Abstract
Introduction: Preterm birth is a major cause of infant mortality. It is unknown whether body mass index (BMI) influences the risk of preterm birth in women, who prenatally use antidepressants.
Materials and methods: The study cohort (N = 6920) consists of all primiparous European born women without previously diagnosed diabetes from the city of Vantaa, Finland, who delivered a singleton child between 2009 and 2015. Data on births, pre-pregnancy BMI and purchases of antidepressants from 12 months before conception until delivery were obtained from Finnish National Registers.
Results: Of the primiparous women, 9.9% used antidepressants. The overall prevalence of preterm birth was 5.2%. In women with a pre-pregnancy BMI <18.5 kg/m2, the Odds Ratio (OR) for preterm birth among antidepressant users compared with those who were non-users was 1.91 (95% confidence intervals [CI] 0.40 to 9.15, adjusted for age, smoking, education, use of fertility treatments and number of previous pregnancies) while in women with a pre-pregnancy BMI ≥30 kg/m2, the OR was 0.53 (95% CI 0.21–1.36), respectively.
Discussion: Primiparous women using antidepressants, who were underweight before conception should be closely monitored and provided tailored care in a maternity clinic to minimize the risk of preterm birth.
Key messages
In primiparous women, one in ten used antidepressant medications before pregnancy and/or during pregnancy.
In primiparous women, the prevalence of preterm birth was 5%.
Underweight primiparous women using antidepressants should be closely monitored and provided tailored care in a maternity clinic.
Keywords: Adiposity, antidepressant, body mass index, cohort, medication, pregnancy, prenatal, preterm birth, primiparous
Introduction
Among women of childbearing age, depressive symptoms are common and in Europe, up to 6% of the pregnant women use antidepressants [1–3].
Several previous meta-analyses have shown an increased risk between use of antidepressants during pregnancy and preterm birth [4–6]. Also, depression and related disorders appear to increase the risk for preterm birth independent of antidepressant use [7]. Few studies have assessed the relationship between pre-pregnancy antidepressant use and risk of preterm birth. An Italian population-based study reported no difference in the prevalence of preterm birth in women using antidepressants during pregnancy compared with women who used antidepressants before pregnancy but stopped during pregnancy [8]. Preterm birth is associated with increased rates of both short- and long-term morbidity for the offspring and is the leading cause of infant mortality [9]. Antidepressants have also been suggested to have adverse effects on the developing foetus, although it is challenging to differentiate between the effects of depression and the use of antidepressant medication [10–12].
Studies focusing on the prenatal use of antidepressants and risk for preterm birth have assessed the relationship with no distinction of maternal body mass index (BMI). It has been shown that both underweight and obesity are risk factors for preterm birth [13–16].
In 2016, we initiated a long-term follow-up study in the city of Vantaa, Finland. The aim of this study is to assess the relationship between the use of antidepressants, as a proxy for depression and related disorders, and risk of preterm birth in primiparous women, simultaneously taking into account the effect of maternal pre-pregnancy BMI.
Patients and methods
This study is an observational cohort study. The study cohort (N = 6920) is composed of all primiparous women who were born in European countries and without previously diagnosed diabetes mellitus from the city of Vantaa, Finland, who gave birth to a singleton child between 1 January 2009 and 31 December 2015. Women with diabetes (n = 47) were excluded because diabetes is associated with a high risk for depression and preterm birth [17,18].
We obtained data on deliveries from the Finnish Medical Birth Register held by the National Institute for Health and Welfare, Finland. The register collects the information on all live births and stillbirths from 22 gestational weeks or 500 grams onwards from all delivery hospitals in Finland. From this source, we obtained data on the participants’ age, pre-pregnancy weight and height, number of previous pregnancies (ectopic pregnancies, induced abortions, or miscarriages) and deliveries, use of infertility treatments, maternal smoking during pregnancy, gestational diabetes mellitus (GDM), hospitalization during pregnancy due to hypertension or vaginal bleeding, preterm birth including reasons for this, sections and stillbirths after 22 weeks of gestation [19]. The Finnish Medical Birth Register has been shown to be of high quality [20].
Preterm birth was defined as birth before 37 + 0 weeks of gestation, moderately preterm birth as birth 32 + 0 to 36 + 6 week of gestation and very preterm birth as birth 22 + 0 to 31 + 6 weeks of gestation, respectively [21].
