Abstract
Proteins of the ADF/cofilin family play a central role in the disassembly of actin filaments, and their activity must be tightly regulated in cells. Recently, the oxidation of actin filaments by the enzyme MICAL1 was found to amplify the severing action of cofilin through unclear mechanisms. Using single filament experiments in vitro, we found that actin filament oxidation by MICAL1 increases, by several orders of magnitude, both cofilin binding and severing rates, explaining the dramatic synergy between oxidation and cofilin for filament disassembly. Remarkably, we found that actin oxidation bypasses the need for cofilin activation by dephosphorylation. Indeed, non‐activated, phosphomimetic S3D‐cofilin binds and severs oxidized actin filaments rapidly, in conditions where non‐oxidized filaments are unaffected. Finally, tropomyosin Tpm1.8 loses its ability to protect filaments from cofilin severing activity when actin is oxidized by MICAL1. Together, our results show that MICAL1‐induced oxidation of actin filaments suppresses their physiological protection from the action of cofilin. We propose that, in cells, direct post‐translational modification of actin filaments by oxidation is a way to trigger their disassembly.
Keywords: actin dynamics, actin‐binding proteins, ADF/cofilin, microfluidics
Subject Categories: Cell Adhesion, Polarity & Cytoskeleton
The oxidation of actin filaments by MICAL1 triggers their rapid disassembly by cofilin‐1, even when cofilin‐1 is at very low concentration or is inhibited by phosphorylation, and when filaments are protected by Tropomyosin 1.8.
Introduction
The controlled assembly and disassembly of actin filaments (F‐actin) is essential for numerous fundamental cellular functions. On one hand, a myriad of actin‐binding proteins (ABPs) has been identified and implicated in filament polymerization and spatial organization, force generation, and actin disassembly. On the other hand, actin post‐translational modifications (PTMs) recently emerged as key players of actin dynamics. But the role and consequences of PTMs at the molecular level often remain mysterious (Varland et al, 2019).
Actin oxidation is one of such important PTMs. The family of oxidoreductases MICAL, in the presence of the coenzyme NADPH and O2, has been identified to oxidize actin, its main target (Hung et al, 2010, 2011; Grintsevich et al, 2017; Frémont et al, 2017a, 2017b; Bai et al, 2020). Here, we focus on MICAL1, one of the three members of the MICAL family in humans. MICAL1 specifically targets F‐actin to oxidize actin Met44 and Met47 (Hung et al, 2011; Grintsevich et al, 2017; Frémont et al, 2017a; Bai et al, 2020). These two residues are part of the “D‐loop”, a region of the actin monomer involved in bridging interstrand and intrastrand actin subunits and thus important for actin filament stability (Dominguez & Holmes, 2011; Grintsevich et al, 2017; Chou & Pollard, 2019). As a consequence, MICAL1‐mediated oxidation strongly accelerates actin filament depolymerization in vitro (Grintsevich et al, 2017; Frémont et al, 2017a; Bai et al, 2020). Oxidation by MICAL1 is selectively reversed by a family of methionine sulfoxide reductases (MsrB; Hung et al, 2013; Lee et al, 2013; Bai et al, 2020). MsrB specifically targets oxidized monomeric actin (G‐actin) and reduces Met44 and Met47, allowing for the repolymerization of actin filaments (Hung et al, 2013; Lee et al, 2013; Bai et al, 2020).
Actin oxidation by MICAL1 plays important roles to regulate actin assembly in muscle organization, axon guidance, and drosophila bristle development (Frémont et al, 2017b; Alto & Terman, 2018). More recently, it has been implicated at the end of cell division, to clear actin filaments from the intercellular bridge, a crucial step for successful abscission (Frémont et al, 2017a). The activity of MICAL1 is balanced with that of MsrB2 which slows down the disassembly of actin structures during cytokinesis (Hung et al, 2013; Lee et al, 2013; Bai et al, 2020).
In vitro, oxidation by MICAL1 accelerates filament depolymerization, but, in contrast to initial reports (Hung et al, 2011), it does not induce filament fragmentation (Grintsevich et al, 2016; Frémont et al, 2017a). In a cellular context, MICAL1 is thus most likely cooperating with other molecular partners to efficiently disassemble actin filaments.
A central regulator of actin filament disassembly is the ubiquitous family of proteins ADF/cofilin (Elam et al, 2013; Hild et al, 2014; Kanellos & Frame, 2016). ADF/cofilin is commonly known to fragment actin filaments and can induce their depolymerization from both filament ends (Wioland et al, 2017, 2019a). Numerous studies described the action of ADF/cofilin at the molecular scale: It binds cooperatively to filaments, forming domains that locally modify actin filament conformation (McGough et al, 1997; Prochniewicz et al, 2005; Huehn et al, 2018, 2020; Wioland et al, 2019b), and induces severing at ADF/cofilin domain boundaries (Suarez et al, 2011; Gressin et al, 2015; Wioland et al, 2017). Nevertheless, on its own, ADF/cofilin cannot account for the very rapid turnover of filaments observed in cells. It can be assisted by various other ABPs, such as cyclase‐associated protein which accelerates the pointed end depolymerization of cofilin‐decorated filaments (Kotila et al, 2019; Shekhar et al, 2019).
Interestingly, Grintsevich et al (2016) recently identified a synergy between dMICAL from drosophila and the human isoform cofilin‐1: When oxidized by dMICAL, filaments are rapidly fragmented by cofilin‐1. The number of cofilin domain boundaries, where severing can occur, results from the balance between the formation of new domains and their growth rate, and it is unclear how these two rates are affected by filament oxidation. In addition, the impact of filament fragilization by oxidation (Grintsevich et al, 2016) on the severing rate per cofilin‐1 domain is unknown.
In vivo, cofilin‐1 is a very abundant protein, reaching tens of µM (Bekker‐Jensen et al, 2017). Its activity is regulated by various factors. A prevalent factor is cofilin‐1 Ser3 phosphorylation by LIM kinases (Arber et al, 1998; Kanellos & Frame, 2016). This phosphorylation strongly and persistently inhibits cofilin‐1 as it reduces its affinity for actin filaments and slows down subsequent severing (Elam et al, 2017). Additionally, cofilin‐1 activity can be restrained by tropomyosins (Tpm; Brayford et al, 2016; Christensen et al, 2017; Gateva et al, 2017; Jansen & Goode, 2019). Tpm is a long dimeric protein which binds and saturates most actin filaments in cells (Meiring et al, 2018). Tpm isoforms regulate specifically the recruitment of other ABPs, and most Tpm isoforms prevent cofilin‐1 binding (Gateva et al, 2017; Gunning & Hardeman, 2017; Jansen & Goode, 2019; Manstein et al, 2019). The interplay between Tpm decoration and post‐translational modifications of actin has never been tested.
Here, we used single filament microfluidics experiments to monitor and quantify the action of cofilin‐1 (Fig 1) and phosphomimetic S3D‐cofilin‐1 (Fig 2) on oxidized filaments compared to standard, non‐oxidized filaments. We next measured whether MICAL1 can oxidize Tpm1.8‐saturated filaments (Fig 3). Finally, we quantified the extent to which Tpm1.8 protects non‐oxidized and oxidized filaments from cofilin‐1‐induced severing (Fig 4). The results lead us to conclude that the oxidation of filaments by MICAL1 is a way to trigger their severing, in spite of their being decorated by tropomyosin, and without requiring the activation of cofilin‐1 (Fig 5).
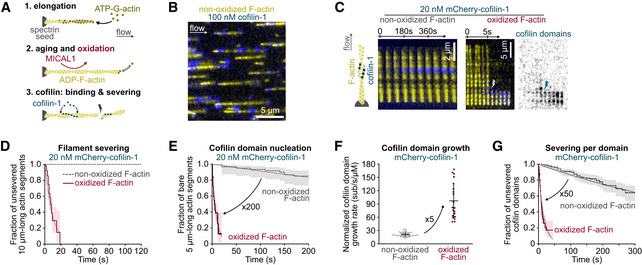
Figure 1. Cofilin at low concentration quickly binds and severs oxidized actin filament.
