Abstract
Background
Otitis media (OM) is a common and potentially serious disease of childhood. Although OM is multifactorial on origin, bacterial infection is a unifying component. Many studies have established a critical role for innate immunity in bacterial clearance and OM resolution. A key component of innate immunity is the recruitment of immune and inflammatory cells, including macrophages.
Methods
To explore the role of macrophages in OM, we evaluated the expression of genes related to macrophage function during a complete episode of acute OM in the mouse caused by middle ear (ME) inoculation with Haemophilus influenzae. We also combined CCR2 deficiency with chlodronate liposome toxicity to deplete macrophages during OM.
Results
Macrophage genes were robustly regulated during OM. Moreover, macrophage depletion enhanced and prolonged the infiltration of neutrophils into the infected ME and increased the persistence of bacterial infection.
Conclusions
The results illustrate the critical role played by macrophages in OM resolution.
Keywords: CCR2, chlodronate liposome, macrophages, otitis media
Macrophages are distributed throughout the body and are crucial for tissue damage restoration. Depletion of CCR2 macrophages combined with chlodronate liposome toxicity to deplete circulating macrophages were used to to explore the function of macrophages in OM resolution and recovery.
Otitis media (OM) is the most common disease of childhood, affecting 90% of children under 5 [1], and it is the primary cause of antibiotic prescription and surgery in children. The annual cost in the United States is estimated to be $6 billion [2]. Although the long-term impacts are controversial, several reports link hearing loss due to persistent OM to deficits in speech, language, and learning abilities [3–5]. In developing countries, OM can have more serious consequences. The World Health Organization estimates that undertreated OM causes 28 000 annual deaths and is responsible for half of the world’s burden of serious hearing loss [6]. Despite the prevalence, cost, and potential sequelae of OM, treatment options have changed relatively little in the past several decades [7].
Unraveling the mechanisms of OM pathogenesis and recovery could lead to new therapies that rely less on antibiotics and surgery. The molecular understanding of OM disease progression has rapidly advanced. Contributions of inflammation, mucins, and cell signaling pathways have been elucidated and defined [8]. Among those, we and others have focused on the role of innate immunity, which plays a central role in both pathobiology and resolution of OM. The innate immune system comprises pattern recognition receptors that respond to pathogen-associated molecular patterns without the need for prior sensitization [9]. The activation of these receptors by pathogen molecules results in the initiation of inflammation and other mechanisms critical not only for the clearance of invading microorganisms and the restoration of tissue homeostasis but also for the activation and sensitization of the adaptive immune system. Deficiencies in any of the innate immune receptors and signaling molecules that have been examined to date in animals have resulted in the persistence of bacterial OM beyond the period normally observed in wild-type (WT) animals. Polymorphisms in several innate immune genes have been linked to OM susceptibility in children [10].
Genes activated by innate immune signaling include those encoding cytokines and chemokines that recruit and activate leukocytes [11], such as neutrophils, monocytes, macrophages, lymphocytes, and natural killer (NK) cells. Infected epithelial cells become the targets of NK cells, whereas neutrophils and macrophages aid in the phagocytic clearance of bacterial pathogens and dead cells [12, 13]. Despite their importance in immune defense, there are few studies that explore the effects of the absence of inflammatory cells in OM.
Macrophages are well recognized as key participants in the resolution of infections [14]. The responses of macrophages triggered by infection comprise 4 interrelated phases: (1) recognition of pathogen-associated molecular patterns by innate immune receptors expressed on/in resident tissue macrophages; (2) increased numbers of proinflammatory (M1) phenotype macrophages, via recruitment of circulating monocytes and/or in situ proliferation, to phagocytize invading pathogens; (3) macrophage bactericidal activity mediated by the expression of proinflammatory cytokines and bactericidal compounds; and (4) conversion to an anti-inflammatory (M2) phenotype to help terminate inflammation and promote tissue repair and healing [15]. Macrophages also participate in cognate immunity. As antigen-presenting cells, they play a major role in the sensitization of T and B cells. Macrophages are commonly observed in the middle ear (ME) before and during OM [16, 17], and they presumably play similar roles. Because there have been few studies to define their role in ME infection, the purpose of this study was to explore the impact that these cells have on OM.
When the monocyte chemotactic protein 1/chemokine (C-C motif) ligand 2 (MCP-1/CCL2) binds to the chemokine receptor CCR2, which is primarily expressed on the surface of monocytes, the monocytes differentiate into macrophages and migrate from the bloodstream to the site of CCR2 expression [18]. Because CCL2 can activate monocytes to become macrophages, the CCL2/CCR2 chemokine family has been a prime target for an investigation into the role of macrophage recruitment [19]. Experiments utilizing CCR2-deficient mouse models have highlighted, for example, the pathogenic activities of monocytes that traverse the blood-brain barrier during inflammation [20]. However, other chemokines can also recruit macrophages, and CCR2 deficiency does not result in the complete abrogation of these cells.
Dichloromethylene bisphosphonate (Cl2MBP) or clodronate, when encapsulated in liposomes, is phagocytosed by macrophages. As the cells degrade the liposomes through fusion with components of the lysosomal pathway, clodronate is released into the interior of the cell where it accumulates to lethal levels. The use of this drug is considered to be a highly efficacious approach for macrophage depletion in mammals [21, 22]. We combined CCR2 deficiency with clodronate treatment, to generate a murine model of OM deficient in macrophages.
METHODS
Animals
CCR2−/− mice (B6.129S4-Ccr2tm1Ifc/J), age-matched WT mice (C57BL/6J), and C57/WB-F1 hybrid mice were obtained from the Jackson Laboratory. All experiments were performed in accordance to the National Institutes of Health guidelines after the approval by the Institutional Animal Care and Use Committee of the Veterans Affairs Medical Center, San Diego, California.
