Abstract
Objective
To estimate the economic burden of systematic lupus erythematous (SLE), stratified by disease severity, in commercially- and Medicaid-insured US populations
Methods
Adults (≥18 years) with SLE treated with antimalarials, selected biologics, immunosuppressants, and systemic glucocorticoids (2010–2014) were identified within the commercial and Medicaid insurance IBM MarketScan® databases (index date = first SLE medication claim). Both cohorts were stratified into mild (receiving antimalarial or glucocorticoid monotherapy ≤5 mg/day) versus moderate/severe SLE (receiving glucocorticoids >5 mg/day, biologic, immunosuppressant, or combination therapy) during a 6-month exposure period. All-cause healthcare utilization and costs were evaluated during the 12 months following the exposure period.
Results
Among 8,231 commercially-insured patients, 32.6% had mild and 67.4% had moderate/severe SLE by our definition. Among 802 Medicaid-insured patients, 25.2% had mild and 74.8% had moderate/severe SLE. Adjusted mean total healthcare costs, excluding pharmacy, for moderate/severe SLE patients were higher than for mild SLE patients in the commercially-insured ($39,021 versus $23,519; p<0.0001) and Medicaid-insured populations ($56,050 versus $44,932; p=0.06). In both SLE severity populations total unadjusted costs were significantly higher among Medicaid-insured than commercially-insured patients.
Conclusion
Commercially-insured patients with treatment suggesting moderate/severe SLE incurred significantly higher adjusted mean healthcare costs, excluding pharmacy, compared with mild SLE patients. While not reaching statistical significance, moderate/severe Medicaid-insured patients had higher costs then mild SLE patients. Total unadjusted healthcare costs were significantly higher among Medicaid-insured than commercially-insured patients. These differential costs are important to consider and monitor when implementing interventions to improve health and reduce healthcare spending for SLE.
Keywords: Systemic lupus erythematosus, healthcare costs, healthcare utilization, severity, medication, autoimmune disease
1. INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by symptoms and complications in multiple organ systems including renal, neurologic, and cardiovascular (1–5). Most patients live with frequent flares or continuous symptoms; less than 10% of patients achieve remission for at least one year (6, 7). SLE is predominantly diagnosed in individuals aged 15–49 years; women account for approximately 90% of cases (8). According to the National Health Interview Survey (2003–2005), SLE affected 161,000 to 322,000 adults in the United States (US) (9).
SLE imposes a substantial economic burden. Most existing research on the economic burden of SLE has described the costs incurred by all SLE patients regardless of disease manifestations (10–13) and some have compared the costs of those with versus without SLE (1, 14–16). Few studies have examined healthcare costs stratified by SLE severity (17–20). A study in a commercially-insured US population (2004–2008) reported that the annualized all-cause medical costs increased substantially as the severity of flares increased (mild: $14,945; moderate: $21,606; severe: $64,578) (17). Although SLE affects those with low socioeconomic status and racial/minorities disproportionately (21, 22), little is known about the healthcare costs among Medicaid-insured patients (15, 23, 24).
Given these knowledge gaps, this claims-based analysis aimed to evaluate the economic burden of SLE by disease severity in the US. Our study goal was to estimate current the healthcare utilization and costs of SLE, stratifed by disease severity, in both commercially-insured and Medicaid-insured populations. We hypothesized that direct healthcare costs would be higher in patients with moderate/severe SLE versus mild disease, and among Medicaid-insured patients versus commercially-insured patients.
2. PATIENTS AND METHODS
2.1. Data source
We conducted a historical, observational cohort study using de-identified administrative medical and pharmacy claims data (2009–2016) from IBM-MarketScan® Commercial Claims and Encounters (commercial) and Medicaid Multi-State (Medicaid) databases. These databases contain enrollment information, inpatient and outpatient medical, and outpatient pharmacy claims data for approximately 90 million individuals with employer-sponsored primary health insurance and >18 million individuals sponsored by Medicaid programs in multiple, geographically-dispersed states across the US (2009–2016). All database records were fully compliant with the Health Insurance Portability and Accountability Act of 1996. Utilizing only de-identified patient records, this protocol was exempted from Institutional Review Board approval.
2.2. Study population
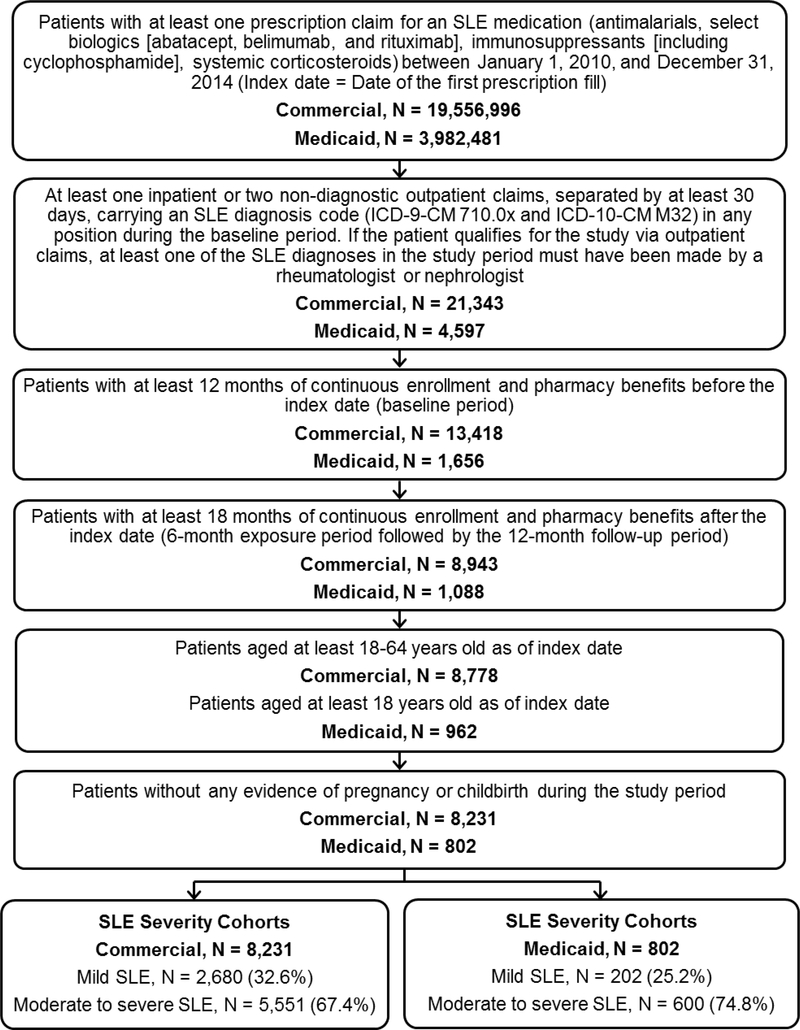
Adults (aged ≥18 years) with at least one prescription claim for an SLE medication (antimalarials, selected biologics [abatacept, belimumab, and rituximab], immunosuppressants [azathioprine, cyclophosphamide, cyclosporine, intravenous immunoglobulin, leflunomide, methotrexate, mycophenolate mofetil/mycophenolic acid, and tacrolimus], and systemic glucocorticoids) between January 1, 2010, and December 31, 2014, were identified (Figure 1). The earliest date of an SLE medication prescription fill was the index date. All patients were required to have ≥1 inpatient or ≥2 non-diagnostic (i.e., not laboratory or radiology, ≥30 days apart) outpatient claims during the 12-month period before the index date carrying an SLE diagnosis code (International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification [ICD-9-CM 710.0x and ICD-10-CM M32]) (1, 15). If the patient qualified for the study via outpatient claims, ≥1 SLE diagnoses must have been made by a rheumatologist or nephrologist (25–27). All patients were required to have continuous health plan coverage for 12 months before and 18 months after the index date, to encompass the baseline, exposure, and follow-up periods. Patients with evidence of pregnancy or childbirth during the study period were excluded.
