Abstract
We discovered a reaction of nitroalkanes with 2-hydrazinylquinolines, 2-hydrazinylpyridines and bis-2,4-dihydrazinylpyrimidines in polyphosphoric acid (PPA) affording 1,2,4-triazolo[4,3-a]quinolines, 1,2,4-triazolo[4,3-a]pyridines and bis[1,2,4]triazolo[4,3-a:4’,3’-c]pyrimidines, respectively. The reaction expands the scope of heterocyclic annulations involving phosphorylated nitronates, believed to be the electrophilic intermediates formed from nitroalkanes in PPA. Several of the synthesized triazoles showed promising anticancer activity by inducing differentiation in neuroblastoma cancer cells. Due to the urgent need for novel differentiation agents for neuroblastoma therapy, this finding warrants further evaluation of this class of compounds against neuroblastoma.
Graphical Abstract
A reaction of nitroalkanes with heterocycles possessing a 2-hydrazinylpyridine moiety leading to triazole-fused polyheterocyclic systems was discovered. One of the obtained heterocycles showed promising anticancer activity by inducing differentiation in neuroblastoma cancer cells.

Introduction
Heterocyclic scaffolds incorporating 1,2,4-triazoles have received a great deal of attention in the medicinal chemistry community due to their status as privileged medicinal scaffolds and their occurrence in bioactive natural products and synthetic pharmaceutical agents.1–5 Specifically, 1,2,4-triazolo[4,3-a]quinolines have been reported to display a wide array of biological activities, including anticonvulsant,6 analgesic,7 antibacterial,8, 9 bromodomain inhibitory,10 histamine H4 receptor antagonistic,11 and cytotoxic,12 to name a few. Selected examples, of biologically active 1,2,4-triazolo[4,3-a]quinolines are shown in Scheme 1. These compounds are generally accessed via cyclization of quinoline substrates bearing hydrazine substituents at C-2, including cyclocondensation of hydrazides13, 14 or oxidative cyclization of hydrazones8 (Scheme 1). The same type of heterocycles can also be accessed by a direct condensation of 2-hydrazinylquinolines with carboxylic acid, but such processes typically require harsh reaction conditions and provide marginal yields.15
Scheme 1.

Examples of biologically active 1,2,4-triazolo[4,3-a]quinolines and main methods utilized for their preparation
In recent years, our laboratories have been exploring the reactivity of nitroalkanes in polyphosphoric acid (PPA), an ionic liquid-like reaction medium prepared by dissolving phosphorous pentoxide in orthophosphoric acid.16 It was demonstrated that PPA converts nitroalkanes, which are commonly used as nucleophiles at the α-carbon, into electrophilic phosphorylated nitronates (A, Scheme 2). We have reported that such species can be effectively employed in reactions with doubly nucleophilic substrates to form benzimidazoles and benzoxazoles (B),17 indoloquinolines (C),18 and oxadiazoles (D).19 Herein, we describe a newly developed synthetic methodology to access triazole-fused heterocycles, such as 1,2,4-triazolo[4,3-a]quinolines (E), using this approach. We also report the results of biological studies, that led to the identification of several triazoles that showed promising anti-tumor activity when tested against neuroblastoma cancer cells.
Scheme 2.

Construction of heterocyclic systems using nitroalkanes as electrophiles
Results and Discussion
Chemistry
Taking into account the binucleophilic nature of 2-hydrazinylquinoline substrates, we wondered if heterocyclic scaffold E could be accessed in the manner similar to the one that was previously demonstrated for 1,3,4-oxadiazoles D (Scheme 2).19 To test this idea, we pre-mixed 86% PPA with 3 equiv. of nitromethane 2a (Scheme 3) at 130 oC and added 2-hydrazinylquinoline 1a in several portions over 1 h. TLC monitoring revealed the completion of the reaction during the next 30 min. Simple aqueous work-up including basification with ammonia and subsequent purification by preparative column chromatography afforded 1,2,4-triazolo[4,3-a]quinoline 3aa as a crystalline solid in 78% yield (Scheme 3). The reaction of unsubstituted quinoline substrate 3a with higher nitroalkanes (2b–e) can also be performed very efficiently providing the corresponding triazoles 3ab–ae in high yields (Scheme 3). The outcome of these reactions was unambiguously confirmed by single crystal X-ray diffraction performed for the structures of 1,2,4-triazolo[4,3-a]quinolines 3ab and 3ad (Fig. 1). It should be pointed out that reaction involving phenylnitromethane (2f) proceeded sluggishly providing very low yield of product 3af. One explanation for this result is reduced electrophilicity of the corresponding phosphorylated nitronate A (R = Ph, Scheme 2) due to resonance effects. Introduction of methyl substituent at C-4 of quinoline substrate was well tolerated, as reactions of 3b provided the corresponding triazoles 3ba–3be in high yields (Scheme 3). Transformation involving ethyl nitroacetate (2g) also proceeded uneventfully allowing for an efficient access to triazole 3bg bearing ester functionality at C-1 (Scheme 3). Next, the reactivity of quinoline substrates 1c,d bearing Br or NO2 substituents at C-6 was examined. Gratifyingly, the reactions involving nitromethane (2a) or higher nitroalkanes (2b–e) as electrophilic components proceeded smoothly, affording the corresponding triazoloquinolines 3ca–3ce and 3da–3de in good to excellent yields (Scheme 3).
Scheme 3.

Synthesis of 1,2,4-triazolo[4,3-a]quinolines using nitroalkanes as electrophiles
Fig 1.

X-ray structures of 1,2,4-triazolo[4,3-a]quinolines 3ab (CCDC # 1994548) and 3ad (CCDC # 1994533). The thermal ellipsoids are shown at 50% probability.
Further demonstration of the reaction scope is shown in Scheme 4 using nitropropane as the electrophilic component. As can be seen, methoxy groups at variable positions are well-tolerated (compounds 3fc, 3gc, 3hc, 3kc). So are aromatic (compounds 3ec, 3fc, 3gc, 3hc) and amide (3jc) groups. Any position on the quinoline ring 3–8 can be substituted successfully. We have also demonstrated that the reaction can be performed on a multigram scale with a reduction of the amount of PPA. Thus, combining 2.67 g of 2c with 1.59 g of hydrazinylquinoline 1a in 10 g of PPA (1.5-fold reduction) gave 1.79 g of 3ac (91%, Scheme 3).
Scheme 4.
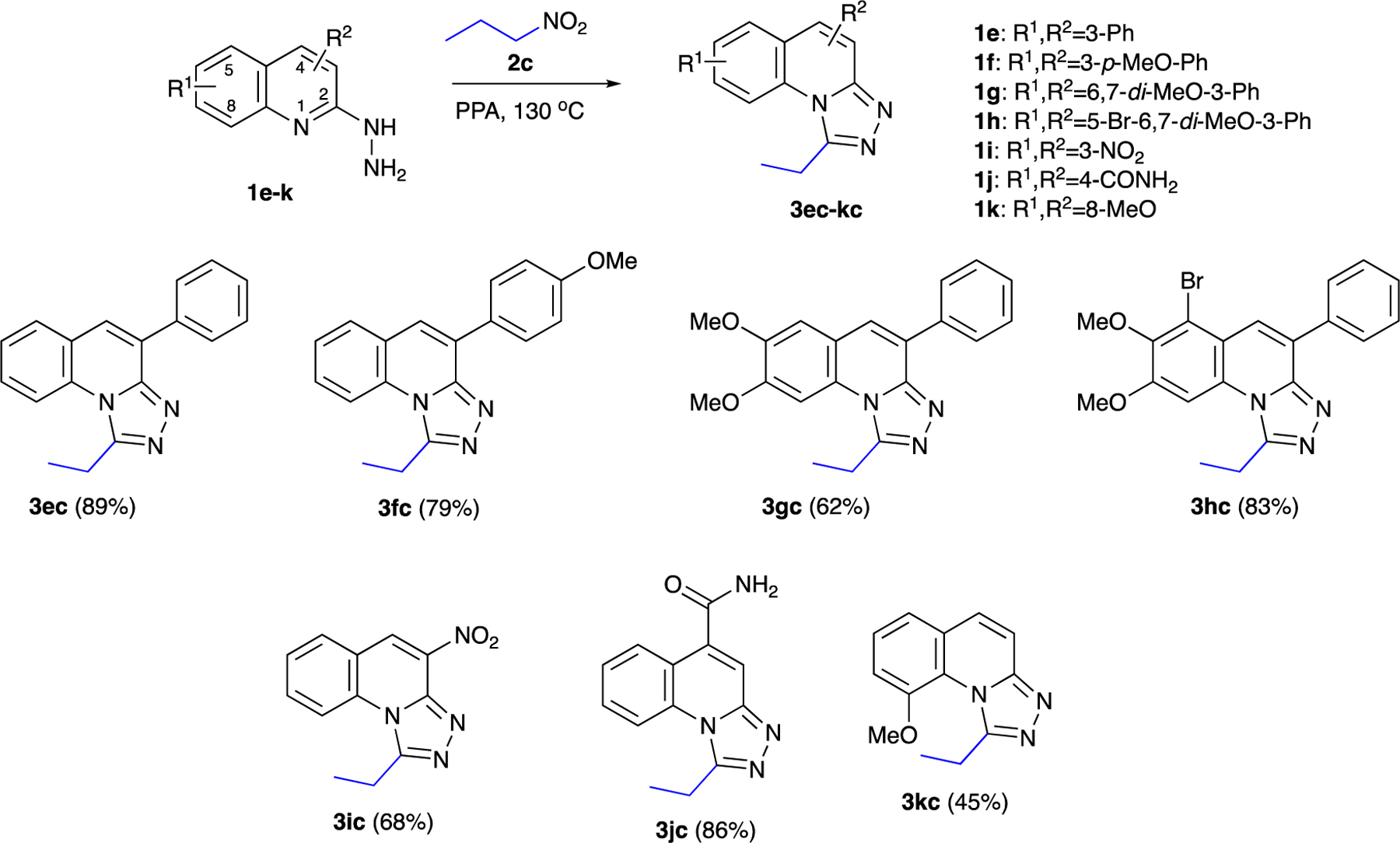
Further diversification in the synthesis of 1,2,4-triazolo[4,3-a]quinolones
After the successful development of the synthetic approach to 1,2,4-triazolo[4,3-a]quinolines, we wondered whether the presence of the entire quinoline heterocyclic system in the starting material is necessary. We reasoned that 2-hydrazinylpyridine (4, Scheme 5a), containing the identical reactive moiety, should also be a good substrate for this transformation. We were pleased to find that the reactions of 4 with nitroalkanes 2a,c successfully provided the corresponding triazolopyridines 5a,c in good yields (Scheme 5a). Again, the reaction tolerates the presence of an aromatic group and ester functionality, affording products 5e and 5g, respectively, albeit in somewhat lower yields (Scheme 5a). Further, a double reaction of dihydrazinylpyrimidine 6 with nitropropane 2c, allowed for the efficient annulation of two triazole rings to yield bis[1,2,4]triazolo-[4,3-a:4’,3’-c]pyrimidines 7c (Scheme 5b). However, the reaction with phenylnitromethane 2f was again low yielding providing 7f in only 7% yield.
Scheme 5.

Synthesis of (a) 1,2,4-triazolo[4,3-a]pyridines and (b) bis[1,2,4]triazolo[4,3-a:4’,3’-c]pyrimidines using nitroalkanes as electrophiles
In a surprising deviation from this established reaction path, our attempt to incorporate a ketone function into the structure of the triazole products by using α-nitroacetophenones resulted in the loss of the carbonyl carbon (Scheme 6). Here, we found that 2 equivalents of nitroacetophenones 8f, 8h and 8i per a hydrazine moiety in 1a, 1c (Scheme 6a) and 6 (Scheme 6b) gave the best results providing excellent yields of triazoles 3af, 3ah, 3ai, 3ci and 7f. Curiously, the successful syntheses of phenyl triazoles 3af and 7f mitigate the low yielding reaction utilizing phenylnitromethane to produce these two compounds (see Schemes 3 and 5b). Finally, we also showed that this procedure can be scaled up and prepared 2.06 g (71%) of 3ai by combining 4.2 g of nitroacetophenone 8i with 1.59 g of hydrazinylquinoline 1a in 10 g of PPA (1.5-fold reduction). The choice of scaling up triazole 3ai was also based on a previous publication reporting cytotoxic activity associated with this compound (see Scheme 1).12 We therefore demonstrated that our methodology can be used for a large scale preparation of 1,2,4-triazolo[4,3-a]quinolines with reported biological activities.
