Abstract
The selection of a Bitemporal (BT) or Right Unilateral (RUL) electrode placement affects the efficacy and side effects of electroconvulsive therapy (ECT). Previous studies have not entirely described the neurobiological underpinnings of such differential effects. Recent neuroimaging research on gray matter (GM) volumes is contributing to understand the mechanism of action of ECT, and could clarify the differential mechanisms of BT and RUL ECT. To assess the whole-brain GM volumetric changes observed after treating subjects with treatment-resistant depression (TRD) with BT or RUL ECT, the authors assessed with magnetic resonance imaging (MRI) 24 subjects with TRD (12 subjects receiving bifrontotemporal ECT and 12 subjects receiving RUL ECT) at two time-points (before the first ECT session and after ECT completion). Subjects receiving BT ECT showed GM volume increases in the bilateral limbic system, but RUL ECT treated subjects showed such GM volume increases limited to the right hemisphere. The authors observed significant differences between the two groups in mid-temporal and subcortical limbic structures in the left hemisphere. These findings highlight that ECT-induced GM volume increases may be specifically observed in the stimulated hemisphere(s). The authors suggest electrode placement may relevantly contribute in clinical settings to develop personalized treatment protocols.
Keywords: Treatment-resistant depression, Bitemporal electroconvulsive therapy, Right unilateral electroconvulsive therapy, Brain Morphometry
INTRODUCTION
Electroconvulsive therapy (ECT) is an effective antidepressant treatment for patients with treatment-resistant depression (TRD) {1}. Therapeutic efficacy and cognitive side effects depend on a number of stimulation parameters (such as electrode placement, pulse width, etc.), but the specific impact of these variables on brain anatomy and physiology are poorly understood. Understanding the effects of treatment parameters on brain biology is a missing critical step towards the rational development of therapeutic innovations, particularly when considering individualization strategies focused on target engagement.
Electrode placement is a critical ECT parameter with both therapeutic and side effects implications. The two most common electrode placements are Bitemporal (BT) and Right Unilateral (RUL or D’Elia electrode placement {2}) ECT. The Food and Drug Administration (FDA) concluded in 2011{3} that BT ECT is the most effective electrode placement. Nevertheless, RUL ECT has been increasingly prevalent in the last decades given its more tolerable profile {4}. Challenging common clinical assumptions, some studies {5-6} suggested that RUL ECT is not inferior in efficacy compared to BT ECT. Altogether, it seems that our knowledge on the comparative efficacy and side effect profile of RUL and BT treatments could be partially revised with additional nuances, and these clinical considerations would benefit from a mechanistic understanding of the distinct biological effects of these treatment parameters.
Gray matter volume increases, mainly in the limbic temporal lobe, have been observed after RUL or BT ECT {7-19}. While most publications have not observed an association between volumetric and clinical changes, some have though not always in the same directions {10, 16, 19, 20}. The nature of these changes may shed light on the mechanism of action of ECT, but current studies do not allow a clear disambiguation between the mechanisms of BT vs. RUL ECT. Indeed, only one recent structural neuroimaging study assessed the effect of RUL vs. bilateral (that is, bitemporal and bifrontal) ECT, but, as opposed to our whole-brain approach, the authors limited its focus to volumetric changes in the hippocampus {20}. Results from this study indicated that electrode placement determines the extent of volume change in right and left hemispheres, with bilateral electrode placement being associated with bilateral hippocampal changes, while RUL electrode placement was preferentially associated with right hippocampal changes. In addition to these volumetric differences, previous neurophysiological studies have already shown that slow-wave activity after RUL ECT was observed in the right hemisphere, whereas with BT ECT slow-wave activity was more prominent over the left hemisphere {21}.
In this study, we aimed to assess the effects of ECT electrode placement, and specifically the differential impact of RUL vs. bifrontotemporal ECT, on whole-brain volumetric changes in subjects with TRD. We hypothesized that despite the common causal association with the ECT-induced generalized seizure, RUL and BT electrode placements would lead to different topographic distributions of the volumetric changes observed after ECT: specifically, subjects treated with BT ECT, in comparison with subjects receiving RUL treatment, would show greater gray matter volume increases in the left hemisphere.
METHODS
Participants
We recruited twenty-four subjects with TRD from 2 research centers. Subjects from the Mood Disorders Inpatient Unit of Bellvitge University Hospital, Barcelona, Spain (N = 12) were 59.17 ± 8.02 years of age, and 50% (n = 6) were men. Subjects from the Department of Psychiatry at Massachusetts General Hospital (MGH), Boston, MA (N = 12) were 42.25 ± 15.78 years of age, and 50% (n = 6) were men. Age and gender were used as covariates for statistical analyses. All subjects met criteria for a major depressive episode according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) {22} and structural clinical interviews: the Structured Clinical Interview for DSM-IV Axis I Disorders-Clinician Version (SCID) for the Bellvitge University Hospital {23} and the Mini International Neuropsychiatric Interview (MINI 6.0) for the MGH cohort {24}. Exclusion criteria included: (i) the presence or past history of a severe medical or neurological disorder, (ii) contraindication to magnetic resonance imaging (MRI) scanning or abnormal MRI upon visual inspection and (iii) a history of ECT during the previous 12 months. Pharmacotherapy was maintained unchanged throughout the ECT protocol with close monitoring for unwanted adverse effects (Table 1 and Table SM1). Moreover, subjects from the Bellvitge University Hospital underwent longitudinal clinical assessment using the Hamilton Rating Scale for Depression (HRSD-21 items) {25} and subjects at the MGH using the Quick Inventory of Depressive Symptomatology (QIDS) {26}. The study was approved by the local ethical review board of each center and was performed in accordance with the Declaration of Helsinki. All participants gave written informed consent after a detailed description of the study.
