Abstract
Alpha-1 antitrypsin deficiency (AATD) has traditionally been associated with the development of early onset panlobular emphysema thought to reflect the direct interstitial damage caused by neutrophil elastase. Since this enzyme is highly sensitive to irreversible inhibition by alpha-1 antitrypsin (AAT), the logic of intravenous augmentation therapy has remained unquestioned and efficacy is supported by both observational studies and formal clinical trials. However, evidence suggests that although AAT augmentation modulates the progression of emphysema, it only slows it down. This raises the issue of whether our long-held beliefs of the cause of the susceptibility to develop emphysema in deficient individuals are correct. There are several aspects of our understanding of the disease that might benefit from a radical departure from traditional thought. This review addresses these concepts and alternative pathways that may be central to progression of emphysema.
Keywords: emphysema, alpha-1 antitrypsin deficiency, small airways disease, proteinases, oxidants
Introduction
The identification of blood and hence, lung deficiency, of alpha-1 antitrypsin (AAT) and the susceptibility to the development of early onset emphysematous chronic obstructive pulmonary disease (COPD)1 was a major step in understanding the pathophysiology of emphysema. An unexpected finding of “emphysematous” change in the lungs of animals exposed to the metalloproteinase papain2 started to close the circle. The concept of an insult in the lungs that leads to an imbalance between protective enzyme inhibitors and destructive enzymes is the basis of the proteinase/antiproteinase hypothesis that persists today.
The subsequent recognition that neutrophil elastase (NE) could replicate many of the clinical features of COPD in animal models and the importance of AAT as the major plasma (and assumed, lung) inhibitor of NE led to extensive literature focused on the NE/AAT balance, factors that affected it and evidence for its involvement in COPD in general with or without the presence of emphysema. Since migrating neutrophils release high levels of NE during migration through endothelium and connective tissue3 it is easy to understand the propensity for AAT deficiency (AATD) to lead to excessive interstitial damage leading to emphysema. Indeed early studies by Damiano and colleagues indicated that the amount of NE in lung connective tissue related to the presence and degree of emphysema.4 In those with normal AAT levels the development of emphysema was thought to relate to an excessive NE load due to increased neutrophil traffic or abnormal neutrophil behaviour as demonstrated by Sapey and colleagues.5 This concept was supported by the observation that neutrophils accumulated in the areas of emphysema seen on positron emission tomography-computed tomography.6
However, as most COPD patients have normal (and assumed protective levels of AAT), evidence for other potentially destructive enzymes (mainly the metalloproteinases) and their inhibitors, the tissue inhibitors of metalloproteinases (TIMPs), has been and continues to be explored7,8 leading to an extensive and ongoing literature. In addition, cysteine proteinases and hence their inhibitors (cystatins), have also been implicated.9,10 This reflects mainly the ability of lung inflammatory cells to produce these proteins in vitro and is supported by evidence of their presence in airways secretions. However, little attention has been given to the clinical patient phenotype and whether these observations were specifically related to emphysema.
These studies centralized on enzymes that had the ability to digest lung elastin since this connective tissue proved central to the pathophysiology of emphysema. NE was so named because of its initial characterization as an elastin degrading enzyme. The initial animal models indicated that elastin degradation was a central event in emphysema models11 and models of elastin synthesis12,13 as well as destruction, supported this. In addition, elastin disorders in patients (though rare) also were associated with emphysema adding further support.14,15 These observations, together with the identification of NE on lung elastin4 and the presence of elastin degradation products in patient studies16 along with animal models preventing elastin re-synthesis,17 provided the final evidence required to perpetuate the elastase/anti-elastase balance (personified by AAT deficiency) as the central process in emphysema development and progression.
