Abstract
Objective:
To understand if presence of mental stress-induced myocardial ischemia (MSIMI) is associated with higher prevalence of cognitive impairment at baseline and its decline over time.
Methods:
A cohort of participants with stable coronary atherosclerosis underwent acute mental stress testing using a series of standardized speech/arithmetic stressors. The stress/rest digital vasomotor response to mental stress (sPAT) was assessed to measure microvascular constriction during mental stress. Patients received 99mTc-sestamibi myocardial perfusion imaging at rest, with mental stress and with conventional (exercise/pharmacological) stress. Cognitive function was assessed both at baseline and at a 2 year follow-up using the Trail Making Test parts A and B and the verbal and visual memory subtests of the Wechsler Memory Scale.
Results:
We studied 486 individuals (72% male, 32.1% Black, 62 ± 9 (mean ± SD) years old). After multivariable adjustment for baseline demographics, risk factors, and medication use, presence of MSIMI was associated with 21% and 20% slower completion of Trail-A and Trail-B, respectively (p for all < 0.01). After a 2-year follow-up period, presence of MSIMI was associated with a 33% slower completion of Trail-B, denoting cognitive decline (B= 0.33, 95% CI, 0.04, 0.62). A lower sPAT, indicating greater vasoconstriction, mediated the association between MSIMI and worsening Trail-B performance by 18.2%. Ischemia with a conventional stress test was not associated with any of the cognitive tests over time.
Conclusion:
MSIMI is associated with slower visuomotor processing and worse executive function at baseline and with greater decline in these abilities over time.
Introduction
Coronary atherosclerosis remains a major cause of morbidity and mortality worldwide.1 The risk of cognitive impairment, especially in the executive domain, has been shown to be higher in those with coronary atherosclerosis compared to those without coronary atherosclerosis.2–8 Executive function is crucial for secondary prevention in patients with coronary atherosclerosis, as lower cognitive flexibility could result in difficulties with regulation of one’s behavior, resulting in missed appointments, inability to make diet changes, and reduced medication adherence.9, 10 The exact mechanisms influencing the maintenance of cognitive performance among persons with coronary atherosclerosis are unclear.
Psychological stress has emerged as a risk factor for both coronary atherosclerosis and cognitive impairment. Acute mental stress impairs various domains of cognitive function, with executive functioning being the component that is most adversely affected by acute stress.11, 12 Acute mental stress can also precipitate myocardial ischemia in individuals with coronary atherosclerosis. Mental stress-induced myocardial ischemia (MSIMI) is associated with a doubling of future cardiovascular events and mortality, similar to ischemia provoked by conventional stress test testing.13 MSIMI, therefore, has been conceptualized as a marker of cardiovascular vulnerability to emotional stress.14 Recently, we have demonstrated that patients with MSIMI show increased brain activation in a number of regions involved in memory and emotion such as the medial frontal gyrus, anterior cingulate, inferior frontal gyrus, and parietal cortex (including inferior parietal lobule and supramarginal gyrus).15 These findings underscore that MSIMI is a phenomenon driven by the physiological response to stress that is regulated in the central nervous system. However, it is unclear if the presence of MSIMI is associated with cognitive impairment; such a link could lend support to the concept that acute psychological stress plays a role in cognitive decline among individuals with coronary atherosclerosis.
MSIMI is thought to reflect coronary and peripheral vasoconstriction, rather than severity of coronary atherosclerosis, which is the underlying mechanism of conventional stress-induced myocardial ischemia (CSIMI).16 Peripheral vasoconstriction can be measured using peripheral arterial tonometry as stress/rest ratio (sPAT), the ratio of the pulse wave amplitude of the digit during mental stress compared to the resting amplitude. sPAT has shown to be an important predictor of MSIMI and future cardiovascular events,17–20 and is directly associated with coronary vasomotion.21, 22
In the current study, we sought to understand the effects of acute mental stress on the cognitive performance of individuals with coronary atherosclerosis by investigating the link between MSIMI and cognitive function, and to evaluate whether stress-induced peripheral microvascular constriction measured with sPAT plays a role. We also sought to contrast the results for MSIMI with those for ischemia provoked by a conventional stress test. We hypothesized that MSIMI is associated with worse cognitive impairment, and that a stronger vasoconstrictive response to acute mental stress would mediate this association.
