Abstract
Background and Aim
Standardization of the sedation protocol during radiofrequency ablation (RFA) in patients with hepatocellular carcinoma (HCC) is needed. This randomized, single‐blind, investigator‐initiated trial compared clinical outcomes during and after RFA using propofol and midazolam, respectively, in patients with HCC.
Methods
Few‐ and small‐nodule HCC patients (≤3 nodules and ≤3 cm) were randomly assigned to either propofol or midazolam. Patient satisfaction was assessed using a 100‐mm visual analog scale (VAS) (1 mm = not at all satisfied, 100 mm = completely satisfied). Sedation recovery rates 1, 2, 3, and 4 h after RFA were evaluated based on Modified Observer's Assessment of Alertness/Sedation (MOAA/S) scores; full recovery was defined as a MOAA/S score of 5.
Results
Between July 2013 and September 2017, 143 patients with HCC were enrolled, and 135 patients were randomly assigned to the treatment group. Compared with midazolam, propofol exhibited similar median procedural satisfaction (propofol: 73.1 mm, midazolam: 76.9 mm, P = 0.574). Recovery rates 1 and 2 h after RFA were higher in the propofol group than in the midazolam group. Meanwhile, recovery rates observed 3 and 4 h after RFA were similar in the two groups. The safety profiles during and after RFA were almost identical in the two groups.
Conclusion
Patient satisfaction was almost identical in patients receiving propofol and midazolam sedation during RFA. Propofol sedation resulted in reduced recovery time compared with midazolam sedation in patients with HCC. The safety profiles of both propofol and midazolam sedation during and after RFA were acceptable.
Keywords: hepatocellular carcinoma, midazolam, propofol, radiofrequency ablation, sedation
The standardization of sedation protocol during radiofrequency ablation (RFA) in patients with hepatocellular carcinoma (HCC) is needed. This randomized, single‐blind, investigator‐initiated trial compared clinical outcomes during and after RFA using propofol and midazolam, respectively, in patients with HCC.
Introduction
Hepatocellular carcinoma (HCC) is a leading cause of cancer‐related death, with 850 000 new cases reported annually worldwide. 1 , 2 Most cases of HCC occur in patients with underlying liver disease, mostly as a result of hepatitis B or C virus infection or alcohol abuse. 3 , 4 Over the past few decades, the importance of early detection of HCC by screening and triaging of high‐risk population has been highlighted, 5 , 6 , 7 and the number of patients with early‐stage HCC receiving curative‐intent treatment has been increasing. 8 , 9
Radiofrequency ablation (RFA) is one of the most common curative local ablation methods for HCC. 8 , 10 Percutaneous modalities using ultrasound guidance have been undergoing a process of refinement since the very beginning (around 2000). 8 , 10 , 11 , 12 , 13 According to guidelines from both east and west, RFA is the best treatment alternative in patients with few and small HCC nodules (≤3 nodules measuring ≤3 cm) who are not eligible for surgical resection. 5 , 6 , 7 Compared with surgical resection, RFA is less invasive, is associated with less morbidity, and requires shorter periods of hospitalization while providing comparable outcomes. 14 , 15 , 16 , 17 , 18 , 19
RFA is generally performed with local anesthesia combined with sedation. Some patients experience severe pain and anxiety during RFA under local anesthesia, which may result in lower patient satisfaction and insufficient tumor ablation. 20 , 21 Sedation using midazolam, which is a traditional intravenous sedative used for percutaneous intervention procedures including RFA, is well known for its modest time to onset of action and modest clearance time. 20 , 21 However, administration of midazolam tends to be associated with agitation, irregular breathing, respiratory depression, and thoracic movement, which may lead to inadequate needle placement and needle tracking and cause an insufficient tumor‐free ablation margin.
With its short time to onset of action and short clearance time, propofol has become the standard intravenous sedative drug for short procedures in digestive and liver disease therapy. 22 , 23 , 24 , 25 , 26 Several randomized controlled trials confirmed the safety and efficacy of propofol sedation compared with midazolam during gastrointestinal endoscopy. 23 , 24 , 25 , 26 , 27 Some of these trials included patients with complicated advanced liver disease. 26 , 27 Recently, the European Society of Gastrointestinal Endoscopy and the European Society of Gastroenterology and Endoscopy Nurses and Associates recommended the use of propofol by nonanesthesiologists for gastrointestinal endoscopy. 28
Standardization of sedation for percutaneous local ablation for HCC is required as it has become a common procedure all over the world. However, to the best of our knowledge, the currently available data are insufficient for generating a consensus on the guidelines of sedation during percutaneous local ablation. The aim of this study was to evaluate the comparative efficacies and safety of propofol and midazolam in HCC patients undergoing RFA.