Table 1.
Concurrent pharmacological regimen of the study samples
| Drugs: % (N) | TRD subjects treated with BT ECT (N=12) | TRD subjects treated with RUL ECT (N=11*) | ||
|---|---|---|---|---|
| Antidepressant | 100 | (12) | 90.9 | (10) |
| Antipsychotics | 75 | (9) | 45.4 | (5) |
| Lithium | 16.7 | (2) | 0 | (0) |
| Anxiolytics | 50 | (6) | 36.4 | (4) |
Abbreviations: TRD, Treatment-resistant Depression; BT, Bitemporal; RUL, Right Unilateral; ECT, Electroconvulsive Therapy.
Data was missing for 1 subject.
Electroconvulsive therapy
The twelve subjects from the Bellvitge University Hospital were treated with bifrontotemporal, brief pulse (0.5–1 ms) ECT, using a Thymatron System IV device (Somatics, Lake Bluff, IL, USA). Anesthesia was induced with intravenous thiopental (2–2.5 mg kg−1) and succinylcholine (0.5 mg kg−1) was used for muscle paralysis. Initial stimulus dose was determined by the half-age method {27} and subsequent dosing was determined according to seizure morphology adequacy. The twelve subjects from the MGH were treated with RUL ECT (D’Elia electrode placement {2}) with ultra-brief and brief pulse (0.3–0.5 ms) using a Mecta Corporation Spectrum 5000Q machine. Anesthesia for the procedure was provided using methohexital (0.8–1.2 mg kg−1) for induction and succinylcholine for muscle relaxation (0.5–1 mg kg−1). Initial stimulus dose was determined by titrating seizure threshold, starting with 19 millicoulombs for women and 38 millicoulombs for men. Subsequent treatments were performed at six times the estimated seizure threshold. During an acute course of ECT, treatments occur thrice a week every other day in both centers.
Image acquisition and preprocessing
All the subjects were scanned two times: before the first ECT session (MRI1) and after the completion of the ECT course (MRI2). A 3.0 T structural T1 – weighted MRI high-resolution structural scan was locally acquired for each participant. Subjects from Bellvitge University Hospital were scanned in a Philips Achieva 3.0 Tesla magnet scanner equipped with an eight-channel phased-array head coil. This center acquired 160 slices with repetition time = 8.1 ms; echo time = 3.7 ms; flip angle = 8º; field of view = 240 × 240 mm; matrix size 256 × 256 pixels; in-plane resolution = 0.94 × 0.94 mm2; slice thickness = 1 mm. Subjects from MGH were scanned at the Martinos Center for Biomedical Imaging using a Siemens Skyra 3.0 Tesla magnet scanner equipped with an 32-channel head coil. This center acquired 156 slices with repetition time = 2530 ms; echo time = 1.69, 3.55, 5.41, 7.27 ms; flip angle = 7º; field of view = 256×256 mm; matrix size 256×256 pixels; in-plane resolution = 1 × 1 mm2; slice thickness = 1 mm.
All the structural MRI data were processed on a Microsoft Windows platform using technical computing software (MATLAB 7.14; The MathWorks, Natick, MA, USA) and Statistical Parametric Mapping (SPM12; The Welcome Department of Imaging Neuroscience, London, UK). The preprocessing consisted of an initial rigid-body within-subject coregistration to the first scan to ensure good starting estimates. This was followed by a pairwise longitudinal registration between the scans of each participant to obtain an average image and a Jacobian difference map. The average image was segmented and the gray matter (GM) voxels were multiplied by the Jacobian difference map to obtain a GM volume change map for each participant. Next, we generated one specific template of both study samples (in Montreal Neurological Institute (MNI) space) using a Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra algorithm {28-29}, which was used to spatially normalize the GM volume change maps. Finally, images were smoothed with a 6 mm full-width at half maximum isotropic Gaussian Kernel.
Statistical analyses
Sociodemographic and clinical data were analyzed with SPSS v.21 (SPSS, Chicago, IL, USA) using nonparametric tests.
We used an independent two-sample model to derive a t-statistic map comparing the GM volume change maps between subjects with TRD treated with bifrontotemporal ECT (BT group) and subjects with TRD treated with right unilateral ECT (RUL group). We initially estimated within-group volumetric changes (using two one-sample t-tests at a p level of p<0.05 (two-tailed), Family-Wise error (FWE) corrected for multiple comparisons across the whole brain) and created a combined mask (adding significant changes from both groups) in which we investigated between-group differences. This approach has been used as a strategy to increase the sensitivity directed towards our main objective (i.e., BT vs. RUL ECT comparison). Age and gender were included as confounding covariates. Statistical significance was set at p<0.05 (two-tailed), FWE corrected for multiple comparisons across all in-mask voxels (i.e., using small-volume correction procedures across all voxels showing volumetric changes in the RUL or the BT group). This mask contained 20592 voxels.