Scheme 6.

Reactions with nitroacetophenones
A putative mechanistic rationale of the featured trasformation is depicted in Scheme 7. We believe that after the initial attack of hydrazine in 1a onto the electrophilic phosphorylated nitronates A, the adducts F undergo elimination of phosphoric acid to give the amidine-type intermediates G (Scheme 7a). The 5-exo-trig intramolecular nucleophilic cyclization of G followed by elimination of phosphorylhydroxylamine then leads to triazoles 3. When nitroacetophenone is used as the source of the electrophilic component, the reaction takes a new path and the intramolecular cyclization occurs through the attack of the quinoline nitrogen onto the hydrazone moiety in J (Scheme 7b). The cyclization product K then undergoes elimination of nitromethane to afford triazole 3af.
Scheme 7.

Proposed mechanisms for (a) formation of 1,2,4-triazolo[4,3-a]quinolines 3 and (b) reaction with nitroacetophenones using 1a and 8f as examples
Biology
Due to a diverse range of biological activities found in this class of compounds, as mentioned in the Introduction, the triazole heterocycles synthesized in the present study were added to a neuroblastoma differentiation screen available in our Department. Neuroblastoma is the 3rd most lethal pediatric cancer responsible for 15% of all childhood cancer-related deaths.20 It is believed to arise from the failure of neural crest precursor cells to differentiate into mature neurons during embryonic development, hence its occurrence primarily in children.21 The idea behind the differentiation therapy is to guide malignant cells to a non-malignant state through the restoration of native biochemical pathways rather than through cell killing. Although chemotherapy and radiation are the major forms of treatment for high-risk patients, the differentiation therapy is employed post-chemotherapy and offers a milder treatment approach to prevent tumor recurrance.22 The standard-of-care differentiation therapy utilizes retinoic acids (RAs), all-trans-RA (ATRA) or 13-cis-RA. However, resistance to RAs is common23, 24 and, in the absence of alternatives, generates an urgent need to discover structurally novel differentiating agents.
Our differentiation screen is based on quantifying neurite outgrowth, which is an established marker of differentiation and can be used to quantify the potency of a compound. Compared with undifferentiated neuroblastoma cells that are characteristically small and substituent at position 3 of the quinoline moiety, indicating that circular with minimal projections, cells treated with ATRA show easily detectable neurite outgrowth, which is quantifiable by measuring its length against the cells body area.25 Fig. 2 shows a scatter plot of normalized neurite lengths associated with the synthesized triazoles. Compounds 3hc, 3ec, 3cb clearly stand out from the other members of this compound collection by an effective induction of neurite outgrowth in neuroblastoma BE(2)-C cells at the concentration of 25 (Fig. 2). Two of these, 3hc and 3ec, are based on the 1,2,4-triazolo[4,3-a]quinolone skeleton, possessing an aromatic further search for more potent compounds may need to include the preparation of compounds having these structural features. The induction of neurite outgrowth can also be observed visually in Fig 3, in which the neurites are highlighted in pink in the images showing neuroblastoma BE(2)-C cells treated with 3hc (Fig. 3b), 3ec (Fig 3c), 3cb (Fig. 3d) and ATRA (Fig. 3e). Fig. 3a shows cells treated with a vehicle control.
Fig. 2.

Effect of compounds on neurite outgrowth in neuroblastoma cell line BE(2)-C. 1,000 cells were treated with the compounds (25 µM) for 4 days. Shown is the scatter plot of normalized neurite lengths, sorted in ascending order, associated with individual compounds. Black dashed line is the mean of all compounds tested. The error bars are derived from 3 replicates.
Fig. 3.

Compounds 3hc, 3ec, 3cb induce neurite outgrowth in BE(2)-C cells. Shown are representative phase-contrast images for cells treated with (a) DMSO control, (b) 3hc (25 µM), (c) 3ec (25 µM), (d) 3cb (25 µM) and (c) ATRA (2.5 µM) for 4 days. Neurites are highlighted in pink and cell bodies in yellow color.
Conclusion
The current investigation expands the spectrum of applications of nitroalkanes in PPA for the construction of heterocycles with useful biological properties. As in our previous work, we believe that these reactions proceed via phosphorylated nitronates readily formed upon pre-mixing of nitro compounds with PPA. These versatile intermediates readily react with bis-nucleophiles to result in heterocyclic annulations, in this case leading to 1,2,4-triazolo[4,3-a]quinolines as well as 1,2,4-triazolo[4,3-a]pyridines and bis[1,2,4]-triazolo[4,3-a:4’,3’-c]pyrimidines. These triazole-based heterocyclic scaffolds are privileged structures in medicinal chemistry as demonstrated in the cited literature and the use of our simple atom economical methodology should further expand their biological applications. In our own evaluation of the synthesized triazoles, we found interesting activity of several triazoloquinolines in inducing differentiation of neuroblastoma cancer cells. Since there is no alternative solution for patients carrying tumors resistant to RAs, the standard-of-care neuroblastoma differentiation therapy agents, the discovery of such effects is exciting and warrants further investigation of compounds possessing these properties as potential anti-neuroblastoma therapies.
Experimental part
General information.
1H and 13C NMR spectra were recorded on a Bruker Avance-III spectrometer (400 or 100 MHz, respectively) equipped with a BBO probe in CDCl3 or DMSO-d6, using TMS as an internal standard. High-resolution mass spectra were obtained using a Bruker Maxis spectrometer (electrospray ionization, MeCN solution, using HCO2Na−HCO2H for calibration). Melting points were measured with a Stuart smp30 apparatus. All reactions were performed in oven-dried flasks equipped with reflux condensers and magnetic stir bars. All reactions were followed by thin-layer chromatography (TLC) using Silufol UV-254 plates, which were visualized under UV light (254 nm), with acetone, hexane/acetone, or hexane/ethanol/acetone mixtures as eluent. Polyphosphoric acid (86%) was obtained by dissolving precise amount of P2O5 in 85% orthophosphoric acid according to the published protocol.26, 27 Hydrazines 1a,28 1b,29 1d,30 1i,31–33, 4,34 6,35 8i,36 6-bromo-2-chloro-4-methylquinoline,37 2-chloro-6,7-dimethoxy-3-phenylquinoline,38 2-chloro-3-(4-methoxyphenyl)-quinoline,39 2-chloro-3-phenylquinoline,40 5-bromo-2-chloro-6,7-dimethoxy-3-phenylquinoline,41 2-chloroquinoline-4-carboxamide,42 2-chloro-8-methoxyquinoline,43 were synthesized according to the literature methods. All other reagents and solvents were purchased from commercial vendors and used as received.
6-Bromo-2-hydrazinyl-4-methylquinoline (1c).
Hydrazine hydrate (80% solution in water, 1.56 g, 25.0 mmol) was added to a solution of 6-bromo-2-chloro-4-methylquinoline (1.28 g, 5.00 mmol) in 5 mL of ethanol. The reaction mixture was stirred at reflux for 8 hours. Then, the mixture was cooled to room temperature, the formed solid presipitate was filtered off and recrystallized from ethanol to afford 1c as a pale orange crystalline solid, m.p. 174–175 °C (ethanol); yield 1.13 g (4.50 mmol, 90%). 1H NMR (400 MHz, DMSO-d6) δ 8.14 (s, 1H), 7.89 (d, J = 1.8 Hz, 1H), 7.58 (dd, J = 8.8, 2.0 Hz, 1H), 7.46 (d, J = 8.8 Hz, 1H), 6.74 (s, 1H), 4.32 (s, 2H), 2.46 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 159.7, 147.0, 143.4, 132.2, 128.4, 126.5, 125.6, 113.8, 112.2, 18.7. FTIR (ZnSe) ν (cm−1): 3269, 3003, 1892, 1750, 1689, 1620, 1559, 1506, 1422, 1341. HRMS (ES TOF, m/z) calcd for C10H11BrN3+ ([M+H]+): 252.0131, found: 252.0121 (3.9 ppm).
2-Hydrazinyl-3-phenylquinoline (1e).
2-chloro-3-phenylquinoline (959 mg, 4.00 mmol), hydrazine hydrate (88% solution in water, 2.3 mL, 40.0 mmol) and ethanol (0.7 mL) were combined in a 30 mL G30 vial and covered with a septum. The vial was placed in a Monowave 300 microwave reactor, and mixture was heated to 160 °C over the course of 5 minutes (power did not exceed 135 watts), after which this temperature was maintained for 1.5 hours (controlled by IR sensor, MW power within 10 watts, 10–15 bar pressure). Resulting mixture was poured into water (50 mL) and extracted with dichloromethane (4 × 20 mL). Combined organic layers were concentrated in vacuo, crude product was purified by silica gel column chromatography (hexanes:ethanol:Et3N, 10:1:0.2, v/v) followed by recrystallization form ethanol to afford 1e as a pale yellow solid, m.p. = 111–112 °C (ethanol); yield 847 mg (3.60 mmol, 90%). Rf = 0.38, hexanes/ethanol/Et3N (5:1:0.1, v/v). 1H NMR (400 MHz, DMSO-d6) δ 7.80 (s, 1H), 7.73 (d, J = 7.7 Hz, 1H), 7.66 (d, J = 8.3 Hz, 1H), 7.56–7.52 (m, 1H), 7.50 (d, J = 4.1 Hz, 4H), 7.46–7.41 (m, 1H), 7.23 (t, J = 7.2 Hz, 1H), 7.02 (br, 1H), 4.45 (br.s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 155.8, 146.5, 136.5, 136.2, 129.3, 128.9 (2C), 128.8 (2C), 128.1, 127.7, 125.4, 124.8, 123.6, 122.1. FTIR (ZnSe) ν (cm−1): 3309, 3253, 3183, 1774, 1615, 1508, 1486, 1244. HRMS (ES TOF, m/z) calcd for C15H14N3+ ([M+H]+): 236.1186, found: 236.1182 (1.6 ppm).
2-Hydrazinyl-3-(4-methoxyphenyl)quinoline (1f).
Product 1f was obtained via method described for compound 1e employing 2-chloro-3-(4-methoxyphenyl)-quinoline (1.076 g, 4.0 mmol), and purified by recrystallization form ethanol. Brown solid, m.p. 132–133 °C (ethanol); yield 853 mg (3.22 mmol, 82%). Rf = 0.55, EtOAc/hexane/Et3N (10:1:0.2, v/v). 1H NMR (400 MHz, DMSO-d6) δ 7.79–7.63 (m, 3H), 7.58–7.48 (m, 1H), 7.47–7.39 (m, 2H), 7.22 (td, J = 7.7, 6.7, 1.0 Hz, 1H), 7.08–7.00 (m, 2H), 7.01 (s, 1H), 4.52 (s, 2H), 3.81 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 159.1, 155.9, 146.2, 135.6, 130.0 (2C), 129.0, 128.5, 127.5, 125.2, 124.6, 123.6, 122.0, 114.3 (2C), 55.1. FTIR (ZnSe) ν (cm−1): 3270, 2928, 2855, 1744, 1611, 1505, 1419, 1246, 1178, 1031. HRMS (ES TOF, m/z) calcd for C16H16N3O+ ([M+H]+): 266.1288, found: 266.1293 (2.1 ppm).
2-Hydrazinyl-6,7-dimethoxy-3-phenylquinoline (1g).
