The genetics underlying severe COVID-19
The immune system is complex and involves many genes, including those that encode cytokines known as interferons (IFNs). Individuals that lack specific IFNs can be more susceptible to infectious diseases. Furthermore, the autoantibody system dampens IFN response to prevent damage from pathogen-induced inflammation. Two studies now examine the likelihood that genetics affects the risk of severe coronavirus disease 2019 (COVID-19) through components of this system (see the Perspective by Beck and Aksentijevich). Q. Zhang et al. used a candidate gene approach and identified patients with severe COVID-19 who have mutations in genes involved in the regulation of type I and III IFN immunity. They found enrichment of these genes in patients and conclude that genetics may determine the clinical course of the infection. Bastard et al. identified individuals with high titers of neutralizing autoantibodies against type I IFN-α2 and IFN-ω in about 10% of patients with severe COVID-19 pneumonia. These autoantibodies were not found either in infected people who were asymptomatic or had milder phenotype or in healthy individuals. Together, these studies identify a means by which individuals at highest risk of life-threatening COVID-19 can be identified.
Science, this issue p. eabd4570, p. eabd4585; see also p. 404
In a large immunological and genomics study of COVID-19 patients, autoantibodies to type 1 interferons correlated with outcomes.
INTRODUCTION
Interindividual clinical variability is vast in humans infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), ranging from silent infection to rapid death. Three risk factors for life-threatening coronavirus disease 2019 (COVID-19) pneumonia have been identified—being male, being elderly, or having other medical conditions—but these risk factors cannot explain why critical disease remains relatively rare in any given epidemiological group. Given the rising toll of the COVID-19 pandemic in terms of morbidity and mortality, understanding the causes and mechanisms of life-threatening COVID-19 is crucial.
RATIONALE
B cell autoimmune infectious phenocopies of three inborn errors of cytokine immunity exist, in which neutralizing autoantibodies (auto-Abs) against interferon-γ (IFN-γ) (mycobacterial disease), interleukin-6 (IL-6) (staphylococcal disease), and IL-17A and IL-17F (mucocutaneous candidiasis) mimic the clinical phenotypes of germline mutations of the genes that encode the corresponding cytokines or receptors. Human inborn errors of type I IFNs underlie severe viral respiratory diseases. Neutralizing auto-Abs against type I IFNs, which have been found in patients with a few underlying noninfectious conditions, have not been unequivocally shown to underlie severe viral infections. While searching for inborn errors of type I IFN immunity in patients with life-threatening COVID-19 pneumonia, we also tested the hypothesis that neutralizing auto-Abs against type I IFNs may underlie critical COVID-19. We searched for auto-Abs against type I IFNs in 987 patients hospitalized for life-threatening COVID-19 pneumonia, 663 asymptomatic or mildly affected individuals infected with SARS-CoV-2, and 1227 healthy controls from whom samples were collected before the COVID-19 pandemic.
RESULTS
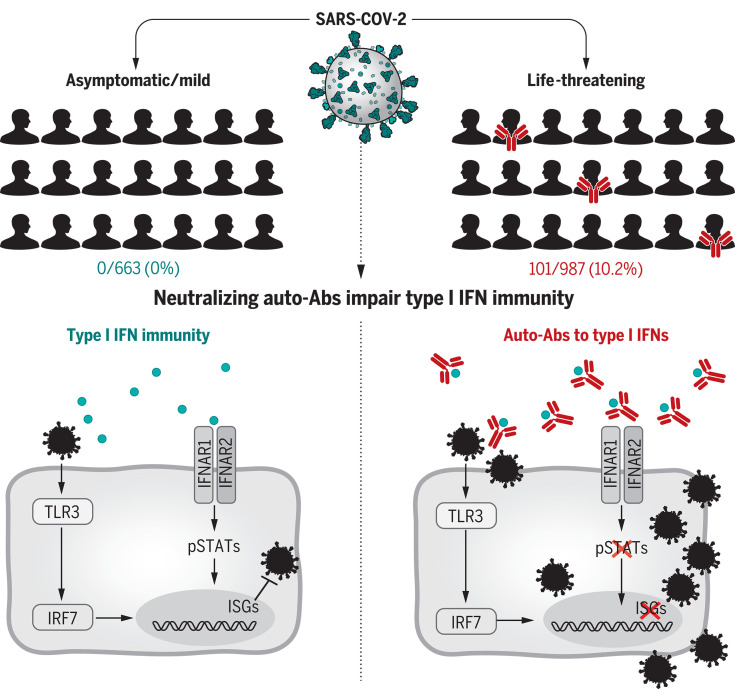
At least 101 of 987 patients (10.2%) with life-threatening COVID-19 pneumonia had neutralizing immunoglobulin G (IgG) auto-Abs against IFN-ω (13 patients), against the 13 types of IFN-α (36), or against both (52) at the onset of critical disease; a few also had auto-Abs against the other three individual type I IFNs. These auto-Abs neutralize high concentrations of the corresponding type I IFNs, including their ability to block SARS-CoV-2 infection in vitro. Moreover, all of the patients tested had low or undetectable serum IFN-α levels during acute disease. These auto-Abs were present before infection in the patients tested and were absent from 663 individuals with asymptomatic or mild SARS-CoV-2 infection (P < 10−16). They were present in only 4 of 1227 (0.33%) healthy individuals (P < 10−16) before the pandemic. The patients with auto-Abs were 25 to 87 years old (half were over 65) and of various ancestries. Notably, 95 of the 101 patients with auto-Abs were men (94%).
CONCLUSION
A B cell autoimmune phenocopy of inborn errors of type I IFN immunity accounts for life-threatening COVID-19 pneumonia in at least 2.6% of women and 12.5% of men. In these patients, adaptive autoimmunity impairs innate and intrinsic antiviral immunity. These findings provide a first explanation for the excess of men among patients with life-threatening COVID-19 and the increase in risk with age. They also provide a means of identifying individuals at risk of developing life-threatening COVID-19 and ensuring their enrolment in vaccine trials. Finally, they pave the way for prevention and treatment, including plasmapheresis, plasmablast depletion, and recombinant type I IFNs not targeted by the auto-Abs (e.g., IFN-β).
Neutralizing auto-Abs to type I IFNs underlie life-threatening COVID-19 pneumonia.
We tested the hypothesis that neutralizing auto-Abs against type I IFNs may underlie critical COVID-19 by impairing the binding of type I IFNs to their receptor and the activation of the downstream responsive pathway. Neutralizing auto-Abs are represented in red, and type I IFNs are represented in blue. In these patients, adaptive autoimmunity impairs innate and intrinsic antiviral immunity. ISGs, IFN-stimulated genes; TLR, Toll-like receptor; IFNAR, IFN-α/β receptor; pSTAT, phosphorylated signal transducers and activators of transcription; IRF, interferon regulatory factor.
Abstract
Interindividual clinical variability in the course of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is vast. We report that at least 101 of 987 patients with life-threatening coronavirus disease 2019 (COVID-19) pneumonia had neutralizing immunoglobulin G (IgG) autoantibodies (auto-Abs) against interferon-ω (IFN-ω) (13 patients), against the 13 types of IFN-α (36), or against both (52) at the onset of critical disease; a few also had auto-Abs against the other three type I IFNs. The auto-Abs neutralize the ability of the corresponding type I IFNs to block SARS-CoV-2 infection in vitro. These auto-Abs were not found in 663 individuals with asymptomatic or mild SARS-CoV-2 infection and were present in only 4 of 1227 healthy individuals. Patients with auto-Abs were aged 25 to 87 years and 95 of the 101 were men. A B cell autoimmune phenocopy of inborn errors of type I IFN immunity accounts for life-threatening COVID-19 pneumonia in at least 2.6% of women and 12.5% of men.
Mycobacteriosis, staphylococcosis, and candidiasis can be driven by monogenic inborn errors of interferon-γ (IFN-γ), interleukin-6 (IL-6), and IL-17A and IL-17F, respectively, or they can be driven by their genetically driven autoimmune phenocopies, with the production of neutralizing autoantibodies (auto-Abs) against these cytokines (1–8). Type I IFNs, first described in 1957, are ubiquitously expressed cytokines that contribute to both innate immunity (through their secretion by plasmacytoid dendritic cells and other leukocytes) and cell-intrinsic immunity (in most if not all cell types) against viral infections (9–13). Their receptors are ubiquitously expressed and trigger the induction of IFN-stimulated genes (ISGs) via phosphorylated STAT1-STAT2-IRF9 trimers (STAT, signal transducers and activators of transcription; IRF, interferon regulatory factor) (14). Neutralizing immunoglobulin G (IgG) auto-Abs against type I IFNs can occur in patients treated with IFN-α2 or IFN-β (15) and exist in almost all patients with autoimmune polyendocrinopathy syndrome type I (APS-1) (16). They are also seen in women with systemic lupus erythematosus (17).
These patients do not seem to suffer from unusually severe viral infections, although human inborn errors of type I IFNs can underlie severe viral diseases, both respiratory and otherwise (18). In 1984, Ion Gresser described a patient with unexplained auto-Abs against type I IFNs suffering from severe chickenpox and shingles (19, 20). More recently, auto-Abs against type I IFNs have been found in a few patients with biallelic, hypomorphic RAG1 or RAG2 mutations and viral diseases including severe chickenpox and viral pneumonias (21). Our attention was drawn to three patients with APS-1, with known preexisting anti–type I IFN auto-Abs, who had life-threatening coronavirus disease 2019 (COVID-19) pneumonia (22) (see detailed case reports in Methods). While searching for inborn errors of type I IFNs (18, 23), we hypothesized that neutralizing auto-Abs against type I IFNs might also underlie life-threatening COVID-19 pneumonia.
Auto-Abs against IFN-α2 and/or IFN-ω in patients with critical COVID-19
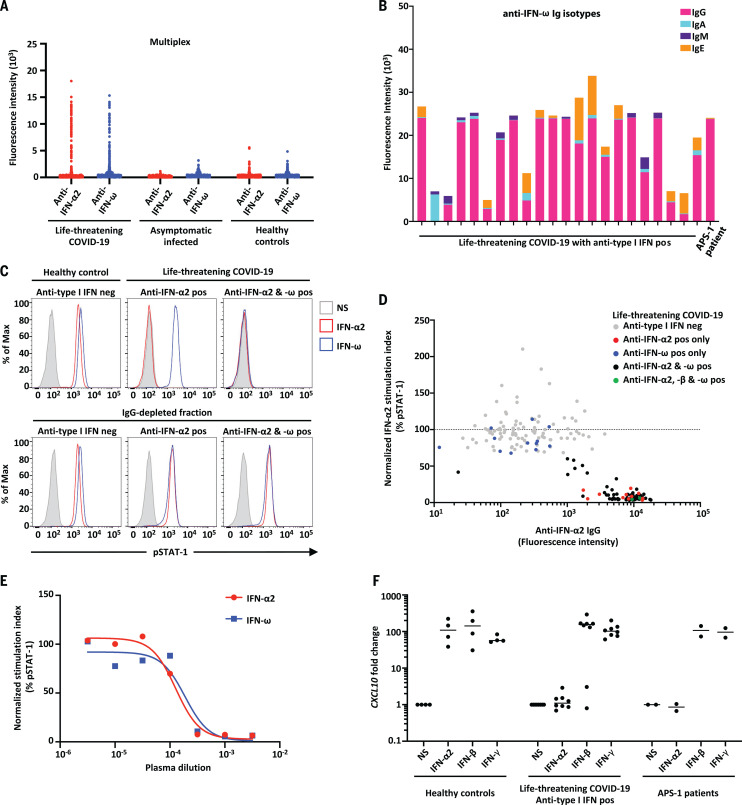
We searched for auto-Abs against type I IFNs in 987 patients hospitalized for life-threatening COVID-19 pneumonia. We also examined 663 individuals infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) presenting asymptomatic infection or mild disease and 1227 healthy controls whose samples were collected before the COVID-19 pandemic. Plasma or serum samples were collected from patients with critical COVID-19 during the acute phase of disease. Multiplex particle-based flow cytometry revealed a high fluorescence intensity (FI) (>1500) for IgG auto-Abs against IFN-α2 and/or IFN-ω in 135 patients (13.7%) with life-threatening COVID-19 (Fig. 1A). We found that 49 of these 135 patients were positive for auto-Abs against both IFN-α2 and IFN-ω, whereas 45 were positive only for auto-Abs against IFN-α2, and 41 were positive only for auto-Abs against IFN-ω.
Fig. 1. Neutralizing auto-Abs against IFN-α2 and/or IFN-ω in patients with life-threatening COVID-19.
(A) Multiplex particle-based assay for auto-Abs against IFN-α2 and IFN-ω in patients with life-threatening COVID-19 (N = 782), in patients with asymptomatic or mild SARS-CoV-2 infection (N = 443), and in healthy controls not infected with SARS-CoV-2 (N = 1160). (B) Anti–IFN-ω Ig isotypes in 23 patients with life-threatening COVID-19 and auto-Abs to type I IFNs. (C) Representative fluorescence-activated cell sorting (FACS) plots depicting IFN-α2– or IFN-ω–induced pSTAT1 in healthy control cells (gated on CD14+ monocytes) in the presence of 10% healthy control or anti–IFN-α2 or anti–IFN-ω auto-Abs–containing patient plasma (top panel) or an IgG-depleted plasma fraction (bottom panel). Max, maximum; neg, negative; pos, positive; NS, not stimulated. (D) Plot of anti–IFN-α2 auto-Ab levels against their neutralization capacity. The stimulation index (stimulated over unstimulated condition) for the plasma from each patient was normalized against that of healthy control plasma from the same experiment. Spearman’s rank correlation coefficient = −0.6805; P < 0.0001. (E) Median inhibitory concentration (IC50) curves representing IFN-α2– and IFN-ω–induced pSTAT1 levels in healthy donor cells in the presence of serial dilutions of patient plasma. The stimulation index (stimulated over unstimulated condition) for patient plasma was normalized against that of 10% healthy control plasma. IFN-α2: IC50 = 0.016%, R2 = 0.985; IFN-ω: IC50 = 0.0353%, R2 = 0.926. R2, coefficient of determination. (F) Neutralizing effect on CXLC10 induction, after stimulation with IFN-α2, IFN-β, or IFN-γ, in the presence of plasma from healthy controls (N = 4), patients with life-threatening COVID-19 and auto-Abs against IFN-α2 (N = 8), and APS-1 patients (N = 2).
We also performed enzyme-linked immunosorbent assay (ELISA), and the results obtained were consistent with those obtained with Luminex technology (fig. S1A). We found that 11 and 14 of 23 patients tested had low levels of IgM and IgA auto-Abs against IFN-ω and IFN-α2, respectively (Fig. 1B and fig. S1B). Auto-Abs against type I IFNs were detected in two unrelated patients for whom we had plasma samples obtained before SARS-CoV-2 infection, which indicates that these antibodies were present before SARS-CoV-2 infection and were not triggered by the infection. As a control, we confirmed that all 25 APS-1 patients tested had high levels of auto-Abs against IFN-α2 and IFN-ω (fig. S1C). Overall, we found that 135 of 987 patients (13.7%) with life-threatening COVID-19 pneumonia had IgG auto-Abs against at least one type I IFN.
The auto-Abs neutralize IFN-α2 and IFN-ω in vitro
We then tested whether auto-Abs against IFN-α2 and IFN-ω were neutralizing in vitro. We incubated peripheral blood mononuclear cells (PBMCs) from healthy controls with 10 ng/mL IFN-α2 or IFN-ω in the presence of plasma from healthy individuals or from patients with auto-Abs. A complete abolition of STAT1 phosphorylation was observed in 101 patients with auto-Abs against IFN-α2 and/or IFN-ω (table S1). The antibodies detected were neutralizing against both IFN-α2 and IFN-ω in 52 of these 101 patients (51%), against only IFN-α2 in 36 patients (36%), and against only IFN-ω in 13 patients (13%) at the IFN-α2 and IFN-ω concentrations tested (Fig. 1, C and D). IgG depletion from patients with auto-Abs restored normal pSTAT1 induction after IFN-α2 and IFN-ω stimulation, whereas the purified IgG fully neutralized this induction (Fig. 1C and fig. S1D). Furthermore, these auto-Abs neutralized high amounts of IFN-α2 (fig. S1E) and were neutralizing at high dilutions (Fig. 1E and fig. S1F). Notably, 15 patients with life-threatening COVID-19 and auto-Abs against IFN-α2 and/or IFN-ω also had auto-Abs against other cytokines [IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-6, IL-10, IL-12p70, IL-22, IL-17A, IL-17F, and/or tumor necrosis factor–β (TNFβ)], only three of which (IL-12p70, IL-22, and IL-6) were neutralizing (in four patients) (fig. S2, A to C). Similar proportions were observed in the other cohorts (fig. S2, D to L).
We also analyzed ISG induction after 2 hours of stimulation with IFN-α2, IFN-β, or IFN-γ in the presence of plasma from healthy individuals or from patients with auto-Abs. With plasma from eight patients with auto-Abs against IFN-α2, the induction of ISG CXCL10 was abolished after IFN-α2 stimulation but maintained after stimulation with IFN-γ (Fig. 1F). We then found that plasma from the five patients with neutralizing auto-Abs neutralized the protective activity of IFN-α2 in Madin–Darby bovine kidney (MDBK) cells infected with vesicular stomatitis virus (VSV) (table S2). Overall, we found that 101 of 987 patients (10.2%)—including 95 men (94%)—with life-threatening COVID-19 pneumonia had neutralizing IgG auto-Abs against at least one type I IFN. By contrast, auto-Abs were detected in only 4 of 1227 healthy controls (0.33%) (Fisher exact test, P < 10−16) and in none of the 663 patients with asymptomatic or mild SARS-CoV-2 infection tested (Fisher exact test, P < 10−16).