Fig 1:
SLE cohort composition, commercially- and Medicaid-insured US patients, 2010–2014.
Abbreviations: ICD-9-CM/ICD-10-CM, International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification; SLE, systemic lupus erythematosus.
2.3. Study periods
The study period was divided into three: 1) the 12 months prior to the index date (baseline period); 2) the six months after the index date (SLE-treatment exposure period); and 3) the 12 months after the exposure period (follow-up period).
2.4. SLE cohorts
We used treatment intensity as a proxy for disease severity given that ICD-9/10 diagnostic codes used in claims do not identify SLE severity status, while pharmacy claims are near complete. Subjects were classified as having mild or moderate/severe SLE based on treatments during the exposure period. Mild SLE was defined as receiving low intensity treatments, including only antimalarial monotherapy or oral glucocorticoid monotherapy at an average ≤5 mg of prednisone/day. Patients treated more intensively with all other SLE immunosuppressive therapies, either alone or in combination, were classified as having moderate/severe SLE.
2.5. Outcomes
2.5.1. Healthcare utilization and costs
All-cause healthcare utilization was measured during the 12-month follow-up period. Specific utilization measures included rates, frequencies, and costs of inpatient hospitalization, emergency room (ER) visits, dialysis-related visits, outpatient office visits, physician administered medications, other outpatient services [i.e. laboratory, radiology, etc], and outpatient pharmacy prescriptions. The subset of outpatient office visits with a rheumatologist were also reported.
Total healthcare costs (medical and pharmacy) during the follow-up period were measured overall and by each service category. As we hypothesized that total healthcare costs would be higher in those with moderate/severe SLE and our definition of moderate/severe SLE was based on greater medication usage, total healthcare costs excluding pharmacy costs were also estimated. Healthcare costs were paid amounts from adjudicated claims, including insurer payments (including coordination of benefits) and out-of-pocket payments (coinsurance, copayments, and deductibles). The payer portion of costs, which excludes out-of-pocket payments, was also reported. All dollar estimates were inflated to 2016 constant US dollars using the Medical Care component of the Consumer Price Index (28).
2.6. Study covariates
Patient demographic characteristics examined at the index date included age, sex, and insurance plan type (comprehensive/preferred provider organization [PPO]/ health maintenance organization/point of service/other). Due to data availability, race (White/Black/Hispanic/other) was reported only among Medicaid-insured patients and urbanicity was reported only among commercially-insured patients. Clinical characteristics measured during the baseline period included the Deyo-Charlson Comorbidity Index (Deyo-CCI) score (29). As lupus nephritis and kidney transplant are important risk factors for SLE-related morbidity and mortality, patients with these conditions were identified. Lupus nephritis was defined as having at least two claims at least 30 days apart with ICD-9-CM diagnosis codes for nephritis, proteinuria, and/or renal failure occurring on or after the index date. Kidney transplant was identified on claims by ICD-9-CM or ICD-10-CM procedure codes, current procedural terminology, Healthcare Common Procedures Coding System, and UB-04 revenue codes. Evidence of clinically relevant comorbid conditions (i.e., avascular necrosis, cancer, cardiovascular disease [cerebrovascular disease, congestive heart failure, myocardial infarction, peripheral vascular disease], cataracts, type 2 diabetes, fibromyalgia, fractures [pathologic and traumatic], glaucoma, hypertension, osteoporosis, and stroke), and the use of selected concomitant medications (i.e., antidepressants, antihypertensives, antidiabetic agents, hormone replacement therapy, and non-steroidal anti-inflammatory drugs [NSAIDs]), were also measured.
2.7. Statistical analyses
All study variables were analyzed descriptively. Results are reported separately for those classified as having mild versus moderate/severe SLE. Categorical measures are presented as counts and percentages. Continuous measures are summarized as means and standard deviations (SD). The statistical significance of differences between the mild and moderate/severe groups within each insurance population and between each insurance population within like severity groups were assessed using chi-squared tests (categorical variables), and two-tailed Student’s t-tests (continuous variables).
Total all-cause medical cost was estimated for mild and moderate/severe SLE patients after adjustment for patient demographic characteristics and comorbid burden. Generalized linear models with gamma-distributed error and log link were specified and fit to the patient data. The dependent variable was total all-cause medical cost and the independent variables included mild vs. moderate/severe SLE, age (18–24,25–34,35–44,45–54,55–64), sex, health plan type, urbanicity (commercial only), race (Medicaid only) and baseline Deyo-CCI. The adjusted dollar cost and incremental expenditure difference between mild and moderate/severe SLE within each insurance population was estimated by the method of recycled predictions based on the fitted models..
3. RESULTS
The final study population consisted of 9,033 SLE patients (Figure 1). Of these, 8,231 were commercially-insured and 802 were Medicaid-insured. Based on exposure period SLE treatment, patients were classified as having moderate/severe SLE (commercial: 67.4%; Medicaid: 74.8%) or mild SLE (commercial: 32.6%; Medicaid: 25.2%).
3.1. Patient characteristics
Table 1 presents the baseline demographic and clinical characteristics of the commercially- and Medicaid-insured study populations by SLE severity. Commercially-insured patients with moderate/severe SLE were slightly younger than patients with mild SLE (46.6 versus 47.7 years old; p<0.001). Over 90% of all commercially-insured patients were women, but the moderate/severe SLE group had a significantly higher proportion of men than the mild SLE group (9.8% versus 7.8%; p=0.004). Over half of all patients were enrolled in a PPO plan, and the distribution of patients among all plan types did vary between the two groups. Approximately 87% of both severity groups resided in an urban setting.
Table 1.