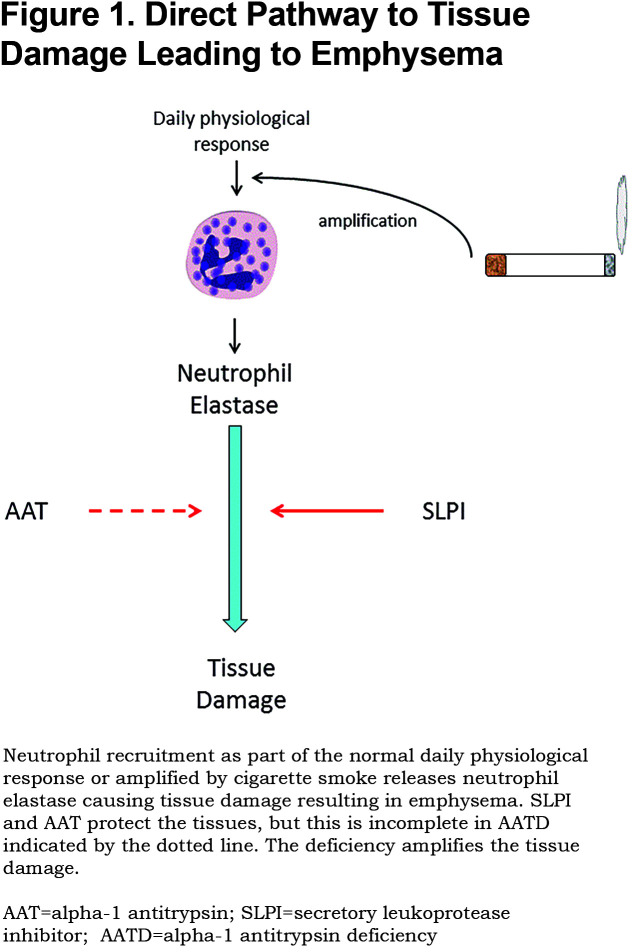
However, although this concept represents a relatively simple pathway (Figure 1) the processes that bring these features together are far from simple even in AATD.
The Neutrophil Elastase/ Alpha-1 Antitrypsin Balance in Alpha-1 Antitrypsin Deficiency
The destruction of lung elastin and its amorphous regeneration (as seen in animal models11) is a complex process in itself. The action takes place in the lung interstitium in the alveolar region. Firstly, this requires recruitment of the major source of NE (the neutrophil) into the lung. This is an ongoing (though low grade) process in health but is enhanced by cigarette smoke18 and becomes a self-perpetuating inflammatory process especially in AATD.19 Migrating neutrophils (PMN) mobilize the store of NE (the azurophil granule) resulting in localization and release at the leading edge.3 The high concentration at release and some retention at the cell surface limits inhibition by lung AAT which is largely derived from plasma by diffusion into the lung. The net effect is some constitutive enzyme activity retained near the cell and connective tissue digestion permitting cell movement. Elastin binding of NE continues its activity still being relatively resistant to inhibition by AAT.20 The remaining free enzyme diffuses away until its concentration equals that of functional AAT; a process called quantum proteolysis.21 This latter process is greatly enhanced when prevailing AAT levels are low, as in AATD.22 Although the lung inhibitor of proteinases, the secretory leucoproteinase inhibitor (SLPI) can provide some interstitial protection against soluble and elastin bound NE,20 it is also finite and insufficient to replace the deficit in AATD. Furthermore, the production of lung SLPI decreases in the presence of inflammation23 and would amplify the process further in AATD. This combination of cells/cell membrane and substrate binding leads to a complex enzyme inhibitor interaction that provides several mechanisms to explain enzyme substrate activity in the lung interstitium. The deficiency of AAT and perhaps its reduced association rate constant for NE (observed in solution), would only enhance enzyme substrate interactions.
Despite these complexities AATD clearly contributes to the destructive potential of NE. The obvious strategy to protect the lung adequately, therefore, was augmentation of plasma and hence, lung AAT, to restore the physiological protective mechanism. It was argued from epidemiological studies of AAT variants that a level around 11uM (almost double that of the average concentration of AAT in the most common genotype, PiZZ deficient variant) was sufficiently protective.24 This was confirmed by in vitro degradation of proteins by neutrophils in the presence of both plasma samples from AAT deficiency variants22 and increasing concentrations of NE inhibitors.21
Augmentation with weekly infusions was able to maintain the nadir levels above this putative protective “threshold”. However, double blind controlled studies (though effective) merely abrogated emphysema progression but did not stop it.25,26,27 This raises the possibility that, despite the evidence, the protective “threshold” is too low to stabilize the emphysema or that non-AAT responsive mechanisms are responsible for the reduced but continued progression.
