Abstract
Deep medicine is rapidly moving towards a high-definition approach for therapeutic management of the patient as an individual given the rapid progress of genome sequencing technologies and machine learning algorithms. While considered a monogenic disease, alpha-1 antitrypsin (AAT) deficiency (AATD) patients present with complex and variable phenotypes we refer to as the “hallmarks of AATD” that involve distinct molecular mechanisms in the liver, plasma and lung tissues, likely due to both coding and non-coding variation as well as genetic and environmental modifiers in different individuals. Herein, we briefly review the current therapeutic strategies for the management of AATD. To embrace genetic diversity in the management of AATD, we provide an overview of the disease phenotypes of AATD patients harboring different AAT variants. Linking genotypic diversity to phenotypic diversity illustrates the potential for sequence-specific regions of AAT protein fold design to play very different roles during nascent synthesis in the liver and/or function in post-liver plasma and lung environments. We illustrate how to manage diversity with recently developed machine learning (ML) approaches that bridge sequence-to-function-to-structure knowledge gaps based on the principle of spatial covariance (SCV). SCV relationships provide a deep understanding of the genotype to phenotype transformation initiated by AAT variation in the population to address the role of genetic and environmental modifiers in the individual. Embracing the complexity of AATD in the population is critical for risk management and therapeutic intervention to generate a high definition medicine approach for the patient.
Keywords: alpha-1 antitrypsin deficiency, phenotypes, genotypes, deep medicine, spatial covariance
Introduction
Recent advances in genome sequencing technology is transforming our understanding and application of genomics to monogenic rare disease, for example, developing molecular therapeutics that target specific genetic variants in cystic fibrosis (CF) and Niemann-Pick C1 (NPC1),1-5 as well as to complex polygenic diseases6,7 such as risk assessment for chronic obstructive pulmonary disease (COPD).8 In addition to genomics, the development of techniques to monitor other personal omics, for example, epigenomics,5,9,10 proteomics,11,12 metabolomics,13 microbiomics,14 environmental exposomics15 and human activity tracking,16 as well as rapid progress of machine learning (ML) analytical tools,1,17,18 is already advancing deep medicine19 at a high-definition20 and high-performance level.21 Here, the patient is viewed as an individual in a population rather than being treated as an average of the population as occurs in conventional medicine.
Alpha-1 antitrypsin deficiency (AATD) is a monogenic familiar disease that is driven by basic and clinical hallmarks (Figure 1), each of which is uniquely influenced by genetic diversity in the population. Each of these hallmarks illustrate the complexity of disease associated with each patient in terms of genetics, tissue pathology (liver, plasma, lung), time of onset, progression and the environment. AATD is caused by genetic variants (alleles) in the SERPINA1 gene which has been identified in all ethnic groups worldwide with a frequency of 1 in every 2500 whites of European descent.22 Thought of as a monogenetic disease, AATD patients present highly variable phenotypes at different time frames with unique mechanisms that involve both gain and loss of function in different tissues (Figure 1). Alpha-1 antitrypsin (AAT) is synthesized in the endoplasmic reticulum (ER) and secreted from the liver through the exocytic pathway in large quantities on a daily basis to maintain a plasma concentration at 1-2 g/L.23,24 A well-established biological function of AAT is its antiprotease activity within the lung that prevents tissue degradation by neutrophil elastase (NE) and is currently the most established genome-wide association study (GWAS) modifier leading to COPD.25 More recently, AAT has been shown to have anti-inflammatory and immunomodulatory functions independent of the antiprotease activity that may contribute to AATD phenotypes.26-29
Approximately 95% of severe AATD cases are caused by the Z allele22,30 where the glutamic acid residue is mutated to lysine residue at position 366 in the AAT polypeptide (sequence numbering including the signal polypeptide sequence). The Z variant leads to misfolding and polymerization of AAT in the ER of hepatocytes which can trigger hallmarks of liver disease phenotypes including chronic hepatitis, cirrhosis and hepatocellular carcinoma31 (Figure 1). Liver disease phenotypes are very heterogeneous in presentation with only about 10%-15% of infants with homozygous Z variant (PiZZ) developing clinically relevant liver disease,32-34 although all homozygotes have some degree of accumulation of misfolded Z protein in the liver35 upon aging and progression of the disease (Figure 1). Epigenetic mechanisms including DNA methylation10 as well as polymorphisms in ER mannosidase 1 gene (MAN1B1)36 impacting glycosylation patterns, contribute to AATD liver phenotypes in subgroup cohorts37 (Figure 1). There is also evidence that inherited traits influencing intracellular proteolysis mechanisms play a role in liver disease susceptibility. Due to the accumulation and degradation of Z-AAT in the ER of hepatocytes, a key hallmark of the disease is that only 10% to 15% of Z-AAT is secreted into the circulation (Figure 1). The loss of AAT function disrupts protease-antiprotease balance in the serum and lung, triggering emphysema and COPD34 (Figure 1). Environmental factors, including cigarette smoke38 and air pollution39,40 contribute to the lung disease phenotype (Figure 1). Moreover, genetic modifiers including, for example, variants in matrix metalloproteinase 1 (MMP1),41 tumor necrosis factor,42 interleukin 10 (IL10),43 iron regulatory binding protein 2 (IREB2) and cholinergic nicotine receptor alpha3 (CHRNA3)44 impact the lung disease phenotypes in AATD (Figure 1). The heterogeneity and complexity of AATD presentation in the clinic indicates the importance of developing a high-definition, deep medicine20 approach for each individual patient based on their genome sequence (Figure 1).
Herein, we briefly review the current therapeutic approaches for AATD. Not surprisingly, all efforts to date nearly exclusively focus on restoration of function of the dominant Z-variant polymerization. Thus, current approaches illustrate potential avenues for intervention for a single variant but fall short of the real problem– diversity in the population which makes it patient-unique. To address this problem, we highlight genotypic diversity of AAT variants and the impact of variation on the hallmarks of AATD (Figure 1) driving clinical presentation. Finally, we discuss the importance of understanding the genotype to phenotype transformation from the perspective of a new paradigm based on the concept of spatial covariance (SCV) in biology to individualize treatment of each patient from a genotype first perspective. In essence, we embrace the concept of “leave no patient behind,” a current goal of the Cystic Fibrosis Foundation,2 as the operational paradigm for AATD treatment.
Current Generic Therapeutic Approaches for Z-Variant Alpha-1 Antitrypsin Deficiency
The vast majority of AATD therapeutic efforts target the dominant Z-variant allele. Here, the patient is treated as an average of the disease etiology of the Z-variant population based on either a reduced level of AAT in plasma and/or progression to endpoint liver/lung damage.
Enzyme Replacement
The current standard of care is based on the principle of enzyme replacement. Here, a quantitative increase in the serum pool of full-length AAT is mediated by intravenous infusion of plasma derived AAT (60mg/kg/wk), elevating transiently serum AAT to 50% of normal.45 Treatment with 120 mg/kg/wk in a recent clinical trial has been shown to approximate a low-normal serum level of AAT with an associated increased antiprotease activity, decreased elastin degradation, and reduced airway inflammation.46 A problem with this approach is that it fails to prevent the inherent accumulation and toxic effects of the expressed Z-variant in the patient.
Chemical and Biochemical Approaches
A number of small-molecule chemical and biochemical approaches are under development for AATD therapy with the goal of fixing the protein misfolding problem in the ER. These efforts largely focus on the aberrant polymerization events that leads to liver pathology and markedly reduces protein secretion into the plasma leading to reduced function in the lung.
One small-molecule chemical approach that addresses Z-variant aggregation in the liver makes use of the Food and Drug Administration (FDA)-approved drug carbamazepine (CBZ) that enhances autophagic pathways thereby reducing Z-AAT polymer intracellular accumulation in multiple cell and mouse models of the disease.47 CBZ is currently in phase 2 clinical trials for individuals with severe liver disease (NCT01379469). 30 Furthermore, the bile compound nor-ursodeoxycholic acid has been shown to clear > 70% of intrahepatic Z-AAT and reduced hepatocellular death through autophagy mechanism, suggesting a novel therapeutic approach for the treatment of liver disease in AATD.48,49
An alternative approach to reduce aggregation involves the FDA-approved drug rapamycin, an mTOR inhibitor, that has been demonstrated to reduce the accumulation of hepatic polymerized Z-AAT and hepatocellular injury in a Z-AAT transgenic mice model.50 Moreover, the generic proteostasis51-53 drug trimethylamine N-oxide (TAMO) has been shown to protect Z-AAT protein from heat-induced polymerization in vitro, although it has no effect on Z-AAT protein secretion or protein re-folding in cell models.54 In contrast, beside inhibiting Z-AAT polymerization and enhancing polymerized Z-AAT degradation, 4-phenylbutyric acid (PBA), as a proteostasis effector of unknown function, has been shown to promote Z-AAT protein secretion and function in both cell model and mouse models.55 However, in 2 small human phase 2 trials in AATD patients, PBA had either no significant change on serum AAT levels, or led to a small increased serum AAT level in 11 PiZZ AATD patients.45
While the above compounds are thought to directly impact proteostasis pathways, histone deacetylase inhibitors (HDACi), including suberoylanilide hydroxamic acid (SAHA), have been shown to increase Z-AAT protein secretion from epithelial cell lines to 50% of wild-type (WT) levels.56 The exact mechanism remains unknown but could reflect epigenetic mechanisms (histone acetylation) leading to changes in the gene expression profile impacting protein folding programs and AAT stability in the ER, or post-translational mechanisms reflecting acetylation/deacetylation of AAT or membrane trafficking pathways facilitating AAT delivery in the cell.56
An alternative chemical strategy is to prevent AAT polymerization using small molecules57,58 and peptides.59,60 AAT polymerization blocking molecules have been developed through virtual ligand screening against the hydrophobic cavity which is formed by s2A β-sheet, helix D and helix E in the AAT structure.57,58 A number of reagents have been identified as potential binders that block Z-AAT polymerization in vitro and in the cell leading to improvement of liver pathology, although delivery of these reagents to intracellular compartments where the problem is initiated remains a major challenge.30,58,61
In contrast to small-molecule chemical approaches impacting folding and stability of the Z variant, a biochemical approach involving the AAT-specific monoclonal antibody 4B12, which interacts with AAT protein by binding a conformation sensitive epitope involving residues Glu32, Glu39, His 43 and Leu 306, has been shown to block AAT polymerization in vitro, providing a potential approach to reduce the aggregation prone phenotype of Z-AAT.62 A single chain-variable-fragment intrabody has been shown to rescue Z-AAT function by both blocking intracellular Z-AAT polymerization and increasing functional Z-AAT protein secretion in cell and animal models, although the physiological mechanism remains a mystery given that intracellular Z-AAT pools in the ER are unlikely to be targeted by exogenous delivery of these reagents. 63 However, these results provide evidence that monoclonal antibodies could be used as a therapeutic approach in treatment of AATD, possibly targeting Z-AAT that is secreted into plasma and in lung tissue to prevent further polymerization.64-66
While all small-molecule chemical- or biochemical-based approaches are preliminary, each of these results raise the hope for small-molecule efforts for Z-variant AATD therapy by targeting mechanistically principles of fold design responsible for stability, monomer-polymer ratio and/or restoration of activity in the liver.45
Genetic Therapy for Alpha-1 Antitrypsin Deficiency
An alternative strategy to the chemical- or biological-based interventions described above, are genetic therapy approaches. Here, the goal is to alter the genotype of the liver (or lung) to replace or convert the variant to a WT genotype in order to directly prevent aggregation disease in the liver and/or provide the lung with endogenously produced AAT to prevent destruction of the lung by NE and related protease activities, thereby reducing the impact of disease hallmarks (Figure 1).