Bacteria
Nontypeable Haemophilus influenzae (NTHi) strain-3655 (biotype II), originally isolated from the ME of an OM patient in St. Louis, Missouri (kindly provided by Asa Melhus, Lund University, Sweden), was used to induce infection of the ME. Inocula were prepared as described previously (in methods from 23, 24).
Clodronate Injection
Clodronate, encapsulated in liposomes (CL), was used to deplete macrophages systemically. Vehicle control liposomes contained phosphate-buffered saline (PBS) only. They were purchased from Liposoma. Each animal received 0.2 mL CL or PBS liposomes intra-abdominally. Injections were done 3 and 1 days before plus 1 and 4 days after from bacterial inoculation of the ME.
Experimental Groups
Wild-type mice, CCR2−/− mice treated with vehicle (CCR2−/−/PBS), and CCR2−/− mice treated with CL liposomes (CCR2−/−/CL) mice were divided into 4 groups of 7 mice each for each experimental time point (0, 2, 3, and 7 days after bacterial inoculation).
Surgery
As described previously, all animals were deeply anesthetized, and approximately 5 µL of an inoculum containing 500–5000 colony-forming units (CFUs) of NTHi were bilaterally injected into the MEs through a puncture in the surgically exposed bulla under standard aseptic conditions [23–25]. Although the initial number of CFUs varied, we have found that bacteria quickly multiply in the ME after inoculation, rapidly reaching 106 CFUs. We have found that initial titer has minimal effect on ME infection [24]. After inoculation, the tympanic membranes were confirmed visually to be intact. Uninoculated animals served as preinfection controls.
Gene Arrays
Groups of 40 C57/WB-F1 mice were used at each of the following time points: without infection (0 hours), 3 hours, 6 hours, 1 day, 2 days, 3 days, 5 days, or 7 days as described previously [11]. After NTHi inoculation, mucosal tissue and effusions were carefully dissected from the MEs. Samples from 20 mice were pooled and homogenized in TRIzol for total ribonucleic acid (RNA) extraction. For each postinfection interval, the procedures were repeated with the remaining 20 mice, generating an independent biological replicate. Labeled probes were hybridized to duplicate Affymetrix MU430_2.0 microarray sets per time point. Gene expression levels were evaluated for differences due to infection using variance-modeled posterior inference (VAMPIRE) [26]. Expression in uninfected MEs was compared to that in NTHi infected MEs at each time point. To assess potential differences between the responses of C57/WB-F1 mice and C57BL/6 mice, the background strain of the CCR2−/− mice, we generated arrays identically using C57 mice for a more limited set of postinfection times: 0 hours, 6 hours, and 2 days, and we assessed the same genes.
Middle Ear Histology
Mice were anesthetized and perfused intracardially with PBS followed by 4% paraformaldehyde. Middle ears were harvested, postfixed overnight, decalcified (8% ethylenediaminetetraacetic acid), processed in paraffin, and then sectioned at 10-µm thickness. Hematoxylin and eosin-stained sections from 6 standardized locations of the ME cavity were digitally recorded and analyzed using Image-Pro [25]. To provide an indirect measure of the number of infiltrating leukocytes, the area occupied by inflammatory cells on the section that displayed the maximum number of cells in the ME cavity, versus the total area of the cavity, was measured to determine the percentage covered by leukocytes. To assess the density of cellular infiltrates and the proportions of neutrophils and macrophages comprising ME infiltrates, the 2 largest clusters of the cellular ME effusion for each ME were photographed (magnification ×400), and the number of cells of each type was counted.
Middle Ear Immunohistochemistry
Paraffin sections were deparaffinized in xylene and rehydrated in a graded ethanol series. Samples were incubated with target retrieval solution pH 6.0 (DAKO) and heated in for 15 minutes at 90°C. The sections were subsequently incubated with blocking solution (2.5% goat serum) for 20 minutes at room temperature (RT), after which the sections were reacted with anti-IBA-1 primary antibody (rabbit; FUJIFILM Wako). The sections were then washed and incubated with rhodamine-conjugated secondary anti-rabbit immunoglobulin G antibody for 2 hours at RT. The sections were mounted with DAPI mounting medium (Vector).
Middle Ear Bacterial Culture
The in vivo clearance of NTHi was assessed in WT, CCR2−/−/PBS mice and CCR2−/−/CL mice. At least 6 ears were sampled at each time point (n = 3–4 mice per time point). A 1-µL loop sample was obtained from the opened ME lumen at 0 hours (uninoculated control) and 2, 3, and 7 days after inoculation. Each loop was streaked successively over 4 quadrants of a chocolate agar plate [27]. A semiquantitative scoring system was used to classify the degree of NTHi colonization per plate, with 0 indicating no CFUs, 1 indicating CFUs in 1 quadrant, 2 indicating CFUs in 2 quadrants, 3 indicating CFUs in 3 quadrants, and 4 indicating CFUs in all 4 quadrants.
Statistical Analysis
Statistical analyses were performed using GraphPad-Prism 5 software. Differences were considered significant at P < .05. Two-way analysis of variance with Bonferroni correction for multiple comparisons was performed on measures of mucosal thickness. Middle ear colonization rates were evaluated by the Student’s t test. Data normality was evaluated using the D’Agostino-Pearson omnibus test. The Mann-Whitney U test was used for data lacking a normal distribution, as for analyses of ME inflammatory cells. Left and right ears in each mouse were considered to be independent of each other, as previously discussed in detail [27], and therefore were analyzed independently.