-
AThree steps of a typical experiment (see also Materials and Methods). Filaments are polymerized with 0.6–1 µM ATP‐G‐actin and aged for 15 min with ATP‐G‐actin at critical concentration (0.1 µM) to maintain the filament length. This solution is supplemented with MICAL1 and NADPH to oxidize filaments. Tpm can also be added at this step to fully decorate filaments (Figs 3 and 4).
-
BFraction (1/17th) of a typical field of view. In a microfluidic chamber, actin filaments (yellow) are anchored by their pointed ends and align with the flow.
-
CTime‐lapse images showing the assembly of cofilin‐1 domains (blue) and subsequent severing of actin filaments (yellow). The time‐lapse of an oxidized filament shows filament severing at 6 s; the first cofilin domain nucleation at 2 s; growth of four individual domains as their fluorescence intensity increases; and severing (lightning symbol) at the top domain, 2 s after its nucleation.
-
DGlobal measurement of the severing of filaments exposed to 20 nM mCherry‐cofilin‐1 from time t = 0 onwards. N = 20 (from one representative experiment) and 50 (1 experiment) for non‐oxidized and oxidized actin filaments, respectively. P‐value = 8 × 10−8 (log‐rank test).
-
ENucleation of the first cofilin domain onto 5 µm long actin segments. Filaments are exposed to 20 nM mCherry‐cofilin‐1 from time t = 0 onwards. N = 60 filaments, from one experiment for each condition. P‐value = 4 × 10−15 (log‐rank test).
-
FGrowth rate of single cofilin domains, normalized by the cofilin concentration. N = 50 (from three independent experiments) and 20 (1 experiment) domains on non‐oxidized and oxidized filaments, respectively. Measurements were obtained using 20 and 100 nM cofilin (non‐oxidized actin) and 10 nM cofilin (oxidized actin). Note that this normalized growth rate does not depend on the cofilin concentration (Appendix Fig S4A). Bars: mean and SD. P‐value = 7 × 10−8 (t‐test).
-
GFilament severing rate at single cofilin domains. Time t = 0 is defined for every domain as the frame on which they nucleate. N = 163 (from two independent experiments) and 203 (3 experiments) cofilin domains on non‐oxidized and oxidized actin filaments, respectively. Measurements were performed at 100 nM cofilin (non‐oxidized F‐actin) and 10, 20, or 30 nM cofilin (oxidized F‐actin). Since the severing rate does not depend on the cofilin concentration (Appendix Fig S4B and (Wioland et al, 2017, 2019a)), data for 10, 20, and 30 nM cofilin on oxidized filaments were pooled. Measurements on non‐oxidized filaments were done at 100 nM mCherry‐cofilin‐1. P‐value = 9 × 10−41 (log‐rank test).
Data information: (D, E, G) Thick solid and dashed lines are survival fractions calculated from the experimental data. Thin gray lines are single exponential fits. 95% confidence intervals are shown as shaded surfaces.
Source data are available online for this figure.
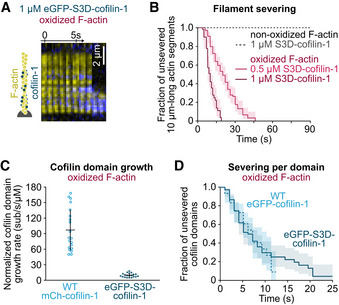
Figure 2. Phospho‐mimic (S3D) cofilin efficiently binds and severs oxidized F‐actin.
-
ATime‐lapse images of eGFP‐S3D‐cofilin‐1 (blue) binding and severing an oxidized actin filament (yellow).
-
BGlobal quantification of the severing events on oxidized and non‐oxidized filaments exposed to unlabeled S3D‐cofilin‐1 from time t = 0 onwards. From top to bottom, N = 50 (from 1 experiment), 50 (1 experiment), 100 (2 experiments) filaments.
-
CGrowth rate of single cofilin domains, normalized by the cofilin concentration. N = 20 domains for WT mCherry‐cofilin‐1 (1 experiment) and eGFP‐S3D‐cofilin‐1 (2 experiments). The growth rate was measured at 10 nM WT cofilin‐1, and 500 nM and 1 µM S3D‐cofilin‐1 (Appendix Fig S4C). Bars: mean and SD. P‐value = 8 × 10−9 (t‐test).
-
DFilament severing at single cofilin domains. Time t = 0 is defined for every domain as the frame on which it nucleates. N = 60 (1 experiment) and 75 (2 experiments) domains of WT and S3D‐cofilin‐1, respectively. Experiments were performed at 10 nM WT eGFP‐cofilin‐1 and 1 µM eGFP‐S3D‐cofilin‐1. P‐value = 0.98 (log‐rank test).
Data information: (B, D) All lines are survival fractions calculated from the experimental data. 95% confidence intervals are shown as shaded surfaces.
Source data are available online for this figure.
Figure 3. MICAL1 can oxidize Tpm‐saturated filaments and does not affect Tpm unbinding.

-
ATime‐lapse images of Tpm‐saturated actin filaments depolymerizing from their barbed end (BE), with or without MICAL1. Depolymerization is initiated at time t = 0 from a non‐oxidized actin filament. The increase in the depolymerization rate reflects the oxidation of actin with time. Tpm is labeled with neonGreen (nGr), and actin is unlabeled.
-
BBarbed end depolymerization rate as a function of the exposure time to either 100 nM nGr‐Tpm1.8 alone (“no MICAL1”) or supplemented with 100 nM MICAL1 and 12 µM NADPH. N = 31 (1 experiment) and 29 (1 experiment) filaments, without and with MICAL1, respectively. Lines: mean velocity. Shaded surfaces: S.D.
-
CTime‐lapse images showing the disassembly of Tpm domains. Filaments were saturated with 100 nM nGr‐Tpm1.8 before the experiment and Tpm was removed from solution at time t = 0 (the solution contains 0.6 µM unlabeled G‐actin to prevent filament depolymerization). PE and BE indicate F‐actin pointed and barbed end, respectively.
-
DDisassembly rate of single Tpm domains, at boundaries located toward the pointed end (PE) and toward the barbed end (BE) of actin filaments. N = 100 (non‐oxidized F‐actin, four experiments) and 50 (oxidized F‐actin, two experiments) domains per direction. Bars: mean and S.D. P‐value (t‐test, comparing oxidized and non‐oxidized filaments) = 0.27 (BE) and 0.12 (PE).
Source data are available online for this figure.
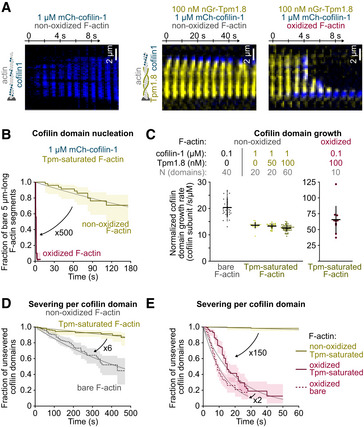
Figure 4. Tpm protects non‐oxidized but not oxidized F‐actin from cofilin‐induced severing.
-
ATime‐lapse images of filaments, oxidized or not, bare or Tpm‐saturated, exposed to 1 µM mCherry‐cofilin‐1, from time t = 0 onwards. Other examples are shown in Appendix Fig S6.
-
BNucleation of the first cofilin domain onto 5 µm long Tpm‐saturated actin filaments. Filaments are exposed to 1 µM mCherry‐cofilin from time t = 0 onwards. N = 50 filaments from one experiment for each condition. P‐value = 4× 10−15 (log‐rank test).