RESULTS
Sociodemographic and clinical characteristics
Twelve subjects with TRD from Bellvitge University Hospital received an acute BT ECT course, averaging 11 ECT sessions per patient (mean ECT sessions ± s.d. = 11.08 ± 1.50). The mean ± s.d. HRSD score prior to ECT initiation was 31.25±9.21 and the mean HRSD ± s.d. score after the completion of the ECT was 2.92± 2.54. At the end of treatment, all subjects fulfilled clinical response criteria (reduction > 50% in HRSD score), and all but one were in clinical remission (HRSD < 8). Moreover, the reduction in depression severity (HRSD score) between MRI1 and MRI2 assessments was significant according to a Wilcoxon signed-rank test (z = −3.062; p = 0.002, two-tailed).
On the other hand, twelve subjects with TRD from MGH received an acute RUL ECT course, averaging 10 ECT sessions per patient (mean ECT sessions ± s.d. = 10.33 ± 2.42). The mean ± s.d. QIDS score prior to ECT initiation was 17.42±3.34 and the mean QIDS ± s.d. score after the completion of the ECT was 11± 4.90. At the end of treatment, four subjects fulfilled clinical response criteria (reduction > 50% in QIDS score), and two were in clinical remission (QIDS < 6). The reduction in depression severity (QIDS score) between MRI1 and MRI2 assessments was significant according to a Wilcoxon signed-rank test (z = −2.671; p = 0.008, two-tailed).
Importantly, we observed a significantly (Mann-Whitney U-test, z = −3.984; p < 0.001, two-tailed) greater reduction in clinical severity in subjects treated with BT ECT (89.26%, as measured by the percentage of change in HRSD scores) than in subjects treated with RUL ECT (33.41%, as measured by the percentage of change in QIDS scores). Conversely, the number of ECT sessions did not significantly differ between groups (Mann-Whitney U-test, z = −1.030; p < 0.303, two-tailed).
Neuroimaging analyses
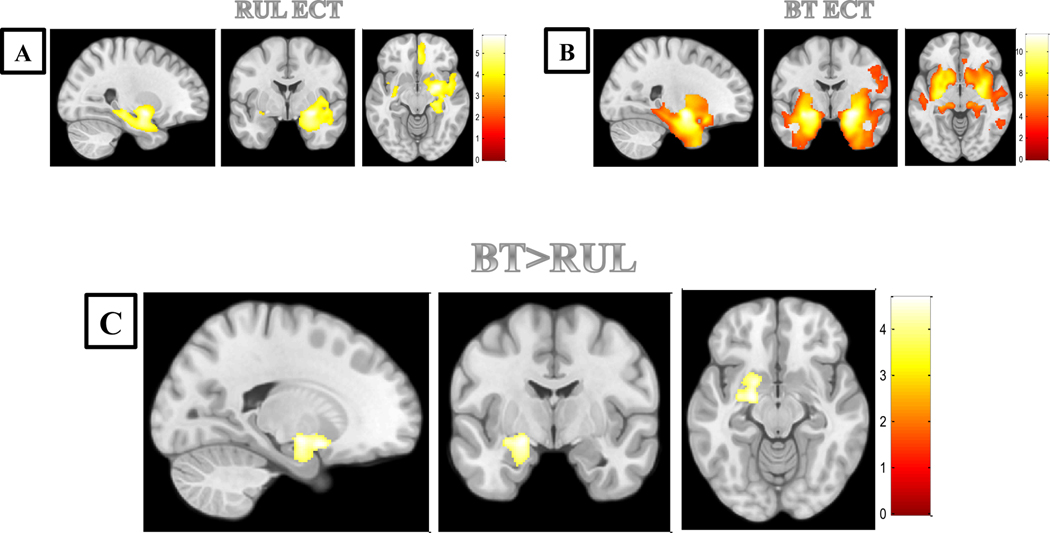
The pattern of volumetric change between MRI1 and MRI2 for each group is shown in Figure 1A and Figure 1B (at a p<0.001, uncorrected, for illustrative purposes). Briefly, in the BT group GM volume increases between both time-points were symmetrically located in the bilateral limbic temporal lobes, the bilateral insula and the bilateral striatum, while in the RUL group, GM volume changes were mainly located in the right limbic temporal lobe. Moreover, both groups showed GM volume increases in the right pregenual anterior cingulate cortex.
Figure 1.
Brain areas showing gray matter volume increases in subjects with treatment-resistant depression treated with bitemporal ECT or right unilateral ECT
A) Volume increases in the right limbic temporal lobe, the right pregenual anterior cingulate cortex, the left putamen and the left insula in subjects with TRD treated with right unilateral ECT. B) Volume increases in the bilateral limbic temporal lobes, the bilateral insula, the bilateral striatum and the right pregenual anterior cingulate cortex in subjects with TRD treated with bitemporal ECT. C) Volume increases in the left limbic system (encompassing the ventral striatum and the amygdala and extending to the ventral hippocampus) in subjects with TRD treated with bitemporal ECT in comparison with subjects receiving right unilateral ECT. Images are in anatomical norm. Sagittal cut is of the right hemisphere. Color bar represents t-value.
Importantly, the direct comparison between subjects with TRD receiving BT and RUL treatment showed that subjects treated with bifrontotemporal ECT, in comparison with subjects receiving right unilateral ECT, displayed a significantly greater GM volume change in left medial-temporal and other subcortical limbic structures, encompassing the ventral striatum (specifically, the ventral putamen portion) and the amygdala, extending to the ventral hippocampus (Table 2 and Figure 1C). These results were significant after adjusting for the number of ECT sessions. Interestingly, the GM volume increase in the left medial-temporal structures was associated with clinical response in a previous study from our group (Cano et al. {19}).