In Finland, the first prenatal clinic visit is common at eight weeks of gestation. During that visit, women’s weight and height is measured, and self-reported pre-pregnancy weight is documented. After delivery, this information is forwarded to the Finnish Medical Birth Register. Based on self-reported pre-pregnancy weight and measured height, BMI was calculated as body weight divided by height squared (kg/m2). We defined women as underweight if their BMI was <18.5 kg/m2, normal weight if their BMI was 18.5–24.9 kg/m2, overweight if their BMI was 25–29.9 kg/m2 and obese, if their BMI was ≥30 kg/m2, respectively [22].
Women were considered as antidepressant users, if they had any purchases of antidepressants within a 12 months period before becoming pregnant and/or during pregnancy. The data on purchases of antidepressants were obtained from the Finnish Social Insurance Institution (Anatomical Therapeutic Chemical Classification System codes N06A or N06C) [23].
Data on highest educational attainment were obtained from Statistics Finland [24].
Ethical aspects
The ethics committee of the Hospital District of Helsinki and Uusimaa, Finland (356/13/03/03/2015, 2 November 2015) and the health authority of the city of Vantaa, Finland, have approved the study. National Institute for Health and Welfare, Finnish Social Insurance Institution, and Statistics Finland gave permission to use register data in the study.
Informed consents were not needed because this is an observational register-based study and no study participants were contacted.
Statistical analyses
Data are presented as means with SD or as counts with percentages. Statistical comparisons between antidepressant users and non-users were made using t-test for continuous variables and Pearson’s chi-square for categorical variables. The distribution of preterm births was assessed by Epps-Singleton two-sample empirical characteristic function test. To investigate the relationships between the main effects (the use of antidepressants and BMI-levels) and their interaction, we applied logistic regression models for the incidence of preterm birth. The models were stratified (five strata) using the propensity score [25]. The propensity score, defined as the conditional probability of using antidepressants, was built by using the logistic regression model with age, cohabiting, smoking, education, BMI, fertility treatments, previous pregnancies, and GDM as predictors. Stata 15.0 (StataCorp LP; College Station, Texas, USA) statistical package was used for the analysis.
Results
Of the primiparous women in our study cohort (n = 6920), 9.9% (n = 688) were using antidepressants, 4.1% (n = 282) used both before and during pregnancy and 0.4% (n = 31) used only during pregnancy. Baseline characteristics of the women are shown in Table 1.
Table 1.
Characteristics of the 6920 primiparous women.
| Non-users of antidepressants n = 6 232 | Users of antidepressantsan = 688 | |
|---|---|---|
| Age (years), mean (SD) | 28.3 (5.1) | 27.9 (5.5) |
| Cohabiting, % | 80.2 | 72.0 |
| Smokersb, % | 17.1 | 31.7 |
| Years of schooling, mean (SD) | 13.4 (2.7) | 12.6 (2.7) |
| Height (cm), mean (SD) | 166 (6) | 165 (6) |
| Weight (kg), mean (SD) | 65.6 (13.4) | 68.5 (14.6) |
| Pre-pregnancy body mass index (kg/m2), mean (SD) | 23.8 (4.5) | 25.0 (5.0) |
| Pre-pregnancy body mass index kg/m2, % | ||
| <18.5 | 4.1 | 2.9 |
| 18.5–24.9 | 67.0 | 56.3 |
| 25.0–29.9 | 19.1 | 24.2 |
| ≥30.0 | 9.8 | 16.6 |
| Previous pregnanciesc, % | ||
| None | 80.9 | 75.3 |
| 1 | 13.7 | 15.3 |
| ≥2 | 5.5 | 9.4 |
| Fertility treatment, % | 8.3 | 5.7 |
| Gestational diabetes mellitus, % | 15.6 | 18.3 |
| Hospitalization due to hypertension, % | 5.8 | 5.4 |
| Hospitalization due to vaginal bleeding, % | 0.4 | 0.0 |
| Induction of labour, % | 25.0 | 27.9 |
| Sections, % | 20.3 | 24.7 |
| Stillbirth after 22 weeks of gestation, % | 0.3 | 0.0 |
| Preterm birth (birth before 37 + 0 weeks of gestation), % | 5.2 | 5.5 |
| Moderately preterm birth (birth 32 + 0 to 36 + 6 weeks of gestation), % | 4.5 | 4.9 |
| Very preterm birth (birth 22 + 0 to 31 + 6 weeks of gestation), % | 0.7 | 0.6 |
Women were considered as antidepressant users, if they had any purchases of antidepressants 12 months or less before becoming pregnant and/or during pregnancy.