Methods
Study Population
The Mental Stress Ischemia Mechanisms and Prognosis Study (MIPS) enrolled stable coronary atherosclerosis individuals from Emory University–affiliated hospitals and clinics between 2011 and 2014. Subjects had a face-to-face clinic visit at baseline and at 2 years. Measures of cognitive function were added to the baseline assessments after the enrolment had already begun. Cognitive function measures were repeated at the 2-year visit. A detailed study protocol has been previously published.23 Presence of coronary atherosclerosis was defined based on a positive nuclear stress test, an abnormal coronary angiogram showing evidence of atherosclerosis, or a history of prior myocardial infarction or revascularization. Exclusion criteria included a history of acute coronary syndrome in the previous week or decompensated heart failure, end-stage kidney disease, a systolic blood pressure higher than 180 mm Hg or diastolic blood pressure higher than 110 mm Hg on the day of the clinic visit, or severe psychiatric conditions such as schizophrenia. Data regarding sociodemographic characteristics, medical history, and medication use were collected using standardized questionnaires, and clinical information was verified by medical record review. This study was approved by the Institutional Review Board of Emory University, and all participants provided informed consent.
Mental Stress Testing
Each participant underwent standardized mental stress testing during the baseline exam, as previously described.15, 23, 24 Briefly, following 30 minutes of resting in a quiet and temperature-controlled (21°C–23°C) room, subjects were asked to imagine a stressful situation in which a close relative had been mistreated in a nursing home. Participants were subsequently asked to prepare a statement for 2 minutes and then they were given 3 minutes to present it in front of an evaluative audience wearing white coats. The mental stress testing procedure was conducted by trained and experienced staff to ensure standardization of the stress-provoking elements.
Cognitive testing
We administered the Trail Making Test parts A and B to evaluate visuomotor sequencing and executive function, respectively.25 During Trail A testing, each individual was instructed to draw lines sequentially connecting 25 encircled numbers distributed on a sheet of paper, whereas during the more complex Trails B testing, the individual was asked to rapidly switch between numbers and letters. The score on each part represents the amount of time (in seconds) required to complete the task. Lower scores on Trails A and B indicate faster completion times and therefore better cognitive function. We also calculated the difference in time between completing Trail-A and Trial-B in order to measure set shifting speed that is not influenced by visuomotor speed only.
We also administered the Visual Reproduction and Verbal Memory subtests of the Wechsler Memory Scale (WMS) according to the Russell revision.26 The Visual Reproduction subtest was used to evaluate immediate and delayed visual memory, and the Verbal Memory subtest was administered to evaluate immediate and delayed verbal declarative memory. For the visual memory testing, participants were asked to reproduce a series of line drawings, each presented for 10 seconds followed by immediate and then 30-minute delayed recall. For the test of immediate verbal memory, each participant was asked to recall the details of a short story immediately after it was read to them and then after a 30-minute delay. Points were assigned, with higher scores indicating better cognitive function.
Digital Blood Flow Measurement via Finger Plethysmography
We used the PAT (Itamar-Medical, Israel) device to measure digital arterial pulse wave amplitude continuously during rest and the mental stress test at the baseline visit, as previously described.18, 21 Briefly, the probe is applied on the index finger and applies a constant subdiastolic pressure over the distal two-thirds of the finger to prevent distal venous blood stasis, unload arterial wall tension, and stabilize the probe to reduce noise. As a result, the changes in pulsatile volume reflect changes in digital arterial blood perfusion. The pulsatile pressure changes from the probe are then registered from a pressure transducer, and fed into a specialized software which filters, amplifies, stores, and analyzes the signal in an operator-independent manner. The baseline pulse wave amplitude at rest was determined by averaging the last 3 minutes of recording that preceded the mental stress test. The pulse wave amplitude during the mental stress test was determined visually as the area of maximum vasoconstriction during the mental stress task. sPAT during mental stress was calculated as the ratio of pulse wave amplitudes during mental stress over the resting baseline, such that a ratio <1 signifies peripheral arterial vasoconstriction during mental stress.
Myocardial perfusion imaging
All participants underwent 3 nuclear 99 m-Tc sestamibi-gated single-photon emission computed-tomography (SPECT) scans at rest, during mental stress and during conventional stress testing (with exercise or pharmacological stress depending on walking capacity). Two experienced nuclear cardiologists performed visual interpretation of the SPECT scans while blinded to clinical data. Any discrepancies in interpretation of images were resolved by consensus. The extent and severity of perfusion defects were quantified at rest and during both mental and conventional stress using a 17-segement model 27, where the severity of the defect was quantified using a 5-point scale from normal (score = 0) to absent perfusion (score = 4). These scores were then summed across the 17 segments in a conventional fashion, yielding a summed stress score for mental and conventional stress test, and a summed rest score for rest, each with a theoretical range of 0 to 68. A summed difference score was also calculated for both mental and conventional stress scans by subtracting the summed rest score from the summed stress score. The summed difference score is a semiquantitative measure of the number and severity of reversible (ischemic) myocardial perfusion defects.28 Presence of myocardial ischemia was defined as a summed total score of ≥ 2 in any segment, or worsening of a preexisting impairment of at least 2 points in a single segment, or worsening of at least 1 point in 2 or more contiguous segments.28
Statistical Analysis
For our analyses, each cognitive test was standardized by subtracting the mean from the raw score and then dividing the result by the standard deviation. This transformation facilitated comparison of the magnitude of association across cognitive function tests in regression models. We performed both linear multivariable regression models to investigate the relationship of mental stress and conventional stress ischemia with cognitive function tests (outcome variables). We also performed similar linear regression analyses with changes in cognitive tests during follow-up as outcome variables. The multivariable models used in the present study were constructed as follows: Model 1: unadjusted; Model 2: adjusted for demographics (age, sex, race, and education), clinical variables (body mass index, hypertension, hyperlipidemia, diabetes mellitus, smoking history, prior myocardial infarction, and heart failure) and medication use (aspirin, statin, angiotensin-converting enzyme inhibitor, and beta-blockers); and Model 3: adjusted for all variables in Model 2 plus sPAT as a continuous variable.