Table 2.
Brain areas showing gray matter volume increases in subjects with treatment-resistant depression treated with bitemporal ECT compared to subjects with treatment-resistant depression treated with right unilateral ECT
| Cluster | x | y | z | t value | df | p valuea | Anatomical location |
|---|---|---|---|---|---|---|---|
| Left limbic system | −20 | 6 | −14 | 4.69 | 20 | 0.027 | Left ventral striatum |
| −21 | −6 | −12 | 4.65 | 20 | 0.029 | Left amygdala |
Abbreviations: df, degrees of freedom. x, y, z coordinates are reported in standard Montreal Neurological Institute (MNI) space.
FWE corrected for multiple comparisons.
DISCUSSION
This is the first study comparing the whole-brain volumetric correlates of BT and RUL ECT in subjects with TRD. In agreement with previous research {7-19}, we observed that ECT is associated with GM volume increases in limbic structures, including the ventral striatum, the amygdala and the hippocampus. Indeed, a large evidence of neuroimaging, neuropathological and lesion analysis studies has highlighted the importance of cortico-limbic circuit disruption in depression {30}. However, our study provides novel insight regarding the GM volume increases associated with ECT electrode placement, since we describe that limbic GM volume increases are specifically observed ipsilateral to the hemisphere(s) stimulated: the pattern of GM change after a BT treatment includes both hemispheres, in comparison with the unilateral right hemisphere changes observed in subjects under RUL ECT. In this sense, our results concur with the recent study by Oltedal et al. {20}, where they observed that while bilateral ECT accounted for similar volume changes in right and left hippocampi, RUL ECT led to more focal effects in the right hippocampus. It is important to note that, although this study also demonstrated a dose-dependent effect of the number of ECT sessions on hippocampal volume, such ECT parameter did not significantly differ between our groups and regressing it out did not change our results. Therefore, our whole-brain analyses show that these changes are not limited to the hippocampus, but extend to other limbic structures critical for negative and positive affective processing and the pathophysiology of depression, such as the amygdala and the ventral striatum {31}.
The literature on BT vs. RUL ECT has shown that the seizure evoked by BT ECT is better generalized throughout the brain {32}. Indeed, current density in unilateral ECT placements is substantially larger in the ipsilateral than in the contralateral hemisphere {33-34}. Accordingly, our brain volumetric findings appear to be related to the specific placement of the electrode(s), and, therefore, likely to be associated to the underlying current density distribution. In addition, although some neuroimaging studies mixed RUL and BT ECT and reported that GM volume increases were not clearly lateralized to the stimulation side {7, 11, 14, 16, 35}, when specifically assessing the brain volumetric effects of RUL ECT {10, 36} or BT ECT {8, 13, 17, 19}, GM volume increases were consistently observed underneath the stimulation side (except for Depping et al. {36}, that limited the analysis to cerebellar volume changes). Overall, our findings and the results from the previous literature clearly support the notion of a direct relationship between electrode placement and lateralization of ECT-induced volumetric increases. Contrary to this line of argument though, it is important to note that, we also observed volumetric increases in the pregenual anterior cingulate cortex that were limited to the right hemisphere both in the BT and the RUL group. Further research is warranted to ascertain the possible mechanisms underlying this finding, which may putatively involve interactions between current density and the specific structural plasticity features of the different brain regions.
Regarding clinical effects, we observed 89.26% of clinical response (as measured by the percentage of change in HRSD scores) in subjects with TRD treated with BT ECT, while subjects treated with RUL ECT only showed a 33.41% of clinical response (as measured by the percentage of change in QIDS scores). These results are in agreement with the conclusions of the FDA in 2011 {3}, indicating that RUL is less effective than BT ECT. While the greater generalization of the evoked seizure in BT ECT {32} has been thought to predict greater clinical response {37-38}, biophysical differences across the two electrode placements, such as induced electric field distribution or intensity in the brain, may be causally linked to the clinical differences between electrode placements. In this sense, the greater capability of BT ECT to induce comparable electric field strength in both hemispheres may be explaining the clinical superiority of this electrode placement {34}. However, despite the characteristically low clinical response of the RUL ECT group, we also observed neuroanatomical changes associated with RUL ECT. In this sense, it is important to note that recent research suggests that volumetric changes should not be necessarily linked to treatment response {20}.
On the other hand, Abrams suggested that slow-wave activity after BT ECT is accentuated on the left hemisphere {21} and that left unilateral (LUL) electrode placement may be as effective as BT ECT {39}, findings that dovetail with the positive association between volume increases in the left hemisphere and clinical improvement observed in our previous study {19}. Therefore, another plausible interpretation of the clinical superiority of BT ECT may be, specifically, the modulation of the left hemisphere. Indeed, other effective brain stimulation treatments for TRD, such as transcranial magnetic stimulation (TMS), primarily target the left hemisphere {40}. Moreover, despite the traditional association between left hemisphere stimulation and verbal memory disruptions, some reports suggest that patients receiving LUL may avoid specific side effects, such as disruption in non-verbal functions {41}. Therefore, LUL may be a reasonable treatment alternative for specific subgroups of patients.