RESULTS
The Expression of Macrophage Genes During Otitis Media
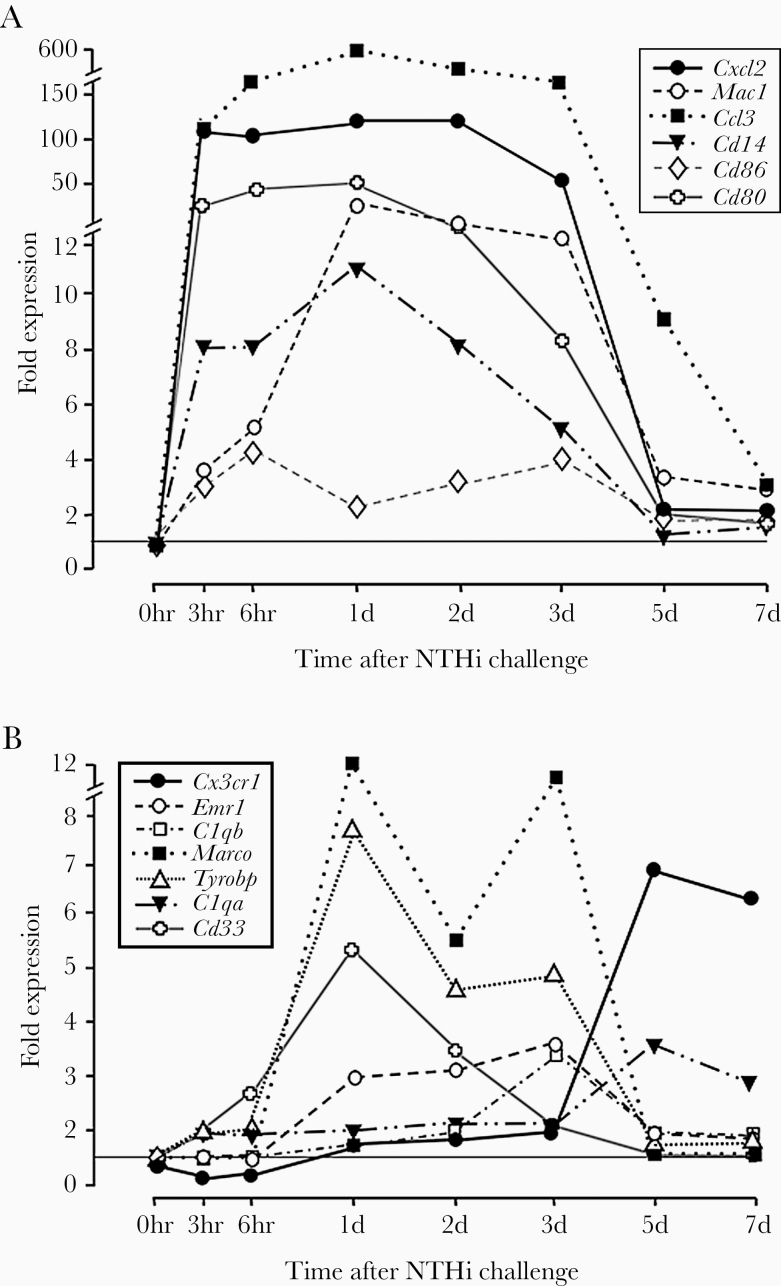
We evaluated genes that are associated with macrophage phenotypes [28] during the course of an episode of acute OM induced by the ME inoculation of NTHi in C57/WB-F1 mice. Genes associated with the proinflammatory M1 macrophage phenotype were strongly regulated early in NTHi-induced OM. As can be seen in Figure 1A, strong upregulation often peaked within hours of bacterial inoculation. In contrast, genes associated with anti-inflammatory M2 phenotypes (Figure 1B) were less strongly upregulated and peaked at later times, from 1 to 5 days after inoculation. Fold expression changes for these genes at 6 hours and 2 days in C57BL/6 mice were highly similar and exhibited the same patterns of expression.
Figure 1.
Regulation of expression for genes related to macrophage phenotype during the course of a complete episode of NTHi-induced otitis media in the mouse. (A) Genes related to the proinflammatory M1 phenotype. (B) Genes related to anti-inflammatory M2 phenotypes.
IBA-1 Documentation of Macrophage Depletion
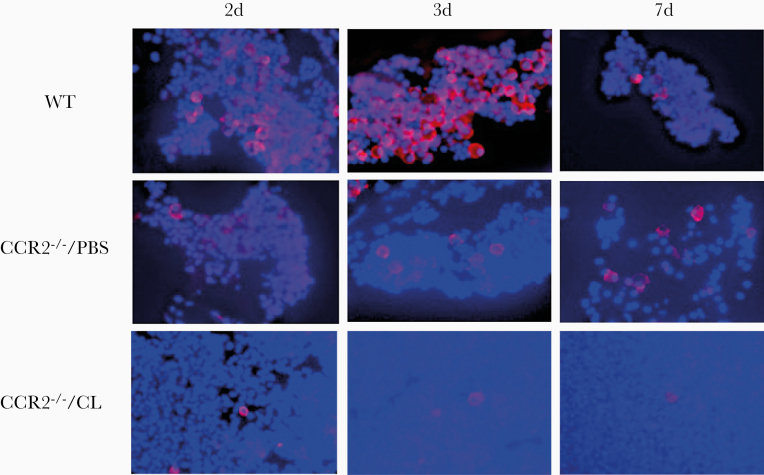
Allograft inflammatory factor 1 (AIF1) is a 17-kDa EF-hand protein that is specifically expressed in macrophage/macroglia and is upregulated during the activation of these cells [28]. In immunohistochemistry, positive staining for AIF-1 was detected in all ME sections. The number of macrophages in the ME lumen was lower in CCR2−/−/PBS than WT mice. However, the MEs of CCR2−/−/CL mice showed very few macrophages. As seen in Figure 2, differences in the ratio between neutrophils (unstained) and macrophages between strains and treatments were readily apparent. The decreased number of macrophages observed at day 7 in WT reflects OM resolution, whereas their persistence on CCR2−/−/PBS and CCR2−/−/CL reflects failure of resolution and recovery of circulating macrophages after the cessation of clodronate treatment.
Figure 2.