Methods
Study design
The present investigator‐initiated, randomized, single‐blind trial was performed in accordance with the good clinical practice guidelines in place at the Chiba University Hospital. The investigators and supporting staff collected the data, and the Clinical Research Center at Chiba University Hospital monitored study conduct. The study was registered with the University Hospital Medical Information Network (UMIN000011443).
All patients receiving RFA were randomly assigned in a 1:1 ratio through a minimization method to either the propofol regimen or the midazolam regimen. The allocation coordinators at the Clinical Research Center enrolled the patients and assigned them to the two trial groups. Allocation factors were RFA history (absent/present), tumor location in nearby intrahepatic vessels (absent/present), and single tumor measuring ≤2 cm (absent/present). Subjects were blinded with regard to the group to which they were assigned.
Inclusion criteria
The inclusion criteria were as follows: age ≥20 years; presence of histologically confirmed or clinically diagnosed HCC (fulfilling the criteria on diagnostic imaging); number of intrahepatic tumors ≤3 and tumor size ≤3 cm; absence of benefit from a treatment of established efficacy (e.g., resection); and presence of Child–Pugh A or B classes, an Eastern Cooperative Oncology Group performance status (ECOG‐PS) of 0 or 1, systolic blood pressure ≥90 mmHg, degree of oxygen saturation ≥95%, hemoglobin ≥8.5 g/dL, white blood cell count ≥2000/mm3, neutrophil count ≥1000/mm3, platelet count ≥50 000/mm3, total bilirubin level ≤3.0 g/dL, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels ≤10 times the upper limits of the normal (ULN), prothrombin time ≤2.3 (international normal ratio), serum albumin ≥2.5 g/dL, and serum–creatinine level ≤1.5 times the ULN.
Exclusion criteria
The key exclusion criteria were as follows: presence of macrovascular invasion and/or extrahepatic metastasis, presence of uncontrolled or significant cardiovascular disease, American Society of Anesthesiologist classification ≥4, body mass index (BMI) ≥30, presence of sleep apnea syndrome, active bacterial infection, and human immunodeficiency virus infection/adult immunodeficiency syndrome.
RFA procedure
The RFA procedure was performed under real‐time ultrasound guidance using a 17‐gauge cool‐tip electrode (Cool‐Tip; RF Ablation System, Covidien, Boulder, Colombia, CO, USA). Under conscious sedation, an electrode was inserted and radiofrequency delivered for 6–15 min for each lesion. As appropriate, intrapleural or intraperitoneal fluid infusion was performed before electrode insertion. A successful RFA was defined as a target lesion—selected at the time of enrollment—confirmed as being completely ablated according to the radiological assessment.
Drug administration
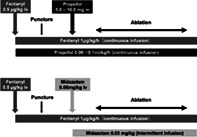
Drug infusion was performed by the attending hepatologists who had been trained by anesthesiologists. The predefined light sedation protocol developed by two anesthesiologists (Natsuko Nozaki‐Taguchi and Shiroh Isono) is shown in Figure S1, Supporting information. A dose of 0.6 mg/kg/h of propofol was administered intravenously when commencing the RFA procedure. After confirming that the needle had been inserted correctly into the tumor, 0.2 mg/kg of propofol was slowly injected intravenously, and the dose was increased to 1.0 mg/kg/h. During ablation, the depth of sedation was monitored using a bispectral index, and the dosage of propofol was adjusted to between 0.3 mg/kg/h and 1.0 mg/kg/h according to the bispectral index 60–80. 22 A total of 0.06 mg/kg of midazolam was administered by intravenous injection directly after confirming correct introduction of the needle of RFA into the tumor. Additional intravenous injections of midazolam at a dose of 0.03 mg/kg were allowed under RFA ablation according to the bispectral index 60–80. After completion of the RFA procedure, 0.5 mg of flumazenil was injected intravenously for recovery from sedation in the midazolam group. To alleviate pain during the procedure, fentanyl was administered during the procedure in both groups. The protocol for fentanyl desensitization was predefined using a simulation program.