In the past decade, a number of delivery vectors have been developed to administer WT AAT to the liver including adenovirus (AVV), retrovirus (RTV) and adeno-associated virus (AAV) vectors.24,67 AVV gene transfer has been used to express human AAT protein in rat lung respiratory epithelium and hepatocytes.68,69 RTV has been used for expression of human WT-AAT mice fibroblasts and dog hepatocytes.70,71 Currently, AAV vectors are the most promising tool for gene delivery given their low toxicity, targeting specificity (liver, muscle, lung), and efficient long-term expression.72 Multiple serotypes have been used in preclinical AATD studies, whereas AAV1, AAV2 and AAVrh.10 have been used in clinical studies.73 To date, AAV1 is considered the vector of choice given sustained expression of AAT, although in phase 2 clinical trials only 2% of the needed therapeutic level was sustained over 5 years, suggesting considerable ground for improvement.74 A major drawback of these efforts is the inability to remove the toxic impact of the Z-variant allele in the patient.67
To relieve the burden of accumulated variant protein-triggering liver damage, another strategy is to reduce the level of misfolded protein in the ER. Small interfering RNA (siRNA) and microRNA have been developed to efficiently silent hepatocyte human Z-AAT (hZ-AAT) protein expression and serum Z-AAT protein level in hZ-AAT mice through delivery by recombinant adeno-associated virus delivery vectors.75 Moreover, antisense oligonucleotides targeting hAAT reduced Z variant plasma levels and reduced intracellular AAT protein accumulation in hZ-AAT transgene expression in mouse models. With long-term treatment, liver disease was reversed and a decrease in liver fibrosis was observed.76 A similar result was observed in human PiZZ transgene expressing mice treated by AAV8 delivering AAT specific shRNA. shRNA decreased 90% of the AAT mutant protein.77 Furthermore, siRNA targeting liver production of Z-AAT has been used in human clinical trials by 2 delivery vectors: ALN-AAT (NCT02503683) and ARC-AAT (NCT02363946) by Alnylam Pharmaceuticals and Arrowhead Research Corporation, respectively.78 Phase 1/2 clinical trials using ALN-AAT have been conducted using homozygous Z-AAT allele patients since 2015. Clinical data shows a nearly 88.9% reduction of circulating Z-AAT.77 In ARC-AAT clinical trials, the vector was tolerated and had sustained reduction of AAT (up to 90%) in a phase 1 trial.30 These studies demonstrate that RNA interference represents a potential therapeutic approach for AATD liver disease but is limited by failing to fix the primary problem–the need for a sufficient level of functional AAT delivery to the plasma to protect against lung disease.
An alternative strategy to disease is to apply gene editing approaches such as CRISPR/Cas9 and related rapidly evolving technologies.79 As a powerful tool for genome modification, gene editing systems have now been used to correct genome variants in multiple rare disease model systems.80-83 Editing vectors transfected into Z-AAT transgenic PiZZ mice provides a proof-of-principle for the approach where hZ-AAT variant expression was reduced, circulating hATT protein levels were increased, and liver fibrosis and protein aggregation reduced.84-86
Cell Therapy for Alpha-1 Antitrypsin Deficiency
While liver transplantation is the end-point strategy for treatment of liver disease, it has severe limitations given the need for donor tissue as well as the need for comprehensive immune suppression to prevent rejection. As an alternative approach to transplantation, AAT protein secretion has been observed by transplantation of hepatocytes from LacZ-transgenic ROSA26 mice into human Z-AAT transgenic mice. These results raised the possibility of hepatocyte transplantation as a therapeutic method for treatment of AATD in humans.87 One possibility is that liver cells differentiated from stem cells from a normal individual found to express normal level of WT-AAT protein88,89 could be used as a transplantation approach, although issues associated with immune rejection and toxic Z-variant load remain. Alternatively, human-induced pluripotent stem cells (hIPSCs) from the PiZZ patients could be differentiated into hepatocyte-like cells which express Z-AAT.90,91 hIPSC-Z-AAT expressing cells show all the hallmarks of disease-disrupted mitochondrial structure, oncogenic protein AKR1B10 expression, upregulated inflammatory genes and induction of the unfolded protein response pathways.92 hIPSC Z-AAT can be corrected by gene editing using a combination of zinc finger nucleases and piggyBac with restored function and AAT secretion. When edited hIPSCs were transplanted into a mouse model of liver injury (Alb-uPA+/+; Rag2-/-; Il2rg-/-) they were distributed throughout the liver leading to AAT secretion.93 Moreover, edited human mesenchymal stem cells generated from patient stem cells have recently been shown to improve liver pathology as reflected in reduced inflammatory response and decreased fibrosis and apoptotic death compared to hZ-AAT-expressing hepatocytes in a mouse model.94
Tackling Genotypic Diversity in Alpha-1 Antitrypsin Deficiency One Patient at a Time
While the Z-variant is the current focus of most therapeutic efforts, the complexity of AATD management is considerably amplified by the large number of variants now detected in the world-wide population through genotyping and genome sequencing efforts.95-99 In the genome aggregation database (gnomAD, v2.1.1),96 which includes the genome sequence information for 141,456 individuals, 601 variants in the SERPINA1 gene are reported. Among these reported variants, 277 are missense variants introducing amino acid residue changes in the polypeptide sequence with 116 being synonymous variants; 88 are in the intron region; 61 are in the untranslated region; 31 are splicing variants and the remaining 28 are deletion, truncation or frameshift. The top-3 most frequent variants are E400D (allele frequency [AF] = 27%), V237A (AF=22%) and R125H (AF=15.6%) which are historically referred to as M alleles (M3, M1 and M4 respectively) to indicate their WT-like activity. The S (AF=2.3%) and Z alleles (AF=1.1%) are fourth and fifth in allele frequency as missense variants, respectively.95 The S allele (E288V) leads to a milder deficiency of AAT in plasma and the heterozygous PiSZ has a lower risk of lung disease than PiZZ individuals.100 However, the clinical impact of the majority of rare variants is poorly understood or completely unknown. Thus, many alleles may be contributing in unknown ways to other features of disease etiology impacting the hallmarks of disease (Figure 1).
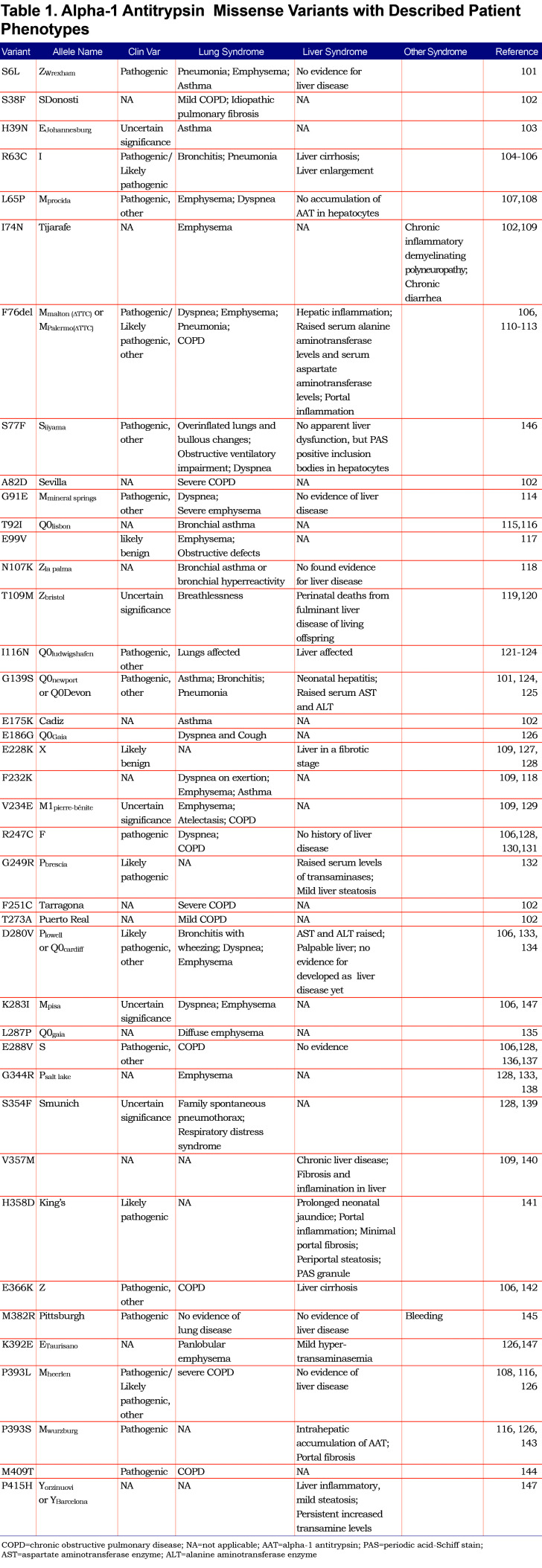
Besides the more common alleles listed above, we examined the distribution of 40 AAT missense variants found in the AATD population contributing to liver and/or lung phenotypes on the AAT structure, to illustrate the potential impact of genetic diversity AAT protein fold design (Table 1101-147; Figure 2, upper panel). Here, patients carrying the variants illustrated in red have been reported to show both liver and lung phenotypes. Patients carrying orange variants have been reported with only the liver-associated phenotype while patients with yellow variants have been reported with only the lung-associated phenotype, whereas variant M382R, which is indicated in light blue, has a unique bleeding phenotype likely impacting the proteases in the coagulation cascade.145,148-150 Variants found in the region of the reaction center loop adjacent to β-sheet A or in β-sheet A (Figure 2A, left panel, oval 1), such as E366K (Z), H358D and V357M, all have liver-associated phenotypes consistent with the known loop-sheet mechanisms leading to the polymerization of AAT in the liver that are predicted to impact AAT stability.61,151,152 Furthermore, deletion of F76 (F76del) and S77F at the back of β-sheet A (Figure 2A, left panel, oval 1) also shows liver disease phenotypes, consistent with the interpretation that they may disrupt the stability of β-sheet A leading to an increase of the polymerization propensity of AAT.146,153,154 Variants in β-sheets B and C (Figure 2A, left panel, oval 2) have more diverse phenotypes, for example, most of them are lung phenotype-associated variants, though some of variants also show liver-associated phenotypes. These variants are close to the ”gate” region which has been shown to allosterically mediate loop-sheet polymerization.147,152,155-158 Surprisingly, the variants localized to the bottom of the molecule (Figure 2A, left panel, oval 3) are exclusively associated with lung disease phenotype, indicating they do not cause aggregation in the liver but could affect secretion. Moreover, mapping these variants on AAT in complex with NE (Figure 2B) reveals that they are near the binding interface to NE, indicating these variants may even exclusively impact anti-protease (NE or other proteases) activity in the plasma or lung. It is possible that the many other variants have uncharacterized functions, for example, they may also contribute to ER-associated degradation promoting AAT deficiency, contribute to stability and/or translocation of AAT from the plasma to the lung, or modulate inflammation-related sensing in the lung.