-
CGrowth rate of single cofilin domains, normalized by the cofilin concentration. Filaments saturated by Tpm during aging (Fig 1A) were then exposed to cofilin, along with 0–100 nM nGr‐Tpm1.8 to test the competition between the two soluble proteins. Note the scale difference for oxidized filaments. Bars: Mean and S.D. Number of experiments, first to last condition: 2, 1, 1, 3, and 1.
-
D, EFilament severing rate at single cofilin domains. Time t = 0 is defined for every domain as the frame on which they nucleate. N = 163 (2 experiments), 180 (4 experiments), 116 (3 experiments), 203 (3 experiments) domains for bare actin (100 nM mCherry‐cofilin‐1), non‐oxidized Tpm‐saturated (1 µM mCherry‐cofilin‐1), oxidized Tpm‐saturated filaments (100 nM mCherry‐cofilin‐1), and bare oxidized actin (10, 20, 30 nM mCherry‐cofilin‐1), respectively.
Data information: (B, D, E) Thick solid and dashed lines are survival fractions calculated from the experimental data. Thin gray lines are single exponential fits. 95% confidence intervals are shown as shaded surfaces.
Source data are available online for this figure.
Figure 5. Actin filament oxidation removes their protections from cofilin‐induced disassembly.
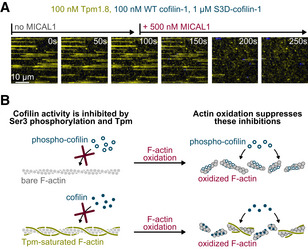
-
ATime‐lapse of actin filaments constantly exposed to nGr‐Tpm1.8 (yellow), WT mCh‐cofilin‐1 (blue) and S3D‐cofilin‐1, and to MICAL1 from time t = 100 s onwards. Here we mixed WT with an excess of S3D‐cofilin‐1 to mimic cellular conditions in which most of cofilin‐1 is inhibited by phosphorylation. While Tpm prevents cofilin from binding to non‐oxidized filaments, MICAL1 rapidly oxidizes actin, allowing cofilin to bind and sever filaments. See also Supp Movie.
-
BSummary of the results. Different mechanisms can simultaneously down regulate the activity of cofilin (here cofilin phosphorylation and protection by Tpm). Actin oxidation is a rapid means to cancel these protections without, for example, the need to activate a large concentration of cofilin.
Results
MICAL1‐induced actin oxidation enhances both cofilin‐1 binding and subsequent severing
In order to dissect the action of cofilin‐1 on oxidized and non‐oxidized filaments, we used a microfluidic setup previously developed in the laboratory (Jégou et al, 2011; Wioland et al, 2020). Briefly, filaments were polymerized from surface‐anchored Spectrin‐actin seeds (Fig 1A and B). Filaments were then aged for 15 min to become fully ADP‐actin filaments. During that time, the actin filaments were either exposed to MICAL1 or not. Using barbed end depolymerization as a readout of actin oxidation (Frémont et al, 2017a), we can estimate that filaments exposed to MICAL1 were fully oxidized during these 15 min (Appendix Fig S1A–D). We thus compared non‐oxidized filaments and fully oxidized filaments (except in Fig 5), as we subsequently exposed them to mCherry‐cofilin‐1. We also verified that the effects we observed on cofilin activity were not amplified further by a longer exposure to MICAL1 (Appendix Fig S1E and F).
All experiments were carried out at pH 7.4 and at room temperature, with rabbit alpha‐skeletal actin (except experiments of Appendix Fig S7, with bovine cytoplasmic beta actin), recombinant human cofilin‐1 and S3D‐cofilin‐1, recombinant human pseudo‐N‐acetylated Tpm1.8, and the recombinant active domain of human MICAL1 (see Materials and Methods for details). We confirmed that cofilin domain nucleation and filament severing occurred uniformly along the filaments and were thus not biased by remaining traces of ADP‐Pi or by tension (Appendix Fig S2; Wioland et al, 2019b).
We first assessed the impact of actin oxidation by MICAL1 on the activity of wild‐type (WT, i.e. non‐phosphorylated) cofilin‐1. At a low concentration of cofilin‐1 (20 nM), we observed no severing on non‐oxidized filaments after 120 s. In contrast, oxidized filaments quickly severed and none remained intact after 20 s (Fig 1C and D), in agreement with previous work (Grintsevich et al, 2016). This accelerated disassembly could be due to either altered cofilin‐1 binding or subsequent accelerated severing. We thus independently measured cofilin‐1 domain nucleation rate, domain growth rate, and filament severing rate per cofilin‐1 domain.
The nucleation rate was estimated by continuously exposing filaments to 20 nM mCherry‐cofilin‐1 and quantifying, over time, the fraction of 5 µm long actin segments with no visible cofilin‐1 domain. While cofilin‐1 domains appeared very slowly on non‐oxidized filaments (less than 20% had a cofilin‐1 domain after 200 s), nearly all oxidized segments presented one or more domains after 20 s. Overall, actin oxidation increases by 200‐fold the nucleation rate of cofilin‐1 domains (from 3 × 10−7/s/sub to 7 × 10−5, Fig 1E). We also confirmed this result by directly measuring the density of cofilin domains over time, which leads to the same 200‐fold increase in the nucleation rate (Appendix Fig S3).
Similarly, we compared the growth rate of single cofilin‐1 domains onto non‐oxidized and oxidized actin filaments. To do so, we used different concentrations of cofilin‐1, high enough for domains to steadily appear and grow, and low enough to limit nucleation and be able to track single domains. While mCherry‐cofilin‐1 domains grow by ~20 sub/s/µM on non‐oxidized filaments, this rate increased 5‐fold, to ~100 sub/s/µM, on oxidized filaments (Fig 1F, Appendix Fig S4). Both nucleation and growth quantifications show that actin oxidation by MICAL1 largely increases the affinity of cofilin‐1 for the filament. As a result, numerous domains are generated, in seconds (Fig 1C).
The global cofilin‐induced filament severing dynamic is the product of the number of cofilin‐1 domains (at the boundary of which severing occurs, Fig 1C) and the severing rate per domain. To quantify the latter, we measured the fraction of unsevered cofilin‐1 domains, from their nucleation to severing or loss (due for example to the severing at another domain). Fits of the survival fraction of domains with a single exponential showed that severing per domain was 50‐fold faster on oxidized filaments (from 2 × 10−3/s to 7 × 10−2/s, Fig 1G).
Actin oxidation by MICAL1 thus accelerates the disassembly of filaments by accelerating both cofilin‐1 recruitment and subsequent severing. In other words, global severing is boosted by the increased severing rate per domain and by the formation of numerous cofilin‐1 domain boundaries where severing occurs.
MICAL1‐oxidized actin filaments are efficiently disassembled by S3D‐cofilin‐1
In vivo, cofilin‐1 is inhibited upon Ser3 phosphorylation by LIM kinases. To study the impact of this inhibition in vitro, we used the common phosphomimetic S3D‐cofilin‐1 (Blanchoin et al, 2000; Elam et al, 2017; Huehn et al, 2020). We first tested the mutant by exposing non‐oxidized filaments to 1 µM unlabeled S3D‐cofilin‐1 (Fig 2B). After 90 s, we did not observe any filament severing event. We also tried to observe the nucleation and growth of cofilin‐1 domains by exposing filaments to 1 µM eGFP‐S3D‐cofilin‐1, but could not observe any binding. As a consequence, we could not measure the severing rate at the boundary of S3D‐cofilin‐1 domains. These observations are consistent with previous work that showed a reduced affinity of phosphomimetic cofilin‐1 to non‐oxidized actin filaments (Elam et al, 2017).