We also performed a mediation analysis with bootstrapping (1,000 bootstrap samples and a 95 percent confidence interval (CI)) to test the statistical effects of sPAT on the association of MSIMI with executive function (SPSS PROCESS macro version 2.16.3).29 This method uses an ordinary least squares or logistic regression-based path framework to estimate direct and indirect effects and produces CIs from bias-corrected bootstrap samples.29 All other statistical analyses were done using Stata 14.0 (StataCorp LP; College Station, T). We used Bonferroni corrections to account for multiple comparisons, with a two-sided p values at < 0.008 being used for significance testing.
Data availability
Anonymized data can be made available on request to the corresponding author.
Results
Between June 23, 2011 and August 5, 2014, 695 patients with coronary atherosclerosis were enrolled. From this initial cohort, measures of cognitive function were introduced after the enrolment had already begun and therefore were available on a subset of 487 individuals who were analyzed in this study. A total of 36 individuals died during the 2-year follow-up period. From the remaining 451 individuals, 289 patients (64%) returned for repeat cognitive testing.
Table 1 shows the characteristics of the study sample. Individuals with MSIMI had higher prevalence of CSIMI and lower sPAT indicating greater vasoconstriction compared to those without MSIMI. Scores for both Trail-A and Trail-B were higher for those with MSIMI compared to those without MSIMI, indicating worse cognitive performance in the attention and executive function domains, respectively (Table 1). There were no other significant differences in baseline demographics, clinical risk factors, medication use, and memory test performance between those with and without MSIMI.
Table 1.
Baseline characteristic of the study population, overall and according to presence of mental stress-induced ischemia.
| All Patients (N=486) | Without Mental Stress-Induced Ischemia (N=409) | With Mental Stress-Induced Ischemia (N=77) | P-value | |
|---|---|---|---|---|
| Demographics | ||||
| Mean age, years (SD) | 62 (9) | 62.6 (9.1) | 62.3 (9.3) | 0.79 |
| Male, N (%) | 350 (72.0) | 290 (70.9) | 60 (77.9) | 0.20 |
| African-American, N (%) | 156 (32.1) | 129 (31.5) | 27 (35.1) | 0.54 |
| Education, years (SD) | 15.1 (3) | 15.0 (3.2) | 15.1 (3.5) | 0.80 |
| Medical History, N (%) | ||||
| Dyslipidemia | 410 (84.4) | 344 (84.1) | 66 (85.7) | 0.72 |
| Current smoking | 72 (14.9) | 61 (15.0) | 11 (14.3) | 0.63 |
| Obesity | 394 (81.6) | 333 (82.0) | 61 (79.2) | 0.56 |
| Hypertension | 387 (79.6) | 325 (79.5) | 62 (80.5) | 0.83 |
| Diabetes mellitus | 156 (32.1) | 138 (33.7) | 18 (23.4) | 0.09 |
| History of heart failure | 66 (13.6) | 60 (14.7) | 6 (7.8) | 0.10 |
| History of myocardial infarction | 176 (36.2) | 143 (35.0) | 33 (42.9) | 0.18 |
| Physical stress-induced ischemia | 158 (32.8) | 103 (25.4) | 55 (72.4) | <0.001 |
| Mental stress related variables | ||||
| sPAT ratio, Mean (SD) | 0.73 (0.34) | 0.75 (0.35) | 0.65 (0.31) | 0.02 |
| Medications, N (%) | ||||
| Aspirin | 411 (84.7) | 342 (83.8) | 69 (89.6) | 0.19 |
| Beta-Blocker | 364 (75.1) | 302 (74.0) | 62 (80.5) | 0.22 |