Role of Proteinase 3
Proteinase 3 (PR3) like NE is a serine proteinase stored within the azurophil granule of PMNs and released upon activation. It can also reproduce many of the features of COPD and emphysema in animal models28 but is more slowly inhibited by AAT and is also partly membrane bound and resistant to inhibition of this site.29 In addition, it is not inhibited by the local lung serine proteinase inhibitor (SLPI) and is stored within the granule at concentrations approximately 3x that of NE.30 For these reasons, its destructive potential would be greater and longer-acting in the vicinity of migrating PMNs.31 Not only would this provide an additional enzyme burden but would tend to be inhibited after NE and nadir levels sufficient to inhibit NE may still fall short of PR3 control. These properties at least partly explain the greater likelihood of detecting active PR3 in the lung secretions of COPD patients with and without AATD.32 This may also provide some explanation for incomplete disease control by AAT augmentation and suggest the need to raise the theoretical AAT threshold to ensure adequate control of both NE and PR3.
However, importantly, this possibility also has implications for the development of specific anti-elastase inhibitors as therapeutic strategies for AATD. A high affinity, irreversible NE inhibitor would remove NE from the local lung environment releasing the remaining AAT to inhibit PR3. On the other hand, a low affinity, reversible inhibitor may have no effect whereas an inhibitor capable of inhibiting both enzymes may prove particularly beneficial. These issues should clearly be considered as part of the drug development strategies for AATD.
Enzymes Other Than Serine Proteinases
As mentioned above there are several other classes of enzymes that have been implicated in the pathophysiology of emphysema because of their potential to cleave elastin and it is possible that these act either in an interactive cascade or independently.33
For instance, NE is known to activate pro metallo proteinases and at the same time inactivate their cognate inhibitors, the TIMPs,33 thereby producing a potential metallo elastase/inhibitor imbalance. Similarly, NE will activated cysteine proteinases whilst inactivating their inhibitors the cystatins33 leading to a cysteine elastase/inhibitor imbalance. This raises the possibility that the enzymes responsible for end organ damage are only indirectly related to NE. The circle is completed by the ability of metallo and cysteine proteinases to inactivate AAT as an inhibitor thereby indirectly facilitating the destructive potential of both NE and PR3. AAT augmentation should of course block the end result, either directly or indirectly, by controlling other proteinases potentially being activate by NE, although this may depend on the rate of activation of the alternative pathways. This sequence of interrelated events would determine the last destructive step and can only be explored by carefully designed projects using specific enzyme class inhibitors. However, if NE orchestrates the downstream effects of other classes of proteinases the cascade should still be abrogated by its inhibition.
Neutrophil Elastase Independent Pathways
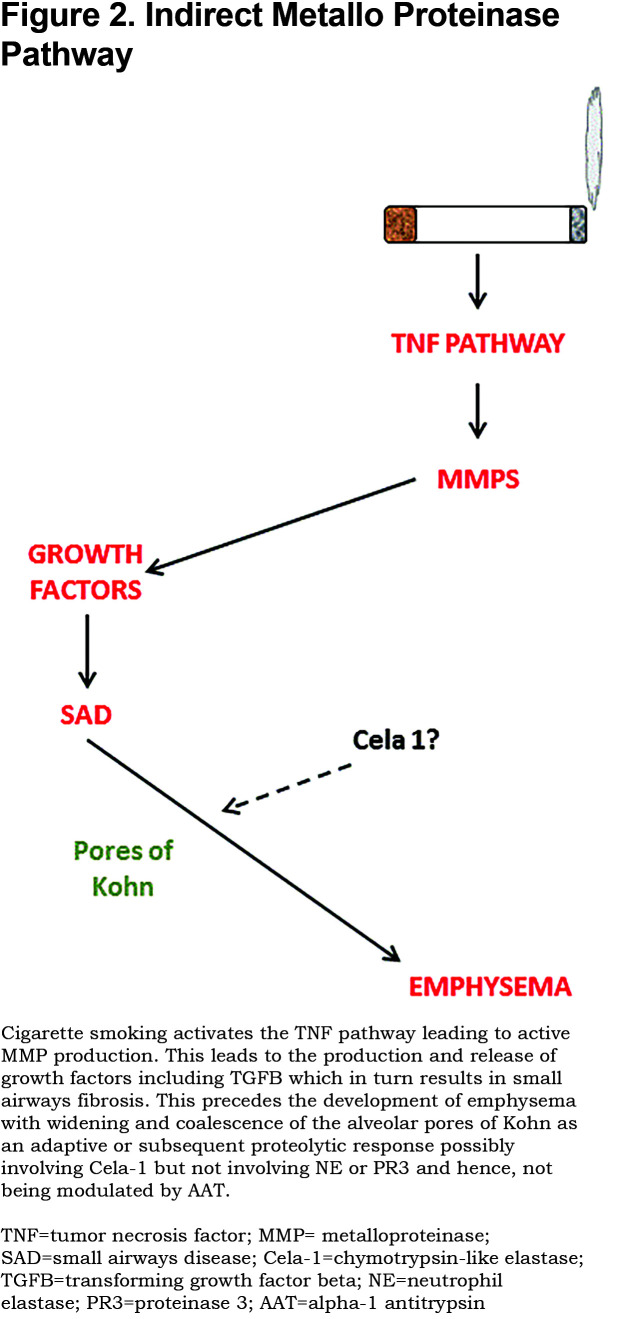
Urokinase-type plasminogen activator (uPA) is expressed by neutrophils (PMN), monocytes, and macrophages. Preformed uPA is stored in and released from the specific granules of PMNs whilst uPA expression is regulated at the transcriptional level in mononuclear phagocytes by pro-inflammatory mediators.34,35 uPA binds to a uPA receptor on phagocytes where it functions as a cell-associated proteinase. The main function of uPA is to convert inactive plasminogen to active plasmin, which cleaves and activates latent growth factors, latent pro matrix metallo proteinases (MMPs), and protease activated receptor-1 (PAR-1) on macrophages, which in turn drives macrophage MMP-12 production.36-39 Thus, by generating plasmin, uPA potentially regulates extracellular matrix degradation (including elastin) by downstream enzyme activation and fibrotic processes in the lung thereby having the potential to lead to both emphysema and small airways narrowing and obstruction. This latter process may result in a further non-proteolytic pathway to the development of emphysema (see below).