Product 1g was obtained via method described for compound 1e employing 2-chloro-6,7-dimethoxy-3-phenylquinoline (1.196 g, 4.00 mmol), and purified by recrystallization form ethanol. Yellow solid, m.p. 187–189 °C (ethanol); yield: 1.015 g (3.44 mmol, 86%). Rf = 0.51, acetone/hexane (1:4, v/v). 1H NMR (400 MHz, DMSO-d6) δ 7.70 (s, 1H), 7.53–7.46 (m, 4H), 7.45–7.37 (m, 1H), 7.21 (s, 1H), 7.13 (s, 1H), 6.67 (s, 1H), 4.33 (br.s, 2H), 3.89 (s, 3H), 3.83 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 155.0, 151.8, 146.2, 142.7, 137.0, 135.2, 128.8 (2C), 128.8 (2C), 127.7, 122.0, 117.7, 106.8, 105.9, 55.5, 55.4. FTIR (ZnSe) ν (cm−1): 3242, 2960, 1767, 1617, 1605, 1501, 1463, 1447, 1433, 1363, 1234, 1204. HRMS (ES TOF, m/z) calcd for C17H18N3O2+ ([M+H]+): 296.1394, found: 296.1402 (2.9 ppm).
5-Bromo-2-hydrazinyl-6,7-dimethoxy-3-phenylquinoline (1h).
Product 1h was obtained via method described for compound 1e employing 5-bromo-2-chloro-6,7-dimethoxy-3-phenylquinoline (1.51 g, 4.00 mmol), and purified by recrystallization from ethanol. Pale brown solid, m.p. = 180–182 °C (ethanol); yield 1.20 g (3.21 mmol, 80%). Rf = 0.34, acetone/hexane (1:3, v/v). 1H NMR (400 MHz, DMSO-d6) δ 7.72 (s, 1H), 7.54–7.35 (m, 5H), 7.19 (s, 1H), 7.16 (s, 1H), 4.44 (s, 2H), 3.95 (s, 3H), 3.78 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 155.8, 154.9, 144.9, 143.1, 136.3, 134.3, 129.0 (2C), 128.8 (2C), 128.2, 123.6, 116.5, 114.5, 106.5, 60.3, 56.1. FTIR (ZnSe) ν (cm−1): 3319, 3246, 2935, 1608, 1550, 1505, 1484, 1461, 1426, 1395, 1230, 1201, 1035, 1019. HRMS (ES TOF, m/z) calcd for C17H17BrN3O2+ ([M+H]+): 374.0499, found 374.0500 (0.5 ppm).
2-Hydrazinylquinoline-4-carboxamide (1j).
Product 1j was obtained according to the literature method by refluxing the mixture of 2-chloroquinoline-4-carboxamide (826.5 mg, 4.00 mmol) and hydrazine hydrate (88% solution in water, 2.3 mL, 40.0 mmol) in ethanol (2 mL) for 30 minutes. Solvent was evaporated in vacuo, the residue was recrystallized from ethanol. Colorless solid, m.p. 209– 213 °C (ethanol); yield: 735 mg (3.64 mmol, 91%). Rf = 0.34, acetone/hexane (1:1, v/v). 1H NMR (400 MHz, DMSO-d6) δ 8.22 (s, 1H), 8.12 (s, 1H), 7.85 (d, J = 8.1 Hz, 1H), 7.73 (s, 1H), 7.58 (d, J = 8.3 Hz, 1H), 7.51 (t, J = 7.6 Hz, 1H), 7.20 (t, J = 7.4 Hz, 1H), 6.89 (s, 1H), 4.38 (br.s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 169.1, 158.5, 148.0, 143.2, 129.4, 125.9, 125.4, 121.7, 119.8, 108.9. FTIR (ZnSe) ν (cm−1): 3326, 3064, 1666, 1590, 1568, 1526, 1489, 1431, 1381, 1307, 1208. HRMS (ES TOF, m/z) calcd for C10H11N4O+ ([M+H]+): 203.0927, found: 203.0938 (5.1 ppm).
2-Hydrazinyl-8-methoxyquinoline (1k).
Product 1k was obtained via method described for compound 1e employing 2-chloro-8-methoxyquinoline (500.0 mg, 2.59 mmol), and purified by silica gel column chromatography (hexanes/ethanol/Et3N, gradient 15:1:1–10:10:1, v/v) followed by recrystallization form ethanol. Brown solid, m.p. = 78–79 °C (ethanol); yield 222 mg (1.16 mmol, 45%). Rf = 0.37, EtOAc/Et3N (20:1, v/v). 1H NMR (400 MHz, DMSO-d6) δ 8.14 (br. s., 1H), 7.86 (d, J = 9.0 Hz, 1H), 7.21 (dd, J = 8.0, 1.2 Hz, 1H), 7.09 (t, J = 7.8 Hz, 1H), 7.00 (dd, J = 7.8, 1.1 Hz, 1H), 6.93 (d, J = 9.0 Hz, 1H), 3.87 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 158.5, 152.9, 138.4, 136.6, 124.1, 121.4, 119.6, 111.1, 109.2, 55.4. FTIR (ZnSe) ν (cm−1): 3304, 2835, 1615, 1487,1423, 1350, 1256, 1097, 1029, 984, 824. HRMS (ES TOF, m/z) calcd for C10H11N3NaO+ ([M+Na]+): 212.0790, found: 212.0794 (2.1 ppm).
General procedure for the synthesis of triazoles 3, 5, and 7 employing nitroalkanes 2 (method A).
Polyphosphoric acid (1.5 g, 86% P2O5) was combined with corresponding nitroalkane 2 (3.00 mmol) in a 10 mL Erlenmeyer flask. The mixture was stirred and heated to 130 °C, then corresponding hydrazine 1, 4 or 6 (1.00 mmol) was added in several portions over a period of one hour. The resulting mixture was stirred at 130 °C, and the reaction progress was monitored by TLC analysis. Upon completion (0.5 – 1.5 hours) the mixture was cooled to rt. Water (5 mL) and 25% aqueous NH4OH solution (3 mL) were added, and the product was extracted with EtOAc (4 × 5 mL). Combined organic extracts were dried over sodium sulfate, filtered, and concentrated in vacuo to afford crude product that was purified by silica gel column chromatography.
General procedure for the synthesis of triazoles 3 and 7 employing α-nitroacetophenone (method B).
Polyphosphoric acid (1.5 g, 86% P2O5) was combined with correcponding α-nitroacetophenone 8 (2.00 mmol for reactions with hydrazines 1 or 4.00 mmol for reaction with 6) in a 10 mL Erlenmeyer flask. This mixture was stirred and heated to 130 °C, then corresponding hydrazine 1 or 6 (1.00 mmol) was added in several portions over a period of one hour. The resulting mixture was stirred at 130 °C, and the reaction progress was monitored by TLC. Upon completion (1 – 1.5 hours), postreaction work up and isolation was performed in the same manner as described for Method A.
[1,2,4]Triazolo[4,3-a]quinoline (3aa).7
Product 3aa was obtained via Method A employing 2-hydrazinylquinoline (1a) (159 mg, 1.00 mmol) and nitromethane (2a) (183 mg, 3.00 mmol), and purified by silica gel column chromatography (ethanol/acetone/hexane, gradient 1:3:6 – 1:4:5, v/v). Dark brown solid, m.p. 170–171 °C (acetone); yield: 132 mg (0.78 mg, 78%). Rf = 0.31, acetone. 1H NMR (400 MHz, DMSO-d6) δ 9.96 (d, J = 0.5 Hz, 1H), 8.39 (d, J = 8.3 Hz, 1H), 7.96 (dd, J = 7.9, 1.0 Hz, 1H), 7.79–7.65 (m, 3H), 7.59–7.53 (dt, J = 7.6, 1.0 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 147.4, 136.4, 132.2, 129.9, 129.5, 129.1, 126.3, 123.0, 116.5, 114.1. FTIR (ZnSe) ν (cm−1): 2997, 2951, 1928, 1780, 1685, 1541, 1515, 1420, 1371, 1239, 1049. HRMS (ES TOF, m/z) calcd for C10H8N3+ ([M+H]+): 170.0713, found: 170.0712 (0.6 ppm).
1-Methyl-[1,2,4]triazolo[4,3-a]quinoline (3ab).44
Product 3ab was obtained via Method A employing 2-hydrazinylquinoline (1a) (159 mg, 1.00 mmol) and nitroethane (2b) (225 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/hexane, gradient 1:1 – pure acetone, v/v). Yellow solid, m.p. 171–172 °C (acetone); yield: 165 mg (0.90 mmol, 90%). Rf = 0.33, acetone. 1H NMR (400 MHz, CDCl3) δ 8.19 (dd, J = 8.5, 0.9 Hz, 1H), 7.78 (dd, J = 7.8, 1.4 Hz, 1H), 7.64 (ddd, J = 8.5, 7.4, 1.4 Hz, 1H), 7.56 (d, J = 9.5 Hz, 1H), 7.52 (ddd, J = 7.8, 7.4, 0.9 Hz, 1H), 7.45 (d, J = 9.5 Hz, 1H), 3.12 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 150.0, 146.4, 132.5, 129.5, 129.4, 129.3, 126.1, 124.6, 115.8, 115.3, 16.1. FTIR (ZnSe) ν (cm−1): 3000, 2864, 2000, 1784, 1765, 1689, 1564, 1409, 1242, 1178, 1057. HRMS (ES TOF, m/z) calcd for C11H9N3Na+ ([M+Na]+): 206.0689, found: 206.0687 (0.9 ppm).
1-Ethyl-[1,2,4]triazolo[4,3-a]quinoline (3ac).
Product 3ac was obtained via Method A employing 2-hydrazinylquinoline (1a) (159 mg, 1.00 mmol) and 1-nitropropane (2c) (267 mg, 3.00 mmol), and purified by silica gel column chromatography (ethanol/acetone/ hexane, gradient 1:3:6 –1:4:5, v/v). Dark brown solid, m.p. 127–128 °C (acetone); yield: 190 mg (0.97 mmol, 97%). Rf = 0.24, ethanol/ acetone/hexane (1:3:6, v/v). 1H NMR (400 MHz, CDCl3) δ 8.18 (dd, J = 8.5, 0.9 Hz, 1H), 7.81 (dd, J = 7.8, 1.4 Hz, 1H), 7.67 (ddd, J = 8.5, 7.4, 1.4 Hz, 1H), 7.62 (d, J = 9.5 Hz, 1H), 7.52 (ddd, J = 7.8, 7.4, 0.9 Hz, 1H), 7.49 (d, J = 9.5 Hz, 1H), 3.48 (q, J = 7.4 Hz, 2H), 1.64 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 151.1, 150.0, 132.3, 129.5, 129.4, 129.4, 126.0, 124.7, 116.1, 115.3, 23.2, 11.4. FTIR (ZnSe) ν (cm−1): 3000, 2940, 1920, 1844, 1772, 1681, 1557, 1428, 1386, 1246, 1049, 943. HRMS (ES TOF, m/z) calcd for C12H12N3+ ([M+H]+): 198.1026, found: 198.1023 (1.6 ppm).
1-Heptyl[1,2,4]triazolo[4,3-a]quinoline (3ad).
Product 3ad was obtained via Method A employing 2-hydrazinylquinoline (1a) (159 mg, 1.00 mmol) and 1-nitrooctane (2b) (477 mg, 3.00 mmol), and purified by silica gel column chromatography (ethanol/acetone/ hexane, gradient 1:1 – pure acetone, v/v). Orange solid, m.p. 79–81 °C (acetone); yield: 227 mg (0.85 mmol, 85%). Rf = 0.32, acetone/ hexane (1:1, v/v). 1H NMR (400 MHz, CDCl3) δ 8.02 (d, J = 8.5 Hz, 1H), 7.69 (d, J = 7.3 Hz, 1H), 7.57 (t, J = 7.8 Hz, 1H), 7.48 (d, J = 9.5 Hz, 1H), 7.42 (t, J = 7.5 Hz, 1H), 7.36 (d, J = 9.5 Hz, 1H), 3.32 (t, J = 7.7 Hz, 2H), 2.00–1.92 (m, 2H), 1.52–1.44 (m, 2H), 1.38–1.29 (m, 2H), 1.29–1.16 (m, 4H), 0.81 (t, J = 6.5 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 150.0, 149.8, 132.2, 129.3, 129.2, 129.0, 125.8, 124.5, 115.9, 115.2, 31.6, 29.4, 29.3, 28.9, 26.6, 22.5, 14.0. FTIR (ZnSe) ν (cm−1): 3022, 2948, 2882, 1678, 1637, 1546, 1496, 1451, 1414, 1249. HRMS (ES TOF, m/z) calcd for C17H22N3+ ([M+H]+): 268.1808, found: 268.1802 (2.4 ppm).
