Abstract
Collagen deposition contributes to both high mammographic density and breast cancer progression. Low stromal PTEN expression has been observed in as many as half of breast tumors and is associated with increases in collagen deposition, however the mechanism connecting PTEN loss to increased collagen deposition remains unclear. Here, we demonstrate that Pten knockout in fibroblasts using an Fsp-Cre;PtenloxP/loxP mouse model increases collagen fiber number and fiber size within the mammary gland. Pten knockout additionally upregulated Sparc transcription in fibroblasts and promoted collagen shuttling out of the cell. Interestingly, SPARC mRNA expression was observed to be significantly elevated in the tumor stroma as compared to the normal breast in several patient cohorts. While SPARC knockdown via shRNA did not affect collagen shuttling, it notably decreased assembly of exogenous collagen. In addition, SPARC knockdown decreased fibronectin assembly and alignment of the extracellular matrix in an in vitro fibroblast-derived matrix model. Overall, these data indicate upregulation of SPARC is a mechanism by which PTEN regulates collagen deposition in the mammary gland stroma.
Introduction
Collagen deposition within the mammary gland impacts both cancer development and progression. It is one of the primary tissue properties contributing to high mammographic density [1], which is one of the greatest risk factors for breast cancer development [2–4]. Furthermore, increasing collagen density in a murine model by mutating type I collagen to prevent cleavage by matrix metalloproteinases increased tumor development in the presence of oncogene expression [5]. In addition to collagen density, increasing collagen stiffness through crosslinking promotes tumor development and progression. Inhibition of the collagen-crosslinking lysyl oxidase (LOX) family in mice decreased fibrosis as well as tumor incidence and tumor size in a mouse model [6], and high lysyl oxidase-like 2 (LOXL2) expression correlates with low overall and metastasis-free survival in breast cancer patients [7]. While a number of single nucleotide polymorphisms have been identified which correlate with high mammographic density, including changes in fibroblast growth factor receptor 2 (FGFR2) and MAP3K1 [8], the molecular mechanisms connecting gene expression changes to collagen accumulation in cases of high mammographic density remain to be elucidated.
Stromal cells, particularly cancer-associated fibroblasts, are responsible for much of the collagen deposition in breast cancer. Low stromal PTEN expression is a widespread phenomenon in breast cancers, and has been observed in as many as half of patients [9]. We have previously shown that low stromal Pten expression correlates with high mammographic density in a small cohort of patients [10]. Knockout of the tumor suppressor Pten in fibroblasts in an Fsp-Cre;PtenloxP/loxP mouse model increased both collagen deposition within the mammary gland and tumor development in mice expressing the Neu oncogene [9], which in combination with the patient data suggests that loss of stromal PTEN may contribute to the development of high mammographic density. However, the mechanism behind increased collagen deposition with PTEN loss is unclear. In this study, Pten knockout did not significantly affect transcriptional levels of fibrillar collagen, suggesting that other parts of the collagen deposition process are altered. Therefore, the question remains as to how Pten regulates this change in collagen deposition. We demonstrate here a new mechanism by which decreased PTEN expression upregulates secreted protein, acidic and rich in cysteine (SPARC) to regulate collagen fiber formation and organization.
SPARC is a matricellular protein which plays a role in collagen deposition and has previously been proposed as a collagen chaperone [11]. Knockout of Sparc in mouse models decreased collagen deposition within the dermis as well as collagen fiber size [12, 13]. Furthermore, SPARC knockdown greatly reduced bleomycin-induced lung fibrosis in a mouse model [14]. In solution, SPARC bound to a variety of different collagens, including collagen I, II, and III, and disrupted spontaneous collagen fibrillogenesis [15]. The mechanism of its effect on collagen secretion and assembly by cells remains to be elucidated.
Here, we demonstrate that Pten knockout in fibroblasts increases collagen secretion and SPARC expression. Furthermore, although SPARC knockdown in these fibroblasts did not affect collagen secretion, it decreased collagen fiber assembly. We further demonstrate that SPARC is important in fibronectin assembly and extracellular matrix alignment. Overall this implicates SPARC as a regulator of extracellular matrix assembly and organization, and suggests that SPARC contributes to the increased collagen deposition observed with PTEN loss in fibroblasts.
Materials and methods
Mouse studies
Wild-type (PtenloxP/loxP) and Pten-/- (Fsp-Cre;PtenloxP/loxP) FVB/N mice were generated and maintained as previously described [9, 16] in compliance with federal and University Laboratory Animal Resources (ULAR) regulations and approved by the Ohio State University Institutional Animal Care and Use Committee under protocol 2007A0120-R1 (PI: MCO). Wild-type FVB/N were purchased from Jackson Laboratories (Bar Harbor, Maine, USA). All mice were 8-10wks of age and at least tenth-generation congenic (N10). Mice were housed in standard 12-hour light/dark cycles in individually ventilated microisolator cages with food and water access ad libitum. Mice were euthanized by carbon dioxide inhalation followed by cervical dislocation. Glands were analyzed from 7 WT mice and 5 Pten-/- mice.
Cell culture
Wild-type (WT; PtenloxP/loxP) and Pten-/- (Fsp-cre;PtenloxP/loxP) murine mammary fibroblasts cell lines were previously generated [9, 10, 17, 18]. Cells were used from passages 30–50 and maintained at 37°C and 5% CO2 in high-glucose DMEM (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS; VWR), 2 mM L-glutamine, 10 U/ml penicillin, and 10 μg/ml streptomycin. FBS was heat-inactivated in a water bath at 55°C for 30 minutes prior to use. Cells were tested for mycoplasma using the PromoKine PCR Mycoplasma Test Kit I/C (VWR) every three months.
Antibodies and reagents
The following antibodies were used in these studies: collagen I (Abcam ab21286, used 1:1000 for WB, 1:250 for IF), SPARC (Cell Signaling 8725, clone D10F10, used 1:1000 for WB), GM130 (BD Biosciences 610822, clone 35/GM130, used 1:250 for IF), GAPDH (Thermo Fisher Scientific NB600502, clone 6C5, used 1:1000 for WB), tensin I (Novus Biologicals NBP1-84129, used 1:100 for IF), horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse (Thermo Fisher Scientific 32460 and 32430, used 1:1000 for WB), and AlexaFluor-conjugated goat anti-rabbit or anti-mouse (Thermo Fisher Scientific A11034 and A21428, used 1:500 for IF). Alexafluor555-conjugated phalloidin (Thermo Fisher Scientific A34055, used 1:40 for IF) and Hoechst 33342 (Thermo Fisher Scientific H3570, used 1:1000 for IF) were used as counterstains.
Second harmonic generation microscopy and collagen quantification
Second harmonic generation microscopy was performed as previously described [10]. In brief, 5 μm sections from the upper mammary gland of 8-week old mice were fixed and paraffin-embedded, then stained in Weigert’s hematoxylin (Sigma-Aldrich) and picrosirius red (Abcam) according to the manufacturer’s instructions. The sections were imaged in 1 μm slices through the depth of the tissue using an Olympus FV1000 MPE microscope with a 25× XLPlan water immersion lens (N.A. 1.05) and a Mai Tai DeepSee Ti:Sapphire laser (Spectra-Physics, Newport Corp.) at 950 nm wavelength. Maximum intensity z-projections of the images were analyzed using CT-Fire software (v1.3, https://loci.wisc.edu/software/ctfire) [19] to determine collagen fiber length and width.
shRNA knockdown
Stable SPARC knockdown lines were produced using MISSION lentiviral transduction particles (Sigma-Aldrich) with a pLKO.1-puro vector, using a multiplicity of infection of 10. Five different shRNA sequences were tested and the two with the highest knockdown efficiency were chosen to carry forward. The shRNA sequences used are as follows: 5’-CCGGCCTAGACAACGACAAGTACATCTCGAGATGTACTTGTCGTTGTCTAGGTTTTTG-3’ (TRCN0000080349, designated shSPARC#1) and 5’-CCGGGAATACATTAACGGTGCTAAACTCGAGTTTAGCACCGTTAATGTATTCTTTTTTG-3’ (TRCN0000418600, designated shSPARC#2). A non-targeting scrambled sequence was used as a control (Sigma-Aldrich SHC016V). Cells were transfected at 30–40% confluence and pretreated with 8 μg/mL polybrene for 45 minutes prior to viral addition. Transfection was performed for 24 hours, then the cells were allowed to recover for 24 hours prior to placing under selection with 5 μg/mL puromycin for 3 days.
qRT-PCR
Cells were plated for 24 hours and then supplemented with 50 μg/mL L-ascorbic acid (Sigma-Aldrich) for a further 24 hours prior to lysis. RNA was isolated using Tri-Reagent (Sigma-Aldrich) using the manufacturer’s instructions, then reverse transcribed using the iScript DNA synthesis kit (Bio-Rad). qRT-PCR was run using primers at 900 nM (Table 1), and results were normalized to the endogenous control, 18S (Thermo Fisher Scientific, primer pair used at 100 nM). Primer sequences were obtained from the Harvard Primer Bank (https://pga.mgh.harvard.edu/primerbank/) and verified using Primer BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/), or designed using Primer BLAST.