MMPs themselves are usually produced as pro-enzymes that can be activated by non-NE mechanisms. This may occur as a result of the actions of other proteinases as well as oxidants in the extracellular space, described as the cysteine switch mechanism of activation.40,41MMPs are generally synthesised following cell activation. However, PMNs also store preformed MMP-8 and MMP-9, in their cytoplasmic granules, being released when PMNs degranulate42 where they become activated. Studies have confirmed increased levels of uPA, and MMPs -1, -2, -8, -9, and -14 in various lung samples from smokers and COPD patients (where there is also an increased oxidant burden) when compared to healthy individuals.43 Activation of these enzymes by non-NE mechanisms provides an alternative route to the direct and indirect development of emphysema. Indeed, the oxidant (proteinase independent activation of MMPs) pathway is supported by animal models demonstrating transgenic animals over-expressing CuZn superoxide dismutase are resistant to both smoke and porcine pancreatic elastase (PPE)- induced emphysema like lesions.44
A series of animal knock-out studies by Churg and colleagues45 has highlighted an alternative pathway via tumor necrosis factor (TNF) and the role of MMPs in activating the TNF receptor. This leads to the up regulation of adhesion molecules and inflammatory cell migration. The whole cascade can be blocked at TNFα level even by AAT that has no proteolytic inhibitory function (see Figure 2) downgrading the conventional PMN serine proteinase pathway. This concept is supported by the association of a functional TNF polymorphism being associated with more prevalent chronic bronchitis and accelerated lung function decline in AATD patients carrying this polymorphism.46
Despite this proteinase cascade leading to connective tissue (and particularly elastin) breakdown, it is possible that the role of nonserine proteinases could be orchestrated via an alternative pathway. The pathogenesis of emphysema has recently been considered in a different light. Classical studies of the pathology of early to late COPD have highlighted the loss of small airways as the first structural change. Although the pathology has not specifically been noted as a feature of AATD, it was assessed in a group of younger patients with a lower smoking history and panacinar emphysema typical of such patients.47Studies from our group have provided physiological and radiological evidence of this sequence of events in cross sectional studies.48
MMPs are responsible for the activation of growth factors leading to small airways abnormalities49 consistent with the above studies. Thereafter, the development of emphysema may reflect a physiological rather than a pathological process. The pores of Kohn are naturally occurring holes that link alveoli. As emphysema progresses, the pores enlarge forming larger airspaces.50 This was elegantly demonstrated by JL Wright in 2001 using a smoking Guinea pig model.51 She demonstrated an increase in the size and irregularity of the pores that preceded any increase in airspace. The process could, therefore, be envisioned as a natural mechanism to enhance collateral ventilation of alveoli that no longer have direct air entry via the serving small airways. Whether such a change relates to proteolytic degradation in the alveolar wall and if so, which proteinases are responsible, remains unknown. However, recent studies in both mice and humans have implicated a further enzyme, Cela-1 that is involved in stretch-dependant elastin remodelling and may represent the downstream effect of loss of small airways. This enzyme is a serine proteinase and should be inhibited by AAT. Nevertheless, it is found in an uninhibited state in the lungs of AATD individuals removed at transplantation.52 Although this alternative pathway could also be at least partly sensitive to AAT inhibition, it may be less responsive than the direct effect on the neutrophil serine proteinase pathway. Clearly, further studies are required to determine whether this alternative pathway is active in AATD and its implications for AAT augmentation.