In a cellular context, it is expected that cofilin‐1 is activated by a phosphatase, e.g. slingshot or chronophin (Kanellos & Frame, 2016). We hypothesized that actin oxidation by MICAL1 might be enough to trigger filament disassembly by S3D‐cofilin‐1. To test this idea, we exposed 10 µm long oxidized actin filament segments to 1 µM unlabeled S3D‐cofilin‐1 and found that all filaments had severed at least once in less than 20 s (Fig 2A and B).
While S3D‐cofilin‐1 efficiently disassembles oxidized actin filaments, it still requires a much higher concentration than WT cofilin‐1 on oxidized filaments (Fig 1C): The global filament severing rate at 1 µM S3D‐cofilin‐1 is comparable to that of 20 nM WT cofilin‐1. In order to clarify this difference, we independently measured the growth rate of phosphomimetic cofilin domains and the severing rate per domain. We found that single eGFP‐S3D‐cofilin‐1 domains grew 10 times slower than WT cofilin‐1 domains onto oxidized filaments, at ~10 sub/s/µM (Fig 2C). Surprisingly, domains of WT or S3D‐cofilin‐1 severed oxidized filaments equally fast (at 0.1/s, Fig 2D).
Overall, actin oxidation bypasses cofilin‐1 activation by allowing S3D‐cofilin‐1 to bind actin filaments and by making filaments vulnerable to cofilin‐induced severing.
MICAL1 can oxidize Tpm1.8‐decorated filaments and does not change Tpm1.8 affinity
In mammals, more than 40 Tpm isoforms are expressed and decorate the majority of actin filaments (Meiring et al, 2018). Each isoform regulates the recruitment and activity of specific ABPs (Gateva et al, 2017; Gunning & Hardeman, 2017; Manstein et al, 2019). Since the activity of MICAL1 has never been tested in vitro on Tpm‐saturated filaments, we first wondered whether Tpm could prevent actin oxidation.
To study this question, we selected a cytosolic, low molecular weight tropomyosin isoform, Tpm1.8. Brayford et al showed that Tpm1.8 regulates actin filaments turnover in vivo and promotes the persistence of the lamellipodia (Brayford et al, 2016; Gateva et al, 2017; Jansen & Goode, 2019). We recently characterized the dynamics of Tpm1.8 domains. We found that Tpm1.8 saturates the two strands of the actin filaments independently and that the domain assembly and disassembly is asymmetric: faster at the domain border closer to the filament barbed end (Bareja et al, 2020).
To detect the oxidation of filaments in our microfluidic setup, we pre‐saturated actin filaments with neonGreen‐Tpm1.8 and measured the barbed end depolymerization rate in the absence of G‐actin in solution, in the presence or absence of MICAL1. Of note, since Tpm hardly binds to fluorescently labeled actin filaments (Gateva et al, 2017; Gunning & Hardeman, 2017; Manstein et al, 2019), we used unlabeled actin in all experiments with Tpm1.8. We confirmed previous results on bare actin filaments, showing that oxidation accelerates barbed end depolymerization about 10‐fold, from ~8 sub/s to ~80 sub/s (Appendix Fig S1A; Grintsevich et al, 2017; Frémont et al, 2017a).
We first measured the depolymerization rate of Tpm1.8‐saturated ADP‐actin filaments in the absence of MICAL1. We found Tpm‐decorated filaments to depolymerize steadily at a slower rate than bare actin filaments (1.8 sub/s vs. 8 sub/s, Fig 3A and B). Tpm1.8 thus appears to have a stabilizing effect, in the absence of other ABPs.
We then continuously exposed the Tpm‐decorated filaments to MICAL1, from time t = 0 onwards. We found that the depolymerization rate increased over time to reach a plateau around 30 sub/s (Fig 3A and B), which is still slower than the barbed end depolymerization of bare oxidized filaments (Grintsevich et al, 2017; Frémont et al, 2017a). We thus concluded that MICAL1 can indeed oxidize Tpm‐decorated filaments and that the oxidation accelerates the barbed end depolymerization rate 15‐fold.
Once filaments have been oxidized, we wondered whether Tpm1.8 domains would be destabilized. We thus measured the disassembly rates of neonGreen‐Tpm1.8 domains in the presence of G‐actin only (to prevent filament depolymerization). As was reported previously, Tpm1.8 disassembly is asymmetric, faster at the domain boundary located toward the filament barbed end (Bareja et al, 2020). We found that Tpm domains disassembled equally fast from non‐oxidized and oxidized filaments (Fig 3C and D). We also measured that Tpm domain growth and Tpm turnover within domains were not affected by oxidation (Appendix Fig S5). Thus, oxidation by MICAL1 has apparently no impact on Tpm affinity.
Tpm1.8 protects non‐oxidized filaments from cofilin‐1 binding and filament severing
Many Tpm isoforms have been shown to inhibit cofilin‐1 binding (Gateva et al, 2017; Jansen & Goode, 2019). In lamellipodia, Tpm1.8 has been shown to regulate filament stability along with cofilin‐1 (Brayford et al, 2016) but their cooperation or competition has never been tested in vitro. We first measured the impact of Tpm1.8 on the activity of cofilin‐1 in the absence of MICAL1.
We first observed that some cofilin‐1 domains could still assemble on Tpm‐saturated filaments, and we confirmed that the mCherry‐cofilin‐1 and neonGreen‐Tpm1.8 fluorescence signals never overlap, as previously observed (Christensen et al, 2017; Gateva et al, 2017; Jansen & Goode, 2019): Cofilin‐1 binding induces the unbinding of Tpm. However, the majority of cofilin‐1 domains nucleate from the free barbed end (Fig 4A and Appendix Fig S6B). A single Tpm1.8 dimer binds along the length of filaments to six actin subunits. The barbed end most likely contains a few bare actin subunits whence cofilin‐1 domains nucleate. We also observed a dramatic decrease in the nucleation rate of cofilin domains within the Tpm‐decorated filament: While bare filaments get fully saturated by cofilin‐1 in about 10 s (at 1 µM mCherry‐cofilin‐1, Fig 4A and Appendix Fig S6A), more than 60% of 5 µm long Tpm‐decorated actin segments are still void of any cofilin‐1 domain after 180 s (Fig 4B).
We then measured the growth rate of single cofilin‐1 domains on bare vs. Tpm‐decorated filaments. Surprisingly, we found the growth rate of cofilin domains to be reduced by only 30% on Tpm1.8‐saturated filaments, compared to bare filaments (~20 sub/s/µM on bare vs. ~13 sub/s/µM on Tpm‐decorated filaments, Fig 4C left). We also measured that the growth rate was barely reduced by the addition of 50 to 100 nM Tpm1.8 in solution (Fig 4C), suggesting that the reduction in cofilin domain growth was predominantly caused by pre‐existing Tpm1.8 domains. Cofilin‐1 domains could grow and thus replace Tpm molecules on the filament, faster than the Tpm off‐rate we measured in the absence of cofilin (Fig 3D). This observation indicates that cofilin does not simply replace departing Tpm molecules: Cofilin accelerates the detachment of Tpm at domain boundaries. Overall, Tpm domains prevent the nucleation of cofilin‐1 domains but they moderately slow down the growth of already established cofilin‐1 domains.
Cofilin‐1‐induced severing occurs at the boundary of cofilin domains, we wondered if the presence of a contiguous Tpm domain might affect the severing rate. We discovered that the severing rate per cofilin‐1 domain was reduced by 6‐fold on Tpm‐saturated filaments (from 2 × 10−3 /s to 3 × 10−4 /s, Fig 4D). Our results show that Tpm1.8 has an impact on every step of cofilin‐1‐induced disassembly and thereby very efficiently maintains filaments.
Oxidation allows the rapid decoration and severing by cofilin‐1 of Tpm1.8‐saturated filaments
Since MICAL1 can oxidize Tpm1.8‐saturated filaments (Fig 3), we tested whether actin oxidation could alter the competition between Tpm and cofilin‐1. We thus polymerized actin filaments and aged them in the presence of both MICAL1 and Tpm. These oxidized, Tpm‐saturated filaments were then exposed to 0.1 to 1 µM mCherry‐cofilin‐1.