Table 1. Primer sequences.
| Gene | F | R |
|---|---|---|
| Sparc | TGGGAGAATTTGAGGACGGTG | GAGTCGAAGGTCTTGTTGTCAT |
| Col1a1 | GCTCCTCTTAGGGGCCACT | CCACGTCTCACCATTGGGG |
| Col1a2 | GGTGAGCCTGGTCAAACGG | ACTGTGTCCTTTCACGCCTTT |
| Col3a1 | CTGTAACATGGAAACTGGGGAAA | CCATAGCTGAACTGAAAACCACC |
| Col4a1 | TCCGGGAGAGATTGGTTTCC | CTGGCCTATAAGCCCTGGT |
| Lox | ACTTCCAGTACGGTCTCCCG | GCAGCGCATCTCAGGTTGT |
| Loxl1 | CGCCCTTCGTAAACCAGTATG | CACCACGGTAGTACACGTAGC |
| Loxl2 | TCGGAGCTTTTCTTCTGGGC | GGAGAGGCAGGAGCCAAAAT |
| Loxl3 | AGGGCCTGGTTGAAGTCAG | CCAGCATACCGCAGACTACG |
| Loxl4 | GTGCCAAGTATGGTCAAGGAG | CCGTTAGAGCCACACTGATCT |
| P4HA1 | AGACCGGCTAACGAGTACAG | CCAACTCACTCCACTCAGTGT |
| P4HA2 | CACCTCCATTGGGCACATGA | GCTCTTAATCTTGGCGAGCTT |
| p4HA3 | AGACGCCCTGGATTACTTGG | GTCTGGGCTGTAGACAAGGAA |
| Plod 1 | AGGATGACGCCAAGCTAGAG | TTGAAGAACTGAGCTGAACGC |
| Plod 2 | GCTGGCAGACAAGTACCCTG | GGGGCATAGCCAATAAAGCC |
| Plod 3 | AAGAAGTTTGTTCAGAGTGGCA | TGAATCCACCAGAGTTGAGGA |
| SOX5 | GTTGGAGCCAGCCGATCATA | ACCTCTCCATCTGTCTCCCC |
| KLF4 | TACCCCTACACTGAGTCCCG | GGAAAGGAGGGTAGTTGGGC |
| Brg1 | ACAAAGACAGCAGCAGTGGA | AGTCCACAGGCTTTCGGATG |
| Snai1 | GAGCCTCCTACCCCTCAGTA | AGCCAGTGGGTTGGCTTTAG |
Western blotting
Cells were plated in 10% heat-inactivated FBS DMEM. After 4 hours, the cells were washed with PBS and the medium replaced with DMEM and 50 μg/mL L-ascorbic acid (Sigma-Aldrich). After 20 hours, the medium was collected and the cells were lysed in RIPA buffer containing 25mM Tris-HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS (Thermo Fisher Scientific), with 1x HALT protease/phosphatase inhibitor cocktail (Thermo Fisher Scientific). The samples were frozen at -70°C until further use. Lysates were centrifuged at 12000RPM for 15 minutes to pellet debris, and the concentration of protein in the supernatant was quantified using the micro BCA assay kit (Thermo Fisher Scientific). The cell culture medium was concentrated using 10 kDa molecular weight cutoff centrifugal filters (Millipore-Sigma). 25 μg of protein was used for each sample of the cell culture lysate, while for the cell culture medium equal volumes of medium was used for each sample. Samples were treated with 100 mM dithiothreitol (Sigma-Aldrich) for 15 minutes, then the Bolt LDS sample buffer (Thermo Fisher Scientific) was added and the samples were heated at 70°C for 10 min. Samples were separated using Bolt 4–12% Bis-Tris Plus gels and Bolt MOPS-SDS running buffer, and transferred onto a PVDF membrane using Bolt Transfer Buffer (Thermo Fisher Scientific). Precision Plus Protein Dual Color Standard (Bio-Rad) was used as a molecular weight ladder. Signal was detected using an HRP-conjugated secondary antibody and chemiluminescent substrate (SuperSignal West Femto for collagen, SuperSignal West Pico PLUS for SPARC and GAPDH, Thermo Fisher Scientific). ImageJ software (National Institutes of Health, v1.43u) was used to perform densitometry.
Immunostaining and imaging
For immunostaining of collagen and GM130, cells were fixed in 4% paraformaldehyde (Electron Microscopy Sciences) for 20 minutes and permeabilized in 0.5% Triton X-100 in PBS for 5 minutes. For immunostaining of tensin, cells were simultaneously fixed and permeabilized using 3% paraformaldehyde with 0.5% Triton-X 100 in PBS for 2 minutes, followed by 3% PFA in PBS only for 30 minutes [20]. All cells were then blocked using 10% normal goat serum in PBS (NGS, Invitrogen) for 30 minutes. Primary antibodies were added at the indicated concentration in 10% NGS in PBS for 1 hour at room temperature, then the samples were washed 3x10 minutes in PBS. Secondary antibodies were added in 10% NGS in PBS for 1 hour at room temperature prior to washing 3x10 minutes in PBS and mounting the samples using ProLong Gold Antifade solution (Life Technologies). Samples were imaged at room temperature using an Olympus FV1000 confocal microscope equipped with a 20× (N.A. 0.85), 40× (N.A. 1.3), or a 60× (N.A. 1.4), oil-immersion objective lens.
Image analysis
A custom CellProfiler routine was written to automatically detect individual cell cytoplasms and extract the integrated density of collagen immunofluorescence for each [21]. Briefly, nuclei were detected using Hoechst stain signal, and cell borders were detected using the phalloidin actin stain. Both signals were thresholded using the minimum cross-entropy method. Nuclei regions were subtracted from cell borders to create individual cytoplasm masks.
Individual fibrillar adhesions were detected using CT-Fire. Briefly, tensin images were thresholded to create a binary mask, and minimum “fiber” length was set to 3.5μm. This cutoff was chosen by generating a histogram of lengths of all detected fibers, which revealed a second small peak above this length (S2 Fig). This length is also similar to previous measurements of average fibrillar adhesion length in fibroblasts [22]. The ratio of number of fibrillar adhesions in each field of view to the total integrated density of tensin signal in the field of view was calculated. Each biological replicate was normalized to the average output of all images taken on that day in order to control for day-to-day variation in signal intensity.
Collagen and fibronectin fibril assembly
For matrix assembly studies, fluorescently labeled collagen (10 μg/mL) or fibronectin (6 μg/mL) was added to cells plated 24 hr after plating. Cells were cultured in growth medium in the absence of ascorbic acid. After 24 hr culture with the fluorescently labeled protein, cells were fixed and stained as described above.
To fluorescently label collagen, 1 mg rat tail type I collagen (1 mg/mL in PBS; Corning) was incubated for 2 hours on ice, rocking, with 12.5 μL of 1M sodium bicarbonate buffer, pH 9, and 12.5 μL of 1 mg/mL NHS-Fluorescein (Fisher Scientific) in DMSO. The collagen was then placed in 8 kDa MWCO dialysis tubing and left in 0.1% acetic acid in PBS for 4 hours at 4°C, stirring, to remove unconjugated NHS-Fluorescein. The labeled collagen was stored at 4°C.
Fibronectin was labeled with NHS-Fluorescein according to previously a published protocol [23], with some minor modifications. Briefly, 10 mg of bovine fibronectin (Millipore-Sigma) was suspended at 1 mg/mL in PBS. Fibronectin was dialyzed in PBS overnight using 8 kDa dialysis tubing, then incubated with 125 μL of 1M sodium bicarbonate buffer, pH 9, and 125 μL of 1 mg/mL NHS-Fluorescein (Fisher Scientific) in DMSO for 2 hours at room temperature, rocking. Labeled fibronectin was separated using PD-10 desalting columns (GE Life Sciences) and stored at -70°C.
Fibroblast-derived matrix production
Fibroblast-derived matrices were produced as previously described [24]. Briefly, cells were plated on gelatin-coated coverslips for 24 hours, then on day 2 and day 4 of the experiment the medium was replaced and supplemented with 50 μg/mL ascorbic acid and 6 μg/mL fluorescently labeled fibronectin. Cells were fixed on day 6 by removing half of the culture medium and replacing it with a solution containing 4% paraformaldehyde and 5% sucrose. Nuclei were stained with Hoechst and the matrices were imaged in 8 μm stacks through the matrix in 1 μm intervals. The images were Z-projected and fiber orientation was quantified using the ImageJ plugin OrientationJ [25] with previously published settings [24]. The mode fiber orientation was set to 0° and matrix alignment was quantified by determining the fraction of fibers falling within 10° or 20° of the mode matrix orientation.