Primary and secondary end‐points
The primary end‐point of the present trial was patient satisfaction, which was assessed with a 100‐mm visual analog scale (VAS) (0 mm = least satisfied and 100 mm = most satisfied). The preplanned secondary end‐points were as follows: achievement rates of sedation according to MOAA/S score (moderate sedation: MOAA/S score ≤4, deep sedation: MOAA/S score ≤2), 25 treatment completion rate, subjective feeling of pain as assessed using a 5‐point scale (0 = least pain and 5 = worst pain), recovery rates of sedation according to MOAA/S score as measured every hour for 4 h after RFA, safety of treatment during the RFA procedure, and safety of treatment after the RFA procedure. The Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 protocol was used for the assessment of adverse events.
Sample size
Superiority of the propofol group over the midazolam group was defined as a 10‐mm higher VAS score for patient satisfaction in the propofol group compared with the midazolam group. Assuming a 70‐mm VAS score for patient satisfaction in the midazolam group, a sample size of 128 patients (propofol group, 64 patients; midazolam group, 64 patients) was required to detect a difference in VAS score of at least 10 mm, using an alfa error of 0.05 and a beta error of 0.20. A total of 140 patients (propofol group, 70 patients; midazolam group, 70 patients) were enrolled in anticipation of the ineligibility of some patients.
Statistical analysis
Pearson's chi‐squared test or Fisher's exact test was used as appropriate. An unpaired t‐test was used to compare means between independent groups. All statistical analyses were conducted using the SAS statistical software (version 9.4, SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
Figure 1 shows the CONSORT flow diagram for the patients included in the present clinical trial. Between July 2013 and September 2017, a total of 143 patients with HCC were enrolled, and 135 patients were randomly assigned to the two treatment groups. All 135 patients received RFA and were included in the final analysis. Thus, a total of 65 patients were randomly assigned to the propofol regimen, while 70 patients were assigned to the midazolam regimen.
Figure 1.
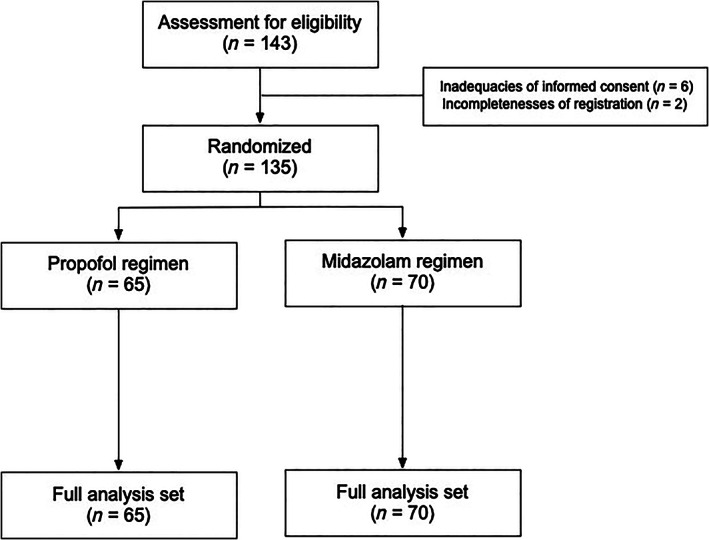
Diagram of consolidated standards of reporting trials.
The median age was 70 years (range, 49–85 years), and 70.4% (95 patients) were male (Table 1). The majority of the patients belonged to the Child–Pugh A category (score 5, 99 patients [73.3%]; score 6, 29 patients [21.5%]). Regarding tumor size and number, 64 patients (47.4%) had single lesions ≤2 cm in size, and 85 patients (63.0%) had tumors located in nearby vessels. More than half of patients (54.8%) had a previous history of RFA. Baseline demographic data and patient characteristics did not differ significantly between the propofol and midazolam regimens (Table 1).
Table 1.
