Dear Editor,
we read with interest the article by AT Hanrath and colleagues in which the Authors showed the association between prior SARS-CoV-2 infection and protection against symptomatic reinfection1. The study confirms the need for a better understanding of the immunological response to SARS-CoV-2 infection. This would permit to better target public health policies such as the vaccination campaign2.
Anti-SARS-CoV-2 antibodies target many of its encoded proteins. In particular, the spike glycoprotein (S) is essential for the virus’ entry and contains the receptor binding domain. The duration and kinetics of anti-S antibodies and the presence of clinical correlates associated with them deserve further longitudinal studies. More data are needed to understand if they are actually protective and a threshold able to correlate with immunity has not yet been established.
On May 13th, our Infectious Diseases Department (Luigi Sacco Hospital, Milan, Italy) activated an outpatient clinic for the long-term follow-up of COVID-19 patients, irrespectively of whether they had been or not hospitalized. The SARS-CoV-2 previous infection was ascertained by a previous positive nasopharyngeal swab or a combination of clinical and epidemiological criteria with a positive serological test. Only patients with a symptom onset or a positive test between February 20th and April 25th, 2020 were included.
The antibody response was tested by DiaSorin's LIAISON—CLIA-S1/S2Ⓡ IgG solution (Saluggia, Italy)3. The choice of an assay targeting S protein was based on previous observation of other epidemic coronaviruses, showing that S protein was highly immunogenic and induced an immune response that was likely to be protective4. The antibody response was tested at the first outpatient visit (T1) set at week 12th (± 3 weeks) after symptoms onset (defined as 2 weeks, average time of clinical course, followed by 2 weeks of quarantine according to public health policy and 8 weeks of follow-up). T2 was set 20 weeks after symptoms onset (± 3 weeks) and T3 was set 32 weeks after symptoms onset (± 3 weeks). Patients were divided according to the WHO classification for COVID-19 severity into mild, moderate, severe and critical5. The between groups comparison for clinical and demographic characteristics were performed with Kruskal-Wallis and chi-square test when appropriated. The baseline values of anti-S antibodies were compared with Kruskal- Wallis and Dunn's test for pairwise multiple comparisons; the overtime trends were estimated by Friedman and Nemenyi test.
The study was approved by the “Comitato Etico Interaziendale Area 1″. All patients signed a written informed consent.
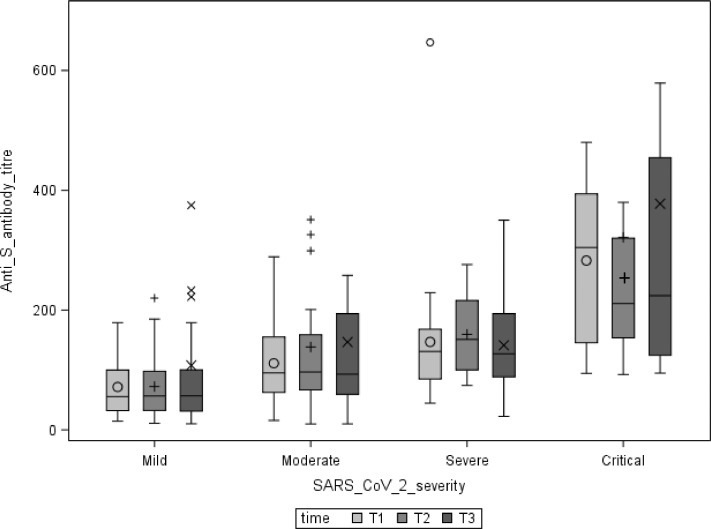
580 patients entered the study, of whom 373 had a T1 diagnosis by molecular testing and 341 (91.4%) developed antibodies. For the purpose of the present analysis, we included a convenience sample of 96 patients who have completed T3 visit assessment. The characteristics of the study population are reported in Table 1 . A correlation between increased COVID-19 severity and higher antibody titer was observed even after adjustment for age and gender. No reduction of the median antibody titre overtime was observed in the mild, moderate, and critical subgroups (Fig. 1 ), whereas a significant reduction of the antibody titer between T2 and T3 was observed in the severe subgroup (p = 0.023). Of note, four patients in at least one detection did not have a positive antibody titer. All of them had a positive nasopharyngeal swab and showed antibody values < 20 UA/ml at every time point. We did not report any case of symptomatic reinfection after 7 months of follow-up.