We first observed that cofilin‐1 domains nucleated very rapidly over the whole filament: Virtually, all 5 µm long oxidized actin segments had at least one visible cofilin‐1 domain in less than 5 s (1 µM mCherry‐cofilin‐1, Fig 4A and Appendix Fig S6C). Oxidation thus increases the nucleation rate on Tpm‐saturated filaments by ~500‐fold (from 1 × 10−6/s/sub to 6 × 10−4/s/sub, Fig 4B and Appendix Fig S3).
We then measured the normalized growth rate of cofilin‐1 domains and similarly found an increase by 5‐fold (from ~13 sub/s/µM to ~65 sub/s/µM) after oxidation of Tpm‐saturated filaments. We noted that the fluorescence signal of neonGreen‐Tpm1.8 and mCherry‐cofilin‐1 did not overlap on oxidized filament either, indicating that cofilin‐1 binding leads to the very quick unbinding of Tpm. Overall, Tpm1.8 decoration does not prevent the rapid binding of cofilin‐1 to oxidized filaments.
Finally, we tested whether Tpm could inhibit the severing of oxidized filaments by cofilin‐1. We found that single cofilin‐1 domain severed Tpm‐decorated oxidized filaments very rapidly, with a half‐life of about 15 s (Fig 4E). Therefore, oxidation by MICAL1 increases the severing rate per cofilin‐1 domain by 150‐fold, compared with non‐oxidized Tpm‐decorated filaments (from 3 × 10−4/s to 5 × 10−2/s). Compared to bare filaments, Tpm moderately slows down the cofilin‐induced severing rate of MICAL1‐oxidized filaments, by less than 2‐fold (from 7 × 10−2/s to 5 × 10−2/s, Fig 4E).
Overall, our results show that MICAL1 makes tropomyosin‐decorated actin filaments extremely vulnerable to cofilin. Oxidation by MICAL1 thus appears as a powerful way to sever filaments, by upending conditions where non‐oxidized filaments would be protected, without specifically removing tropomyosins and without activating large amounts of cofilin. To illustrate this idea, we sought to monitor the effect of MICAL1 activation in what would be a likely physiological situation, where filaments are decorated by tropomyosin and most of the available cofilin is phosphorylated. We thus polymerized unlabeled non‐oxidized actin filaments and first exposed them to 100 nM neonGreen‐Tpm1.8, and a mixture of 100 nM WT mCherry‐cofilin‐1 and 1 µM unlabeled S3D‐cofilin‐1 (Fig 5A). We observed that Tpm rapidly decorated the actin filaments, preventing cofilin‐1 binding and filament severing. After 100 s, the filaments were intact. We then mimicked the activation of a significant amount of MICAL1, that would trigger the rapid oxidation of the filaments, by adding 500 nM MICAL1 to the solution. Over the next 100 s, MICAL1 oxidized the Tpm‐saturated filaments, allowing cofilin‐1 to assemble into domains that rapidly fragmented all the filaments.
Discussion
We found that oxidation by MICAL1 makes filaments vulnerable to cofilin‐1‐induced disassembly in conditions that would otherwise be of little consequence (Fig 5B). Trace amounts of active cofilin‐1, or larger amounts of phosphomimetic S3D‐cofilin‐1, which are harmless to actin filaments, readily disassemble them as soon as they are oxidized by MICAL1 (Figs 1, 2 and 5). Filament decoration by Tropomyosin Tpm1.8, which offers an efficient protection against cofilin‐1‐induced disassembly, becomes inefficient as soon as the filaments are oxidized (Figs 3, 4, 5).
Our experiments were performed using alpha‐skeletal actin, but we have confirmed the effect of MICAL1‐induced oxidation on cofilin activity for filaments made mostly of cytoplasmic beta actin (Appendix Fig S7). Our conclusions may thus apply to most cell types. We have also verified that the effects we observed on filaments following their exposure to MICAL1 were not due to potential traces of MICAL1 remaining bound to the filaments (Appendix Fig S8).
Filament oxidation by MICAL1 can thus be viewed as a way to abolish different protections from cofilin‐induced disassembly. Interestingly, the temporary protection offered by actin’s ADP‐Pi content, which prevents cofilin from binding to freshly assembled filament regions (Suarez et al, 2011), is also suppressed by MICAL1‐induced oxidation (Grintsevich et al, 2017). MICAL1‐induced oxidation thus appears to favor the action of cofilin in various situations.
Remarkably, these multiple effects, which upend the regulation of actin disassembly, are due to the modification of only two residues in the D‐loop of actin, Met44 and Met47 (Hung et al, 2011). As shown previously, these modifications weaken intersubunit bonds and, as a consequence, filaments depolymerize faster (Grintsevich et al, 2017; Frémont et al, 2017a) and are easier to fragment by mechanical shearing (Grintsevich et al, 2016). Here, our experiments apply negligible mechanical stress to the filaments, and we further confirm that oxidized filaments barely sever without cofilin. As with non‐oxidized filaments, severing occurs at the boundaries of cofilin domains and preferably at the pointed end boundary of cofilin domains. These observations suggest that filament oxidation does not alter the nature of the severing mechanism and that the weakening of intersubunit bonds may suffice to explain the spectacular 50‐fold increase in the severing rate at cofilin domain boundaries (Fig 1G).
In addition, the oxidation of the D‐loop of actin favors the binding of cofilin and especially the binding of the first cofilin molecules (Fig 1E), leading to the formation of many cofilin domains and domain boundaries, thereby further increasing the occurrence of severing events. Structural data show that, when cofilin binds to an actin filament, the N‐terminal region of cofilin contacts the vicinity of actin’s D‐loop (Blanchoin et al, 2000; Galkin et al, 2011; Elam et al, 2017; Huehn et al, 2020). Local changes induced by oxidation in the D‐loop (Grintsevich et al, 2017) may thus explain how cofilin binding is favored. Further, this local interaction is known to be impaired by modifications on Ser3 of cofilin (Huehn et al, 2020) and our results suggest that it may be restored by the oxidation of the D‐loop of actin (Fig 2). The oxidation of actin’s D‐loop may also induce global modifications of the filament conformation and stiffness, which have yet to be measured and which could contribute to favor cofilin binding.
It appears that most Tpm isoforms studied so far inhibit cofilin‐induced disassembly of non‐oxidized filaments, to different extents (Gateva et al, 2017; Jansen & Goode, 2019). Here, we show that Tpm1.8 can efficiently prevent the nucleation of new cofilin domains and that, in addition, it strongly reduces severing at cofilin‐Tpm domain boundaries (Fig 4). Tpm binding and MICAL1‐induced oxidation appear unaffected by each other (Fig 3), consistent with the observation that Tpm binds actin filaments along their positively charged groove, away from the D‐loop (von der Ecken et al, 2015). Nonetheless, Tpm1.8 prevents the formation of cofilin domains on non‐oxidized actin filaments and is no longer able to do so when the D‐loop is oxidized (Fig 4). It is thus likely that more global effects, such as perhaps changes in filament stiffness induced by Tpm binding (Greenberg et al, 2008), also play a role and need to be explored further.
Our results illustrate how factors of different natures can come together to regulate actin assembly (Jégou & Romet‐Lemonne, 2016). The interplay between ABPs and mechanical factors, in particular, is currently under intense scrutiny (Schramm et al, 2017; Harris et al, 2018; Zimmermann & Kovar, 2019; Wioland et al, 2019b; Jegou & Romet‐Lemonne, 2020; Suzuki et al, 2020). Today, PTMs of actin are emerging as a key regulatory factor of actin assembly (Varland et al, 2019) and how they modify interactions with ABPs is mostly unknown. Here, we have quantified the role of the direct PTM of actin by oxidation as a critical regulatory mechanism that controls actin disassembly, in synergy with well‐established ABPs. In the future, we expect that more studies will go beyond the simple combination of ABPs, to decipher the multifactorial regulation of the cytoskeleton.