Baseline demographic and clinical characteristics of commercially- and Medicaid-insured patients with SLE, 2010–2014a
| Commercially-insured (n =
8,231) |
Medicaid-insured (n =
802) |
|||
|---|---|---|---|---|
| Characteristics | Moderate/severe SLE (n = 5,551) | Mild SLE (n = 2,680) | Moderate/severe SLE (n = 600) | Mild SLE (n = 202) |
| Age, years, mean ± SD | 46.6 ± 10.6b,c | 47.7 ± 10.2b | 41.4 ± 12.4b,c | 46.5 ± 9.9b |
| Sex | ||||
| Female | 5,007 (90.2)d | 2,470 (92.2)d | 547 (91.2) | 185 (91.6) |
| Insurance plan type | ||||
| Comprehensive | 158 (2.9)c,d | 84 (3.1)c,d | 327 (54.5)c | 117 (57.9)c |
| PPO | 3,191 (57.5)c,d | 1,487 (55.5)c,d | 0 (0.0)c | 0 (0.0)c |
| HMO | 420 (7.6)c,d | 214 (8.0)c,d | 120 (20.0)c | 27 (13.4)c |
| POS | 783 (14.1)c,d | 345 (12.9)c,d | 153 (25.5)c | 58 (28.7)c |
| Other | 999 (18.0)c,d | 550 (20.5)c,d | 0 (0.0)c | 0 (0.0)c |
| Race | ||||
| White | - | - | 162 (27.0) | 73 (36.1) |
| Black | - | - | 348 (58.0) | 102 (50.5) |
| Hispanic | - | - | 19 (3.2) | 7 (3.5) |
| Other | - | - | 71 (11.8) | 20 (9.9) |
| Population density | ||||
| Urban | 4,809 (86.6) | 2,320 (86.6) | - | - |
| Rural | 730 (13.2) | 347 (13.0) | - | - |
| Unknown | 12 (0.2) | 13 (0.5) | - | - |
| Deyo-CCI, mean ± SD | 1.8 ± 1.4b,c | 1.5 ± 1.1b,c | 2.5 ± 2.0b,c | 2.4 ± 2.3b,c |
| Renal involvement | ||||
| Lupus nephritis | 1,121 (20.2)b,c | 216 (8.1)b,c | 205 (34.2)b,c | 38 (18.8)b,c |
| Kidney transplant | 37 (0.7)b | 1 (0.0)b | 4 (0.7) | 0 (0.0) |
| Comorbidities | ||||
| Avascular necrosis | 99 (1.8)c,d | 25 (0.9)d | 30 (5.0)c,d | 2 (1.0)d |
| Cancer | 288 (5.2) | 125 (4.7) | 36 (6.0) | 9 (4.5) |
| Cardiovascular disease | 739 (13.3)b,c | 276 (10.3)b,c | 219 (36.5)c | 79 (39.1)c |
| Cerebrovascular disease | 352 (6.3)c | 160 (6.0)c | 80 (13.3)c | 37 (18.3)c |
| Congestive heart failure | 252 (4.5)b,c | 69 (2.6)b,c | 124 (20.7)c | 35 (17.3)c |
| Myocardial infarction | 121 (2.2)c | 44 (1.6)c | 37 (6.2)c,d | 21 (10.4)c,d |
| Peripheral vascular disease | 154 (2.8)c | 57 (2.1)c | 45 (7.5)c | 17 (8.4)c |
| Cataracts | 394 (7.1) | 166 (6.2) | 47 (7.8) | 8 (4.0) |
| Type 2 diabetes | 572 (10.3)b,c | 185 (6.9)b,c | 168 (28.0)c | 55 (27.2)c |
| Fibromyalgia | 891 (16.1)c,d | 368 (13.7)c,d | 161 (26.8)c,d | 69 (34.2) c,d |
| Fractures | 308 (5.5)b | 86 (3.2)b,c | 37 (6.2) | 18 (8.9)c |
| Pathologic fractures | 37 (0.7)b | 2 (0.1)b | 4 (0.7) | 1 (0.5) |
| Traumatic fractures | 296 (5.3)b | 86 (3.2)b,c | 35 (5.8) | 18 (8.9)c |
| Glaucoma | 353 (6.4) | 142 (5.3)e | 30 (5.0) | 4 (2.0%)e |
| Hypertension | 2,324 (41.9)b,c | 910 (34.0)b,c | 425 (70.8)c | 149 (73.8)c |
| Osteoporosis | 467 (8.4)b | 132 (4.9)b | 54 (9.0) | 11 (5.4) |
| Stroke | 129 (2.3)c | 55 (2.1)c | 35 (5.8)c | 18 (8.9)c |
| Concomitant medications | ||||
| Antidepressants | 1,976 (35.6)c,d | 864 (32.2)c,d | 298 (49.7)c,d | 123 (60.9)c,d |
| Antihypertensives | 2,808 (50.6)b,c | 1,067 (39.8)b,c | 353 (58.8)c | 121 (59.9)c |
| Antidiabetic agents | 447 (8.1)b,c | 150 (5.6)b,c | 107 (17.8)c | 46 (22.8)c |
| Hormone replacement therapy | 733 (13.2) | 328 (12.2) | 63 (10.5) | 22 (10.9) |
| NSAIDs | 2,082 (37.5)c | 993 (37.1)c | 289 (48.2)c | 111 (55.0)c |
Values are N (%) unless otherwise noted. Statistical comparisons were performed (1) between the two severity groups in each payer group, (2) between the two payers for each severity. Only the differences reaching statistical significance (p<0.05) were displayed using specific superscripts.
p<0.001, moderate to severe versus mild SLE within either the commercially-insured or Medicaid population.
p<0.001, moderate/severe commercially-insured versus moderate/severe Medicaid or mild commercially insured versus mild Medicaid.
p<0.05, moderate to severe versus mild SLE within either the commercially-insured or Medicaid population.
p<0.05, mild commercially-insured versus mild Medicaid.
Abbreviations: CCI, Charlson comorbidity index; HMO, health maintenance organization; NSAIDs, non-steroidal anti-inflammatory drugs; POS, point of service; PPO, preferred provider organization; SD, standard deviation; SLE, systemic lupus erythematosus.
Among Medicaid-insured patients, a higher proportion had moderate/severe SLE, by our definition, than among commercially-insured patients (commercial: 67.4% versus Medicaid: 74.8%; p<0.001). Medicaid-insured patients with moderate/severe SLE were younger than those with mild SLE (41.4 versus 46.5 years old; p<0.001) and >90% of the SLE patients in both severity groups were women. The majority of the Medicaid-insured SLE patients were Black, and about a third were White.
Commercially-insured patients had significantly lower mean Deyo-CCI scores than their Medicaid-insured counterparts in both severity cohorts (moderate/severe: 1.8 versus 2.5; mild: 1.5 versus 2.4; both p<0.001)
Lupus nephritis was approximately twice as common during the baseline period among patients with moderate/severe than mild SLE in both insurance cohorts (commercial: 20.2% versus 8.1%; Medicaid: 34.2% versus 18.8%; both p<0.001). Furthermore, Medicaid-insured patients were more likely to be diagnosed with lupus nephritis than commercially-insured patients regardless of SLE severity (both p<0.001).
Most of the comorbid conditions during the baseline period occurred more frequently in the moderate/severe SLE group than the mild SLE group, among commercially-insured patients. In contrast, Medicaid-insured patients had few significant differences in the prevalence of baseline comorbidities between the two SLE severity groups. Among Medicaid-insured patients, avascular necrosis occurred more frequently in the moderate/severe SLE patients (p=0.012), whereas fibromyalgia and myocardial infarction occurred more frequently in the mild SLE patients (both p<0.05). Medicaid-insured patients were more likely to have evidence of cardiovascular disease, type 2 diabetes, hypertension, and stroke during the baseline period than the commercially-insured patients (for all p<0.001). Medicaid-insured patients were also more likely to have taken antidepressants, antihypertensives, antidiabetics, and NSAIDs than the commercially-insured patients (p<0.001).
3.2. SLE treatments
Table 2 presents SLE treatment during the exposure period. Commercially-insured patients were more likely than Medicaid-insured to have used an antimalarial among both the mild SLE patients (78.6% versus 36.6%; p<0.001) and moderate/severe SLE patients (62.2% versus 50.7%; p<0.001). While there was not a significant difference in the use of a biologic therapy between commercially-insured and Medicaid-insured moderate/severe patients, commercially-insured moderate/severe patients were significantly more likely to have used an immunosuppressant (51.5% versus 39.2%; p<0.001). In contrast, commercially-insured patients were significantly less likely to have used a systemic glucocorticoid than Medicaid-insured patients. While the pattern was evident in both severity cohorts, Medicaid-insured mild patients were almost three times as likely to have used a systemic glucocorticoid than their commercially-insured counterparts (commercial: 21.4% versus Medicaid: 63.4%; p<0.001).
Table 2.