Including those who stopped during pregnancy.
Including ectopic pregnancies, induced abortions, or miscarries.
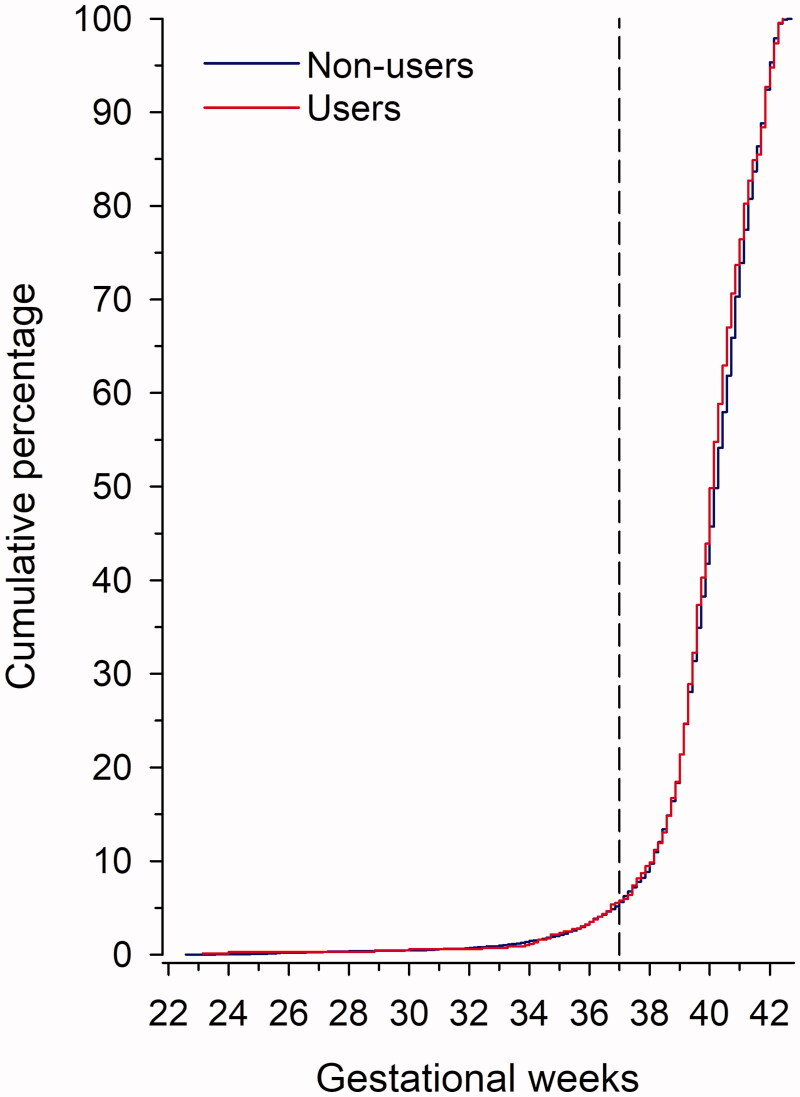
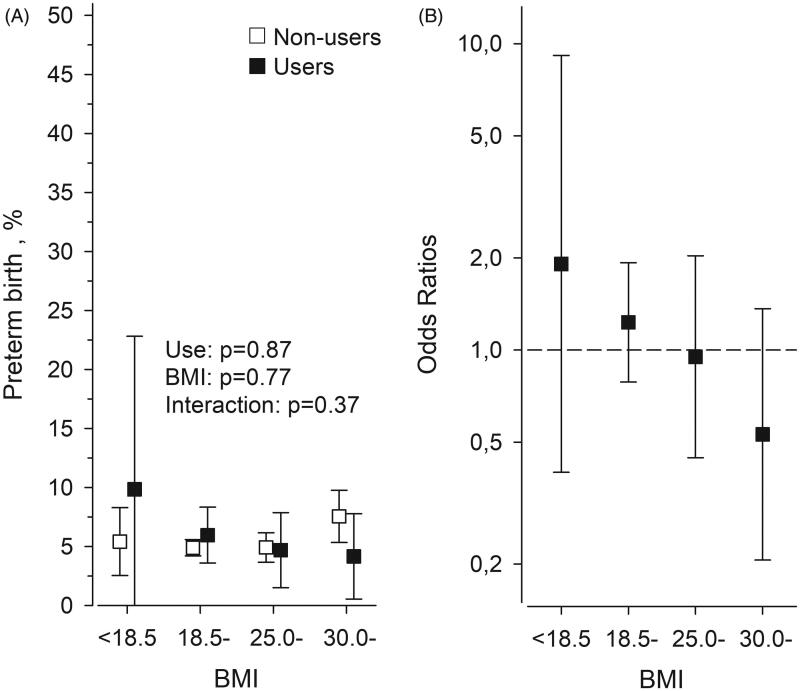
The overall prevalence of preterm birth was 5.2%. Use of antidepressants was not associated with duration of gestation (p = .32) (Figure 1). Table 2 shows the risk of preterm birth between women not using antidepressants and women who used antidepressants before becoming pregnant or used only during pregnancy or used both before and during pregnancy. Prevalence of preterm birth is illustrated in Figure 2(A) according to the use of antidepressants and pre-pregnancy BMI. In underweight women, the Odds Ratio (OR) for preterm birth among antidepressant users compared with non-users was 1.94 (95% confidence intervals [CI] 0.41–9.32) when adjusted for covariates and in obese women, the OR was 0.50 (95% CI 0.10–1.30), respectively (Figure 2(B)).
