Abstract
Coronavirus disease 2019 (COVID‐19) is a novel disorder caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Although it mainly affects the respiratory system, the pancreas could also become the virus' target. The issue regarding pancreatic involvement in COVID‐19 has been raised by several researchers. They found increased serum amylase and/or lipase in COVID‐19 patients, which suggested pancreatic injury. We aimed to critically review the evidence to provide insights and to answer the very question of the possibility of pancreatic injury. Current evidence shows that increased amylase and/or lipase is not necessarily a pancreatic injury in COVID‐19 patients. Those increased enzymes might also be found in other clinical conditions.
Keywords: amylases, COVID‐19, lipase, pancreas
Coronavirus disease 2019 (COVID‐19) is a novel disorder caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Although it mainly affects respiratory system, the issue regarding pancreatic involvement in COVID‐19 has been raised by several researchers. Current evidence shows that increased serum amylase and/or lipase is not necessarily an indicator of pancreatic injury in COVID‐19 patients. Those increased enzymes might also be found in other clinical conditions.
Introduction
On 31 December 2019, a group of patients with pneumonia of unknown cause was reported in Wuhan City, Hubei Province, China. The etiology was identified as severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), and the disease was named 2019 novel coronavirus (nCoV) pneumonia, which was later renamed as coronavirus disease 2019 (COVID‐19). Due to its rapid spreading outside China, involving 114 countries worldwide, the World Health Organization (WHO) declared COVID‐19 a pandemic on 11 March 2020. 1 , 2
Initially, physicians recognized respiratory symptoms and signs as the sole clinical manifestations in COVID‐19 patients, but recent mounting evidence showed that clinical presentations might be expanding beyond the typical presentation. These include skin, neurologic, ophthalmologic, cardiovascular, gastrointestinal, hepatic, and hematologic manifestations. 3 , 4 , 5 Pancreatic clinical presentation has also been reported by several authors. 6 , 7 , 8 Here, we will further discuss and review the suggested pancreatic involvement in COVID‐19 patients.
The phenomenon of increased serum amylase and/or lipase in COVID‐19 patients
Emerging data revealed increased serum amylase and/or lipase in COVID‐19 patients. Liu et al. 6 studied 121 laboratory‐confirmed COVID‐19 patients in Wuhan, China. They reported that one patient (1.85%) from 54 mild cases had increased serum amylase and lipase. Twelve patients of 67 (17.91%) severe cases had increased serum amylase, and 11 of 67 (16.41%) severe cases had increased serum lipase. In a radiologic study, there were only 5 of 67 severe patients (7.46%) who had focal swelling of the pancreas or dilatation of the pancreatic duct. There were no patients with pancreatic necrosis. 6
Liu et al. 6 also proposed the hypothesis of SARS‐CoV‐2‐induced pancreatic injury. They explored RNA sequencing data from the Genotype Tissue Expression (GTEx) database to investigate the expression of angiotensin converting enzyme 2 (ACE‐2) receptors in normal human pancreas. Those receptors are recognized as the entry point of SARS‐CoV‐2 into human cells. They discovered that ACE‐2 receptors are found in both pancreatic exocrine and endocrine tissues. Hikmet et al. 9 performed an immunohistochemistry examination of human tissues, and they were able to show ACE‐2 protein expression in human cells, including pancreas. They demonstrated that the expression of ACE‐2 protein in pancreas was 1.6 NX (NX is the consensus normalized expression). For comparison, the expression of ACE‐2 protein in the small intestines was high (122 NX). These findings suggested the possibility of a pathophysiologic mechanism of SARS‐CoV‐2‐induced pancreatic injury. However, further studies are needed to confirm the postulation.
Another study by Wang et al. 7 from Wuhan, China, focused on 52 COVID‐19 pneumonia patients, and they found increased serum amylase and/or lipase in 9 subjects (17.3%). In those nine patients, the mean value of amylase (normal value 0–90 unit/L) was 115 ± 25 unit/liter and of lipase (normal value 0–70 unit/L) was 71 ± 34 unit/L. The mean value of creatinine (normal value 0.7–1.2 mg/dL) in those nine patients was 1.1 ± 0.4 mg/dL, whereas the mean value of creatinine in other patients (without increased amylase and/or lipase) was 0.7 ± 0.2 mg/dL. The authors did not provide data of clinical manifestations, such as abdominal pain, nor the imaging modalities.
McNabb‐Baltar et al. 8 from Massachusetts, USA, reported hyperlipasemia in 9 patients (12.7%) from a total of 71 patients with confirmed COVID‐19. The mean value of lipase was 151.8 U/L ± 148.4 U/L on hospital admission. Two subjects (2.8%) had a lipase value over than three times the upper limit of normal (>180 U/L) without clinical symptoms of acute pancreatitis. Digestive symptoms were found among those nine patients, including anorexia in six (66.7%) patients, nausea in five (55.6%) patients, diarrhea in five (55.6%) patients, and abdominal discomfort in three (33.3%) patients. Three patients underwent an abdominal computed tomography (CT) scan. In the first patient with Crohn's disease, the abdominal CT scan showed inflammation in ileocolonic anastomosis. The second patients had mild fat stranding around the pancreas and gallbladder without signs of pancreatitis. The third patient had a normal abdominal CT scan. 8 The authors did not report the serum amylase value. The comparison of those three studies is provided in Table 1.