Applying Deep Medicine to Individualizing Treatment in Alpha-1 Antitrypsin Deficiency
We now appreciate that the genome encodes key, evolutionary conserved information that reflects protein fold design principles that are largely unknown, yet define the functional requirements driving natural selection in response to a changing environment.1
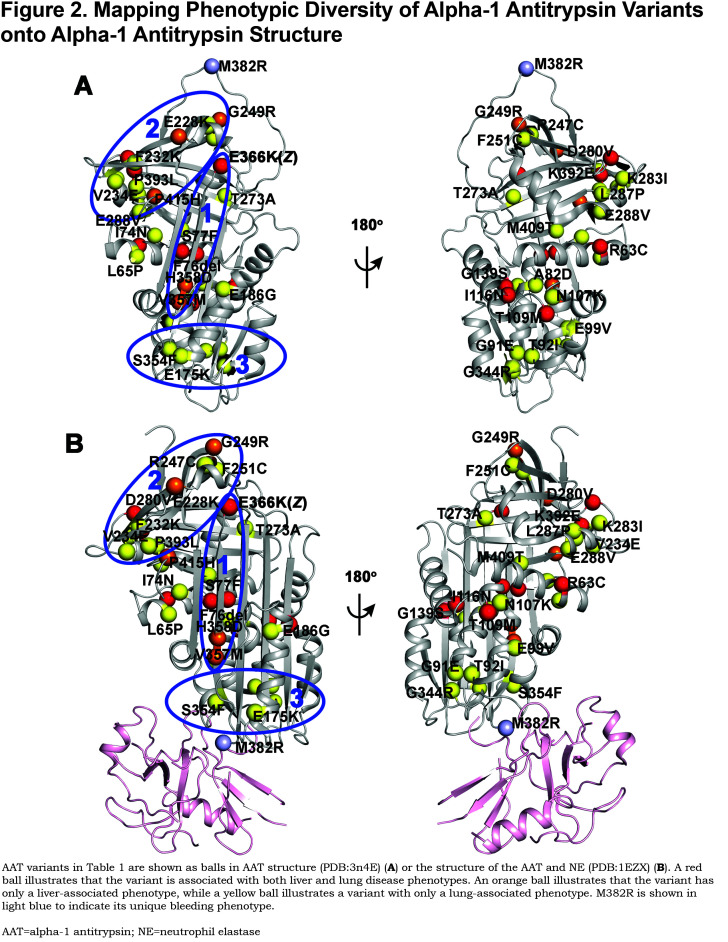
To appreciate how the genome encodes these protein fold design principles, the Balch laboratory has developed variation spatial profiling (VSP) based on the principle of SCV1 (Figure 3A). VSP is a Gaussian process regression (GPR)-based machine learning (GPR-ML)1 approach that re-describes central dogma in the context of SCV matrices that link sequence-to-function-to-structure.1 Here, SCV relationships are defined by a sparse collection of variants found in the extant human population for any protein (such as the 40 variants found in AAT) (Figure 2). We refer to these known variants as trusted or fiduciary reporters, as they record the evolving genomic rules driving protein fold design. Using these variants to train the hidden layers using GPR-ML (Figure 3A), we generate sequence-to-function descriptions of the entire protein fold, that we refer to as “phenotype landscapes”1 (Figure 3A and Figure 3B). Phenotype landscapes can be generated by using the sparse collection of variants from any protein to build SCV relationships based on their sequence position in the genome relative to functional features. For example, we have pioneered application of SCV using the rare disease protein cystic fibrosis transmembrane conductance regulator (CFTR). CFTR, like AAT, traffics through the secretory pathway. At the cell surface, instead of being secreted like AAT, it functions as a Cl- channel function (Figure 3A and Figure 3B)158 When defective in disease in response to over 600 variants in the population,3,159 patients present with markedly diverse clinical phenotypes, even for the same prominent F508del allele,2,3,160 These known relationships are then used to predict the function of all uncharacterized residues in the protein sequence design (Figure 3A and Figure 3B) that is displayed as a phenotype landscape and which can be mapped to protein structure at atomic resolution (detailed methods are explained in Wang et al1) (Figures Figure 3A and Figure 3C). Phenotype landscapes are very robust in design, allowing the construction of phenotype landscapes that measure and predict the protein sequence response on a residue-by-residue basis to a broad spectrum of basic and clinical functional features, and in response to the changing environment that drives onset and progression of human disease.1,161
As an example of the utility of SCV relationships in understanding therapeutic development and with the goal of understanding the impact of genetic diversity on management of AAT in the clinic, we generated phenotype landscapes based on 63 CFTR variants1 to reveal the function of all residues across the entire CFTR sequence, illustrating that some variants effect ER export, while others only impact chloride channel function at the cell surface (Figure 3B,left panel). Whereas the most common variant, F508del, prevents ER export, the rare CFTR variant G551D was the basis for the first FDA-approved personalized therapy for CF–the drug ivacaftor (Figure 3B and Figure 3C).159,162 G551D is a gating mutation that does not affect trafficking to the cell surface, as observed for some AAT variants that are efficiently secreted yet lack function (Figure 2). We have shown, using SCV relationships, that 63% of CFTR residues found in the disease population could be corrected by ivacaftor (Figure 3C).1 More recently, in combination with other compounds targeting different features of the CFTR folding trajectory based on our current knowledge of the genotype impact on CFTR function,1,3,159 a new triple combination of drugs improved the response of even the most severe F508del variant,160,163-165 indicating that nearly all variants may be differentially accessible to correction in the clinical setting based on a complete understanding of SCV relationships contributing to disease. A second example are the rare variants found in the NPC1 protein (600 patients world-wide) responsible for NPC1 disease which maintains cholesterol homeostasis in all cell types.166,167 Over 300 variants are now known to contribute to the differential onset and progression of cholesterol mismanagement leading to early onset neurodegenerative disease.4 Application of GRP-ML to NPC1 disease, using around 50 variants triggering disease, reveals that both proteostasis4 and epigenetic5 approaches can be used to correct cholesterol deficiencies of many but not all variants, revealing the power of SCV relationships in addressing the individualized nature of human disease where each patient in essence is unique and therefore requires a comprehensive understanding of disease phenotype.
Managing Alpha-1 Antitrypsin from a Spatial Covariance Perspective
AAT is a metastable protein and, like the CFTR transmembrane channel that goes through rapid open and closed cycles for chloride conduction, is a remarkable example of a conformation that has evolved to be sufficiently stable to support its synthesis in the ER of the liver through the exocytic pathway and secretion to plasma,151 while still maintaining its flexibility to perform in post-liver environments with its structural acrobatics that prevent the function of NE (or other proteases) in the lung in response to a large conformational change of molecule.152,161,168 This dynamic conformational change is also, from a genetic diversity perspective, the Achilles heel of the disease.
Given the above, it is now apparent that different AAT variants will need to be understood in the context of their basic fold design principles from both basic and clinical sequence-to-function perspectives that can be captured by profiling SCV relationships.4 For example, the plot of variants on the known structure of native and NE-bound AAT (Figure 2) already suggests that different variants and their projected SCV relationships defining the AAT fold will have functions that are likely independent of one another, not only given the physical spatial differences associated with their sequence position, but the contribution of a given variant for a particular known and/or unknown function in the complex environments of host (i.e., liver versus plasma versus lung). For example, the liver is involved in nascent synthesis and management of fold monomer solubility at high concentrations in the ER for efficient secretion that is differentially challenged by different variants, whereas the post-liver function in plasma and/or lung environments is critical for inhibitor activity driving disease presentation and progression in the clinic (Figure 2).
Thus, by selectively applying knowledge of genetic diversity found in the extant AATD population through building SCV relationships based on GPR-ML, it may become possible to tailor therapeutic development and management to specific alleles that are unique to an individual. Such a “genotype first” approach will allow us to specifically and differentially, mitigate the hallmarks of AATD in an individualized, deep medicine approach (Figure 1).
Summary and Perspective
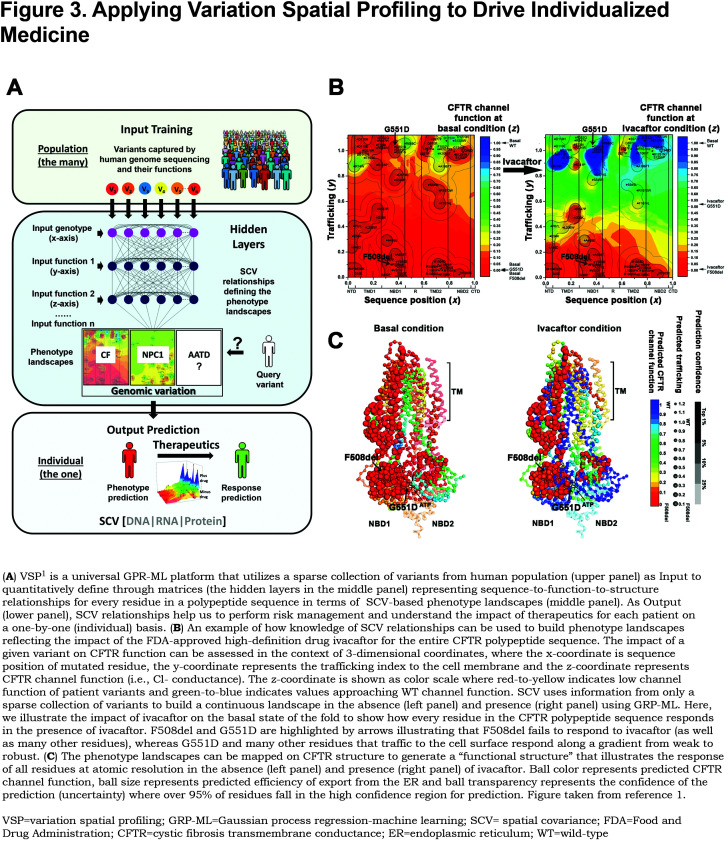
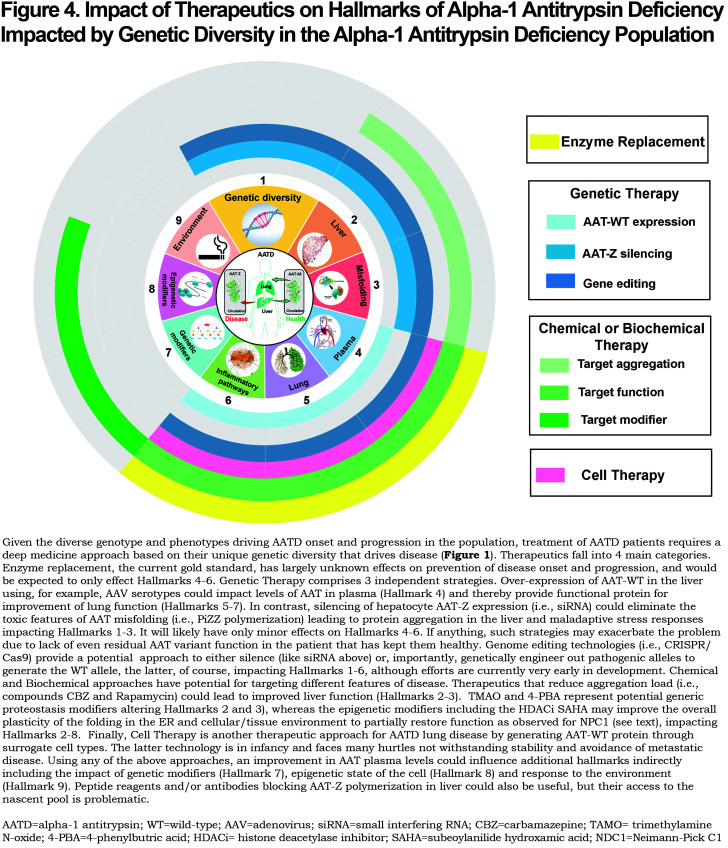
What is the best approach to manage AATD? Current approaches are illustrated in the context of hallmarks of AATD (Figure 1) and genetic diversity in the population with the genotype of the individual we posit being the ultimate gauge determining the basis for therapeutic management (Figure 4).19-21
Genetic approaches are currently at the forefront of personalized approaches (Figure 4). For example, silencing of variant expression through RNA interference technology is the farthest along in clinical trials for Z-variant liver disease with a strong likelihood of success in the near term.78 The limitations of this focused strategy include the lack of a means to restore expression of WT ATT. In contrast, gene editing, now possible through multiple approaches including CRISPR-Cas9 improved probes, still remains at its infancy for human use, particularly in terms of targeting efficiency and off-target effects that could exacerbate disease and/or trigger other unexpected pathologies. A key limitation of both of these technologies is not only expense but deciding when to initiate treatment, particularly as a pre-emptive strike before tissue damage in either the liver or lung is beyond repair. As an alternative to handling genetic diversity, patient-specific stem and iPSC cell supplementation or replacement strategies provide a very different and potentially powerful approach, but will certainly require considerable advances in current technologies given our poor understanding of their stability in the patient and malignancy potential, the nature of the tissue niche to which they need to be targeted and maintained, and proper management of the level of expression. Moreover, this approach lacks the ability to remove the toxic threat of the AAT variant driving disease, a concern inherent in current enzyme replacement approaches as well as the genetic therapies discussed above.