Auto-Abs against all 13 IFN-α subtypes in patients with auto-Abs to IFN-α2
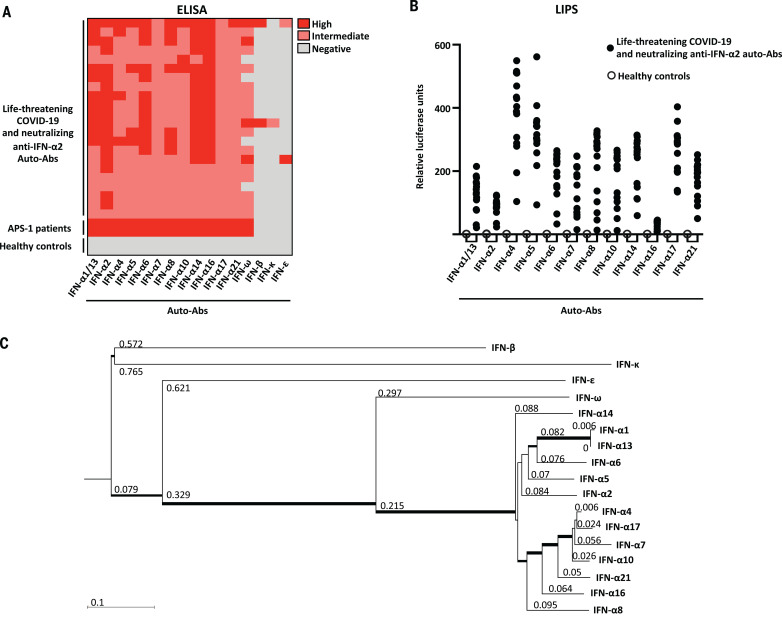
We investigated whether patients with neutralizing auto-Abs against IFN-α2 only or those with neutralizing auto-Abs against IFN-α2 and IFN-ω also had auto-Abs against the other 15 type I IFNs. ELISA showed that all patients tested (N = 22) with auto-Abs against IFN-α2 also had auto-Abs against all 13 IFN-α subtypes (IFN-α1, -α2, -α4, -α5, -α6, -α7, -α8, -α10, -α13, -α14, -α16, -α17, and -α21), whereas only 2 of the 22 patients tested had auto-Abs against IFN-β, 1 had auto-Abs against IFN-κ, and 2 had auto-Abs against IFN-ε (Fig. 2A). The auto-Abs against IFN-β had neutralizing activity against IFN-β (Fig. 1D). We confirmed that all of the patients had auto-Abs against all 13 subtypes of IFN-α by testing the same samples using luciferase-based immunoprecipitation assay (LIPS) (Fig. 2B). For IFN-β, we also screened the whole cohort in a multiplex assay. We found that 19 of 987 (1.9%) patients had auto-Abs against IFN-β and that all of them were in our cohort of severe COVID-19 individuals with neutralizing auto-Abs against IFN-α and/or IFN-ω. Of these patients with auto-Abs against IFN-β, only two were neutralizing against IFN-β (Fig. 1, D and F).
Fig. 2. Auto-Abs against the different type I IFN subtypes.
(A) ELISA for auto-Abs against the 13 different IFN-α subtypes, IFN-ω, IFN-β, IFN-κ, and IFN-ε in patients with life-threatening COVID-19 and auto-Abs against IFN-α2 (N = 22), APS-1 patients (N = 2), and healthy controls (N = 2). (B) LIPS for the 12 different IFN-α subtypes tested in patients with auto-Abs against IFN-α2 (N = 22) and healthy controls (N = 2). (C) Neighbor-joining phylogenetic tree of the 17 human type I IFN proteins. Horizontal branches are drawn to scale (bottom left, number of substitutions per site). Thinner, intermediate, and thicker internal branches have bootstrap support of <50, ≥50, and >80%, respectively. The bootstrap value for the branch separating IFN-ω from all IFN-α subtypes is 100%.
Ten of the 17 genes encoding type I IFNs (IFN-α2, -α5, -α6, α8, -α13, -α14, -α21, -β, -ω, and -κ), have undergone strong negative selection, which suggests that they play an essential role in the general population. By contrast, the other seven IFN loci in the human genome often carry loss-of-function alleles (24). Moreover, the 13 IFN-α subtypes and IFN-ω are more-closely related to each other than they are to the other three IFNs (IFN-β, IFN-ε, and IFN-κ), which are structurally and phylogenetically more distant (Fig. 2C). Thus, all patients with neutralizing auto-Abs against IFN-α2 that we tested (N = 22) had auto-Abs against all 13 IFN-α subtypes, and 3 of the 22 patients tested (14%) had auto-Abs against 14 or more type I IFNs.
The auto-Abs neutralize IFN-α2 against SARS-CoV-2 in vitro and IFN-α in vivo
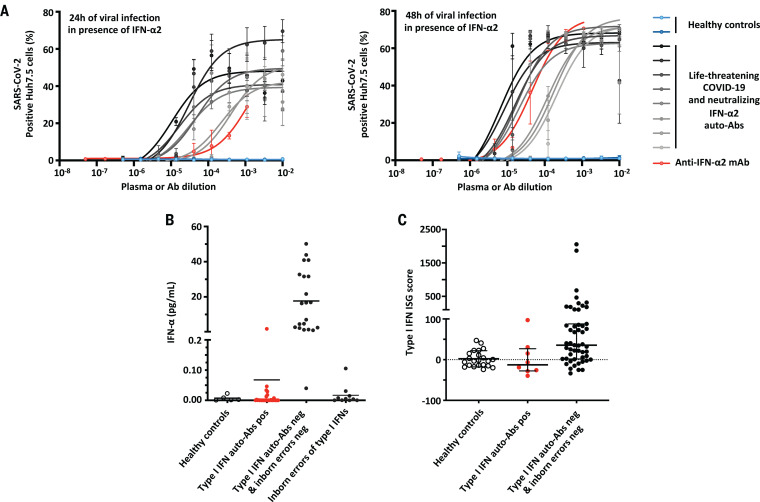
Plasma from eight patients with neutralizing auto-Abs against type I IFN also neutralized the ability of IFN-α2 to block the infection of Huh7.5 cells with SARS-CoV-2 (Fig. 3A). Plasma from two healthy controls or from seven SARS-CoV-2–infected patients without auto-Abs did not block the protective action of IFN-α2 (Fig. 3A and fig. S3A). These data provide compelling evidence that the patients’ blood carried sufficiently large amounts of auto-Abs to neutralize the corresponding type I IFNs and block their antiviral activity in vitro, including that against SARS-CoV-2.
Fig. 3. Enhanced SARS-CoV-2 replication, despite the presence of IFN-α2, in the presence of plasma from patients with auto-Abs against IFN-α2 and low in vivo levels of IFN-α.
(A) SARS-CoV-2 replication—measured 24 hours (left) and 48 hours (right) after infection—in Huh7.5 cells treated with IFN-α2 in the presence of plasma from patients with life-threatening COVID-19 and neutralizing auto-Abs against IFN-α2 (N = 8); a commercial anti–IFN-α2 antibody; or control plasma (N = 2). (B) IFN-α levels in the plasma or serum of patients with neutralizing auto-Abs (N = 41), healthy controls (N = 5), COVID-19 patients without auto-Abs (N = 21), and patients with life-threatening COVID-19 and loss-of-function (LOF) variants (N = 10), as assessed by Simoa ELISA. (C) z-scores for type I IFN gene responses in whole blood of COVID-19 patients with (N = 8) or without (N = 51) neutralizing auto-Abs, or healthy uninfected controls (N = 22). The median ± interquartile range is shown. z-scores were significantly lower for patients with neutralizing auto-Abs compared with patients without auto-Abs (Mann-Whitney test, P = 0.01).
We also found that all 41 patients with neutralizing auto-Abs against the 13 types of IFN-α tested had low (one patient) or undetectable (40 patients) levels of the 13 types of IFN-α in their plasma during the course of the disease (Fig. 3B) (25, 26). Type I IFNs may be degraded and/or bound to the corresponding circulating auto-Abs. The presence of circulating neutralizing auto-Abs against IFN-α is, therefore, strongly associated with low serum IFN-α levels (Fisher exact test, P < 10−6). Consistently in patients with neutralizing auto-Abs against IFN-α2, the baseline levels of type I IFN–dependent transcripts were low, whereas they were normal for nuclear factor κB (NF-κB)–dependent transcripts (Fig. 3C and fig. S3B). Overall, our findings indicate that the auto-Abs against type I IFNs present in patients with life-threatening COVID-19 were neutralizing in vitro and in vivo.
Pronounced excess of men in patients with auto-Abs against type I IFNs
There was a pronounced excess of male patients (95 of 101; 94%) with critical COVID-19 pneumonia and neutralizing auto-Abs against type I IFNs. This proportion of males was higher than that observed in patients with critical COVID-19 without auto-Abs (75%; Fisher exact test, P = 2.5 × 10−6), and the proportion was much higher than that in male patients in the asymptomatic or pauci-symptomatic cohort (28%; Fisher exact test, P < 10−6) (Table 1, Fig. 4A, and fig. S4A). Further evidence for X-chromosome linkage was provided by the observation that one of the seven women with auto-Abs and life-threatening COVID-19 had X chromosome–linked incontinentia pigmenti (IP), in which cells activate only a single X chromosome (cells having activated the X chromosome bearing the null mutation in NEMO dying in the course of development) (27). The prevalence of auto-Abs against type I IFNs in the general population was estimated at 0.33% (0.015 to 0.67%) in a sample of 1227 healthy individuals—a value much lower than that in patients with life-threatening COVID-19 pneumonia, by a factor of at least 15.
Table 1. Sex and age distribution of patients with critical COVID-19 with and without auto-Abs.
Ages and sexes of the patients and controls and information about auto-Abs against IFN-α2 and IFN-ω, presented by age and sex. Dashes in rightmost column indicate data not available. OR, odds ratio; CI, confidence interval.
|
Life-threatening COVID-19 |
N total |
N auto-Abs positive (percentage) |
OR [95% CI] | P value* |
| Sex | ||||
| Female | 226 | 6 (2.6%) | 1 | – |
| Male | 761 | 95 (12.5%) | 5.22 [2.27 – 14.80] | 2.5 × 10−6 |
| Age | ||||
| <65 years | 602 | 51 (8.5%) | 1 | – |
| ≥65 years | 385 | 50 (13.0%) | 1.61 [1.04 – 2.49] | 0.024 |
*P values were derived from Fisher’s exact test, as implemented in R (https://cran.r-project.org/).
Fig. 4. Demographic and ethnic information about the patients and controls.
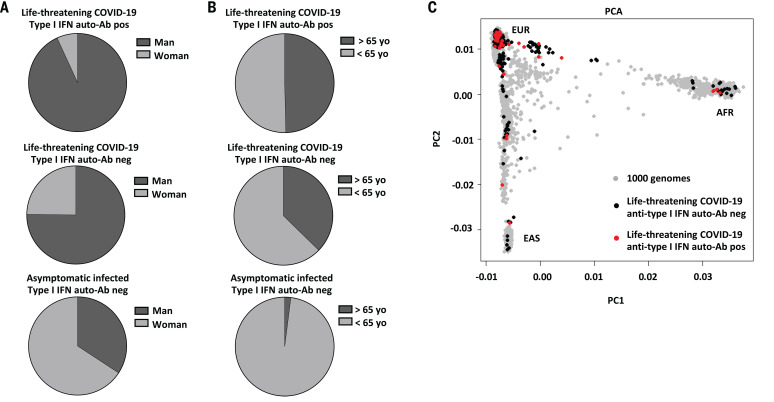
(A) Gender distribution in patients with life-threatening COVID-19 and auto-Abs to type I IFNs, patients with life-threatening COVID-19 and without auto-Abs to type I IFNs, and individuals with asymptomatic or mild SARS-CoV-2. (B) Age distribution in patients with life-threatening COVID-19 and auto-Abs to type I IFNs, patients with life-threatening COVID-19 and without auto-Abs to type I IFNs, and individuals with asymptomatic or mild SARS-CoV-2. yo, years old. (C) PCA on 49 patients with life-threatening COVID-19 and auto-Abs against type I IFNs. EUR, Europeans; AFR, Africans; EAS, East-Asians.
The patients with auto-Abs were also slightly older than the rest of our cohort (49.5% of patients positive for auto-Abs were over 65 years of age versus 38% for the rest of the cohort; P = 0.024), which suggests that the frequency of circulating anti–type I IFNs auto-Abs increases with age (Table 1 and Fig. 4B). However, auto-Abs were found in patients aged from 25 to 87 years (fig. S4B). Principal components analysis (PCA) was performed on data from 49 patients: 34 Europeans, 5 North Africans, 4 sub-Saharan Africans, 2 patients from the Middle East, 2 South Asians, 1 East Asian, and 1 South American (Fig. 4C). Large-scale studies will be required to determine the frequency of such auto-Abs in humans of different sexes, ages, and ancestries. Finally, the presence of auto-Abs was associated with a poor outcome, with death occurring in 37 of the 101 patients (36.6%) (table S1).
Neutralizing auto-Abs to type I IFNs are causative of critical COVID-19
There are multiple lines of evidence to suggest that the neutralizing auto-Abs against type I IFNs observed in these 101 patients preceded infection with SARS-CoV-2 and accounted for the severity of disease. First, the two patients for whom testing was performed before COVID-19 were found to have auto-Abs before infection. Second, three patients with APS-1 known to have neutralizing auto-Abs against type I IFN immunity before infection also had life-threatening COVID-19 (22) (supplementary methods). Third, we screened a series of 32 women with IP and found that a quarter of them had auto-Abs against type I IFNs, including one who developed critical COVID-19 (fig. S1C). Fourth, there is a marked bias in favor of men, which suggests that the production of auto-Abs against type I IFNs—whether driven by germ line or somatic genome—may be X chromosome–linked and therefore preexisting to infection.
Moreover, IFN-α subtypes were undetectable during acute disease in the blood of patients with auto-Abs against IFN-α, which suggests a preexisting or concomitant biological impact in vivo. It is also unlikely that patients could break self-tolerance and mount high titers of neutralizing IgG auto-Abs against type I IFN within only 1 or even 2 weeks of infection. Finally, inborn errors of type I IFNs underlying life-threatening COVID-19 in other previously healthy adults—including autosomal recessive IFN-α/β receptor subunit 1 (IFNAR1) deficiency—have also been reported in an accompanying paper (18). Collectively, these findings suggest that auto-Abs against type I IFNs are a cause and not a consequence of severe SARS-Cov-2 infection, although their titers and affinity may be enhanced by the SARS-CoV-2–driven induction of type I IFNs. They also provide an explanation for the major sex bias seen in patients with life-threatening COVID-19 and perhaps also for the increase in risk with age.
Conclusion
We report here that at least 10% of patients with life-threatening COVID-19 pneumonia have neutralizing auto-Abs against type I IFNs. With our accompanying description of patients with inborn errors of type I IFNs and life-threatening COVID-19 (18), this study highlights the crucial role of type I IFNs in protective immunity against SARS-CoV-2. These auto-Abs against type I IFNs were clinically silent until the patients were infected with SARS-CoV-2—a poor inducer of type I IFNs (28)—which suggests that the small amounts of IFNs induced by the virus are important for protection against severe disease. The neutralizing auto-Abs against type I IFNs, like inborn errors of type I IFN production, tip the balance in favor of the virus, which results in devastating disease with insufficient, and even perhaps deleterious, innate and adaptive immune responses.
Our findings have direct clinical implications. First, SARS-CoV-2–infected patients can be screened to identify individuals with auto-Abs at risk of developing life-threatening pneumonia. Such patients recovering from life-threatening COVID-19 should also be excluded from donating convalescent plasma for ongoing clinical trials, or at least they should be tested before their plasma donations are accepted (29). Second, this finding paves the way for preventive or therapeutic intervention, including plasmapheresis, monoclonal Abs depleting plasmablasts, and the specific inhibition of type I IFN–reactive B cells (30). Finally, in this patient group, early treatment with IFN-α is unlikely to be beneficial; however, treatment with injected or nebulized IFN-β may have beneficial effects, as auto-Abs against IFN-β appear to be rare in patients with auto-Abs against type I IFNs.
Materials and methods
Subjects and samples
We enrolled 987 patients with proven life-threatening (critical) COVID-19, 663 asymptomatic or pauci-symptomatic individuals with proven COVID-19, and 1227 healthy controls in this study. All subjects were recruited following protocols approved by local Institutional Review Boards (IRBs). All protocols followed local ethics recommendations and informed consent was obtained when required.
COVID-19 disease severity was assessed in accordance with the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia. The term life-threatening COVID-19 pneumonia describes pneumonia in patients with critical disease, whether pulmonary, with mechanical ventilation [continuous positive airway pressure (CPAP), bilevel positive airway pressure (BIPAP), intubation, or high-flow oxygen], septic shock, or damage to any other organ requiring admission in the intensive care unit (ICU). The individuals with asymptomatic or mild SARS-CoV-2 infection were individuals infected with SARS-CoV-2 who remained asymptomatic or developed mild, self-healing, ambulatory disease with no evidence of pneumonia. The healthy controls were individuals who had not been exposed to SARS-CoV-2.