Table 1.
Comparison of demographic and clinical characteristic of COVID‐19 patients with increased serum amylase and/or lipase among three studies 6 , 7 , 8
| Liu et al. 6 (n = 121; 13 patients with increased serum amylase and/or lipase) | Wang et al. 7 (n = 52; 9 patients with increased serum amylase and/or lipase) | McNabb‐Baltar et al. 8 (n = 71; 9 patients with increased serum lipase) | |
|---|---|---|---|
| Demographic | |||
| Age (year) (±SD) | 44 in nonsevere and 62 (53‐69) in severe type† | 55 ± 15 | 62.4 ± 15.4 |
| Gender: female (%) | 1 (7.7) in nonsevere and 6 (46.1) in severe type | 3 (33.3) | 5 (55.6) |
| GI symptoms | |||
| Nausea |
Not available Not available Not available 2 (15.3) |
Not available | 5 (55.6) |
| Anorexia | 1 (11) | 6 (66.7) | |
| Abdominal discomfort | Not available | 3 (33.3) | |
| Diarrhea | 1 (11) | 5 (55.6) | |
| Laboratory findings | |||
| Serum lipase (U/L) |
56 in nonsevere and 31(24‐48) in severe type 76 in nonsevere and 62 (59‐121) in severe type 0.43 in nonsevere and 0.6 (0.48‐0.79) in severe type 45 in nonsevere and 31 (20‐78) in severe type 37 in nonsevere and 34 (31‐51) in severe type |
71 ± 34‡ | 151.8 ± 148.4 |
| Serum amylase (U/L) | 115 ± 25‡ | Not available | |
| Creatinine (mg/dL) | 1.09 ± 0.39‡ | 1.48 ± 1.00 | |
| ALT (IU/L) | 49 ± 51‡ | Not available | |
| AST (IU/L) | 52 ± 28‡ |
Not available |
|
| Radiologic findings |
Normal: 8 (3.62%) Enlargement/dilation: 5 (4.13%)§ |
No radiologic examinations were performed in this study | Only three patients with hyperlipasemia underwent abdominal CT imaging. They did not meet the criteria for pancreatitis |
Age in Liu's study was presented as median with youngest and oldest age, while in other studies, age was presented in mean and SD.
The data were collected in COVID‐19 patients with increased serum amylase and/or lipase.
In Liu et al.'s study, only 13 patients underwent imaging evaluation.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; COVID‐19, coronavirus disease 2019; CT, computed tomography; GI, gastrointestinal; SD, standard deviation.
Barlass et al. 10 from Chicago, USA, studied 294 admitted COVID‐19 patients, and 83 of them were checked for serum lipase. As many as 14 from those 83 patients (16.8%) had increased serum lipase over three times the upper limit normal (ULN). With regard to ICU admission, 32.8% patients with serum lipase level lower than three times the ULN (group 1) were admitted compared to a much higher 92.9% patients with serum lipase over three times the ULN (group 2), and the difference was significant (P < 0.001). We also found that 23.5% patients in group 1 were intubated compared to the much higher 78.6% in group 2 (P = 0.0002).
Increased serum amylase and/or lipase in COVID‐19: Critical point of view
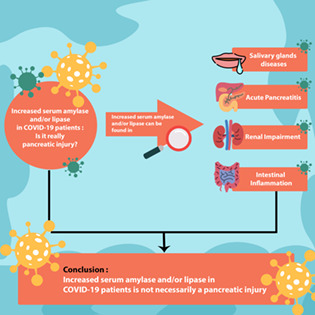
Those three studies should be interpreted cautiously, mainly because increased serum amylase and/or lipase are not exclusive indicators of pancreatic injury. 11 We acknowledge that serum amylase and/or lipase is the end result of equilibrium between its synthesis and clearance from the blood. Amylase is formed by the pancreas and salivary glands. Consequently, elevated serum amylase might be caused by not only pancreatic injury or inflammation but also salivary gland diseases. Chen et al. 12 demonstrated 4 patients with positive SARS‐CoV‐2 RNA based on saliva among 31 COVID‐19 patients. They obtained the saliva specimen from the orificium of the salivary glands. These facts suggest that SARS‐CoV‐2 might infect the salivary glands and might cause hyperamylasemia, as found in Liu et al. 6 and Wang et al. 7 study.
Furthermore, hyperamylasemia can be identified in other conditions such as alcoholism, lactic acidosis, anorexia nervosa, amylase‐producing tumor, bowel infarction, bowel perforation, and diarrhea. Patients diagnosed with COVID‐19 may present with acidosis and diarrhea, thus causing elevated serum amylase. Diarrhea or intestinal inflammation increases the absorption of amylase and lipase in the bowel lumen, with further absorption into blood. Amylase is cleared by the reticuloendothelial system (RES) and kidney. 11 , 13 It explains that hyperamylasemia can be found in kidney injury due to its reduced excretion.