Traction force microscopy
Traction force microscopy was used to measure the contractility of the fibroblasts at individual cell level as described previously [26]. Cells were maintained under normal culture conditions. TFM substrates were prepared with polyacrylamide (PA) on 22 mm glass coverslips using an 8% acrylamide and 0.04% bis-acrylamide solution (BioRad) polymerized with 10% ammonium persulphate and tetramethylethylenediamine (TEMED). The gels were embedded with 0.5 μm-diameter red fluorescent carboxylate modified beads (Invitrogen) and coated with 50 μg/mL bovine collagen type I (Advanced BioMatrix Inc.). Cells were seeded (~1000/gel) in their respective treatment media and allowed to adhere overnight. After 24 hours, isolated single cells were imaged using phase contrast microscopy to identify cell boundaries, while fluorescent images of bead positions were obtained before and after cell detachment by trypsinization. Images were then analyzed to compute the bead displacement along the cell boundary using correlation-based particle image velocimetry in MATLAB (MathWorks). The traction stress field on the gel surface was calculated using the 3D finite element software COMSOL Multiphysics where the measured bead displacements under the cell were applied as a boundary condition, and the resulting stress field was analyzed for average traction and net contractile moment [26].
Patient datasets
Results from this screen were compared to several publicly available stromal gene expression datasets including those generated by Finak et al [27] Gene Expression Omnibus (GEO):GSE9014, Karnoub et al [28] GEO:GSE8977, and Ma et al [29] GEO:GSE14548 for comparison between normal and breast tumor-associated stroma expression. mRNA expression levels were compared by t-test.
Statistical analysis
Statistical testing was done using GraphPad Prism v7 and v8. Comparisons between two groups were performed using a two-tailed unpaired student’s t-test. Comparisons between more than two samples were performed using a one-way ANOVA followed by Tukey’s posttest. Comparison of collagen fiber length and fiber width between conditions was done by pooling fibers from all samples into a histogram and using a two-sample Kolmogorov-Smirnov test to examine differences in sample distribution. Differences were considered significant at p<0.05.
Results
Pten knockout increases collagen fiber formation and fiber size in the mammary gland
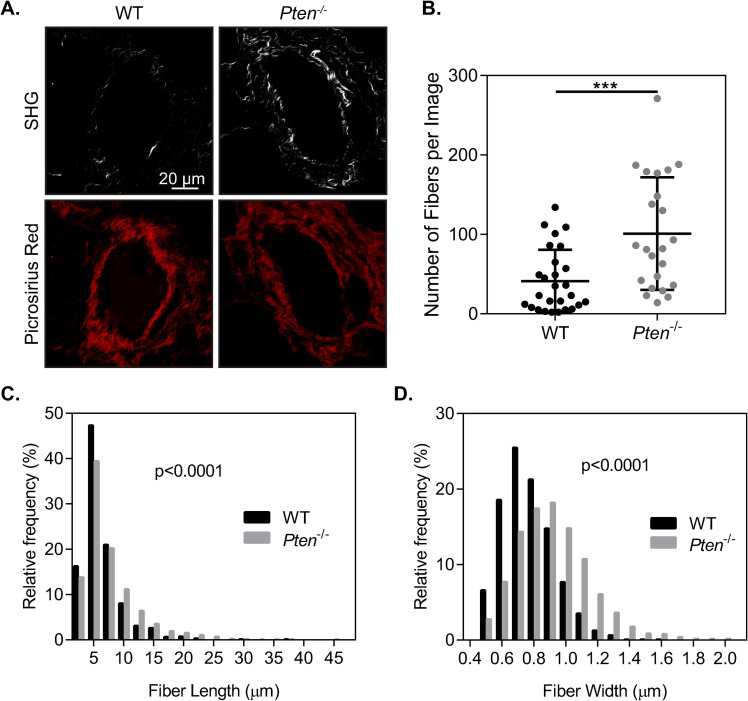
While PTEN is well known for its role as a tumor suppressor, it has more recently been identified as a regulator of extracellular matrix deposition and organization. Increases in collagen deposition within the mammary gland have previously been reported with Pten knockout using an Fsp-Cre;PtenloxP/loxP mouse model, particularly increases in collagen I [9]. To characterize the changes in collagen within the mammary gland, second harmonic generation microscopy was used to image collagen I surrounding ducts within the mammary gland of mice with WT or Pten-/- fibroblasts (Fig 1A). There was a significant increase in the number of collagen fibers surrounding mammary ducts in the Pten-/- glands (Fig 1B). Collagen fiber width and length were quantified using CT-Fire software [19] and plotted as a histogram (Fig 1C and 1D). There was a small but statistically significant shift in the distribution toward longer fiber lengths, as well as a significant (p<0.0001) shift in the distribution of fiber width, indicating an increase in collagen fiber size in the Pten-/- mammary glands. Overall, this suggests that there may be changes in collagen fibrillogenesis with Pten knockout, although it remains unclear whether this is due to changes in collagen production or assembly.
Fig 1. Pten knockout increases collagen fiber formation.
(A) Second harmonic generation (SHG) and picrosirius red staining surrounding ducts in murine mammary tissue sections. SHG images are shown above their corresponding picrosirius red images. (B) Quantification of the number of collagen fibers in SHG images, shown as mean+SD with individual values plotted, n = 32 ducts imaged across 7 WT mice, 23 ducts imaged across 5 Pten-/- mice. (C) Histogram of collagen fiber length in SHG images. (D) Histogram of collagen fiber width in SHG images. ***p<0.001.
PTEN loss alters collagen processing
Deposition of a collagen matrix is regulated at multiple levels, including collagen synthesis, secretion, and fiber assembly. However, it is unknown which of these levels of regulation are impacted by Pten, leading to the observed increased collagen deposition by Pten-/- fibroblasts in the mammary gland. Collagen is initially synthesized as a propeptide which requires hydroxylation for protein folding and transportation from the endoplasmic reticulum (ER) to the Golgi apparatus [30]. After secretion into the extracellular space, proteases such as BMP-1 [31] and members of the ADAMTS family [32] cleave the C- and N-terminal peptides and trigger self-assembly of collagen fibers [33, 34]. More recently, cell contractility and α2β1 integrin engagement [35], as well as assembly of a pre-existing fibronectin matrix [36] have been shown to contribute to collagen fiber assembly.
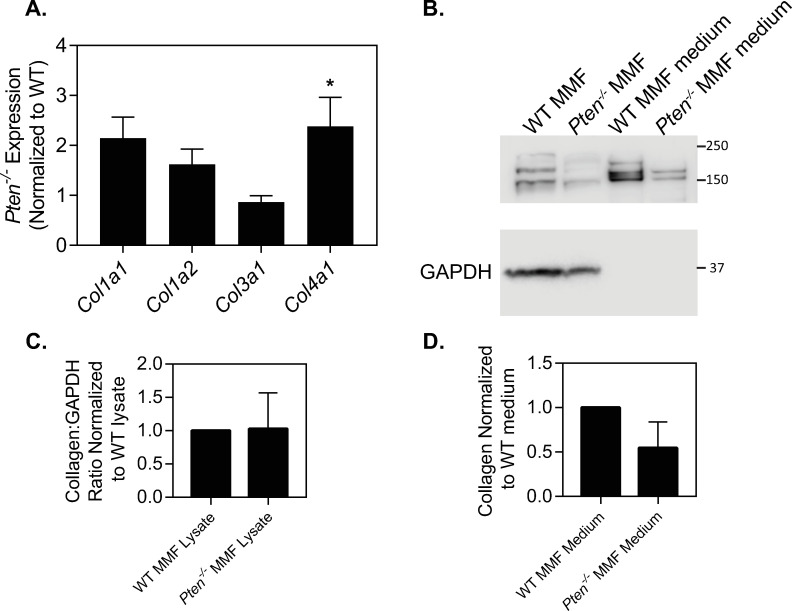
To determine which part of this collagen processing pathway is altered with Pten knockout, transcriptional levels of collagen genes were first examined in Pten-/- murine mammary fibroblasts as compared to WT cells (Fig 2A). While the fibrillar collagen genes Col1a1, Col1a2, or Col3a1 were not significantly different between the two cell types, Col4a1 mRNA levels were significantly increased with Pten knockout. Given the changes in collagen fiber number and size observed within the mammary tissue, the lack of large changes in fibrillar collagen mRNA levels suggests that collagen deposition is altered post-transcriptionally. Collagen protein expression was analyzed by western blot in WT and Pten-/- fibroblasts supplemented with 50 μg/mL ascorbic acid for 20 hr. Ascorbic acid is an essential cofactor for prolyl-4-hydroxylases to hydroxylate collagen as part of the folding process prior to leaving the ER; in the absence of ascorbic acid, collagen is retained in the ER [30, 37]. Collagen protein expression was measured in the cell lysate and concentrated cell culture medium to observe both intracellular and extracellular collagen levels (Fig 2B, full blot in S1 Fig). Samples were treated with dithiothreitol to ensure solubilization of the collagen. While expression was somewhat variable, protein expression of collagen was not statistically different between the WT and Pten-/- fibroblasts in the cell lysate or culture medium at this time point (Fig 2C and 2D). Collagen immunofluorescence of cells cultured with ascorbic acid for 20 hr showed no visible collagen deposited extracellularly at this time point (S3 Fig).