SLE treatments during exposure perioda
| Commercially-insured (n = 8,231) | Medicaid-insured (n = 802) | |||
|---|---|---|---|---|
| Moderate/severe SLE (n = 5,551) | Mild SLE (n = 2,680) | Moderate/severe SLE (n = 600) | Mild SLE (n = 202) | |
| Antimalarials | 3,455 (62.2)b,c | 2,106 (78.6)b,c | 304 (50.7)b,c | 74 (36.6)b,c |
| Chloroquine | 28 (0.5) | 9 (0.3) | 4 (0.7) | 1 (0.5) |
| Hydroxychloroquine | 3,429 (61.8)b,c | 2,099 (78.3)b,c | 301 (50.2)b,c | 74 (36.6)b,c |
| Biologic therapies | 113 (2.0)b | 0 (0.0)b | 18 (3.0)d | 0 (0.0)d |
| Abatacept | 29 (0.5)b | 0 (0.0)b | 3 (0.5) | 0 (0.0) |
| Belimumab | 11 (0.2)d | 0 (0.0)d | 2 (0.3) | 0 (0.0) |
| Rituximab | 74 (1.3)b,e | 0 (0.0)b | 14 (2.3) d,e | 0 (0.0)d |
| Immunosuppressants | 2,858 (51.5)b,c | 0 (0.0)b | 235 (39.2)b,c | 0 (0.0)b |
| Azathioprine | 772 (13.9)b,e | 0 (0.0)b | 62 (10.3)b,e | 0 (0.0)b |
| Cyclophosphamide | 101 (1.8)b,e | 0 (0.0)b | 19 (3.2)d,e | 0 (0.0)d |
| Cyclosporine | 61 (1.1)b | 0 (0.0)b | 3 (0.5) | 0 (0.0) |
| IV immunoglobulin | 51 (0.9)b | 0 (0.0)b | 5 (0.8) | 0 (0.0) |
| Leflunomide | 143 (2.6)b,e | 0 (0.0)b | 4 (0.7)e | 0 (0.0) |
| Methotrexate | 904 (16.3)b,c | 0 (0.0)b | 52 (8.7)b,c | 0 (0.0)b |
| Mycophenolate mofetil | 1,052 (19.0)b | 0 (0.0)b | 110 (18.3)b | 0 (0.0)b |
| Tacrolimus | 125 (2.3)b | 0 (0.0)b | 10 (1.7) | 0 (0.0) |
| Systemic glucocorticoids | 4,773 (86.0)b,c | 574 (21.4)b,c | 562 (93.7)b,c | 128 (63.4)b,c |
| Prednisone | 3,481 (62.7)b,c | 380 (14.2)b,c | 450 (75.0)b,c | 94 (46.5)b,c |
| Prednisolone | 13 (0.2)e | 2 (0.1) | 6 (1.0)e | 1 (0.5) |
| Hydrocortisone | 84 (1.5)b | 3 (0.1)b | 15 (2.5)d | 0 (0.0)d |
| Oral methylprednisolone | 765 (13.8)b | 221 (8.2)b,c | 75 (12.5)d | 40 (19.8)c,d |
| IV methylprednisolone | 1,204 (21.7)b,e | 0 (0.0)b | 164 (27.3)b,e | 0 (0.0)b |
| Dexamethasone | 598 (10.8)b,c | 19 (0.7)b | 96 (16.0)b,c | 2 (1.0)b |
Values are N (%) assessed during the first six months following the index date (inclusive of the index date). Statistical comparisons were performed (1) between the two severity groups in each payer group, (2) between the two payers for each severity. Only the differences reaching statistical significance (p<0.05) were displayed using specific superscripts.
p<0.001, moderate/severe versus mild SLE within either the commercially-insured or Medicaid population.
p<0.001, moderate/severe commercially-insured versus moderate/severe Medicaid or mild commercially-insured versus mild Medicaid.
p<0.05, moderate/severe versus mild SLE within either the commercially-insured or Medicaid population.
p<0.05, moderate/severe commercially-insured versus moderate/severe Medicaid.
Abbreviations: IV, intravenous; SLE, systemic lupus erythematosus.
3.3. Healthcare utilization
Table 3 summarizes healthcare utilization in commercially- and Medicaid-insured populations during the follow-up period. There were significant differences in several utilization measures between mild and moderate/severe SLE patients within each insurance cohort. Among the commercially-insured patients, the average number of all-cause inpatient admissions and outpatient visits and services (ER, outpatient office visits, rheumatology outpatient visits, dialysis-related outpatient visits, physician-administered medications, and other outpatient services) per patient, was significantly higher for patients with moderate/severe SLE compared with mild SLE (all p<0.05). Individuals with moderate/severe SLE had a significantly longer average duration of stay per admission than those with mild SLE (4.4 versus 4.0 days; p<0.001). Patients with moderate/severe SLE also had significantly more outpatient pharmacy claims per person, both for all medications (75.3 versus 50.8; p<0.001) and specifically for SLE medications (16.0 versus 8.2; p<0.001).
Table 3.
Healthcare utilization during follow-up perioda
| Commercially-insured (n =
8,231) |
Medicaid-insured (n =
802) |
|||
|---|---|---|---|---|
| Moderate/severe SLE (n = 5,551) | Mild SLE (n = 2,680) | Moderate/severe SLE (n = 600) | Mild SLE (n = 202) | |
| Inpatient admissions | ||||
| Patients with an admission, N (%) | 1,434 (25.8) c,d | 395 (14.7)c,d | 342 (57.0)d | 112 (55.4)d |
| Admissions per patient | 0.5 ± 1.3c,d | 0.3 ± 0.9c,d | 1.8 ± 2.9d | 1.8 ± 3.1d |
| Length of stay per admission (all admissions) | 4.4 ± 4.1c,e | 4.0 ± 3.7c,d | 4.8 ± 3.9e | 5.2 ± 5.8d |
| Outpatient visits and services | ||||
| ER visits | ||||
| Patients with a visit, N (%) | 2,502 (45.1)c,d | 933 (34.8)c,d | 499 (83.2)d | 167 (82.7)d |
| Visits per patient | 1.3 ± 3.5c,d | 0.8 ± 1.9c,d | 6.5 ± 10.9d | 6.0 ± 13.3d |
| Outpatient office visits | ||||
| Patients with a visit, N (%) | 5,525 (99.5)e | 2,667 (99.5)d | 593 (98.8)e | 197 (97.5)d |
| Outpatient office visits per patient | 21.2 ± 13.9c | 15.2 ± 10.3c,d | 21.9 ± 14.7f | 18.4 ± 13.1d,f |
| Rheumatology outpatient visits | ||||
| Patients with a visit, N (%) | 4378 (78.9)d | 2153 (80.3)d | 123 (20.5)d,f | 22 (10.9)d,f |
| Visits per patient | 4.4 ± 4.0c,d | 3.0 ± 2.5c,d | 0.9 ± 2.1c,d | 0.3 ± 1.0c,d |
| Dialysis-related outpatient visits | ||||
| Patients with a visit, N (%) | 123 (2.2)d,f | 34 (1.3)f | 31 (5.2)d | 5 (2.5) |
| Visits per patient | 2.2 ± 23.1f | 1.0 ± 12.4f | 1.6 ± 10.3 | 0.7 ± 5.1 |
| Physician-administered medications | ||||
| Patients with a medication, N (%) | 2,486 (44.8)c | 347 (12.9)c | 284 (47.3)c | 35 (17.3)c |
| Medications per patient | 1.9 ± 5.0c | 0.2 ± 1.0c,e | 1.9 ± 4.5c | 0.4 ± 1.3 c,e |
| SLE medications per patientb | 0.0 ± 2.0d | 0.0 ± 0.0 | 1.0 ± 2.0c,d | 0.0 ± 0.0c |
| Other outpatient services (e.g., laboratory, radiology) | ||||
| Patients with other service, N (%) | 5,542 (99.8)f | 2,668 (99.6)f | 600 (100.0) | 202 (100.0) |
| Services per patient | 124.9 ± 120.9c,d | 80.5 ± 80.0c,d | 189.6 ± 170.6d | 179.1 ± 196.8d |
| Outpatient pharmacy | ||||
| Patients with a claim, N (%) | 5,475 (98.6)c,e | 2,680 (100.0)c | 600 (100.0)e | 202 (100.0) |
| Claims per patient | 75.3 ± 55.4c,d | 50.8 ± 41.5c,d | 135.3 ± 94.3d | 131.8 ± 89.8d |
| Claims for SLE medications per patient | 16.0 ± 12.0c | 8.2 ± 5.9c,d | 17.0 ± 13.5c | 6.0 ± 5.9 c,d |
Values are mean ± SD (calculated among all patients), unless otherwise noted. Statistical comparisons were performed (1) between the two severity groups in each payer group, (2) between the two payers for each severity. Only the differences reaching statistical significance (p<0.05) were displayed using specific superscripts.