| Statins | 418 (86.2) | 352 (86.3) | 66 (85.7) | 0.89 |
| Angiotensin-converting enzyme inhibitors | 239 (49.3) | 194 (47.5) | 45 (58.4) | 0.10 |
| Memory Function, mean (SD) | ||||
| Trail-A (seconds) | 43 (19) | 41 (17) | 46 (24) | 0.007 |
| Trail-B (seconds) | 101 (49) | 99 (46) | 111 (59) | 0.002 |
| Immediate Verbal Memory (score points) | 10.4 (3.3) | 10.5 (3.3) | 9.9 (3.1) | 0.55 |
| Delayed Verbal Memory (score points) | 7.4 (3.7) | 7.6 (3.8) | 6.9 (3.3) | 0.29 |
| Immediate Visual Reproduction (score points) | 8.8 (4.7) | 8.8 (4.7) | 8.1 (4.3) | 0.24 |
| Delayed Visual Reproduction (score points) | 8.3 (4.7) | 8.5 (4.8) | 7.7 (4.5) | 0.55 |
sPAT: peripheral arterial tonometry as stress/rest ratio with higher sPAT indicating greater vasoconstriction
Association between ischemia and cognitive function
As shown in Table 2, unadjusted analyses indicated that presence of MSIMI was positively associated with Trial-A and Trail-B scores (Model 1), indicating that those with MSIMI had worse cognitive function than those without MSIMI. These associations remained significant after adjusting for baseline demographics, clinical variables and medication use (Model 2). In this model, presence of MSIMI was associated with 21% and 20% higher (i.e., slower) Trail-A, and Trail-B scores, respectively (P<0.01 for both). However, further adjustment for sPAT attenuated this association for both Trial-A and Trail-B scores (Model 3). Similarly, MSIMI was positively associated with set shifting speed calculated as the difference in seconds between Trail-A and Trail-B scores. After adjusting for variables in Model 2, presence of MSIMI was associated with 16% higher scores in set shifting (B 0.16, 95% CI 0.06- 0.39). There were no significant associations between MSIMI and memory function tests (Table 2). In contrast to MSIMI, CSIMI was not significantly associated with any of the cognitive function measures (Table 2).
Table 2.
Association between ischemia (provoked by either mental stress or conventional stress) and memory performance tests at baseline
| Variables* | Trail Making tests | Verbal Memory | Visual Memory | |||
|---|---|---|---|---|---|---|
| Trail-A | Trail-B | Immediate | Delayed | Immediate | Delayed | |
| B (95% CI) | ||||||
| Mental Stress-Induced ischemia | ||||||
| Model 1 | 0.23 (0.09, 0.47)1 | 0.22 (0.02, 0.47) | −0.15 (−0.40, 0.08) | −0.17 (−0.42, 0.06) | −0.15 (−0.40, 0.08) | −0.16 (−0.40, 0.08) |
| Model 2 | 0.21 (0.02, 0.46) | 0.20 (0.01, 0.43) | −0.12 (−0.35, 0.11) | −0.14 (−0.37, 0.09) | −0.12 (−0.36, 0.10) | −0.13 (−0.37, 0.09) |
| Model 3 | 0.12 (−0.10, 0.26) | 0.16 (−0.08, 0.42) | −0.18 (−0.45, 0.07) | −0.17 (−0.43, 0.09) | −0.08 (−0.33, 0.16) | −0.09 (−0.34, 0.16) |
| Conventional Stress-Induced ischemia | ||||||
| Model 1 | 0.12 (−0.06, 0.32) | 0.02 (−0.14, 0.20) | −0.04 (−0.28, 0.19) | −0.08 (−0.27, 0.10) | −0.05 (−0.23, 0.13) | −0.04 (−0.23, 0.14) |
| Model 2 | 0.10 (−0.06, 0.28) | 0.01 (−0.17, 0.18) | −0.03 (−0.25, 0.15) | −0.08 (−0.26, 0.10) | −0.05 (−0.23, 0.12) | −0.05 (−0.24, 0.12) |
| Model 3 | 0.07 (−0.11, 0.27) | 0.01 (−0.16, 0.17) | −0.02 (−0.23, 0.17) | −0.05 (−0.25, 0.15) | −0.02 (−0.22, 0.19) | −0.05 (−0.25, 0.14) |
Memory performance tests modeled as standard deviation (SD) units.