The hypothesis that hyperamylasemia was not always associated with pancreatic injury was shown in Wang et al. 7 's study. They reported that all nine COVID‐19 patients with pancreatic injury (increased serum amylase) had significantly increased creatinine level compared to COVID‐19 patients without pancreatic injury. In addition, one patient with hyperamylasemia had diarrhea. 7 As described previously, renal impairment and diarrhea are two contributing factors of the elevated serum amylase value. Currently, there are no studies evaluating serum pancreatic‐type amylase levels in COVID‐19 patients.
Lipase is synthesized by the pancreas and excreted by the kidney. Elevated serum lipase level could be seen in kidney diseases due to decreased lipase excretion. Serum lipase can be increased when the patient experiences diarrhea or bowel inflammation. 11 In Wang et al.'s study, diarrhea and impaired kidney function were found to be higher in the elevated lipase group compared to the normal group. 7 In McNabb‐Baltar et al.'s study, lipase was increased in 12.7% of patients, but there were no symptoms and signs of acute pancreatitis. In one patient, the elevated serum lipase might be caused by the ileocolonic anastomosis inflammation. 8
Novel insights were provided by Barlass et al. 10 They studied the serum lipase level and found that elevated serum lipase level was significantly related to the rate of ICU admission and intubation. However, it should be noted that increased lipase can be discovered in critically ill patients due to possible pancreatic hypoperfusion and not necessarily acute pancreatitis. They did not perform an abdominal CT scan to confirm acute pancreatitis in those patients. The severity of acute pancreatitis should be determined by the information obtained from the CT scan and not by serum amylase because the previous reports have shown that there was no correlation between serum amylase level and the severity of pancreatitis.
Conclusion
For now, we conclude that increased amylase and/or lipase is not necessarily an indicator of pancreatic injury in COVID‐19 patients. Those increased enzymes might be found in other clinical conditions. In the future, studies should be conducted to confirm the presence of pancreatic injury or clinically relevant pancreatitis in COVID‐19 patients. More data, such as clinical symptoms, and imaging modalities, such as abdominal contrast‐enhanced CT scan, are required to evaluate the specific pancreatic inflammation in COVID‐19 patients.
Acknowledgment
The authors are grateful to Oemar Ichsan, MD, Vita Budi Damayanti, and Prima Setya Haryanto for the positive contribution.
Declaration of conflict of interest: The authors declare that there were no conflicts of interest or financial ties to disclose.
References
- 1. World Health Organization . Coronavirus Disease (COVID‐19) Pandemic Cited 29 Apr 2020. Available from URL: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2. Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, evaluation and treatment coronavirus (COVID‐19) In: StatPearls. Treasure Island, FL: StatPearls Publishing, 2020. Cited 29 Apr 2020. Available from URL: http://www.ncbi.nlm.nih.gov/books/NBK554776/. [PubMed] [Google Scholar]
- 3. Susilo A, Rumende CM, Pitoyo CW et al Coronavirus disease 2019: Tinjauan Literatur Terkini. J. Penyakit Dalam Indones. 2020; 7: 45–67. [Google Scholar]
- 4. Goyal P, Choi JJ, Pinheiro LC et al Clinical characteristics of Covid‐19 in New York City. N. Engl. J. Med. 2020; 382: 2372–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lodigiani C, Iapichino G, Carenzo L et al Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020; 191: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 Expression in Pancreas May Cause Pancreatic Damage After SARS‐CoV‐2 Infection. Clin Gastroenterol Hepatol. 2020; 18: 2128–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang F, Wang H, Fan J, Zhang Y, Wang H, Zhao Q. Pancreatic injury patterns in patients with COVID‐19 pneumonia. Gastroenterology. 2020; 159: 369–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McNabb‐Baltar J, Jin DX, Grover AS et al Lipase elevation in patients with COVID‐19. Am. J. Gastroenterol. 2020; 115: 1286–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hikmet F, Méar L, Edvinsson Å, Micke P, Uhlén M, Lindskog C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020; 16: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barlass U, Wiliams B, Dhana K et al Marked elevation of lipase in COVID‐19 disease: a cohort study. Clin. Transl. Gastroenterol. 2020; 11: e00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De‐Madaria E, Siau K, Cárdenas‐Jaén K. Increased amylase and lipase in patients with COVID‐19 pneumonia: don't blame the pancreas just yet! Gastroenterology. 2020. 10.1053/j.gastro.2020.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen L, Zhao J, Peng J et al Detection of 2019‐nCoV in saliva and characterization of oral symptoms in COVID‐19 patients. SSRN Electron. J. 10.2139/ssrn.3556665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vege SS. Approach to the patient with elevated serum amylase or lipase. Uptodate. 2020. [cited 2020 Apr 29]. Available from: https://www.uptodate.com/contents/approach-to-the-patient-with-elevated-serum-amylase-or-lipase [Google Scholar]