Fig 2. Pten knockout does not change collagen expression.
(A) Normalized collagen mRNA expression, n = 5+SD. (B) Western blot of collagen I, with GAPDH used as a loading control for cell lysates. Individual bands likely represent procollagen, processing intermediates, and alpha subunit chains. (C) Quantification of collagen in cell lysates, normalized to WT. n = 3+SD. (D) Quantification of collagen in culture medium, normalized to WT. n = 3+SD. *p<0.05.
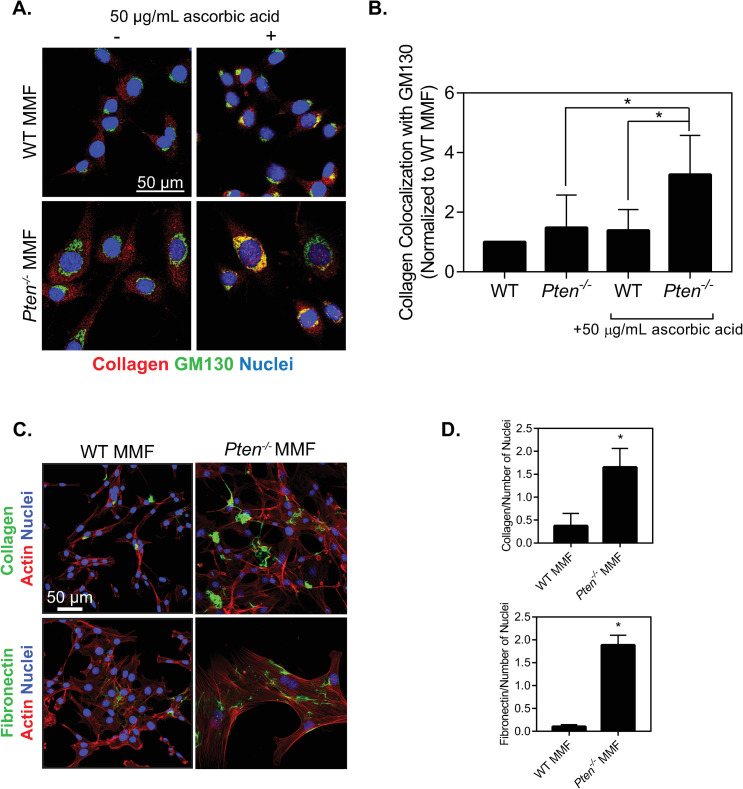
Collagen expression can also be regulated by secretion and the shuttling of collagen from the ER to the Golgi apparatus and into the extracellular space. To observe changes in collagen transport within the cell, cells were plated for 24 hours and supplemented with ascorbic acid for 1 hour prior to fixation. The cells were then stained for collagen and GM130, a Golgi marker (Fig 3A). In the absence of ascorbic acid, collagen appeared to be retained in the endoplasmic reticulum and showed no noticeable colocalization with the Golgi in either cell type, as expected since ascorbic acid is necessary for collagen shuttling from the ER. In the presence of ascorbic acid, the Pten-/- cells had significantly increased collagen colocalization with the Golgi as compared to either the WT cells or the Pten-/- cells without ascorbic acid supplementation (Fig 3B). In contrast, ascorbic acid addition did not noticeably increase collagen colocalization with GM130 in WT cells, suggesting that collagen remained in the ER. These data indicate that collagen shuttling from the ER to the Golgi is increased with Pten knockout [10].
Fig 3. Pten knockout alters collagen shuttling and fibrillogenesis.
(A) Immunofluorescence images of WT and Pten-/- MMF plated for 24 hours and cultured with or without 50 μg/mL ascorbic acid for 1 hour, then fixed and stained for collagen, GM130, and nuclei. (B) Quantification of collagen colocalization with GM130, determined by Manders’ correlation coefficient and normalized to the WT condition. n = 5+SD. *p<0.05. (C) Immunofluorescence images of cells incubated with fluorescently labeled collagen and fibronectin for 24 hours, then fixed and stained for actin and nuclei. (D) Quantification of the area of each image covered by collagen fibers normalized to number of nuclei in each image. (E) Quantification of the area of each image covered by fibronectin fibers normalized to number of nuclei in each image. n = 3+SD. *p<0.05 relative to WT.
We next hypothesized that Pten may also be altering collagen fibrillogenesis after collagen secretion by the cell. To test this, fluorescently labeled type I collagen and fluorescently labeled fibronectin were added exogenously to cells in the absence of ascorbic acid, to isolate assembly by the cells from the ability of these cells to produce and secrete these proteins (Fig 3C). Fibrillogenesis was quantified using ImageJ to examine the area within each image covered by collagen and fibronectin, relative to the number of nuclei in the image (Fig 3D and 3E).
PTEN loss alters collagen processing genes
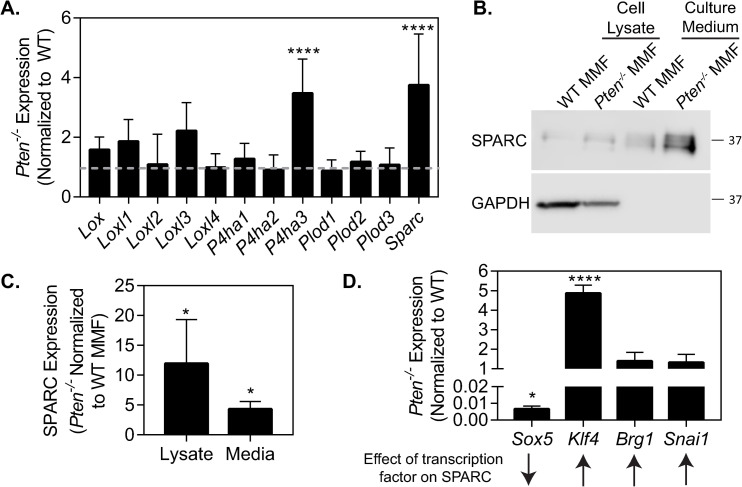
In order to further elucidate the changes to collagen processing with Pten knockout, we used RT-PCR to examine mRNA expression of different genes involved in collagen processing. The LOX family is involved in collagen crosslinking outside of the cell [38], while prolyl-4-hydroxylases (P4ha1, -2, and -3) and lysyl hydroxylases (Plod1, -2, and -3) hydroxylate procollagen to induce correct folding and are necessary for procollagen to leave the ER [37]. SPARC is important for collagen deposition and its attenuation via siRNA significantly decreases fibrosis [14] but the exact mechanism of its effect on collagen is not yet understood. P4ha3 and Sparc were significantly upregulated in Pten-/- fibroblasts compared to WT fibroblasts (Fig 4A). P4HA3 hydroxylates collagen in a similar manner as the other two prolyl-4-hydroxylase isoforms, but is typically expressed at much lower levels in most adult tissues [39]. As the role of prolyl-4-hydroxylases in collagen synthesis is well-characterized [40–42], we chose to examine the role of SPARC in Pten knockout-induced collagen deposition. Pten-/- fibroblasts showed a notable (~12-fold) increase in SPARC expression and in SPARC secretion (~4-fold) into the extracellular space versus WT cells (Fig 4B, quantified in 4C, full blot in S1 Fig). As SPARC appeared to be increased transcriptionally, the expression of several known transcription factors affecting SPARC was examined by RT-PCR (Fig 4D). The expression of Sox5, a known transcriptional repressor of Sparc [43], was decreased ~150-fold in Pten-/- cells. Of several known factors which directly transcribe Sparc [44–46], the expression of Klf4 was significantly increased.
Fig 4. Pten loss upregulates SPARC expression.