Table 1.
Demographic, clinical and immunological characteristics of the COVID-19 patients according to the maximum disease severity (WHO categories).
| Overall | Mild | Moderate | Severe | Critical | p-value | |
|---|---|---|---|---|---|---|
| N = 96 | n = 31 | n = 34 | n = 23 | n = 8 | ||
| Gender male, n (%) | 50 (52.1) | 14 (45.2) | 16 (47.1) | 17 (73.9) | 3 (37.5) | 0.111 |
| Age, median [IQR] | 55.00 [46.25, 62.25] |
54.00 [37.00, 60.50] |
56.00 [50.25, 58.75] |
57.00 [50.00, 63.00] |
59.50 [40.75, 69.50] |
0.542 |
| Hospitalization, n (%) | 60 (62.5) | 6 (19.35) | 23 (67.6) | 23 (100) | 8 (100) | |
| Treatment with steroid, n (%) | 0 (0.0) | 0 (0.0) | 1 (4.3) | 3 (37.5) | <0.001 | |
| 4 (4.1) | ||||||
| Treatment with Tocilizumab/Sarilu mab/Baricitinib, n (%) | 2 (2.1) | 0 (0.0) | 1 (2.9) | 0 (0.0) | 1 (12.5) | 0.137 |
| Number of comorbidities, n (%) | ||||||
| 0 | 41(42.7) | 17 (54.8) | 14 (41.2) | 5 (21.7) | 5 (62.5) | 0,093 |
| 1 | 37 (38.5) | 12 (38.7) | 14 (41.2) | 11 (47.8) | 0 (0.0) | |
| 2 | 13 (13.5) | 1 (3.2) | 4 (11.8) | 6 (26.1) | 2 (25.0) | |
| ≥3 | 5 (5.2) | 1 (3.2) | 2 (5.9) | 1 (4.3) | 1 (12.5) | |
| Immune System Disorders, n (%) | 7 (7.3) | 2 (6.5) | 2 (5.9) | 3 (13.0) | 0 (0.0) | 0.5962 |
| Days from symptoms onset to T1, median [IQR] | 83.50 [72.75, 97.00] |
85.00 [75.00, 99.00] |
82.50 [76.00, 95.75] |
78.00 [70.00, 99.50] |
84.50 [72.00, 89.50] |
0.902 |
| Antibody title at T1 UA/mL, median [IQR] | 95.20 [54.83, 152.25] |
55.60 [34.45, 99.50] |
95.10 [63.02, 150.50] |
131.00 [87.75, 160.50] |
304.50 [151.25, 372.50] |
<0.001 |
| Days from symptoms onset to T2, median [IQR] | 130.00 [123.00, 136.00] |
131.00 [128.00, 135.00] |
126.50 [122.25, 131.00] |
131.00 [123.00, 137.00] |
125.50 [117.00, 136.75] |
0.205 |
| Antibody title at T2) UA/mL, median [IQR] | 99.35 [63.23, 176.50] |
56.70 [32.30, 96.75] |
96.65 [66.88, 158.75] |
151.00 [101.00, 214.50] |
211.00 [162.75, 290.00] |
<0.001 |
| Days from symptoms onset to T3, median [IQR] |
217.00 [214.00, 224.00] |
219.00 [215.00, 224.50] |
216.50 [213.50, 221.00] |
215.00 [214.00, 222.50] |
218.00 [214.00, 228.00] |
0.598 |
| Antibody title at T3 UA/mL, median [IQR] | 94.60 [56.40, 190.25] |
56.90 [31.90, 99.40] |
92.90 [59.85, 191.50] |
127.00 [89.45, 191.50] |
224.00 [134.75, 392.25] |
0.001 |
a > 15 UA/Ml = positive; 12–15 UA/mL = undetermined; <12 UA/mL = negative List of abbreviations: n, number; IQR, Inter Quartile Range; T, Time point.
Fig. 1.