Immunohistochemistry of macrophages in middle ear (ME) effusions. The macrophage marker AIF1 (allograft inflammatory factor 1) is labeled with rhodamine, and cell nuclei are labeled with DAPI. AIF1-negative cells are primarily neutrophils. The decrease in ME macrophages for CCR2−/−/PBS mice is readily apparent. The figure also illustrates the scarcity of macrophages, as well as the persistence of substantial cellular infiltrate at 7 days (7d) after NTHi inoculation, in the CCR2−/−/CL MEs.
Mucosal Hyperplasia
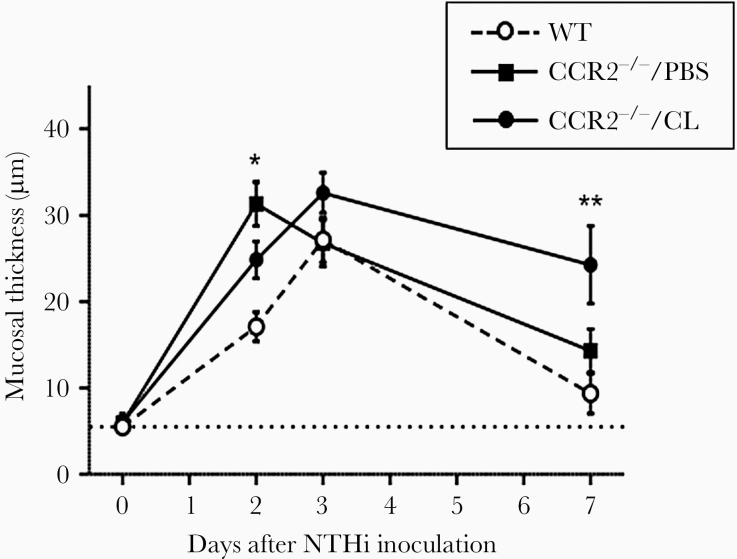
Hyperplasia of the ME mucosa is illustrated in Figure 3. When exposed to NTHi, WT mice exhibited characteristic, robust mucosal hyperplasia that reached a maximum on day 3 after inoculation. By day 7, mucosal thickness was no longer different from preinfection values, although there was a nonsignificant tendency for increased thickness. CCR2−/−/PBS mice exhibited significantly greater mucosal thickness than WT mice on day 2 but not at subsequent times. The mucosal thickness of CCR2−/−/CL mice was significantly greater than that in WT mice on day 7, suggesting delayed resolution of OM.
Figure 3.
The mucosal thickness of the middle ear (ME) during infection. CCR2−/−/PBS mice showed a thicker mucosa at 2 days after NTHi inoculation than did wild-type (WT) mice. The ME mucosa of CCR2−/−/CL mice remained thick even when the mucosa of WT and CCR2−/−/PBS mice returned to its original thickness. N = 6 ears per time point (*, P < .01 and **, P < .01; 2-way analysis of variance).
Persistent Leukocyte Recruitment to the Middle Ear in the Absence of Macrophage
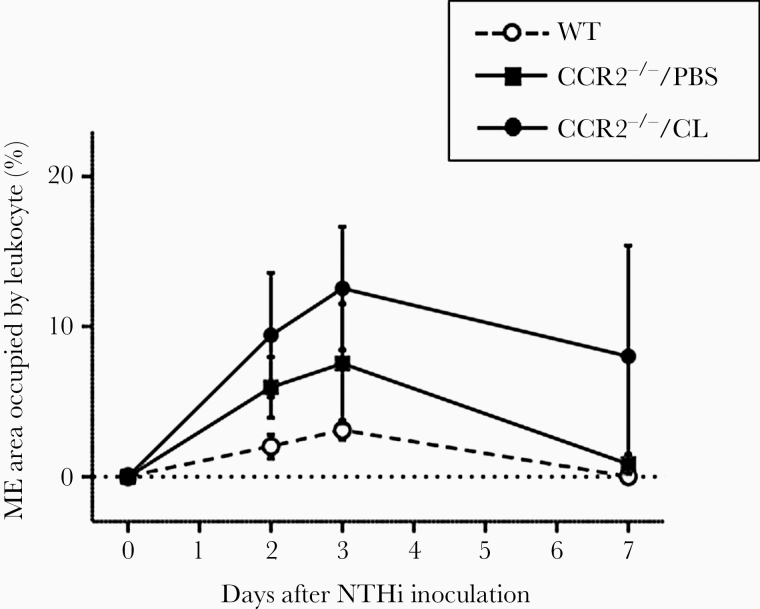
In WT mice, the area of the ME lumen occupied by infiltrating leukocytes was highest on days 2 and 3 after NTHi inoculation. The level of infiltration of inflammatory cells into the MEs of CCR2−/− mice, with PBS or CL liposome treatment, was higher than that in WT mice, but due to high variability the differences were not statistically significant (Figure 4).
Figure 4.
The mean middle ear (ME) areas covered by inflammatory cells. Leukocyte infiltration peaked at days 3 after NTHi inoculation and then decreased. Although CCR2−/−/PBS MEs, and especially CCR2−/−/CL MEs, exhibited higher levels of inflammatory cell infiltration than wild-type (WT) MEs that was sustained through day 7 after bacterial inoculation, the differences were not statistically significant.