Bold indicates P<0.008
Model 1 unadjusted; Model 2 adjusted for demographics (age, sex, race, and education), clinical variables (body mass index, hypertension, hyperlipidemia, diabetes mellitus, smoking history, prior myocardial infarction, and heart failure) and medication use (aspirin, statin, angiotensin-converting enzyme inhibitor, and beta-blockers); Model 3 adjusted for all variables in Model 2 plus sPAT
In order to understand the directionality of the association between ischemia and cognitive function, we examined whether either ischemia measures were associated with changes in cognitive function after 2 years of follow-up. Follow-up assessments of cognitive function were available in 289 participants. Patients with MSIMI at baseline were found to have worsening Trail-B scores at follow up (change in Trail-B, 6 seconds, P=0.02) while those with a negative mental stress-induced ischemia did not show a significant decline (change in Trail-B, 2 seconds, P=0.19). In a multivariable regression analysis adjusted for baseline demographics, clinical variables and medication use, presence of MSIMI was associated with 33% larger increase in Trail-B score, signifying a slower completion of Trail-B test over time (worse function),compared with absence of MSIMI (Table 3). There were no significant associations between MSIMI and changes in other memory performance measures. CSIMI was not associated with changes in any of the cognitive tests over time (Table 3).
Table 3.
Association between ischemia (provoked by either mental stress or conventional stress) and changes in memory performance tests during 2-year follow-up
| Variable | Mental Stress-Induced Ischemia | Conventional Stress-Induced Ischemia |
|---|---|---|
| B (95% CI) |
||
| Change in Trail A | 0.05 (−0.39, 0.29) | 0.15 (−0.09, 0.41) |
| Change in Trail B | 0.33 (0.04, 0.62) | 0.12 (−0.07, 0.38) |
| Change in Immediate Logical | −0.07 (−0.41, 0.23) | −0.02 (−0.27, 0.22) |
| Change in Delayed Logical | −0.06 (−0.39, 0.27) | −0.02 (−0.29, 0.25) |
| Change in Immediate Visual | −0.004 (−0.33, 0.34) | −0.01 (−0.26, 0.23) |
| Change in Delayed Visual | −0.11 (−0.48, 0.22) | −0.06 (−0.32, 0.18) |
B represents the magnitude of change (in SD units) over a 2-year period for each cognitive test in the presence of mental or conventional stress-induced ischemia, compared with absence of the respective type of ischemia.
Model adjusted for demographics (age, sex, race, and education), clinical variables (body mass index, hypertension, hyperlipidemia, diabetes mellitus, smoking history, prior myocardial infarction, and heart failure) and medication use (aspirin, statin, angiotensin-converting enzyme inhibitor, and beta-blockers)
Mediation Analysis
We performed mediation analysis to examine whether the pathway linking MSIMI to worse Trail-B scores after 2 years of follow-up was mediated by lower sPAT (greater vasoconstriction). As shown in Figure 1, sPAT significantly mediated the association of MSIMI with changes in Trail-B scores by 18.2%.
Figure 1. Mediation analysis linking mental stress-induced myocardial ischemia and worse executive function over time through peripheral vasoconstriction.
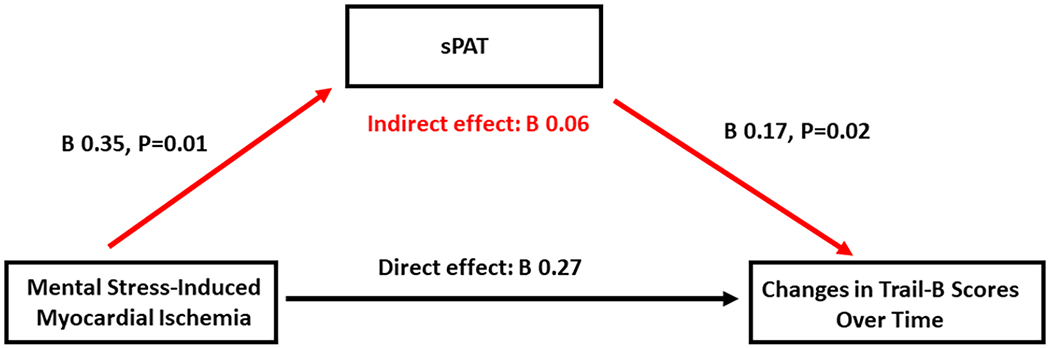
Total and indirect effects of sPAT on the association between mental stress-induced ischemia and worsening executive function. Indirect effect= 0.35*0.17. This pathway accounted for 18.2% of the total effect (indirect effect/(indirect effect + direct effect) x100).
Model adjusted for demographics (age, sex, race, and education), clinical variables (body mass index, hypertension, hyperlipidemia, diabetes mellitus, smoking history, prior myocardial infarction, and heart failure) and medication use (aspirin, statin, angiotensin-converting enzyme inhibitor, and beta-blockers).
Discussion
In a cohort of individuals with coronary atherosclerosis, we showed that MSIMI, but not CSIMI, is associated with worse cognitive performance in the domains of attention and executive functioning. These associations remained significant after adjusting for baseline demographics, clinical variables and medication use. Moreover, we found that MSIMI is also associated with a more rapid decline in executive function over time. Finally, mediation analysis showed that at least part of this association is mediated by higher peripheral vasoconstriction during mental stress. These findings suggest that among individuals with coronary atherosclerosis, those who exhibit MSIMI are at a higher risk of cognitive impairment and, in particular, lower executive function, and that sympathetically-mediated vasoconstriction during stress plays a role.