We propose that, in cells, the local activation of MICAL1 is a powerful way to trigger the severing of actin filaments, by turning innocuous concentrations of active and inactive cofilins into a lethal mix for filaments and by making tropomyosins powerless to protect the filaments (Fig 5). Several ABPs are known to assist cofilin to rapidly disassemble filaments, and they may also be sensitive to the change in the redox state of actin filaments.
Materials and Methods
Proteins purification and labeling
Skeletal muscle actin was purified from rabbit muscle acetone powder following the protocol described in (Wioland et al, 2017), adapted from the original protocol (Spudich & Watt, 1971). Actin was labeled on accessible surface lysines of F‐actin, with Alexa‐488 or Alexa‐568 succinimidyl ester (Life Technologies). To limit artifacts from the fluorophore, we used a labeling fraction of 12 %, except in experiments with Tpm where filaments were unlabeled.
Cytoplasmic beta actin from bovine spleen was a gift from the laboratory of Pekka Lappalainen (University of Helsinki). It was purified as described in (Segura & Lindberg, 1984).
Spectrin‐actin seeds were purified from human erythrocytes as described in Wioland et al (2017), based on the original protocol by Casella et al (1986).
Mouse cofilin‐1 (Uniprot: P18760) and S3D‐cofilin‐1, unlabeled or fluorescently tagged with eGFP and mCherry at their N‐terminus, were purified as described previously in (Kremneva et al, 2014).
neonGreen‐Tpm1.8 was constructed as a protein with N‐terminal His6‐tag followed by mNeonGreen and a peptide linker (GGGSGGGSGTAS) fused to the N‐terminus of Tpm1.8, as described in Bareja et al (2020). The alanine–serine fused directly at the N‐terminus of Tpm1.8 mimics its acetylation.
Since the full‐length MICAL1 is auto‐inhibited, we only used the catalytic domain of human MICAL1, purified as described in Frémont et al (2017a). It was always supplemented with its cofactor nicotinamide adenine dinucleotide phosphate (NADPH, 12 µM).
Buffer
We performed all experiments in F‐buffer (5 mM Tris–HCl pH 7.4, 50 mM KCl, 1 mM MgCl2, 0.2 mM EGTA, 0.2 mM ATP, 10 mM DTT, and 1 mM DABCO).
Microfluidics chamber
Experiments were performed in poly dimethyl siloxane (PDMS, Sylgard) microfluidics chambers, 20 µm in height, 800 µm in width, and about 1 cm long. Chambers had a cross‐shape with three inlets, merging into a central channel, leading to the outlet (Jégou et al, 2011).
Coverslips were washed and sonicated with Hellmanex (30‐min sonication), 1 M KOH (30 min), and pure ethanol (30 min) and thoroughly rinsed with dH20 between each step. Coverslips were finally dried with pressurized air and exposed to UV along with PDMS chambers to allow for a tight binding.
Chamber functionalization and passivation
Twenty µL of the following solutions was directly injected into the chamber with a pipette. First, 18 pM spectrin‐actin seeds left for 30 to 60 s for seeds to adhere non‐specifically to the glass surface, followed by PLL‐PEG (1 mg/ml, 1 h), casein (8 mg/ml, 15 min), and BSA (50 mg/ml, 15 min) to passivate the surface.
Filament elongation and experiment
Experiments were performed in three steps (Fig 1A):
Filament elongation: polymerized from seeds with 0.6 to 1 µM ATP‐G‐actin for about 10 min. Filaments are anchored to the surface by their pointed end only.
Filament aging, oxidation, and Tpm saturation: Filaments are then aged for 15 min with a solution of ATP‐G‐actin at critical concentration, ~0.1 µM. This results in more than 99.9% of the subunits in an ADP form (Jégou et al, 2011). Actin subunits can also be simultaneously oxidized during this step by supplementing the solution with typically 50 nM MICAL1 and 12 µM NADPH, resulting in nearly 100% oxidized actin subunits (Frémont et al, 2017a). Simultaneously, filaments can be saturated with 100 nM Tpm1.8.
Filament or Tpm domain disassembly: Filaments are finally exposed to buffer, in the presence or absence of WT or S3D‐cofilin‐1.
Acquisition
The microfluidic setup was placed on a Nikon TiE or TE2000 inverted microscope, equipped with a 60× oil immersion objective and an optional additional 1.5× magnification. We either used TIRF, HiLo or epifluorescence depending on the background fluorophore concentration in solution. The TiE microscope was controlled by Metamorph, illuminated in TIRF or HiLo by 100 mW tunable lasers (ILAS2, Gataca Systems), and images were acquired by an Evolve EMCCD camera (Photometrics). The TE2000 microscope was controlled by micromanager (Edelstein et al, 2014), illuminated with a 120W Xcite lamp (Lumen dynamics), and images were acquired by an sCMOS Orca‐Flash4.0 camera (Hamamatsu).
Analysis
“Filament severing”: A set of randomly picked 10 µm long actin segments was monitored from time t = 0 when cofilin (or buffer) was first injected. We measured the time at which each segment either severs or is lost, due for example to severing somewhere else on the filament (e.g., at the anchor). We calculated the survival fraction from this set of severing or loss times, using a standard Kaplan–Meier method. Survival fraction, confidence intervals, and P‐values (log‐rank test) were calculated using the Python package Lifelines. Survival fractions were fitted with a single exponential on Excel (Microsoft Office) to obtain reaction rates (shown as thin continuous gray lines on plots).
“Severing per cofilin domain”: We tracked single cofilin domains, from the frame on which they appear (nucleation) which defines t = 0. We then followed the same procedure as for “filament severing”.
-
“Cofilin domain nucleation”: 5 µm long actin segments were tracked from t = 0 when cofilin was injected. We then measured the frame on which the first cofilin domain nucleates or the segment is lost. The survival fraction was calculated with the same Kaplan–Meier method described above for “filament severing”.
Alternatively, we calculated the density D of cofilin domains over time. At each frame t, D was calculated as D(t) = D(t−1) + Ndom(t)/(Nfil(t)*L) where Ndom is the number of domains that nucleated on frame t, Nfil the number of intact (unsevered) filaments at frame t, and L the length of those filaments (here L = 5 µm).
Fitting either the survival fraction (single exponential, on Excel) or the density D (linear function, on Excel) yielded comparable nucleation rates.
“Cofilin domain growth”: The growth rate was measured by two different methods. When large domains can assemble (i.e. when nucleation is limited), the growth rate is simply the sum of the rates toward both ends, measured manually on kymographs made with ImageJ. When many domains nucleate simultaneously and rapidly merge, we measured the fluorescence intensity of a single domain spreading over 3–5 pixels. The intensity was normalized by that on a filament fully saturated with cofilin. We previously showed that the two methods yield comparable results (Wioland et al, 2017).
“Tpm disassembly”: We measured the disassembly rate of Tpm domains toward the two filament ends, directly on kymographs. As the two filament strands can be decorated by Tpm independently (Bareja et al, 2020), we only analyzed single domains (i.e. with one strand decorated only) which had a better fluorescence signal to noise ratio.
“Tpm chase experiment”: To follow the replacement of neonGreen‐Tpm1.8 by mCherry‐Tpm1.8, we measured the total neonGreen fluorescence along actin filaments, from which background fluorescence was subtracted. This net fluorescence was normalized for each filament to 1 at time t = 0 when mCherry‐Tpm1.8 was injected. The decrease in neonGreen fluorescence was not due to photobleaching, since it was not homogeneous along the filaments but rather occurred in gaps, reflecting the dissociation of Tpm domains.