This study has a number of limitations. Most importantly, there is a perfect collinear association between ECT electrode placement and scanner type, as these studies were conducted independently (i.e., without possibility for a priori randomization) and our results stem from a post-hoc analysis of the merged datasets. While these confounders might introduce nuisance between-group variance, the longitudinal processing applied to our data allowed us to minimize such putative inter-scan confounding effects: subjects from the different scanners were never directly compared and, instead, we exclusively compared the gray matter volume change maps which were obtained from each individual patient. Moreover, the anatomical correspondence between stimulation side and structural changes would be difficult to explain by differences in scanner-specific artifacts, and suggest that findings were indeed consequence of ECT treatment. Nevertheless, a prospective study with a priori randomization would be needed to fully confirm our results. Second, we cannot determine what effect, if any, concurrent pharmacological treatment had on our results, although in an attempt to minimize this confounding effect, pharmacological treatment was not modified throughout the entire ECT course. Third, the putative differences between the standardized scales used in each site (i.e., HRSD and QIDS) in ascertaining remission from depression could have compromised our clinical findings. Finally, other ECT parameters in addition to electrode placement differed between study groups (i.e., dosing method and pulse width). Therefore, our results should probably be better understood as depicting the neurobiological correlates of two different treatment approaches differing in various associated factors, of which electrode placement is the primary driver of other differences (e.g., BT ECT will be associated with lower stimulation intensity and greater therapeutic response). Nevertheless, despite being unable to specifically control for these other factors, due to their collinear association with electrode placement, our findings provide a framework to interpret previous results and stimulate further research on the relationships between ECT parameters with changes in brain biology and clinical efficacy in a prospective controlled manner.
In conclusion, our findings indicate that brain volumetric changes associated with ECT may be ipsilateral to the stimulation side(s) (i.e., RUL ECT triggered GM volume increases limited to the right hemisphere while BT ECT unleashed bilateral GM volume increases). Despite its diffuse biophysical properties, the biological effects of ECT are more specific than previously considered, and this specificity is parameter-dependent. Since the lateralization of GM volume changes may be related with the clinical effects of ECT (i.e., efficacy and side effects), our results highlight the importance of carefully considering electrode placement in clinical settings in order to develop tailored treatment protocols, which may include RUL, BT or even LUL approaches. Moreover, clinical research should also carefully consider electrode placement in order to properly interpret the results from studies aiming at unraveling the mechanisms of action of ECT.
Supplementary Material
Table 3.
Medical Exams and Results of Local and Remotely Evaluated Cohorts
| Local (n=16) | Remote (n=56) | P Value | |||||
|---|---|---|---|---|---|---|---|
| Brain MRI, N. (%) | 14 | (87.5) | 48 | (85.7) | 0.8555 | ||
| Normal MRI, N. (%) | 4 | (25.0) | 18 | (32.1) | 0.6021 | ||
| Abnormal MRI, N. (%) | 9 | (56.3) | 29 | (51.8) | |||
| Routine EEG, N. (%) | 12 | (75.0) | 32 | (57.1) | 0.1963 | ||
| Abnormal rEEG, N. (%) | 4 | (25.0) | 11 | (34.4) | 0.8954 | ||
| Epileptiform Activity, N. (%) | 0.7735 | ||||||
| None, N. (%) | 9 | (56.3) | 23 | (41.1) | |||
| Spikes, N. (%) | 0 | (0.0) | 1 | (1.8) | |||
| Sharps, N. (%) | 1 | (6.3) | 2 | (3.6) | |||
| Slowing, N. (%) | 0 | (0.0) | 2 | (3.6) | |||
| Generalized Epileptiform Discharge, N. (%) |
2 | (12.5) | 2 | (3.6) | |||
| Ambulatory EEG, N. (%) | 5 | (31.3) | 5 | (8.9) | 0.0228* | ||
| Abnormal aEEG, N. (%) | 1 | (20.0) | 2 | (40.0) | 0.1819 | ||
| Epileptiform Activity | 0.2439 | ||||||
| None, N. (%) | 4 | (25.0) | 3 | (5.4) | |||
| Slowing, N. (%) | 0 | (0.0) | 1 | (1.8) | |||
| Video EEG, N. (%) | 14 | (87.5) | 52 | (92.9) | 0.4941 | ||
| Abnormal vEEG, N. (%) | 4 | (28.6) | 13 | (25.0) | 0.8813 | ||
| Epileptiform Activity, N. (%) | 0.5296 | ||||||
| None, N. (%) | 10 | (62.5) | 41 | (73.2) | |||
| Spikes, N. (%) | 0 | (0.0) | 2 | (3.6) | |||
| Sharps, N. (%) | 1 | (6.25) | 0 | (0.0) | |||
| Sowing, N. (%) | 0 | (0.0) | 1 | (1.8) | |||
| Generalized Epileptiform Discharge, N. (%) |
1 | (6.3) | 2 | (3.6) | |||
| Other, N. (%) | 0 | (0.0) | 1 | (1.8) | |||
|
Elemental Neuro Exam
Abnormal, N. (%) |
12 | (75) | 32 | (57) | 0.3166 | ||
Abbreviations: MRI, Magnetic Resonance Imaging; EEG, Electroencephalography; rEEG, Routine EEG; aEEG, Ambulatory EEG; vEEG, Video EEG; N., Number.
, Indicates statistically significant differences between local and remote groups.
Table 4.