Figure 1.
Distribution of preterm births at different gestational weeks in primiparous women who were non-users (n = 6263) or users (n = 688) of antidepressants. Women were considered as antidepressant users if they had any purchases of antidepressants within a 12-month period before becoming pregnant and/or during pregnancy.
Table 2.
Risk of preterm birth between primiparous women without the use of antidepressants and women who used antidepressants before becoming pregnant or used only during pregnancy or used both before and during pregnancy.
| Model 1, Odds Ratio (95% CI) | Model 2, Odds Ratio (95% CI) | |
|---|---|---|
| No use of antidepressants (n = 6323) | Reference (1.00) | Reference (1.00) |
| Use of antidepressants only before pregnancy (n = 5919) | 0.87 (0.53–1.44) | 0.85 (0.51–1.41) |
| Use of antidepressants only during pregnancy (n = 31) | 1.26 (0.30–5.31) | 1.18 (0.28–4.96) |
| Use of antidepressants both before and during pregnancy (n = 282) | 1.31 (0.81–2.12) | 1.25 (0.77–2.03) |
| p-value for linearity .39 | p-value for linearity .53 |
Model 1: unadjusted model.
Model 2: stratified (five strata) using the propensity score. Propensity score includes the following variables: age, cohabiting, smoking, education, body mass index, fertility treatments, previous pregnancies and gestational diabetes mellitus.
CI: confidence intervals.
Figure 2.
(A) Prevalence of preterm birth in primiparous women, who were antidepressants users or non-users divided according to BMI. (B) Odds Ratio for preterm birth among antidepressants users with different BMIs compared with those, who were non-users with the same BMI. Women were considered as antidepressant users, if they had any purchase of antidepressants within a 12-month period before becoming pregnant and/or during pregnancy. Logistic regression models were used for both Figures (A,B). The models were stratified (five strata) using the propensity score. Propensity score includes the following variables: age, cohabiting, smoking, education, fertility treatments, previous pregnancies and gestational diabetes mellitus. BMI: body mass index.
Discussion
In the present study, 10% of the primiparous women used antidepressants during a 12-month period prior to conception, while 5% used antidepressants during pregnancy. In primiparous women using antidepressants, a decreasing trend for the risk of preterm birth with increasing BMI was observed. To the best of our knowledge, this is the first study to evaluate the association between the use of antidepressants, maternal BMI and preterm birth.
Our study finding of use of antidepressants in primiparous women is in line with previous observations from Europe [2,3]. Further, we observed that women who used antidepressants were more often obese compared with non-users endorsing previous studies [26]. In our study cohort, the prevalence of preterm birth was 5%, which is in line with the level of preterm births on a nationwide level in Finland [27]. In other parts of Europe, the prevalence of preterm birth varies between 5 and 11% [28].
In general, in primiparous women, most preterm births occur without any clinical known risk factors [13]. Risk factors known to predict preterm birth include a history of cervical operative procedures, a shortened cervical length, GDM or pre-eclampsia [13]. Further, several studies have shown that low pre-pregnancy BMI is a risk factor for preterm birth [13–16]. With regard to obesity, previous study findings are not consistent showing both neutral and increased risk of preterm birth [13–16].