Our results showed that at baseline, individuals with MSIMI had worse executive function compared to those without MSIMI. These differences were indicated by a 12% slower completion of the Trail-B tests in those with MSIMI compared to those without MSIMI, and were independent of baseline demographics and cardiovascular risk factors. These results suggest that patients with coronary atherosclerosis who develop myocardial ischemia with a mental stress challenges are also vulnerable to impairments in the executive function domain.
In addition to the differences in baseline executive function, the group with MSIMI also showed a more pronounced decline in executive function over time than the group without MSIMI. During a 2-year follow-up period, those with MSIMI showed 33% slower completion of the Trail-B scores compared to those without MSIMI. These findings suggest that individuals with MSIMI are also at higher risk of worsening executive function over time.
In our study, MSIMI was only associated with worse executive functioning, and no significant associations were found between MSIMI and other cognitive domains including visual and verbal memory. Our findings are in line with previous reports showing that among cognitive domains, executive function is most affected in those with coronary atherosclerosis.2–8 Structural brain studies have shown that white matter microstructural integrity is positively associated with executive functioning, but not with other domains of cognitive function,in those with coronary atherosclerosis.30 These findings emphasize the need to develop strategies to prevent cognitive impairment, especially in the executive functioning domain, in this vulnerable population.
Our results demonstrated that those with MSIMI had higher peripheral vasoconstriction (lower sPAT) during mental stress compared to those without MSIMI. This is similar to previous reports that have shown sPAT to be an important predictor of MSIMI.17–20 Moreover, our mediation analyses revealed that sPAT partly explained the association between MSIMI and worsening of executive function. These findings is in line with our previous study showing that sPAT is associated with mental stress-induced brain activity changes in areas of the brain that are involved in executive functioning such as the inferior frontal lobe, medial prefrontal cortex (anterior cingulate) and cuneus.31 These findings suggest that vasoconstriction could play a role in the link between MSIMI and worsening cognitive function.
We did not find any associations between CSIMI and impairments in any cognitive domains. Follow-up cognitive studies also did not reveal any associations between CSIMI and cognitive decline over time. These findings could be attributed to the differences in pathophysiological mechanisms between MSIMI and CSIMI.14 Conventional stress testing is accompanied by dilatation of both the epicardial coronary arteries and the peripheral vasculature.32, 33 In the presence of coronary stenosis, the demand for vasodilation of post-stenotic segments at rest is increased to maintain flow.33 This reduces the vasodilatory capacity during exercise, predisposing the post-stenotic coronary vessels to ischemia during conventional stress testing.34 In contrast to conventional stress testing, mental stress increases both coronary and peripheral vasculature resistance. These findings further support the notion that the sympathetically mediated vasoconstriction with mental stress is a key factor in cognitive decline among individuals with coronary atherosclerosis.
The major strengths of our study included the prospective design and the inclusion of a large cohort of individuals with stable coronary atherosclerosis who underwent both mental and conventional stress testing, as well as cognitive and vascular reactivity measurements. A limitation included the relatively short duration of follow-up which may not have been sufficient to observe cognitive decline in domains other than executive function. However, we were still able to show an association between MSIMI and worsening executive function. Also, we did not include a control group free of coronary atherosclerosis in this study. While MSIMI is only relevant to individuals with a diagnosis of coronary artery disease, it would be important to understand if similar vasoconstrictive responses to mental stress in absence of coronary atherosclerosis could help identify those at risk of cognitive impairment. Follow-up cognitive measures were only available for a subgroup of individuals. This may have introduced selection bias, as sicker individuals with worse cognitive performance may have been less likely to participate in the repeat in-person interview. However, it is likely that this loss of potentially more impaired individuals would have biased the study estimates towards the null. Another limitation is that, while we measured peripheral vasoconstriction as an indication of the intensity of sympathetic response to stress, peripheral vasoconstriction has multifactorial origins and may not solely be a representation of heightened adrenergic responses to acute mental stress. Furthermore, although our measure of acute stress in the laboratory is a strength, we did not have measures of chronic stress. However, the implication is that acute stress responses may repeat during everyday life and thus, in addition to acute effects, mental stress testing may index potential cumulative, chronic effects. Finally, the association between MSIMI and cognitive impairment could be a manifestation of systemic atherosclerosis affecting both cardiac and brain vasculature. However, the lack of an association between CSIMI and cognitive impairment suggests that systemic atherosclerosis may not have a prominent role in this association.