Symptom Scales of Local and Remotely Evaluated Cohorts
| Local (n=16) | Remote (n=56) | P Value | |||||
|---|---|---|---|---|---|---|---|
| BDI, Mean [95% CI] | 25.0 | [23.0,27.0] | 28.9 | [27.7,30.1] | 0.0016* | ||
| BAI, Mean [95% CI] | 23.3 | [21.4,25.3] | 28.5 | [27.3,29.7] | <0.001* | ||
| GAF, Mean [95% CI] ^ | 51.1 | [48.6,53.6] | 50.4 | [49.1,51.7] | 0.6470 | ||
| CAGE, Mean [95% CI] | 0.6 | [0.3,1.1] | 0.7 | [0.5,1.0] | 0.6655 | ||
| PCL-S, Mean [95% CI] | 57.2 | [55.1,59.3] | 56.8 | [55.5,58.2] | 0.7719 | ||
| HLOC, Mean [95% CI] a | 57.3 | [53.2,61.3] | 53.7 | [51.6,55.8] | 0.1250 | ||
| QOLIE, Mean [95% CI] a, ^ | 37.6 | [34.7,40.6] | 35.8 | [34.4,37.3] | 0.2753 | ||
| FAD | |||||||
| Problem Solving, Mean [95% CI] | 2.4 | [1.8,3.0] | 2.1 | [1.8,2.5] | 0.4270 | ||
| Communication, Mean [95% CI] | 2.4 | [1.8,2.9] | 2.2 | [1.9,2.5] | 0.6463 | ||
| Roles, Mean [95% CI] | 2.4 | [1.8,2.5] | 2.2 | [1.9,2.5] | 0.5695 | ||
| Affective Responsiveness, Mean [95% CI] | 2.4 | [1.8,3.0] | 2.3 | [2.0,2.6] | 0.7811 | ||
| Affective Involvement, Mean [95% CI] | 2.3 | [1.7,2.9] | 2.2 | [1.9,2.5] | 0.6704 | ||
| Behavior Control, Mean [95% CI] | 1.9 | [1.3,2.5] | 1.8 | [1.5,2.1] | 0.7456 | ||
| Global Functioning, Mean [95% CI] | 2.3 | [1.7,2.9] | 2.1 | [1.8,2.4] | 0.4596 | ||
| SCL90 a | |||||||
| Somatization, Mean [95% CI] | 70.0 | [63.2,76.8] | 71.7 | [69.1, 74.3] | 0.6430 | ||
| Obsessive-Compulsive, Mean [95% CI] | 72.0 | [64.6,79.4] | 74.3 | [71.5,77.1] | 0.5596 | ||
| Interpersonal-Sensitivity, Mean [95% CI] | 63.8 | [54.3,73.3] | 68.6 | [65.1,72.2] | 0.3454 | ||
| Depression, Mean [95% CI] | 63.8 | [56.1,71.6] | 73.0 | [70.1,75.9] | 0.0309* | ||
| Anxiety, Mean [95% CI] | 62.2 | [53.3,71.1] | 72.9 | [69.5,76.3] | 0.0279* | ||
| Hostility, Mean [95% CI] | 63.8 | [54.1,73.6] | 66.7 | [63.1,70.4] | 0.5764 | ||
| Phobic Anxiety, Mean [95% CI] | 66.7 | [57.3,76.1] | 71.2 | [67.7,74.8] | 0.3642 | ||
| Paranoid Ideation, Mean [95% CI] | 59.2 | [49.7,68.6] | 63.7 | [60.1,67.3] | 0.3695 | ||
| Psychoticism, Mean [95% CI] | 67.3 | [60.0,74.6] | 72.2 | [69.4,74.9] | 0.2196 | ||
| Global Severity Index, Mean [95% CI] | 70.8 | [64.2,77.5] | 74.9 | [72.4,77.4] | 0.2576 | ||
| Positive Symptom Distress Index, Mean [95% CI] | 67.8 | [61.2,74.4] | 68.5 | [66.0,71.0] | 0.8500 | ||
| SF36 a, ^ | |||||||
| Physical Functioning, Mean [95% CI] | 47.5 | [44.6,50.4] | 40.3 | [38.8,41.7] | <0.001* | ||
| Pain, Mean [95% CI] | 32.7 | [30.0,35.4] | 33.7 | [32.3,35.2] | 0.4932 | ||
| General Health, Mean [95% CI] | 32.0 | [29.4,34.8] | 36.3 | [34.8,37.7] | 0.0083* | ||
| Vitality, Mean [95% CI] | 27.5 | [25.0,30.2] | 27.3 | [26.0,28.6] | 0.8761 | ||
| Social Functioning, Mean [95% CI] | 39.6 | [36.8,42.5] | 37.8 | [36.3,39.3] | 0.2605 | ||
| Mental Health, Mean [95% CI] | 44.7 | [31.8, 57.5] | 43.5 | [36.7, 50.2] | 0.8673 | ||
| Mini-Mental State Exam ^ Mean [95%CI] | 27.3 | [22,30] | 28.0 | [25,30] | 0.1345 | ||
scores are reported as T scores.
For all assessments, except those marked with an up carrot, a higher score indicates a worse condition.
, Indicates statistically significant differences between local and remote groups.
Table 5.