Baseline demographic data and patient characteristics
| Propofol regimen (n = 65) | Midazolam regimen (n = 70) | P | |
|---|---|---|---|
| Gender, male [n (%)] | 45 (69.2) | 50 (71.4) | 0.851 |
| Age, ≥70 years [n (%)] | 39 (60.0) | 38 (54.3) | 0.121 |
| HBs‐Ag, positive [n (%)] | 11 (16.9) | 10 (14.3) | 0.813 |
| HCV‐Ab, positive [n (%)] | 40 (61.5) | 47 (67.1) | 0.590 |
| ECOG‐PS 0 [n (%)] | 58 (89.2) | 64 (91.4) | 0.774 |
| Child–Pugh score [n (%)] | |||
| 5 | 48 (73.8) | 51 (72.9) | 0.795 |
| 6 | 14 (21.5) | 15 (21.4) | |
| 7 | 1 (1.5) | 3 (4.3) | |
| 8 | 2 (3.1) | 1 (1.4) | |
| Tumor number [n (%)] | |||
| 1 | 45 (69.2) | 49 (70.0) | 0.834 |
| 2 | 17 (26.2) | 16 (22.9) | |
| 3 | 3 (4.6) | 5 (7.1) | |
| Maximum tumor size in mm [mean (SD)] | 17.4 (5.9) | 16.4 (5.6) | 0.319 |
| Single nodule and ≤20 mm [n (%)] | 30 (46.2) | 34 (48.6) | 0.863 |
| AFP value, ng/mL [mean (SD)] | 51.8 (175.4) | 78.7 (332.1) | 0.553 |
| Tumor location, nearby vessels [n (%)] | 41 (63.1) | 44 (62.9) | 1.000 |
| Past history of RFA [n (%)] | 30 (46.2) | 35 (50.0) | 0.864 |
Abbreviations: AFP, alpha‐fetoprotein; ECOG‐PS, Eastern Cooperative Oncology Group performance status; HBs‐Ag, hepatitis B surface antigen; HCV‐Ab, hepatitis C virus antibody; RFA, radiofrequency ablation.
VAS scores after RFA procedure
The results for the primary end‐point are shown in Figure 2. Mean VAS scores in the propofol and midazolam arms were 73.1 (SD, 35.5) and 76.9 (SD, 29.1), respectively, with no significant difference between the two groups (P = 0.574). In addition, the proportion of VAS score ratings ≥70 mm in both groups did not differ significantly (propofol, 72.7%; midazolam, 75.4%; P = 0.835).
Figure 2.
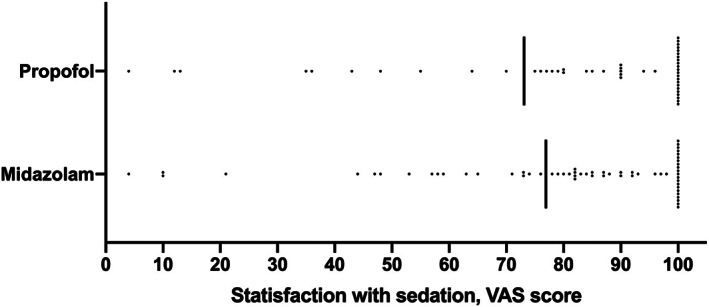
Satisfaction with sedations assessed by visual analog scale (VAS) score.
Procedure, depth of sedation, and recovery time
Of the 65 patients in the propofol arm, 58 patients (89.2%) successfully completed treatment, with all target lesions being ablated. Likewise, successful RFA was achieved for 64 of the 70 patients (91.4%) in the midazolam arm. The achievement rates of moderate and deep sedation according to MOAA/S scores are displayed in Figure 3a. We observed high frequency rates of achieving moderate and deep sedation in both arms (moderate sedation, P = 0.587; deep sedation, P = 1.000). Figure 3b demonstrates the results of pain assessment during the RFA procedure according to the 5‐point scale scores. There was no significant difference between the two groups (P = 0.198). After the RFA procedure, MOAA/S scores were assessed every hour for 4 h. The distribution of MOAA/S scores is shown in Figure 4. Recovery rates observed 1 and 2 h after RFA procedure were higher in the propofol group than in the midazolam group.
Figure 3.