In conclusion, our study demonstrated that myocardial ischemia induced by mental stress, but not by conventional stress testing, is associated, both cross-sectionally and longitudinally, with worse cognitive performance primarily in the executive function domain. This association was partly explained by a greater peripheral vasoconstrictive response during mental stress. These findings suggest that stress-induced vascular responses play a role in the pathogenesis of cognitive decline in patients with coronary atherosclerosis.
Highlights.
Mental stress-induced myocardial ischemia is associated with slower visuomotor processing.
Mental stress-induced myocardial ischemia is associated with worse executive function
This association exists both at baseline and during follow-up.
This association is partly explained by greater peripheral vasoconstriction.
Acknowledgments
Funding:
This work was supported by grants P01 HL101398, R01 HL109413, R01HL109413-02S1, R01HL125246, K24HL077506, K24 MH076955, UL1TR000454, KL2TR000455, K23HL127251, K12HD085850, L30HL148912 and T32HL130025 from the National Institutes of Health.
Appendix 1.
| Name | Location | Contribution |
|---|---|---|
| Kasra Moazzami | Emory University, Atlanta | Design and conceptualized study; analyzed the data; drafted the manuscript for intellectual content |
| Samaah Sullivan | Emory University, Atlanta | Interpreted the data; revised the manuscript for intellectual content |
| Bruno B. Lima | Emory University, Atlanta | Interpreted the data; revised the manuscript for intellectual content |
| Jeong Hwan Kim | Emory University, Atlanta | Interpreted the data; revised the manuscript for intellectual content |
| Muhammad Hammadah | Emory University, Atlanta | Interpreted the data; revised the manuscript for intellectual content |
| Zakaria Almuwaqqat | Emory University, Atlanta | Interpreted the data; revised the manuscript for intellectual content |
| Amit J. Shah | Emory University, Atlanta | Interpreted the data; revised the manuscript for intellectual content |
| Ihab Hajjar | Emory University, Atlanta | Interpreted the data; revised the manuscript for intellectual content |
| Felicia C. Goldstein | Emory University, Atlanta | Interpreted the data; revised the manuscript for intellectual content |
| Allan I. Levey | Emory University, Atlanta | Interpreted the data; revised the manuscript for intellectual content |
| J. Douglas Bremner | Emory University, Atlanta | Interpreted the data; revised the manuscript for intellectual content |
| Arshed A. Quyyumi | Emory University, Atlanta | Interpreted the data; revised the manuscript for intellectual content |
| Viola Vaccarino | Emory University, Atlanta | Design and conceptualized study |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure Statement: None
References
- 1.Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 2.Stefanidis KB, Askew CD, Greaves K, Summers MJ. The Effect of Non-Stroke Cardiovascular Disease States on Risk for Cognitive Decline and Dementia: A Systematic and Meta-Analytic Review. Neuropsychol Rev 2018;28:1–15. [DOI] [PubMed] [Google Scholar]
- 3.Schievink SHJ, van Boxtel MPJ, Deckers K, van Oostenbrugge RJ, Verhey FRJ, Kohler S. Cognitive changes in prevalent and incident cardiovascular disease: a 12-year follow-up in the Maastricht Aging Study (MAAS). Eur Heart J 2017. [DOI] [PubMed] [Google Scholar]
- 4.Singh-Manoux A, Britton AR, Marmot M. Vascular disease and cognitive function: evidence from the Whitehall II Study. J Am Geriatr Soc 2003;51:1445–1450. [DOI] [PubMed] [Google Scholar]
- 5.Singh-Manoux A, Sabia S, Lajnef M, et al. History of coronary heart disease and cognitive performance in midlife: the Whitehall II study. Eur Heart J 2008;29:2100–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie W, Zheng F, Yan L, Zhong B. Cognitive Decline Before and After Incident Coronary Events. J Am Coll Cardiol 2019;73:3041–3050. [DOI] [PubMed] [Google Scholar]
- 7.Zheng L, Mack WJ, Chui HC, et al. Coronary artery disease is associated with cognitive decline independent of changes on magnetic resonance imaging in cognitively normal elderly adults. J Am Geriatr Soc 2012;60:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eggermont LH, de Boer K, Muller M, Jaschke AC, Kamp O, Scherder EJ. Cardiac disease and cognitive impairment: a systematic review. Heart 2012;98:1334–1340. [DOI] [PubMed] [Google Scholar]
- 9.Stilley CS, Bender CM, Dunbar-Jacob J, Sereika S, Ryan CM. The impact of cognitive function on medication management: three studies. Health Psychol 2010;29:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naik AD, Dyer CB, Kunik ME, McCullough LB. Patient autonomy for the management of chronic conditions: a two-component re-conceptualization. Am J Bioeth 2009;9:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hidalgo V, Pulopulos MM, Salvador A. Acute psychosocial stress effects on memory performance: Relevance of age and sex. Neurobiol Learn Mem 2019;157:48–60. [DOI] [PubMed] [Google Scholar]