1-Benzyl-[1,2,4]triazolo[4,3-a]quinoline (3ae).
Product 3ae was obtained via Method A employing 2-hydrazinylquinoline (1a) (159 mg, 1.00 mmol) and (2-nitroethyl)benzene (2e) (453 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/hexane, gradient 1:3 – 1:1, v/v). Pale yellow solid, m.p. 172–174 °C (acetone); yield: 238 mg, 92%. Rf = 0.31, acetone/hexane (1:1, v/v). 1H NMR (400 MHz, CDCl3) δ: 7.99 (dd, J = 7.7, 1.4 Hz, 1H), 7.76 (dd, J = 7.4, 1.9 Hz, 1H), 7.67 (d, J = 9.5 Hz, 1H), 7.52 (d, J = 9.5 Hz, 1H), 7.50–7.42 (m, 2H), 7.30 (t, J = 7.3 Hz, 2H), 7.25–7.18 (m, 3H), 4.92 (s, 2H). 13C NMR (101 MHz, CDCl3) δ: 150.5, 147.9, 135.3, 131.8, 129.7, 129.4, 129.4, 129.2 (2C), 128.2 (2C), 127.4, 126.1, 124.7, 116.7, 115.3, 34.7. FTIR (ZnSe) (cm−1): 2917, 2864, 1920, 1780, 1644, 1560, 1447, 1318, 1239, 1072, 996. HRMS (ES TOF, m/z) calcd for C17H14N3+ ([M+H]+): 260.1182, found: 260.1185 (1.2 ppm).
1-Phenyl-[1,2,4]triazolo[4,3-a]quinoline (3af).45
Product 3af was independently obtained via Method A employing 2-hydrazinyl-quinoline (1a) (159 mg, 1.00 mmol) and (nitromethyl)benzene (2а) (411 mg, 3.00 mmol). Yield: 29 mg, (0.12 mmol, 12%). Alternatively, the same compound was obtained via and Method B employing 2-hydrazineylquinoline (1a) (159 mg, 1.00 mmol) and α-nitroacetophenone (8f) (330 mg, 2.00 mmol). Yield 210 mg (0.86 mmol, 86%). In each case, crude product was purified by silica gel column chromatography (acetone/ hexane, gradient 1:2 – 3:2, v/v). The titled material was obtained as orange solid, m.p. 135–137 °C (acetone); Rf = 0.34, acetone/hexane (3:2, v/v). 1H NMR (400 MHz, CDCl3) δ 7.77 (dd, J = 7.8, 1.4 Hz, 1H), 7.69–7.64 (m, 3H), 7.62–7.54 (m, 4H), 7.52 (d, J = 8.6 Hz, 1H), 7.43 (ddd, J = 7.8, 7.2, 0.9 Hz, 1H), 7.31 (ddd, J = 8.6, 7.2, 1.4 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ: 149.9, 149.1, 131.9, 130.6, 130.0 (2C), 129.9, 129.5, 129.4, 129.2 (2C), 129.1, 126.2, 124.7, 116.8, 115.1. FTIR (ZnSe) ν (cm−1): 3188, 3062, 2924, 2858, 1666, 1623, 1448, 1399, 1221, 980. HRMS (ES TOF, m/z) calcd for C16H12N3+ ([M+H]+): 246.1026, found: 246.1024 (0.6 ppm).
1-(4-Methoxyphenyl)-[1,2,4]triazolo[4,3-a]quinoline (3ah).
Product 3ah was obtained via Method B employing 2-hydrazinylquinoline (1a) (159 mg, 1.00 mmol) and 1-(4-methoxyphenyl)-2-nitroethan-1-one (8h) (390 mg, 2.00 mmol). Product was purified by silica gel column chromatography (acetone/hexanes, gradient 1:2 – 3:2, v/v). Colorless solid, m.p. = 135–137 °C (acetone); yield 267 mg (0.97 mmol, 97%). Rf 0.29, acetone/hexanes (3:2, v/v). 1H NMR (400 MHz, DMSO-d6) δ 7.11 (d, J = 7.3 Hz, 1H), 6.93 (d, J = 9.6 Hz, 1H), 6.85 (d, J = 9.5 Hz, 1H), 6.77 (d, J = 8.6 Hz, 2H), 6.68–6.53 (m, 3H), 6.33 (d, J = 8.7 Hz, 2H), 3.03 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 160.7, 149.0, 148.3, 131.5, 131.4(2C), 129.8, 129.5, 129.0, 126.0, 124.2, 121.5, 115.9, 114.6, 114.5(2C), 55.4. FTIR (ZnSe) ν (cm−1): 3172, 3078, 2548, 2048, 1680, 1613, 1563, 1536, 1479, 1444, 1400. HRMS (ES TOF, m/z) calcd for C17H14N3O+ ([M+H]+): 276.1131, found 276.1135 (1.5 ppm).
1-(2-Nitrophenyl)-[1,2,4]triazolo[4,3-a]quinoline (3ai).
Product 3ai was obtained via Method B employing 2-hydrazinylquinoline (1a) (159 mg, 1.00 mmol) and 2-nitro-1-(2-nitrophenyl)ethan-1-one (8i) (420 mg, 2.00 mmol). Product was purified by silica gel column chromatography (acetone/hexane, gradient 1:2 – 1:1, v/v). Yellow solid, m.p. = 210–212 °C (acetone); yield 211 mg (0.73 mmol, 73%). Rf = 0.37, hexane/acetone (1:1, v/v). 1H NMR (400 MHz, DMSO-d6) δ 8.43 (dd, J = 7.7, 1.7 Hz, 1H), 7.96–7.85 (m, 2H), 7.83 (d, J = 7.1 Hz, 1H), 7.80–7.73 (m, 2H), 7.65 (d, J = 9.6 Hz, 1H), 7.46 (t, J = 7.6 Hz, 1H), 7.37–7.29 (m, 1H), 7.15 (d, J = 8.5 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 149.1, 148.1, 144.4, 135.1, 133.3, 132.8, 131.2, 130.4, 129.9, 129.8, 126.4, 125.6, 124.4, 124.0, 115.2, 114.5. FTIR (ZnSe) ν (cm−1): 3342, 2989, 2124, 1732, 1654, 1377, 1249, 1051, 1026, 993, 823. HRMS (ES TOF, m/z) calcd for C16H11N4O2+ ([M+H]+): 291.0880, found: 291.0877 (1.1 ppm).
5-Methyl-[1,2,4]triazolo[4,3-a]quinoline (3ba).
Product 3ba was obtained via Method A employing 2-hydrazinyl-4-methylquinoline (1b) (173 mg, 1.00 mmol) and nitromethane (2a) (183 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/ hexane, gradient 1:2 – pure acetone, v/v). Dark brown solid, m.p. 227–228 °C (acetone); yield: 150 mg (0.82 mmol, 82%). Rf = 0.3, acetone. 1H NMR (400 MHz, CDCl3) δ 9.21 (s, 1H), 8.00 (d, J = 8.2 Hz, 1H), 7.95 (dd, J = 8.1, 0.8 Hz, 1H), 7.72 (ddd, J = 8.2, 7.2, 1.1 Hz, 1H), 7.60 (ddd, J = 8.1, 7.2, 0.8 Hz, 1H), 7.53 (s, 1H), 2.64 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 148.1, 136.9, 134.3, 130.2, 129.8, 126.4, 126.2, 124.2, 115.8, 113.5, 19.6. FTIR (ZnSe) ν (cm−1): 1931, 1753, 1704, 1568, 1504, 1371, 1288, 1246, 1061. HRMS (ES TOF, m/z) calcd for C11H10N3+ ([M+H]+): 184.0869, found: 184.0870 (0.3 ppm).
1,5-Dimethyl-[1,2,4]triazolo[4,3-a]quinoline (3bb).46
Product 3bb was obtained via Method A employing 2-hydrazinyl-4-methylquinoline (1b) (173 mg, 1.00 mmol) and nitroethane (2b) (225 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/ hexane, gradient 1:2 – pure acetone, v/v). Yellow solid, m.p. 194–195 °C (acetone); yield: 190 mg (0.86 mmol, 86%). Rf = 0.3, acetone/ hexane/ethanol (1:3:1, v/v). 1H NMR (400 MHz, CDCl3) δ 8.24 (d, J = 8.5 Hz, 1H), 7.93 (dd, J = 8.1, 1.1 Hz, 1H), 7.69 (ddd, J = 8.5, 7.3, 1.1 Hz, 1H), 7.59 (dd, J = 8.1, 7.3 Hz, 1H), 7.49 (s, 1H), 3.13 (s, 3H), 2.60 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 149.4, 146.0, 137.0, 132.3, 129.4, 126.2, 126.2, 125.2, 116.1, 113.9, 19.8, 16.3. FTIR (ZnSe) ν (cm−1): 3031, 1920, 1780, 1681, 1628, 1560, 1504, 1458, 1416, 1371, 1246. HRMS (ES TOF, m/z) calcd for C12H12N3+ ([M+H]+): 198.1026, found: 198.1028 (1.3 ppm).
1-Ethyl-5-methyl-[1,2,4]triazolo[4,3-a]quinoline (3bc).
Product 3bc was obtained via Method A employing 2-hydrazinyl-4-methylquin-oline (1b) (173 mg, 1.00 mmol) and 1-nitropropane (2c) (267 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/ hexane, gradient 1:2 – pure acetone, v/v). Dark brown solid, m.p. 152–154 °C (acetone); yield: 187 mg (0.87 mmol, 87%). Rf = 0.28, acetone. 1H NMR (400 MHz, CDCl3) δ 8.20 (d, J = 8.5 Hz, 1H), 7.94 (dd, J = 8.1, 1.2 Hz, 1H), 7.70 (ddd, J = 8.5, 7.4, 1.2 Hz, 1H), 7.59 (ddd, J = 8.1, 7.4, 0.8 Hz, 1H), 7.53 (s, 1H), 3.47 (q, J = 7.3 Hz, 2H), 2.61 (s, 3H), 1.63 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 150.7, 148.6, 139.0, 131.9, 130.0, 126.7, 126.5, 125.4, 116.6, 113.1, 23.5, 19.9, 11.3. FTIR (ZnSe) ν (cm−1): 2980, 2888, 2363, 2337, 1737, 1712, 1511, 1454, 1384, 1240, 1170, 1056, 991. HRMS (ES TOF, m/z) calcd for C13H14N3+ ([M+H]+): 212.1182, found: 212.1183 (0.3 ppm).
1-Heptyl-5-methyl[1,2,4]triazolo[4,3-a]quinoline (3bd).
Product 3bd was obtained via Method A employing 2-hydrazinyl-4-methylquinoline (1b) (173 mg, 1.00 mmol) and 1-nitrooctane (2b) (477 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/hexane, gradient 1:2 – 1:1, v/v). Orange solid, m.p. 121–123 °C (acetone); yield: 244 mg (0.85 mmol, 85%). Rf = 0.53, acetone. 1H NMR (400 MHz, CDCl3) δ 8.16 (d, J = 8.4 Hz, 1H), 7.93 (dd, J = 8.0, 0.9 Hz, 1H), 7.69 (ddd, J = 8.4, 7.4, 0.9 Hz, 1H), 7.58 (dd, J = 8.0, 7.4 Hz, 1H), 7.50 (s, 1H), 3.41 (t, J = 7.7 Hz, 2H), 2.60 (s, 3H), 2.10–1.94 (m, 2H), 1.60–1.48 (m, 2H), 1.46–1.36 (m, 2H), 1.33–1.13 (m, 4H), 0.87 (t, J = 6.5 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 149.8, 149.4, 136.8, 132.2, 129.4, 126.2, 126.1, 125.4, 116.4, 114.1, 31.8, 29.7, 29.5, 29.1, 26.7, 22.7, 19.8, 14.2. FTIR (ZnSe) ν (cm−1): 3039, 2956, 2874, 2841, 1682, 1637, 1566, 1509, 1459. HRMS (ES TOF, m/z) calcd for C18H24N3+ ([M+H]+): 282.1965, found: 282.1971 (2.3 ppm).
