | 2.7 (1.9–4.6) | 3.3 (1.9–5.1) | 0.48 | |
| Lymphocytic count (/μL) | 1.6 (1.1.-2) | 1 (0.8–1.4) | 0.0001 | |
| Lymphocytes % | 33 (21–41) | 24 (12–35.5) | 0.011 | |
| Neutrophil % | 60 (48–69) | 69.5 (56–77.7) | 0.011 | |
| Platelets (10³/cmm) | 220 (170–253) | 225 (187–282) | 0.32 | |
| Urea (mg/dl) | 22 (20–28) | 36 (20–48) | 0.011 | |
| Creatinine (mg/dl) | 0.8 (0.7–0.9) | 0.9 (0.7–1.1) | 0.019 | |
| ALT (U/l) | 21 (15–39) | 26 (18–35) | 0.33 | |
| AST (U/l) | 20 (16–31) | 36 (20–43) | 0.01 | |
| CRP (mg/L) | 10 (2–28) | 37.5 (21–103) | 0.0001 | |
| D-Dimer (μg/mL) | 0.4 (0.3–0.7) | 0.4 (0.3–0.8) | 0.94 | |
| Ferritin (ng/mL) | 150 (0.3–0.7) | 374 (224–673) | 0.0001 | |
| Severity of infection [n (%)] | Moderate | 51 (100%) | 23 (71.8%) | 0.0001 |
| Severe | 0 (0%) | 9 (28.2%) | ||
19 patients with controlled hypertension, 16 patients were diabetic, 10 patients with ischemic heart disease.
Chronic lung disease included 5 patients with chronic obstructive pulmonary disease, 2 patients with bronchial asthma, 1 patient with idiopathic pulmonary fibrosis and 1 patient with sarcoidosis. BMI: body mass index, IQR: interquartile range, Hb: hemoglobin, TLC: total leucocyte count, NLR: Neutrophil leucocyte ratio, ALT: alanine transaminase, AST: aspartate aminotransferase, CRP: C-reactive protein.
3.3. Severity of COVID-19 infection in relation to outcome
All patients in group I had moderate COVID-19 infection whereas group II included 23 (71.8%) patients of moderate infection and 9 (28.2%) with severe infection showing a statistical significance with p value (0.0001).
3.4. Laboratory characteristics in relation to outcome
There was statistically significant difference between the 2 groups in certain labs as NLR lymphocytic count, lymphocytes %, Neutrophil %, Urea (mg/dl), Creatinine, AST, CRP and ferritin as outlined in Table (2).
3.5. Radiological characteristics in relation to outcome
Initial chest CT of pure consolidation/mixed consolidation and GGOs pattern was statistically significant as regarding development of post COVID-19 persistent pulmonary lesions, (P = 0.035) Table (3) and Fig. (1) .
Table 3.
Chest CT findings of the studied groups.
| All (n = 83) | Group I (n = 51) | Group II (n = 32) | P -value | ||
|---|---|---|---|---|---|
|
Initial chest CT Findings |
Pure GGOs pattern/GGOs and linear opacities | 38 (45.8%) | 28 (54.9%) | 10 (31.2%) | 0.035 |
| Pure Consolidation pattern/mixed consolidation and GGOs | 45 (54.2%) | 23 (45.1%) | 22 (68.8%) | ||
|
Distribution |
Unilateral distribution | 7 (8.4%) | 5 (9.8%) | 2 (6.2%) | 0.571 |
| Bilateral distribution | 76 (91.6%) | 46 (90.1%) | 30 (93.8%) | ||
CT: computed tomography, GGOs: ground glass opacifications.
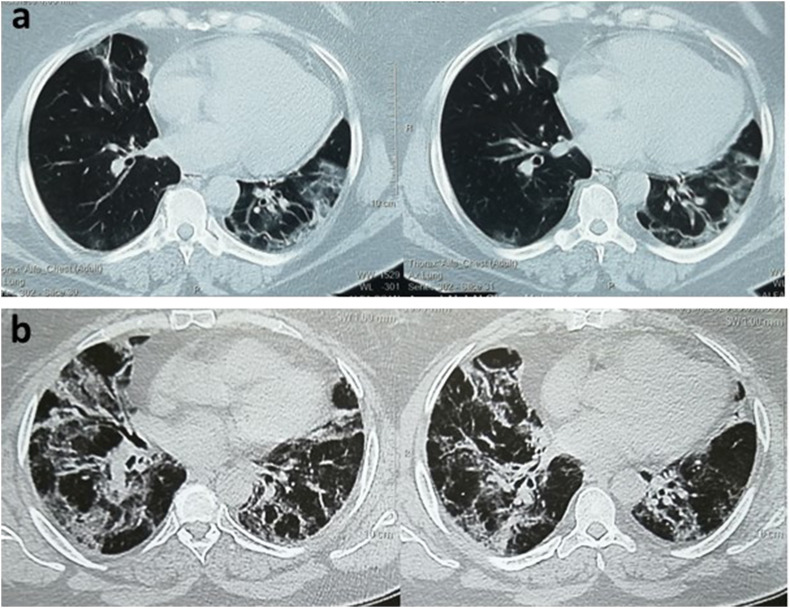
Fig. 1.