(A) mRNA expression of collagen-processing proteins. n = 5+SD. (B) Western blotting for SPARC expression, with GAPDH as a loading control. (C) Quantification of SPARC expression in cell lysate and culture medium, normalized to WT. n = 3+SD. (D) mRNA expression of transcription factors known to affect SPARC. n = 3+SD. *p<0.05, ****p<0.0001
SPARC knockdown does not affect collagen secretion by Pten-/- fibroblasts
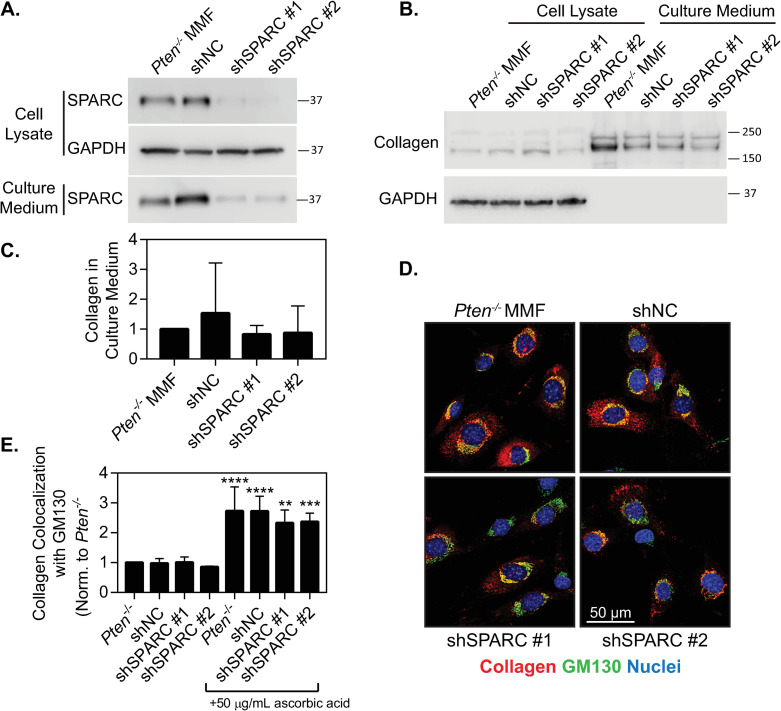
To examine the effects of SPARC on collagen trafficking, SPARC was knocked down in Pten-/- fibroblasts using shRNA. Analysis of the knockdown via Western blot showed ~80% knockdown in in both the cell lysate and in the culture medium in two lines (Fig 5A, full blot shown in S1 Fig, quantified in S4 Fig). To determine how SPARC knockdown affected collagen secretion, collagen protein expression in the cell lysate and culture medium were measured using a Western blot. (Fig 5B, full blot in S1 Fig). Although we hypothesized that SPARC knockdown would decrease collagen secretion, no collagen retention was visible within the cell and collagen levels in the culture medium were similar across cell lines (Fig 5C). Similarly, we examined collagen colocalization with the Golgi apparatus in the presence of ascorbic acid, and saw no significant change in colocalization, although all cell lines showed a significant response with ascorbic acid addition (Fig 5D and 5E). Overall, this indicates that SPARC does not play a notable role as a collagen chaperone in this model. We therefore hypothesized that the change in collagen shuttling to the Golgi with Pten knockout may be due to the observed increase in P4ha3 levels (Fig 4A).
Fig 5. SPARC knockdown does not affect collagen shuttling.
(A) Western blot verifying SPARC knockdown in both the cell lysate and the culture medium, with GAPDH as a loading control. (B) Western blot for collagen I in the cell lysate and culture medium with SPARC knockdown. (C) Quantification of collagen expression in the culture medium with SPARC knockdown, normalized to Pten-/- MMF. n = 3+SD, with no significant difference observed between conditions. (D) Immunofluorescence images of MMF plated for 24 hours and cultured with 50 μg/mL ascorbic acid for 1 hour, then fixed and stained for collagen, GM130, and nuclei. (E) Quantification of collagen colocalization with GM130, determined by Manders’ correlation coefficient and normalized to the Pten-/- parental condition. n = 3+SD. **p<0.01, ***p<0.001, ****p<0.0001 compared to the same cell line without ascorbic acid treatment.
Pten knockout-induced upregulation of SPARC promotes ECM assembly
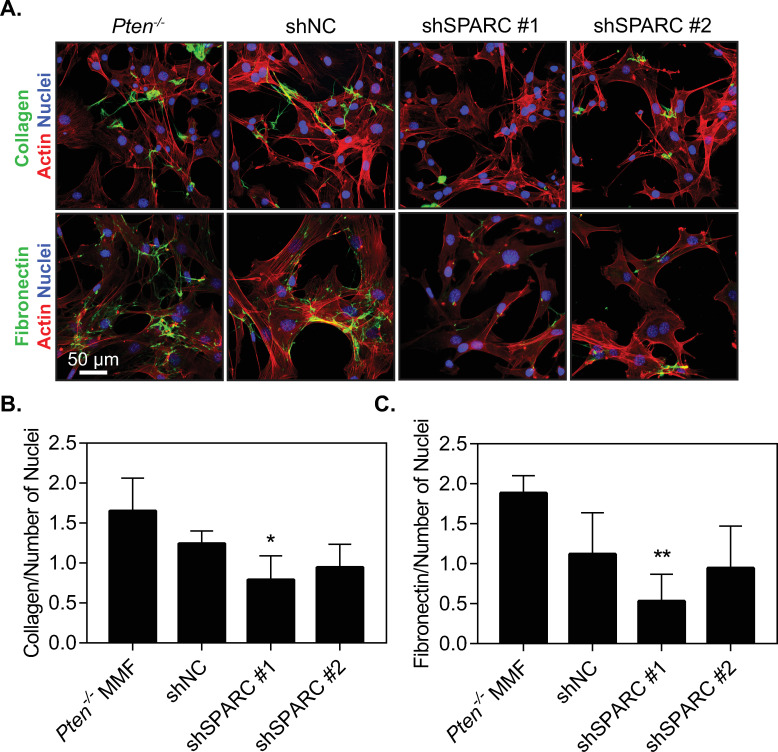
As SPARC expression did not appear to affect collagen trafficking out of the cell, we therefore hypothesized that SPARC may be affecting collagen fibrillogenesis after collagen secretion by the cell as SPARC is a known regulator of collagen fibrillogenesis [12, 13, 15, 47]. To test this hypothesis, fluorescently labeled type I collagen was added exogenously to cells in the absence of ascorbic acid, to isolate collagen assembly by the cells from the ability of these cells to produce and secrete collagen (Fig 6A). Fibrillogenesis was quantified using ImageJ to examine the area within each image covered by collagen, relative to the number of nuclei to control for cellularity in each image (Fig 6B). Cells modified with shSPARC#1 significantly decreased fiber formation relative to the parental Pten-/- line. The second SPARC shRNA resulted in more variable fiber formation, but the average was similar to that of the first shRNA sequence. This suggests that SPARC contributes to the increased ability of Pten-/- cells to form collagen fibers.
Fig 6. SPARC expression induced by Pten knockout promotes collagen and fibronectin fiber formation.
(A) Immunofluorescence images of cells incubated with fluorescently labeled collagen and fibronectin for 24 hours, then fixed and stained for actin and nuclei. (B) Quantification of the area of each image covered by collagen fibers normalized to number of nuclei in each image. (C) Quantification of the area of each image covered by fibronectin fibers normalized to number of nuclei in each image. n = 3+SD. *p<0.05 relative to Pten-/-. **p<0.01 relative to Pten-/-.
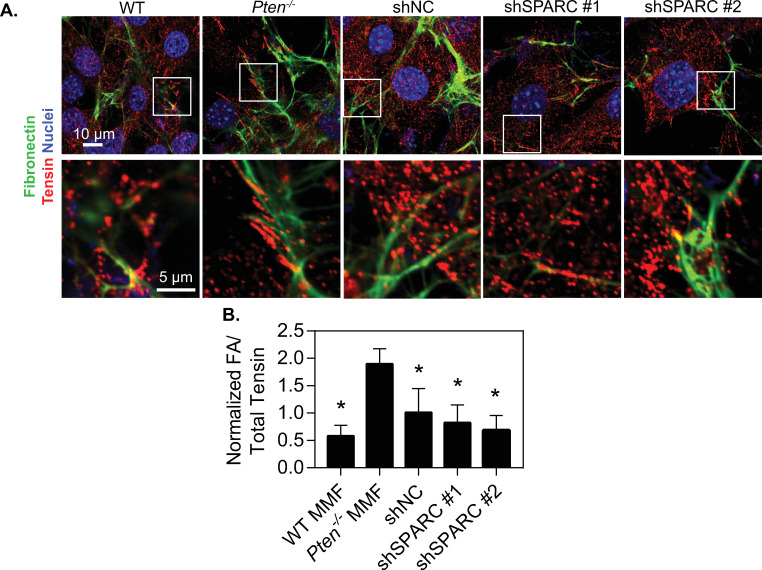
To determine whether the effects of SPARC on matrix deposition are specific to collagen or also affect other matrix proteins, murine mammary fibroblasts were incubated with fluorescently labeled fibronectin in a similar manner to the collagen studies. After 24 hours, there was a notable decrease in fibronectin assembly by SPARC knockdown there were notable increases in fiber formation by Pten-/- fibroblasts as compared to WT fibroblasts, which were abrogated (Fig 6A). Quantification of the fiber area relative to nuclear area in each image revealed a similar trend to collagen (Fig 6C), indicating that SPARC likely plays a role in overall matrix assembly. One possible mechanism SPARC may influence matrix assembly is through fibrillar adhesions, which are critical for the assembly of FN and collagen fibrils [48, 49]. Immunofluorescence staining of the fibrillar adhesion marker tensin I indicated an increase in the number of fibrillar adhesions in Pten-/- as compared to the WT fibroblasts, and knockdown of SPARC decreased the number of fibrillar adhesions (Fig 7).
Fig 7. SPARC expression induced by Pten knockout promotes fibrillar adhesion formation.