- 12.Schoofs D, Wolf OT, Smeets T. Cold pressor stress impairs performance on working memory tasks requiring executive functions in healthy young men. Behav Neurosci 2009;123:1066–1075. [DOI] [PubMed] [Google Scholar]
- 13.Wei J, Rooks C, Ramadan R, et al. Meta-analysis of mental stress-induced myocardial ischemia and subsequent cardiac events in patients with coronary artery disease. Am J Cardiol 2014;114:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arri SS, Ryan M, Redwood SR, Marber MS. Mental stress-induced myocardial ischaemia. Heart 2016;102:472–480. [DOI] [PubMed] [Google Scholar]
- 15.Bremner JD, Campanella C, Khan Z, et al. Brain Correlates of Mental Stress-Induced Myocardial Ischemia. Psychosom Med 2018;80:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaccarino V, Sullivan S, Hammadah M, et al. Mental Stress-Induced-Myocardial Ischemia in Young Patients With Recent Myocardial Infarction: Sex Differences and Mechanisms. Circulation 2018;137:794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goor DA, Sheffy J, Schnall RP, et al. Peripheral arterial tonometry: a diagnostic method for detection of myocardial ischemia induced during mental stress tests: a pilot study. Clin Cardiol 2004;27:137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassan M, York KM, Li H, et al. Usefulness of peripheral arterial tonometry in the detection of mental stress-induced myocardial ischemia. Clin Cardiol 2009;32:E1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammadah M, Alkhoder A, Al Mheid I, et al. Hemodynamic, catecholamine, vasomotor and vascular responses: Determinants of myocardial ischemia during mental stress. Int J Cardiol 2017;243:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JH, Almuwaqqat Z, Hammadah M, et al. Peripheral Vasoconstriction During Mental Stress and Adverse Cardiovascular Outcomes in Patients With Coronary Artery Disease. Circ Res 2019;125:874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramadan R, Sheps D, Esteves F, et al. Myocardial ischemia during mental stress: role of coronary artery disease burden and vasomotion. J Am Heart Assoc 2013;2:e000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammadah M, Kim JH, Al Mheid I, et al. Coronary and Peripheral Vasomotor Responses to Mental Stress. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammadah M, Al Mheid I, Wilmot K, et al. The Mental Stress Ischemia Prognosis Study: Objectives, Study Design, and Prevalence of Inducible Ischemia. Psychosom Med 2017;79:311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheps DS, McMahon RP, Becker L, et al. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: Results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation 2002;105:1780–1784. [DOI] [PubMed] [Google Scholar]
- 25.Filskov SB, Goldstein SG. Diagnostic validity of the Halstead-Reitan neuropsychological battery. J Consult Clin Psychol 1974;42:382–388. [DOI] [PubMed] [Google Scholar]
- 26.Russell EW. A multiple scoring method for the assessment of complex memory functions. Journal of Consulting and Clinical Psychology 1975;43:800–809. [Google Scholar]
- 27.Garcia EV, Faber TL, Cooke CD, Folks RD, Chen J, Santana C. The increasing role of quantification in clinical nuclear cardiology: the Emory approach. J Nucl Cardiol 2007;14:420–432. [DOI] [PubMed] [Google Scholar]
- 28.Holly TA, Abbott BG, Al-Mallah M, et al. Single photon-emission computed tomography. J Nucl Cardiol 2010;17:941–973. [DOI] [PubMed] [Google Scholar]
- 29.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 2008;40:879–891. [DOI] [PubMed] [Google Scholar]
- 30.Santiago C, Herrmann N, Swardfager W, et al. White Matter Microstructural Integrity Is Associated with Executive Function and Processing Speed in Older Adults with Coronary Artery Disease. Am J Geriatr Psychiatry 2015;23:754–763. [DOI] [PubMed] [Google Scholar]
- 31.Shah A, Chen C, Campanella C, et al. Brain correlates of stress-induced peripheral vasoconstriction in patients with cardiovascular disease. Psychophysiology 2019;56:e13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev 2008;88:1009–1086. [DOI] [PubMed] [Google Scholar]
- 33.Bache RJ, Cobb FR. Effect of maximal coronary vasodilation on transmural myocardial perfusion during tachycardia in the awake dog. Circ Res 1977;41:648–653. [DOI] [PubMed] [Google Scholar]
- 34.Spaan JA. Mechanical determinants of myocardial perfusion. Basic Res Cardiol 1995;90:89–102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data can be made available on request to the corresponding author.