Plasma and serum samples from the patients and controls were frozen at −20°C immediately after collection. The fluid-phase LIPS assay was used to determine the levels of antibodies against the SARS-CoV-2 nucleoprotein and spike protein, as has been previously described (31).
Detection of anti-cytokine auto-Abs
Multiplex particle-based assay
Serum and plasma samples were screened for auto-Abs against 18 targets in a multiplex particle-based assay, in which magnetic beads with differential fluorescence were covalently coupled to recombinant human proteins. Patients with an FI of >1500 for IFN-α2 or IFN-β or >1000 for IFN-ω were tested for blocking activity, as were patients positive for another cytokine.
ELISA
ELISA was performed as previously described (5). In brief, ELISA plates were coated with recombinant human interferon-α (rhIFN-α) or rhIFN-ω and incubated with 1:50 dilutions of plasma samples from the patients or controls. A similar protocol was used when testing for 12 subtypes of IFN-α.
LIPS
Levels of auto-Abs against IFN-α subtypes were measured with LIPS, as previously described (32). IFN-α1, IFN-α2, IFN-α4, IFN-α5, IFN-α6, IFN-α7, IFN-α8, IFN-α10, IFN-α14, IFN-α16, IFN-α17, and IFN-α21 sequences were transfected in HEK293 cells, and the IFN-α-luciferase fusion proteins were collected in the tissue culture supernatant. For autoantibody screening, serum samples were incubated with protein G agarose beads, and we then added 2 × 106 luminescence units (LU) of antigen and incubated. Luminescence intensity was measured. The results are expressed in arbitrary units (AU), as a fold-difference relative to the mean of the negative control samples.
Functional evaluation of anti-cytokine auto-Abs
The blocking activity of anti–IFN-α and anti–IFN-ω auto-Abs was determined by assessing STAT1 phosphorylation in healthy control cells after stimulation with the appropriate cytokines in the presence of 10% healthy control or patient serum or plasma.
We demonstrated that the IFN-α and IFN-ω blocking activity observed was due to auto-Abs and not another plasma factor, by depleting IgG from the plasma with a protein G column Without eluting the IgG, the flow-through fraction (IgG-depleted) was then collected and compared with total plasma in the phospho-STAT1 assay.
The blocking activity of anti–IFN-γ, –GM-CSF, –IFN-λ1, –IFN-λ2, –IFN-λ3, –IL-6, –IL-10, –IL-12p70, –IL-22, –IL-17A, –IL-17F, -TNFα, and -TNFβ antibodies was assessed with the assays outlined in table S3, as previously reported (21).
For the neutralization of ISG induction, PBMCs were left unstimulated or were stimulated for 2 hours with 10 ng/mL IFN-α or 10 ng/mL IFN-γ in a final volume of 100 μL. Real-time quantitative polymerase chain reaction (RT-qPCR) analysis was performed with Applied Biosystems Taqman assays for CXCL10, and the β-glucuronidase (GUS) housekeeping gene for normalization. Results are expressed according to the ΔΔCt method, as described by the manufacturer’s kit.
Phylogenetic reconstruction
Protein sequences were aligned with the online version of MAFFT v7.471 software (33), using the L-INS-i strategy (34) and the BLOSUM62 scoring matrix for amino acid substitutions. Phylogenetic tree reconstruction was performed by the neighbor-joining method (35) with the substitution model (36). Low-confidence branches (<50%) are likely to be due to gene conversion events between IFNA genes, as previously reported (24, 37). The tree was then visualized (38). Very similar results were obtained with the corresponding DNA sequences (37, 39).
Statistical analysis
Comparison of proportions were performed using a Fisher exact test, as implemented in R (https://cran.r-project.org/). PCA was performed with Plink v1.9 software on whole-exome and whole-genome sequencing data with the 1000 Genomes (1kG) Project phase 3 public database as a reference.
Simoa
Serum IFN-α concentrations were determined with Simoa technology, as previously described (40, 41), with reagents and procedures obtained from the Quanterix Corporation.
VSV assay
The seroneutralization assay was performed as previously described (42). In brief, the incubation of IFN-α2 with MDBK cells protects the cultured cells against the cytopathic effect of VSV. The titer of anti–IFN-α antibodies was defined as the last dilution causing 50% cell death.
SARS-CoV-2 experiment
SARS-CoV-2 strain USA-WA1/2020 was obtained from BEI Resources and amplified in Huh7.5 hepatoma cells at 33°C. Viral titers were measured on Huh7.5 cells in a standard plaque assay. Plasma samples or a commercial anti–IFN-α2 antibody were serially diluted and incubated with 20 pM recombinant IFN-α2 for 1 hour at 37°C (starting concentrations: plasma samples = 1/100 and anti–IFN-α2 antibody = 1/1000). The cell culture medium was then removed and replaced with the plasma– or antibody–IFN-α2 mixture. The plates were incubated overnight, and the plasma– or antibody–IFN-α2 mixture was removed by aspiration. The cells were washed once with phosphate-buffered saline (PBS) to remove potential anti–SARS-CoV-2 neutralizing antibodies, and fresh medium was then added. Cells were then infected with SARS-CoV-2 by directly adding the virus to the wells. Cells infected at a high multiplicity of infection (MOI) were incubated at 37°C for 24 hours, whereas cells infected at a low MOI were incubated at 33°C for 48 hours. The cells were fixed with 7% formaldehyde, stained for SARS-CoV-2 with an anti-N antibody, imaged, and analyzed as previously described (43).
Nanostring
For the NanoString assay, total RNA was extracted from whole blood samples collected in PaxGene tubes. The expression of selected genes was determined by NanoString methods and a 28-gene type I IFN score was calculated (44).
Acknowledgments
We thank the patients, their families, and healthy donors for placing their trust in us. We thank the French Incontinentia pigmenti association for their help and support. We thank Y. Nemirovskaya, D. Papandrea, M. Woollett, D. Liu, C. Rivalain, and C. Patissier for administrative assistance; D. Kapogiannis (National Institute on Aging) for providing healthy donor samples; and S. Xirasager, J. Barnett, X. Cheng, S. Weber, J. Danielson, B. Garabedian, and H. Matthews for their assistance in this study. We also thank R. Apps, B. Ryan, and Y. Belkaid of the CHI for their assistance. We thank the CRB-Institut Jérôme Lejeune, CRB-BioJeL, Paris, France, for their assistance. We thank M. C. García Guerrero; I. Erkizia; E. Grau; M. Massanella from IrsiCaixa AIDS Research Institute, Badalona, Spain; and J. Guitart from the Department of Clinical Genetics, University Hospital Germans Trias i Pujol, Badalona, Spain, for providing samples. We also thank J. Dalmau from IrsiCaixa for assistance. Funding: The Laboratory of Human Genetics of Infectious Diseases is supported by the Howard Hughes Medical Institute, The Rockefeller University, the St. Giles Foundation, the National Institutes of Health (NIH) (R01AI088364), the National Center for Advancing Translational Sciences (NCATS), NIH Clinical and Translational Science Award (CTSA) program (UL1 TR001866), a Fast Grant from Emergent Ventures, the Mercatus Center at George Mason University, the Yale Center for Mendelian Genomics and the GSP Coordinating Center funded by the National Human Genome Research Institute (NHGRI) (UM1HG006504 and U24HG008956), the French National Research Agency (ANR) under the Investments for the Future program (ANR-10-IAHU-01), the Integrative Biology of Emerging Infectious Diseases Laboratory of Excellence (ANR-10-LABX-62-IBEID), the French Foundation for Medical Research (FRM) (EQU201903007798), the FRM and ANR GENCOVID project (ANRS-COV05), the Square Foundation, Grandir – Fonds de solidarité pour l’enfance, the SCOR Corporate Foundation for Science, the Institut Institut National de la Santé et de la Recherche Médicale (INSERM), and the University of Paris. Samples from San Raffaele Hospital were obtained through the Covid-BioB project and by healthcare personnel of San Raffaele Hospital, San Raffaele Telethon Institute for Gene Therapy (SR-TIGET) clinical laboratory and clinical research unit, funded by the Program Project COVID-19 OSR-UniSR and Fondazione Telethon. The French COVID Cohort Study Group was sponsored by INSERM and supported by the REACTing consortium and by a grant from the French Ministry of Health (PHRC 20-0424). The Cov-Contact Cohort was supported by the REACTing consortium, the French Ministry of Health, and the European Commission (RECOVER WP 6). The Milieu Intérieur Consortium was supported by the French Government’s Investissement d’Avenir program, Laboratoire d’Excellence Milieu Intérieur grant (ANR-10-LABX-69-01) (primary investigators: L.Q.-M. and D.Du.). The Simoa experiment was supported by the PHRC-20-0375 COVID-19 grant “DIGITAL COVID” (primary investigator: G.G.). S.G.T. is supported by a Leadership 3 Investigator Grant awarded by the National Health and Medical Research Council of Australia and a COVID19 Rapid Response Grant awarded by UNSW Sydney. C.R.-G. and colleagues were supported by the Instituto de Salud Carlos III (COV20_01333 and COV20_01334, Spanish Ministry of Science and Innovation RTC-2017-6471-1; AEI/FEDER, UE) and Cabildo Insular de Tenerife (CGIEU0000219140 and “Apuestas científicas del ITER para colaborar en la lucha contra la COVID-19”). S.T.-A. and A.B. were supported by ANR-20-COVI-0064 (primary investigator: A.Be.). This work is supported by the French Ministry of Health “Programme Hospitalier de Recherche Clinique Inter regional 2013,” by the Contrat de Plan Etat-Lorraine and FEDER Lorraine, and by a public grant overseen by the French National Research Agency (ANR) as part of the second Investissements d’Avenir program FIGHT-HF (reference no. ANR-15-RHU-0004) and by the French PIA project “Lorraine Université d’Excellence” (reference no. ANR-15-IDEX-04-LUE) (45); and biobanking is performed by the Biological Resource Center Lorrain BB-0033-00035. This study was supported by the Fonds IMMUNOV, for Innovation in Immunopathology; by a grant from the Agence National de la Recherche (ANR-flash Covid19 “AIROCovid” to F.R.-L.); and by the FAST Foundation (French Friends of Sheba Tel Hashomer Hospital). Work in the Laboratory of Virology and Infectious Disease was supported by NIH grants P01AI138398-S1, 2U19AI111825, and R01AI091707-10S1; a George Mason University Fast Grant; and the G. Harold and Leila Y. Mathers Charitable Foundation. The Amsterdam UMC Covid-19 Biobank was supported by grants from the Amsterdam Corona Research Fund, the Dr. C.J. Vaillant Fund, and the Netherlands Organization for Health Research and Development [ZonMw; NWO-Vici-Grant (grant no. 918·19·627 to D.v.d.B.)]. This work was also supported by the Division of Intramural Research of the National Institute of Dental Craniofacial Research and the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and by Regione Lombardia, Italy (project “Risposta immune in pazienti con COVID-19 e comorbidita”). The opinions and assertions expressed herein are those of the author(s) and do not necessarily reflect the official policy or position of the Uniformed Services University or the Department of Defense. J.H. holds an Institut Imagine M.D.-Ph.D. fellowship from the Fondation Bettencourt Schueller. J.R. is supported by the INSERM Ph.D. program (“poste d’accueil Inserm”). P.Ba. was supported by the French Foundation for Medical Research (FRM, EA20170638020) and the M.D.-Ph.D. program of the Imagine Institute (with the support of the Fondation Bettencourt-Schueller). We thank the Association “Turner et vous” for their help and support. Sample processing at IrsiCaixa was possible thanks to the crowdfunding initiative YoMeCorono. D.C.V. is supported by the Fonds de la recherche en santé du Québec clinician-scientist scholar program. K.K. was supported by the Estonian Research Council grant PUT1367. We thank the GEN-COVID Multicenter Study (https://sites.google.com/dbm.unisi.it/gen-covid). We thank the NIAID Office of Cyber Infrastructure and Computational Biology, Bioinformatics and Computational Biosciences Branch (contract no. HHSN316201300006W/HHSN27200002 to MSC, Inc.), the Operations Engineering Branch for developing the HGRepo system to enable streamlined access to the data, and the NCI Advanced Biomedical Computational Science (ABCS) for data transformation support. Biomedical Advanced Research and Development Authority was supported under contract no. HHSO10201600031C (to J.H.). Financial support was provided by the National Institute of Allergy and Infectious Diseases (NIAID) K08AI135091; the Burroughs Wellcome Fund CAMS; the Clinical Immunology Society; and the American Academy of Allergy, Asthma, and Immunology. Author contributions: P.Ba., L.B.R., Q.Z., E.M., H.-H.H., Y.Z., K.Dor., Q.P., J.R., V.B., J.Ma., E.S., L.H., P.P., L.L., L.B., S.T.-A., K.Dob., A.A.d.J., A.Be., L.P., D.D., E.S.H., J.S.T., R.G.-M., K.K., A.P., S.-Y.Z., S.M.H., G.G., E.J., C.M.R., L.D.N., H.C.S., and J.-L.C. performed or provided supervision of experiments, generated and analyzed data, and contributed to the manuscript by providing figures and tables. J.L.P., G.K., B.B., Y.S., R.Y., A.B., K.B., R.P.L., M.M., A.C., and L.A. performed computational analysis of data. P.Ba., A.K., E.C., Y.T.-L., A.N.S., O.M.D., M.S.A., A.A., G.C., V.L., F.C., M.V., D.M.S., J.H., B.T., D.Du., L.Q.-M., D.v.d.B., L.R., D.C.V., S.G.T., F.H., D.Da., T.H.M., P.Br., J.M.-P., M.C.N., S.B.-D., C.R.-G., G.V., A.J.O., J.Gu, P.D.B., J.I.C., A.B., L.R.B., M.D.’A., P.Bo., P.R., F.R.-L., F.F., M.V.U., L.I., A.S., S.P., E.Q.-R., C.R., R.C., D.M., A.L., G.L.M., X.D., J.Gh., M.S.L., and G.G. evaluated and recruited patients to COVID and/or control cohorts of patients. P.Ba. and J.-L.C. wrote the manuscript. J.-L.C. supervised the project. All authors edited the manuscript. Competing interests: H.C.S. is adjunct faculty at the University of Pennsylvania. J.-L.C. is listed as an inventor on patent application U.S. 63/055,155 filed by The Rockefeller University that encompasses aspects of this publication. R.P.L. is a nonexecutive director of Roche and its subsidiary Genentech. The authors declare no other competing interests. Data and materials availability: All data are available in the manuscript or in the supplementary materials. Plasma, cells, and genomic DNA are available from J.-L.C. or D.C.V. under a material transfer agreement with The Rockefeller University and the Research Institute-McGill University Health Centre. Huh7.5 cells are available on request from C.M.R. under a material transfer agreement with The Rockefeller University and Apath, LLC. Materials and reagents used are almost exclusively commercially available and nonproprietary. Requests for materials derived from human samples may be made available, subject to any underlying restrictions on such samples. J.-L.C. can make material transfer agreements available through The Rockefeller University. This work is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/. This license does not apply to figures/photos/artwork or other content included in the article that is credited to a third party; obtain authorization from the rights holder before using such material.
Supplementary Materials
science.sciencemag.org/content/370/6515/eabd4585/suppl/DC1
Supplementary Materials and Methods
Figs. S1 to S4
Tables S1 to S3
Data S1
HGID Lab
Andrés Augusto Arias1,3, Bertrand Boisson1,2, Soraya Boucherit2, Jacinta Bustamante1,2, Marwa Chbihi2, Jie Chen1, Maya Chrabieh2, Tatiana Kochetkov1, Tom Le Voyer2, Dana Liu1, Yelena Nemirovskaya1, Masato Ogishi1, Dominick Papandrea1, Cécile Patissier2, Franck Rapaport1, Manon Roynard2, Natasha Vladikine2, Mark Woollett1, Peng Zhang1
1St. Giles Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, NY, USA. 2Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U1163, Necker Hospital for Sick Children, Paris, France. 3School of Microbiology and Group of Primary Immunodeficiencies, University of Antioquia UdeA, Medellín, Colombia.
NIAID-USUHS Immune Response to COVID Group
Anuj Kashyap1, Li Ding1, Marita Bosticardo1, Qinlu Wang2, Sebastian Ochoa1, Hui Liu1, Samuel D. Chauvin3, Michael Stack1, Galina Koroleva4, Neha Bansal5, Clifton L. Dalgard6,7, Andrew L. Snow8
1Laboratory of Clinical Immunology and Microbiology, Division of Intramural Research, NIAID, NIH, Bethesda, MD, USA. 2Bioinformatics and Computational Biosciences Branch, NIAID Office of Cyber Infrastructure and Computational Biology, NIAID, NIH, Bethesda, MD, USA. 3Laboratory of Immune System Biology, Division of Intramural Research, NIAID, NIH, Bethesda, MD, USA. 4NIH Center for Human Immunology, NIH, Bethesda, MD, USA. 5Multiscale Systems Biology Section, Laboratory of Immune System Biology, NIAID, NIH, Bethesda, MD, USA. 6PRIMER, Uniformed Services University of the Health Sciences, Bethesda, MD, USA. 7Department of Anatomy, Physiology & Genetics, Uniformed Services University of the Health Sciences, Bethesda, MD, USA. 8Department of Pharmacology & Molecular Therapeutics, Uniformed Services University of the Health Sciences, Bethesda, MD, USA.