1-Benzyl-5-methyl-[1,2,4]triazolo[4,3-a]quinoline (3be).
Product 3be was obtained via Method A employing 2-hydrazinyl-4-methylquinoline (1b) (173 mg, 1.00 mmol) and (2-nitroethyl)benzene (2e) (453 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/hexane, gradient 1:2 – pure acetone, v/v). Yellow-brown solid, m.p. 125–126 °C (acetone); yield: 236 mg (0.90 mmol, 90%). Rf = 0.26, acetone. 1H NMR (400 MHz, CDCl3) δ 8.00–7.95 (m, 1H), 7.89–7.82 (m, 1H), 7.51–7.43 (m, 3H), 7.28 (t, J = 7.3 Hz, 2H), 7.24–7.16 (m, 3H), 4.88 (s, 2H), 2.59 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 150.3, 147.5, 136.5, 135.3, 131.6, 129.2 (2C), 129.1, 128.2 (2C), 127.3, 125.92, 125.88, 125.2, 116.9, 114.3, 34.8, 19.8. FTIR (ZnSe) ν (cm−1): 3028, 2884, 1685, 1632, 1519, 1453, 1414, 1383, 1252, 1178. HRMS (ES TOF, m/z) calcd for C18H16N3+ ([M+H]+): 274.1339, found: 274.1341 (0.9 ppm).
Ethyl 5-methyl[1,2,4]triazolo[4,3-a]quinoline-1-carboxylate (3bg).
Product 3bg was obtained via Method A employing 2-hydrazinyl-4-methylquinoline (1b) (173 mg, 1.00 mmol) and ethyl 2-nitroacetate (2g) (399 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/hexane, gradient 1:2 – 1:1, v/v). Orange solid, m.p. 103–105 °C (acetone); yield: 232 mg, 91%. Rf = 0.49, acetone/ hexane (1:1, v/v). 1H NMR (400 MHz, CDCl3) δ 8.88 (d, J = 8.5 Hz, 1H), 8.00 (d, J = 8.0 Hz, 1H), 7.74 (dd, J = 8.5, 7.8 Hz, 1H), 7.71–7.63 (m, 2H), 4.64 (q, J = 7.1 Hz, 2H), 2.70 (s, 3H), 1.55 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 159.7, 150.5, 141.6, 140.2, 131.2, 129.9, 127.4, 125.9, 125.4, 119.7, 113.3, 63.5, 19.9, 14.3. FTIR (ZnSe) ν (cm−1): 2930, 2851, 1716, 1640, 1435, 1323, 1201. HRMS (ES TOF, m/z) calcd for C14H13N3NaO2+ ([M+Na]+): 278.0900, found: 278.0905 (1.8 ppm).
7-Bromo-5-methyl-[1,2,4]triazolo[4,3-a]quinoline (3ca).
Product 3ca was obtained via Method A employing 6-bromo-2-hydrazinyl-4-methylquinoline (1c) (252 mg, 1.00 mmol) and nitromethane (2a) (183 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/hexane, gradient 1:2 –1:1, v/v). Dark violet solid, m.p. 280–282 °C (acetone); yield: 166 mg (0.63 mmol, 63%). Rf = 0.48, acetone. 1H NMR (400 MHz, DMSO-d6) δ 9.91 (s, 1H), 8.40 (d, J = 8.8 Hz, 1H), 8.15 (d, J = 2.0 Hz, 1H), 7.98 (dd, J = 8.8, 2.0 Hz, 1H), 7.63 (s, 1H), 2.57 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 147.1, 136.3, 135.9, 132.4, 129.2, 128.4, 125.4, 119.0, 118.9, 114.0, 18.8. FTIR (ZnSe) ν (cm−1): 3110, 2921, 2857, 1947, 1837, 1750, 1674, 1651, 1564, 1469, 1375. HRMS (ES TOF, m/z) calcd for C11H9BrN3+ ([M+H]+): 261.9974, found: 261.9968 (2.3 ppm).
7-Bromo-1,5-dimethyl-[1,2,4]triazolo[4,3-a]quinoline (3cb).
Product 3cb was obtained via Method A employing 6-bromo-2-hydrazinyl-4-methylquinoline (1c) (252 mg, 1.00 mmol) and nitroethane (2b) (225 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/ethanol, gradient 8:1 –4:1, v/v). Colorless solid, m.p. 172–174 °C (acetone); yield: 258 mg (0.93. mmol, 93%). Rf = 0.32, acetone/ethanol (4:1, v/v). 1H NMR (400 MHz, CDCl3) δ 8.12 (d, J = 9.0 Hz, 1H), 8.06 (d, J = 2.0 Hz, 1H), 7.80 (dd, J = 9.0, 2.0 Hz, 1H), 7.55 (s, 1H), 3.12 (s, 3H), 2.60 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 149.2, 146.4, 136.0, 132.3, 131.1, 129.0, 127.1, 119.9, 117.6, 115.2, 19.8, 16.2. FTIR (ZnSe) ν (cm−1): 2929, 2864, 1931, 1840, 1769, 1674, 1666, 1628, 1560, 1507, 1428. HRMS (ES TOF, m/z) calcd for C12H11BrN3+ ([M+H]+): 276.0131, found: 276.0136 (2.0 ppm).
7-Bromo-1-ethyl-5-methyl-[1,2,4]triazolo[4,3-a]quinoline (3cc).
Product 3cc was obtained via Method A employing 6-bromo-2-hydrazinyl-4-methylquinoline (1c) (252 mg, 1.00 mmol) and 1-nitropropane (2c) (267 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/hexane, gradient 1:2 – pure acetone, v/v). Colorless solid, m.p. 184–186 °C (acetone); yield: 116 mg (0.80 mmol, 80%). Rf = 0.26, acetone. 1H NMR (400 MHz, CDCl3) δ: 8.01 (d, J = 9.0 Hz, 1H),7.99 (d, J = 2.1 Hz, 1H), 7.74 (dd, J = 9.0, 2.1 Hz, 1H), 7.46 (s, 1H), 3.39 (q, J = 7.3 Hz, 2H), 2.54 (s, 3H), 1.61 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ: 150.8, 149.5, 135.5, 132.1, 131.1, 128.9, 127.2, 119.6, 117.9, 115.5, 23.4, 19.7, 11.4. FTIR (ZnSe) v (cm−1): 2978, 2940, 2861, 2366, 2343, 1920, 1780, 1674, 1553, 1507, 1428, 1375. HRMS (ES TOF, m/z) calcd for C13H13BrN3+ ([M+H]+): 290.0287, found: 290.0285 (0.6 ppm).
7-Bromo-1-heptyl-5-methyl[1,2,4]triazolo[4,3-a]quinoline (3cd).
Product 3cd was obtained via Method A employing 6-bromo-2-hydrazineyl-4-methylquinoline (1c) (252 mg, 1.00 mmol) and 1-nitrooctane (2b) (477 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/hexane, gradient 1:2 – pure acetone, v/v). Pale yellow solid, m.p. 115–116 °C (acetone); yield: 351 mg (0.98 mmol, 98%). Rf = 0.29, acetone/hexane (1:1, v/v). 1H NMR (400 MHz, CDCl3) δ 8.00–7.96 (m, 2H), 7.75 (d, J = 8.5 Hz, 1H), 7.45 (s, 1H), 3.35 (t, J = 7.4 Hz, 2H), 2.54 (s, 3H), 2.10–1.89 (m, 2H), 1.58–1.48 (m, 2H), 1.45–1.34 (m, 2H), 1.36–1.20 (m, 4H), 0.87 (t, J = 6.32 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 149.7, 149.5, 134.7, 131.8, 131.0, 128.7, 127.0, 119.3, 117.7, 115.6, 31.7, 29.0, 29.4, 29.0, 26.5, 22.6, 19.5, 14.0. FTIR (ZnSe) ν (cm−1): 3004, 2955, 2845, 1776, 1681, 1663, 1568, 1526, 1466, 1420. HRMS (ES TOF, m/z) calcd for C18H23BrN3+ ([M+H]+): 360.1070, found: 360.1073 (1.0 ppm).
1-Benzyl-5-methyl-7-bromo-[1,2,4]triazolo[4,3-a]quinoline (3ce).
Product 3ce was obtained via Method A employing 2-hydrazinyl-4-methylquinoline (1b) (173 mg, 1.00 mmol) and (2-nitroethyl)benzene (2e) (453 mg, 3.00 mmol), purified by silica gel column chromatography (acetone/hexane, gradient 1:2 – pure acetone, v/v). Colorless solid, m.p. 221–223 °C (acetone); yield: 272 mg (0.77 mmol, 77%). Rf = 0.22, acetone/hexane (1:1, v/v). 1H NMR (400 MHz, CDCl3) δ 8.09 (d, J = 2.2 Hz, 1H), 7.95 (d, J = 9.1 Hz, 1H), 7.87 (s, 1H), 7.69 (dd, J = 9.1, 2.2 Hz, 1H), 7.36–7.27 (m, 3H), 7.16 (d, J = 6.9 Hz, 2H), 4.91 (s, 2H), 2.66 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 148.0, 147.5, 133.7, 133.2, 129.7, 129.6 (2C), 129.1, 128.1 (2C), 128.0, 127.0, 121.1, 120.3, 118.8, 113.4, 34.6, 19.9. FTIR (ZnSe) ν (cm−1): 3000, 2864, 1776, 1655, 1553, 1507, 1379, 1254, 1053, 1004. HRMS (ES TOF, m/z) calcd for C18H15BrN3+ ([M+H]+): 352.0444, found: 352.0443 (0.2 ppm).
7-Bromo-5-methyl-1-(2-nitrophenyl)-[1,2,4]triazolo[4,3-a]quinoline (3ci).
Product 3ci was obtained via Method B employing 6-bromo-2-hydrazinyl-4-methylquinoline (1c) (159 mg, 1.00 mmol) and 2-nitro-1-(2-nitrophenyl)ethan-1-one (8i) (420 mg, 2.00 mmol). Product was purified by silica gel column chromatography (acetone/hexanes, gradient 1:2 – 1:1, v/v). Yellow solid, m.p. = 263– 264 °C (acetone); yield 79 mg (0.70 mmol, 70%). Rf = 0.54, acetone. 1H NMR (400 MHz, DMSO-d6) δ 8.46 (dd, J = 7.8, 1.3 Hz, 1H), 8.21 (d, J = 2.0 Hz, 1H), 8.12–8.00 (m, 2H), 7.96 (dd, J = 7.2, 1.6 Hz, 1H), 7.77 (s, 1H), 7.69 (dd, J = 9.0, 2.1 Hz, 1H), 7.15 (d, J = 9.0 Hz, 1H), 2.64 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 148.8, 148.1, 144.2, 136.7, 135.2, 133.3, 132.9, 132.4, 130.1, 128.8, 126.3, 125.7, 124.1, 119.1, 117.6, 114.4, 19.0. FTIR (ZnSe) ν (cm−1): 2980, 2820, 1613, 1573, 1512, 1493, 1447, 1388, 1132, 743. HRMS (ES TOF, m/z) calcd for C17H12BrN4O2+ ([M+H]+): 383.0133, found: 383.0138 (1.3 ppm).
5-Methyl-7-nitro-[1,2,4]triazolo[4,3-a]quinoline (3da).
Product 3da was obtained via Method A employing 2-hydrazinyl-4-methyl-6-nitroquinoline (1d) (218 mg, 1.00 mmol) and nitromethane (2a) (183 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/hexane, gradient 1:2 – 1:1, v/v). Dark purple solid, m.p. 324–327 °C (acetone, with decomposion); yield: 138 mg (0.60 mmol, 60%). Rf = 0.30, acetone/hexane (1:1, v/v). 1H NMR (400 MHz, DMSO-d6) δ 10.04 (s, 1H), 8.77 (d, J = 2.2 Hz, 1H), 8.68 (d, J = 9.1 Hz, 1H), 8.63 (dd, J = 9.1, 2.2 Hz, 1H), 7.77 (s, 1H), 2.68 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 147.4, 144.9, 137.0, 136.6, 134.0, 124.3, 123.9, 121.8, 118.5, 114.9, 18.7. FTIR (ZnSe) ν (cm−1): 3099, 2989, 1916, 1787, 1685, 1564, 1534, 1511, 1469, 1390, 1201. HRMS (ES TOF, m/z) calcd for C11H8N4NaO2+ ([M+Na]+): 251.0539, found: 251.0538 (0.7 ppm).