Physician-administered SLE medications (outpatient) include antimalarials, selected biologics [abatacept, belimumab, and rituximab], immunosuppressants [azathioprine, cyclophosphamide, cyclosporine, intravenous immunoglobulin, leflunomide, methotrexate, mycophenolate mofetil/mycophenolic acid, and tacrolimus], and systemic glucocorticoids.
p<0.001, moderate/severe versus mild SLE within either the commercially-insured or Medicaid population.
p<0.001, moderate/severe commercially-insured versus moderate/severe Medicaid or mild commercially-insured versus mild Medicaid.
p<0.05, moderate/severe commercially-insured versus moderate/severe Medicaid or mild commercially-insured versus mild Medicaid.
p<0.05, moderate/severe versus mild SLE within either the commercially-insured or Medicaid population.
Abbreviations: ER, emergency room; SD, standard deviation; SLE, systemic lupus erythematosus.
Among the Medicaid-insured population, however, only mean number of outpatient office visits (21.9 versus 18.4; p=0.003), rheumatology outpatient visits (0.9 versus 0.3; p<0.001), and physician-administered medications (1.9 versus 0.4; p<0.001) were higher among those with moderate/severe SLE. The mean number of outpatient pharmacy claims was also comparable between patients with moderate/severe and those with mild SLE, but moderate/severe patients had more outpatient pharmacy claims for SLE medications (17.0 versus 6.0; p<0.001).
When comparing commercially-insured patients with Medicaid-insured patients of the same SLE severity, similar patterns emerge for the moderate/severe and mild patients. Commercially-insured patients had fewer inpatient admissions (moderate/severe: 0.5 versus 1.8; mild: 0.3 versus 1.8; both p<0.001), ER visits (moderate/severe: 1.3 versus 6.5; mild: 0.8 versus 6.0; both p<0.001), other outpatient services (moderate/severe: 124.9 versus 189.6; mild: 80.5 versus 179.1; both p<0.001), and outpatient pharmacy claims (moderate/severe: 75.3 versus 135.3; mild: 50.8 versus 131.8; both p<0.001) than Medicaid-insured SLE patients.
3.4. Healthcare costs
3.4.1. Unadjusted healthcare costs
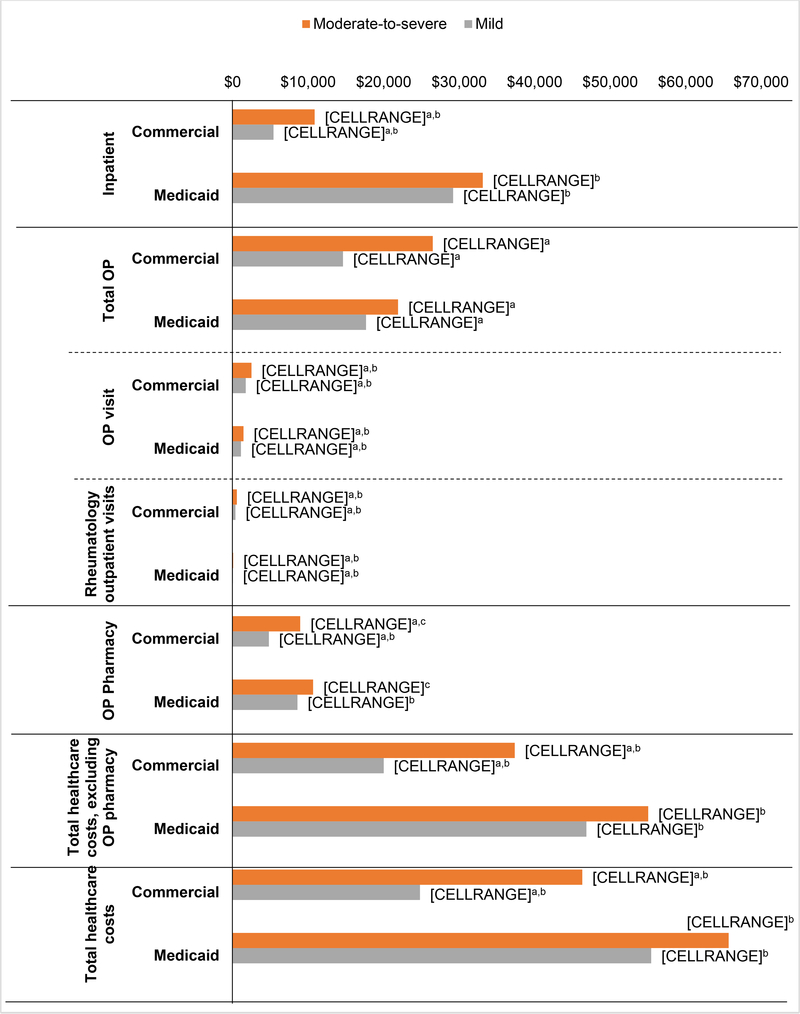
Figure 2 and Supplementary table 1 summarize the unadjusted healthcare costs of the study population during the 12-month follow-up period. Among commercially-insured patients, the unadjusted mean total healthcare costs, which included both medical and pharmacy costs, were significantly higher for patients with moderate/severe compared with patients with mild SLE ($46,302 versus $24,801; p<0.001). The payer portion of the total unadjusted healthcare costs was $42,554 and $22,113 respectively (p<0.001). The total healthcare costs during the follow-up period were still higher for patients with moderate/severe SLE than for mild SLE when excluding the outpatient pharmacy costs (total: $37,354 versus $20,010; payer portion: $34,889 versus $18,198; both p<0.001). The largest driver of higher costs among commercially-insured moderate/severe SLE patients were other outpatient services (moderate/severe: $18,292, 39.5% of total; mild: $10,877, 43.9% of total), which captured laboratory and radiology costs, followed by the costs of inpatient admissions (moderate/severe: $10,860, 23.5%; mild: $5,403, 21.8%) and outpatient pharmacy costs (moderate/severe: $8,948, 19.3%; mild: $4,791, 19.3%).
Fig 2:
Unadjusted healthcare costs during follow-up period.
Statistical comparisons were performed (1) between the two severity groups in each payer group, (2) between the two payers for each severity. Only the differences reaching statistical significance (p<0.05) were displayed using specific superscripts. Total outpatient cost is the sum of ER cost, outpatient visit, dialysis-related outpatient visit, physician-administered medications, and other outpatient services.
ap<0.001, moderate/severe versus mild SLE within either the commercially-insured or Medicaid population.
bp<0.001, moderate/severe commercially-insured versus moderate/severe Medicaid or mild commercially-insured versus mild Medicaid.
cp<0.05, moderate/severe commercially-insured versus moderate/severe Medicaid.
Abbreviations: ER, emergency room; OP, outpatient; SD, standard deviation.