COVID Clinicians
Jorge Abad1, Sergio Aguilera-Albesa2, Ozge Metin Akcan3, Ilad Alavi Darazam4, Juan C. Aldave5, Miquel Alfonso Ramos6, Seyed Alireza Nadji7, Gulsum Alkan8, Jerome Allardet-Servent9, Luis M. Allende10, Laia Alsina11, Marie-Alexandra Alyanakian12, Blanca Amador-Borrero13, Zahir Amoura14, Arnau Antolí15, Sevket Arslan16, Sophie Assant17, Terese Auguet18, Axelle Azot19, Fanny Bajolle20, Aurélie Baldolli21, Maite Ballester22, Hagit Baris Feldman23, Benoit Barrou24, Alexandra Beurton25, Agurtzane Bilbao26, Geraldine Blanchard-Rohner27, Ignacio Blanco1, Adeline Blandinières28, Daniel Blazquez-Gamero29, Marketa Bloomfield30, Mireia Bolivar-Prados31, Raphael Borie32, Ahmed A. Bousfiha33, Claire Bouvattier34, Oksana Boyarchuk35, Maria Rita P. Bueno36, Jacinta Bustamante20, Juan José Cáceres Agra37, Semra Calimli38, Ruggero Capra39, Maria Carrabba40, Carlos Casasnovas41, Marion Caseris42, Martin Castelle43, Francesco Castelli44, Martín Castillo de Vera45, Mateus V. Castro36, Emilie Catherinot46, Martin Chalumeau47, Bruno Charbit48, Matthew P. Cheng49, Père Clavé31, Bonaventura Clotet50, Anna Codina51, Fatih Colkesen52, Fatma Colkesen53, Roger Colobran 54, Cloé Comarmond55, Angelo G. Corsico56, David Dalmau57, David Ross Darley58, Nicolas Dauby59, Stéphane Dauger60, Loic de Pontual61, Amin Dehban62, Geoffroy Delplancq63, Alexandre Demoule64, Antonio Di Sabatino65, Jean-Luc Diehl66, Stephanie Dobbelaere67, Sophie Durand68, Waleed Eldars69, Mohamed Elgamal70, Marwa H. Elnagdy71, Melike Emiroglu72, Emine Hafize Erdeniz73, Selma Erol Aytekin74, Romain Euvrard75, Recep Evcen76, Giovanna Fabio40, Laurence Faivre77, Antonin Falck42, Muriel Fartoukh78, Morgane Faure79, Miguel Fernandez Arquero80, Carlos Flores81, Bruno Francois82, Victoria Fumadó83, Francesca Fusco84, Blanca Garcia Solis85, Pascale Gaussem86, Juana Gil-Herrera87, Laurent Gilardin88, Monica Girona Alarcon89, Mónica Girona-Alarcón89, Jean-Christophe Goffard90, Funda Gok91, Rafaela González-Montelongo92, Antoine Guerder93, Yahya Gul94, Sukru Nail Guner94, Marta Gut95, Jérôme Hadjadj96, Filomeen Haerynck97, Rabih Halwani98, Lennart Hammarström99, Nevin Hatipoglu100, Elisa Hernandez-Brito101, María Soledad Holanda-Peña102, Juan Pablo Horcajada103, Sami Hraiech104, Linda Humbert105, Alejandro D. Iglesias106, Antonio Íñigo-Campos92, Matthieu Jamme107, María Jesús Arranz108, Iolanda Jordan109, Fikret Kanat110, Hasan Kapakli111, Iskender Kara112, Adem Karbuz113, Kadriye Kart Yasar114, Sevgi Keles115, Yasemin Kendir Demirkol116, Adam Klocperk117, Zbigniew J. Król118, Paul Kuentz119, Yat Wah M. Kwan120, Jean-Christophe Lagier121, Yu-Lung Lau122, Fleur Le Bourgeois60, Yee-Sin Leo123, Rafael Leon Lopez124, Daniel Leung122, Michael Levin125, Michael Levy60, Romain Lévy20, Zhi Li48, Agnes Linglart126, José M. Lorenzo-Salazar92, Céline Louapre127, Catherine Lubetzki127, Charles-Edouard Luyt128, David C. Lye129, Davood Mansouri130, Majid Marjani131, Jesus Marquez Pereira132, Andrea Martin133, David Martínez Pueyo134, Javier Martinez-Picado135, Iciar Marzana136, Alexis Mathian14, Larissa R. B. Matos36, Gail V. Matthews137, Julien Mayaux138, Jean-Louis Mège139, Isabelle Melki140, Jean-François Meritet141, Ozge Metin142, Isabelle Meyts143, Mehdi Mezidi144, Isabelle Migeotte145, Maude Millereux146, Tristan Mirault147, Clotilde Mircher68, Mehdi Mirsaeidi148, Abián Montesdeoca Melián149, Antonio Morales Martinez150, Pierre Morange151, Clémence Mordacq105, Guillaume Morelle152, Stéphane Mouly13, Adrián Muñoz-Barrera92, Cyril Nafati153, João Farela Neves154, Lisa F. P. Ng155, Yeray Novoa Medina156, Esmeralda Nuñez Cuadros157, J. Gonzalo Ocejo-Vinyals158, Zerrin Orbak159, Mehdi Oualha20, Tayfun Özçelik160, Qiang Pan Hammarström161, Christophe Parizot138, Tiffany Pascreau162, Estela Paz-Artal163, Rebeca Pérez de Diego85, Aurélien Philippe164, Quentin Philippot78, Laura Planas-Serra165, Dominique Ploin166, Julien Poissy167, Géraldine Poncelet42, Marie Pouletty168, Paul Quentric138, Didier Raoult139, Anne-Sophie Rebillat68, Ismail Reisli169, Pilar Ricart170, Jean-Christophe Richard171, Nadia Rivet28, Jacques G. Rivière172, Gemma Rocamora Blanch15, Carlos Rodrigo1, Carlos Rodriguez-Gallego173, Agustí Rodríguez-Palmero174, Carolina Soledad Romero175, Anya Rothenbuhler176, Flore Rozenberg177, Maria Yolanda Ruiz del Prado178, Joan Sabater Riera15, Oliver Sanchez179, Silvia Sánchez-Ramón180, Agatha Schluter165, Matthieu Schmidt181, Cyril E. Schweitzer182, Francesco Scolari183, Anna Sediva184, Luis M. Seijo185, Damien Sene13, Sevtap Senoglu114, Mikko R. J. Seppänen186, Alex Serra Ilovich187, Mohammad Shahrooei62, David Smadja188, Ali Sobh189, Xavier Solanich Moreno15, Jordi Solé-Violán190, Catherine Soler191, Pere Soler-Palacín133, Yuri Stepanovskiy192, Annabelle Stoclin193, Fabio Taccone145, Yacine Tandjaoui-Lambiotte194, Jean-Luc Taupin195, Simon J. Tavernier196, Benjamin Terrier197, Caroline Thumerelle105, Gabriele Tomasoni198, Julie Toubiana47, Josep Trenado Alvarez199, Sophie Trouillet-Assant200, Jesús Troya201, Alessandra Tucci202, Matilde Valeria Ursini84, Yurdagul Uzunhan203, Pierre Vabres204, Juan Valencia-Ramos205, Ana Maria Van Den Rym85, Isabelle Vandernoot206, Hulya Vatansev207, Valentina Vélez-Santamaria41, Sébastien Viel166, Cédric Vilain208, Marie E. Vilaire68, Audrey Vincent34, Guillaume Voiriot209, Fanny Vuotto105, Alper Yosunkaya91, Barnaby E. Young123, Fatih Yucel210, Faiez Zannad211, Mayana Zatz36, Alexandre Belot212*
1University Hospital and Research Institute “Germans Trias i Pujol”, Badalona, Spain. 2Navarra Health Service Hospital, Pamplona, Spain. 3Division of Pediatric Infectious Diseases, Necmettin Erbakan University, Meram Medical Faculty, Konya, Turkey. 4Department of Infectious Diseases, Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran. 5Hospital Nacional Edgardo Rebagliati Martins, Lima, Peru. 6Parc Sanitari Sant Joan de Déu, Sant Boi de Llobregat, Barcelona, Spain. 7Virology Research Center, National institutes of Tuberculosis and Lung diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran. 8Division of Pediatric Infectious Diseases, Faculty of Medicine, Selcuk University, Konya, Turkey. 9Intensive Care Unit, Hôpital Européen, Marseille, France. 10Immunology Department, University Hospital 12 de Octubre, Research Institute imas12, Complutense University, Madrid, Spain. 11Hospital Sant Joan de Déu, Barcelona, Spain. 12Department of Biological Immunology, Necker Hospital for Sick Children, APHP and INEM, Paris, France. 13Internal Medicine Department, Hôpital Lariboisière, APHP; Université de Paris, Paris, France. 14Internal Medicine Department, Pitié-Salpétrière Hospital, Paris, France. 15Hospital Universitari de Bellvitge, Barcelona, Spain. 16Division of Clinical Immunology and Allergy, Necmettin Erbakan University, Meram Medical Faculty, Konya, Turkey. 17Joint Research Unit, Hospices Civils de Lyon-bio Mérieux, Hospices Civils de Lyon, Lyon Sud Hospital, Lyon, France. 18Hospital Universitario de Tarragona Joan XXIII, Universitat Rovira i Virgili (URV), IISPV, Tarragona, Spain. 19Private practice, Paris, France. 20Necker Hospital for Sick Children, AP-HP, Paris, France. 21Department of Infectious Diseases, CHU de Caen, Caen, France. 22Consorcio Hospital General Universitario, Valencia, Spain. 23The Genetics Institute, Tel Aviv Sourasky Medical Center and Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel. 24Department of Urology, Nephrology, Transplantation, APHP-SU, Sorbonne Université, INSERM U 1082, Paris, France. 25Service de Médecine Intensive–Réanimation et Pneumologie, APHP Hôpital Pitié–Salpêtrière, Paris, France. 26Cruces University Hospital, Bizkaia, Spain. 27Paediatric Immunology and Vaccinology Unit, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland. 28Hematology, Georges Pompidou Hospital, APHP, Paris, France. 29Pediatric Infectious Diseases Unit, Instituto de Investigación 12 de Octubre (imas12), Hospital Universitario 12 de Octubre, Madrid, Spain. 30Department of Immunology, Motol University Hospital, 2nd Faculty of Medicine, Charles University, Department of Pediatrics, Thomayer’s Hospital, 1st Faculty of Medicine, Charles University, Prague, Czech Republic. 31Centro de Investigación Biomédica en Red de Enfermedades Hepàticas y Digestivas (Ciberehd), Hospital de Mataró, Consorci Sanitari del Maresme, Mataró, Spain. 32Service de Pneumologie, Hopital Bichat, APHP, Paris, France. 33Clinical Immunology Unit, Pediatric Infectious Disease Department, Faculty of Medicine and Pharmacy, Averroes University Hospital, LICIA Laboratoire d'immunologie clinique, d'inflammation et d'allergie, Hassann Ii University, Casablanca, Morocco. 34Endocrinology Unit, APHP Hôpitaux Universitaires Paris-Sud, Le Kremlin-Bicêtre, France. 35Department of Children's Diseases and Pediatric Surgery, I.Horbachevsky Ternopil National Medical University, Ternopil, Ukraine. 36Human Genome and Stem-Cell Research Center, University of São Paulo, São Paulo, Brazil. 37Hospital Insular, Las Palmas de Gran Canaria, Spain. 38Division of Critical Care Medicine, Department of Anesthesiology and Reanimation, Konya State Hospital, Konya, Turkey. 39MS Center, Spedali Civili, Brescia, Italy. 40Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy. 41Bellvitge University Hospital, L'Hospitalet de Llobregat, Barcelona, Spain. 42Hopital Robert Debré, Paris, France. 43Pediatric Immuno-hematology Unit, Necker Enfants Malades Hospital, AP-HP, Paris, France. 44Department of Infectious and Tropical Diseases, University of Brescia, ASST Spedali Civili di Brescia, Brescia, Italy. 45Doctoral Health Care Center, Canarian Health System, Las Palmas de Gran Canaria, Spain. 46Hôpital Foch, Suresnes, France. 47Necker Hospital for Sick Children, Paris University, AP-HP, Paris, France. 48Pasteur Institute, Paris, France. 49McGill University Health Centre, Montreal, Canada. 50University Hospital and Research Institute “Germans Trias i Pujol”, IrsiCaixa AIDS Research Institute, UVic-UCC, Badalona, Spain. 51Clinical Biochemistry, Pathology, Paediatric Neurology and Molecular Medicine Departments and Biobank, Institut de Recerca Sant Joan de Déu and CIBERER-ISCIII, Esplugues, Spain. 52Division of Clinical Immunology and Allergy, Department of Internal Medicine, Necmettin Erbakan University, Meram Medical Faculty, Konya, Turkey. 53Department of Infectious Diseases and Clinical Microbiology, Konya Training and Research Hospital, Konya, Turkey. 54Hospital Universitari Vall d’Hebron, Barcelona, Spain. 55Pitié-Salpêtrière Hospital, Paris, France. 56Respiratory Diseases Division, IRCCS Policlinico San Matteo Foundation and University of Pavia, Pavia, Italy. 57Fundació Docència i Recerca Mútua Terrassa, Barcelona, Spain. 58UNSW Medicine, St Vincent's Clinical School; Department of Thoracic Medicine, St Vincent's Hospital Darlinghurst, Sidney, Australia. 59CHU Saint-Pierre, Université Libre de Bruxelles (ULB), Brussels, Belgium. 60Pediatric Intensive Care Unit, Robert-Debré University Hospital, APHP, Paris, France. 61Sorbonne Paris Nord, Hôpital Jean Verdier, APHP, Bondy, France. 62Specialized Immunology Laboratory of Dr. Shahrooei, Sina Medical Complex, Ahvaz, Iran. 63Centre de génétique humaine, CHU Besançon, Besançon, France. 64Sorbonne Université Médecine and APHP Sorbonne Université site Pitié-Salpêtrière, Paris, France. 65Department of Internal Medicine, Fondazione IRCCS Policlinico San Matteo, University of Pavia, Pavia, Italy. 66Intensive Care Unit, Georges Pompidou Hospital, APHP, Paris, France. 67Department of Pneumology, AZ Delta, Roeselare, Belgium. 68Institut Jérôme Lejeune, Paris, France. 69Department of Microbiology and Immunology, Faculty of Medicine, Mansoura University, Mansoura, Egypt. 70Department of Chest, Faculty of Medicine, Mansoura University, Mansoura, Egypt. 71Department of Medical Biochemistry and Molecular Biology, Faculty of Medicine, Mansoura University, Mansoura, Egypt. 72Faculty of Medicine, Division of Pediatric Infectious Diseases, Selcuk University, Konya, Turkey. 73Division of Pediatric Infectious Diseases, Ondokuz Mayıs University, Samsun, Turkey. 74Necmettin Erbakan University, Meram Medical Faculty, Division of Pediatric Allergy and Immunology, Konya, Turkey. 75Centre Hospitalier Fleyriat, Bourg-en-Bresse, France. 76Division of Clinical Immunology and Allergy, Department of Internal Medicine, Necmettin Erbakan University, Meram Medical Faculty, Konya, Turkey. 77Centre de Génétique, CHU Dijon, Dijon, France. 78APHP Tenon Hospital, Paris, France. 79Sorbonne Universités, UPMC University of Paris, Paris, France. 80Department of Clinical Immunology, Hospital Clínico San Carlos, Madrid, Spain. 81Genomics Division, Instituto Tecnológico y de Energías Renovables (ITER), Santa Cruz de Tenerife, Spain; CIBER de Enfermedades Respiratorias, Instituto de Salud Carlos III, Madrid, Spain; Research Unit, Hospital Universitario N.S. de Candelaria, Santa Cruz de Tenerife, Spain; Instituto de Tecnologías Biomédicas (ITB), Universidad de La Laguna, San Cristóbal de La Laguna, Spain. 82CHU Limoges and Inserm CIC 1435 & UMR 1092, Limoges, France. 83Infectious Diseases Unit, Department of Pediatrics, Hospital Sant Joan de Déu, Barcelona, Spain; Institut de Recerca Sant Joan de Déu, Spain; Universitat de Barcelona (UB), Barcelona, Spain. 84Institute of Genetics and Biophysics ‘Adriano Buzzati-Traverso’, IGB-CNR, Naples, Italy. 85Laboratory of Immunogenetics of Human Diseases, IdiPAZ Institute for Health Research, La Paz Hospital, Madrid, Spain. 86Hematology, APHP, Hopital Européen Georges Pompidou and Inserm UMR-S1140, Paris, France. 87Hospital General Universitario and Instituto de Investigación Sanitaria "Gregorio Marañón", Madrid, Spain. 88Bégin military Hospital, Bégin, France. 89Pediatric Intensive Care Unit, Hospital Sant Joan de Déu, Barcelona, Spain. 90Department of Internal Medicine, Hôpital Erasme, Université Libre de Bruxelles, Brussels, Belgium. 91Division of Critical Care Medicine, Department of Anesthesiology and Reanimation, Necmettin Erbakan University, Meram Medical Faculty, Konya, Turkey. 92Genomics Division, Instituto Tecnológico y de Energías Renovables (ITER), Santa Cruz de Tenerife, Spain. 93Assistance Publique Hôpitaux de Paris, Paris, France. 94Division of Allergy and Immunology, Necmettin Erbakan University, Meram Medical Faculty, Konya, Turkey. 95CNAG-CRG, Centre for Genomic Regulation (CRG), Barcelona Institute of Science and Technology (BIST); Universitat Pompeu Fabra (UPF), Barcelona, Spain. 96Department of Internal Medicine, National Reference Center for Rare Systemic Autoimmune Diseases, AP-HP, APHP-CUP, Hôpital Cochin, Paris, France. 97Ghent University Hospital, Ghent, Belgium. 