Summary
The simple NE/AAT axis has long been central to our understanding of the pathophysiology of emphysema, especially in AATD. However, the process is clearly complex and failure of augmentation using the current dosage regimen to block emphysema progression completely suggests alternative processes play a role and this can only be explored and defined by specific antagonistic interventions. Because of the function of AAT as a serine proteinase inhibitor and the assumed central role of enzymes with the ability to cleave elastin in the emphysema process, this review has concentrated on this axis. It should be noted that the array of putative destructive enzymes with a role in lung disease is becoming more extensive and covered recently in the review by Taggart and colleagues together with their target substrates.53 How non-elastases interact in the inflammatory pathway specific to AATD and emphysema remains speculative but warrants continued consideration in the light of specific interventions and the clinical response.
Abbreviations
alpha-1 antitrypsin deficiency, AATD; alpha-1 antitrypsin, AAT; chronic obstructive pulmonary disease, COPD; neutrophil elastase, NE; tissue inhibitors of metallo proteinases, TIMPs; migrating neturophils, PMN; secretaory leucoproteinase inhibitor, SLPI; proteinase 3, PR3; urokinase-type plasminogen activator, uPA; matrix metallo proteinases, MMPs; protease activated receptor-1, PAR-1; porcine pancreatic elastase, PPE; tumor necrosis factor, TNF; chymotrypsin-like elastase, Cela-1
References
- 1.Laurell C-B,Eriksson S. The electrophoretic alpha-1-globulin pattern of serum in alpha-1-antitrypsin deficiency. Scand J Clin Lab Invest. 1963;15(2):132-140. doi: https://doi.org/10.3109/00365516309051324 [Google Scholar]
- 2.Gross P,Babyak MA,Tolker E,Kaschak M. Enzymatically produced pulmonary emphysema; A preliminary report. J Occup Med. 1964;6:481-484. [PubMed] [Google Scholar]
- 3.Cepinskas G1,Sandig M,Kvietys PR. PAF-induced elastase-dependent neutrophil transendothelial migration is associated with the mobilization of elastase to the neutrophil surface and localization to the migrating front. J Cell Sci. 1999;112:1937-1945. [DOI] [PubMed] [Google Scholar]
- 4.Damiano VV,Tsang A,Kucich U,et al. Immunolocalization of elastase in human emphysematous lungs. J Clin Invest. 1986;78(2):482-493. doi: https://doi.org/10.1172/JCI112600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sapey E,Stockley JA,Greenwood H,et al. Behavioral and structural differences in migrating peripheral neutrophils from patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183(9):1176-1186. doi: https://doi.org/10.1164/rccm.201008-1285OC [DOI] [PubMed] [Google Scholar]
- 6.Subramanian DR,Jenkins L,Edgar R,Quraishi N,Stockley RA,Parr DG. Assessment of pulmonary neutrophilic inflammation in emphysema by quantitative positron emission tomography. Am J Respir Crit Care Med. 2012;186(11):1125-1132. doi: https://doi.org/10.1164/rccm.201201-0051OC [DOI] [PubMed] [Google Scholar]
- 7.Ostridge K,Williams N,Kim V,et al. Relationship between pulmonary matrix metalloproteinases and quantitative CT markers of small airways disease and emphysema in COPD. Thorax. 2016;71(2):126-132. doi: https://doi.org/10.1136/thoraxjnl-2015-207428 [DOI] [PubMed] [Google Scholar]
- 8.Lo CY,Huang HY,He JR,et al. Increased matrix metalloproteinase-9 to tissue inhibitor of metalloproteinase-1 ratio in smokers with airway hyperresponsiveness and accelerated lung function decline. Int J Chron Obstruct Pulmon Dis. 2018;13:1135-1144. doi: https://doi.org/10.2147/COPD.S161257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lesser M,Padilla ML,Cardozo C. Induction of emphysema in hamsters by intratracheal instillation of cathepsin B. Am Rev Respir Dis. 1992;145(3):661-668. doi: https://doi.org/10.1164/ajrccm/145.3.661 [DOI] [PubMed] [Google Scholar]