“Barbed end depolymerization”: Filament barbed end position was measured on every frame with a custom build algorithm, coded in Python. The mean depolymerization rate at a time t was obtained by pooling the barbed end position of all filaments over contiguous seven frames (centered around time t). The position over time was fitted with a linear function whose slope yielded the depolymerization rate.
“Oxidation rate”: The barbed depolymerization rate was fitted over time to estimate the oxidation rate. We used a minimal model with two hypotheses: MICAL1 oxidizes actin subunits with a single constant rate, and the depolymerization rate only depends on the oxidation status of the terminal actin subunit. As a consequence , where is the depolymerization rate after t seconds of oxidation, and the depolymerization rate of a non‐oxidized and fully oxidized filament, respectively, and the oxidation rate for a given MICAL1 concentration.
“Cofilin domain distribution”: To test whether domains were homogeneously distributed along the filament length, we measured the distance di between newly nucleated cofilin domains i and the filament barbed end (up to the six first domains per filament, two in average). We then computed the cumulative distribution function as , where w(i) is the weight assigned to the event i. The weight depends on the distance di and filament length L distribution: w(i) = 1/Nfil(di), where Nfil(di) is the number of analyzed filaments of length L > di (Wioland et al, 2019b). An homogenous distribution of cofilin domains then results in a linear cumulative distribution function F.
“Filament severing event distribution”: The spatial distribution of the severing events was assessed by calculating a similar cumulative distribution function, except that the weight was defined as w(i) = Li / Nfil(di), where Li is the length of the filament that severed (Wioland et al, 2019b). We only recorded the first severing event for each filament.
Statistics
Individual filaments were selected for analysis with no prior knowledge of their behavior. We only excluded filaments sticking to the surface and, during analysis, photo‐induced pauses occurring during depolymerization (Niedermayer et al, 2012).
All experiments were performed at least twice, and at least one representative movie was analyzed.
P‐values for cofilin and Tpm domain growth and F‐actin oxidation rates were calculated with a Welch t‐test. We used Python function “scipy.stats.ttest_ind” with unequal variance.
P‐values for survival fractions were calculated with a log‐rank test using the Python package Lifelines.
Author contributions
HW, SF, AE, AJ, and GRL conceived the project. SF and BG produced and purified proteins. HW carried out the experiments, analyzed the data, and generated the figures. AE, AJ, and GRL supervised the work and secured funding. HW and GRL wrote the manuscript with input from all authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Review Process File
Source Data for Figure 1
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Acknowledgements
This work was supported by the CNRS, Institut Pasteur, the ANR (grant RedoxActin to AE and GRL) and the ERC (grant StG‐679116 to AJ). The authors thank Till Boecking for the plasmid of neonGreen‐Tpm1.8, as well as Tommi Kotila and Pekka Lappalainen for cytoplasmic actin.
EMBO reports (2021) 22: e50965.
Contributor Information
Antoine Jégou, Email: antoine.jegou@ijm.fr.
Guillaume Romet‐Lemonne, Email: romet@ijm.fr.
Data availability
This study includes no primary dataset deposited in external repositories.
References
- Alto LT, Terman JR (2018) MICALs. Curr Biol 28: R538–R541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P (1998) Regulation of actin dynamics through phosphorylation of cofilin by LIM‐kinase. Nature 393: 805–809 [DOI] [PubMed] [Google Scholar]
- Bai J, Wioland H, Advedissian T, Cuvelier F, Romet‐Lemonne G, Echard A (2020) Actin reduction by MsrB2 is a key component of the cytokinetic abscission checkpoint and prevents tetraploidy. Proc Natl Acad Sci USA 117: 4169–4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareja I, Wioland H, Janco M, Nicovich PR, Jégou A, Romet‐Lemonne G, Walsh J, Böcking T (2020) Dynamics of Tpm1.8 domains on actin filaments with single molecule resolution. Mol Biol Cell 31: 2452–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekker‐Jensen DB, Kelstrup CD, Batth TS, Larsen SC, Haldrup C, Bramsen JB, Sørensen KD, Høyer S, Ørntoft TF, Andersen CL et al (2017) An optimized shotgun strategy for the rapid generation of comprehensive human proteomes. Cell Syst 4: 587–599.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchoin L, Robinson RC, Choe S, Pollard TD (2000) Phosphorylation of Acanthamoeba actophorin (ADF/cofilin) blocks interaction with actin without a change in atomic structure. J Mol Biol 295: 203–211 [DOI] [PubMed] [Google Scholar]
- Brayford S, Bryce NS, Schevzov G, Haynes EM, Bear JE, Hardeman EC, Gunning PW (2016) Tropomyosin promotes lamellipodial persistence by collaborating with Arp2/3 at the leading edge. Curr Biol 26: 1312–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella JF, Maack DJ, Lin S (1986) Purification and initial characterization of a protein from skeletal muscle that caps the barbed ends of actin filaments. J Biol Chem 261: 10915–10921 [PubMed] [Google Scholar]
- Chou SZ, Pollard TD (2019) Mechanism of actin polymerization revealed by cryo‐EM structures of actin filaments with three different bound nucleotides. Proc Natl Acad Sci USA 116: 4265–4274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JR, Hocky GM, Homa KE, Morganthaler AN, Hitchcock‐DeGregori SE, Voth GA, Kovar DR (2017) Competition between Tropomyosin, Fimbrin, and ADF/Cofilin drives their sorting to distinct actin filament networks. eLife 6: e23152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez R, Holmes KC (2011) Actin structure and function. Annu Rev Biophys 40: 169–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Ecken J, Müller M, Lehman W, Manstein DJ, Penczek PA, Raunser S (2015) Structure of the F‐actin‐tropomyosin complex. Nature 519: 114–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein AD, Tsuchida MA, Amodaj N, Pinkard H, Vale RD, Stuurman N (2014) Advanced methods of microscope control using μManager software. J Biol Methods 1: e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elam WA, Kang H, De la Cruz EM (2013) Biophysics of actin filament severing by cofilin. FEBS Lett 587: 1215–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elam WA, Cao W, Kang H, Huehn A, Hocky GM, Prochniewicz E, Schramm AC, Negrón K, Garcia J, Bonello TT et al (2017) Phosphomimetic S3D‐Cofilin binds but only weakly severs actin filaments. J Biol Chem 292: 19565–19579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frémont S, Hammich H, Bai J, Wioland H, Klinkert K, Rocancourt M, Kikuti C, Stroebel D, Romet‐Lemonne G, Pylypenko O et al (2017a) Oxidation of F‐actin controls the terminal steps of cytokinesis. Nat Commun 8: 14528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frémont S, Romet‐Lemonne G, Houdusse A, Echard A (2017b) Emerging roles of MICAL family proteins–from actin oxidation to membrane trafficking during cytokinesis. J Cell Sci 130: 1509–1517 [DOI] [PubMed] [Google Scholar]
- Galkin VE, Orlova A, Kudryashov DS, Solodukhin A, Reisler E, Schröder GF, Egelman EH (2011) Remodeling of actin filaments by ADF/cofilin proteins. Proc Natl Acad Sci USA 108: 20568–20572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gateva G, Kremneva E, Reindl T, Kotila T, Kogan K, Gressin L, Gunning PW, Manstein DJ, Michelot A, Lappalainen P (2017) Tropomyosin Isoforms specify functionally distinct actin filament populations in vitro. Curr Biol 27: 705–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg MJ, Wang C‐LA, Lehman W, Moore JR (2008) Modulation of actin mechanics by caldesmon and tropomyosin. Cell Motil Cytoskeleton 65: 156–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressin L, Guillotin A, Guérin C, Blanchoin L, Michelot A (2015) Architecture dependence of actin filament network disassembly. Curr Biol 25: 1437–1447 [DOI] [PubMed] [Google Scholar]