Small-molecule chemical/biochemical approaches also hold great promise to manage genetic diversity in the population that is unique for the individual (Figure 4), particularly if screened for effectiveness in the context of genetic diversity of the AATD population.1,4,5 However, they are generally limited by their associated dosing and safety issues in complex environments of the human, often reflecting an inability to target the drug to the site of interest. Ivacaftor treatment for G551D and the predicted 63% of CFTR residues that are likely to respond to the drug based on GRP-ML (Figure 3), as well as the more recent triple drug combination for management of CF,2,160,165 are pioneering examples of a personalized genotype specific strategy that targets different variants through their impact on the protein fold to minimize off-target effects. The Balch laboratory has proposed1,4,5 that by understanding how human evolution works to manage diversity leading to fitness in the population in response to function of the protein fold,1,4,5 we will be able to “pre-train” a high throughput screening drug development program based on genetic diversity to “pre-tune” the therapeutic approach in advance of clinical efforts.
Given the above, the first challenge we posit will be to lay the groundwork for precision disease management in the context of genetic diversity in AATD by understanding the impact of given variants in the context of the operation of the full-length fold, as reported by detailed natural history and clinical perspectives of the AATD patient population sequenced using whole-genome sequencing technologies (i.e., AllofUS).169 Additional detailed biochemical/functional characterization of AAT variants are essential to understand the comprehensive genotype to phenotype landscape of variant AAT in response to different tissue environments as we have shown for CFTR1 and NPC14,5 from genetic, epigenetic and therapeutic perspectives. We anticipate a major component of AATD treatment in the future will be a genotype first approach revealed by whole-genome sequencing efforts that help us to understand not only an individual’s AAT genotype but integrate that genotype with the many divergent basic and clinical features of disease reflected in the genetic diversity of the rest of the genome that make each one of us unique. These data will allow us to computationally formulate a common multi-dimensional framework to prioritize disease features that need to be corrected as well as their short- and long-term impact on the patient.
Put in another way, success will come from not only a deep understanding of the phenotype transformation driving disease in response to a patient’s unique AAT genotype, but will likely be influenced by multiple non-AAT alleles that contribute to the modifier environment that is, like AAT specific alleles, also unique to each individual harboring disease. Characterizing this complex interplay of patient specific genomics with their unique phenomics (Figure 4) in the context of environmental factors such as smoking, air quality and life-style will contribute to a more globalized understanding of each patient as an individual for personalized treatment. Digitizing AATD in the context of multi-dimensional layers of -omics matrices as performed using GRP-ML and rapidly advancing deep learning technologies18,20 we predict will bring a new era of personalized medicine to AATD patient health and disease management. These are not insurmountable goals given the rapid advances now beginning to drive the future of deep medicine,19one that embraces the need for active patient involvement in all aspects of therapeutic development, as the SCV strategy is about “this is me.”1
Abbreviations
alpha-1 antitrypsin, AAT; alpha-1 antitrypsin deficiency, AATD; machine learning, ML; spatial covariance, SCV; cystic fibrosis, CF; Neimann-Pick C1, NCP1; chronic obstructive pulmonary disease, COPD; endoplasmic reticulum, ER; neutrophil elastase, NE; genome-wide association study, GWAS; carbamazepine, CBZ; trimethylamine N-oxide, TAMO; 4-phenylbutric acid, PBA; histone deacetylase inhibitor, HDACi; suberoylanilide hydroxamic acid, SAHA; wild-type, WT; adenovirus, AVV; retrovirus, RTV; adeno-associated virus, AAV; small interfering RNA, siRNA; human Z-AAT, hZ-ATT; human-induced pluripotent stems cells, hIPSCs; human mesenchymal stem cells, hMSCs; allele frequency, AF; variation spatial profiling, VSP; Gaussian process regression, GPR; cystic fibrosis transmembrane conductance regulator, CFTR
Funding Statement
WEB is supported by a grant from the National Institutes of Heath (HL141810). JT is supported by the Alpha-1 Foundation. The meeting leading to this perspective was generously sponsored by the Alpha-1 Foundation.
References
- 1.Wang C,Balch WE. Bridging genomics to phenomics at atomic resolution through variation spatial profiling. Cell Rep. 2018;24(8):2013-2028. doi: https://doi.org/10.1016/j.celrep.2018.07.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell SC,Mall MA,Gutierrez H,et al. The future of cystic fibrosis care: a global perspective. Lancet Respir Med. 2019;8(1):65-124. doi: https://doi.org/10.1016/S2213-2600(19)30337-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCague AF,Ranaigh KS,Pellicore MJ,et al. Correlating cystic fibrosis transmembrane conductance regulator function with clinical features to inform precision treatment of cystic fibrosis. Am J Respir Crit Care Med. 2019;199(9):1116-1126. doi: https://doi.org/10.1164/rccm.201901-0145OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang C,Scott SM,Sun S,et al. Individualized management of genetic diversity in Niemann-pick c1 through modulation of the hsp70 chaperone system. Hum Mol Genet. 2019;29(1):1-19. doi: https://doi.org/10.1093/hmg/ddz215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C,Scott SM,Subramarian K,et al. Quantitating the epigenetic transformation contributing to cholesterol homeostasis using gaussian process. Nat Commun. 2019;10:5052. doi: https://doi.org/10.1038/s41467-019-12969-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young AI,Benonisdottir S,Przeworski M,Kong A. Deconstructing the sources of genotype-phenotype associations in humans. Science. 2019;365(6460):1396-1400. doi: https://doi.org/10.1126/science.aax3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeggini E,Gloyn AL,Barton AC,Wain LV. Translational genomics and precision medicine: Moving from the lab to the clinic. Science. 2019;365(6460):1409-1413. doi: https://doi.org/10.1126/science.aax4588 [DOI] [PubMed] [Google Scholar]
- 8.Shrine N,Guyatt AL,Erzrumlacglu M,et al. New genetic signals for lung function highlight pathways and chronic obstructive pulmonary disease associations across multiple ancestries. Nat Genet. 2019;51:481-493. doi: https://doi.org/10.1038/s41588-018-0321-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Bisceglie AM,Teckman J. Liver disease due to alpha-1 antitrypsin deficiency: are we surprised that it is more complex than we thought?. Hepatology. 2019;70(1):5-7. doi: https://doi.org/10.1002/hep.30694 [DOI] [PubMed] [Google Scholar]
- 10.Wang L,Marck GW,Hlady RA,et al. Alpha-1 antitrypsin deficiency liver disease, mutational homogeneity modulated by epigenetic heterogeneity with links to obesity. Hepatology. 2019;70(1):51-66. doi: https://doi.org/10.1002/hep.30526 [DOI] [PubMed] [Google Scholar]
- 11.Geyer PE,Kurlak NA,Pichler G,Holdt LM,Teupser D,Mann M,et al. Plasma proteome profiling to assess human health and disease. Cell Syst. 2016;2(3):185-195. doi: https://doi.org/10.1016/j.cels.2016.02.015 [DOI] [PubMed] [Google Scholar]
- 12.Pankow S,Bamberger C,Calzolari D,et al. F508 CFTR interactome remodelling promotes rescue of cystic fibrosis. Nature. 2015;528:510-516. doi: https://doi.org/10.1038/nature15729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diao W,Laabaki WW,Han MK,et al. Disruption of histidine and energy homeostasis in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2019;14:2015-2025. doi: https://doi.org/10.2147/COPD.S210598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Integrative HMP Research Network Consortium; The Integrative Human Microbiome Project. Nature. 2019;569:641-648. doi: https://doi.org/10.1038/s41586-019-1238-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang C,Wang X,Li X,et al. Dynamic human environmental exposome revealed by longitudinal personal monitoring. Cell. 2018;175(1):277-291. doi: https://doi.org/10.1016/j.cell.2018.08.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schussler-Fiorenza Rose SM,Contrepois K,Moneghetti KJ,et al. A longitudinal big data approach for precision health. Nat Med. 2019;25:792-804. doi: https://doi.org/10.1038/s41591-019-0414-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J,Li X,Zhang S,Snyder M. Gene-environment interaction in the era of precision medicine. Cell. 2019;177(1):38-44. doi: https://doi.org/10.1016/j.cell.2019.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou J,Huss M,Abid A,Mohammadi P,Torkamani A,Telenti A. A primer on deep learning in genomics. Nat Genet. 2019;51:12-18. doi: https://doi.org/10.1038/s41588-018-0295-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topol E. Deep Medicine: How Artificial Intelligence Can Make Healthcare Human Again. Basic Books, Inc;. 2019. [Google Scholar]
- 20.Torkamani A,Andersen KG,Steinhubl SR,Topol EJ. High-definition medicine. Cell. 2017;170(5):828-843. doi: https://doi.org/10.1016/j.cell.2017.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25:44-56. doi: https://doi.org/10.1038/s41591-018-0300-7 [DOI] [PubMed] [Google Scholar]