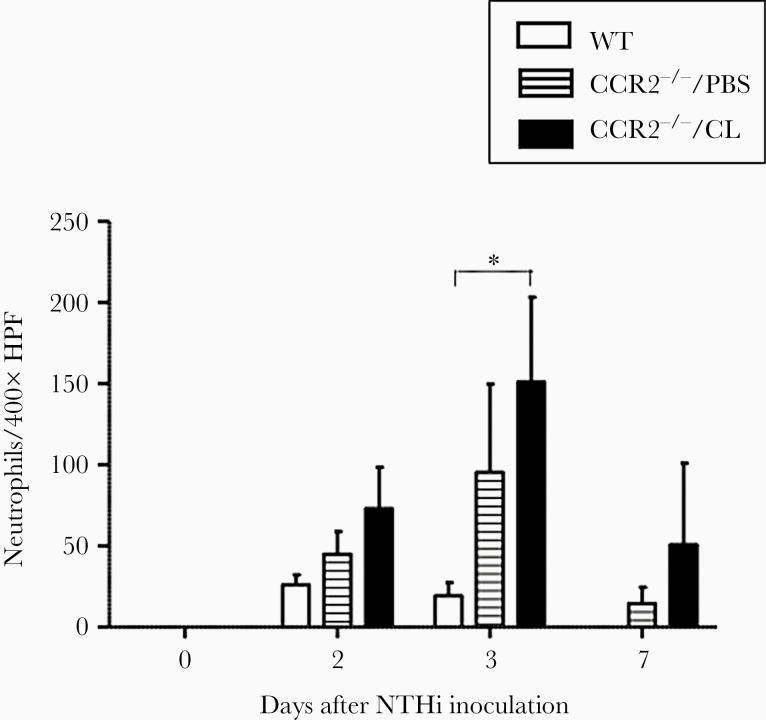
As shown in Figure 5, neutrophil influx into WT MEs was observed on days 2 and 3, but was not present at day 7. In CCR2−/−/PBS MEs, neutrophil counts increased compared with those in WT mice. On day 3, the number of neutrophils was significantly higher than WT in the MEs of CCR2−/−/CL mice. Although neutrophils were essentially absent from the MEs of WT animals at day 7, neutrophils persisted in CCR2−/−/PBS and CCR2−/−/CL MEs at day 7.
Figure 5.
The numbers of neutrophils in a ×400 field of middle ear (ME) infiltrate. In wild-type (WT) ME, the number of neutrophils was highest at day 2 of NTHi inoculation, and neutrophils cleared from the ME by day 7. In CCR2−/−/PBS mice, the number of neutrophils was highest at day 3. There were significantly more neutrophils in the ME infiltrates of the CCR2−/−/CL mice at day 3 than in WT mice. (*, P < .05, 2-way analysis of variance). HPF, high-power field.
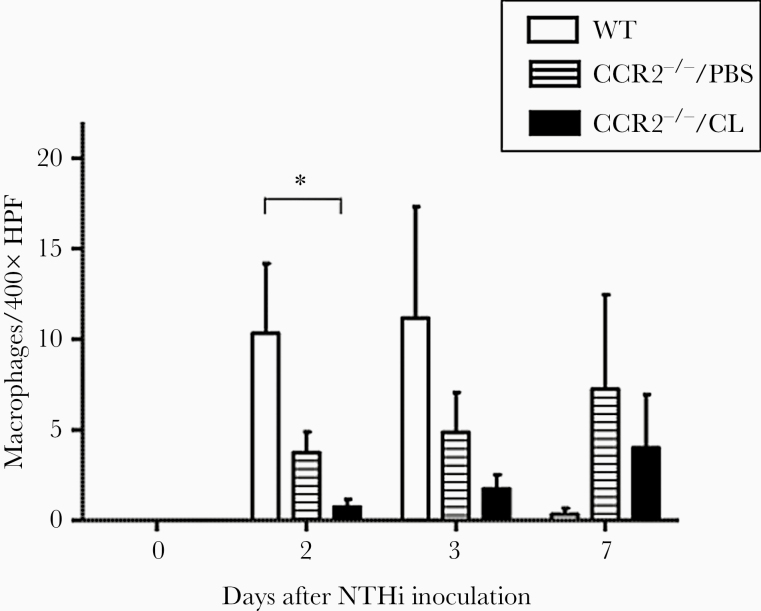
Macrophages were observed in the ME cavities of both strains by day 2 after infection but in much smaller numbers than neutrophils (Figure 6). In CCR2−/−/PBS mice, macrophage counts were lower than those in WT mice on days 2 and 3. On day 2, the number of macrophages was significantly lower in CCR2−/−/CL than in WT MEs (P = .0136 by Student t test). However, macrophage counts were higher in CCR2−/−/PBS and CCR2−/−/CL than in WT MEs on day 7.
Figure 6.
The numbers of macrophages in a 400× field of middle ear (ME) infiltrate. In wild-type (WT) MEs, the number of macrophages peaked at 2–3 days, and very few were observed at 7 days. Substantially fewer macrophages were observed in CCR2−/−/CL MEs than WT MEs on days 2 (P < .05, 2-way analysis of variance) and 3 after infection. On day 7, few macrophages were observed in WT MEs, consistent with the resolution of otitis media, but macrophages remained in the MEs of CCR2−/−/PBS and CCR2−/−/CL mice. HPF, high-power field.
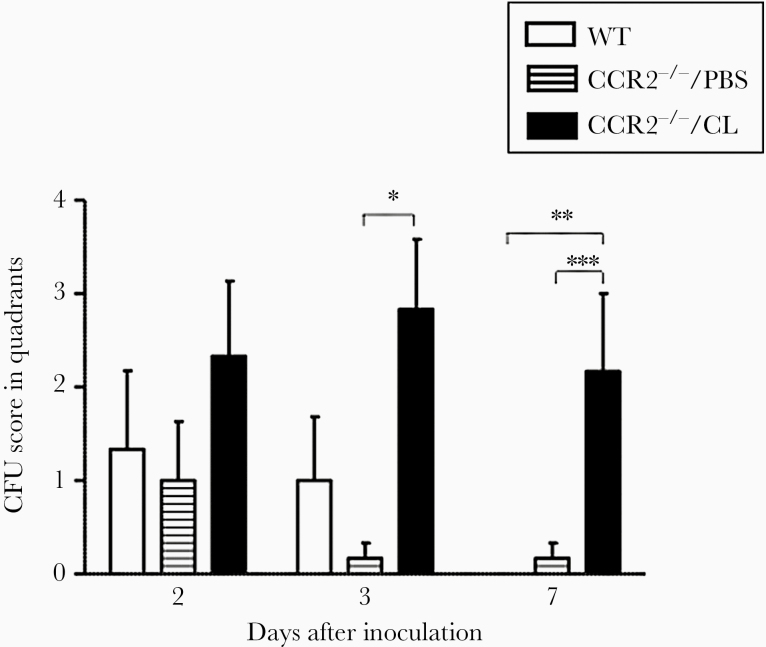
Bacterial Clearance Is Delayed in CCR2−/− Mice Treated With Clodronate Encapsulated in Liposomes
We assessed ME bacterial clearance in WT and CCR2−/− mice (Figure 7). As expected, MEs of neither CCR2-deficient nor WT mice showed bacterial colonies before challenge with NTHi. At 2 days, MEs of WT and PBS or CL-treated CCR2−/− mice showed similar culture positivity. By day 3, CCR2−/−/PBS showed a decreased colony score compared with CCR2−/−/CL, but bacterial colonies in WT and CCR2−/−/CL were maintained. By day 7, no NTHi were recovered from WT mouse MEs. Moreover, only 1 quadrant in a single plate had colonies out of 6 CCR2−/−/PBS. In contrast, colony scores were maintained at a high level in CCR2−/−/CL on days 3 and 7. Thus, the persistent inflammation seen in the MEs of CCR2−/−/CL mice, as evidenced by mucosal hyperplasia and leukocyte infiltration, was associated with an impaired capacity to clear NTHi.