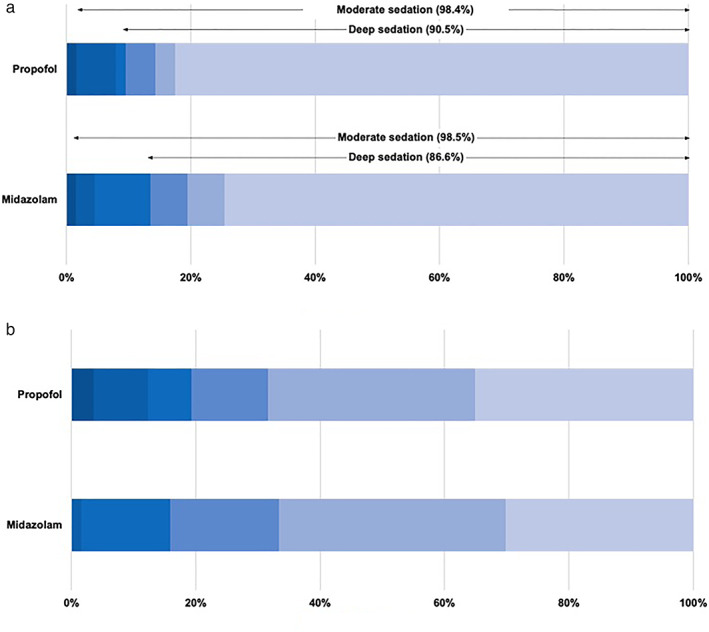
Depths of sedation (a) and pain assessments (b) during radiofrequency ablation. (a): ( ), 5; (
), 5; ( ), 4; (
), 4; ( ), 3; (
), 3; ( ), 2; (
), 2; ( ), 1; (
), 1; ( ), 0. (b): (
), 0. (b): ( ), 5; (
), 5; ( ), 4; (
), 4; ( ), 3; (
), 3; ( ), 2; (
), 2; ( ), 1; (
), 1; ( ), 0.
), 0.
Figure 4.
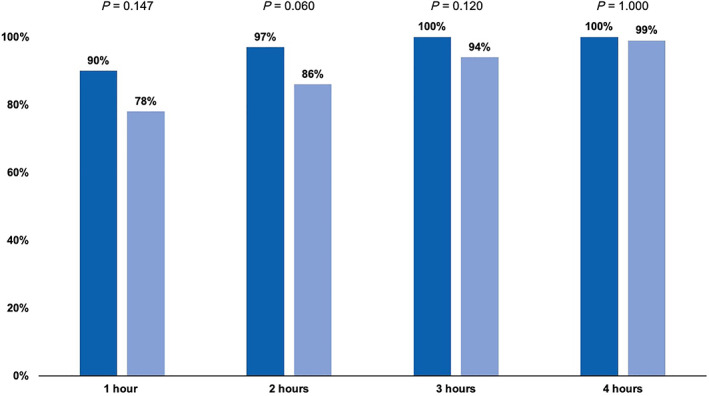
Frequencies of fully recover after radiofrequency ablation according to Modified Observer's Assessment of Alertness/Sedation score. ( ), Propofol; (
), Propofol; ( ), midazolam.
), midazolam.
Safety evaluation under and after RFA treatment
We monitored blood pressure, heart rate, and oxygen saturation every 2 min during the RFA procedure (Table 2). Maximum systolic and diastolic pressures were significantly higher in the propofol group than in the midazolam group. Table 3 displays data pertaining to adverse events according to the CTCAE v4.0 before and after RFA treatments. The most common adverse events during the RFA procedure were hypertension, abdominal pain, nausea, and vomiting. On the other hand, increased AST, hypoalbuminemia, anemia, increased blood bilirubin, and decreased platelet counts were the most common adverse events observed after RFA. The frequency of severe hypertension during the RFA procedure was significantly higher in the propofol group compared with the midazolam group. No significant differences in other adverse events were observed between the two groups.
Table 2.
Vital signs during radiofrequency ablation
| Propofol regimen | Midazolam regimen | ||||
|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | P | |
| Systolic blood pressure (mmHg) | |||||
| Maximum | 165 (25) | 127–259 | 154 (22) | 112–209 | 0.006 |
| Minimum | 94 (17) | 64–158 | 90 (15) | 53–131 | 0.237 |
| Diastolic blood pressure (mmHg) | |||||
| Maximum | 124 (24) | 64–177 | 114 (22) | 61–170 | 0.022 |
| Minimum | 75 (14) | 42–107 | 69 (16) | 19–107 | 0.034 |
| Heart rate (/min) | |||||
| Maximum | 83 (15) | 52–122 | 85 (16) | 56–130 | 0.631 |
| Minimum | 68 (13) | 40–100 | 69 (13) | 41–115 | 0.623 |
| Oxygen saturation (%) | |||||
| Minimum | 92 (6) | 72–100 | 94 (3) | 80–100 | 0.015 |
| Oxygen flow rate, liter/min | |||||
| Maximum | 2.1 (0.5) | 2.0–5.0 | 2.1 (0.4) | 2.0–5.0 | 0.928 |
Table 3.