In primiparous women using antidepressants, we observed a decreasing trend for the risk of preterm birth with increasing BMI. We can only speculate about the factors underlying the observation. Antidepressants are commonly prescribed for treating a variety of mental disorders and other diseases; in a general practice setting almost half of the prescriptions are for depression, one-fifth for anxiety or panic disorders, one-tenth for sleeping disorders, while the rest are prescriptions for diseases such as obsessive-compulsive disorders, eating disorders, migraine and neuropathic pain [29]. Possibly, in these above-mentioned disorders, maternal stress might influence the hypothalamic-pituitary-adrenal-axis and induce an elevation in concentrations of cortisol, which is a regulator for the placenta to stimulate a release of a corticotrophin-releasing hormone [30]. Elevated levels of the placental corticotrophin-releasing hormone, in turn, have been shown to increase the risk of preterm birth [30]. Further, in Western underweight primiparous women with the prenatal use of antidepressants, nutritional factors might at least partly explain the elevated risk for preterm birth [15]. According to current care guidelines, pregnant women with physical or mental disorders should be provided specially tailored support and guidance, such as nutritional and mental well-being counselling [31,32]. In Finland, the public prenatal clinics offer comprehensive and high-quality care [33]. This may partly explain why obese primiparous women with antidepressant medication had a lower risk for preterm birth compared with obese women without antidepressant medication – assuming that the users of antidepressants might have had a more holistic pregnancy care and follow-up by healthcare professionals. This, in turn, might have a preventive effect on the development of other complications such as hypertensive disorders and gestational diabetes, some other known risk factors for preterm birth.
Study strengths and limitations
Our study cohort is comprehensive; all primiparous women from the city of Vantaa, the fourth biggest city with 220,000 inhabitants in Finland, without previously diagnosed diabetes mellitus and who were born in European countries, were included in this study. Further, data on purchases of antidepressants are encompassing; the Finnish Social Insurance Institution collects data on all reimbursed purchases of antidepressants in Finland. Data on deliveries are based on data from the Finnish Medical Birth Register, which has been found to be of high quality [20]. Further, the information on maternal anthropometry is based on medical records documented by healthcare professionals.
The study has some limitations. We have information on the purchases of antidepressants, but we are missing the information about the underlying reasons and diagnoses of the purchases, the doses of antidepressants used and whether the medication has been used as prescribed. In our study population, only a few primiparous underweight or obese women used antidepressants, which should be kept in mind when considering the generalizability of our study observations. Furthermore, the generalizability of our study observations might be limited, as all the study participants were women with a European background.
In conclusion
Among primiparous women, with a mean age of 28 years, one in ten used antidepressants. Pre-pregnancy underweight women with antidepressant medication should be closely controlled and provided individualized care in order to minimize the risk of preterm birth.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Mladovsky P, Allin S, Masseria C, et al. Health in the European Union, trends and analysis; 2009. [cited 2018 Jul 2]. Available from: www.euro.who.int/__data/assets/pdf_file/0003/98391/E93348.pdf.
- 2.Bakker MK, Kolling P, van den Berg PB, et al. Increase in use of selective serotonin reuptake inhibitors in pregnancy during the last decade, a population-based cohort study from the Netherlands. Br J Clin Pharmacol. 2008;65:600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munk-Olsen T, Gasse C, Laursen TM. Prevalence of antidepressant use and contacts with psychiatrists and psychologists in pregnant and postpartum women. Acta Psychiatr Scand. 2012;125:318–324. [DOI] [PubMed] [Google Scholar]
- 4.Ross LE, Grigoriadis S, Mamisashvili L, et al. Selected pregnancy and delivery outcomes after exposure to antidepressant medication: a systematic review and meta-analysis. JAMA Psychiatry. 2013;70:436–443. [DOI] [PubMed] [Google Scholar]