Among patients with Medicaid insurance, the unadjusted mean total healthcare costs were not significantly different between the moderate/severe SLE patients (total: $65,687; payer portion: $65,431) and those with mild SLE (total: $55,427; payer portion: $55,225). The total healthcare costs excluding the outpatient pharmacy costs during the follow-up period were $55,031 for patients with moderate/severe SLE, of which the payer portion was $54,919, compared with $46,854 for patients with mild SLE, of which the payer portion was $46,786. The single largest driver of total costs was inpatient admission costs, which accounted for $33,123 (50.4%) of the total healthcare costs in patients with moderate/severe SLE and $29,195 (52.7%) for those with mild SLE. The only significant differences in costs between the Medicaid-insured moderate/severe SLE patients and mild SLE patients were in outpatient office visits ($1,437 versus $1,099; p<0.001) and rheumatology outpatient visits ($70 versus $27; p<0.001).
Commercially-insured patients had significantly lower unadjusted total healthcare costs than Medicaid-insured patients among both moderate/severe SLE patients ($46,302 versus $65,687) and those with mild SLE ($24,801 versus $55,427, both p<0.001). In both the SLE severity populations, costs of inpatient (moderate/severe: $10,860 versus $33,123; mild: $5,403 versus $29,125, both p<0.001), ER (moderate/severe: $1,324 versus $2,496, p=0.004; mild: $695 versus $2,444, p<0.001), and outpatient pharmacy services (moderate/severe: $8,948 versus $10,655, p=0.022; mild: $4,791 versus $8,573, p<0.001) were significantly lower in the commercially-insured cohorts than the Medicaid-insured cohorts, whereas outpatient visit costs (moderate/severe: $2,485 versus $1,437; mild: $1,729 versus $1,099, both p<0.001) and rheumatology outpatient visit costs (moderate/severe: $551 versus $70; mild: $359 versus $27, both p<0.001) were significantly higher in the commercially-insured cohorts as compared to the Medicaid-insured cohorts.
3.4.2. Multivariable-adjusted healthcare costs
Table 4 summarizes the adjusted total healthcare costs, with and without outpatient pharmacy costs included, of the study population during the 12-month follow-up period (full regression results in Supplemental Tables 2–5). In the commercially-insured cohort, the mean adjusted total all-cause healthcare costs incurred over 12 months of follow-up for patients with moderate/severe SLE were $19,244 (95% CI: $16,933 to $21,675) higher than those for patients with mild SLE (p<0.0001). When excluding pharmacy costs, adjusted healthcare costs during the follow-up period were $15,502 (95% CI: $13,392 to $17,734) higher among moderate/severe SLE patients as compared to mild SLE patients within the commercially-insured population (p<0.0001).
Table 4.
Adjusted healthcare costs during follow-up perioda
| Group | Mean | Difference in means | Lower 95% CI | Upper 95% CI | P value |
|---|---|---|---|---|---|
| Healthcare costs | |||||
| Commercially-insured | |||||
| Mild SLE | $28,298 | –$19,244 | –$16,933 | –$21,675 | <0.0001 |
| Moderate/severe SLE | $47,542 | ||||
| Medicaid-insured | |||||
| Mild SLE | $53,329 | –$13,605 | –$412 | –$30,041 | 0.04 |
| Moderate/severe SLE | $66,935 | ||||
| Healthcare costs excluding pharmacy costs | |||||
| Commercially-insured | |||||
| Mild SLE | $23,519 | –$15,502 | –$13,392 | –$17,734 | <0.0001 |
| Moderate/severe SLE | $39,021 | ||||
| Medicaid-insured | |||||
| Mild SLE | $44,932 | –$11,118 | $536 | –$25,832 | 0.06 |
| Moderate/severe SLE | $56,050 | ||||
Values are derived from the models adjusted for age, sex, health plan type, urbanicity (Commercial only), race (Medicaid only), and mean baseline Deyo-CCI.
Abbreviations: CCI, Charlson Comorbidity Index; CI, confidence interval; SLE, systemic lupus erythematosus.
Among the Medicaid-insured patients, the mean adjusted total healthcare costs incurred over 12 months of follow-up for patients with moderate/severe SLE were $13,605 (95% CI: $412 to $30,041; p=0.04) higher than those for patients with mild SLE. While differences in adjusted healthcare costs, excluding pharmacy, during follow-up did not reach statistical significance (p=0.06), they were $11,118 (95% CI: -$536 to $25,832) higher among moderate/severe compared to mild SLE patients within the Medicaid-insured population.
4. DISCUSSION
This multi-payer analysis demonstrates that adjusted annual healthcare costs, excluding pharmacy, were $15,502 and $11,118 higher for patients with moderate/severe SLE than those with mild SLE among the commercially-insured and Medicaid-insured, respectively. This difference in costs was statistically significant among the commercially-insured but did not reach statistical significance among Medicaid-insured patients. The statistical comparison between severity cohorts in the Medicaid-insured patients was limited by the small cohort sizes, which were approximately a tenth of the commercially-insured cohorts and had they been larger, a significant difference may have been observed. Additionally, the narrower cost difference between SLE severity cohorts in the Medicaid-insured population is potentially because the use of treatment intensity as a proxy for disease severity is imperfect. Although, in general, SLE patients with more mild disease tend to be on monotherapy with antimalarials or low-dose glucocorticoids, use of medications to define disease severity may have resulted in misclassification in cases where individuals with severe disease were undertreated or those with mild disease were treated aggressively. It is likely that the risk of misclassification by disease severity is higher in the Medicaid-insured population where poor access or lower quality of care may result in greater undertreatment.
The current analysis also adds a direct comparison between commercial and Medicaid insurance populations of SLE patients with similar severity. Due to the fundamental and unresolvable differences in demographic data availability, patient characteristics, healthcare delivery systems, and payment models between insurance populations, univariable, rather than multivariable, comparisons were made within like severity groups. Furthermore, as the differences between these insurance groups are also fundamental to understanding their total costs, adjusting for these differences would not provide a useful interpretation of the data.
Healthcare utilization and unadjusted costs were much higher for Medicaid-insured patients than for commercially-insured patients with similar SLE severity. When comparing Medicaid-insured patients to commercially-insured patients, the mean 12-month unadjusted total costs were 123.5% higher for patients with mild SLE and 41.9% higher for patients with moderate/severe SLE. Cost differences of this scale are important in planning for future healthcare expenditures and setting priorities for allocating healthcare resources. There are several potential reasons for the higher costs in the Medicaid patients, including disease heterogeneity resulting in greater disease burden and the striking differences in SLE treatments received by the two insurance populations. Commercially-insured patients with mild SLE were approximately three times as likely to be on antimalarial monotherapy as on low dose systemic glucocorticoids. In the Medicaid-insured cohort, however, mild SLE patients were twice as likely to be receiving low dose systemic glucocorticoids as antimalarial monotherapy. Given the protective effects of hydroxychloroquine and the deleterious effects of long-term glucocorticoid use (30, 31), these findings suggest gaps in care for the Medicaid-insured population.
