Abstract
Removal of chloroquine from national malaria formularies can lead to the reversion of resistant Plasmodium falciparum to wild-type. We report a steep decline in chloroquine-resistant P falciparum within 10 years of national discontinuation of chloroquine monotherapy in Zimbabwe. Drug resistance surveillance is a vital component of malaria control programs, and the experience with chloroquine in Zimbabwe and elsewhere in sub-Saharan Africa is illustrative of the potentially rapid and dramatic impact of drug policy on antimalarial resistance.
Keywords: chloroquine, drug resistance, malaria, Plasmodium falciparum, Zimbabwe
Within 10 years after national discontinuation of chloroquine monotherapy for Plasmodium falciparum malaria in Zimbabwe, the frequency of chloroquine resistance declined from 67% to 3% and was undetected 5 years later.
Chloroquine (CQ) resistance was first described in Plasmodium falciparum, a causative agent of malaria, in 1961 [1]. The following decade, it appeared in sub-Saharan Africa and expanded rapidly with enormous public health consequences. By the turn of the century, CQ monotherapy was no longer tenable for falciparum malaria on the continent, and national programs replaced it with multidrug regimens, foremost artemisinin-based combination therapies (ACTs). Within years of retiring CQ, reports emerged of the re-establishment of susceptible parasites [2], although in a heterogeneous distribution at varying rates that likely reflected differences in malaria endemicity, vector populations, choice of first-line ACT (eg, amodiaquine, part of the combination artesunate-amodiaquine, is a structural relative of CQ), or CQ continuation that is unsanctioned or on account of coendemic vivax malaria [3, 4].
Plasmodium falciparum resistance to CQ is via drug efflux from the parasite’s digestive vacuole, mediated in African strains by the K76T mutation in the chloroquine resistance transporter gene (pfcrt) [5]. Molecular surveillance of CQ resistance relies on detection of pfcrt mutants [6].
In 2003, owing to escalating CQ resistance [7], Zimbabwe replaced CQ monotherapy first with the combination CQ-sulfadoxine/pyrimethamine and then in 2008 with artemether-lumefantrine (an ACT). Chloroquine was withdrawn elsewhere in sub-Saharan Africa in similar time frames, and nearby countries Zambia, Mozambique, Malawi, and Uganda have since documented widespread reversion to pfcrt wild-type, although these studies were generally limited by small sample size and geographic range with no truly systematic assessment of CQ resistance in the full parasite population [2, 8–10]. In contrast, countries in west Africa generally find sustained prevalence of mutant pfcrt as long as 8–9 years on average after CQ cessation [11].
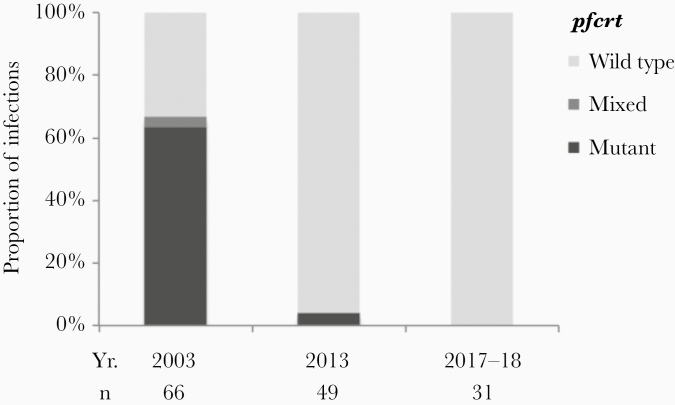
We genotyped pfcrt in P falciparum parasites collected over the course of community-based malaria surveys in 2003, 2013, and 2017–2018 to investigate the shift over time in K76T allele frequencies. Existing literature documented high prevalence of pfcrt K76T-resistant mutants in historical Zimbabwean samples or returned travelers to Zimbabwe over 20 years ago [12]. To the best of our knowledge, this is the first published report of the re-establishment in Zimbabwe of CQ-susceptible P falciparum.