(A) Top row: Immunofluorescence images of cells incubated with fluorescently labeled fibronectin for 24 hours, then fixed and stained for tensin and nuclei. Inset boxes indicate areas of interest, magnified in bottom row. (B) Quantification of the number of fibrillar adhesions (FAs) per field of view (FOV) relative to the total amount of tensin signal present. n = 3+SD. #p<0.05 relative to Pten-/-.
Matrix assembly is regulated in part by cell contractility, and SPARC has previously been observed to regulate cell contractility pathways through ILK [50]. Furthermore, we have previously shown that Pten knockout in fibroblasts increases extracellular matrix alignment through increases in cell contractility [10]. We therefore hypothesized that changes in traction forces might be mediating the effects of SPARC on matrix alignment. The traction forces generated by these cells were examined using traction force microscopy (S6 Fig). Surprisingly, although Pten knockout significantly increased the average traction force as well as the net contractile moment generated by cells, SPARC knockdown did not significantly affect either of these metrics. This suggests that SPARC plays a role in matrix assembly and matrix alignment independent of traction-force mediated matrix assembly.
SPARC knockdown mitigates Pten knockout-induced matrix alignment
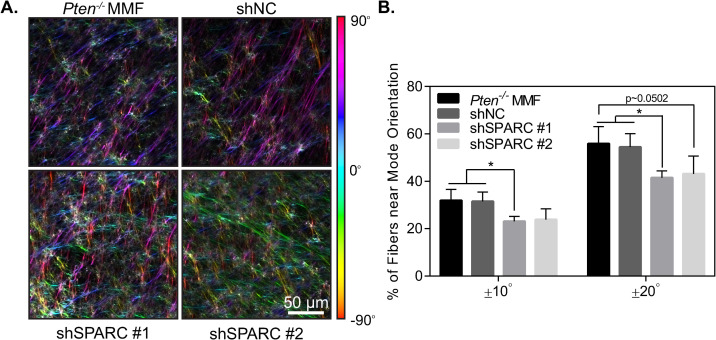
Extracellular matrix alignment surrounding tumors is thought to provide highways for cancer cell invasion away from the primary tumor and is an independent negative prognostic factor in breast cancer patients [51, 52]. Pten knockout in mammary fibroblasts has previously been shown to increase matrix alignment both in vivo and in vitro [10]. As SPARC knockdown abrogated increases in both collagen and fibronectin fiber formation caused by Pten knockout, we hypothesized that SPARC would additionally play a role in matrix organization. To test this hypothesis, fibroblast-derived extracellular matrices were generated and imaged using confocal microscopy (Fig 8A). The orientation of the fibers was quantified using OrientationJ software [25] and alignment was determined by taking the fraction of fibers falling within 10° or 20° of the mode orientation for each image. Using this metric, a higher fraction of fibers falling within this range signifies a more highly aligned matrix. There was a significant decrease in matrix alignment with the first SPARC shRNA, while the second shRNA sequence showed a similar trend but did not reach statistical significance (Fig 8B). Overall, this suggests that SPARC contributes to alignment of the ECM by Pten-/- cells.
Fig 8. SPARC knockdown decreases extracellular matrix alignment.
(A) Fibroblast-derived extracellular matrices, color-coded by fiber orientation. (B) Quantification of fibroblast-derived matrix alignment, n = 3+SD.
SPARC expression is upregulated in the tumor microenvironment
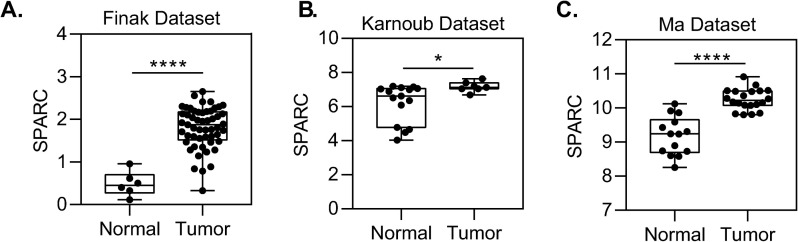
While low stromal PTEN expression has previously been observed in breast cancer patients [9], changes in SPARC expression in the stroma of human breast tumors had not been investigated. Using several patient datasets that specifically examined gene expression in the breast stroma (Finak et al [27], Karnoub et al [28], and Ma et al [29]), the expression of SPARC in normal and tumor breast stroma was queried. SPARC was consistently upregulated in the tumor stroma as compared to the normal stroma in each of these datasets (p 9A, 9B and 9C), underscoring its potential to contribute to collagen deposition in the tumor microenvironment.
Fig 9. SPARC expression is upregulated in the tumor microenvironment.
Stromal gene expression of SPARC in normal or breast cancer patients from (A) Finak et al, (B) Karnoub et al, or (C) Ma et al. *p<0.05, ***p<0.001, ****p<0.0001.
Discussion
Collagen deposition is one of the defining characteristics of high mammographic density and plays a significant role in both tumor formation and cancer progression. We have previously shown that low stromal PTEN expression is associated with increases in mammographic density [10] and stromal Pten knockout increased collagen deposition in the mammary gland in a mouse model [9]. However, it remained unclear how PTEN loss altered collagen synthesis pathways. Here, we show that Pten loss contributes to collagen shuttling out of the cell as well as collagen fiber formation, and that the increases in collagen fiber formation are dependent on increased expression of SPARC. Although SPARC has previously been suggested to act as a collagen chaperone [11], in this Pten knockout model SPARC did not affect collagen shuttling. The effects of SPARC on collagen deposition may primarily impact fibrillogenesis, as seen here, or other changes in collagen shuttling due to Pten knockout in this model may be masking the effects of SPARC. Collagen shuttling may be dependent on hydroxylation by P4HA3, as we observed significant upregulation of mRNA expression of this enzyme in the PTEN null cells. Another potential mechanism by which SPARC may regulate collagen deposition is by modulating the activity of transglutaminases, a family of proteins involved in extracellular collagen fiber assembly [53]. In dental tissue, transglutaminase crosslinking of collagen was found to be greater in SPARC‐null tissues in comparison to WT, suggesting that SPARC bound to collagen may reduce accessibility of collagen to TG modification [54]. TG expression has also been shown to play an important role in the motile and invasive phenotype of metastatic breast cancer [55] and to regulate interactions between cancer cells and the extracellular matrix [56, 57]. Higher levels of tissue TG have been also observed in metastatic lymph node tumors as compared to the primary breast tumors [58], further supporting the role of TG in cancer metastasis.
SPARC expression is widely associated with breast cancer, as approximately 75% of invasive breast cancers show strong SPARC expression, while in normal mammary tissue 92% of samples show undetectable or poorly detectable expression [59]. The role of SPARC in breast cancer remains in question, as it has been linked both to increased progression [60] and decreased cancer cell proliferation [61, 62]. In triple negative breast cancer patients, high SPARC expression correlates with poor overall survival [63]. These differential effects may also be linked to diverging roles of SPARC in the tumor as compared to the stroma, as stromal expression but not tumor expression has been linked to poor patient outcomes in pancreatic ductal adenocarcinoma [64]. Here, we show that stromal expression of SPARC is significantly upregulated in breast cancer as compared to the normal breast in several different patient cohorts. Further studies to compare the effects of SPARC in the tumor and the stroma are warranted.
Consistent with the idea that stromal SPARC expression may promote cancer progression, SPARC knockdown in fibroblasts decreased ECM fiber formation and alignment in our study, and these factors play a large role in tumor progression. Periductal collagen alignment is associated with high mammographic density [10, 65] and collagen alignment perpendicular to the tumor edge can form “highways” for cancer cell invasion [51]. The formation of perpendicularly-oriented collagen fibers is an independent prognostic factor in breast cancer patients [52] and we have previously observed that Pten knockout in fibroblasts increases the formation of these fibers surrounding MMTV-Neu-generated tumors in mice [10]. Therefore, SPARC expression in fibroblasts may support cancer progression, despite its apparent antiproliferative effects in cancer cells. We suggest that stromal SPARC plays a role in regulating ECM production and alignment in breast cancer, and that further work should be done to characterize the role that SPARC plays in the stroma as compared to the tumor.
Supporting information
Dotted line indicates threshold used for minimum length of fibrillar adhesions.
(TIF)
(TIF)
(A) Quantification of SPARC expression in the cell, normalized to GAPDH as a loading control. (B) Quantification of SPARC in the culture medium. n = 3+SD, *p<0.05, **p<0.01.
(TIF)
(A) Collagen fibers assembled by fibroblasts. (B) Fibronectin fibers assembled by fibroblasts.
(TIF)
(A) Average traction force per cell, shown as mean±SD with values of individual cells plotted. (B) Net contractile moment per cell. n = 25–46 cells. *p<0.05, ***p<0.001, ****p<0.0001.