98Sharjah Institute of Medical Research, College of Medicine, University of Sharjah, Sharjah, United Arab Emirates. 99Department of Biosciences and Nutrition, SE14183, Huddinge, Karolinska Institutet, Stockholm, Sweden. 100Pediatric Infectious Diseases Unit, Bakirkoy Dr. Sadi Konuk Training and Research Hospital, University of Health Sciences, Istanbul, Turkey. 101Department of Immunology, Hospital Universitario de Gran Canaria Dr. Negrín, Canarian Health System, Las Palmas de Gran Canaria, Spain. 102Intensive Care Unit, Marqués de Valdecilla Hospital, Santander, Spain. 103Hospital del Mar, Parc de Salut Mar, Barcelona, Spain. 104Intensive Care Unit, APHM, Marseille, France. 105CHU Lille, Lille, France. 106Department of Pediatrics, Columbia University, New York, NY, USA. 107Centre Hospitalier Intercommunal Poissy Saint Germain en Laye, Poissy, France. 108Fundació Docència i Recerca Mútua Terrassa, Barcelona, Spain. 109Hospital Sant Joan de Déu, Kids Corona Platfform, Barcelona, Spain. 110Selcuk University, Faculty of Medicine, Chest Diseases Department, Konya, Turkey. 111Division of Allergy and Immunology, Balikesir Ataturk City Hospital, Balikesir, Turkey. 112Division of Critical Care Medicine, Selcuk University, Faculty of Medicine, Konya, Turkey. 113Division of Pediatric Infectious Diseases, Prof. Dr. Cemil Tascıoglu City Hospital, Istanbul, Turkey. 114Departments of Infectious Diseases and Clinical Microbiology, Bakirkoy Dr. Sadi Konuk Training and Research Hospital, University of Health Sciences, Istanbul, Turkey. 115Meram Medical Faculty, Necmettin Erbakan University, Meram Medical Faculty, Konya, Turkey. 116Health Sciences University, Umraniye Education and Research Hospital, Istanbul, Turkey. 117Department of Immunology, 2nd Faculty of Medicine, Charles University and University Hospital in Motol, Prague, Czech Republic. 118Central Clinical Hospital of Ministry of the Interior and Administration in Warsaw, Warsaw, Poland. 119Oncobiologie Génétique Bioinformatique, PC Bio, CHU Besançon, Besançon, France. 120Paediatric Infectious Disease Unit, Hospital Authority Infectious Disease Center, Princess Margaret Hospital, Hong Kong (Special Administrative Region), China. 121Aix Marseille Univ, IRD, MEPHI, IHU Méditerranée Infection, Marseille, France. 122Department of Paediatrics and Adolescent Medicine, The University of Hong Kong, Hong Kong, China. 123National Centre for Infectious Diseases, Singapore. 124Hospital Universitario Reina Sofía, Cordoba, Spain. 125Imperial College, London, UK. 126Endocrinology and diabetes for children, AP-HP, Bicêtre Paris-Saclay Hospital, Le Kremlin-Bicêtre, France. 127Neurology Unit, APHP Pitié-Salpêtrière Hospital, Paris University, Paris, France. 128Intensive Care Unit, APHP Pitié-Salpêtrière Hospital, Paris University, Paris, France. 129National Centre for Infectious Diseases; Tan Tock Seng Hospital; Yong Loo Lin School of Medicine; Lee Kong Chian School of Medicine, Singapore. 130Department of Clinical Immunology and Infectious Diseases, National Research Institute of Tuberculosis and Lung Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran. 131Clinical Tuberculosis and Epidemiology Research Center, National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran. 132Hospital Sant Joan de Déu and University of Barcelona, Barcelona, Spain. 133Pediatric Infectious Diseases and Immunodeficiencies Unit, Hospital Universitari Vall d’Hebron, Vall d'Hebron Research Institute, Vall d’Hebron Barcelona Hospital Campus, Universitat Autònoma de Barcelona (UAB), Barcelona, Spain. 134Hospital Universitari Mutua de Terrassa, Universitat de Barcelona, Barcelona, Spain. 135IrsiCaixa AIDS Research Institute, ICREA, UVic-UCC, Research Institute “Germans Trias i Pujol”, Badalona, Spain. 136Department of Laboratory, Cruces University Hospital, Barakaldo, Bizkaia, Spain. 137University of New South Wales, Darlinghurst, NSW, Australia. 138APHP Pitié-Salpêtrière Hospital, Paris, France. 139Aix-Marseille University, APHM, Marseille, France. 140Robert Debré Hospital, Paris, France. 141APHP Cohin Hospital, Paris, France. 142Necmettin Erbakan University Meram Faculty of Medicine Department of Pediatric Infectious Diseases, Konya, Turkey. 143University Hospitals Leuven, Leuven, Belgium. 144Hospices Civils de Lyon, Hôpital de la Croix-Rousse, Lyon, France. 145Hôpital Erasme, Brussels, Belgium. 146CH Gonesse, Gonesse, France. 147Vascular Medicine, Georges Pompidou Hospital, APHP, Paris, France. 148Division of Pulmonary and Critical Care, University of Miami, Miami, FL, USA. 149Guanarteme Health Care Center, Canarian Health System, Las Palmas de Gran Canaria, Spain. 150Regional University Hospital of Málaga, Málaga, Spain. 151Aix-Marseille Université, Marseille, France. 152Department of General Paediatrics, Hôpital Bicêtre, AP-HP, University of Paris Saclay, Le Kremlin-Bicêtre, France. 153CHU de La Timone, Marseille, France. 154Centro Hospitalar Universitário de Lisboa Central, Lisbon, Portugal. 155Infectious Diseases Horizontal Technlogy Centre, A*STAR; Singapore Immunology Network, A*STAR, Singapore. 156Department of Pediatrics, Complejo Hospitalario Universitario Insular-Materno Infantil, Canarian Health System, Las Palmas de Gran Canaria, Spain. 157Regional University Hospital of Málaga, Málaga, Spain. 158Hospital Universitario Marqués de Valdecilla, Santander, Spain. 159Ataturk University Medical Faculty, Erzurum, Turkey. 160Bilkent University, Department of Molecular Biology and Genetics, Ankara, Turkey. 161Department of Laboratory Medicine, Karolinska Institutet, SE14186, Stockholm, Sweden. 162L'Hôpital Foch, Suresnes, France. 163Department of Immunology, Hospital Universitario 12 de Octubre, Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), Madrid, Spain. 164APHP Hôpitaux Universitaires Paris-Sud, Le Kremlin-Bicêtre, France. 165Neurometabolic Diseases Laboratory, IDIBELL-Hospital Duran i Reynals, Barcelona; CIBERER U759, ISCiii, Madrid, Spain. 166Hospices Civils de Lyon, Lyon, France. 167Université de Lille, Inserm U1285, CHU Lille, Paris, France. 168Department of General Pediatrics, University Hospital Robert Debré, APHP, Paris, France. 169Necmettin Erbakan University, Konya, Turkey. 170Germans Trias i Pujol Hospital, Badalona, Spain. 171Medical Intensive Care Unit, Hopital de la Croix-Rousse, Hospices Civils de Lyon, Lyon, France. 172Pediatric Infectious Diseases and Immunodeficiencies Unit, Hospital Universitari Vall d’Hebron, Vall d'Hebron Research Institute, Vall d’Hebron Barcelona Hospital Campus, Barcelona, Spain. 173Department of Immunology, Hospital Universitario de Gran Canaria Dr. Negrín, Canarian Health System, Las Palmas de Gran Canaria, Spain; University Fernando Pessoa Canarias, Las Palmas de Gran Canaria, Spain. 174Neurometabolic Diseases Laboratory, IDIBELL-Hospital Duran i Reynals, Barcelona, Spain. 175Consorcio Hospital General Universitario, Valencia, Spain. 176APHP Hôpitaux Universitaires Paris-Sud, Paris, France. 177Virology Unit, Université de Paris, Cohin Hospital, APHP, Paris, France. 178Hospital San Pedro, Logroño, Spain. 179Respiratory medicine, Georges Pompidou Hospital, APHP, Paris, France. 180Department of Immunology, Hospital Clínico San Carlos, Madrid, Spain. 181Service de Médecine Intensive Réanimation, Institut de Cardiologie, Hopital Pitié-Salpêtrière, Paris, France. 182CHRU de Nancy, Hôpital d'Enfants, Vandoeuvre, France. 183Chair of Nephrology, University of Brescia, Brescia, Italy. 184Department of Immunology, 2nd Faculty of Medicine, Charles University and Motol University Hospital, Prague, Czech Republic. 185Clínica Universidad de Navarra, Madrid, Spain. 186HUS Helsinki University Hospital, Children and Adolescents, Rare Disease Center, and Inflammation Center, Adult Immunodeficiency Unit, Majakka, Helsinki, Finland. 187Fundació Docència i Recerca Mútua Terrassa, Terrassa, Spain. 188Hopital Européen Georges Pompidou, Paris, France. 189Department of Pediatrics, Faculty of Medicine, Mansoura University, Mansoura, Egypt. 190Critical Care Unit, Hospital Universitario de Gran Canaria Dr. Negrín, Canarian Health System, Las Palmas de Gran Canaria, Spain. 191CHU de Saint Etienne, Saint-Priest-en-Jarez, France. 192Shupyk National Medical Academy for Postgraduate Education, Kiev, Ukraine. 193Gustave Roussy Cancer Campus, Villejuif, France. 194Intensive Care Unit, Avicenne Hospital, APHP, Bobigny, France. 195Laboratory of Immunology and Histocompatibility, Saint-Louis Hospital, Paris University, Paris, France. 196Department of Internal Diseases and Pediatrics, Primary Immune Deficiency Research Laboratory, Centre for Primary Immunodeficiency Ghent, Jeffrey Modell Diagnosis and Research Centre, Ghent University Hospital, Ghent, Belgium. 197Department of Internal Medicine, Université de Paris, INSERM, U970, PARCC, F-75015, Paris, France. 198First Division of Anesthesiology and Critical Care Medicine, University of Brescia, ASST Spedali Civili di Brescia, Brescia, Italy. 199Intensive Care Department, Hospital Universitari MutuaTerrassa, Universitat Barcelona, Terrassa, Spain. 200Hospices Civils de Lyon, Lyon Sud Hospital, Lyon, France. 201Infanta Leonor University Hospital, Madrid, Spain. 202Hematology Department, ASST Spedali Civili di Brescia, Brescia, Italy. 203Pneumologie, Hôpital Avicenne, APHP, INSERM U1272, Université Sorbonne Paris Nord, Bobigny, France. 204Dermatology Unit, Laboratoire GAD, INSERM UMR1231 LNC, Université de Bourgogne, Dijon, France. 205University Hospital of Burgos, Burgos, Spain. 206Center of Human Genetics, Hôpital Erasme, Université Libre de Bruxelles, Brussels, Belgium. 207Department of Chest Diseases, Necmettin Erbakan University, Meram Medical Faculty, Konya, Turkey. 208CHU de Caen, Caen, France. 209Sorbonne Université, Service de Médecine Intensive Réanimation, Hôpital Tenon, Assistance Publique-Hôpitaux de Paris, Paris, France. 210General Intensive Care Unit, Konya Training and Research Hospital, Konya, Turkey. 211CHU de Nancy, Nancy, France. 212University of Lyon, CIRI, INSERM U1111, National Referee Centre RAISE, Pediatric Rheumatology, HFME, Hospices Civils de Lyon, Lyon, France. *Leader of the COVID-Clinicians group.
COVID-STORM Clinicians
Giuseppe Foti1, Giacomo Bellani1, Giuseppe Citerio1, Ernesto Contro1, Alberto Pesci2, Maria Grazia Valsecchi3, Marina Cazzaniga4
1Department of Emergency, Anesthesia and Intensive Care, School of Medicine and Surgery, University of Milano-Bicocca, San Gerardo Hospital, Monza, Italy. 2Department of Pneumology, School of Medicine and Surgery, University of Milano-Bicocca, San Gerardo Hospital, Monza, Italy. 3Center of Bioinformatics and Biostatistics, School of Medicine and Surgery, University of Milano-Bicocca, San Gerardo Hospital, Monza, Italy. 4Phase I Research Center, School of Medicine and Surgery, University of Milano-Bicocca, San Gerardo Hospital, Monza, Italy.
Imagine COVID Group
Christine Bole-Feysot1, Stanislas Lyonnet1*, Cécile Masson1, Patrick Nitschke1, Aurore Pouliet1, Yoann Schmitt1, Frederic Tores1, Mohammed Zarhrate1
1Imagine Institute, Université de Paris, INSERM UMR 1163, Paris, France. *Leader of the Imagine COVID Group.
French COVID Cohort Study Group
Laurent Abel1, Claire Andrejak2, François Angoulvant3, Delphine Bachelet4, Romain Basmaci5, Sylvie Behillil6, Marine Beluze7, Dehbia Benkerrou8, Krishna Bhavsar4, François Bompart9, Lila Bouadma4, Maude Bouscambert10, Mireille Caralp11, Minerva Cervantes-Gonzalez12, Anissa Chair4, Alexandra Coelho13, Camille Couffignal4, Sandrine Couffin-Cadiergues14, Eric D’ortenzio12, Charlene Da Silveira4, Marie-Pierre Debray4, Dominique Deplanque15, Diane Descamps16, Mathilde Desvallées17, Alpha Diallo18, Alphonsine Diouf13, Céline Dorival8, François Dubos19, Xavier Duval4, Philippine Eloy4, Vincent V. E. Enouf20, Hélène Esperou21, Marina Esposito-Farese4, Manuel Etienne22, Nadia Ettalhaoui4, Nathalie Gault4, Alexandre Gaymard10, Jade Ghosn4, Tristan Gigante23, Isabelle Gorenne4, Jérémie Guedj24, Alexandre Hoctin13, Isabelle Hoffmann4, Salma Jaafoura21, Ouifiya Kafif4, Florentia Kaguelidou25, Sabina Kali4, Antoine Khalil4, Coralie Khan17, Cédric Laouénan4, Samira Laribi4, Minh Le4, Quentin Le Hingrat4, Soizic Le Mestre18, Hervé Le Nagard24, François-Xavier Lescure4, Yves Lévy26, Claire Levy-Marchal27, Bruno Lina10, Guillaume Lingas24, Jean Christophe Lucet4, Denis Malvy28, Marina Mambert13, France Mentré4, Noémie Mercier18, Amina Meziane8, Hugo Mouquet20, Jimmy Mullaert4, Nadège Neant24, Marion Noret29, Justine Pages30, Aurélie Papadopoulos21, Christelle Paul18, Nathan Peiffer-Smadja4, Ventzislava Petrov-Sanchez18, Gilles Peytavin4, Olivier Picone31, Oriane Puéchal12, Manuel Rosa-Calatrava10, Bénédicte Rossignol23, Patrick Rossignol32, Carine Roy4, Marion Schneider4, Caroline Semaille12, Nassima Si Mohammed4, Lysa Tagherset4, Coralie Tardivon4, Marie-Capucine Tellier4, François Téoulé8, Olivier Terrier10, Jean-François Timsit4, Théo Treoux4, Christelle Tual33, Sarah Tubiana4, Sylvie van der Werf34, Noémie Vanel35, Aurélie Veislinger33, Benoit Visseaux16, Aurélie Wiedemann26, Yazdan Yazdanpanah36
1Inserm UMR 1163, Paris, France. 2CHU Amiens, Amiens, France. 3Hôpital Necker, Paris, France. 4Hôpital Bichat, Paris, France. 5Hôpital Louis Mourrier, Colombes, France. 6Institut Pasteur, Paris, France. 7F-CRIN Partners Platform, AP-HP, Université de Paris, Paris, France. 8Inserm UMR 1136, Paris, France. 9Drugs for Neglected Diseases Initiative, Geneva, Switzerland. 10Inserm UMR 1111, Lyon, France. 11Inserm Transfert, Paris, France. 12REACTing, Paris, France. 13Inserm UMR 1018, Paris, France. 14Inserm, Pôle Recherche Clinique, France. 15CIC 1403 Inserm-CHU Lille, Paris, France. 16Université de Paris, IAME, INSERM UMR 1137, AP-HP, University Hospital Bichat Claude Bernard, Virology, F-75018 Paris, France. 17Inserm UMR 1219, Bordeaux, France. 18ANRS, Paris, France. 19CHU Lille, Lille, France. 20Pasteur Institute, Paris, France. 21Inserm sponsor, Paris, France. 22Rouen - SMIT, France. 23FCRIN INI-CRCT, Nancy, France. 24Inserm UMR 1137, Paris, France. 25Centre d'Investigation Clinique, Inserm CIC1426, Hôpital Robert Debré, Paris, France. 26Inserm UMR 955, Créteil, France; Vaccine Research Instiute (VRI), Paris, France. 27F-CRIN INI-CRCT, Paris, France. 28Bordeaux - SMIT, France. 29RENARCI, Annecy, France. 30Hôpital Robert Debré, Paris, France. 31Colombes - Louis Mourier - Gynécologie, France. 32University of Lorraine, Plurithematic Clinical Investigation Centre Inserm CIC-P; 1433, Inserm U1116, CHRU Nancy Hopitaux de Brabois, F-CRIN INI-CRCT; (Cardiovascular and Renal Clinical Trialists), Nancy, France. 33Inserm CIC-1414, Rennes, France. 34Institut Pasteur, UMR 3569 CNRS, Université de Paris, Paris, France. 35Hôpital la timone, Marseille, France. 36Paris - Bichat - SMIT, France.