Developmental History of Local and Remotely Evaluated Cohortsa
| Local (n=16) | Remote (n=56) | P Value | |||
|---|---|---|---|---|---|
| Physical Trauma | 8 | (50.0) | 28 | (50.0) | 0.9490 |
| As a Child, N. (%) | 6 | (37.5) | 25 | (44.6) | 0.5900 |
| As an Adult, N. (%) | 4 | (25.0) | 9 | (16.1) | 0.3849 |
| Verbal Trauma | 4 | (25.0) | 23 | (41.1) | 0.2635 |
| As a Child, N. (%) | 4 | (25.0) | 23 | (41.1) | 0.1953 |
| As an Adult, N. (%) | 1 | (6.3) | 1 | (1.8) | 0.3411 |
| Emotional Trauma | 8 | (50.0) | 28 | (50.0) | 0.9191 |
| As a Child, N. (%) | 6 | (37.5) | 24 | (42.9) | 0.8056 |
| As an Adult, N. (%) | 4 | (25.0) | 11 | (19.6) | 0.5873 |
| Sexual Trauma | 6 | (37.5) | 23 | (41.1) | 0.8992 |
| As a Child, N. (%) | 5 | (31.3) | 18 | (32.1) | 0.9494 |
| As an Adult, N. (%) | 3 | (18.8) | 10 | (17.9) | 0.9182 |
| Traumatic Brain Injury, N. (%) | 9 | (56.3) | 40 | (71.4) | 0.2876 |
Abbreviations: N., Number.
Acknowledgements
This study was supported in part by the NIMH (R01 MH112737-01) and the Harvard Medical School Dupont-Warren award (to JAC), the Carlos III Health Institute (PS09/01961; EC08/00134 and CIBER-CB06/03/0034), FEDER funds, “A way to build Europe”, and by the Agency of University and Research Funding Management of the Catalan Government (AGAUR; 2014SGR1672 and 2017SGR1292). MC is supported by a grant from the Spanish Ministry for Education, Culture and Sport (FPU13/02141). IMZ is supported by a P-FIS grant (FI17/00294) from the Carlos III Health Institute. OCR is supported by a postdoctoral “PERIS” contract from the Catalan Government (SLT006/17/00236). CSM is funded by a Miguel Servet contract from the Carlos III Health Institute (CPII16/00048).
Footnotes
Conflicts of interest
JAC serves in the scientific advisory board for Apex Neuroscience. The rest of authors declared no competing interest to disclose.
References
- 1.Lisanby SH: Electroconvulsive therapy for depression. N Engl J Med 2007; 357:1939–1945. [DOI] [PubMed] [Google Scholar]
- 2.d’Elia G: Unilateral electroconvulsive therapy. Acta Psychiatr Scand Suppl 1970; 215:1–98. [PubMed] [Google Scholar]
- 3.Food and Drug Administration: Meeting to Discuss the Classification of Electroconvulsive Therapy Devices (ECT). 2011; http://emord.com/blawg/wp-content/uploads/2016/08/ECT-Petition-Exhibits-Part-1.pdf (accessed February 21 2018).
- 4.Semkovska M, Keane D, Babalola O, et al. : Unilateral brief-pulse electroconvulsive therapy and cognition: effects of electrode placement, stimulus dosage and time. J Psychiatr Res 2011; 45:770–780. [DOI] [PubMed] [Google Scholar]
- 5.Sackeim HA, Prudic J, Devanand DP, et al. : A Prospective, Randomized, Double-blind Comparison of Bilateral and Right Unilateral Electroconvulsive Therapy at Different Stimulus Intensities. Arch Gen Psychiatry 2000; 57:425–434. [DOI] [PubMed] [Google Scholar]
- 6.Semkovska M, Landau S, Dunne R, et al. : Bitemporal Versus High-Dose Unilateral Twice-Weekly Electroconvulsive Therapy for Depression (EFFECT-Dep): A Pragmatic, Randomized, Non-Inferiority Trial. Am J Psychiatry 2016; 173:408–417. [DOI] [PubMed] [Google Scholar]
- 7.Nordanskog P, Dahlstrand U, Larsson MR, et al. : Increase in hippocampal volume after electroconvulsive therapy in patients with depression: a volumetric magnetic resonance imaging study. J ECT 2010; 26:62–67. [DOI] [PubMed] [Google Scholar]
- 8.Tendolkar I, van Beek M, van Oostrom I, et al. : Electroconvulsive therapy increases hippocampal and amygdala volume in therapy refractory depression: a longitudinal pilot study. Psychiatry Res 2013; 214:197–203. [DOI] [PubMed] [Google Scholar]
- 9.Abbott CC, Jones T, Lemke NT, et al. : Hippocampal structural and functional changes associated with electroconvulsive therapy response. Transl Psychiatry 2014; 4:e483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dukart J, Regen F, Kherif F, et al. : Electroconvulsive therapy-induced brain plasticity determines therapeutic outcome in mood disorders. Proc Natl Acad Sci USA 2014; 111:1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nordanskog P, Larsson MR, Larsson EM, et al. : Hippocampal volume in relation to clinical and cognitive outcome after electroconvulsive therapy in depression. Acta Psychiatr Scand 2014; 129:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouckaert F, De Winter FL, Emsell L, et al. : Grey matter volume increase following electroconvulsive therapy in patients with late life depression: a longitudinal MRI study. J Psychiatry Neurosci 2015; 41:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ota M, Noda T, Sato N, et al. : Effect of electroconvulsive therapy on gray matter volume in major depressive disorder. J Affect Disord 2015; 186:186–191. [DOI] [PubMed] [Google Scholar]
- 14.Bouckaert F, Dols A, Emsell L, et al. : Relationship between hippocampal volume, serum BDNF, and depression severity following electroconvulsive therapy in late-life depression. Neuropsychopharmacol 2016; 41:2741–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jorgensen A, Magnusson P, Hanson LG, et al. : Regional brain volumes, diffusivity, and metabolite changes after electroconvulsive therapy for severe depression. Acta Psychiatr Scand 2016; 133:154–164. [DOI] [PubMed] [Google Scholar]