(TIF)
Acknowledgments
Images presented in this report were generated using the instruments and services at the Campus Microscopy and Imaging Facility, The Ohio State University.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
Funding for this work was provided by the College of Engineering, The Ohio State University and The James Comprehensive Cancer Center as startup funds to JLL, which also supported the salary of CEJ, JTS, and JMZ. JTS received salary support from the Ohio State University Presidential Postdoctoral Scholars Program. SNG, STS, and JLL received salary support from the Ohio State University. Images presented in this report were generated using the instruments and services at the Campus Microscopy and Imaging Facility, The Ohio State University. This facility is supported in part by NIH cancer center grant P30 CA016058 to The Ohio State University, National Cancer Institute, Bethesda, MD. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Li T, Sun L, Miller N, Nicklee T, Woo J, Hulse-Smith L, et al. The Association of Measured Breast Tissue Characteristics with Mammographic Density and Other Risk Factors for Breast Cancer. Cancer Epidemiol Prev Biomark. 2005;14: 343–349. 10.1158/1055-9965.EPI-04-0490 [DOI] [PubMed] [Google Scholar]
- 2.Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ. Mammographic densities and breast cancer risk. Cancer Epidemiol Prev Biomark. 1998;7: 1133–1144. [PubMed] [Google Scholar]
- 3.Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, et al. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005;6: 798–808. 10.1016/S1470-2045(05)70390-9 [DOI] [PubMed] [Google Scholar]
- 4.Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K. Population-Attributable Risk Proportion of Clinical Risk Factors for Breast Cancer. JAMA Oncol. 2017;3: 1228–1236. 10.1001/jamaoncol.2016.6326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6: 11 10.1186/1741-7015-6-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell. 2009;139: 891–906. 10.1016/j.cell.2009.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker HE, Chang J, Cox TR, Lang G, Bird D, Nicolau M, et al. LOXL2-Mediated Matrix Remodeling in Metastasis and Mammary Gland Involution. Cancer Res. 2011;71: 1561–1572. 10.1158/0008-5472.CAN-10-2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Easton DF, Pooley KA, Dunning AM, Pharoah PDP, Thompson D, Ballinger DG, et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. 2007;447: 1087–1093. 10.1038/nature05887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461: 1084–1091. 10.1038/nature08486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones CE, Hammer AM, Cho Y, Sizemore GM, Cukierman E, Yee LD, et al. Stromal PTEN Regulates Extracellular Matrix Organization in the Mammary Gland. Neoplasia. 2019;21: 132–145. 10.1016/j.neo.2018.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinek N, Shahab J, Sodek J, Ringuette M. Is SPARC an Evolutionarily Conserved Collagen Chaperone? J Dent Res. 2007;86: 296–305. 10.1177/154405910708600402 [DOI] [PubMed] [Google Scholar]
- 12.Bradshaw AD, Puolakkainen P, Wight TN, Helene Sage E, Dasgupta J, Davidson JM. SPARC-Null Mice Display Abnormalities in the Dermis Characterized by Decreased Collagen Fibril Diameter and Reduced Tensile Strength. J Invest Dermatol. 2003;120: 949–955. 10.1046/j.1523-1747.2003.12241.x [DOI] [PubMed] [Google Scholar]
- 13.Rentz TJ, Poobalarahi F, Bornstein P, Sage EH, Bradshaw AD. SPARC Regulates Processing of Procollagen I and Collagen Fibrillogenesis in Dermal Fibroblasts. J Biol Chem. 2007;282: 22062–22071. 10.1074/jbc.M700167200 [DOI] [PubMed] [Google Scholar]
- 14.Wang J-C, Lai S, Guo X, Zhang X, de Crombrugghe B, Sonnylal S, et al. Attenuation of fibrosis in vitro and in vivo with SPARC siRNA. Arthritis Res Ther. 2010;12: R60 10.1186/ar2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giudici C, Raynal N, Wiedemann H, Cabral WA, Marini JC, Timpl R, et al. Mapping of SPARC/BM-40/Osteonectin-binding Sites on Fibrillar Collagens. J Biol Chem. 2008;283: 19551–19560. 10.1074/jbc.M710001200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trimboli AJ, Fukino K, de Bruin A, Wei G, Shen L, Tanner SM, et al. Direct Evidence for Epithelial-Mesenchymal Transitions in Breast Cancer. Cancer Res. 2008;68: 937–945. 10.1158/0008-5472.CAN-07-2148 [DOI] [PubMed] [Google Scholar]
- 17.Bronisz A, Godlewski J, Wallace JA, Merchant AS, Nowicki MO, Mathsyaraja H, et al. Reprogramming of the tumour microenvironment by stromal PTEN-regulated miR-320. Nat Cell Biol. 2011;14: 159–167. 10.1038/ncb2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sizemore G, Balakrishnan S, Hammer A, Thies K, Trimboli A, Wallace J, et al. Stromal PTEN inhibits the expansion of mammary epithelial stem cells through Jagged-1. Oncogene. 2017;36: 2297–2308. 10.1038/onc.2016.383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bredfeldt JS, Liu Y, Pehlke CA, Conklin MW, Szulczewski JM, Inman DR, et al. Computational segmentation of collagen fibers from second-harmonic generation images of breast cancer. J Biomed Opt. 2014;19: 016007 10.1117/1.JBO.19.1.016007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zamir E, Katz BZ, Aota S, Yamada KM, Geiger B, Kam Z. Molecular diversity of cell-matrix adhesions. J Cell Sci. 1999;112: 1655–1669. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7: R100 10.1186/gb-2006-7-10-r100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking Cell-Matrix Adhesions to the Third Dimension. Science. 2001;294: 1708–1712. 10.1126/science.1064829 [DOI] [PubMed] [Google Scholar]
- 23.Pankov R, Momchilova A. Fluorescent Labeling Techniques for Investigation of Fibronectin Fibrillogenesis (Labeling Fibronectin Fibrillogenesis). Extracellular Matrix Protocols. Humana Press; 2009. pp. 261–274. 10.1007/978-1-59745-413-1_18 [DOI] [PubMed] [Google Scholar]
- 24.Franco-Barraza J, Beacham D, Amatangelo MD, Cukierman E. Preparation of Extracellular Matrices Produced by Cultured and Primary Fibroblasts. Curr Protoc Cell Biol. 2016;71: 109.1–10.9.34. 10.1002/cpcb.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rezakhaniha R, Agianniotis A, Schrauwen JTC, Griffa A, Sage D, Bouten CVC, et al. Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy. Biomech Model Mechanobiol. 2011;11: 461–473. 10.1007/s10237-011-0325-z [DOI] [PubMed] [Google Scholar]
- 26.Zielinski R, Mihai C, Kniss D, Ghadiali SN. Finite Element Analysis of Traction Force Microscopy: Influence of Cell Mechanics, Adhesion, and Morphology. J Biomech Eng. 2013;135: 071009–071009–9. 10.1115/1.4024467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14: 518–527. 10.1038/nm1764 [DOI] [PubMed] [Google Scholar]
- 28.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449: 557–563. 10.1038/nature06188 [DOI] [PubMed] [Google Scholar]
- 29.Ma X-J, Dahiya S, Richardson E, Erlander M, Sgroi DC. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res. 2009;11: R7 10.1186/bcr2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walmsley AR, Batten MR, Lad U, Bulleid NJ. Intracellular Retention of Procollagen within the Endoplasmic Reticulum Is Mediated by Prolyl 4-Hydroxylase. J Biol Chem. 1999;274: 14884–14892. 10.1074/jbc.274.21.14884 [DOI] [PubMed] [Google Scholar]
- 31.Hartigan N, Garrigue-Antar L, Kadler KE. Bone Morphogenetic Protein-1 (BMP-1). Identification of the domain structure for procollagen C-proteinase activity. J Biol Chem. 2003;278: 18045–18049. 10.1074/jbc.M211448200 [DOI] [PubMed] [Google Scholar]
- 32.Fernandes RJ, Hirohata S, Engle JM, Colige A, Cohn DH, Eyre DR, et al. Procollagen II Amino Propeptide Processing by ADAMTS-3. Insights on dermatosparaxis. J Biol Chem. 2001;276: 31502–31509. 10.1074/jbc.M103466200 [DOI] [PubMed] [Google Scholar]
- 33.Leung MK, Fessler LI, Greenberg DB, Fessler JH. Separate amino and carboxyl procollagen peptidases in chick embryo tendon. J Biol Chem. 1979;254: 224–232. [PubMed] [Google Scholar]