1,5-Dimethyl-7-nitro-[1,2,4]triazolo[4,3-a]quinoline (3db).
Product 3db was obtained via Method A employing 2-hydrazinyl-4-methyl-6-nitroquinoline (1d) (218 mg, 1.00 mmol) and nitroethane (2b) (225 mg, 3.00 mmol), and purified by silica gel column chromatography (gradient acetone/hexane 1:1 – ethanol/acetone/hexane, 1:2:2, v/v). Yellow solid, m.p. >300 °C; yield: 234 mg (0.96 mmol, 96%). Rf = 0.31, ethanol/acetone/hexane (1:4:5, v/v). 1H NMR (400 MHz, DMSO-d6) δ 8.72 (d, J = 2.2 Hz, 1H), 8.57 (d, J = 9.3 Hz, 1H), 8.54 (dd, J = 9.3, 2.2 Hz, 1H), 7.71 (d, J = 0.9 Hz, 1H), 3.07 (s, 3H), 2.65 (d, J = 0.9 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 149.1, 147.5, 144.8, 137.0, 135.9, 125.4, 124.2, 121.8, 119.0, 115.6, 19.2, 15.8. FTIR (ZnSe) ν (cm−1): 3114, 3034, 2932, 2857, 1844, 1780, 1697, 1560, 1515, 1401, 1356, 1220, 1057. HRMS (ES TOF, m/z) calcd for C12H11N4O2+ ([M+H]+): 243.0877, found: 243.0874 (1.2 ppm).
1-Ethyl-5-methyl-7-nitro-[1,2,4]triazolo[4,3-a]quinoline (3dc).
Product 3dc was obtained via Method A employing 2-hydrazinyl-4-methyl-6-nitroquinoline (1d) (218 mg, 1.00 mmol) and 1-nitropropane (2c) (267 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/hexane, gradient 1:2 – pure acetone, v/v). Yellow solid, m.p. 236–238 °C (acetone); yield: 230 mg (0.89 mmol, 89%). Rf = 0.31, acetone. 1H NMR (400 MHz, CDCl3) δ 8.83 (d, J = 2.4 Hz, 1H), 8.54 (dd, J = 9.3, 2.4 Hz, 1H), 8.36 (d, J = 9.3 Hz, 1H), 7.65 (s, 1H), 3.49 (q, J = 7.3 Hz, 2H), 2.71 (s, 3H), 1.67 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 151.1, 149.6, 144.8, 136.0, 135.7, 126.0, 123.7, 121.8, 117.3, 116.5, 23.5, 19.7, 11.2. FTIR (ZnSe) ν (cm−1): 1670, 1613, 1545, 1511, 1443, 1394, 1352, 1307, 1231, 1159. HRMS (ES TOF, m/z) calcd for C13H13N4O2+ ([M+H]+): 257.1033, found: 257.1032 (0.3 ppm).
1-Heptyl-5-methyl-7-nitro-[1,2,4]triazolo[4,3-a]quinoline (3dd).
Product 3dd was obtained via Method A employing 2-hydrazinyl-4-methyl-6-nitroquinoline (1d) (218 mg, 1.00 mmol) and 1-nitrooctane (2b) (477 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/hexane, gradient 1:2 – pure acetone, v/v). Dark brown solid, m.p. 131–133 °C (acetone); yield: 268 mg (0.82 mmol, 82%). Rf = 0.34, acetone/hexane (1:1, v/v). 1H NMR (400 MHz, CDCl3) δ 8.86 (d, J = 2.4 Hz, 1H), 8.58 (dd, J = 9.1, 2.4 Hz, 1H), 8.36 (d, J = 9.1 Hz, 1H), 7.74 (s, 1H), 3.46 (t, J = 7.3 Hz, 2H), 2.74 (s, 3H), 2.08–2.05 (m, 2H), 1.61–1.54 (m, 2H), 1.43–1.40 (m, 2H), 1.36–1.28 (m, 4H), 0.89 (t, J = 6.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 150.2, 149.6, 144.7, 135.8, 135.5, 126.0, 123.6, 121.7, 117.2, 116.6, 31.7, 29.6, 29.4, 29.0, 26.5, 22.6, 19.6, 14.1. FTIR (ZnSe) ν (cm−1): 2864, 1776, 1681, 1655, 1545, 1515, 1469, 1333, 1250, 1114. HRMS (ES TOF, m/z) calcd for C18H23N4O2+ ([M+H]+): 327.1816, found: 327.1815 (0.2 ppm).
1-Benzyl-5-methyl-7-nitro-[1,2,4]triazolo[4,3-a]quinoline (3de).
Product 3de was obtained via Method A employing 2-hydrazinyl-4-methyl-6-nitroquinoline (1d) (218 mg, 1.00 mmol) and (2-nitroethyl)-benzene (2e) (453 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/hexane, gradient 1:2 – pure acetone, v/v). Yellow solid, m.p. 246–248 °C (acetone); yield: 246 mg (0.77 mmol, 77%). Rf = 0.36, acetone/hexane (1:1, v/v). 1H NMR (400 MHz, CDCl3) δ 8.84 (d, J = 2.4 Hz, 1H), 8.41 (dd, J = 9.3, 2.4 Hz, 1H), 8.24 (d, J = 9.3 Hz, 1H), 7.91 (s, 1H), 7.40–7.27 (m, 3H), 7.19 (d, J = 7.1 Hz, 2H), 4.98 (s, 2H), 2.77 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 148.5, 148.2, 145.5, 134.4, 133.4, 129.9, 129.7 (2C), 128.2, 128.1 (2C), 126.0, 124.5, 122.0, 118.6, 114.6, 34.8, 20.0. FTIR (ZnSe) ν (cm−1): 3038, 2944, 1909, 1772, 1689, 1617, 1568, 1549, 1519, 1428, 1390, 1337. HRMS (ES TOF, m/z) calcd for C18H15N4O2+ ([M+H]+): 319.1190, found: 319.1191 (0.4 ppm).
1-Ethyl-4-phenyl-[1,2,4]triazolo[4,3-a]quinoline (3ec).
Product 3ec was obtained via Method A employing 2-hydrazinyl-3-phenylquinoline (3e) (235 mg, 1.00 mmol) and 1-nitropropane (2c) (267 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/hexane, gradient 1:2 – 1:1, v/v). Orange solid, m.p. = 73–74 °C (acetone); yield 242 mg (0.89 mmol, 89%). Rf = 0.46, acetone/ hexane (1:1, v/v). 1H NMR (400 MHz, CDCl3) δ 8.14 (d, J = 8.4 Hz, 1H), 8.06 (d, J = 7.3 Hz, 2H), 7.80 (d, J = 7.7 Hz, 1H), 7.61 (t, J = 7.7 Hz, 1H), 7.54 (s, 1H), 7.48 (t, J = 7.6 Hz, 3H), 7.42 (t, J = 7.2 Hz, 1H), 3.49 (q, J = 7.3 Hz, 2H), 1.63 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 151.5, 149.5, 135.1, 131.6, 129.5, 128.98(2C), 128.96 (2C), 128.6 (2C), 128.4, 126.6, 126.0, 125.1, 115.8, 23.2, 11.6. FTIR (ZnSe) ν (cm−1): 3050, 2977, 2879, 1709, 1606, 1529, 1494, 1444, 1332, 1227. HRMS (ES TOF, m/z) calcd for C18H16N3+ ([M+H]+): 274.1339, found: 274.1342 (1.3 ppm).
1-Ethyl-4-(4-methoxyphenyl)-[1,2,4]triazolo[4,3-a]quinoline (3fc).
Product 3fc was obtained via Method A employing 2-hydrazinyl-3-(4-methoxyphenyl)quinoline (1f) (265 mg, 1.00 mmol) and 1-nitropropane (2c) (267 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/hexane, gradient 1:2 – 1:1, v/v). Yellow solid, m.p. = 146–147 °C (acetone); yield 239 mg, (0.79 mmol, 79%). Rf = 0.42, hexane/acetone (1:1, v/v). 1H NMR (400 MHz, CDCl3) δ 8.19 (d, J = 8.5 Hz, 1H), 8.07 (d, J = 8.7 Hz, 2H), 7.83 (d, J = 8.4 Hz, 1H), 7.65 (dd, J = 8.4, 7.5 Hz, 1H), 7.55 (s, 1H), 7.52 (dd, J = 8.5, 7.5 Hz, 1H), 7.03 (d, J = 8.7 Hz, 2H), 3.87 (s, 3H), 3.54 (q, J = 7.3 Hz, 2H), 1.65 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 160.3, 151.4, 149.6, 131.3, 130.2 (2C), 129.3, 128.6, 127.9, 127.4, 126.0, 125.6, 125.3, 115.8, 114.0 (2C), 55.4, 23.1, 11.5. FTIR (ZnSe) ν (cm−1): 2857, 1751, 1648, 1558, 1507, 1474, 1262, 1180, 1105, 1028, 989, 828. HRMS (ES TOF, m/z) calcd for C19H18N3O+ ([M+H]+): 304.1444, found: 304.1447 (0.8 ppm).
1-Ethyl-7,8-dimethoxy-4-phenyl-[1,2,4]triazolo[4,3-a]quinoline (3gc).
Product 3gc was obtained via Method A employing 2-hydrazinyl-6,7-dimethoxy-3-phenylquinoline (1g) (295 mg, 1.00 mmol) and 1-nitropropane (2c) (267 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/hexane, gradient 1:2 – 1:1, v/v). Colorless solid, m.p. 197–198 °C (acetone); yield: 218 mg (0.62 mmol, 62%). Rf = 0.49, acetone/hexane (1:1, v/v). 1H NMR (400 MHz, DMSO-d6) δ 8.12 (dd, J = 8.7, 1.9 Hz, 2H), 7.89 (s, 1H), 7.71 (s, 1H), 7.58 (s, 1H), 7.53 (t, J = 7.5 Hz, 2H), 7.45 (t, J = 7.3 Hz, 1H), 3.99 (s, 3H), 3.89 (s, 3H), 3.56 (q, J = 7.3 Hz, 2H), 1.52 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 150.6, 150.6, 149.0, 147.6, 135.7, 129.0 (2C), 128.8 (2C), 128.8, 126.9, 126.2, 124.4, 118.5, 110.5, 99.9, 56.3, 56.2, 22.4, 11.9. FTIR (ZnSe) ν (cm−1): 2921, 2844, 1625, 1533, 1489, 1454, 1379, 1252, 1229, 1152. HRMS (ES TOF, m/z) calcd for C20H20N3O2+ ([M+H]+): 334.1550, found: 334.1541 (2.8 ppm).
6-Bromo-1-ethyl-7,8-dimethoxy-4-phenyl-[1,2,4]triazolo[4,3-a]quinoline (3hc).
Product 3hc was obtained via Method A employing 5-bromo-2-hydrazinyl-6,7-dimethoxy-3-phenylquinoline (1h) (374 mg, 1.00 mmol) and 1-nitropropane (2c) (267 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/hexane, gradient 1:4 – 1:2, v/v). Colorless solid, m.p. = 191– 193 °C (acetone); yield: 341 mg,(0.83 mmol, 83%). Rf = 0.26, acetone/hexane (1:3, v/v). 1H NMR (400 MHz, DMSO-d6) δ 8.05 (d, J = 7.1 Hz, 2H), 7.89 (s, 1H), 7.83 (s, 1H), 7.58–7.43 (m, 3H), 4.08 (s, 3H), 3.85 (s, 3H), 3.59 (d, J = 7.0 Hz, 2H), 1.53 (t, J = 6.7 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 153.6, 151.0, 148.4, 144.7, 134.9, 129.0, 128.9, 128.7 (2C), 128.6 (2C), 125.8, 124.6, 117.4, 117.4, 100.1, 60.3, 56.4, 22.2, 11.3. FTIR (ZnSe) ν (cm−1): 2935, 1601, 1519, 1475, 1447, 1400, 1361, 1255, 1243, 1026, 708. HRMS (ES TOF, m/z) calcd for C20H19BrN3O2+ ([M+H]+): 412.0655, found 412.0653 (0.6 ppm).