- 22.Blanco I,Bueno P,Diego I,et al. Alpha-1 antitrypsin Pi*Z gene frequency and Pi*ZZ genotype numbers worldwide: an update. Int J Chron Obstruct Pulmon Dis. 2017;12:561-569. doi: https://doi.org/10.2147/COPD.S125389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franciosi AN,Carroll TP,McElvaney NG. Pitfalls and caveats in alpha-1 antitrypsin deficiency testing: a guide for clinicians. Lancet Respir Med. 2019;7(12):1059-1067. doi: https://doi.org/10.1016/S2213-2600(19)30141-9 [DOI] [PubMed] [Google Scholar]
- 24.Patel D,Teckman JH. Alpha-1 antitrypsin deficiency liver disease. Clin Liver Dis. 2018;22(4):643-655. doi: https://doi.org/10.1016/j.cld.2018.06.010 [DOI] [PubMed] [Google Scholar]
- 25.Ragland MF,Benway CJ,Lutz SM,et al. Genetic advances in chronic obstructive pulmonary disease. Insights from COPDGene. Am J Respir Crit Care Med. 2019;200(6):677-690. doi: https://doi.org/10.1164/rccm.201808-1455SO [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonigk D,Al-Omari M,Maegel L,et al. Anti-inflammatory and immunomodulatory properties of alpha-1 antitrypsin without inhibition of elastase. Proc Natl Acad Sci U S A. 2013;110(37):15007-15012. doi: https://doi.org/10.1073/pnas.1309648110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai X,Bai A,Honda JR,et al. Alpha-1 antitrypsin enhances primary human macrophage immunity against non-tuberculous mycobacteria. Front Immunol. 2019;10:1417. doi: https://doi.org/10.3389/fimmu.2019.01417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wanner A. Alpha-1 antitrypsin as a therapeutic agent for conditions not associated with alpha-1 antitrypsin deficiency. In: Wanner A, Sandhous RA eds. Alpha-1 Antitrypsin: Role in Health and Disease Springer International Publishing. 2016:141-155. doi: https://doi.org/10.1007/978-3-319-23449-6_8 [Google Scholar]
- 29.Zhou T,Huang Z,Zhu X,et al. Alpha-1 antitrypsin attenuates m1 microglia-mediated neuroinflammation in retinal degeneration. Front Immunol. 2018;9:1202. doi: https://doi.org/10.3389/fimmu.2018.01202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lomas DA. New therapeutic targets for alpha-1 antitrypsin deficiency. Chronic Obstr Pulm Dis. 2018;5(4):233-243. doi: https://doi.org/10.15326/jcopdf.5.4.2017.0165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Serres FJ,Blanco I. Prevalence of alpha-1 antitrypsin deficiency alleles PI*S and PI*Z worldwide and effective screening for each of the five phenotypic classes PI*MS, PI*MZ, PI*SS, PI*SZ, and PI*ZZ: a comprehensive review. Ther Adv Respir Dis. 2012;6(5):277-295. doi: https://doi.org/10.1177/1753465812457113 [DOI] [PubMed] [Google Scholar]
- 32.Sveger T. Liver disease in alpha-1 antitrypsin deficiency detected by screening of 200,000 infants. N Engl J Med. 1976;294:1316-1321. doi: https://doi.org/10.1056/NEJM197606102942404 [DOI] [PubMed] [Google Scholar]
- 33.Townsend SA,Edgar RG,Ellis PR,Kantas D,Newsome PN,Turner AM. Systematic review: the natural history of alpha-1 antitrypsin deficiency, and associated liver disease. Aliment Pharmacol Ther. 2018;47(7):877-885. doi: https://doi.org/10.1111/apt.14537 [DOI] [PubMed] [Google Scholar]
- 34.Greene CM,Marciniak SJ,Teckman J,et al. Alpha-1 antitrypsin deficiency. Nat Rev Dis Primers. 2016;2:6051. doi: https://doi.org/10.1038/nrdp.2016.51 [DOI] [PubMed] [Google Scholar]
- 35.Hamesch K,Madorfer M,Pereira VM,et al. Liver fibrosis and metabolic alterations in adults with alpha-1 antitrypsin deficiency caused by the Pi*ZZ mutation. Gastroenterology. 2019;157(3):705-719. doi: https://doi.org/10.1053/j.gastro.2019.05.013 [DOI] [PubMed] [Google Scholar]
- 36.Pan S,Huang L,McPerhson J,et al. Single nucleotide polymorphism-mediated translational suppression of endoplasmic reticulum mannosidase I modifies the onset of end-stage liver disease in alpha1-antitrypsin deficiency. Hepatology. 2009;50(1):275-281. doi: https://doi.org/10.1002/hep.22974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chappell S,Guetta-Baranes T,Hadzic N,Stockley R,Kalsheker N. Polymorphism in the endoplasmic reticulum mannosidase I (MAN1B1) gene is not associated with liver disease in individuals homozygous for the Z variant of the alpha1-antitrypsin protease inhibitor (PiZZ individuals). Hepatology. 2009;50(4):1315-1316. doi: https://doi.org/10.1002/hep.23170 [DOI] [PubMed] [Google Scholar]
- 38.O'Brien ME,Pennycooke K,Carroll TP,et al. The impact of smoke exposure on the clinical phenotype of alpha-1 antitrypsin deficiency in Ireland: exploiting a national registry to understand a rare disease. COPD. 2015;12 (Suppl 1):2-9. doi: https://doi.org/10.3109/15412555.2015.1021913 [DOI] [PubMed] [Google Scholar]
- 39.Wood AM,Harrison RM,Semple S,Ayres JG,Stockley RA. Outdoor air pollution is associated with disease severity in alpha-1 antitrypsin deficiency. Eur Respir J. 2009;34(2):346-353. doi: https://doi.org/10.1183/09031936.00087908 [DOI] [PubMed] [Google Scholar]
- 40.Wood AM,Harrison RM,Semple S,Ayres JG,Stockley RA. Outdoor air pollution is associated with rapid decline of lung function in alpha-1 antitrypsin deficiency. Occup Environ Med. 2010;67(8):556-561. doi: https://doi.org/10.1136/oem.2009.047589 [DOI] [PubMed] [Google Scholar]
- 41.McAloon CJ,Wood AM,Gough SC,Stockley RA. Matrix metalloprotease polymorphisms are associated with gas transfer in alpha-1 antitrypsin deficiency. Ther Adv Respir Dis. 2009;3(1):23-30. doi: https://doi.org/10.1177/1753465809102263 [DOI] [PubMed] [Google Scholar]
- 42.Wood AM,Simmonds MJ,Bayley DL,Newby PR,Gough SC,Stockley RA. The TNF alpha gene relates to clinical phenotype in alpha-1 antitrypsin deficiency. Respir Res. 2008;9:52. doi: https://doi.org/10.1186/1465-9921-9-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demeo DL,Campbell EJ,Barker AF,et al. IL10 polymorphisms are associated with airflow obstruction in severe alpha1-antitrypsin deficiency. Am J Respir Cell Mol Biol. 2008;38(1):114-120. doi: https://doi.org/10.1165/rcmb.2007-0107OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim WJ,Wood AM,Barker AF,et al. Association of IREB2 and CHRNA3 polymorphisms with airflow obstruction in severe alpha-1 antitrypsin deficiency. Respir Res. 2012;13:16. doi: https://doi.org/10.1186/1465-9921-13-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pye A,Turner AM. Experimental and investigational drugs for the treatment of alpha-1 antitrypsin deficiency. Expert Opin Investig Drugs. 2019;28(10):1-12. doi: https://doi.org/10.1080/13543784.2019.1672656 [DOI] [PubMed] [Google Scholar]
- 46.Campos MA,Geraghty P,Holt G,et al. The biological effects of double-dose alpha-1 antitrypsin augmentation therapy. A pilot clinical trial. Am J Respir Crit Care Med. 2019;200(3):318-326. doi: https://doi.org/10.1164/rccm.201901-0010OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hidvegi T,Ewing M,Hale P,et al. An autophagy-enhancing drug promotes degradation of mutant alpha-1 antitrypsin Z and reduces hepatic fibrosis. Science. 2010;329(598):229-232. doi: https://doi.org/10.1126/science.1190354 [DOI] [PubMed] [Google Scholar]
- 48.Tang Y,Blomenkamp KS,Fickert P,Trauner M,Teckman JH. NorUDCA promotes degradation of alpha-1 antitrypsin mutant Z protein by inducing autophagy through AMPK/ULK1 pathway. PLoS One. 2018;13(8):e0200897. doi: https://doi.org/10.1371/journal.pone.0200897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang Y,Fickert P,Trauner M,Marcus N,BlomenKamp K,Teckman J. Autophagy induced by exogenous bile acids is therapeutic in a model of alpha-1-AT deficiency liver disease. Am J Physiol Gastrointest Liver Physiol. 2016;311(1):G156-165. doi: https://doi.org/10.1152/ajpgi.00143.2015 [DOI] [PubMed] [Google Scholar]
- 50.Kaushal S,Annamali M,Blomenkamp K,et al. Rapamycin reduces intrahepatic alpha-1 antitrypsin mutant Z protein polymers and liver injury in a mouse model. Exp Biol Med (Maywood). 2010;235(6):700-709. doi: https://doi.org/10.1258/ebm.2010.009297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balch WE,Morimoto RI,Dillin A,Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319(5865):916-919. doi: https://doi.org/10.1126/science.1141448 [DOI] [PubMed] [Google Scholar]
- 52.Hipp MS,Kasturi P,Hartl FU. The proteostasis network and its decline in ageing. Nat Rev Mol Cell Biol. 2019;20:421-435. doi: https://doi.org/10.1038/s41580-019-0101-y [DOI] [PubMed] [Google Scholar]
- 53.Sala AJ,Bott LC,Morimoto RI. Shaping proteostasis at the cellular, tissue, and organismal level. J Cell Biol. 2017;216(5):1231-1241. doi: https://doi.org/10.1083/jcb.201612111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Devlin GL,Parfrey H,Tew DJ,Lomas DA,Bottomley SP. Prevention of polymerization of M and Z alpha-1 antitrypsin (alpha-1-AT) with trimethylamine N-oxide. Implications for the treatment of alpha-1-AT deficiency. Am J Respir Cell Mol Biol. 2001;24(6):727-732. doi: https://doi.org/10.1165/ajrcmb.24.6.4407 [DOI] [PubMed] [Google Scholar]
- 55.Burrows JA,Willis LK,Perlmutter DH. Chemical chaperones mediate increased secretion of mutant alpha-1 antitrypsin (alpha-1 AT) Z: a potential pharmacological strategy for prevention of liver injury and emphysema in alpha-1 AT deficiency. Proc Natl Acad Sci U S A. 2000;97(4):1796-1801. doi: https://doi.org/10.1073/pnas.97.4.1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bouchecareilh M,Hutt DM,Szajner P,Flotte TR,Balch WE. Histone deacetylase inhibitor (HDACi) suberoylanilide hydroxamic acid (SAHA)-mediated correction of alpha-1 antitrypsin deficiency. J Biol Chem. 2012;287:38265-38278. doi: https://doi.org/10.1074/jbc.M112.404707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parfrey H,Mahadeva R,Ravenhill NA,et al. Targeting a surface cavity of alpha-1 antitrypsin to prevent conformational disease. J Biol Chem. 2003;278:33060-33066. doi: https://doi.org/10.1074/jbc.M302646200 [DOI] [PubMed] [Google Scholar]