Anti S antibody persistence overtime. ○ mean/outliers of Anti-S antibody titres at T1 by severity groups; + mean/outliers of Anti-S antibody titres at T2 by severity groups; x mean/outliers of Anti-S antibody titres at T3 by severity groups.
At the beginning of the vaccine era, it is mandatory to better understand the immune response to SARS-CoV-2 infection. A study by Huang et al. on 94 patients showed the durability of anti-S IgG without reduction over 6 months of follow-up6. Another cohort of 121 patients, tested with Mount-Sinai Anti-S IgG ELISA by Wajnberg et al., presented similar rates of persistence overtime (approximately 5 months) despite a significant reduction of antibodies titer7. Our data are consistent with the persistence overtime of anti-Spike antibodies although a slight reduction of the titer was observed in the severe group after 5 months as shown in Fig. 1. However, at T3 the median values observed were above the positivity cut-off in all the subgroups of patients, maintaining a direct correlation with COVID-19 severity. This relationship has already been highlighted in the acute phase studies8 and our data add information on midterm follow-up (approximately 7 months) according to COVID-19 severity. Our observation of no symptomatic reinfection is in line with the low rate of reinfection reported in the literature suggesting a likely immunity provided by the natural infection which persist in the midterm1. Furthermore, some of the recently developed vaccines base their theoretical efficacy on the production of on the S1+S2 protein antibodies9. Therefore, studies are needed to assess whether the effectiveness of vaccines in preventing infections is associated with the production of anti-S antibodies. This should help to understand whether it is useful to vaccinate people recovered from COVID-19 and possibly after how long.
Our study presents several limitations. First, sample size was small especially in the subgroup of patients with critical disease; second, the patients belong to the first pandemic period in which the therapeutic interventions were different from the current standard of care. In the end, we did not perform neutralization tests. In particular, the neutralization activity of anti-S IgG is yet to be defined. There are plenty of assays of antibodies against different SARS- CoV-2 proteins and the standardization of observations is therefore tricky. Moreover, the comparison with in vitro neutralization assays is difficult to estimate. Furthermore, it could be speculated that in vivo protection is given by a concomitance of factors that act simultaneously, including humoral and cell-mediated immunity. We therefore believe that is urgent to clarify the relationship between IgG against various viral targets (such as anti-S IgG, widely distributed on a large scale) and clinical protection on large series.
In conclusion, the vast majority of COVID-19 recovered patients maintained a detectable anti-S titre after 7 months from SARS-CoV-2 infection irrespectively of disease severity. Studies are urgently needed to clarify if subjects recovered from COVID-19 with an anti-S titre would gain additional benefit from COVID-19 vaccination.
Acknowledgements
A special thanks to Mrs. Simona Bocchio for her silent but fundamental help.
References
- 1.Hanrath A.T., Payne B.A., Duncan C.J. Prior SARS-CoV-2 infection is associated with protection against symptomatic reinfection. 2020. doi:10.1016/j.jinf.2020.12.023 [DOI] [PMC free article] [PubMed]
- 2.Why decoding the immune response to COVID matters for vaccines. Nature. 2020;586(7830):473–474. doi: 10.1038/d41586-020-02943-9. [DOI] [PubMed] [Google Scholar]
- 3.LIAISON Ⓡ SARS-CoV-2 S1/S2 IgG The fully automated serology test for the detection of SARS-CoV-2 IgG Antibodies. https://www.diasorin.com/sites/default/files/allegati/liaisonr_sars-cov-2_s1s2_igg_brochure.pdf.pdf (accessed on 20 Jan, 2021)
- 4.Kim D.S., Rowland-Jones S., Gea-Mallorquí E. Will SARS-CoV-2 Infection Elicit Long-Lasting Protective or Sterilising Immunity? implications for vaccine strategies (2020) Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.571481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical Management of COVID-19. https://www.who.int/publications/i/item/clinical-management-of-covid-19. https://www.who.int/publications/i/item/clinical-management-of-covid-19 (accessed on 20 Jan, 2021)
- 6.Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;0(0) doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wajnberg A., Amanat F., Firpo A., et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y., Zhang L., Sang L., et al. Kinetics of viral load and antibody response in relation to COVID-19 severity. J Clin Invest. 2020;130(10):5235–5244. doi: 10.1172/JCI138759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]