- 34.Kadler KE, Hojima Y, Prockop DJ. Assembly of collagen fibrils de novo by cleavage of the type I pC-collagen with procollagen C-proteinase. Assay of critical concentration demonstrates that collagen self-assembly is a classical example of an entropy-driven process. J Biol Chem. 1987;262: 15696–15701. [PubMed] [Google Scholar]
- 35.Li S, Van Den Diepstraten C, D’souza SJ, Chan BMC, Pickering JG. Vascular Smooth Muscle Cells Orchestrate the Assembly of Type I Collagen via α2β1 Integrin, RhoA, and Fibronectin Polymerization. Am J Pathol. 2003;163: 1045–1056. 10.1016/s0002-9440(10)63464-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saunders JT, Schwarzbauer JE. Fibronectin matrix as a scaffold for procollagen proteinase binding and collagen processing. Mol Biol Cell. 2019;30: 2218–2226. 10.1091/mbc.E19-03-0140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Canty EG, Kadler KE. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci. 2005;118: 1341–1353. 10.1242/jcs.01731 [DOI] [PubMed] [Google Scholar]
- 38.Cox TR, Bird D, Baker A-M, Barker HE, Ho MW-Y, Lang G, et al. LOX-Mediated Collagen Crosslinking Is Responsible for Fibrosis-Enhanced Metastasis. Cancer Res. 2013;73: 1721–1732. 10.1158/0008-5472.CAN-12-2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kukkola L, Hieta R, Kivirikko KI, Myllyharju J. Identification and Characterization of a Third Human, Rat, and Mouse Collagen Prolyl 4-Hydroxylase Isoenzyme. J Biol Chem. 2003;278: 47685–47693. 10.1074/jbc.M306806200 [DOI] [PubMed] [Google Scholar]
- 40.Gorres KL, Raines RT. Prolyl 4-hydroxylase. Crit Rev Biochem Mol Biol. 2010;45: 106–124. 10.3109/10409231003627991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kivirikko KI, Myllylä R, Pihlajaniemi T. Protein hydroxylation: prolyl 4-hydroxylase, an enzyme with four cosubstrates and a multifunctional subunit. FASEB J. 1989;3: 1609–1617. 10.1096/fasebj.3.5.2537773 [DOI] [PubMed] [Google Scholar]
- 42.Pihlajaniemi T, Kivirikko KI. Prolyl 4-hydroxylase and its role in collagen synthesis. J Hepatol. 1991;13: S2–S7. 10.1016/0168-8278(91)90002-s [DOI] [PubMed] [Google Scholar]
- 43.Huang D-Y, Lin Y-T, Jan P-S, Hwang Y-C, Liang S-T, Peng Y, et al. Transcription factor SOX-5 enhances nasopharyngeal carcinoma progression by down-regulating SPARC gene expression. J Pathol. 2008;214: 445–455. 10.1002/path.2299 [DOI] [PubMed] [Google Scholar]
- 44.Zhou Y, Hofstetter WL, He Y, Hu W, Pataer A, Wang L, et al. KLF4 inhibition of lung cancer cell invasion by suppression of SPARC expression. Cancer Biol Ther. 2010;9: 507–513. 10.4161/cbt.9.7.11106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu YZ, Heravi M, Thuraisingam T, Marco SD, Muanza T, Radzioch D. Brg-1 mediates the constitutive and fenretinide-induced expression of SPARC in mammary carcinoma cells via its interaction with transcription factor Sp1. Mol Cancer. 2010;9: 210 10.1186/1476-4598-9-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hung J-Y, Yen M-C, Jian S-F, Wu C-Y, Chang W-A, Liu K-T, et al. Secreted protein acidic and rich in cysteine (SPARC) induces cell migration and epithelial mesenchymal transition through WNK1/snail in non-small cell lung cancer. Oncotarget. 2017;8: 63691–63702. 10.18632/oncotarget.19475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bradshaw AD. The role of SPARC in extracellular matrix assembly. J Cell Commun Signal. 2009;3: 239–246. 10.1007/s12079-009-0062-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pankov R, Cukierman E, Katz B-Z, Matsumoto K, Lin DC, Lin S, et al. Integrin Dynamics and Matrix AssemblyTensin-Dependent Translocation of α5β1 Integrins Promotes Early Fibronectin Fibrillogenesis. J Cell Biol. 2000;148: 1075–1090. 10.1083/jcb.148.5.1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zamir E, Katz M, Posen Y, Erez N, Yamada KM, Katz B-Z, et al. Dynamics and segregation of cell–matrix adhesions in cultured fibroblasts. Nat Cell Biol. 2000;2: 191–196. 10.1038/35008607 [DOI] [PubMed] [Google Scholar]
- 50.Barker TH, Baneyx G, Cardó-Vila M, Workman GA, Weaver M, Menon PM, et al. SPARC Regulates Extracellular Matrix Organization through Its Modulation of Integrin-linked Kinase Activity. J Biol Chem. 2005;280: 36483–36493. 10.1074/jbc.M504663200 [DOI] [PubMed] [Google Scholar]
- 51.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4: 38 10.1186/1741-7015-4-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, et al. Aligned Collagen Is a Prognostic Signature for Survival in Human Breast Carcinoma. Am J Pathol. 2011;178: 1221–1232. 10.1016/j.ajpath.2010.11.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosset EM, Trombetta‐eSilva J, Hepfer G, Chen P, Yao H, Bradshaw AD. Inhibition of transglutaminase activity in periodontitis rescues periodontal ligament collagen content and architecture. J Periodontal Res. 2020;55: 107–115. 10.1111/jre.12694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trombetta‐eSilva J, Rosset EA, Hepfer RG, Wright GJ, Baicu C, Yao H, et al. Decreased Mechanical Strength and Collagen Content in SPARC-Null Periodontal Ligament Is Reversed by Inhibition of Transglutaminase Activity. J Bone Miner Res. 2015;30: 1914–1924. 10.1002/jbmr.2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mangala LS, Fok JY, Zorrilla-Calancha IR, Verma A, Mehta K. Tissue transglutaminase expression promotes cell attachment, invasion and survival in breast cancer cells. Oncogene. 2007;26: 2459–2470. 10.1038/sj.onc.1210035 [DOI] [PubMed] [Google Scholar]
- 56.Aeschlimann D, Thomazy V. Protein Crosslinking in Assembly and Remodelling of Extracellular Matrices: The Role of Transglutaminases. Connect Tissue Res. 2000;41: 1–27. 10.3109/03008200009005638 [DOI] [PubMed] [Google Scholar]
- 57.Zemskov EA, Janiak A, Hang J, Waghray A, Belkin AM. The role of tissue transglutaminase in cell-matrix interactions. Front Biosci J Virtual Libr. 2006;11: 1057–1076. 10.2741/1863 [DOI] [PubMed] [Google Scholar]
- 58.Mehta K, Fok J, Miller FR, Koul D, Sahin AA. Prognostic Significance of Tissue Transglutaminase in Drug Resistant and Metastatic Breast Cancer. Clin Cancer Res. 2004;10: 8068–8076. 10.1158/1078-0432.CCR-04-1107 [DOI] [PubMed] [Google Scholar]
- 59.Bellahcéne A, Castronovo V. Increased Expression of Osteonectin and Osteopontin, Two Bone Matrix Proteins, in Human Breast Cancer. Am J Pathol. 1995;146: 6 [PMC free article] [PubMed] [Google Scholar]
- 60.Jones C, Mackay A, Grigoriadis A, Cossu A, Reis-Filho JS, Fulford L, et al. Expression Profiling of Purified Normal Human Luminal and Myoepithelial Breast Cells: Identification of Novel Prognostic Markers for Breast Cancer. Cancer Res. 2004;64: 3037–3045. 10.1158/0008-5472.can-03-2028 [DOI] [PubMed] [Google Scholar]
- 61.Koblinski JE, Kaplan-Singer BR, VanOsdol SJ, Wu M, Engbring JA, Wang S, et al. Endogenous Osteonectin/SPARC/BM-40 Expression Inhibits MDA-MB-231 Breast Cancer Cell Metastasis. Cancer Res. 2005;65: 7370–7377. 10.1158/0008-5472.CAN-05-0807 [DOI] [PubMed] [Google Scholar]
- 62.Nagai MA, Gerhard R, Fregnani JHTG, Nonogaki S, Rierger RB, Netto MM, et al. Prognostic value of NDRG1 and SPARC protein expression in breast cancer patients. Breast Cancer Res Treat. 2011;126: 1–14. 10.1007/s10549-010-0867-2 [DOI] [PubMed] [Google Scholar]
- 63.Zhu A, Yuan P, Du F, Hong R, Ding X, Shi X, et al. SPARC overexpression in primary tumors correlates with disease recurrence and overall survival in patients with triple negative breast cancer. Oncotarget. 2016;7: 76628–76634. 10.18632/oncotarget.10532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Infante JR, Matsubayashi H, Sato N, Tonascia J, Klein AP, Riall TA, et al. Peritumoral Fibroblast SPARC Expression and Patient Outcome With Resectable Pancreatic Adenocarcinoma. J Clin Oncol. 2007;25: 319–325. 10.1200/JCO.2006.07.8824 [DOI] [PubMed] [Google Scholar]
- 65.McConnell JC, O’Connell OV, Brennan K, Weiping L, Howe M, Joseph L, et al. Increased peri-ductal collagen micro-organization may contribute to raised mammographic density. Breast Cancer Res. 2016;18: 5 10.1186/s13058-015-0664-2 [DOI] [PMC free article] [PubMed] [Google Scholar]