Adverse events during and after radiofrequency ablation
| Propofol regimen | Midazolam regimen | P | ||||
|---|---|---|---|---|---|---|
| Any grade | Grade 3 ≤ | Any grade | Grade 3 ≤ | Any grade | Grade 3 ≤ | |
| During radiofrequency ablation [n (%)] | ||||||
| Hypertension | 50 (76.9) | 35 (53.9) | 50 (71.4) | 25 (35.7) | 0.557 | 0.039 |
| Abdominal pain | 10 (15.4) | 0 (0) | 14 (20.0) | 2 (2.9) | 0.509 | 0.497 |
| Nausea | 8 (12.3) | 0 (0) | 8 (11.4) | 0 (0) | 1.000 | — |
| Vomiting | 6 (9.2) | 0 (0) | 5 (7.1) | 0 (0) | 0.758 | — |
| Vasovagal reaction | 2 (3.1) | 2 (3.1) | 4 (5.7) | 4 (5.7) | 0.682 | 0.682 |
| Apnea | 4 (6.2) | 4 (6.2) | 2 (2.9) | 2 (2.9) | 0.428 | 0.428 |
| Intra‐abdominal hemorrhage | 3 (4.6) | 1 (1.5) | 1 (1.5) | 0 (0) | 0.109 | 0.482 |
| After radiofrequency ablation [n (%)] | ||||||
| AST increase | 58 (89.2) | 22 (33.9) | 60 (85.7) | 25 (35.7) | 0.610 | 0.858 |
| Hypoalbuminemia | 27 (41.5) | 0 (0) | 36 (51.4) | 0 (0) | 0.301 | |
| Anemia | 25 (38.5) | 2 (3.1) | 33 (47.1) | 0 (0) | 0.385 | 0.230 |
| Blood bilirubin increase | 17 (26.2) | 2 (3.1) | 35 (50.0) | 0 (0) | 0.005 | 0.230 |
| Platelet count decrease | 22 (33.9) | 6 (9.2) | 22 (31.4) | 5 (7.1) | 0.855 | 0.758 |
| White blood cell decrease | 9 (13.9) | 2 (3.1) | 10 (14.3) | 0 (0) | 1.000 | 0.230 |
| Anorexia | 10 (15.4) | 0 (0) | 8 (11.4) | 0 (0) | 0.614 | — |
| Fever | 6 (9.2) | 0 (0) | 8 (11.4) | 0 (0) | 0.781 | — |
| Abdominal pain | 6 (9.2) | 0 (0) | 5 (7.1) | 0 (0) | 0.758 | — |
| Hypertension | 3 (4.6) | 2 (3.1) | 2 (2.9) | 0 (0) | 0.672 | 0.230 |
Listed adverse events defined by the National Cancer Institution Common Terminology Criteria (version 4.0).
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMY, amylase; AST, aspartate aminotransferase; GGT, gamma‐glutamyl transpeptidase.
Discussion
The present investigator‐initiated, single‐blind, randomized trial analyzed the clinical outcomes in HCC patients who received RFA with the use of either propofol or midazolam for gastroenterologist‐administered moderate sedation. Faster recovery times after RFA were observed when propofol was used for sedation compared with midazolam; however, overall satisfaction, which was the primary end‐point, did not differ between the two groups. Moreover, the safety profiles in both study arms were almost identical during and after RFA. We believe that our results can inform the development of a standard protocol for sedation during RFA in patients with HCC, which is highly warranted. 20 , 21
Full recovery rates as observed 1 and 2 h after RFA according to MOAA/S score were higher in the propofol arm compared with the midazolam arm. On the other hand, full recovery rates 3 and 4 h after RFA were almost identical between two groups. Our results thereby suggested that patients using propofol recovered earlier than those receiving midazolam. Although similar observations have been published in several studies in various treatment procedures related to endoscopy, 25 , 29 to the best of our knowledge, the present study is the first to identify faster recovery times using propofol compared with midazolam for RFA in HCC patients using a well‐designed randomized controlled trial.