The Milieu Intérieur Consortium
Laurent Abel1, Andres Alcover2, Hugues Aschard2, Kalla Astrom3, Philippe Bousso2, Pierre Bruhns2, Ana Cumano2, Caroline Demangel2, Ludovic Deriano2, James Di Santo2, Françoise Dromer2, Gérard Eberl2, Jost Enninga2, Jacques Fellay4, Ivo Gomperts-Boneca2, Milena Hasan2, Serge Hercberg5, Olivier Lantz6, Hugo Mouquet2, Etienne Patin2, Sandra Pellegrini2, Stanislas Pol7, Antonio Rausell8, Lars Rogge2, Anavaj Sakuntabhai2, Olivier Schwartz2, Benno Schwikowski2, Spencer Shorte2, Frédéric Tangy2, Antoine Toubert9, Mathilde Touvier10, Marie-Noëlle Ungeheuer2, Matthew L. Albert11*, Darragh Duffy2*, Lluis Quintana-Murci2*
1INSERM U1163, University of Paris, Imagine Institute, Paris, France. 2Pasteur Institute, Paris, France. 3Lund University, Lund, Sweden. 4EPFL, Lausanne, Switzerland. 5Université Paris 13, Paris, France. 6Curie Institute, Paris, France. 7Cochin Hospital, Paris, France. 8INSERM UMR 1163 – Institut Imagine, Paris, France. 9Hôpital Saint-Louis, Paris, France. 10Sorbonne Paris Nord University, Inserm U1153, Inrae U1125, Cnam, Nutritional Epidemiology Research Team (EREN), Bobigny, France. 11In Sitro, San Francisco, CA, USA. *Co-coordinators of The Milieu Intérieur Consortium. Additional information can be found at: www.milieuinterieur.fr/en.
CoV-Contact Cohort
Loubna Alavoine1, Karine K. A. Amat2, Sylvie Behillil3, Julia Bielicki4, Patricia Bruijning5, Charles Burdet6, Eric Caumes7, Charlotte Charpentier8, Bruno Coignard9, Yolande Costa1, Sandrine Couffin-Cadiergues10, Florence Damond8, Aline Dechanet11, Christelle Delmas10, Diane Descamps8, Xavier Duval1, Jean-Luc Ecobichon1, Vincent Enouf3, Hélène Espérou10, Wahiba Frezouls1, Nadhira Houhou11, Emila Ilic-Habensus1, Ouifiya Kafif11, John Kikoine11, Quentin Le Hingrat8, David Lebeaux12, Anne Leclercq1, Jonathan Lehacaut1, Sophie Letrou1, Bruno Lina13, Jean-Christophe Lucet14, Denis Malvy15, Pauline Manchon11, Milica Mandic1, Mohamed Meghadecha16, Justina Motiejunaite17, Mariama Nouroudine1, Valentine Piquard11, Andreea Postolache11, Caroline Quintin1, Jade Rexach1, Layidé Roufai10, Zaven Terzian11, Michael Thy18, Sarah Tubiana1, Sylvie van der Werf3, Valérie Vignali1, Benoit Visseaux8, Yazdan Yazdanpanah14
1Centre d'Investigation Clinique, Inserm CIC 1425, Hôpital Bichat Claude Bernard, APHP, Paris, France. 2IMEA Fondation Léon M'Ba, Paris, France. 3Institut Pasteur, UMR 3569 CNRS, Université de Paris, Paris, France. 4University of Basel Children’s Hospital, Basel, Switzerland. 5Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, Netherlands. 6Université de Paris, IAME, Inserm UMR 1137, F-75018, Paris, France; Hôpital Bichat Claude Bernard, APHP, Paris, France. 7Hôpital Pitiè Salpétriere, APHP, Paris, France. 8Université de Paris, IAME, INSERM UMR 1137, AP-HP, University Hospital Bichat Claude Bernard, Virology, F-75018 Paris, France. 9Santé Publique France, Saint Maurice, France. 10Pole Recherche Clinique, Inserm, Paris, France. 11Hôpital Bichat Claude Bernard, APHP, Paris, France. 12APHP, Paris, France. 13Virpath Laboratory, International Center of Research in Infectiology, Lyon University, INSERM U1111, CNRS UMR 5308, ENS, UCBL, Lyon, France . 14IAME Inserm UMR 1138, Hôpital Bichat Claude Bernard, APHP, Paris, France. 15Service des Maladies Infectieuses et Tropicales, Groupe Pellegrin, Place Amélie-Raba-Léon, Bordeaux, France. 16Hôpital Hotel Dieu, APHP, Paris, France. 17Service des explorations fonctionnelles, Hôpital Bichat - Claude Bernard, APHP, Paris, France. 18Center for Clinical Investigation, Assistance Publique-Hôpitaux de Paris, Bichat-Claude Bernard University Hospital, Paris, France.
Amsterdam UMC Covid-19 Biobank
Michiel van Agtmael1, Anna Geke Algera2, Frank van Baarle2, Diane Bax3, Martijn Beudel4, Harm Jan Bogaard5, Marije Bomers1, Lieuwe Bos2, Michela Botta2, Justin de Brabander6, Godelieve Bree6, Matthijs C. Brouwer4, Sanne de Bruin2, Marianna Bugiani7, Esther Bulle2, Osoul Chouchane1, Alex Cloherty3, Paul Elbers2, Lucas Fleuren2, Suzanne Geerlings1, Bart Geerts8, Theo Geijtenbeek9, Armand Girbes2, Bram Goorhuis1, Martin P. Grobusch1, Florianne Hafkamp9, Laura Hagens2, Jorg Hamann10, Vanessa Harris1, Robert Hemke11, Sabine M. Hermans1, Leo Heunks2, Markus W. Hollmann8, Janneke Horn2, Joppe W. Hovius1, Menno D. de Jong12, Rutger Koning4, Niels van Mourik2, Jeaninne Nellen1, Frederique Paulus2, Edgar Peters1, Tom van der Poll1, Benedikt Preckel8, Jan M. Prins1, Jorinde Raasveld2, Tom Reijnders1, Michiel Schinkel1, Marcus J. Schultz2, Alex Schuurman13, Kim Sigaloff1, Marry Smit2, Cornelis S. Stijnis1, Willemke Stilma2, Charlotte Teunissen14, Patrick Thoral2, Anissa Tsonas2, Marc van der Valk1, Denise Veelo8, Alexander P. J. Vlaar15, Heder de Vries2, Michèle van Vugt1, W. Joost Wiersinga1, Dorien Wouters16, A. H. (Koos) Zwinderman17, Diederik van de Beek18*
1Department of Infectious Diseases, Amsterdam UMC, Amsterdam, Netherlands. 2Department of Intensive Care, Amsterdam UMC, Amsterdam, Netherlands. 3Experimental Immunology, Amsterdam UMC, Amsterdam, Netherlands. 4Department of Neurology, Amsterdam UMC, Amsterdam Neuroscience, Amsterdam, Netherlands. 5Department of Pulmonology, Amsterdam UMC, Amsterdam, Netherlands. 6Department of Infectious Diseases, Amsterdam UMC, Amsterdam, Netherlands. 7Department of Pathology, Amsterdam UMC, Amsterdam, Netherlands. 8Department of Anesthesiology, Amsterdam UMC, Amsterdam, Netherlands. 9Department of Experimental Immunology, Amsterdam UMC, Amsterdam, Netherlands. 10Amsterdam UMC, Netherlands Biobank Core Facility, Amsterdam UMC, Amsterdam, Netherlands. 11Department of Radiology, Amsterdam UMC, Amsterdam, Netherlands. 12Department of Medical Microbiology, Amsterdam UMC, Amsterdam, Netherlands. 13Department of Internal Medicine, Amsterdam UMC, Amsterdam, Netherlands. 14Neurochemical Laboratory, Amsterdam UMC, Amsterdam, Netherlands. 15Deparment of Intensive Care, Amsterdam UMC, Amsterdam, Netherlands. 16Department of Clinical Chemistry, Amsterdam UMC, Amsterdam, Netherlands. 17Department of Clinical Epidemiology, Biostatistics and Bioinformatics, Amsterdam UMC, Amsterdam, Netherlands. 18Department of Neurology, Amsterdam UMC, Amsterdam, Netherlands. *Leader of the AMC consortium.
COVID Human Genetic Effort
Laurent Abel1, Alessandro Aiuti2, Saleh Al Muhsen3, Fahd Al-Mulla4, Mark S. Anderson5, Andrés Augusto Arias6, Hagit Baris Feldman7, Dusan Bogunovic8, Alexandre Bolze9, Anastasiia Bondarenko10, Ahmed A. Bousfiha11, Petter Brodin12, Yenan Bryceson12, Carlos D. Bustamante13, Manish Butte14, Giorgio Casari15, Samya Chakravorty16, John Christodoulou17, Elizabeth Cirulli9, Antonio Condino-Neto18, Megan A. Cooper19, Clifton L. Dalgard20, Joseph L. DeRisi21, Murkesh Desai22, Beth A. Drolet23, Sara Espinosa24, Jacques Fellay25, Carlos Flores26, Jose Luis Franco27, Peter K. Gregersen28, Filomeen Haerynck29, David Hagin30, Rabih Halwani31, Jim Heath32, Sarah E. Henrickson33, Elena Hsieh34, Kohsuke Imai35, Yuval Itan8, Timokratis Karamitros36, Kai Kisand37, Cheng-Lung Ku38, Yu-Lung Lau39, Yun Ling40, Carrie L. Lucas41, Tom Maniatis42, Davoud Mansouri43, Laszlo Marodi44, Isabelle Meyts45, Joshua D. Milner46, Kristina Mironska47, Trine Mogensen48, Tomohiro Morio49, Lisa F. P. Ng50, Luigi D. Notarangelo51, Giuseppe Novelli52, Antonio Novelli53, Cliona O'Farrelly54, Satoshi Okada55, Tayfun Ozcelik56, Rebeca Perez de Diego57, Anna M. Planas58, Carolina Prando59, Aurora Pujol60, Lluis Quintana-Murci61, Laurent Renia62, Alessandra Renieri63, Carlos Rodríguez-Gallego64, Vanessa Sancho-Shimizu65, Vijay Sankaran66, Kelly Schiabor Barrett9, Mohammed Shahrooei67, Andrew Snow68, Pere Soler-Palacín69, András N. Spaan70, Stuart Tangye71, Stuart Turvey72, Furkan Uddin73, Mohammed J. Uddin74, Diederik van de Beek75, Sara E. Vazquez76, Donald C. Vinh77, Horst von Bernuth78, Nicole Washington9, Pawel Zawadzki79, Helen C. Su51*, Jean-Laurent Casanova80*
1INSERM U1163, University of Paris, Imagine Institute, Paris, France. 2San Raffaele Telethon Institute for Gene Therapy, IRCCS Ospedale San Raffaele, Milan, Italy. 3King Saud University, Riyadh, Saudi Arabia. 4Dasman Diabetes Institute, Department of Genetics and Bioinformatics, Dasman, Kuwait. 5University of California, San Francisco, San Francisco, CA, USA. 6Universidad de Antioquia, Group of Primary Immunodeficiencies, Antioquia, Colombia. 7The Genetics Institute, Tel Aviv Sourasky Medical Center and Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel. 8Icahn School of Medicine at Mount Sinai, New York, NY, USA. 9Helix, San Mateo, CA, USA. 10Shupyk National Medical Academy for Postgraduate Education, Kiev, Ukraine. 11Clinical Immunology Unit, Pediatric Infectious Disease Department, Faculty of Medicine and Pharmacy, Averroes University Hospital, LICIA Laboratoire d'immunologie clinique, d'inflammation et d'allergie, Hassann Ii University, Casablanca, Morocco. 12Karolinska Institute, Stockholm, Sweden. 13Stanford University, Stanford, CA, USA. 14University of California, Los Angeles, CA, USA. 15Medical Genetics, IRCCS Ospedale San Raffaele, Milan, Italy. 16Department of Pediatrics and Children’s Healthcare of Atlanta, Emory University, Atlanta, GA, USA. 17Murdoch Children's Research Institute, Victoria, Australia. 18University of São Paulo, São Paulo, Brazil. 19Washington University School of Medicine, St. Louis, MO, USA. 20The American Genome Center; Uniformed Services University of the Health Sciences, Bethesda, MD, USA. 21University of California San Francisco; Chan Zuckerberg Biohub, San Francisco, CA, USA. 22Bai Jerbai Wadia Hospital for Children, Mumbai, India. 23 School of Medicine and Public Health, University of Wisconsin, Madison, WI, USA. 24Instituto Nacional de Pediatria (National Institute of Pediatrics), Mexico City, Mexico. 25Swiss Federal Institute of Technology Lausanne, Lausanne, Switzerland. 26Research Unit, Hospital Universitario Nuestra Señora de Candelaria, Canarian Health System, Santa Cruz de Tenerife, Spain. 27University of Antioquia, Medellín, Colombia. 28Feinstein Institute for Medical Research, Northwell Health USA, Manhasset, NY, USA. 29Department of Paediatric Immunology and Pulmonology, Centre for Primary Immunodeficiency Ghent (CPIG), PID Research Laboratory, Jeffrey Modell Diagnosis and Research Centre, Ghent University Hospital, Edegem, Belgium. 30The Genetics Institute Tel Aviv Sourasky Medical Center, Tel Aviv, Israel. 31Sharjah Institute of Medical Research, College of Medicine, University of Sharjah, Sharjah, United Arab Emirates. 32Institute for Systems Biology, Seattle, WA, USA. 33Children's Hospital of Philadelphia, Philadelphia, PA, USA. 34Anschutz Medical Campus, Aurora, CO, USA. 35Riken, Tokyo, Japan. 36Hellenic Pasteur Institute, Athens, Greece. 37University of Tartu, Tartu, Estonia. 38Chang Gung University, Taoyuan County, Taiwan. 39The University of Hong Kong, Hong Kong, China. 40Shanghai Public Health Clinical Center, Fudan University, Shanghai, China. 41Yale School of Medicine, New Haven, CT, USA. 42New York Genome Center, New York, NY, USA. 43Shahid Beheshti University of Medical Sciences, Tehran, Iran. 44Semmelweis University Budapest, Budapest, Hungary. 45KU Leuven, Department of Immunology, Microbiology and Transplantation, Leuven, Belgium. 46Columbia University Medical Center, New York, NY, USA. 47University Clinic for Children's Diseases, Skopje, North Macedonia. 48Aarhus University, Aarhus, Denmark. 49Tokyo Medical & Dental University Hospital, Tokyo, Japan. 50Singapore Immunology Network, Singapore. 51National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA. 52Department of Biomedicine and Prevention, University of Rome “Tor Vergata,” Rome, Italy. 53Bambino Gesù Children's Hospital, Rome, Italy. 54Trinity College, Dublin, Ireland. 55Hiroshima University, Hiroshima, Japan. 56Bilkent University, Ankara, Turkey. 57Laboratory of Immunogenetics of Human Diseases, Innate Immunity Group, IdiPAZ Institute for Health Research, La Paz Hospital, Madrid, Spain. 58IIBB-CSIC, IDIBAPS, Barcelona, Spain. 59Faculdades Pequeno Príncipe e Instituto de Pesquisa Pelé Pequeno Príncipe, Curitiba, Brazil. 60Neurometabolic Diseases Laboratory, IDIBELL - Hospital Duran I Reynals; Catalan Institution for Research and Advanced Studies (ICREA); CIBERER U759, ISCiii Madrid Spain, Barcelona, Spain. 61Institut Pasteur (CNRS UMR2000) and Collège de France, Paris, France. 62Infectious Diseases Horizontal Technology Center and Singapore Immunology Network, Agency for Science Technology (A*STAR), Singapore. 63Medical Genetics, University of Siena, Italy; Genetica Medica, Azienda Ospedaliero-Universitaria Senese, GEN-COVID Multicenter Study, Italy. 64Hospital Universitario de Gran Canaria Dr Negrín, Canarian Health System, Canary Islands, Spain. 65Imperial College London, London, UK. 66Boston Children's Hospital, Harvard Medical School, Boston, MA, USA. 67Saeed Pathobiology and Genetic Laboratory, Tehran, Iran. 68Uniformed Services University of the Health Sciences (USUHS), Bethesda, MD, USA. 69Hospital Universitari Vall d'Hebron, Barcelona, Spain. 70University Medical Center Utrecht, Amsterdam, Netherlands. 71Garvan Institute of Medical Research, Sydney, Australia. 72The University of British Columbia, Vancouver, Canada. 73Holy Family Red Crescent Medical College; Centre for Precision Therapeutics, NeuroGen Children's Healthcare; Genetics and Genomic Medicine Centre, NeuroGen Children's Healthcare, Dhaka, Bangladesh. 74Mohammed Bin Rashid University of Medicine and Health Sciences, College of Medicine, Dubai, United Arab Emirates; The Centre for Applied Genomics, Department of Genetics and Genome Biology, The Hospital for Sick Children, Toronto, Ontario, Canada. 75Amsterdam UMC, University of Amsterdam, Department of Neurology, Amsterdam Neuroscience, Amsterdam, Netherlands. 76University of California, San Francisco, San Francisco, CA, USA. 77McGill University Health Centre, Montreal, Canada. 78Charité - Berlin University Hospital Center, Berlin, Germany. 79Molecular Biophysics Division, Faculty of Physics, A. Mickiewicz University, Uniwersytetu Poznanskiego 2, Poznań, Poland. 80The Rockefeller University, Howard Hughes Medical Institute, Necker Hospital, New York, NY, USA. *Leaders of the COVID Human Genetic Effort.