- 58.Mallya M,Phillips RL,Saldanha SA,et al. Small molecules block the polymerization of Z alpha1-antitrypsin and increase the clearance of intracellular aggregates. J Med Chem. 2007;50(22):5357-5363. doi: https://doi.org/10.1021/jm070687z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mahadeva R,Dafforn TR,Carrell RW,Lomas DA. 6-mer peptide selectively anneals to a pathogenic serpin conformation and blocks polymerization. Implications for the prevention of Z alpha(1)-antitrypsin-related cirrhosis. J Biol Chem. 2002;277:6771-6774. doi: https://doi.org/10.1074/jbc.C100722200 [DOI] [PubMed] [Google Scholar]
- 60.Zhou A,Stein PE,Huntington JA,et al. How small peptides block and reverse serpin polymerisation. J Mol Biol. 2004;342(3):931-941. doi: https://doi.org/10.1016/j.jmb.2004.07.078 [DOI] [PubMed] [Google Scholar]
- 61.Gooptu B,Lomas DA. Conformational pathology of the serpins: themes, variations, and therapeutic strategies. Annu Rev Biochem. 2009;78:147-176. doi: https://doi.org/10.1146/annurev.biochem.78.082107.133320 [DOI] [PubMed] [Google Scholar]
- 62.Motamedi-Shad N,Jagger AM,Liedtke M,et al. An antibody that prevents serpin polymerisation acts by inducing a novel allosteric behaviour. Biochem J. 2016;473:3269-3290. doi: https://doi.org/10.1042/BCJ20160159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ordonez A,Perez J,Tan L,et al. A single-chain variable fragment intrabody prevents intracellular polymerization of Z alpha-1 antitrypsin while allowing its antiproteinase activity. FASEB J. 2015;29(6):2667-2678. doi: https://doi.org/10.1096/fj.14-267351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fra A,Cosmi F,Ordonez A,et al. Polymers of Z alpha-1 antitrypsin are secreted in cell models of disease. Eur Respir J. 2016;47(3):1005-1009. doi: https://doi.org/10.1183/13993003.00940-2015 [DOI] [PubMed] [Google Scholar]
- 65.Tan L,Dickens JA,DeMeo DL,et al. Circulating polymers in alpha-1 antitrypsin deficiency. Eur Respir J. 2014;43(5):1501-1504. doi: https://doi.org/10.1183/09031936.00111213 [DOI] [PubMed] [Google Scholar]
- 66.Mulgrew AT,Taggert CC,Lawless MW,et al. Z alpha-1 antitrypsin polymerizes in the lung and acts as a neutrophil chemoattractant. Chest. 2004;125(5):1952-1957. doi: https://doi.org/10.1378/chest.125.5.1952 [DOI] [PubMed] [Google Scholar]
- 67.Stiles KM,Sondhi D,Kaminsky SM,De BP,Rosenberg JB,Crystal RG. Intrapleural gene therapy for alpha-1 antitrypsin deficiency-related lung Disease. Chronic Obstr Pulm Dis. 2018;5(4):244-257. doi: https://doi.org/10.15326/jcopdf.5.4.2017.0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosenfeld MA,Siegfried W,Yoshimura K,et al. Adenovirus-mediated transfer of a recombinant alpha-1 antitrypsin gene to the lung epithelium in vivo. Science. 1991;252:431-434. doi: https://doi.org/10.1126/science.2017680 [DOI] [PubMed] [Google Scholar]
- 69.Jaffe HA,Daniel C,Longenecker G,et al. Adenovirus-mediated in vivo gene transfer and expression in normal rat liver. Nat Genet. 1992;1:372-378. doi: https://doi.org/10.1038/ng0892-372 [DOI] [PubMed] [Google Scholar]
- 70.Garver R Jr.,Chytil A,Courtney M,Crystal RG. Clonal gene therapy: transplanted mouse fibroblast clones express human alpha 1-antitrypsin gene in vivo. Science. 1987;237:762-764. doi: https://doi.org/10.1126/science.3497452 [DOI] [PubMed] [Google Scholar]
- 71.Kay MA,Baley P,Rothenberg S,et al. Expression of human alpha 1-antitrypsin in dogs after autologous transplantation of retroviral transduced hepatocytes. Proc Natl Acad Sci U S A. 1992;89(1):89-93. doi: https://doi.org/10.1073/pnas.89.1.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Daya S,Berns KI. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 2008;21(4):583-593. doi: https://doi.org/10.1128/CMR.00008-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chiuchiolo MJ,Crystal RG. Gene therapy for alpha-1 antitrypsin deficiency lung disease. Ann Am Thorac Soc. 2016;13(Suppl 4):S352-369. doi: https://doi.org/10.1513/AnnalsATS.201506-344KV [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mueller C,Gernoux G,Gruntman AM,et al. 5-Year expression and neutrophil defect repair after gene therapy in alpha-1 antitrypsin deficiency. Mol Ther. 2017;25(6):1387-1394. doi: https://doi.org/10.1016/j.ymthe.2017.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mueller C,Tang Q,Gruntman A,et al. Sustained miRNA-mediated knockdown of mutant AAT with simultaneous augmentation of wild-type AAT has minimal effect on global liver miRNA profiles. Mol Ther. 2012;20(3):590-600. doi: https://doi.org/10.1038/mt.2011.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guo S,Booten SL,Aghajan M,et al. Antisense oligonucleotide treatment ameliorates alpha-1 antitrypsin-related liver disease in mice. J Clin Invest. 2014;124(1):251-261. doi: https://doi.org/10.1172/JCI67968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li C,Xiao P,Gray SJ,Weinberg MS,Samulski RJ. Combination therapy utilizing shRNA knockdown and an optimized resistant transgene for rescue of diseases caused by misfolded proteins. Proc Natl Acad Sci U S A. 2011;108:14258-14263. doi: https://doi.org/10.1073/pnas.1109522108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turner AM,Stolk J,Bals R,et al. Hepatic-targeted RNA interference provides robust and persistent knockdown of alpha-1 antitrypsin levels in ZZ patients. J Hepatol. 2018;69(2):378-384. doi: https://doi.org/10.1016/j.jhep.2018.03.012 [DOI] [PubMed] [Google Scholar]
- 79.Pickar-Oliver A,Gersbach CA. The next generation of CRISPR-Cas technologies and applications. Nat Rev Mol Cell Biol. 2019;20:490-507. doi: https://doi.org/10.1038/s41580-019-0131-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu L,Xu L,Wang J,et al. CRISPR-edited stem cells in a patient with HIV and acute lymphocytic leukemia. N Engl J Med. 2019;381:1240-1247. doi: https://doi.org/10.1056/NEJMoa1817426 [DOI] [PubMed] [Google Scholar]
- 81.Yin H,Xue W,Anderson DG. CRISPR-Cas: a tool for cancer research and therapeutics. Nat Rev Clin Onco. 2019;l(16):281-295. doi: https://doi.org/10.1038/s41571-019-0166-8 [DOI] [PubMed] [Google Scholar]
- 82.Tian X,Gut Patel S,Bode AM,Lee M-H,Bong Z. eCRISPR/Cas9 - An evolving biological tool kit for cancer biology and oncology. NPJ Precis Oncol. 2019;3:8. doi: https://doi.org/10.1038/s41698-019-0080-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eid A,Mahfouz MM. Genome editing: the road of CRISPR/Cas9 from bench to clinic. Exp Mol Med. 2016;48. doi: https://doi.org/10.1038/emm.2016.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Song CQ,Wang D,Jiang T,et al. In vivo genome editing partially restores alpha-1 antitrypsin in a murine model of AAT deficiency. Hum Gene Ther. 2018;29(8):853-860. doi: https://doi.org/10.1089/hum.2017.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bjursell M,Porritt MJ,Ericson E,et al. Therapeutic genome editing with CRISPR/CAS9 in a humanized mouse model ameliorates alpha-1 antitrypsin deficiency phenotype. EBioMedicine. 2018;29:104-111. doi: https://doi.org/10.1016/j.ebiom.2018.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shen S,Sanchez ME,Blomenkamp K,et al. Amelioration of alpha-1 antitrypsin deficiency diseases with genome editing in transgenic mice. Hum Gene Ther. 2018;29(8):861-873. doi: https://doi.org/10.1089/hum.2017.227 [DOI] [PubMed] [Google Scholar]
- 87.Ding J,Yannam GR,Roy-Chowhury N,et al. Spontaneous hepatic repopulation in transgenic mice expressing mutant human alpha-1 antitrypsin by wild-type donor hepatocytes. J Clin Invest. 2011;121(5):1930-1934. doi: https://doi.org/10.1172/JCI45260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saito K,Yoshikawa M,Ouji Y,et al. Promoted differentiation of cynomolgus monkey ES cells into hepatocyte-like cells by co-culture with mouse fetal liver-derived cells. World J Gastroenterol. 2006;12(42):6818-6827. doi: https://doi.org/10.3748/wjg.v12.i42.6818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou QJ,Xiang L-X,Shao J-Z,et al. In vitro differentiation of hepatic progenitor cells from mouse embryonic stem cells induced by sodium butyrate. J Cell Biochem. 2007;100(1):29-42. doi: https://doi.org/10.1002/jcb.20970 [DOI] [PubMed] [Google Scholar]
- 90.Rashid ST,Corbineau S,Hannan N,et al. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J Clin Invest. 2010;120(9):3127-3136. doi: https://doi.org/10.1172/JCI43122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wilson AA,Ying L,Liesa M,et al. Emergence of a stage-dependent human liver disease signature with directed differentiation of alpha-1 antitrypsin-deficient iPS cells. Stem Cell Reports. 2015;4(5):873-885. doi: https://doi.org/10.1016/j.stemcr.2015.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Segeritz CP,Rashid ST,de Brito MC,et al. hiPSC hepatocyte model demonstrates the role of unfolded protein response and inflammatory networks in alpha-1 antitrypsin deficiency. J Hepatol. 2018;69(4):851-860. doi: https://doi.org/10.1016/j.jhep.2018.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yusa K,Rashid ST,Strick-Marchand H,et al. Targeted gene correction of alpha-1 antitrypsin deficiency in induced pluripotent stem cells. Nature. 2011;478:391-394. doi: https://doi.org/10.1038/nature10424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baligar P,Kochat V,Arindkar SK,et al. Bone marrow stem cell therapy partially ameliorates pathological consequences in livers of mice expressing mutant human alpha-1 antitrypsin. Hepatology. 2017;65(4):1319-1335. doi: https://doi.org/10.1002/hep.29027 [DOI] [PubMed] [Google Scholar]
- 95.Giacopuzzi E,Laffranchi M,Berardelli R,et al. Real-world clinical applicability of pathogenicity predictors assessed on SERPINA1 mutations in alpha-1 antitrypsin deficiency. Hum Mutat. 2018;39(9):1203-1213. doi: https://doi.org/10.1002/humu.23562 [DOI] [PubMed] [Google Scholar]