Figure 7.
Recovery of NTHi from the middle ear (ME). Bacterial recovery from wild-type (WT) mouse MEs was robust on days 2 and 3 after inoculation, but no bacteria were found in the MEs of WT mice on day 7. More bacteria were recovered from the MEs of CCR2−/−/CL mice than from CCR2−/−/PBS mice on days 3 and 7, and from WT mice on day 7. N = 6 to 8 ears per time point (*, P = .006, **, P = .026, and ***, P = .04 by Student t test). CFU, colony-forming units.
DISCUSSION
Gene Expression and Macrophage Phenotypes
Resident macrophages have been well documented in the ME mucosa before OM [16], and macrophages are a major component of cellular effusions in the ME during OM [17]. Our gene expression data suggest that these cells, and others that are recruited into the ME, rapidly adopt a proinflammatory phenotype and play a key role in the initiation of innate immune defense. They express inflammatory cytokines that attack bacteria and chemokines that attract additional leukocytes. This M1 phenotypic expression pattern continues for several days. However, as OM progresses, gene expression suggests that some macrophages adopt an M2 phenotype, following the normal progression of this cell type during infection [15]. These cells presumably contribute to OM resolution and the return of ME homeostasis. Although the upregulation of M2 phenotypic genes is less robust than that of M1 genes, it should be noted that expression was compared with that in the preinfection ME. In this state, resident ME macrophages are highly likely to be of an anti-inflammatory phenotype, providing higher basal expression for M2-related genes.
It will be noted that our array data were generated in C57/WB-F1 mice [29]. This was done to reduce any potential effects of recessive mutations that are common in inbred strains. Therefore, we used F1-hybrids between 2 Mus musculus strains that are not closely related. However, most knockout models are on the C57BL/6 background, with minimal contributions from the 129-strain that supplied the embryonic stem cells used in recombination, due to an extensive number of backcrosses to the C57 background. This raises the possibility that our array data might not match C57 strain responses. Therefore, we confirmed the transcriptional responses of the genes presented in Figure 1A and B, using the same infection model in C57BL/6 mice, although in a more limited set of time points (0 hours, 6 hours, and 2 days). The C57BL/6 results were highly similar to our C57/WB-F1 data, suggesting no significant differences in transcriptional responses between the 2 mouse models.
Macrophage Depletion in the Middle Ear
The results of our depletion strategy demonstrate that although lack of CCR2 reduces macrophage entry into the ME during OM, treatment of CCR2-deficient animals with clodronate-loaded liposomes resulted in almost completely elimination of this cell type from OM. Only after CL treatment was ended did macrophages again begin to enter the ME of CCR2−/−/CL mice.
Lack of Macrophages Affects Otitis Media
Macrophage depletion had complex effects upon the ME response to infection. Some aspects of ME inflammation were enhanced in mice lacking macrophages, including mucosal hyperplasia and neutrophil recruitment at some postinfection intervals. Other aspects were decreased, especially OM resolution, in that recovery from acute OM was significantly delayed with macrophage depletion: bacteria, inflammation, and leukocytes persisted significantly longer in CCR2−/−/CL animals. It is interesting to note that even though CCR2−/−/PBS mice did not show a significant reduction in ME macrophages during OM, they were affected in OM phenotype when compared with WT mice. Mucosal hyperplasia was enhanced in CCR2−/−/PBS early in OM. This is before the entry of many macrophages into the ME lumen in WT mice, but single-cell RNA-sequencing data indicate that a significant number of macrophages are present in the ME mucosa before infection [30]. This suggests that either the nonsignificant reduction in macrophages observed in these mice (see Figures 2 and 6) was sufficient to have an effect on OM, or there were functional differences in resident and extravasating macrophages due to the lack of this chemokine receptor.
Macrophage depletion has been shown to have diverse effects in studies of other systems. It has accelerated disease progression in the lung, lower airway, gingiva, central nervous system, and peritoneum [31–36]. It has resulted in increased numbers of neutrophils in most studies. A decrease in the NK cells has also been reported [33], as has an inhibition of the inflammatory bone absorption [31]. Therefore, it is not surprising that macrophage depletion had complex effects upon the course of OM dynamics.
Although macrophage depletion increased some aspects of pathogenesis and delayed recovery, OM pathogenesis remained present in CCR2−/−/CL mice. This included mucosal hyperplasia and neutrophil recruitment to the ME cavity. These data indicate that other cell types are also critically involved in the pathophysiology of this disease. Other cell types present in the ME express innate immune receptors, including epithelial and stromal cells [29, 37], and are presumably responsible for stimulating mucosal hyperplasia and neutrophil recruitment. Thus, although macrophages play a major role in OM resolution, they are only 1 player in the ME cellular response to infection.