METHODS
The study was conducted in the lowlands of Mutasa District, a mountainous area in eastern Zimbabwe with unstable and epidemic malaria transmission. The Mutasa District was selected from a panel of 5 districts in Manicaland Province that together contributed more than half of the total burden of malaria in Zimbabwe. Peak malaria transmission season is January to May. Three community-based, cross-sectional surveys were carried out each month from January to May 2003 (n = 408), every other month from January to November 2013 (n = 373), and over 2 years every other month from January to December 2017–2018 (n = 767) as previously described [13]. In brief, households in the study area were enumerated using Quickbird satellite images acquired from DigitalGlobe Services Inc. (Denver, CO) for random selection from sampling grids in ArcGIS 9.2 (Redlands, CA). Adults and children of randomly selected households who provided informed consent, or whose legal guardians provided informed consent, were included. Blood microscopy (all years) and rapid diagnostic test (2013 and 2017–2018 surveys; Paracheck-Pf; Orchid Biomedical Systems, dramapur, India) were performed. Participants with positive results were referred to the local health facility for treatment according to national guidelines. For molecular testing, finger prick capillary blood was collected on Whatman 903 filter paper, dried overnight, and stored with desiccant at −20°C.
Plasmodium falciparum deoxyribonucleic acid (DNA) was extracted from dried blood spots on filter paper by the Chelex method. Plasmodium falciparum DNA isolates were genotyped at the CQ resistance-conferring pfcrt codon 76 using nested polymerase chain reaction (PCR) and restriction enzyme digestion [6]. Laboratory assays were performed at the National Institute of Health Research in Harare and Africa University in Mutare. Proportions and their 95% confidence intervals (CIs) were calculated. Data were compared using the χ 2 test (infection prevalence) or Fisher’s exact test (pfcrt genotype) across all sampling periods for an overall comparison and between sampling periods for pairwise comparisons in Stata 14.0 (StataCorp LLC, College Station, TX).
The 2003 cross-sectional study was approved by the Medical Research Council of Zimbabwe (MRCZ). The 2013 and 2017–2018 studies were approved by MRCZ and The Johns Hopkins Bloomberg School of Public Health Institutional Review Board as part of the Southern and Central Africa International Centers of Excellence for Malaria Research.
RESULTS
Participants across the 3 surveys were similar in terms of sex and age distribution, except a slight majority were female in the latest survey (Table 1). In 2003, 16% (66 of 408) had PCR-detected parasitemia compared to 13% (49 of 373) in 2013 and 4% (31 of 767) in 2017–2018. Parasite prevalence was significantly lower in 2017–2018 compared to 2003 and 2013 but not significantly different in 2013 compared to 2003. The frequency of pfcrt K76T alleles (mixed plus mutant) in 2003 was 67% (95% CI, 55%–77%) compared to 3% (95% CI, 1.1%–14%) in 2013 and 0% (95% CI, 0.0%–7.3%) in 2017–2018 (Figure 1). All others were wild-type.
Table 1.
Demographics of Study Participantsa
| Characteristic | 2003, n = 408 | 2013, n = 373 | 2017–2018, n = 767 |
|---|---|---|---|
| Female sex | 214 (52) | 191 (51) | 445 (58) |
| Age (Years) | |||
| <5 | 73 (18) | 51 (14) | 127 (17) |
| 5–20 | 119 (29) | 140 (38) | 248 (32) |
| 21–40 | 138 (34) | 112 (30) | 217 (28) |
| 41–60 | 61 (15) | 45 (12) | 109 (14) |
| 61–80 | 15 (4) | 21 (6) | 58 (8) |
| >80 | 2 (<1) | 4 (1) | 8 (1) |
| Parasite prevalence | 66 (16) | 49 (13) | 31 (4) |
aData are numbers (%).
Figure 1.
Comparisons of cross-sectional surveys conducted in 2003, 2013, and 2017–2018 in Mutasa District, Zimbabwe identified reversion of Plasmodium falciparum to the pfcrt wild-type with the withdrawal of chloroquine monotherapy in 2003.