Contributor Information
Collaborators: Andrés Augusto Arias, Bertrand Boisson, Soraya Boucherit, Jacinta Bustamante, Marwa Chbihi, Jie Chen, Maya Chrabieh, Tatiana Kochetkov, Tom Le Voyer, Dana Liu, Yelena Nemirovskaya, Masato Ogishi, Dominick Papandrea, Cécile Patissier, Franck Rapaport, Manon Roynard, Natasha Vladikine, Mark Woollett, Peng Zhang, Anuj Kashyap, Li Ding, Marita Bosticardo, Qinlu Wang, Sebastian Ochoa, Hui Liu, Samuel D. Chauvin, Michael Stack, Galina Koroleva, Neha Bansal, Clifton L. Dalgard, Andrew L. Snow, Jorge Abad, Sergio Aguilera-Albesa, Ozge Metin Akcan, Ilad Alavi Darazam, Juan C. Aldave, Miquel Alfonso Ramos, Seyed Alireza Nadji, Gulsum Alkan, Jerome Allardet-Servent, Luis M. Allende, Laia Alsina, Marie-Alexandra Alyanakian, Blanca Amador-Borrero, Zahir Amoura, Arnau Antolí, Sevket Arslan, Sophie Assant, Terese Auguet, Axelle Azot, Fanny Bajolle, Aurélie Baldolli, Maite Ballester, Hagit Baris Feldman, Benoit Barrou, Alexandra Beurton, Agurtzane Bilbao, Geraldine Blanchard-Rohner, Ignacio Blanco, Adeline Blandinières, Daniel Blazquez-Gamero, Marketa Bloomfield, Mireia Bolivar-Prados, Raphael Borie, Ahmed A. Bousfiha, Claire Bouvattier, Oksana Boyarchuk, Maria Rita P. Bueno, Jacinta Bustamante, Juan José Cáceres Agra, Semra Calimli, Ruggero Capra, Maria Carrabba, Carlos Casasnovas, Marion Caseris, Martin Castelle, Francesco Castelli, Martín Castillo de Vera, Mateus V. Castro, Emilie Catherinot, Martin Chalumeau, Bruno Charbit, Matthew P. Cheng, Père Clavé, Bonaventura Clotet, Anna Codina, Fatih Colkesen, Fatma Colkesen, Roger Colobran, Cloé Comarmond, Angelo G. Corsico, David Dalmau, David Ross Darley, Nicolas Dauby, Stéphane Dauger, Loic de Pontual, Amin Dehban, Geoffroy Delplancq, Alexandre Demoule, Antonio Di Sabatino, Jean-Luc Diehl, Stephanie Dobbelaere, Sophie Durand, Waleed Eldars, Mohamed Elgamal, Marwa H. Elnagdy, Melike Emiroglu, Emine Hafize Erdeniz, Selma Erol Aytekin, Romain Euvrard, Recep Evcen, Giovanna Fabio, Laurence Faivre, Antonin Falck, Muriel Fartoukh, Morgane Faure, Miguel Fernandez Arquero, Carlos Flores, Bruno Francois, Victoria Fumadó, Francesca Fusco, Blanca Garcia Solis, Pascale Gaussem, Juana Gil-Herrera, Laurent Gilardin, Monica Girona Alarcon, Mónica Girona-Alarcón, Jean-Christophe Goffard, Funda Gok, Rafaela González-Montelongo, Antoine Guerder, Yahya Gul, Sukru Nail Guner, Marta Gut, Jérôme Hadjadj, Filomeen Haerynck, Rabih Halwani, Lennart Hammarström, Nevin Hatipoglu, Elisa Hernandez-Brito, María Soledad Holanda-Peña, Juan Pablo Horcajada, Sami Hraiech, Linda Humbert, Alejandro D. Iglesias, Antonio Íñigo-Campos, Matthieu Jamme, María Jesús Arranz, Iolanda Jordan, Fikret Kanat, Hasan Kapakli, Iskender Kara, Adem Karbuz, Kadriye Kart Yasar, Sevgi Keles, Yasemin Kendir Demirkol, Adam Klocperk, Zbigniew J. Król, Paul Kuentz, Yat Wah M. Kwan, Jean-Christophe Lagier, Yu-Lung Lau, Fleur Le Bourgeois, Yee-Sin Leo, Rafael Leon Lopez, Daniel Leung, Michael Levin, Michael Levy, Romain Lévy, Zhi Li, Agnes Linglart, José M. Lorenzo-Salazar, Céline Louapre, Catherine Lubetzki, Charles-Edouard Luyt, David C. Lye, Davood Mansouri, Majid Marjani, Jesus Marquez Pereira, Andrea Martin, David Martínez Pueyo, Javier Martinez-Picado, Iciar Marzana, Alexis Mathian, Larissa R. B. Matos, Gail V. Matthews, Julien Mayaux, Jean-Louis Mège, Isabelle Melki, Jean-François Meritet, Ozge Metin, Isabelle Meyts, Mehdi Mezidi, Isabelle Migeotte, Maude Millereux, Tristan Mirault, Clotilde Mircher, Mehdi Mirsaeidi, Abián Montesdeoca Melián, Antonio Morales Martinez, Pierre Morange, Clémence Mordacq, Guillaume Morelle, Stéphane Mouly, Adrián Muñoz-Barrera, Cyril Nafati, João Farela Neves, Lisa F. P. Ng, Yeray Novoa Medina, Esmeralda Nuñez Cuadros, J. Gonzalo Ocejo-Vinyals, Zerrin Orbak, Mehdi Oualha, Tayfun Özçelik, Qiang Pan Hammarström, Christophe Parizot, Tiffany Pascreau, Estela Paz-Artal, Rebeca Pérez de Diego, Aurélien Philippe, Quentin Philippot, Laura Planas-Serra, Dominique Ploin, Julien Poissy, Géraldine Poncelet, Marie Pouletty, Paul Quentric, Didier Raoult, Anne-Sophie Rebillat, Ismail Reisli, Pilar Ricart, Jean-Christophe Richard, Nadia Rivet, Jacques G. Rivière, Gemma Rocamora Blanch, Carlos Rodrigo, Carlos Rodriguez-Gallego, Agustí Rodríguez-Palmero, Carolina Soledad Romero, Anya Rothenbuhler, Flore Rozenberg, Maria Yolanda Ruiz del Prado, Joan Sabater Riera, Oliver Sanchez, Silvia Sánchez-Ramón, Agatha Schluter, Matthieu Schmidt, Cyril E. Schweitzer, Francesco Scolari, Anna Sediva, Luis M. Seijo, Damien Sene, Sevtap Senoglu, Mikko R. J. Seppänen, Alex Serra Ilovich, Mohammad Shahrooei, David Smadja, Ali Sobh, Xavier Solanich Moreno, Jordi Solé-Violán, Catherine Soler, Pere Soler-Palacín, Yuri Stepanovskiy, Annabelle Stoclin, Fabio Taccone, Yacine Tandjaoui-Lambiotte, Jean-Luc Taupin, Simon J. Tavernier, Benjamin Terrier, Caroline Thumerelle, Gabriele Tomasoni, Julie Toubiana, Josep Trenado Alvarez, Sophie Trouillet-Assant, Jesús Troya, Alessandra Tucci, Matilde Valeria Ursini, Yurdagul Uzunhan, Pierre Vabres, Juan Valencia-Ramos, Ana Maria Van Den Rym, Isabelle Vandernoot, Hulya Vatansev, Valentina Vélez-Santamaria, Sébastien Viel, Cédric Vilain, Marie E. Vilaire, Audrey Vincent, Guillaume Voiriot, Fanny Vuotto, Alper Yosunkaya, Barnaby E. Young, Fatih Yucel, Faiez Zannad, Mayana Zatz, Alexandre Belot, Giuseppe Foti, Giacomo Bellani, Giuseppe Citerio, Ernesto Contro, Alberto Pesci, Maria Grazia Valsecchi, Marina Cazzaniga, Christine Bole-Feysot, Stanislas Lyonnet, Cécile Masson, Patrick Nitschke, Aurore Pouliet, Yoann Schmitt, Frederic Tores, Mohammed Zarhrate, Laurent Abel, Claire Andrejak, François Angoulvant, Delphine Bachelet, Romain Basmaci, Sylvie Behillil, Marine Beluze, Dehbia Benkerrou, Krishna Bhavsar, François Bompart, Lila Bouadma, Maude Bouscambert, Mireille Caralp, Minerva Cervantes-Gonzalez, Anissa Chair, Alexandra Coelho, Camille Couffignal, Sandrine Couffin-Cadiergues, Eric D’ortenzio, Charlene Da Silveira, Marie-Pierre Debray, Dominique Deplanque, Diane Descamps, Mathilde Desvallées, Alpha Diallo, Alphonsine Diouf, Céline Dorival, François Dubos, Xavier Duval, Philippine Eloy, Vincent V. E. Enouf, Hélène Esperou, Marina Esposito-Farese, Manuel Etienne, Nadia Ettalhaoui, Nathalie Gault, Alexandre Gaymard, Jade Ghosn, Tristan Gigante, Isabelle Gorenne, Jérémie Guedj, Alexandre Hoctin, Isabelle Hoffmann, Salma Jaafoura, Ouifiya Kafif, Florentia Kaguelidou, Sabina Kali, Antoine Khalil, Coralie Khan, Cédric Laouénan, Samira Laribi, Minh Le, Quentin Le Hingrat, Soizic Le Mestre, Hervé Le Nagard, François-Xavier Lescure, Yves Lévy, Claire Levy-Marchal, Bruno Lina, Guillaume Lingas, Jean Christophe Lucet, Denis Malvy, Marina Mambert, France Mentré, Noémie Mercier, Amina Meziane, Hugo Mouquet, Jimmy Mullaert, Nadège Neant, Marion Noret, Justine Pages, Aurélie Papadopoulos, Christelle Paul, Nathan Peiffer-Smadja, Ventzislava Petrov-Sanchez, Gilles Peytavin, Olivier Picone, Oriane Puéchal, Manuel Rosa-Calatrava, Bénédicte Rossignol, Patrick Rossignol, Carine Roy, Marion Schneider, Caroline Semaille, Nassima Si Mohammed, Lysa Tagherset, Coralie Tardivon, Marie-Capucine Tellier, François Téoulé, Olivier Terrier, Jean-François Timsit, Théo Treoux, Christelle Tual, Sarah Tubiana, Sylvie van der Werf, Noémie Vanel, Aurélie Veislinger, Benoit Visseaux, Aurélie Wiedemann, Yazdan Yazdanpanah, Laurent Abel, Andres Alcover, Hugues Aschard, Kalla Astrom, Philippe Bousso, Pierre Bruhns, Ana Cumano, Caroline Demangel, Ludovic Deriano, James Di Santo, Françoise Dromer, Gérard Eberl, Jost Enninga, Jacques Fellay, Ivo Gomperts-Boneca, Milena Hasan, Serge Hercberg, Olivier Lantz, Hugo Mouquet, Etienne Patin, Sandra Pellegrini, Stanislas Pol, Antonio Rausell, Lars Rogge, Anavaj Sakuntabhai, Olivier Schwartz, Benno Schwikowski, Spencer Shorte, Frédéric Tangy, Antoine Toubert, Mathilde Touvier, Marie-Noëlle Ungeheuer, Matthew L. Albert, Darragh Duffy, Lluis Quintana-Murci, Loubna Alavoine, Karine K. A. Amat, Sylvie Behillil, Julia Bielicki, Patricia Bruijning, Charles Burdet, Eric Caumes, Charlotte Charpentier, Bruno Coignard, Yolande Costa, Sandrine Couffin-Cadiergues, Florence Damond, Aline Dechanet, Christelle Delmas, Diane Descamps, Xavier Duval, Jean-Luc Ecobichon, Vincent Enouf, Hélène Espérou, Wahiba Frezouls, Nadhira Houhou, Emila Ilic-Habensus, Ouifiya Kafif, John Kikoine, Quentin Le Hingrat, David Lebeaux, Anne Leclercq, Jonathan Lehacaut, Sophie Letrou, Bruno Lina, Jean-Christophe Lucet, Denis Malvy, Pauline Manchon, Milica Mandic, Mohamed Meghadecha, Justina Motiejunaite, Mariama Nouroudine, Valentine Piquard, Andreea Postolache, Caroline Quintin, Jade Rexach, Layidé Roufai, Zaven Terzian, Michael Thy, Sarah Tubiana, Sylvie van der Werf, Valérie Vignali, Benoit Visseaux, Yazdan Yazdanpanah, Michiel van Agtmael, Anna Geke Algera, Frank van Baarle, Diane Bax, Martijn Beudel, Harm Jan Bogaard, Marije Bomers, Lieuwe Bos, Michela Botta, Justin de Brabander, Godelieve Bree, Matthijs C. Brouwer, Sanne de Bruin, Marianna Bugiani, Esther Bulle, Osoul Chouchane, Alex Cloherty, Paul Elbers, Lucas Fleuren, Suzanne Geerlings, Bart Geerts, Theo Geijtenbeek, Armand Girbes, Bram Goorhuis, Martin P. Grobusch, Florianne Hafkamp, Laura Hagens, Jorg Hamann, Vanessa Harris, Robert Hemke, Sabine M. Hermans, Leo Heunks, Markus W. Hollmann, Janneke Horn, Joppe W. Hovius, Menno D. de Jong, Rutger Koning, Niels van Mourik, Jeaninne Nellen, Frederique Paulus, Edgar Peters, Tom van der Poll, Benedikt Preckel, Jan M. Prins, Jorinde Raasveld, Tom Reijnders, Michiel Schinkel, Marcus J. Schultz, Alex Schuurman, Kim Sigaloff, Marry Smit, Cornelis S. Stijnis, Willemke Stilma, Charlotte Teunissen, Patrick Thoral, Anissa Tsonas, Marc van der Valk, Denise Veelo, Alexander P. J. Vlaar, Heder de Vries, Michèle van Vugt, W. Joost Wiersinga, Dorien Wouters, A. H. (Koos) Zwinderman, Diederik van de Beek, Laurent Abel, Alessandro Aiuti, Saleh Al Muhsen, Fahd Al-Mulla, Mark S. Anderson, Andrés Augusto Arias, Hagit Baris Feldman, Dusan Bogunovic, Alexandre Bolze, Anastasiia Bondarenko, Ahmed A. Bousfiha, Petter Brodin, Yenan Bryceson, Carlos D. Bustamante, Manish Butte, Giorgio Casari, Samya Chakravorty, John Christodoulou, Elizabeth Cirulli, Antonio Condino-Neto, Megan A. Cooper, Clifton L. Dalgard, Joseph L. DeRisi, Murkesh Desai, Beth A. Drolet, Sara Espinosa, Jacques Fellay, Carlos Flores, Jose Luis Franco, Peter K. Gregersen, Filomeen Haerynck, David Hagin, Rabih Halwani, Jim Heath, Sarah E. Henrickson, Elena Hsieh, Kohsuke Imai, Yuval Itan, Timokratis Karamitros, Kai Kisand, Cheng-Lung Ku, Yu-Lung Lau, Yun Ling, Carrie L. Lucas, Tom Maniatis, Davoud Mansouri, Laszlo Marodi, Isabelle Meyts, Joshua D. Milner, Kristina Mironska, Trine Mogensen, Tomohiro Morio, Lisa F. P. Ng, Luigi D. Notarangelo, Giuseppe Novelli, Antonio Novelli, Cliona O'Farrelly, Satoshi Okada, Tayfun Ozcelik, Rebeca Perez de Diego, Anna M. Planas, Carolina Prando, Aurora Pujol, Lluis Quintana-Murci, Laurent Renia, Alessandra Renieri, Carlos Rodríguez-Gallego, Vanessa Sancho-Shimizu, Vijay Sankaran, Kelly Schiabor Barrett, Mohammed Shahrooei, Andrew Snow, Pere Soler-Palacín, András N. Spaan, Stuart Tangye, Stuart Turvey, Furkan Uddin, Mohammed J. Uddin, Diederik van de Beek, Sara E. Vazquez, Donald C. Vinh, Horst von Bernuth, Nicole Washington, Pawel Zawadzki, Helen C. Su, and Jean-Laurent Casanova
References and Notes
- 1.Casanova J.-L., Abel L., The human genetic determinism of life-threatening infectious diseases: Genetic heterogeneity and physiological homogeneity? Hum. Genet. 139, 681–694 (2020). 10.1007/s00439-020-02184-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Döffinger R., Helbert M. R., Barcenas-Morales G., Yang K., Dupuis S., Ceron-Gutierrez L., Espitia-Pinzon C., Barnes N., Bothamley G., Casanova J.-L., Longhurst H. J., Kumararatne D. S., Autoantibodies to interferon-γ in a patient with selective susceptibility to mycobacterial infection and organ-specific autoimmunity. Clin. Infect. Dis. 38, e10–e14 (2004). 10.1086/380453 [DOI] [PubMed] [Google Scholar]
- 3.Höflich C., Sabat R., Rosseau S., Temmesfeld B., Slevogt H., Döcke W.-D., Grütz G., Meisel C., Halle E., Göbel U. B., Volk H.-D., Suttorp N., Naturally occurring anti–IFN-γ autoantibody and severe infections with Mycobacterium cheloneae and Burkholderia cocovenenans. Blood 103, 673–675 (2004). 10.1182/blood-2003-04-1065 [DOI] [PubMed] [Google Scholar]
- 4.Kampmann B., Hemingway C., Stephens A., Davidson R., Goodsall A., Anderson S., Nicol M., Schölvinck E., Relman D., Waddell S., Langford P., Sheehan B., Semple L., Wilkinson K. A., Wilkinson R. J., Ress S., Hibberd M., Levin M., Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-γ. J. Clin. Invest. 115, 2480–2488 (2005). 10.1172/JCI19316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puel A., Picard C., Lorrot M., Pons C., Chrabieh M., Lorenzo L., Mamani-Matsuda M., Jouanguy E., Gendrel D., Casanova J.-L., Recurrent staphylococcal cellulitis and subcutaneous abscesses in a child with autoantibodies against IL-6. J. Immunol. 180, 647–654 (2008). 10.4049/jimmunol.180.1.647 [DOI] [PubMed] [Google Scholar]