Macrophage function is established in response to microenvironmental signals, which drive the acquisition of polarized programs whose extremes are simplified in the M1 and M2 dichotomy [38]. Our gene array data suggest that macrophage depletion may have preferentially affected macrophages of the M1 phenotype, because depletion was maximal early in OM when M1 phenotype genes are normally dominant (Figure 1). Lack of macrophage bactericidal activity likely contributed to the persistence of bacteria in the MEs of CCR2−/−/CL mice, which in turn would result in delayed recovery from mucosal hyperplasia (Figure 3). Because macrophages began to enter the ME in larger numbers at this time point, due to cessation of CL treatment, increased inflammatory response to bacteria could have played a role. Alternatively, reduction in the tissue repair and recovery actions of M2 macrophages may have delayed return of the mucosa to its normal thickness.
CONCLUSIONS
The underlying molecular mechanisms by which acute OM proceeds to chronic OM are incompletely understood. However, there is substantial evidence to suggest that defects in innate immunity contribute to OM persistence and OM proneness [29, 37, 39]. In general, macrophages are the cell type that expresses the majority of innate immune molecules [15], suggesting that they are important to the first line of defense of the ME against infection. In addition, although primary deficiencies of macrophage function in human disease are relatively uncommon, there is increasing evidence that even subtle genetic changes in macrophage function contribute to altered responses to acute infections [40]. Therefore, it seems possible that defects or differences in macrophage function could contribute to OM proneness in humans. Supporting this hypothesis, Leichtle et al [41] found that deficiency in tumor necrosis factor-alpha (TNFA) caused phagocytic and intracellular killing defects in macrophages, and that TNFA-deficient mice exhibited persistent OM. They showed that CCL3 treatment not only restored the phagocytic and intracellular killing activity of macrophages deficient in TNFA, but also hastening the recovery of experimental OM in TNFA-deficient mice. Because polymorphisms in the tnfa gene have been associated with OM proneness in humans [39], they could also be linked to altered macrophage function. Finally, Mittal et al [42] observed that Pseudomonas aeruginosa enters and multiplies inside both human and mouse primary macrophages. Because the survival of bacteria inside macrophages leads to evasion of killing and lack of pathogen clearance by phagocytes, this could contribute to the persistence of infection in chronic, suppurative OM, in which P aeruginosa is often involved.
Notes
Acknowledgments
Disclaimer. The authors declare that they have no competing interests.
Financial support. This work was funded by grants from the National Institutes of Health/National Institute on Deafness and Other Communication Disorders (DC000129, DC012595, DC014801) and the Research Service of the VA (1I1BX001205). This work was also funded by the Gyeongsang National University Fund for Professors on Sabbatical Leave, 2017.
Potential conflicts of interest. A. F. R. is a cofounder of Otonomy Inc., serves as a member of the Scientific Advisory Board, and holds an equity position in the company. The UCSD Committee on Conflict of Interest has approved this relationship. Otonomy, Inc. played no part in the research reported here. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Pichichero ME Ten-year study of acute otitis media in Rochester, NY. Pediatr Infect Dis J 2016; 35:1027–32. [DOI] [PubMed] [Google Scholar]
- 2. Ahmed S, Shapiro NL, Bhattacharyya N. Incremental health care utilization and costs for acute otitis media in children. Laryngoscope 2014; 124:301–5. [DOI] [PubMed] [Google Scholar]
- 3. Friel-Patti S, Finitzo-Hieber T, Conti G, Brown KC. Language delay in infants associated with middle ear disease and mild, fluctuating hearing impairment. Pediatr Infect Dis 1982; 1:104–9. [DOI] [PubMed] [Google Scholar]
- 4. Teele DW, Klein JO, Chase C, Menyuk P, Rosner BA. Otitis media in infancy and intellectual ability, school achievement, speech, and language at age 7 years. Greater Boston Otitis Media Study Group. J Infect Dis 1990; 162:685–94. [DOI] [PubMed] [Google Scholar]
- 5. Shriberg LD, Friel-Patti S, Flipsen P Jr, Brown RL. Otitis media, fluctuant hearing loss, and speech-language outcomes: a preliminary structural equation model. J Speech Lang Hear Res 2000; 43:100–20. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization. Chronic suppurative otitis media. 2004. Available at: http://www.who.int/pbd/deafness/activities/hearing_care/otitis_media.pdf. Accessed 22 January 2020.