- 5.Huybrechts KF, Sanghani RS, Avorn J, et al. Preterm birth and antidepressant medication use during pregnancy: a systematic review and meta-analysis. PLoS One. 2014;9:e92778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tak CR, Job KM, Schoen-Gentry K, et al. The impact of exposure to antidepressant medications during pregnancy on neonatal outcomes: a review of retrospective database cohort studies. Eur J Clin Pharmacol. 2017;73:1055–1069. [DOI] [PubMed] [Google Scholar]
- 7.Staneva A, Bogossian F, Pritchard M, et al. The effects of maternal depression, anxiety, and perceived stress during pregnancy on preterm birth: a systematic review. Women Birth. 2015;28:179–193. [DOI] [PubMed] [Google Scholar]
- 8.Cantarutti A, Merlino L, Monzani E, et al. Is the risk of preterm birth and low birth weight affected by the use of antidepressant agents during pregnancy? A population-based investigation. PLoS One. 2016;11:e0168115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slattery MM, Morrison JJ. Preterm delivery. Lancet. 2002;360:1489–1497. [DOI] [PubMed] [Google Scholar]
- 10.Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinette MG, Wax JR. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstet Gynecol. 2010;115:188–189. author reply 189. [DOI] [PubMed] [Google Scholar]
- 12.Liu C, Cnattingius S, Bergstrom M, et al. Prenatal parental depression and preterm birth: a national cohort study. BJOG. 2016;123:1973–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dekker GA, Lee SY, North RA, et al. Risk factors for preterm birth in an international prospective cohort of nulliparous women. PLoS One. 2012;7:e39154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Jongh BE, Paul DA, Hoffman M, et al. Effects of pre-pregnancy obesity, race/ethnicity and prematurity. Matern Child Health J. 2014;18:511–517. [DOI] [PubMed] [Google Scholar]
- 15.Liu P, Xu L, Wang Y, et al. Association between perinatal outcomes and maternal pre-pregnancy body mass index. Obes Rev. 2016;17:1091–1102. [DOI] [PubMed] [Google Scholar]
- 16.Ju AC, Heyman MB, Garber AK, et al. Maternal obesity and risk of preterm birth and low birthweight in Hawaii PRAMS, 2000–2011. Matern Child Health J. 2018;22:893–902. [DOI] [PubMed] [Google Scholar]
- 17.Roy T, Lloyd CE. Epidemiology of depression and diabetes: a systematic review. J Affect Disord. 2012;142:S8–S21. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Zhang M, Tian H, et al. Preterm birth and risk of type 1 and type 2 diabetes: systematic review and meta-analysis. Obes Rev. 2014;15:804–811. [DOI] [PubMed] [Google Scholar]
- 19.THL. Medical birth register; 2015[cited 2018 Mar 16]. Available from: http://www.thl.fi/en/statistics/parturients.
- 20.Gissler M, Teperi J, Hemminki E, et al. Data quality after restructuring a national medical registry. Scand J Soc Med. 1995;23:75–80. [DOI] [PubMed] [Google Scholar]
- 21. WHO: recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Modifications recommended by FIGO as amended October 14, 1976. Acta Obstet Gynecol Scand 1977;56:247–253. [PubMed] [Google Scholar]
- 22.WHO. Global Database on Body Mass Index; 2018. [cited 2018 Jul 10]. Available from: www.who.int/bmi/index.jsp.
- 23.WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD index; 2017. [Accessed 2018 Mar 16]. Available from: https://whocc.no/atc_ddd_index.
- 24.STAT. Koulutusaste; 2016. [cited 2018 Mar 16]. Available from: http://www.stat.fi/meta/luokitukset/koulutus/001-2016/kuvaus.
- 25.Guo S, Fraser MW. Propensity score analysis: statistical methods and applications. London, United Kingdom: SAGE Publications; 2010. [Google Scholar]
- 26.Rajan TM, Menon V. Psychiatric disorders and obesity: a review of association studies. J Postgrad Med. 2017;63:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.THL. Perinataalitilasto – synnyttäjät, synnytytkset ja vastasyntyneet 2016; 2017. [cited 2018 May 5]. Available from: www.thl.fi/statistics.
- 28.Euro-Peristat. European Perinatal Health Report. The Health and care of pregnant women and babies in Europe in 2010; 2013. [cited 2018 May 5]. Available from: www.europeristat.com.
- 29.Gardarsdottir H, Heerdink ER, van Dijk L, et al. Indications for antidepressant drug prescribing in general practice in the Netherlands. J Affect Disord. 2007;98:109–115. [DOI] [PubMed] [Google Scholar]
- 30.Sandman CA, Glynn L, Schetter CD, et al. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27:1457–1463. [DOI] [PubMed] [Google Scholar]
- 31.Gunatilake RP, Perlow JH. Obesity and pregnancy: clinical management of the obese gravida. Am J Obstet Gynecol. 2011;204:106–119. [DOI] [PubMed] [Google Scholar]
- 32.ACOG Practice Bulletin No 156 Obesity in pregnancy. Obstet Gynecol. 2015; 126:e112–e126. [DOI] [PubMed] [Google Scholar]
- 33.THL. Äitiysneuvola; 2018. [cited 2018 May 6]. Available from: https://thl.fi/web/lapset-nuoret-ja-perheet.