1-Ethyl-4-nitro-[1,2,4]triazolo[4,3-a]quinoline (3ic).
Product 3ic was obtained via Method A employing 2-hydrazineyl-3-nitroquinoline (1i) (204 mg, 1.00 mmol) and 1-nitropropane (2c) (267 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/hexane, gradient 1:5 – 1:1, v/v). Brown solid, m.p. 219–221 °C (ethanol); yield: 165 mg (0.68 mmol, 68%). Rf = 0.22, EtOAc. 1H NMR (400 MHz, DMSO-d6) δ 8.96 (d, J = 3.4 Hz, 1H), 8.40 (d, J = 4.9 Hz, 1H), 8.32 (d, J = 6.4 Hz, 1H), 7.98 (t, J = 7.5 Hz, 1H), 7.73 (t, J = 6.1 Hz, 1H), 3.51 (d, J = 4.3 Hz, 2H), 1.51 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 152.3, 142.5, 134.9, 133.7, 133.3, 132.4, 129.6, 126.8, 121.4, 117.0, 22.3, 11.1. FTIR (ZnSe) ν (cm−1): 2991, 2943, 1740, 1633, 1607, 1568, 1505, 1451, 1383, 1320, 1283, 1243. HRMS (ES TOF, m/z) calcd for C12H11N4O2+ ([M+H]+): 243.0877, found: 243.0882 (2.1 ppm).
1-Ethyl-[1,2,4]triazolo[4,3-a]quinoline-5-carboxamide (3jc).
Product 3jc was obtained via Method A employing 2-hydrazinylquinoline-4-carboxamide (1j) (202 mg, 1.00 mmol) and 1-nitropropane (2c) (267 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/ethanol, gradient: pure acetone – 1:2, v/v). Colorless solid, m.p. 302–303 °C (ethanol). yield: 206 mg (0.86 mmol, 86%). Rf = 0.23, acetone; Rf = 0.69, acetone/ethanol (1:2, v/v). 1H NMR (400 MHz, DMSO-d6) δ 8.34 (d, J = 8.5 Hz, 1H), 8.30 (s, 1H), 8.23 (dd, J = 8.2, 1.5 Hz, 1H), 7.89 (s, 1H), 7.82–7.72 (m, 2H), 7.61 (t, J = 7.7 Hz, 1H), 3.49 (q, J = 7.3 Hz, 2H), 1.49 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 168.1, 151.3, 148.1, 135.1, 132.1, 129.9, 127.5, 126.0, 121.3, 117.0, 113.6, 22.4, 11.2. FTIR (ZnSe) ν (cm−1): 3319, 3166, 2970, 1673, 1561, 1449, 1431, 1383, 1322, 1233. HRMS (ES TOF, m/z) calcd for C13H13N4O+ ([M+H]+): 241.1084, found: 241.1087 (1.3 ppm). HRMS (ES TOF, m/z) calcd for C13H12N4NaO+ ([M+Na]+): 263.0903, found: 263.0904 (0.2 ppm).
1-Ethyl-9-methoxy-[1,2,4]triazolo[4,3-a]quinoline (3kc).
Product 3kc was obtained via Method A employing 2-hydrazinyl-8-methoxyquinoline (1k) (189 mg, 1.00 mmol) and 1-nitropropane (2c) (267 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/hexane, gradient: pure acetone–1:2, v/v). Brown oil; yield 102 mg (0.45 mmol, 45%). Rf = 0.43, acetone. 1H NMR (400 MHz, DMSO-d6) δ 7.64 (d, J = 9.4 Hz, 1H), 7.59 (d, J = 9.5 Hz, 1H), 7.57–7.51 (m, 2H), 7.41 (dd, J = 7.4, 2.1 Hz, 1H), 4.02 (s, 3H), 3.33 (q, J = 7.4 Hz, 2H), 1.26 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 153.5, 150.2, 149.2, 129.0, 127.0, 126.8, 121.8, 120.7, 115.4, 112.0, 55.5, 23.8, 12.5. FTIR (ZnSe) ν (cm−1): 2974, 1736, 1652, 1571, 1474, 1412, 1319, 1277, 1101, 954, 814. HRMS (ES TOF, m/z) calcd for C13H14N3O+ ([M+H]+): 228.1131, found: 228.1136 (2.2 ppm).
[1,2,4]Triazolo[4,3-a]pyridine (5a).45
Product 5a was obtained via Method A employing 2-hydrazinylpyridine (4) (109 mg, 1.00 mmol) and nitromethane (2a) (183 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/hexane, gradient 1:1 – pure acetone, v/v). Yellow oil; yield: 83 mg (0.70 mmol, 70%). Rf = 0.38, acetone/hexane (1:1, v/v). 1H NMR (400 MHz, CDCl3) δ 8.86 (s, 1H), 8.20 (d, J = 6.9 Hz, 1H), 7.63 (dd, J = 9.3, 0.6 Hz, 1H), 7.16 (ddd, J = 9.3, 6.6, 0.6 Hz, 1H), 6.76 (t, J = 6.6 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 149.2, 135.7, 127.8, 123.8, 115.9, 114.1. FTIR (ZnSe) ν (cm−1): 3113, 2985, 2886, 1736, 1649, 1546, 1517, 1455, 1368, 1253. HRMS (ES TOF, m/z) calcd for C6H6N3+ ([M+H]+): 120.0556, found: 120.0554 (2.2 ppm).
3-Ethyl-[1,2,4]triazolo[4,3-a]pyridine (5c).48
Product 5c was obtained via Method A employing 2-hydrazinylpyridine (4) (109 mg, 1.00 mmol) and 1-nitropropane (2c) (267 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/hexane, gradient 1:1 – pure acetone, v/v). Yellow solid, m.p. 121–123 °C (acetone); yield: 99 mg (0.71 mmol, 71%). Rf = 0.28, acetone/hexane (1:1, v/v). 1H NMR (400 MHz, CDCl3) δ 7.87 (d, J = 8.0 Hz, 1H), 7.70 (d, J = 8.0 Hz, 1H), 7.19 (t, J = 8.7 Hz, 1H), 6.81 (t, J = 8.0 Hz, 1H), 3.07 (d, J = 7.5 Hz, 2H), 1.49 (t, J = 7.5 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 149.9, 147.8, 126.5, 121.8, 116.6, 113.5, 18.2, 10.9. FTIR (ZnSe) ν (cm−1): 2993, 2890, 1641, 1521, 1467, 1393, 1228, 1142, 1051. HRMS (ES TOF, m/z) calcd for C8H10N3+ ([M+H]+): 148.0869, found: 148.0871 (1.0 ppm).
3-Benzyl[1,2,4]triazolo[4,3-a]pyridine (5e).47
Product 5e was obtained via Method A employing 2-hydrazinylpyridine (4) (109 mg, 1.00 mmol) and (2-nitroethyl)benzene (2e) (453 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/hexane, gradient 1:1 – pure acetone, v/v). Yellow solid, m.p. 164–166 °C (acetone); yield: 75 mg (0.41 mmol, 41%). Rf = 0.26, acetone/hexane (1:1, v/v). 1H NMR (400 MHz, CDCl3) δ: 7.76–7.71 (m, 2H), 7.33–7.17 (m, 6H), 6.72 (t, J = 6.7 Hz, 1H), 4.55 (s, 2H). 13C NMR (101 MHz, CDCl3) δ: 150.2, 145.3, 134.5, 129.1 (2C), 128.4 (2C) , 127.4, 127.0, 122.3, 116.5, 113.7, 31.3. FTIR (ZnSe) ν (cm−1): 3107, 3050, 2884, 2078, 1777, 1694, 1650, 1611, 1563, 1501, 1471, 1431. HRMS (ES TOF, m/z) calcd for C13H12N3+ ([M+H]+): 210.1026, found: 210.1027 (0.5 ppm).
Ethyl [1,2,4]triazolo[4,3-a]pyridine-3-carboxylate (5g).
Product 5g was obtained via Method A employing 2-hydrazinylpyridine (4) (109 mg, 1.00 mmol) and 2-nitroacetate (2g) (399 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/hexane, gradient 1:1 – pure acetone, v/v). Pale orange solid, m.p. 120–122 °C (acetone); yield: 80 mg (0.42 mmol, 42%). Rf = 0.32, acetone/hexane (1:1, v/v). 1H NMR (400 MHz, CDCl3) δ 9.14 (d, J = 7.0 Hz, 1H), 7.94 (d, J = 9.2 Hz, 1H), 7.48 (dd, J = 8.6, 7.3 Hz, 1H), 7.11 (t, J = 7.3 Hz, 1H), 4.54 (q, J = 7.1 Hz, 2H), 1.47 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 158.4, 151.4, 137.8, 129.5, 126.0, 116.6, 116.2, 62.5, 14.4. FTIR (ZnSe) ν (cm−1): 3003, 2924, 2858, 1980, 1699, 1636, 1455, 1316, 1237, 1052, 762. HRMS (ES TOF, m/z) calcd for C9H10N3O 2+ ([M+H]+): 192.0768, found: 192.0769 (0.9 ppm).
3,9-Diethyl-5-methylbis[1,2,4]triazolo[4,3-a:4’,3’-c]pyrimidine (7c).
Product 7c was obtained via Method A employing 2,4-dihydrazinyl-6-methylpyrimidine (6) (154 mg, 1.00 mmol) and 1-nitropropane (2c) (267 mg, 3.00 mmol), and purified by silica gel column chromatography (acetone/hexane, gradient 1:1 – pure acetone, v/v). Off-white solid, m.p. 204–205 °C (acetone); yield: 168 mg (0.73 mmol, 73%). Rf = 0.44, acetone. 1H NMR (400 MHz, CDCl3) δ 6.80 (s, 1H), 3.49 (q, J = 7.5 Hz, 2H), 3.21 (q, J = 7.4 Hz, 2H), 2.77 (s, 3H), 1.52 (dt, J = 10.3, 7.4 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 152.0, 150.9, 146.5, 143.2, 135.7, 101.8, 22.0, 20.3, 19.7, 11.9, 11.6. FTIR (ZnSe) ν (cm−1): 2984, 2879, 1781, 1724, 1698, 1650, 1545, 1506, 1466, 1431, 1379, 1340. HRMS (ES TOF, m/z) calcd for C11H15N6+ ([M+H]+): 231.1353, found: 231.1354 (0.4 ppm).
5-Methyl-3,9-diphenylbis([1,2,4]triazolo)[4,3-a:4’,3’-c]pyrimidine (7f).
Product 7f was obtained via Method A employing 2,4-dihydrazinyl-6-methylpyrimidine (6) (154 mg, 1.00 mmol) and (nitromethyl)benzene (2f) (411 mg, 3.00 mmol), yield: 23 mg, (0.07 mmol, 7%). Alternatively, the same compound was obtained via Method B employing 2,4-dihydrazineyl-6-methylpyrimidine (6) (154 mg, 1.00 mmol) and α-nitroacetophenone (8f) (660 mg, 4.00 mmol), 228 mg (0.70 mmol, 70%). In each case the crude product was purified by silica gel column chromatography (acetone/hexane, gradient 1:2 – 1:1, v/v). Orange solid, m.p. 135–137 °C (acetone); Rf = 0.71, acetone/hexane (1:1, v/v). 1H NMR (400 MHz, CDCl3) δ 8.38 (dd, J = 6.5, 3.1 Hz, 2H), 7.66–7.59 (m, 3H), 7.57 (d, J = 6.7 Hz, 2H), 7.54–7.50 (m, 3H), 6.92 (s, 1H), 2.25 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 165.9, 149.9, 149.1, 144.5, 138.4, 131.3, 131.0 (2C), 130.8, 129.6, 128.9 (2C), 128.7 (2C), 127.7 (2C) , 127.6, 103.1, 20.7. FTIR (ZnSe) ν (cm−1): 2930, 2864, 1745, 1660, 1445, 1397, 1247. 973. HRMS (ES TOF, m/z) calcd for C19H14N6Na+ ([M+Na]+): 349.1172, found: 349.1179 (1.8 ppm).