We also found a notable difference between the sources of healthcare costs between the Medicaid- and commercially-insured populations. Inpatient admissions were the largest single driver of costs in the Medicaid-insured population, in both the mild and moderate/severe SLE patient groups, while outpatient costs were the largest drivers among the commercially-insured patients. Medicaid-insured patients were more than twice as likely to have an inpatient admission and had at least three times more inpatient admissions per patient, resulting in hospitalization costs at least three times higher than commercially-insured patients. Differences in inpatient costs are particularly striking considering commercial insurers pay an estimated 75% more than Medicaid for similar inpatient stays (32), although a comparison of costs for similar inpatient stays between the two insurers was not performed in this study. Overall, commercially-insured patients had lower resource utilization and lower costs; one exception to this was that approximately 80% of commercially-insured patients saw a rheumatologist, as compared with only 10%–20% of Medicaid-insured patients. Eligibility and benefits for Medicaid, the US Federal-state jointly administered healthcare insurance for the poor, vary substantially state-to-state. Past studies have highlighted suboptimal healthcare and poor outcomes among SLE patients insured with Medicaid (33–36). The current study re-demonstrates that Medicaid-insured SLE patients have a high comorbidity burden and are potentially receiving substandard care; they receive lower than expected antimalarial prescriptions and fewer rheumatology visits, while they have high costs, driven in large part by inpatient admissions. Previous studies have shown that low adherence to medications in the Medicaid-insured population results in higher subsequent healthcare utilization (34). This study also found that among Medicaid patients, moderate/severe SLE patients were on average younger than mild SLE patients, likely as a result of Medicaid’s skew towards younger patients, who are more likely to have childhood or early adult-onset SLE that has not entered remission. The relative severity of childhood and early adult-onset SLE results in disability, continued enrolment in Medicaid, and further enrichment of young moderate/severe SLE patients. Greater disease burden, poorer access to care, lower quality of care, and social determinants of health that contribute to low medication adherence and more acute care utilization are all possible drivers of higher costs in the Medicaid-insured population.
These data add to a growing body of work describing the high economic burden associated with SLE(15, 16, 18, 19, 24, 37) and provide contemporary estimates of costs for economic evaluations in this condition. In a 2004–2008 study of commercially-insured patients’ insurance claims, the unadjusted annualized medical costs were $14,945, $21,606, and $64,578 (Medical-care inflation adjusted to 2016 USD: $19,041, $27,527, and $82,275) for SLE patients with mild, moderate, and severe flares, respectively, during follow-up (17). Similar findings were reported by other studies conducted in a cohort of US managed care patients with SLE (18), and among patients with Medicaid health coverage (15, 24). In a past study of Medicaid-insured patients (1999–2005), the mean annual medical costs for newly diagnosed SLE patients during their first year of treatment was $16,089 (2016: $16,089), which steadily increased to $23,860 (2016: $34,227) by year 5 (24). In a study of Medicaid-insured patients from 2002 to 2009, Kan et al. reported that the annual costs of SLE patients exceeded those of matched controls without SLE by $10,984 (2016: $13,560), with costs per flare ranging from $129 (2016: $159) for a mild flare to $11,716 (2016: $14,464) for a severe flare (15). The results from this current study, which are higher than previous estimates in the same populations, expand upon and update these prior analyses in light of changes in healthcare costs during the past decade.
The current study has limitations that merit consideration. First, as previously mentioned, our use of treatment intensity as a proxy for disease severity may be imperfect, with a greater likelihood of misclassification in the Medicaid cohort. While Garris et al developed an algorithm to classify SLE disease severity using administrative claims data (18), it is limited as accurate ascertainment of SLE activity and damage (38), disease characteristics that influence severity, require clinical data that are not available in claims databases. Second, we compared claims data for commercially-insured and Medicaid-insured SLE patients, but they may have differing underlying SLE manifestations, as this information is not available in the claims data employed. The data available for this analysis under the terms of the standard de-identified data use agreement did not include non-SLE comparison patients, provider information, race/ethnicity or region of residence, precluding comparisons involving these variables. Third, the much smaller sample size of the Medicaid-insured cohort potentially limited our ability to observe significant differences between the Medicaid-insured severity cohorts. Further, the potential for misclassification of SLE status/treatments, covariables, or study outcomes is present, as patients were identified using de-identified administrative claims data, which are subject to data coding limitations and data entry error. Additionally, in the Medicaid analysis a small portion of healthcare claims could be missing among patients dually enrolled in Medicare. However, as only five patients were dually eligible for both Medicare and Medicaid and only the small subset of their costs that were paid entirely by Medicare would be missing, the impact of this missingness is very small. Finally, results of the analyses may not be generalizable to SLE patients with other types of coverage (e.g. Medicare or Veterans Administration insurance), or to the uninsured, newly diagnosed, or untreated SLE patients.
5. CONCLUSIONS
In summary, the findings of the current study suggest that SLE imposes substantial resource and cost burdens. Healthcare utilization and costs were influenced by disease severity, with considerably higher costs incurred by patients with moderate/severe versus mild SLE among the commercially-insured. Furthermore, costs were also influenced by type of insurance with higher costs observed among the Medicaid versus commercially-insured for patients with similar severity. Costs, such as those estimated here, should be tracked as they are an important component of evaluating new interventions for SLE that aim to improve outcomes and reduce economic burden.
Supplementary Material
8. ACKNOWLEDGMENTS
This study was funded by AstraZeneca. Medical writing support was provided by Santosh Tiwari, Ph.D and Swathi Malla, M.Pharm. of IBM Watson Health, in accordance with Good Publication Practice guidelines (www.ismpp.org/gpp3) and funded by AstraZeneca.
6. DECLARATION OF FUNDING
This study was funded by AstraZeneca and conducted by IBM Watson Health (formerly Truven Health Analytics). Dr. Costenbader is supported by NIH K24 AR066109.
7. DISCLOSURES OF INTEREST
Dr. A.E. Clarke has received consulting fees from AstraZeneca, Bristol-Myers Squibb, and Exagen Diagnostics. Dr. J. Yazdany has received consulting fees from AstraZeneca and has received an investigator-initiated research grant from Pfizer. Dr. K. Costenbader has received consulting fees from AstraZeneca, Merck & Co, and GlaxoSmithKline. Dr. S. Kabadi, is an employee of AstraZeneca. Dr. K. Griffing was employed by AstraZeneca at the time this study was conducted. I. Winer is an employee of IBM Watson Health, which was compensated by AstraZeneca for conducting this research. Dr. E. Durden was employed by IBM Watson Health at the time this study was conducted.