DISCUSSION
We conducted molecular surveillance of CQ-resistant P falciparum in Zimbabwe after national discontinuation of CQ monotherapy and found dramatic reversion to the wild-type within a 10-year window. Zimbabwe joins its southern and eastern African neighbors in documenting the return of CQ-susceptible P falciparum within its borders, most similar in scale to that seen in Malawi and Uganda [2, 9]. This contrasts starkly with west Africa where, despite phasing out CQ in the early 2000s, pfcrt mutants remain prevalent: 56% in Cameroon (2012), 35% in Nigeria (2015–2016), and 45%–64% in Mali (2016) [11].
Our earlier work in Zimbabwe identified a return to some communities of CQ efficacy over the period 1995–2003, predating the withdrawal of CQ [14]. Communities that underwent indoor residual spraying (IRS) had lower odds of CQ treatment failure than those without IRS, hinting that the repopulation of CQ-sensitive parasites is driven in part by factors independent of selective drug pressure [14]. Differences in the rates of re-establishment of CQ sensitivity across Africa speak to this point as well. The interplay among drug pressure (including the deselective pressure of lumefantrine), altered parasite fitness, complexity of infections, human host premunition, and differential transit through different vector species could dictate the spatial patterns and pace of CQ resensitization [3, 4]. Understanding these general principles is crucial to developing successful drug containment strategies as the world now wrestles with the spread of ACT resistance.
In this regard, the experience of CQ provides important historical context. Similar to the artemisinin derivatives in use today, CQ was initially prized for its presumed high barrier to resistance given its broad mechanism of action [15]. For decades, it was deployed in malaria control and global eradication efforts. The earliest clinical confirmation of CQ-resistant malaria was in 2 nonimmune travelers to an endemic area [1], auguring a march of resistant parasites across continents. Three years ago, similar reports were made of returned travelers to Africa who failed treatment with artemether-lumefantrine, the ACT in widest use today [16].
The practicable relevance of the return of CQ-sensitive P falciparum is less clear. Should CQ be reinstated in select areas for certain indications? Does the cumulative evidence foster an argument for a “crop field” rotation approach to antimalarial formularies? Sustained efficacy in sub-Saharan Africa of well tolerated and more potent alternatives to CQ, a lately robust development pipeline for new classes of antimalarials, and concerns for cross-resistance to structural relatives of CQ suggest the answers are currently no. However, its potential role in multidrug regimens might be reasonably explored in regions where resistance is no longer found.
There were limitations to this study. The design provided little temporal granularity, and during the interval 2003–2013 there was a period of CQ combined with antifolate antimalarials, insecticide-treated bed net distributions, ongoing IRS campaigns, and possibly other unmeasured factors that may have influenced the tempo of the re-establishment of CQ-sensitive parasites. The sample size for the latest study period, during which no resistant parasites were identified, may have been insufficient to detect resistant parasites circulating in small numbers. Our results pertain to a limited geographic area, and they may not hold for elsewhere in Zimbabwe, although they are consistent with prior observations made across sub-Saharan Africa that suggest a contiguous map of CQ sensitivity regionally and a continuum continentally.
CONCLUSIONS
The return of CQ-susceptible P falciparum to Zimbabwe has precedent in nearby Malawi and elsewhere, but it was not foreordained. Knowledge garnered from approximately 9 decades of experience with CQ might prove valuable to current efforts against the spread of multidrug-resistant malaria.
Notes
Acknowledgments. We thank the study participants for their generous cooperation and the field team for conducting community mobilization and data collection. We also thank the Biomedical Research and Training Institute for facilitating sample collections and the National Institute of Health Research for their laboratory support.
Author contributions. S. Mh. conceived the study, supervised data acquisition, performed data analysis and interpretation, and prepared the first draft of the manuscript. Z. M.-Z. performed the laboratory assays, data analysis, and contributed to the manuscript. N. Mu., C. M., T. X. G., A. M., and G. M. assisted with laboratory assays, data entry and processing, and critically reviewed the manuscript. S. Mu., L. G., S. L. M., and P. M. were involved in study conceptualization and design, data interpretation, and manuscript preparation. T. K. and N. M. provided laboratory oversight and manuscript review. W. J. M., program director of the Southern and Central Africa International Centers of Excellence for Malaria Research, contributed to overall study design, data interpretation, and manuscript review. M. M. I. supervised data analysis and data interpretation and prepared the manuscript.