- Grintsevich EE, Yesilyurt HG, Rich SK, Hung R‐J, Terman JR, Reisler E (2016) F‐actin dismantling through a redox‐driven synergy between Mical and cofilin. Nat Cell Biol 18: 876–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grintsevich EE, Ge P, Sawaya MR, Yesilyurt HG, Terman JR, Zhou ZH, Reisler E (2017) Catastrophic disassembly of actin filaments via Mical‐mediated oxidation. Nat Commun 8: 2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning PW, Hardeman EC (2017) Tropomyosins. Curr Biol 27: R8–R13 [DOI] [PubMed] [Google Scholar]
- Harris AR, Jreij P, Fletcher DA (2018) Mechanotransduction by the actin cytoskeleton: converting mechanical stimuli into biochemical signals. Annu Rev Biophys 47: 617–631 [Google Scholar]
- Hild G, Kalmár L, Kardos R, Nyitrai M, Bugyi B (2014) The other side of the coin: functional and structural versatility of ADF/cofilins. Eur J Cell Biol 93: 238–251 [DOI] [PubMed] [Google Scholar]
- Huehn A, Cao W, Elam WA, Liu X, De La Cruz EM, Sindelar CV (2018) The actin filament twist changes abruptly at boundaries between bare and cofilin‐decorated segments. J Biol Chem 293: 5377–5383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huehn AR, Bibeau JP, Schramm AC, Cao W, De La Cruz EM, Sindelar CV (2020) Structures of cofilin‐induced structural changes reveal local and asymmetric perturbations of actin filaments. Proc Natl Acad Sci USA 117: 1478–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung R‐J, Yazdani U, Yoon J, Wu H, Yang T, Gupta N, Huang Z, van Berkel WJH, Terman JR (2010) Mical links semaphorins to F‐actin disassembly. Nature 463: 823–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung R‐J, Pak CW, Terman JR (2011) Direct redox regulation of F‐actin assembly and disassembly by Mical. Science 334: 1710–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung R‐J, Spaeth CS, Yesilyurt HG, Terman JR (2013) SelR reverses Mical‐mediated oxidation of actin to regulate F‐actin dynamics. Nat Cell Biol 15: 1445–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen S, Goode BL (2019) Tropomyosin Isoforms differentially tune actin filament length and disassembly. Mol Biol Cell 30: 671–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jégou A, Niedermayer T, Orbán J, Didry D, Lipowsky R, Carlier M‐F, Romet‐Lemonne G (2011) Individual actin filaments in a microfluidic flow reveal the mechanism of ATP hydrolysis and give insight into the properties of profilin. PLoS Biol 9: e1001161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jégou A, Romet‐Lemonne G (2016) Single filaments to reveal the multiple flavors of actin. Biophys J 110: 2138–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegou A, Romet‐Lemonne G (2020) The many implications of actin filament helicity. Semin Cell Dev Biol 102: 65–72 [DOI] [PubMed] [Google Scholar]
- Kanellos G, Frame MC (2016) Cellular functions of the ADF/cofilin family at a glance. J Cell Sci 129: 3211–3218 [DOI] [PubMed] [Google Scholar]
- Kotila T, Wioland H, Enkavi G, Kogan K, Vattulainen I, Jégou A, Romet‐Lemonne G, Lappalainen P (2019) Mechanism of synergistic actin filament pointed end depolymerization by cyclase‐associated protein and cofilin. Nat Commun 10: 5320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremneva E, Makkonen MH, Skwarek‐Maruszewska A, Gateva G, Michelot A, Dominguez R, Lappalainen P (2014) Cofilin‐2 controls actin filament length in muscle sarcomeres. Dev Cell 31: 215–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BC, Péterfi Z, Hoffmann FW, Moore RE, Kaya A, Avanesov A, Tarrago L, Zhou Y, Weerapana E, Fomenko DE et al (2013) MsrB1 and MICALs regulate actin assembly and macrophage function via reversible stereoselective methionine oxidation. Mol Cell 51: 397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manstein DJ, Meiring JCM, Hardeman EC, Gunning PW (2019) Actin‐tropomyosin distribution in non‐muscle cells. J Muscle Res Cell Motil 41: 11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough A, Pope B, Chiu W, Weeds A (1997) Cofilin changes the twist of F‐actin: implications for actin filament dynamics and cellular function. J Cell Biol 138: 771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiring JCM, Bryce NS, Wang Y, Taft MH, Manstein DJ, Liu Lau S, Stear J, Hardeman EC, Gunning PW (2018) Co‐polymers of actin and tropomyosin account for a major fraction of the human actin cytoskeleton. Curr Biol 28: 2331–2337.e5 [DOI] [PubMed] [Google Scholar]
- Niedermayer T, Jegou A, Chièze L, Guichard B, Helfer E, Romet‐Lemonne G, Carlier M‐F, Lipowsky R (2012) Intermittent depolymerization of actin filaments is caused by photo‐induced dimerization of actin protomers. Proc Natl Acad Sci USA 109: 10769–10774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochniewicz E, Janson N, Thomas DD, De la Cruz EM (2005) Cofilin increases the torsional flexibility and dynamics of actin filaments. J Mol Biol 353: 990–1000 [DOI] [PubMed] [Google Scholar]
- Schramm AC, Hocky GM, Voth GA, Blanchoin L, Martiel J‐L, De La Cruz EM (2017) Actin filament strain promotes severing and cofilin dissociation. Biophys J 112: 2624–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura M, Lindberg U (1984) Separation of non‐muscle isoactins in the free form or as profilactin complexes. J Biol Chem 259: 3949–3954 [PubMed] [Google Scholar]
- Shekhar S, Chung J, Kondev J, Gelles J, Goode BL (2019) Synergy between Cyclase‐associated protein and Cofilin accelerates actin filament depolymerization by two orders of magnitude. Nat Commun 10: 5319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich JA, Watt S (1971) The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin‐troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem 246: 4866–4871 [PubMed] [Google Scholar]
- Suarez C, Roland J, Boujemaa‐Paterski R, Kang H, McCullough BR, Reymann A‐C, Guérin C, Martiel J‐L, De la Cruz EM, Blanchoin L (2011) Cofilin tunes the nucleotide state of actin filaments and severs at bare and decorated segment boundaries. Curr Biol 21: 862–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki EL, Chikireddy J, Dmitrieff S, Guichard B, Romet‐Lemonne G, Jégou A (2020) Geometrical constraints greatly hinder Formin mDia1 activity. Nano Lett 20: 22–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varland S, Vandekerckhove J, Drazic A (2019) Actin post‐translational modifications: the Cinderella of cytoskeletal control. Trends Biochem Sci 44: 502–516 [DOI] [PubMed] [Google Scholar]
- Wioland H, Guichard B, Senju Y, Myram S, Lappalainen P, Jégou A, Romet‐Lemonne G (2017) ADF/Cofilin accelerates actin dynamics by severing filaments and promoting their depolymerization at both ends. Curr Biol 27: 1956–1967.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wioland H, Jegou A, Romet‐Lemonne G (2019a) Quantitative variations with pH of actin depolymerizing factor/Cofilin’s multiple actions on actin filaments. Biochemistry 58: 40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wioland H, Jegou A, Romet‐Lemonne G (2019b) Torsional stress generated by ADF/cofilin on cross‐linked actin filaments boosts their severing. Proc Natl Acad Sci USA 116: 2595–2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wioland H, Suzuki E, Cao L, Romet‐Lemonne G, Jegou A (2020) The advantages of microfluidics to study actin biochemistry and biomechanics. J Muscle Res Cell Motil 41: 175–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann D, Kovar DR (2019) Feeling the force: Formin’s role in mechanotransduction. Curr Opin Cell Biol 56: 130–140 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Review Process File
Source Data for Figure 1
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Data Availability Statement
This study includes no primary dataset deposited in external repositories.