Abbreviations
- CI
confidence interval
- Deyo-CCI
Deyo-Charlson Comorbidity Index
- ER
emergency room
- ICD-9/10-CM
International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification
- NSAIDs
non-steroidal anti-inflammatory drugs
- PPO
preferred provider organization
- SLE
systemic lupus erythematosus
- SD
standard deviation
9. REFERENCES
- 1.Garris C, Shah M, Farrelly E. The prevalence and burden of systemic lupus erythematosus in a medicare population: retrospective analysis of medicare claims. Cost Eff Resour Alloc. 2015;13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joseph FG, Scolding NJ. Neurolupus. Pract Neurol. 2010;10(1):4–15. [DOI] [PubMed] [Google Scholar]
- 3.Palatinus A, Adams M. Thrombosis in systemic lupus erythematosus. Semin Thromb Hemost. 2009;35(7):621–9. [DOI] [PubMed] [Google Scholar]
- 4.Skamra C, Ramsey-Goldman R. Management of cardiovascular complications in systemic lupus erythematosus. Int J Clin Rheumtol. 2010;5(1):75–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD. Manifestations of systemic lupus erythematosus. Maedica. 2011;6(4):330. [PMC free article] [PubMed] [Google Scholar]
- 6.Urowitz MB, Feletar M, Bruce IN, Ibanez D, Gladman DD. Prolonged remission in systemic lupus erythematosus. J Rheumatol. 2005;32(8):1467–72. [PubMed] [Google Scholar]
- 7.Györi N, Giannakou I, Chatzidionysiou K, Magder L, Van Vollenhoven RF, Petri M. Disease activity patterns over time in patients with SLE: analysis of the Hopkins Lupus Cohort. Lupus science & medicine. 2017;4(1):e000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Somers EC, Marder W, Cagnoli P, Lewis EE, DeGuire P, Gordon C, et al. Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis & rheumatology. 2014;66(2):369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis and rheumatism. 2008;58(1):15–25. [DOI] [PubMed] [Google Scholar]
- 10.Meacock R, Dale N, Harrison MJ. The humanistic and economic burden of systemic lupus erythematosus : a systematic review. Pharmacoecon. 2013;31(1):49–61. [DOI] [PubMed] [Google Scholar]
- 11.Panopalis P, Clarke AE, Yelin E. The economic burden of systemic lupus erythematosus. Best Pract Res Clin Rheumatol. 2012;26(5):695–704. [DOI] [PubMed] [Google Scholar]
- 12.Slawsky KA, Fernandes AW, Fusfeld L, Manzi S, Goss TF. A structured literature review of the direct costs of adult systemic lupus erythematosus in the US. Arthritis Care Res (Hoboken). 2011;63(9):1224–32. [DOI] [PubMed] [Google Scholar]
- 13.Turchetti G, Yazdany J, Palla I, Yelin E, Mosca M. Systemic lupus erythematosus and the economic perspective: a systematic literature review and points to consider. Clin Exp Rheumatol. 2012;30(4 Suppl 73):S116–22. [PMC free article] [PubMed] [Google Scholar]
- 14.Carls G, Li T, Panopalis P, Wang S, Mell AG, Gibson TB, et al. Direct and indirect costs to employers of patients with systemic lupus erythematosus with and without nephritis. J Occup Environ Med. 2009;51(1):66–79. [DOI] [PubMed] [Google Scholar]
- 15.Kan HJ, Song X, Johnson BH, Bechtel B, O’Sullivan D, Molta CT. Healthcare utilization and costs of systemic lupus erythematosus in Medicaid. Biomed Res Int. 2013;2013:808391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narayanan S, Wilson K, Ogelsby A, Juneau P, Durden E. Economic burden of systemic lupus erythematosus flares and comorbidities in a commercially insured population in the United States. J Occup Environ Med. 2013;55(11):1262–70. [DOI] [PubMed] [Google Scholar]
- 17.Oglesby A, Durden E, Narayanan S, Juneau P, Wilson K. Economic burden of systemic lupus erythematosus (SLE) in a commercially insured population in the United States. Value in Health. 2013;16(3):A126. [DOI] [PubMed] [Google Scholar]
- 18.Garris C, Jhingran P, Bass D, Engel-Nitz NM, Riedel A, Dennis G. Healthcare utilization and cost of systemic lupus erythematosus in a US managed care health plan. J Med Econ. 2013;16(5):667–77. [DOI] [PubMed] [Google Scholar]
- 19.Clarke AE, Urowitz MB, Monga N, Hanly JG. Costs associated with severe and nonsevere systemic lupus erythematosus in Canada. Arthritis Care Res (Hoboken). 2015;67(3):431–6. [DOI] [PubMed] [Google Scholar]
- 20.Doria A, Amoura Z, Cervera R, Khamastha MA, Schneider M, Richter J, et al. Annual direct medical cost of active systemic lupus erythematosus in five European countries. Ann Rheum Dis. 2014;73(1):154–60. [DOI] [PubMed] [Google Scholar]
- 21.Dall’Era M, Cisternas MG, Snipes K, Herrinton LJ, Gordon C, Helmick CG. The Incidence and Prevalence of Systemic Lupus Erythematosus in San Francisco County, California: The California Lupus Surveillance Project. Arthritis Rheumatol. 2017;69(10):1996–2005. [DOI] [PubMed] [Google Scholar]
- 22.Izmirly PM, Wan I, Sahl S, Buyon JP, Belmont HM, Salmon JE, et al. The Incidence and Prevalence of Systemic Lupus Erythematosus in New York County (Manhattan), New York: The Manhattan Lupus Surveillance Program. Arthritis Rheumatol. 2017;69(10):2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldman CH, Hiraki LT, Liu J, Fischer MA, Solomon DH, Alarcon GS, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000–2004. Arthritis and rheumatism. 2013;65(3):753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li T, Carls GS, Panopalis P, Wang S, Gibson TB, Goetzel RZ. Long-term medical costs and resource utilization in systemic lupus erythematosus and lupus nephritis: a five-year analysis of a large medicaid population. Arthritis and rheumatism. 2009;61(6):755–63. [DOI] [PubMed] [Google Scholar]
- 25.Bernatsky S, Joseph L, Pineau C, Tamblyn R, Feldman D, Clarke A. A population-based assessment of systemic lupus erythematosus incidence and prevalence—results and implications of using administrative data for epidemiological studies. Rheumatology. 2007;46(12):1814–8. [DOI] [PubMed] [Google Scholar]
- 26.Hahn BH, Mcmahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis care & research. 2012;64(6):797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moores KG, Sathe NA. A systematic review of validated methods for identifying systemic lupus erythematosus (SLE) using administrative or claims data. Vaccine. 2013;31:K62–K73. [DOI] [PubMed] [Google Scholar]
- 28.Consumer Price Index. U.S. Bureau of Labor Statistics; 2016. [Google Scholar]
- 29.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. [DOI] [PubMed] [Google Scholar]
- 30.Apostolopoulos D, Morand EF. It hasn’t gone away: the problem of glucocorticoid use in lupus remains. Rheumatol. 2017;56(suppl_1):i114–i22. [DOI] [PubMed] [Google Scholar]
- 31.Floris A, Piga M, Mangoni AA, Bortoluzzi A, Erre GL, Cauli A. Protective effects of hydroxychloroquine against accelerated atherosclerosis in systemic lupus erythematosus. Mediators Inflamm. 2018;2018:3424136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selden TM, Karaca Z, Keenan P, White C, Kronick R. The growing difference between public and private payment rates for inpatient hospital care. Health Affairs. 2015;34(12):2147–50. [DOI] [PubMed] [Google Scholar]
- 33.Chen SK, Barbhaiya M, Fischer MA, Guan H, Lin TC, Feldman CH, et al. Lipid testing and statin prescription among Medicaid recipients with systemic lupus erythematosus, diabetes mellitus and the general Medicaid population. Arthritis Care Res (Hoboken). 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feldman CH, Yazdany J, Guan H, Solomon DH, Costenbader KH. Medication nonadherence is associated with increased subsequent acute care utilization among medicaid beneficiaries with systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2015;67(12):1712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin TC, Marmor MF, Barbhaiya M, Guan H, Chen SK, Feldman CH, et al. Baseline retinal examinations among SLE patients newly initiating hydroxychloroquine in a U.S. Medicaid SLE Population, 2000–2010. Arthritis Care Res (Hoboken). 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yazdany J, Feldman CH, Liu J, Ward MM, Fischer MA, Costenbader KH. Quality of care for incident lupus nephritis among Medicaid beneficiaries in the United States. Arthritis Care Res (Hoboken). 2014;66(4):617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furst DE, Clarke A, Fernandes AW, Bancroft T, Gajria K, Greth W, et al. Resource utilization and direct medical costs in adult systemic lupus erythematosus patients from a commercially insured population. Lupus. 2013;22(3):268–78. [DOI] [PubMed] [Google Scholar]
- 38.Romero-Diaz J, Isenberg D, Ramsey-Goldman R. Measures of adult systemic lupus erythematosus: updated version of British Isles Lupus Assessment Group (BILAG 2004), European Consensus Lupus Activity Measurements (ECLAM), Systemic Lupus Activity Measure, Revised (SLAM-R), Systemic Lupus Activity Questionnaire for Population Studies (SLAQ), Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), and Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.