- 96.Karczewski KJ,Francioli LC,Tiao G,et al. Variation across 141,456 human exomes and genomes reveals the spectrum of loss-of-function intolerance across human protein-coding genes. bioRxiv. 2019;531210. doi: https://doi.org/10.1101/531210 [Google Scholar]
- 97.Bycroft C,Freeman C,Petkova D,et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203-209. doi: https://doi.org/10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Strange C,Monk R,Schwarz L,Walker D,Kumbhare S,Bieko T. The United States Alpha-1 Foundation Research Registry: genesis, impact and future. COPD. 2015;12(Suppl 1):42-45. doi: https://doi.org/10.3109/15412555.2015.1021914 [DOI] [PubMed] [Google Scholar]
- 99.Stockley RA. Antitrypsin Deficiency Assessment and Programme for Treatment (ADAPT): the United Kingdom Registry. COPD. 2015;2(Suppl 1):63-68. doi: https://doi.org/10.3109/15412555.2015.1021911 [DOI] [PubMed] [Google Scholar]
- 100.Green CE,Vayalapra S,Hampson JA,et al. PiSZ alpha-1 antitrypsin deficiency (AATD): pulmonary phenotype and prognosis relative to PiZZ AATD and PiMM COPD. Thorax. 2015;70(10):939-945. doi: https://doi.org/10.1136/thoraxjnl-2015-206906 [DOI] [PubMed] [Google Scholar]
- 101.Graham A,Kalsheker NA,Bamforth FJ,Newton CR,Markham AF. Molecular characterisation of two alpha-1-antitrypsin deficiency variants: proteinase inhibitor (Pi) Null(Newport) (Gly115----Ser) and (Pi) Z Wrexham (Ser-19----Leu). Hum Genet. 1990;85:537-540. doi: https://doi.org/10.1007/BF00194233 [DOI] [PubMed] [Google Scholar]
- 102.Matamala N,Lara B,Gomez-Mariano G,et al. Characterization of novel missense variants of SERPINA1 gene causing alpha-1 antitrypsin deficiency. Am J Respir Cell Mol Biol. 2018;58(6):706-716. doi: https://doi.org/10.1165/rcmb.2017-0179OC [DOI] [PubMed] [Google Scholar]
- 103.Mahadeva R,Gaillard M,Pillay V,Halkas A,Lomas D. Characterization of a new variant of alpha(1)-antitrypsin E(Johannesburg) (H15N) in association with asthma. Hum Mutat. 2001;17(2):156. doi: https://doi.org/10.1002/1098-1004(200102)17:2%3c156::AID-HUMU19%3e3.0.CO;2-Y [DOI] [PubMed] [Google Scholar]
- 104.Mahadeva R,Change W-SW,Dafforn TR,et al. Heteropolymerization of S, I, and Z alpha-1 antitrypsin and liver cirrhosis. J Clin Invest. 1999;103(7):999-1006. doi: https://doi.org/10.1172/JCI4874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baur X,Bencze K. Study of familial alpha-1 proteinase inhibitor deficiency including a rare proteinase inhibitor phenotype (IZ).I. Alpha-1-phenotyping and clinical investigations. Respiration. 1987;51:188-195. doi: https://doi.org/10.1159/000195201 [DOI] [PubMed] [Google Scholar]
- 106.Zorzetto M,Russi E,Senn O,et al. SERPINA1 gene variants in individuals from the general population with reduced alpha-1 antitrypsin concentrations. Clin Chem. 2008;54(8):331-1338. doi: https://doi.org/10.1373/clinchem.2007.102798 [DOI] [PubMed] [Google Scholar]
- 107.Takahashi H,Nukiwa T,Satoh K,et al. Characterization of the gene and protein of the alpha-1 antitrypsin "deficiency" allele M procida. J Biol Chem. 1988;263(30):15528-15534. [PubMed] [Google Scholar]
- 108.Prins J,van der Meiden BB,Kraaijenhagen RJ,Wielders JP. Inherited chronic obstructive pulmonary disease: new selective-sequencing workup for alpha-1 antitrypsin deficiency identifies 2 previously unidentified null alleles. Clin Chem. 2008;54(1):101-107. doi: https://doi.org/10.1373/clinchem.2007.095125 [DOI] [PubMed] [Google Scholar]
- 109.Kueppers F,Dunbrack RL Jr.,Kim J,Sanders CL. Protein modeling to assess the pathogenicity of rare variants of SERPINA1 in patients suspected of having alpha-1 antitrypsin Deficiency. BMC Med Genet. 2019;20:125. doi: https://doi.org/10.1186/s12881-019-0852-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Suh-Lailam BB,Procter M,Krautscheid P,et al. Challenging identification of a novel PiISF and the rare PiMmaltonZ alpha-1 antitrypsin deficiency variants in two patients. Am J Clin Pathol. 2014;141(5):742-746. doi: https://doi.org/10.1309/AJCPR7EIQS8PIMLV [DOI] [PubMed] [Google Scholar]
- 111.Lara B,Martinez-Delgado B,Torres ML,Marin-Arguedas S,Bustamante A,Miravittles M. Alpha-1 antitrypsin deficiency associated with the Mattawa variant. Arch Bronconeumol. 2013;49(12):548-550. doi: https://doi.org/10.1016/j.arbres.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 112.Curiel DT,Holmes MD,Okayama H,et al. Molecular basis of the liver and lung disease associated with the alpha-1 antitrypsin deficiency allele Mmalton. J Biol Chem. 1989;264(23):13938-13945. [PubMed] [Google Scholar]
- 113.Allen MB,Ward AM,Perks WH. Alpha-1 antitrypsin deficiency due to MMaltonZ phenotype: case report and family study. Thorax. 1986;41:568-570. doi: https://doi.org/10.1136/thx.41.7.568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Curiel DT,Vogelmeier C,Hubbard RC,Stier LE,Crystal RG. Molecular basis of alpha-1 antitrypsin deficiency and emphysema associated with the alpha-1 antitrypsin M mineral springs allele. Mol Cell Biol. 1990;10(1):47-56. doi: https://doi.org/10.1128/MCB.10.1.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Faber JP,Poller W,Weidinger S,et al. Identification and DNA sequence analysis of 15 new alpha-1 antitrypsin variants, including two PI*Q0 alleles and one deficient PI*M allele. Am J Hum Genet. 1994;55(6):1113-1121. [PMC free article] [PubMed] [Google Scholar]
- 116.Poller W,Merklein F,Schneider-Rasp S,et al. Molecular characterisation of the defective alpha-1 antitrypsin alleles PI Mwurzburg (Pro369Ser), Mheerlen (Pro369Leu), and Q0lisbon (Thr68Ile). Eur J Hum Genet. 1999;7:321-331. doi: https://doi.org/10.1038/sj.ejhg.5200304 [DOI] [PubMed] [Google Scholar]
- 117.Miranda E,Ferrarotti I,Berardelli R,et al. The pathological Trento variant of alpha-1-antitrypsin (E75V) shows nonclassical behaviour during polymerization. FEBS J. 2017;284(13):2110-2126 . doi: https://doi.org/10.1111/febs.14111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.de Seynes C,Ged C,deVernuil H,Chollet N,Balduyck M,Raherison C. Identification of a novel alpha-1 antitrypsin variant. Respir Med Case Rep. 2017;20:64-67. doi: https://doi.org/10.1016/j.rmcr.2016.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lovegrove JU,Jeremiah S,Gillet GT,Temple IK,Povey S,Whitehouse DB. A new alpha-1 antitrypsin mutation, Thr-Met 85, (PI Zbristol) associated with novel electrophoretic properties. Ann Hum Genet. 1997;61(5):385-391. doi: https://doi.org/10.1046/j.1469-1809.1997.6150385.x [DOI] [PubMed] [Google Scholar]
- 120.Bates KJ,Puxley M,Hill M,et al. A patient with the rare alpha-1 antitrypsin variant (Z)bristol in compound heterozygosity with the Z mutation. Ann Clin Biochem. 50:618-621. doi: https://doi.org/10.1177/0004563213484303 [DOI] [PubMed] [Google Scholar]
- 121.DeMeo DL,Silverman EK. Alpha-1 antitrypsin deficiency. 2: genetic aspects of alpha (1)-antitrypsin deficiency: phenotypes and genetic modifiers of emphysema risk. Thorax. 2004;59(3):259-264. doi: https://doi.org/10.1136/thx.2003.006502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Frazier GC,Siewertsen MA,Hofker MH,Brubacher MG,Cox DW. A null deficiency allele of alpha-1 antitrypsin, QOludwigshafen, with altered tertiary structure. J Clin Invest. 1990;86(6):1878-188. doi: https://doi.org/10.1172/JCI114919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stoller JK,Aboussouan LS. Alpha-1 antitrypsin deficiency. Lancet. 2005;365(9478):2225-2236. doi: https://doi.org/10.1016/S0140-6736(05)66781-5 [DOI] [PubMed] [Google Scholar]
- 124.Ferrarotti I,Carroll TP,Ottaviani S,et al. Identification and characterisation of eight novel SERPINA1 Null mutations. Orphanet J Rare Dis. 2014;9:172. doi: https://doi.org/10.1186/s13023-014-0172-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bamforth FJ,Kalsheker NA. Alpha-1 antitrypsin deficiency due to Pi null: clinical presentation and evidence for molecular heterogeneity. J Med Genet. 1988;25(2):83-87. doi: https://doi.org/10.1136/jmg.25.2.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Silva D,Oliveira MJ,Guimares M,Lima R,Games S,Seixas S. Alpha-1 antitrypsin (SERPINA1) mutation spectrum: three novel variants and haplotype characterization of rare deficiency alleles identified in Portugal. Respir Med. 2016;116:8-18. doi: https://doi.org/10.1016/j.rmed.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 127.Lodewyckx L,Vanderyer C,Vandervorst C,Van Steenbergen W,Raus J,Michrels L. Mutation detection in the alpha-1 antitrypsin gene (PI) using denaturing gradient gel electrophoresis. Hum Mutat. 2001;18(3):243-250. doi: https://doi.org/10.1002/humu.1180 [DOI] [PubMed] [Google Scholar]
- 128.Joly P,Francina P,Lacan J,Heraut J,Chapuis-Cellier C. Place de l'analyse génotypique en complément du phénotype et du dosage de l'α-1 antitrypsine sérique. Ann Biol Clin (Paris). 2011;69:571-576. doi: https://doi.org/10.1684/abc.2011.0613 [DOI] [PubMed] [Google Scholar]
- 129.Joly P,Lacan P,Chapuis-Cellier C,Garcia C,Bererd M,Fraancina A. Molecular characterization of 7 new alpha-1 antitrypsin (A1AT) variants including two with an associated deficient phenotype. Clin Chim Acta. 2014;427:21-22. doi: https://doi.org/10.1016/j.cca.2013.09.017 [DOI] [PubMed] [Google Scholar]
- 130.Okayama H,Brantly M,Holmes M,Crystal RG. Characterization of the molecular basis of the alpha-1 antitrypsin F allele. Am J Hum Genet. 1991;48:1154-1158. [PMC free article] [PubMed] [Google Scholar]