- 7. Simon F, Haggard M, Rosenfeld RM, et al. International consensus (ICON) on management of otitis media with effusion in children. Eur Ann Otorhinolaryngol Head Neck Dis 2018; 135:33–9. [DOI] [PubMed] [Google Scholar]
- 8. Preciado D, Granath A, Lin J, et al. Panel 8: report on recent advances in molecular and cellular biochemistry. Otolaryngol Head Neck Surg 2017; 156:106–13. [DOI] [PubMed] [Google Scholar]
- 9. Beutler B Innate immunity and the new forward genetics. Best Pract Res Clin Haematol 2016; 29:379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurabi A, Pak K, Ryan AF, Wasserman SI. Innate immunity: orchestrating inflammation and resolution of otitis media. Curr Allergy Asthma Rep 2016; 16:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hernandez M, Leichtle A, Pak K, Webster NJ, Wasserman SI, Ryan AF. The transcriptome of a complete episode of acute otitis media. BMC Genomics 2015; 16:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mogensen TH Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 2009; 22:240–73, Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Medzhitov R, Janeway C Jr. Innate immunity. N Engl J Med 2000; 343:338–44. [DOI] [PubMed] [Google Scholar]
- 14. Byrne AJ, Mathie SA, Gregory LG, Lloyd CM. Pulmonary macrophages: key players in the innate defence of the airways. Thorax 2015; 70:1189–96. [DOI] [PubMed] [Google Scholar]
- 15. Zhang L, Wang CC. Inflammatory response of macrophages in infection. Hepatobiliary Pancreat Dis Int 2014; 13:138–52. [DOI] [PubMed] [Google Scholar]
- 16. Takahashi M, Peppard J, Harris JP. Immunohistochemical study of murine middle ear and Eustachian tube. Acta Otolaryngol 1989; 107:97–103. [DOI] [PubMed] [Google Scholar]
- 17. Lim DJ, Lewis DM, Schram JL, Birck HG. Otitis media with effusion. cytological and microbiological correlates. Arch Otolaryngol 1979; 105:404–12. [DOI] [PubMed] [Google Scholar]
- 18. Behfar S, Hassanshahi G, Nazari A, Khorramdelazad H. A brief look at the role of monocyte chemoattractant protein-1 (CCL2) in the pathophysiology of psoriasis. Cytokine 2018; 110:226–31. [DOI] [PubMed] [Google Scholar]
- 19. Murray PJ Immune regulation by monocytes. Semin Immunol 2018; 35:12–8. [DOI] [PubMed] [Google Scholar]
- 20. Klueh U, Czajkowski C, Ludzinska I, Qiao Y, Frailey J, Kreutzer DL. Impact of CCL2 and CCR2 chemokine/receptor deficiencies on macrophage recruitment and continuous glucose monitoring in vivo. Biosens Bioelectron 2016; 86:262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Van Rooijen N, Sanders A. Kupffer cell depletion by liposome-delivered drugs: comparative activity of intracellular clodronate, propamidine, and ethylenediaminetetraacetic acid. Hepatology 1996; 23:1239–43. [DOI] [PubMed] [Google Scholar]
- 22. Fink K, Ng C, Nkenfou C, Vasudevan SG, van Rooijen N, Schul W. Depletion of macrophages in mice results in higher dengue virus titers and highlights the role of macrophages for virus control. Eur J Immunol 2009; 39:2809–21. [DOI] [PubMed] [Google Scholar]
- 23. Melhus A, Hermansson A, Prellner K. Nontypeable and encapsulated Haemophilus influenzae yield different clinical courses of experimental otitis media. Acta Otolaryngol 1994; 114:289–94. [DOI] [PubMed] [Google Scholar]
- 24. Melhus A, Ryan AF. A mouse model for acute otitis media. APMIS 2003; 111:989–94. [DOI] [PubMed] [Google Scholar]
- 25. Ebmeyer J, Furukawa M, Pak K, et al. Role of mast cells in otitis media. J Allergy Clin Immunol 2005; 116:1129–35. [DOI] [PubMed] [Google Scholar]
- 26. Hsiao A, Ideker T, Olefsky JM, Subramaniam S. VAMPIRE microarray suite: a web-based platform for the interpretation of gene expression data. Nucleic Acids Res 2005; 33:W627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deniffel D, Nuyen B, Pak K, et al. Otitis media and nasopharyngeal colonization in ccl3(-/-) mice. Infect Immun 2017; 85:e0014817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gautier EL, Shay T, Miller J, et al. ; Immunological Genome Consortium Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 2012; 13:1118–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hernandez M, Leichtle A, Pak K, et al. Myeloid differentiation primary response gene 88 is required for the resolution of otitis media. J Infect Dis 2008; 198:1862–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ryan AF, Nasamran CA, Pak K, et al. Single-cell transcriptomes reveal a complex cellular landscape in the middle ear and differential capacities for acute response to infection. Front Genet 2020; 11:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lam RS, O’Brien-Simpson NM, Lenzo JC, et al. Macrophage depletion abates Porphyromonas gingivalis-induced alveolar bone resorption in mice. J Immunol 2014; 193:2349–62. [DOI] [PubMed] [Google Scholar]
- 32. Saini Y, Wilkinson KJ, Terrell KA, et al. Neonatal pulmonary macrophage depletion coupled to defective mucus clearance increases susceptibility to pneumonia and alters pulmonary immune responses. Am J Respir Cell Mol Biol 2016; 54:210–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pribul PK, Harker J, Wang B, et al. Alveolar macrophages are a major determinant of early responses to viral lung infection but do not influence subsequent disease development. J Virol 2008; 82:4441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abe C, Tanaka S, Ihara F, Nishikawa Y. Macrophage depletion prior to Neospora caninum infection results in severe neosporosis in mice. Clin Vaccine Immunol 2014; 21:1185–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ghoneim HE, Thomas PG, McCullers JA. Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections. J Immunol 2013; 191:1250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iannacone M, Moseman EA, Tonti E, et al. Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus. Nature 2010; 465:1079–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leichtle A, Hernandez M, Pak K, et al. TLR4-mediated induction of TLR2 signaling is critical in the pathogenesis and resolution of otitis media. Innate Immun 2009; 15:205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sica A, Erreni M, Allavena P, Porta C. Macrophage polarization in pathology. Cell Mol Life Sci 2015; 72:4111–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Emonts M, Veenhoven RH, Wiertsema SP, et al. Genetic polymorphisms in immunoresponse genes TNFA, IL6, IL10, and TLR4 are associated with recurrent acute otitis media. Pediatrics 2007; 120:814–23. [DOI] [PubMed] [Google Scholar]
- 40. Bowdish DME, Gordon S. Macrophage function disorders. In: Encyclopedia of Life Sciences. Chichester, UK: John Wiley & Sons, Ltd; 2009: pp 1–12. [Google Scholar]
- 41. Leichtle A, Hernandez M, Ebmeyer J, et al. CC chemokine ligand 3 overcomes the bacteriocidal and phagocytic defect of macrophages and hastens recovery from experimental otitis media in TNF-/- mice. J Immunol 2010; 184:3087–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mittal R, Lisi CV, Kumari H, et al. Otopathogenic Pseudomonas aeruginosa enters and survives inside macrophages. Front Microbiol 2016; 7:1828. [DOI] [PMC free article] [PubMed] [Google Scholar]