- 6.Puel A., Döffinger R., Natividad A., Chrabieh M., Barcenas-Morales G., Picard C., Cobat A., Ouachée-Chardin M., Toulon A., Bustamante J., Al-Muhsen S., Al-Owain M., Arkwright P. D., Costigan C., McConnell V., Cant A. J., Abinun M., Polak M., Bougnères P.-F., Kumararatne D., Marodi L., Nahum A., Roifman C., Blanche S., Fischer A., Bodemer C., Abel L., Lilic D., Casanova J.-L., Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J. Exp. Med. 207, 291–297 (2010). 10.1084/jem.20091983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kisand K., Bøe Wolff A. S., Podkrajsek K. T., Tserel L., Link M., Kisand K. V., Ersvaer E., Perheentupa J., Erichsen M. M., Bratanic N., Meloni A., Cetani F., Perniola R., Ergun-Longmire B., Maclaren N., Krohn K. J. E., Pura M., Schalke B., Ströbel P., Leite M. I., Battelino T., Husebye E. S., Peterson P., Willcox N., Meager A., Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J. Exp. Med. 207, 299–308 (2010). 10.1084/jem.20091669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ku C.-L., Chi C.-Y., von Bernuth H., Doffinger R., Autoantibodies against cytokines: Phenocopies of primary immunodeficiencies? Hum. Genet. 139, 783–794 (2020). 10.1007/s00439-020-02180-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isaacs A., Lindenmann J., Virus interference. I. The interferon. Proc. R. Soc. Lond. B 147, 258–267 (1957). 10.1098/rspb.1957.0048 [DOI] [PubMed] [Google Scholar]
- 10.Isaacs A., Lindenmann J., Valentine R. C., Virus interference. II. Some properties of interferon. Proc. R. Soc. Lond. B 147, 268–273 (1957). 10.1098/rspb.1957.0049 [DOI] [PubMed] [Google Scholar]
- 11.Gresser I., Wherefore interferon? J. Leukoc. Biol. 61, 567–574 (1997). 10.1002/jlb.61.5.567 [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann H.-H., Schneider W. M., Rice C. M., Interferons and viruses: An evolutionary arms race of molecular interactions. Trends Immunol. 36, 124–138 (2015). 10.1016/j.it.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Weerd N. A., Vivian J. P., Lim S. S., Huang S. U.-S., Hertzog P. J., Structural integrity with functional plasticity: What type I IFN receptor polymorphisms reveal. J. Leukoc. Biol. 108, 909–924 (2020). 10.1002/JLB.2MR0420-152R [DOI] [PubMed] [Google Scholar]
- 14.Darnell J. E. Jr., ., STATs and gene regulation. Science 277, 1630–1635 (1997). 10.1126/science.277.5332.1630 [DOI] [PubMed] [Google Scholar]
- 15.Vallbracht A., Treuner J., Flehmig B., Joester K. E., Niethammer D., Interferon-neutralizing antibodies in a patient treated with human fibroblast interferon. Nature 289, 496–497 (1981). 10.1038/289496a0 [DOI] [PubMed] [Google Scholar]
- 16.Meager A., Visvalingam K., Peterson P., Möll K., Murumägi A., Krohn K., Eskelin P., Perheentupa J., Husebye E., Kadota Y., Willcox N., Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLOS Med. 3, e289 (2006). 10.1371/journal.pmed.0030289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panem S., Check I. J., Henriksen D., Vilcek J., Antibodies to alpha-interferon in a patient with systemic lupus erythematosus. J. Immunol. 129, 1–3 (1982). [PubMed] [Google Scholar]
- 18.Zhang Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J., Ogishi M., Sabli I. K. D., Hodeib S., Korol C., Rosain J., Bilguvar K., Ye J., Bolze A., Bigio B., Yang R., Augusto Arias Sierra A., Zhou Q., Zhang Y., Onodi F., Korniotis S., Karpf L., Philippot Q., Chbihi M., Bonnet-Madin L., Dorgham K., Smith N., Schneider W. M., Razooky B. S., H.-H. Hoffmann, Michailidis E., Moens L., Han J. E., Lorenzo L., Bizien L., Meade P., Neehus A.-L., Ugurbil A. C., Corneau A., Kerner G., Zhang P., Rapaport F., Seeleuthner Y., Manry J., Masson C., Schmitt Y., Schlüter A., Le Voyer T., Khan T., Li J., Fellay J., Roussel L., Shahrooei M., Alosaimi M. F., Mansouri D., Al-Saud H., Al-Mulla F., Almourfi F., Al-Muhsen S. Z., Alsohime F., Al Turki S., Hasanato R., van de Beek D., Biondi A., Bettini L. R., D’Angio M., Bonfanti P., Imberti L., Sottini A., Paghera S., Quiros-Roldan E., Rossi C., Oler A. J., Tompkins M. F., Alba C., Vandernoot I., Goffard J.-C., Smits G., Migeotte I., Haerynck F., Soler-Palacin P., Martin-Nalda A., Colobran R., Morange P.-E., Keles S., Çölkesen F., Ozcelik T., Yasar K. K., Senoglu S., Karabela Ş. N., Rodríguez-Gallego C., Novelli G., Hraiech S., Tandjaoui-Lambiotte Y., Duval X., Laouenan C., COVID-STORM Clinicians, COVID Clinicians, Imagine COVID group, French COVID Cohort Study Group, CoV-Contact Cohort, Amsterdam UMC Covid-19 Biobank, COVID Human Genetic Effort, NIAID-USUHS/TAGC COVID Immunity Group, Snow A. L., Dalgard C. L., Milner J., Vinh D. C., Mogensen T. H., Marr N., Spaan A. N., Boisson B., Boisson-Dupuis S., Bustamante J., Puel A., Ciancanelli M., Meyts I., Maniatis T., Soumelis V., Amara A., Nussenzweig M., García-Sastre A., Krammer F., Pujol A., Duffy D., Lifton R., Zhang S.-Y., Gorochov G., Béziat V., Jouanguy E., Sancho-Shimizu V., Rice C. M., Abel L., Notarangelo L. D., Cobat A., Su H. C., Casanova J.-L., Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 370, eabd4570 (2020). 10.1126/science.abd4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pozzetto B., Mogensen K. E., Tovey M. G., Gresser I., Characteristics of autoantibodies to human interferon in a patient with varicella-zoster disease. J. Infect. Dis. 150, 707–713 (1984). 10.1093/infdis/150.5.707 [DOI] [PubMed] [Google Scholar]
- 20.Casanova J.-L., Ion Gresser. J. Interferon Cytokine Res. 39, 317–320 (2019). 10.1089/jir.2018.29015.mem [DOI] [Google Scholar]
- 21.Walter J. E., Rosen L. B., Csomos K., Rosenberg J. M., Mathew D., Keszei M., Ujhazi B., Chen K., Lee Y. N., Tirosh I., Dobbs K., Al-Herz W., Cowan M. J., Puck J., Bleesing J. J., Grimley M. S., Malech H., De Ravin S. S., Gennery A. R., Abraham R. S., Joshi A. Y., Boyce T. G., Butte M. J., Nadeau K. C., Balboni I., Sullivan K. E., Akhter J., Adeli M., El-Feky R. A., El-Ghoneimy D. H., Dbaibo G., Wakim R., Azzari C., Palma P., Cancrini C., Capuder K., Condino-Neto A., Costa-Carvalho B. T., Oliveira J. B., Roifman C., Buchbinder D., Kumanovics A., Franco J. L., Niehues T., Schuetz C., Kuijpers T., Yee C., Chou J., Masaad M. J., Geha R., Uzel G., Gelman R., Holland S. M., Recher M., Utz P. J., Browne S. K., Notarangelo L. D., Broad-spectrum antibodies against self-antigens and cytokines in RAG deficiency. J. Clin. Invest. 125, 4135–4148 (2015). 10.1172/JCI80477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beccuti G., Ghizzoni L., Cambria V., Codullo V., Sacchi P., Lovati E., Mongodi S., Iotti G. A., Mojoli F., A COVID-19 pneumonia case report of autoimmune polyendocrine syndrome type 1 in Lombardy, Italy: Letter to the editor. J. Endocrinol. Invest. 43, 1175–1177 (2020). 10.1007/s40618-020-01323-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casanova J.-L., Su H. C., COVID Human Genetic Effort , A Global Effort to Define the Human Genetics of Protective Immunity to SARS-CoV-2 Infection. Cell 181, 1194–1199 (2020). 10.1016/j.cell.2020.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manry J., Laval G., Patin E., Fornarino S., Itan Y., Fumagalli M., Sironi M., Tichit M., Bouchier C., Casanova J.-L., Barreiro L. B., Quintana-Murci L., Evolutionary genetic dissection of human interferons. J. Exp. Med. 208, 2747–2759 (2011). 10.1084/jem.20111680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trouillet-Assant S., Viel S., Gaymard A., Pons S., Richard J.-C., Perret M., Villard M., Brengel-Pesce K., Lina B., Mezidi M., Bitker L., Belot A., COVID HCL Study group , Type I IFN immunoprofiling in COVID-19 patients. J. Allergy Clin. Immunol. 146, 206–208.e2 (2020). 10.1016/j.jaci.2020.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., Péré H., Charbit B., Bondet V., Chenevier-Gobeaux C., Breillat P., Carlier N., Gauzit R., Morbieu C., Pène F., Marin N., Roche N., Szwebel T.-A., Merkling S. H., Treluyer J.-M., Veyer D., Mouthon L., Blanc C., Tharaux P.-L., Rozenberg F., Fischer A., Duffy D., Rieux-Laucat F., Kernéis S., Terrier B., Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 369, 718–724 (2020). 10.1126/science.abc6027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris A., Collins J., Vetrie D., Cole C., Bobrow M., X inactivation as a mechanism of selection against lethal alleles: Further investigation of incontinentia pigmenti and X linked lymphoproliferative disease. J. Med. Genet. 29, 608–614 (1992). 10.1136/jmg.29.9.608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanco-Melo D., Nilsson-Payant B. E., Liu W.-C., Uhl S., Hoagland D., Møller R., Jordan T. X., Oishi K., Panis M., Sachs D., Wang T. T., Schwartz R. E., Lim J. K., Albrecht R. A., tenOever B. R., Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 181, 1036–1045.e9 (2020). 10.1016/j.cell.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein S. L., Pekosz A., Park H.-S., Ursin R. L., Shapiro J. R., Benner S. E., Littlefield K., Kumar S., Naik H. M., Betenbaugh M., Shrestha R., Wu A. A., Hughes R. M., Burgess I., Caturegli P., Laeyendecker O., Quinn T. C., Sullivan D. J., Shoham S., Redd A. D., Bloch E. M., Casadevall A., Tobian A. A. R., Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J. Clin. Invest. 142004 (2020). 10.1172/JCI142004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang T. T., Ravetch J. V., Functional diversification of IgGs through Fc glycosylation. J. Clin. Invest. 129, 3492–3498 (2019). 10.1172/JCI130029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burbelo P. D., Riedo F. X., Morishima C., Rawlings S., Smith D., Das S., Strich J. R., Chertow D. S., Davey R. T. Jr.., Cohen J. I., Sensitivity in Detection of Antibodies to Nucleocapsid and Spike Proteins of Severe Acute Respiratory Syndrome Coronavirus 2 in Patients With Coronavirus Disease 2019. J. Infect. Dis. 222, 206–213 (2020). 10.1093/infdis/jiaa273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer S., Woodward M., Hertel C., Vlaicu P., Haque Y., Kärner J., Macagno A., Onuoha S. C., Fishman D., Peterson H., Metsküla K., Uibo R., Jäntti K., Hokynar K., Wolff A. S. B., APECED patient collaborative, Krohn K., Ranki A., Peterson P., Kisand K., Hayday A., AIRE-Deficient Patients Harbor Unique High-Affinity Disease-Ameliorating Autoantibodies. Cell 166, 582–595 (2016). 10.1016/j.cell.2016.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katoh K., Rozewicki J., Yamada K. D., MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 20, 1160–1166 (2019). 10.1093/bib/bbx108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katoh K., Kuma K., Toh H., Miyata T., MAFFT version 5: Improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 33, 511–518 (2005). 10.1093/nar/gki198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saitou N., Nei M., The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425 (1987). 10.1093/oxfordjournals.molbev.a040454 [DOI] [PubMed] [Google Scholar]
- 36.Jones D. T., Taylor W. R., Thornton J. M., The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 8, 275–282 (1992). 10.1093/bioinformatics/8.3.275 [DOI] [PubMed] [Google Scholar]
- 37.Woelk C. H., Frost S. D. W., Richman D. D., Higley P. E., Kosakovsky Pond S. L., Evolution of the interferon alpha gene family in eutherian mammals. Gene 397, 38–50 (2007). 10.1016/j.gene.2007.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han M. V., Zmasek C. M., phyloXML: XML for evolutionary biology and comparative genomics. BMC Bioinformatics 10, 356 (2009). 10.1186/1471-2105-10-356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pestka S., Krause C. D., Walter M. R., Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 202, 8–32 (2004). 10.1111/j.0105-2896.2004.00204.x [DOI] [PubMed] [Google Scholar]
- 40.Rissin D. M., Kan C. W., Campbell T. G., Howes S. C., Fournier D. R., Song L., Piech T., Patel P. P., Chang L., Rivnak A. J., Ferrell E. P., Randall J. D., Provuncher G. K., Walt D. R., Duffy D. C., Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 28, 595–599 (2010). 10.1038/nbt.1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathian A., Mouries-Martin S., Dorgham K., Devilliers H., Barnabei L., Ben Salah E., Cohen-Aubart F., Garrido Castillo L., Haroche J., Hie M., Pineton de Chambrun M., Miyara M., Sterlin D., Pha M., Lê Thi Huong D., Rieux-Laucat F., Rozenberg F., Gorochov G., Amoura Z., Monitoring Disease Activity in Systemic Lupus Erythematosus With Single-Molecule Array Digital Enzyme-Linked Immunosorbent Assay Quantification of Serum Interferon-α. Arthritis Rheumatol. 71, 756–765 (2019). 10.1002/art.40792 [DOI] [PubMed] [Google Scholar]
- 42.Lebon P., Ponsot G., Aicardi J., Goutières F., Arthuis M., Early intrathecal synthesis of interferon in herpes encephalitis. Biomedicine 31, 267–271 (1979). [PubMed] [Google Scholar]
- 43.Robbiani D. F., Gaebler C., Muecksch F., Lorenzi J. C. C., Wang Z., Cho A., Agudelo M., Barnes C. O., Gazumyan A., Finkin S., Hägglöf T., Oliveira T. Y., Viant C., Hurley A., Hoffmann H.-H., Millard K. G., Kost R. G., Cipolla M., Gordon K., Bianchini F., Chen S. T., Ramos V., Patel R., Dizon J., Shimeliovich I., Mendoza P., Hartweger H., Nogueira L., Pack M., Horowitz J., Schmidt F., Weisblum Y., Michailidis E., Ashbrook A. W., Waltari E., Pak J. E., Huey-Tubman K. E., Koranda N., Hoffman P. R., West A. P. Jr.., Rice C. M., Hatziioannou T., Bjorkman P. J., Bieniasz P. D., Caskey M., Nussenzweig M. C., Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 584, 437–442 (2020). 10.1038/s41586-020-2456-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim H., de Jesus A. A., Brooks S. R., Liu Y., Huang Y., VanTries R., Montealegre Sanchez G. A., Rotman Y., Gadina M., Goldbach-Mansky R., Development of a Validated Interferon Score Using NanoString Technology. J. Interferon Cytokine Res. 38, 171–185 (2018). 10.1089/jir.2017.0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferreira J. P., Girerd N., Bozec E., Mercklé L., Pizard A., Bouali S., Eby E., Leroy C., Machu J.-L., Boivin J.-M., Lamiral Z., Rossignol P., Zannad F., Cohort Profile: Rationale and design of the fourth visit of the STANISLAS cohort: a familial longitudinal population-based cohort from the Nancy region of France. Int. J. Epidemiol. 47, 395–395j (2018). 10.1093/ije/dyx240 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
science.sciencemag.org/content/370/6515/eabd4585/suppl/DC1
Supplementary Materials and Methods
Figs. S1 to S4
Tables S1 to S3
Data S1