- 131.Ringenbach MR,Banta E,Snyder MR,Craig TJ,Ishmael FT. A challenging diagnosis of alpha-1 antitrypsin deficiency: identification of a patient with a novel F/Null phenotype. Allergy Asthma Clin Immunol. 2011;7:18. doi: https://doi.org/10.1186/1710-1492-7-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Medicina D,Montani N,Fra AM,et al. Molecular characterization of the new defective P(brescia) alpha-1 antitrypsin allele. Hum Mutat. 2009;30(8):E771-781. doi: https://doi.org/10.1002/humu.21043 [DOI] [PubMed] [Google Scholar]
- 133.Bornhorst JA,Calderon FRO,Procter M,Tang W,Ashwood ER,Mao R. Genotypes and serum concentrations of human alpha-1 antitrypsin "P" protein variants in a clinical population. J Clin Pathol. 2007;60(10):1124-1128. doi: https://doi.org/10.1136/jcp.2006.042762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Graham A,Kalsheker NA,Newton CR,Bamforth FJ,Powell SJ,Markham AT. Molecular characterisation of three alpha-1 antitrypsin deficiency variants: proteinase inhibitor (Pi) nullcardiff (Asp256----Val); PiMmalton (Phe51----deletion) and PiI (Arg39----Cys). Hum Genet. 1989;84:55-58. doi: https://doi.org/10.1007/BF00210671 [DOI] [PubMed] [Google Scholar]
- 135.Oliveira MJ,Seixas S,Ladeira I,et al. Alpha-1 antitrypsin deficiency caused by a novel mutation (p. Leu263Pro): Pi*ZQ0gaia - Q0gaia allele. Rev Port Pneumol. 2015;21(6):341-343. doi: https://doi.org/10.1016/j.rppnen.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 136.Curiel DT,Chytil A,Courtney M,Crystal RG. Serum alpha-1 antitrypsin deficiency associated with the common S-type (Glu264----Val) mutation results from intracellular degradation of alpha-1 antitrypsin prior to secretion. J Biol Chem. 1989;264:10477-10486. [PubMed] [Google Scholar]
- 137.Ferrarotti I,Thun GA,Zorzetto M,et al. Serum levels and genotype distribution of alpha-1 antitrypsin in the general population. Thorax. 2012;67(8):669-674. doi: https://doi.org/10.1136/thoraxjnl-2011-201321 [DOI] [PubMed] [Google Scholar]
- 138.Ljujic M,Divac Rankov A,Kojic S,Miranda E,Radojkovic D. Functional analysis of novel alpha-1 antitrypsin variants G320R and V321F. Mol Biol Rep. 2014;41:6133-6141. doi: https://doi.org/10.1007/s11033-014-3492-z [DOI] [PubMed] [Google Scholar]
- 139.Greene DN,Procter M,Krautscheid P,Mao R,Lyon E,Grenache DG. Alpha-1 antitrypsin deficiency in fraternal twins born with familial spontaneous pneumothorax. Chest. 2012;141(1):239-241. doi: https://doi.org/10.1378/chest.11-0104 [DOI] [PubMed] [Google Scholar]
- 140.Arora NK,Arora S,Ahuja A,et al. Alpha-1 antitrypsin deficiency in children with chronic liver disease in North India. Indian Pediatr. 2010;47:1015-1023. doi: https://doi.org/10.1007/s13312-010-0174-3 [DOI] [PubMed] [Google Scholar]
- 141.Miranda E,Perez J,Ekeowa UI,et al. A novel monoclonal antibody to characterize pathogenic polymers in liver disease associated with alpha-1 antitrypsin deficiency. Hepatology. 2010;52(3):1078-1088. doi: https://doi.org/10.1002/hep.23760 [DOI] [PubMed] [Google Scholar]
- 142.Kidd VJ,Wallace RB,Itakura K,Woo SL. Alpha-1 antitrypsin deficiency detection by direct analysis of the mutation in the gene. Nature. 1983;304:230-234. doi: https://doi.org/10.1038/304230a0 [DOI] [PubMed] [Google Scholar]
- 143.Seixas S,Lopes AI,Rocha J,et al. Association between the defective Pro369Ser mutation and in vivo intrahepatic alpha-1 antitrypsin accumulation. J Med Genet. 2001;38(7):472-474. doi: https://doi.org/10.1136/jmg.38.7.472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hernandez Perez JM,Ramos Diaz R,Fumero Garcia S,Perez JA. Description of alpha-1 antitrypsin deficiency associated with PI*Q0ourem allele in La Palma Island (Spain) and a genotyping assay for its detection. Arch Bronconeumol. 2015;51(1):e1-3. doi: https://doi.org/10.1016/j.arbres.2014.01.011 [DOI] [PubMed] [Google Scholar]
- 145.Vidaud D,Emmerich J,Alhenc-Gelas M,Yvart J,Fiessinger N,Aiach M. Met 358 to Arg mutation of alpha-1 antitrypsin associated with protein C deficiency in a patient with mild bleeding tendency. J Clin Invest. 1992;89(5):1537-1543. doi: https://doi.org/10.1172/JCI115746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Seyama K,Nukiwa T,Takabe K,Takahashi H,Miyake K,Kira S. Siiyama (serine 53 (TCC) to phenylalanine 53 (TTC)). A new alpha-1 antitrypsin-deficient variant with mutation on a predicted conserved residue of the serpin backbone. J Biol Chem. 1991;266(19):12627-12632. [PubMed] [Google Scholar]
- 147.Fra AM,Gooptu B,Ferrarotti I,et al. Three new alpha-1 antitrypsin deficiency variants help to define a C-terminal region regulating conformational change and polymerization. PLoS One. 2012;7:e38405. doi: https://doi.org/10.1371/journal.pone.0038405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lewis JH,Iammarino RM,Spero JA,Hasiba U. Antithrombin Pittsburgh: an alpha1-antitrypsin variant causing hemorrhagic disease. Blood. 1978;51:129-137. doi: https://doi.org/10.1182/blood.V51.1.129.129 [PubMed] [Google Scholar]
- 149.Owen MC,Brennan SO,Lewis JH,Carrell RW. Mutation of antitrypsin to antithrombin. Alpha-1 antitrypsin Pittsburgh (358 Met leads to Arg), a fatal bleeding disorder. N Engl J Med. 1983;309:694-698. doi: https://doi.org/10.1056/NEJM198309223091203 [DOI] [PubMed] [Google Scholar]
- 150.Emmerich J,Alhenc-Gelas M,Gandrille S,Guicket C,Fiessinger JN,Hiach M. Mechanism of protein C deficiency in a patient with arginine 358 alpha-1 antitrypsin (Pittsburgh mutation): role in the maintenance of hemostatic balance. J Lab Clin Med. 1995;125(4):531-539. [PubMed] [Google Scholar]
- 151.Gooptu B,Dickens JA,Lomas DA. The molecular and cellular pathology of alpha (1)-antitrypsin deficiency. Trends Mol Med. 2014;20:116-127. doi: https://doi.org/10.1016/j.molmed.2013.10.007 [DOI] [PubMed] [Google Scholar]
- 152.Gooptu B,Lomas DA. Misfolding and polymerization of alpha-1 antitrypsin: conformational pathology and therapeutic targeting. In: Wanner A, Sandhous RA, eds. Alpha-1 Antitrypsin: Role in Health and Disease Springer International Publishing; . 2016:31-52. [Google Scholar]
- 153.Lomas DA,Finch JT,Seyama K,Nukiwa T,Carrell RW. Alpha-1 antitrypsin Siiyama (Ser53-->Phe). Further evidence for intracellular loop-sheet polymerization. J Biol Chem. 1993;268:15333-15335. [PubMed] [Google Scholar]
- 154.Lomas DA,Elliott PR,Sidhar SK,et al. Alpha-1 antitrypsin Mmalton (Phe52-deleted) forms loop-sheet polymers in vivo. Evidence for the C sheet mechanism of polymerization. J Biol Chem. 1995;270:6864-16870. doi: https://doi.org/10.1074/jbc.270.28.16864 [DOI] [PubMed] [Google Scholar]
- 155.Wardell MR,et al. Preparative induction and characterization of L-antithrombin: a structural homologue of latent plasminogen activator inhibitor-1. Biochemistry. 1997;36:13133-13142. doi: https://doi.org/10.1021/bi970664u [DOI] [PubMed] [Google Scholar]
- 156.Yamasaki M,Sendall TJ,Pearce MC,Whisstock JC,Huntington JA. Molecular basis of alpha-1 antitrypsin deficiency revealed by the structure of a domain-swapped trimer. EMBO Rep. 2011;12(10):1011-1017. doi: https://doi.org/10.1038/embor.2011.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Accurso FJ,Rowe SM,Clancy JP,et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363(21):1991-2003. doi: https://doi.org/10.1056/NEJMoa0909825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Amaral MD,Balch WE. Hallmarks of therapeutic management of the cystic fibrosis functional landscape. J Cyst Fibros. 2015;14(6):687-699. doi: https://doi.org/10.1016/j.jcf.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Han ST,Rab A,Pellicore MJ,et al. Residual function of cystic fibrosis mutants predicts response to small molecule CFTR modulators. JCI Insight. 2018;3(14):e121159. doi: https://doi.org/10.1172/jci.insight.121159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Keating D,Marigowda G,Burr L,et al. VX-445-Tezacaftor-Ivacaftor in patients with cystic gibrosis and one or two Phe508del alleles. N Engl J Med. 2018;379:1612-1620. doi: https://doi.org/10.1056/NEJMoa1807120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang C,Balch WE. Managing the adaptive proteostatic landscape: restorying resilience in alpha-1 antitrypsin deficiency. In: Wanner A, Sandhous RA, eds. Alpha-1 Antitrypsin: Role in Health and Disease Springer International Publishing;. 2016:53-83. [Google Scholar]
- 162.Ramsey BW,Davies J,McElvaney G,et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365(18):1663-1672. doi: https://doi.org/10.1056/NEJMoa1105185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wainwright CE,Elborn S,Ramsey BW,et al. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373:220-231. doi: https://doi.org/10.1056/NEJMoa1409547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rowe SM,Rowe SM,Daines C,Ringshausen FC,et al. Tezacaftor-ivacaftor in residual-function heterozygotes with cystic fibrosis. N Engl J Med. 2017;377:2024-2035. doi: https://doi.org/10.1056/NEJMoa1709847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Davies JC,Moskowitz SM,Brown C,et al. VX-659-tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med. 2018;379:1599-1611. doi: https://doi.org/10.1056/NEJMoa1807119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Arenas F,Garcia-Ruiz C,Fernandez-Checa JC. Intracellular cholesterol trafficking and impact in neurodegeneration. Front Mol Neurosci. 2017;10:382. doi: https://doi.org/10.3389/fnmol.2017.00382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vanier MT. Niemann-Pick diseases. Handb Clin Neurol. 2013;113:1717-1721. doi: https://doi.org/10.1016/B978-0-444-59565-2.00041-1 [DOI] [PubMed] [Google Scholar]
- 168.Gershenson A,Gierasch LM,Pastore A,Radford SE. Energy landscapes of functional proteins are inherently risky. Nat Chem Biol. 2014;10:884-891. doi: https://doi.org/10.1038/nchembio.1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.National Institutes of Health (NIH) All of Me Research Program. NIH website. Updated 2020 Accessed July 2020 https://allofus.nih.gov/ [Google Scholar]