- 10.Burnett D,Crocker J,Stockley RA. Cathepsin B-like cysteine proteinase activity in sputum and immunohistologic identification of cathepsin B in alveolar macrophages. Am Rev Respir Dis. 1983;128(5):915-919. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn C,Yu SY,Chraplyvy M,Linder HE,Senior RM. The induction of emphysema with elastase. II. Changes in connective tissue. Lab Invest. 1976;34372-34380. [PubMed] [Google Scholar]
- 12.Kuhn C,Starcher B. The effect of lathyrogens on the evolution of elastase-induced emphysema. Am Rev Respir Dis. 1980;122:453-460. [DOI] [PubMed] [Google Scholar]
- 13.Niewoehner DE,Hoidal JR. Lung fibrosis or emphysema: Divergent responses to a common injury. Science. 1982;217(4557):359-360. doi: https://doi.org/10.1126/science.7089570 [DOI] [PubMed] [Google Scholar]
- 14.Turner-Stokes L,Turton C,Pope FM,Green M. Emphysema and cutis laxa. Thorax. 1983;38:790-795. doi: https://doi.org/10.1136/thx.38.10.790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh TA,Gopagondanahalli KR,Malhotra A. Williams-Beuren syndrome and congenital lobar emphysema: uncommon association with common pathology?. Case Rep Pediatr. 2017:3480980. doi: https://doi.org/10.1155/2017/3480980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kucich U,Christner P,Lippmann M,Fein A,Goldberg A,Kimbel P,Weinbaum G,Rosenbloom J. Immunologic measurement of elastin-derived peptides in human serum. Am Rev Respir Dis. 1983;127(2):S28-30. [DOI] [PubMed] [Google Scholar]
- 17.Kuhn C, III,Starcher BC. The effect of lathyrogens on the evolution of elastase-induced emphysema. Am Rev Respir Dis. 1980;122(3):453-460. [DOI] [PubMed] [Google Scholar]
- 18.Churg A,Zay K,Shay S,et al. Acute cigarette smoke-induced connective tissue breakdown requires both neutrophils and macrophage metalloelastase in mice. Am J Respir Cell Mol Biol. 2002;27(3):368-374. doi: https://doi.org/10.1165/rcmb.4791 [DOI] [PubMed] [Google Scholar]
- 19.Stockley RA. Alpha- 1 antitrypsin review. Clin Chest Med. 2014;35(1):39-50. doi: https://doi.org/10.1016/j.ccm.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 20.Morrison HM,Welgus HG,Stockley RA,Burnett D,Campbell EJ. Inhibition of human leukocyte elastase bound to elastin: relative ineffectiveness and two mechanisms of inhibitory activity. Am J Respir Cell Mol Biol. 1990;2(3):263-269. doi: https://doi.org/10.1165/ajrcmb/2.3.263 [DOI] [PubMed] [Google Scholar]
- 21.Liou TG,Campbell EJ. Quantum proteolysis resulting from release of single granules by neutrophils: a novel, non-oxidative mechanism of extracellular proteolytic activity. J Immunol. 1996;157:2624-2631. [PubMed] [Google Scholar]
- 22.Campbell EJ,Campbell MA,Boukedes SS,Owen CA. Quantum proteolysis by neutrophils: implications for pulmonary emphysema in alpha-1 antitrypsin deficiency. J Clin Invest. 1999;104(3):337-344. doi: https://doi.org/10.1172/JCI6092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill AT,Campbell EJ,Bayley DL,Hill SL,Stockley RA. Evidence for excessive bronchial inflammation during an acute exacerbation of chronic obstructive pulmonary disease in patients with alpha (1)-antitrypsin deficiency (PiZ). Am J Respir Crit Care Med. 1999;160(6):1968-1975. doi: https://doi.org/10.1164/ajrccm.160.6.9904097 [DOI] [PubMed] [Google Scholar]