No significant superior overall satisfaction regarding sedation during RFA using propofol was observed when compared with the use of midazolam. Regarding other procedures, such as an upper gastrointestinal endoscopy, Levitzky et al. reported that propofol sedation by adequately trained endoscopists resulted in superior patient satisfaction compared with midazolam sedation during upper gastrointestinal endoscopy. 26 All patients included in their study were outpatients undergoing upper gastrointestinal endoscopy. On the other hand, all of the patients included in the present study were hospitalized. Taken together, outpatients who need to return home on the same day may feel satisfied about fast recovery. From the point of view of medical staff, achieving faster full recovery after RFA should be conducive to reducing the workload related to post‐RFA patient monitoring.
The present study assessed complications during and after RFA separately. To the best of our knowledge, this article was the first to report on safety during RFA with moderate sedation. Our results showed that hypertension was the most common adverse event in both of the groups. Other common adverse events during RFA included abdominal pain, nausea, and vomiting. Meanwhile, we observed only a limited number of vasovagal reactions and cases of apnea, which are well‐known adverse events related to the use of sedative agents.
In the present study, sedation by either propofol or midazolam was controlled by hepatologists. Deep sedation rates in the propofol and midazolam groups amounted to 88.5 and 90.5%, respectively. Rates of complete painlessness in the propofol and the midazolam groups were only 32.5 and 35.1%, respectively. Hence, for some patients in both groups, it was not possible to achieve successful sedation. Taken together, we judged that hepatologists, who are not specialists of anesthesiology, might have had some difficulties in controlling the sedation possibly due to fear of oversedation, although they had been trained by anesthesiologists before taking control of the sedative agents. As hepatologist‐administered sedation has been widely used in several procedures, including RFA, standardization of education programs for nonspecialists in anesthesiology is strongly required.
According to the primary end‐point and sample size calculation that we set up based on the data of the retrospective cohort in our institution, we were unable to prove the superiority of sedation by propofol compared with by midazolam. If the trial design is reconsidered by experts including biostatisticians, we may be able to show the result that shows the superiority of propofol to midazolam during RFA in patients with HCC.
In conclusion, propofol sedation during RFA by hepatologists resulted in faster full recovery time compared with midazolam sedation in patients with HCC; however, post‐RFA patient satisfaction was similar in the two groups. As the safety profiles of propofol and midazolam sedation during and after RFA were acceptable, deep sedation during RFA should be considered a feasible option by hepatologists for patients with HCC. Therefore, a standard education program for hepatologists who perform sedation in daily practice is strongly required.
Supporting information
Figure S1. Sedation protocols of this study.
Acknowledgments
We thank the following people for their contributions to the data management: Michiko Hanawa, Yoko Hattori, Mayumi Negishi, and Makiko Matsui.
Declaration of conflict of interest: None.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Llovet JM, Zucman‐Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M. Hepatocellular carcinoma. Nat. Rev. Dis. Primers. 2016; 14: 16018. [DOI] [PubMed] [Google Scholar]
- 3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 4. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018; 391: 1301–14. [DOI] [PubMed] [Google Scholar]
- 5. Kokudo N, Takemura N, Hasegawa K et al Clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2017 (4th JSH‐HCC guidelines) 2019 update. Hepatol. Res. 2019; 49: 1109–13. [DOI] [PubMed] [Google Scholar]
- 6. European Association for the Study of the Liver, European Association for the Study of the Liver . EASL clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 2018; 69: 182–236. [DOI] [PubMed] [Google Scholar]
- 7. Omata M, Cheng AL, Kokudo N et al Asia‐Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol. Int. 2017; 11: 317–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shiina S, Tateishi R, Arano T et al Radiofrequency ablation for hepatocellular carcinoma: 10‐year outcome and prognostic factors. Am. J. Gastroenterol. 2012; 107: 569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shindoh J, Makuuchi M, Matsuyama Y et al Complete removal of the tumor‐bearing portal territory decreases local tumor recurrence and improves disease‐specific survival of patients with hepatocellular carcinoma. J. Hepatol. 2016; 64: 594–600. [DOI] [PubMed] [Google Scholar]