- 16.Joshi SH, Espinoza RT, Pirnia T, et al. : Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry 2016; 79:282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu H, Li X, Zhao W, et al. : Electroconvulsive therapy-induced brain structural and functional changes in major depressive disorders: a longitudinal study. Med Sci Monit 2016; 22:4577–4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sartorius A, Demirakca T, Böhringer A, et al. : Electroconvulsive therapy increases temporal gray matter volume and cortical thickness. Eur Neuropsychopharmacol 2016; 26:506–517. [DOI] [PubMed] [Google Scholar]
- 19.Cano M, Martínez-Zalacaín I, Bernabéu-Sanz Á, et al. : Brain volumetric and metabolic correlates of electroconvulsive therapy for treatment-resistant depression: a longitudinal neuroimaging study. Transl Psychiatry 2017; 7:e1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oltedal L, Narr KL, Abbott C, et al. : Volume of the human hippocampus and clinical response following electroconvulsive therapy. Biol Psychiatry 2018; S0006–3223:31534–31538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abrams R, Dornbush RL, Feldstein S, et al. : Unilateral and bilateral electroconvulsive therapy. Effects on depression, memory, and the electroencephalogram. Arch Gen Psychiatry 1972; 27:88–91. [DOI] [PubMed] [Google Scholar]
- 22.American Psychiatric Association (ed): Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR), 4th edition Washington, DC, APA, 2000. [Google Scholar]
- 23.First MB, Spitzer RL, Gibbon M, et al. (ed): Structured Clinical Interview for DSMIV Axis I Disorders – Clinician Version (SCID-CV). Washington, DC, American Psychiatric Press, 1997. [Google Scholar]
- 24.Sheehan DV, Lecrubier Y, Sheehan KH, et al. : The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 20:22–33. [PubMed] [Google Scholar]
- 25.Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rush AJ, Trivedi MH, Ibrahim HM, et al. : The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003; 54:573–583. [DOI] [PubMed] [Google Scholar]
- 27.Petrides G, Fink M: The “half-age”stimulation strategy for ECT dosing. Convuls Ther 1996; 12:138–146. [PubMed] [Google Scholar]
- 28.Ashburner J: A fast diffeomorphic image registration algorithm. Neuroimage 2007; 38:95–113. [DOI] [PubMed] [Google Scholar]
- 29.Ashburner J, Friston KJ: Computing average shaped tissue probability templates. Neuroimage 2009; 45:333–341. [DOI] [PubMed] [Google Scholar]
- 30.Drevets WC, Price JL, Furey ML: Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 2008; 213:93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price JL, Drevets WC: Neurocircuitry of mood disorders. Neuropsychopharmacology 2010; 35:192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swartz CM, Larson G: Generalization of the effects of unilateral and bilateral ECT. Am J Psychiatry 1986; 143:1040–1041. [DOI] [PubMed] [Google Scholar]
- 33.Sackeim HA: The efficacy of electroconvulsive therapy in the treatment of major depressive disorder, in The Limits of Biological Treatments for Psychological Distress: Comparisons with Psychotherapy and Placebo, edited by Fisher S, Greenberg RP. New York, Lawrence Erlbaum Associates, 1989, pp 275–308. [Google Scholar]
- 34.Lee WH, Deng ZD, Kim TS, et al. : Regional electric field induced by electroconvulsive therapy in a realistic finite element head model: influence of white matter anisotropic conductivity. Neuroimage 2012; 59:2110–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wade BS, Joshi SH, Njau S, et al. : Effect of Electroconvulsive Therapy on Striatal Morphometry in Major Depressive Disorder. Neuropsychopharmacology 2016; 41:2481–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Depping MS, Nolte HM, Hirjak D, et al. : Cerebellar volume change in response to electroconvulsive therapy in patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry 2017; 73:31–35. [DOI] [PubMed] [Google Scholar]
- 37.Ottosson JO: Experimental studies of the mode of action of electroconvulsive therapy: Introduction. Acta Psychiatr Scand Suppl 1960; 35:5–6. [PubMed] [Google Scholar]
- 38.Swartz CM, Nelson AI: Rational Electroconvulsive Therapy Electrode Placement. Psychiatry (Edgmont) 2005; 2:37–43. [PMC free article] [PubMed] [Google Scholar]
- 39.Abrams R (ed): Electroconvulsive therapy, 4th edition, New York, Oxford University Press, 2002. [Google Scholar]
- 40.O’Reardon JP, Solvason HB, Janicak PG, et al. : Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry 2007; 62:1208–1216. [DOI] [PubMed] [Google Scholar]
- 41.Kellner CH, Farber KG, Chen XR, et al. : A systematic review of left unilateral electroconvulsive therapy. Acta Psychiatr Scand 2017; 136:166–176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.