Financial support. This work was funded by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health as part of the International Centers of Excellence for Malaria Research (U19AI089680) and the Johns Hopkins Malaria Research Institute. M. M. I. is supported by the National Institutes of Health (K23AI139343), the Sherrilyn and Ken Fisher Center for Environmental Infectious Disease at Johns Hopkins University, and the Burroughs Wellcome Fund-American Society of Tropical Medicine and Hygiene Postdoctoral Fellowship in Tropical Infectious Diseases.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Moore DV, Lanier JE. Observations on two Plasmodium falciparum infections with an abnormal response to chloroquine. Am J Trop Med Hyg 1961; 10:5–9. [DOI] [PubMed] [Google Scholar]
- 2. Kublin JG, Cortese JF, Njunju EM, et al. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J Infect Dis 2003; 187:1870–5. [DOI] [PubMed] [Google Scholar]
- 3. Mharakurwa S, Sialumano M, Liu K, Scott A, Thuma P. Selection for chloroquine-sensitive Plasmodium falciparum by wild Anopheles arabiensis in Southern Zambia. Malar J 2013; 12:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sisowath C, Petersen I, Veiga MI, et al. In vivo selection of Plasmodium falciparum parasites carrying the chloroquine-susceptible pfcrt K76 allele after treatment with artemether-lumefantrine in Africa. J Infect Dis 2009; 199:750–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krogstad DJ, Gluzman IY, Kyle DE, et al. Efflux of chloroquine from Plasmodium falciparum: mechanism of chloroquine resistance. Science 1987; 238:1283–5. [DOI] [PubMed] [Google Scholar]
- 6. Djimdé A, Doumbo OK, Steketee RW, Plowe CV. Application of a molecular marker for surveillance of chloroquine-resistant falciparum malaria. Lancet 2001; 358:890–1. [DOI] [PubMed] [Google Scholar]
- 7. Mharakurwa S, Mugochi T. Chloroquine-resistant falciparum malaria in an area of rising endemicity in Zimbabwe. J Trop Med Hyg 1994; 97:39–45. [PubMed] [Google Scholar]
- 8. Mwanza S, Joshi S, Nambozi M, et al. The return of chloroquine-susceptible Plasmodium falciparum malaria in Zambia. Malar J 2016; 15:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Asua V, Vinden J, Conrad MD, et al. Changing molecular markers of antimalarial drug sensitivity across Uganda. Antimicrob Agents Chemother 2019; 63:e01818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gupta H, Macete E, Bulo H, et al. Drug-resistant polymorphisms and copy numbers in Plasmodium falciparum, Mozambique, 2015. Emerg Infect Dis 2018; 24:40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ocan M, Akena D, Nsobya S, et al. Persistence of chloroquine resistance alleles in malaria endemic countries: a systematic review of burden and risk factors. Malar J 2019; 18:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Durand R, Jafari S, Bouchaud O, Ralaimazava P, Keundjian A, Le Bras J. Plasmodium falciparum: pfcrt and DHFR mutations are associated with failure of chloroquine plus proguanil prophylaxis in travelers. J Infect Dis 2001; 184:1633–4. [DOI] [PubMed] [Google Scholar]
- 13. Mharakurwa S, Mutambu SL, Mberikunashe J, Thuma PE, Moss WJ, Mason PR; Southern Africa ICEMR Team Changes in the burden of malaria following scale up of malaria control interventions in Mutasa District, Zimbabwe. Malar J 2013; 12:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mharakurwa S, Mutambu SL, Mudyiradima R, Chimbadzwa T, Chandiwana SK, Day KP. Association of house spraying with suppressed levels of drug resistance in Zimbabwe. Malar J 2004; 3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schmidt LH Chemotherapy of the drug-resistant malarias. Annu Rev Microbiol 1969; 23:427–54. [DOI] [PubMed] [Google Scholar]
- 16. Sutherland CJ, Lansdell P, Sanders M, et al. pfk13-independent treatment failure in four imported cases of Plasmodium falciparum malaria treated with artemether-lumefantrine in the United Kingdom. Antimicrob Agents Chemother 2017; 61: e02382. [DOI] [PMC free article] [PubMed] [Google Scholar]