- 10. Rossi S, Di Stasi M, Buscarini E et al Percutaneous radio frequency interstitial thermal ablation in the treatment of small hepatocellular carcinoma. Cancer J. Sci. Am. 1995; 1: 73–81. [PubMed] [Google Scholar]
- 11. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio‐frequency ablation versus ethanol injection. Radiology. 1999; 210: 655–61. [DOI] [PubMed] [Google Scholar]
- 12. Shiina S, Teratani T, Obi S et al A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005; 129: 122–30. [DOI] [PubMed] [Google Scholar]
- 13. Tateishi R, Shiina S, Teratani T. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005; 103: 1201–9. [DOI] [PubMed] [Google Scholar]
- 14. Hasegawa K, Kokudo N, Makuuchi M et al Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J. Hepatol. 2013; 58: 724–9. [DOI] [PubMed] [Google Scholar]
- 15. Chen MS, Li JQ, Zheng Y et al A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann. Surg. 2006; 243: 321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feng K, Yan J, Li X et al A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J. Hepatol. 2012; 57: 794–802. [DOI] [PubMed] [Google Scholar]
- 17. Fang Y, Chen W, Liang X et al Comparison of long‐term effectiveness and complications of radio‐frequency ablation with hepatectomy for small hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2014; 29: 193–200. [DOI] [PubMed] [Google Scholar]
- 18. Huang J, Yan L, Cheng Z et al A randomized trial comparing radio‐frequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann. Surg. 2010; 252: 903–12. [DOI] [PubMed] [Google Scholar]
- 19. Izumi N, Hasegawa K, Nishioka Y et al A multicenter randomized controlled trial to evaluate the efficacy of surgery vs. radiofrequency ablation for small hepatocellular carcinoma (SURF trial). J. Clin. Oncol. 2019; 37 (15_suppl): 4002. [Google Scholar]
- 20. Sato K, Taniki N, Kanazawa R et al Efficacy and safety of deep sedation in percutaneous radiofrequency ablation for hepatocellular carcinoma. Adv. Ther. 2019; 36: 344–54. [DOI] [PubMed] [Google Scholar]
- 21. Joung KW, Choi SS, Jang DM et al Comparative effects of dexmedetomidine and propofol on US‐guided radiofrequency ablation of hepatic neoplasm under monitored anesthesia care: a randomized controlled study. Medicine (Baltimore). 2015; 94: e1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lera dos Santos ME, Maluf‐Filho F, Chaves DM et al Deep sedation during gastrointestinal endoscopy: propofol‐fentanyl and midazolam‐fentanyl regimens. World J. Gastroenterol. 2013; 19: 3439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Correia LM, Bonilha DQ, Gomes GF et al Sedation during upper GI endoscopy in cirrhotic outpatients: a randomized, controlled trial comparing propofol and fentanyl with midazolam and fentanyl. Gastrointest. Endosc. 2011; 73: 45–51. [DOI] [PubMed] [Google Scholar]
- 24. Akbulut UE, Saylan S, Sengu B, Akcali GE, Erturk E, Cakir M. A comparison of sedation with midazolam‐ketamine versus propofol‐fentanyl during endoscopy in children: a randomized trial. Eur. J. Gastroenterol. Hepatol. 2017; 29: 112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levitzky BE, Lopez R, Dumot JA, Vargo JJ. Moderate sedation for elective upper endoscopy with balanced propofol versus fentanyl and midazolam alone: a randomized clinical trial. Endoscopy. 2012; 44: 13–20. [DOI] [PubMed] [Google Scholar]
- 26. Ahmed SA, Selim A, Hawash N et al Randomized controlled study comparing use of propofol plus fentanyl versus midazolam plus fentanyl as sedation in diagnostic endoscopy in patients with advanced liver disease. Int. J. Hepatol. 2017; 2017: 8462756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Watanabe K, Hikichi T, Takagi T et al Propofol is a more effective and safer sedative agent than midazolam in endoscopic injection sclerotherapy for esophageal varices in patients with liver cirrhosis: a randomized controlled trial. Fukushima J. Med. Sci. 2018; 64: 133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dumonceau JM, Riphaus A, Schreiber F et al Non‐anesthesiologist administration of propofol for gastrointestinal endoscopy: European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates Guideline–updated June 2015. Endoscopy. 2015; 47: 1175–89. [DOI] [PubMed] [Google Scholar]
- 29. Wang Z, Hu Z, Dai T. The comparison of propofol and midazolam for bronchoscopy: a meta‐analysis of randomized controlled studies. Medicine (Baltimore). 2018; 97: e12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Sedation protocols of this study.