- 24.Gadek JE,Klein HK,Holland PV,Crystal RG. Replacement therapy of alpha-1 antitrypsin deficiency. Reversal of protease-antiprotease imbalance within the alveolar structures of PiZ subjects. J Clin Invest. 1981;68(5):1158-1165. doi: https://doi.org/10.1172/JCI110360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dirksen A,Dijkman JH,Madsen F,et al. A randomized clinical trial of alpha- 1 antitrypsin augmentation therapy. Am J Respir Crit Care Med. 1999;160(5):1468-1472. doi: https://doi.org/10.1164/ajrccm.160.5.9901055 [DOI] [PubMed] [Google Scholar]
- 26.Dirksen A,Piitulainen E,Parr DG,et al. Exploring the role of CT densitometry: a randomised study of augmentation therapy in alpha-1 antitrypsin deficiency. Eur Respir J. 2009;33(6):1345-1353. doi: https://doi.org/10.1183/09031936.00159408 [DOI] [PubMed] [Google Scholar]
- 27.Chapman KR,Burdon JG,Piitulainen E,et al. Intravenous augmentation treatment and lung density in severe alpha-1 antitrypsin deficiency (RAPID): a randomised, double-blind placebo-controlled trial. Lancet. 2015;386(9991):360-368. doi: https://doi.org/10.1016/S0140-6736(15)60860-1 [DOI] [PubMed] [Google Scholar]
- 28.Kao RC,Wehner NG,Skubitz KM,et al. Proteinase 3. A distinct human polymorphonuclear leukocyte proteinase that produces emphysema in hamsters. J Clin Invest. 1988;82(6):1963-1973. doi: https://doi.org/10.1172/JCI113816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owen CA. Leukocyte cell surface proteinases: regulation of expression, functions, and mechanisms of surface localization. Int J Biochem Cell Biol. 2008;40(6-7):1246-1272. doi: https://doi.org/10.1016/j.biocel.2008.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell EJ,Campbell MA,Owen CA. Bioactive proteinase 3 on the cell surface of human neutrophils: quantification, catalytic activity, and susceptibility to inhibition. J Immunol. 2000;165(6):3366-3374. doi: https://doi.org/10.4049/jimmunol.165.6.3366 [DOI] [PubMed] [Google Scholar]
- 31.Sinden NJ,Baker MJ,Smith DJ,et al. α-1-antitrypsin variants and the proteinase/antiproteinase imbalance in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2015;308(2):L179-90. doi: https://doi.org/10.1152/ajplung.00179.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinden NJ,Stockley RA. Proteinase 3 activity in sputum from subjects with alpha-1-antitrypsin deficiency and COPD. Eur Respir J. 2013;41(5):1042-1050. doi: https://doi.org/10.1183/09031936.00089712 [DOI] [PubMed] [Google Scholar]
- 33.Sullivan AL,Stockley RA. Proteinases and COPD. In:. Chronic Obstructive Pulmonary Disease. BlackwellPublishingLtd;2007:349-366. doi: https://doi.org/10.1002/9780470755976.ch31 [Google Scholar]
- 34.Granelli-Peperno A,Vassalli J-D,Reich E. Secretion of plasminogen activator by human polymorphonuclear leukocytes. J Exp Med. 1977;146(6):1693-1706. doi: https://doi.org/10.1084/jem.146.6.1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vassalli J-D,Sappino AP,Belin D. The plasminogen activator/plasmin system. J Clin Invest. 1991;88(4):1067-1072. doi: https://doi.org/10.1172/JCI115405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saksela O,Rifkin DB. Cell-associated plasminogen activation: Regulation and physiological functions. Ann Rev Cell Biol. 1988;4:93-126. doi: https://doi.org/10.1146/annurev.cb.04.110188.000521 [DOI] [PubMed] [Google Scholar]
- 37.Taipale J,Koli K,Keski-Oja J. Release of transforming growth factorbeta1 from the pericellular matrix of cultured fibroblasts and fibrosarcoma cells by plasmin and thrombin. J Biol Chem. 1992;26735:25378-25384. [PubMed] [Google Scholar]
- 38.Raza SL,Nehring LC,Shapiro SD,et al. Proteinase activated receptor-1 regulation of macrophage elastase secretion by serine proteinases. J Biol Chem. 2000;52:41243-41250. doi: https://doi.org/10.1074/jbc.M005788200 [DOI] [PubMed] [Google Scholar]