Procedure for gram-scale synthesis of triazole 3ai.
Polyphosphoric acid (10 g, 86% P2O5) was combined with 2-nitro-1-(2-nitrophenyl)ethan-1-one (8i) (4.2 g, 20.00 mmol) in a 25 mL Erlenmeyer flask. This mixture was stirred and heated to 130 °C, then 2-hydrazinylquinoline (1a) (1.59 g, 10.00 mmol) was added in several portions over a period of one hour. The resulting mixture was stirred at 130 °C, and the reaction progress was monitored by TLC analysis. Upon completion (1 – 1.5 hours) the mixture was cooled to rt. Water (50 mL) and 25% aqueous NH4OH solution (30 mL) were added, and the product was extracted with EtOAc (4 × 50 mL). Combined organic extracts were dried over sodium sulfate, filtered, and concentrated in vacuo to afford crude product that was purified by silica gel column chromatography (acetone/hexane, gradient 1:2 – 1:1, v/v). Yield 2.06 g (7.10 mmol, 71%).
Procedure for gram-scale synthesis of triazole 3ac.
Polyphosphoric acid (10 g, 86% P2O5) was combined with 1-nitropropane (2c) (2.67 g, 30.00 mmol) in a 25 mL Erlenmeyer flask. The mixture was stirred and heated to 130 °C, then 2-hydrazinylquinoline 1a (1.59 g, 10.00 mmol) was added in several portions over a period of one hour. The resulting mixture was stirred at 130 °C, and the reaction progress was monitored by TLC analysis. Upon completion (0.5 – 1.5 hours) the mixture was cooled to rt. Water (50 mL) and 25% aqueous NH4OH solution (30 mL) were added, and the product was extracted with EtOAc (4 × 50 mL). Combined organic extracts were dried over sodium sulfate, filtered, and concentrated in vacuo to afford crude product that was purified by silica gel column chromatography (ethanol/acetone/ hexane, gradient 1:3:6 –1:4:5, v/v). Yield 1.79 g (9.08 mmol, 91 %).
Detection of neurite outgrowth.
Human neuroblastoma cell line BE(2)-C cells were grown in DMEM / F-12 (Corning Cellgro) supplemented with 10% Equafetal bovine serum (Atlas Biologicals). 1,000 cells were plated and treated in 96-well plates with different concentration of compounds in triplicates for 4 days. Cell images were taken under 20X magnification using IncuCyte ZOOM Live Cell Imaging System (Essen BioScience). Neurite lengths were analyzed in the acquired images using the neurite definition defined by NeuroTrack system (Essen BioScience) as described previously.25
Supplementary Material
Acknowledgements
This work was supported by the Russian Foundation for Basic Research (grant #18–33-20021 mol_a_ved) and the Ministry of Education and Science of the Russian Federation (grant #0795–2020-0031). AK and LD acknowledge the National Institutes of Health (grants 1R15CA227680–01A1 and 1R15CA213199–01A1).
Footnotes
Publisher's Disclaimer: This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication.
Publisher's Disclaimer: Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. We will replace this Accepted Manuscript with the edited and formatted Advance Article as soon as it is available.
Electronic Supplementary Information (ESI) available: Copies of 1H and 13C NMR spectra and crystallographic data for CCDC 199548 and 1994533. See DOI: 10.1039/d0ob01007c
Conflicts of interest
There are no conflicts to declare.
References and notes
- 1.Mulakayala N, Reddy Ch U, Iqbal J and Pal M, Tetrahedron, 2010, 66, 4919–4938. [Google Scholar]
- 2.Lima CGS, Ali A, van Berkel SS, Westermann B and Paixao MW, Chem. Commun, 2015, 51, 10784–10796. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z, Liu Z, Cao G, Li H, Ren H, Adv. Synth. Catal. 2017, 359, 202–224. [Google Scholar]
- 4.Li C-S, An C-Y, Li X-M, Gao S-S, Cui C-M, Sun H-F and Wang B-G, J. Nat. Prod, 2011, 74, 1331–1334. [DOI] [PubMed] [Google Scholar]
- 5.Zhou X, Xu T, Wen K, Yang X-W, Xu S-H and Liu Y, Biosci., Biotechnol., Biochem, 2010, 74, 1089–1091. [DOI] [PubMed] [Google Scholar]
- 6.Guo L-J, Wei C-X, Jia J-H, Zhao L-M and Quan Z-S, Eur. J. Med. Chem, 2009, 44, 954–958. [DOI] [PubMed] [Google Scholar]
- 7.Savini L, Chiasserini L, Pellerano C, Filippelli W and Falcone G, Farmaco, 2001, 56, 939–945. [DOI] [PubMed] [Google Scholar]
- 8.Sadana AK, Mirza Y, Aneja KR and Prakash O, Eur. J. Med. Chem, 2003, 38, 533–536. [DOI] [PubMed] [Google Scholar]
- 9.Kumar M, Kumar V and Gupta GK, Med. Chem. Res, 2015, 24, 1857–1868. [Google Scholar]
- 10.Myrianthopoulos V, Gaboriaud-Kolar N, Tallant C, Hall M-L, Grigoriou S, Brownlee PM, Fedorov O, Rogers C, Heidenreich D, Wanior M, Drosos N, Mexia N, Savitsky P, Bagratuni T, Kastritis E, Terpos E, Filippakopoulos P, Muller S, Skaltsounis A-L, Downs JA, Knapp S and Mikros E, J. Med. Chem, 2016, 59, 8787–8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko K, Kim H-J, Ho P-S, Lee SO, Lee J-E, Min C-R, Kim YC, Yoon J-H, Park E-J, Kwon Y-J, Yun J-H, Yoon D-O, Kim J-S, Park W-S, Oh S-S, Song Y-M, Cho W-K, Morikawa K, Lee K-J and Park C-H, J. Med. Chem, 2018, 61, 2949–2961. [DOI] [PubMed] [Google Scholar]
- 12.Zheng H, Wang K, Zhang W and Liu R, Synth. Commun, 2015, 45, 2849–2856. [Google Scholar]
- 13.Yanborisova OA and Konshin ME, Khim. Geterotsikl. Soedin, 1991, 493–496.
- 14.Wang H, Robl JA, Hamann LG, Simpkins L, Golla R, Li Y-X, Seethala R, Zvyaga T, Gordon DA and Li JJ, Bioorg. Med. Chem. Lett, 2011, 21, 4146–4149. [DOI] [PubMed] [Google Scholar]
- 15.Upadhayaya RS, Shinde PD, Sayyed AY, Kadam SA, Bawane AN, Poddar A, Plashkevych O, Foeldesi A and Chattopadhyaya J, Org. Biomol. Chem, 2010, 8, 5661–5673. [DOI] [PubMed] [Google Scholar]
- 16.Popp FD and McEwen WE, Chem. Rev. (Washington, DC, U. S.), 1958, 58, 321–401. [Google Scholar]
- 17.Aksenov AV, Smirnov AN, Aksenov NA, Bijieva AS, Aksenova IV and Rubin M, Org. Biomol. Chem, 2015, 13, 4289–4295. [DOI] [PubMed] [Google Scholar]
- 18.Aksenov NA, Aksenov AV, Kornienko A, De Carvalho A, Mathieu V, Aksenov DA, Ovcharov SN, Griaznov GD and Rubin M, RSC Adv, 2018, 8, 36980–36986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aksenov AV, Khamraev V, Aksenov NA, Kirilov NK, Domenyuk DA, Zelensky VA and Rubin M, RSC Adv, 2019, 9, 6636–6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colon NC and Chung DH, Adv. Pediatr, 2011, 58, 297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kholodenko IV, Kalinovsky DV, Doronin II, Deyev SM, Kholodenko RV, Doronin II, Kholodenko RV and Deyev SM, J Immunol Res, 2018, 2018, 7394268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de The H, Nat. Rev. Cancer, 2018, 18, 117–127. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds CP, Curr Oncol Rep, 2000, 2, 511–518. [DOI] [PubMed] [Google Scholar]
- 24.Chlapek P, Slavikova V, Mazanek P, Sterba J and Veselska R, Int. J. Mol. Sci, 2018, 19, 132/131–132/114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Z, Ma X, Hsiao T-H, Lin G, Kosti A, Yu X, Suresh U, Chen Y, Tomlinson GE, Pertsemlidis A and Du L, Oncotarget, 2014, 5, 2499–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huhti AL and Gartaganis PA, Can. J. Chem, 1956, 34, 785–797. [Google Scholar]
- 27.Uhlig F, Angew. Chem, 1954, 66, 435–436. [Google Scholar]
- 28.Calatayud DG, Lopez-Torres E and Mendiola MA, Eur. J. Inorg. Chem, 2013, 2013, 80–90. [DOI] [PubMed] [Google Scholar]
- 29.Marckwald W and Meyer E, Ber, 1900, 33, 1885–1895. [Google Scholar]
- 30.Ishikawa M and Kikkawa I, Yakugaku Zasshi (J. Pharm. Soc. Jpn.), 1955, 75, 36–39. [Google Scholar]
- 31.Schwendt G and Glasnov T, Monatshefte für Chemie-Chemical Mon, 2017, 148, 69–75. [Google Scholar]
- 32.Lindsay AC and Sperry J, Synlett, 2013, 24, 461–464. [Google Scholar]
- 33.Fleet GWJ and Fleming I, J. Chem. Soc. C Org, 1969, 13, 1758–1763. [Google Scholar]
- 34.Gandikota NM, Bolla RS, Viswanath IVK and Bethi S, Asian J. Chem, 2017, 29, 1920–1924. [Google Scholar]
- 35.Karp VK and Portnyagina VA, Pharmaceutical Chemistry Journal, 1970, 4, 390–393. [Google Scholar]
- 36.Wlodarczyk N, Simenel C, Delepierre M, Barale JC and Janin YL, Synthesis (Stuttg), 2011, 2011, 934–942. [Google Scholar]
- 37.Seter, Isr. J. Chem, 1966, 4, 7–22. [Google Scholar]
- 38.Gazit A, App H, McMahon G, Chen J, Levitzki A and Bohmer FD, J. Med. Chem, 1996, 39, 2170–2177. [DOI] [PubMed] [Google Scholar]
- 39.Blackburn TP, Cox B, Guildford AJ, Le Count DJ, Middlemiss DN, Pearce RJ and Thornber CW, J. Med. Chem, 1987, 30, 2252–2259. [DOI] [PubMed] [Google Scholar]
- 40.Hino K, Nagai Y and Uno H, Chem. Pharm. Bull, 1987, 35, 2819–2824. [DOI] [PubMed] [Google Scholar]
- 41.Davis SE, Rauckman BS, Chan JH and Roth B, J. Med. Chem, 1989, 32, 1936–1942. [DOI] [PubMed] [Google Scholar]
- 42.Büchi J, Hurni H and Lieberherr R, Helv. Chim. Acta, 1949, 32, 1806–1814. [Google Scholar]
- 43.Tagawa Y, Yamashita H, Nomura M and Goto Y, ChemInform, 1999, 30, 2379–2387. [Google Scholar]
- 44.Potts KT, Bhattacharyya J, Smith SL, Ihrig AM and Girard CA, J. Org. Chem, 1972, 37, 4410–4415. [Google Scholar]
- 45.Ye Z, Ding M, Wu Y, Li Y, Hua W and Zhang F, Green Chem, 2018, 20, 1732–1737. [Google Scholar]
- 46.Kidwai M, Goel Y and Kumar R, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem, 1998, 37B, 174–179. [Google Scholar]
- 47.Reddy Lonka M, Zhang J, Gogula T and Zou H, Org. Biomol. Chem, 2019, 17, 7455–7460. [DOI] [PubMed] [Google Scholar]
- 48.Nakka M, Tadikonda R, Rayavarapu S, Sarakula P and Vidavalur S, Synthesis, 2015, 47, 517–525. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