Chest CT of a 60-year-old female with severe COVID-19 (a) CT images demonstrate bilateral subpleural patchy and ground glass opacities at time of presentation; (b) Follow up CT images after 3 weeks show pulmonary residuals with wide spread consolidations, bilateral reticulations and traction bronchiectasis.
3.6. Predictors of COVID-19 infection persistent pulmonary lesions outcome
A logistic regression was performed to detect the effects of age, sex, BMI, CRP, ferritin, lymphocyte count as well as initial chest CT pattern on the probability of participants to progress to persistent pulmonary lesions as a sequela of COVID-19 disease. The logistic regression model was statistically significant, χ2 = 2.6, p < .0001. The model explained 64% (Nagelkerke R2) of the variance in persistent pulmonary lesions as a sequela of COVID-19 disease and correctly classified 86.7% of cases. Males were 7 times more likely to exhibit post COVID-19 persistent pulmonary lesions than females. Those with consolidation/mixed consolidation and GGOs patterns was 5 times more likely to exhibit persistent pulmonary lesions than those with ground glass appearance. With one unit increase in the BMI, CRP, ferritin the odds of exhibiting persistent pulmonary lesions tend to increase by 12.5%, 1.2%, 0.3% respectively whereas decreasing lymphocytic count by one unit was associated with a 75% increase in the odds of exhibiting persistent pulmonary lesions while the age was not statistically significant. Table (4) .
Table 4.
Binary logistic regression analysis for the significant predictors of post COVID-19 organizing pneumonia.
| B | S.E. | OR | 95% C.I for OR |
P-Value | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age | 0.042 | 0.027 | 1.043 | 1.043 | 0.989 | 0.119 |
| Gender: male | 1.906 | 0.906 | 6.727 | 6.727 | 1.139 | 0.035 |
| BMI | 0.118 | 0.060 | 1.125 | 1.125 | 1.000 | 0.050 |
| CRP | 0.012 | 0.006 | 1.012 | 1.001 | 1.023 | 0.040 |
| Ferritin | 0.003 | 0.001 | 1.003 | 1.000 | 1.005 | 0.030 |
| Lymphocyte count | −1.372 | 0.579 | 0.253 | .082 | 0.788 | 0.018 |
| Initial chest CT pattern: patchy opacity | 1.606 | 0.729 | 4.984 | 1.195 | 20.791 | 0.028 |
| Constant | −8.063 | 2.783 | 0.004 | |||
B: regression coefficient, SE: standard error, OR: odds ratio, CI: confidence interval.
4. Discussion
The aim of our study is to identify the occurrence of persistent pulmonary lesions as one of the sequelae of COVID-19 and to detect clinical, laboratory and radiological predictors of these persistent lesions.
In our study, 38.5% of the study population had tendency to have persistent pulmonary lesions, persistent pulmonary symptoms and hypoxia after 3 weeks of follow up. It is worth mentioning that those patients were older, mostly males with higher BMI, and more prevalence of comorbid illness than patients who showed near complete resolution as evidenced by their chest CT.
Several previous studies have already established that advanced age, male gender and the presence of comorbid illness are considered as risk factors associated with adverse COVID-19 disease outcome.13, 14, 15
As this may be related to the fact that age advancement is associated with the reduction in both cell mediated function and humoral immune response as well as cellular homeostasis impairment thus reducing the body's capability to mount proper response to external stimuli including viruses.16 , 17 Our finding that older age was associated with adverse disease outcome was consistent with other reports.18
Similarly those who have an already compromised functioning organ as in those with comorbid illness are not capable of maintaining proper viral response hence further loading the body and as COVID-19 disease progress body oxygen decreases, contributing to more organ dysfunction.19
With regard to gender, our observation that the disease is more prevalent in males has already been reported by a previous study,20 researchers assumed that the sex difference is possibly related to the higher ACE2 receptor expression that has been recognized as a receptor for SARS CoV-2 and the lack of estrogen and X chromosome protection, hence weakening immune responses in males to viral respiratory diseases, thus signifying that males are more susceptible to increased severity of infection.21 , 22 In our study, males were 6.7 times more likely to have persistent pulmonary lesions than females.