- 39.Churg A,Wang R,Wang X,et al. Effect of an MMP-9/MMP-12 inhibitor on smoke-induced emphysema and airway remodelling in guinea pigs. Thorax. 2007;628:706-713. doi: https://doi.org/10.1136/thx.2006.068353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy G,Stanton H,Cowell S,et al. Mechanisms for pro matrix metalloproteinase activation. APMIS. 1999;107(1-6):38-44. doi: https://doi.org/10.1111/j.1699-0463.1999.tb01524.x [DOI] [PubMed] [Google Scholar]
- 41.Fu X,Kassim SY,Parks WC,et al. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem. 2001;27644:41279-41287. doi: https://doi.org/10.1074/jbc.M106958200 [DOI] [PubMed] [Google Scholar]
- 42.Owen CA,Campbell EJ. The cell biology of leukocyte-mediated proteolysis. J Leukoc Biol. 1999;65:137-150. doi: https://doi.org/10.1002/jlb.65.2.137 [DOI] [PubMed] [Google Scholar]
- 43.Owen CA. Roles for proteinases in the pathogenesis of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3(2):253-268. https://doi.org/10.2147/COPD.S2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foronjy RF,Mirochnitchenko O,Propokenko O,et al. Superoxide dismutase expression attenuates cigarette smoke- or elastase-generated emphysema in mice. Am J Respir Crit Care Med. 2006;173(6):623-631. doi: https://doi.org/10.1164/rccm.200506-850OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Churg A,Wang X,Wang RD,et al. Alpha1-antitrypsin suppresses TNF-alpha and MMP-12 production by cigarette smoke-stimulated macrophages. Am J Respir Cell Mol Biol. 2007;37(2):144-151. doi: https://doi.org/10.1165/rcmb.2006-0345OC [DOI] [PubMed] [Google Scholar]
- 46.Sapey E,Wood AM,Ahmad A,Stockley RA. Tumor necrosis factor-{alpha} rs361525 polymorphism is associated with increased local production and downstream inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182(2):192-199. doi: https://doi.org/10.1164/rccm.200912-1846OC [DOI] [PubMed] [Google Scholar]
- 47.McDonough JE,Yuan R,Suzuki M,et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365:1567-1575. doi: https://doi.org/10.1056/NEJMoa1106955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stockley JA,Ismail AM,Hughes SM,et al. Maximal mid-expiratory flow detects early lung disease in α1-antitrypsin deficiency. Eur Respir J. 2017;49(3):1602055. doi: https://doi.org/10.1183/13993003.02055-2016 [DOI] [PubMed] [Google Scholar]
- 49.Churg A,Tai H,Coulthard T,et al. Cigarette smoke drives small airway remodeling by induction of growth factors in the airway wall. Am J Respir Crit Care Med. 2006;174(12):1327-1334. doi: https://doi.org/10.1164/rccm.200605-585OC [DOI] [PubMed] [Google Scholar]
- 50.Yoshikawa A,Sato S,Tanaka T,et al. Breakdown of lung framework and an increase in pores of Kohn as initial events of emphysema and a cause of reduction in diffusing capacity. Int J Chron Obstruct Pulmon Dis. 2016;11:2287-2294. doi: https://doi.org/10.2147/COPD.S114281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wright JL. The importance of ultramicroscopic emphysema in cigarette smoke-induced lung disease. Lung. 2001;179:71-81. doi: https://doi.org/10.1007/s004080000048 [DOI] [PubMed] [Google Scholar]
- 52.Joshi R,Heinz A,Fan Q,et al. Role for Cela1 in postnatal lung remodelling and alpha-1 antitrypsin deficient emphysema. Am J Respir Cell Mol Biol. 2018;59(2):167-178. doi: https://doi.org/10.1165/rcmb.2017-0361OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taggart C,Mall MA,Lalmanach G,et al. Protean proteases: at the cutting edge of lung diseases. Eur Respir J. 2017;49(2):pii-1501200. doi: https://doi.org/10.1183/13993003.01200-2015 [DOI] [PubMed] [Google Scholar]