Likewise, Obesity aggravates the severity of respiratory diseases through inducing a low grade chronic inflammatory state evidenced by the upregulated pro-inflammatory cytokines, adipokines and interleukin 6 (IL-6) levels over expression,23, 24, 25 coupled with rapid severe disease progression through dysregulated immune response and impaired T cell memory function.26 Our finding presuming that one unit increase in the BMI increase the risk of persistent pulmonary lesions by 12.5%.
Using lymphocytopenia to stipulate insights regarding COVID-19 prognosis is reported in several studies.18 Several possible mechanisms hypothesizing the occurrence of lymphocytopenia in COVID-19 patients were presumed as that SARS-COV-2 may either induce direct infection or cytokine-mediated lymphocyte destruction as well as increased lymphocytic migration to the lungs.27 So, acknowledging lymphocytopenia as one of the potential predictors to severe inflammation and tissue injury was mandatory which in turn influenced the tissue reparative process explaining our finding that decreasing lymphocytic count by one unit was associated with a 75% increase in the odds of exhibiting post COVID-19 persistent pulmonary lesions.
Similarly, other biomarkers that mirror the inflammatory state of the body and contribute to weakening the immune responses28 are also potential predictors of the disease, CRP and ferritin should be the first in line to be considered. Acute inflammation is associated with an evident increase in the C-reactive protein (CRP) levels denotes tissue injury.29 Also increased serum ferritin levels via inflammation triggers a sequence of events where higher ferritin levels signals more tissue damage.30 The present study revealed that a unit increase in the CRP and ferritin levels increases the risk of progression to persistent pulmonary lesions development by 1.2%, 0.3% respectively.
Authors of this study postulate that lymphopenia, high CRP and ferritin levels in patients who tend to have persistent pulmonary lesions imply an inflammatory profile that appears to be associated with consolidation present in their initial CT. This suggests more pronounced tissue injury and delayed healing process with a protracted disease course.
These findings echo the latest report, that severe patients with affection of more than half of their lungs presented with higher CRP level and neutrophil count, lower lymphocyte count, and more consolidations on CT31 Confirming that consolidations are linked with adverse disease outcome.32
Regarding the initial chest CT pattern, pure consolidation or mixed consolidation/GGOs were likely to develop persistent pulmonary lesions indicating progressive pulmonary involvement and signaling likelihood pulmonary residuals on follow up CT as well as suggesting a protracted disease course. On the other hand the initial chest CT pattern with pure GGOs or GGOs/linear opacities were likely to heal by near complete resolution of the radiological lesions indicating a downgraded reparative course of the disease.
Putting in mind the prognostic implications of these indicators that might contribute in predicting the radiological sequelae in COVID-19 cases we assume that initial chest CT pattern showing pure consolidation or mixed consolidation/GGOs patterns has tendency to have persistent pulmonary lesions by 4.98 times especially upon considering the findings of Hani et al.3
5. Conclusion
38.5% of moderate and severe COVID-19 patients tend to have pulmonary residuals that are likely to respond to corticosteroids. Independent predictors of persistent pulmonary lesions as one of the sequelae of COVID-19 disease are male gender, high BMI, initial chest CT of consolidation and mixed consolidation/GGOs patterns, lymphocytopenia, high serum CRP and ferritin levels.
References
- 1.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hani C., Trieu N.H., Saab I. COVID-19 pneumonia: a review of typical CT findings and differential diagnosis. Diagnostic and interventional imaging. 2020;101:263–268. doi: 10.1016/j.diii.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan C.H., Faraji F., Prajapati D.P., Ostrander B.T., DeConde A.S. Self-reported olfactory loss associates with outpatient clinical course in COVID-19. International Forum of Allergy & Rhinology. 2020;10:821–831. doi: 10.1002/alr.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das K.M., Lee E.Y., Jawder S.E. Acute Middle East respiratory syndrome coronavirus: temporal lung changes observed on the chest radiographs of 55 patients. Am J Roentgenol. 2015;205(3):267–S274. doi: 10.2214/AJR.15.14445. [DOI] [PubMed] [Google Scholar]
- 6.Hui D.S., Joynt G.M., Wong K.T. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60(5):401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J., Zheng X., Tong Q. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J Med Virol. 2020 May;92(5):491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ojo A.S., Balogun S.A., Williams O.T., Ojo O.S. Pulmonary fibrosis in COVID-19 survivors: predictive factors and risk reduction strategies. Pulmonary Medicine. 2020 Aug 10:2020. doi: 10.1155/2020/6175964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization . 2020. Clinical Management of COVID-19: Interim Guidance; p. 62. [Google Scholar]
- 10.National Institutes of Health . 2020. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. [PubMed] [Google Scholar]
- 11.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Muller N.L., Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2005;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 12.Liu D., Zhang W., Pan F. The pulmonary sequalae in discharged patients with COVID-19: a short-term observational study. Respir Res. 2020;21:1–7. doi: 10.1186/s12931-020-01385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J., Zheng Y., Gou X. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory Medicine. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J., Yu M., Tong S., Liu L.Y., Tang L.V. Predictive factors for disease progression in hospitalized patients with coronavirus disease 2019 in Wuhan, China. J Clin Virol. 2020;127:104392. doi: 10.1016/j.jcv.2020.104392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohammadzadeh A., Mirza-Aghazadeh-Attari M., Hallaj S. Crosstalk between P53 and DNA damage response in ageing. DNA Repair. 2019;80:8–15. doi: 10.1016/j.dnarep.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Opal S.M., Girard T.D., Ely E.W. The immunopathogenesis of sepsis in elderly patients. Clin Infect Dis. 2005;41(suppl 7):S504–S512. doi: 10.1086/432007. [DOI] [PubMed] [Google Scholar]
- 18.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirza-Aghazadeh-Attari M., Zarrintan A., Nezami N. Predictors of coronavirus disease 19 (COVID-19) pneumonitis outcome based on computed tomography (CT) imaging obtained prior to hospitalization: a retrospective study. Emerg Radiol. 2020:1–9. doi: 10.1007/s10140-020-01833-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schurz H., Salie M., Tromp G., Hoal E.G., Kinnear C.J., Möller M. The X chromosome and sex-specific effects in infectious disease susceptibility. Hum Genom. 2019;13(1):2. doi: 10.1186/s40246-018-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J., Ji H., Zheng W. Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17β-oestradiol-dependent and sex chromosome-independent. Biol Sex Differ. 2010;1(1):6. doi: 10.1186/2042-6410-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Heredia F.P., Gómez-Martínez S., Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71(2):332–338. doi: 10.1017/S0029665112000092. [DOI] [PubMed] [Google Scholar]
- 24.Hegde V., Dhurandhar N.V. Microbes and obesity—interrelationship between infection, adipose tissue and the immune system. Clin Microbiol Infect. 2013;19(4):314–320. doi: 10.1111/1469-0691.12157. [DOI] [PubMed] [Google Scholar]
- 25.Simonnet A., Chetboun M., Poissy J. LICORN and the Lille COVID-19 and Obesity study group. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muscogiuri G., Pugliese G., Barrea L., Savastano S., Colao A. Comentary: obesity: the “Achilles heel” for COVID-19? Metab Clin Exp. 2020;108:154251. doi: 10.1016/j.metabol.2020.154251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall J.C., Charbonney E., Gonzalez P.D. The immune system in critical illness. Clin Chest Med. 2008;29(4):605–616. doi: 10.1016/j.ccm.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Xiang N., Havers F., Chen T. Use of national pneumonia surveillance to describe influenza A (H7N9) virus epidemiology, China, 2004–2013. Emerg Infect Dis. 2013;19(11):1784. doi: 10.3201/eid1911.130865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenne C.N., Liao S., Singh B. Neutrophils: multitasking first responders of immunity and tissue homeostasis. Cell Tissue Res. 2018;371(3):395–397. doi: 10.1007/s00441-018-2802-5. [DOI] [PubMed] [Google Scholar]
- 30.Kell D.B., Pretorius E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metall. 2014;6(4):748–773. doi: 10.1039/c3mt00347g. [DOI] [PubMed] [Google Scholar]
- 31.Ruch Y., Kaeuffer C., Ohana M. CT lung lesions as predictors of early death or ICU admission in COVID-19 patients. Clin Microbiol Infect. 2020;26(10):1417. doi: 10.1016/j.cmi.2020.07.030. e5-1417.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan M., Yin W., Tao Z., Tan W., Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PloS One. 2020;15(3) doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]