Abstract
Background
Previous studies have established an association between low birthweight (LBW) and future kidney disease, but few have explored the progression of kidney dysfunction through the pediatric years leading up through adolescence and young adulthood.
Methods
To better understand the temporal effects of birthweight on kidney disease progression, we conducted a retrospective cohort study comparing the glomerular filtration rate (GFR) between LBW (<2500 grams) and normal birthweight (NBW) infants who were admitted to the neonatal intensive care unit (NICU) at our institution from 1992 to 2006.
Results
Age at follow-up ranged 1–26 years old. GFR was found to be significantly lower in participants born with LBW than those born with NBW, with a mean difference of 5.5 mL/min/1.73m2 (P < 0.01). These differences were found in the adolescent and young adult age group over 9 years of age, specifically in the extremely low birthweight group (ELBW) whose birthweight was less than 1000 grams.
Conclusions
We recommend screening for CKD in ELBW individuals starting at the age of 9 years old, regardless of their previous medical history.
Keywords: CKD, ESRD, low birthweight, low nephron endowment, prematurity
INTRODUCTION
As a result of medical advances in the past 30 years, low birth-weight (LBW) and preterm infants now have a better chance of surviving to adulthood than ever before [1]. About 90% of the infants with birthweights <1500 g survive to be discharged from the neonatal intensive care unit (NICU) and ∼60% of survivors leave the NICU without any major neonatal morbidity [2]. However, as the first few generations of these NICU graduates reach adulthood, there is concern for their increased risk for chronic kidney disease (CKD) associated with LBW and extreme prematurity [3].
While nephron formation begins as early as 9 weeks gestation [4], the majority of nephrons are formed within the last trimester and completed at 36 weeks gestation [5]. Extreme prematurity results in a gross deficit of nephrons. Neonates who are born at 23 weeks, for example, will miss 12–13 weeks of nephron development compared with their normal-gestation counterparts. Although there is evidence for continuing nephrogenesis for another 40 days post-gestation [6], the nephrons that are formed in an ex utero environment are subject to an increased number of histological abnormalities, including larger than normal glomeruli and reduced podocytes [7]. Nephrologist Barry Brenner proposed that those with a lower nephron endowment can initially maintain a normal glomerular filtration rate (GFR) but eventually suffer from maladaptive processes, such as chronic hyperfiltration injury, leading to hypertension and proteinuria [8].
Multiple studies have already demonstrated the relationship between LBW and kidney disease. In a systematic review that included 31 cohort or case–control studies, White et al. [9] found that individuals who were born with LBW had a 70% greater risk of developing CKD later in life. Extremely LBW (ELBW) infants <1000 g were not included in the meta-analysis, but other more inclusive studies exhibited similar findings. A study of >2 million Norwegian children found that those born below the 10th percentile had a relative risk of 1.7 for end-stage renal disease (ESRD) when compared with those born in the 10th–90th percentile [10]. Similar results were reproduced in the USA, with LBW infants having an odds ratio of 1.4 for developing ESRD when compared with those born with normal birthweight (NBW) [11].
While these studies have highlighted the long-term renal consequences in LBW neonates, few studies have investigated the progression of kidney dysfunction through the pediatric years leading up to young adulthood. One study demonstrated an increase in microalbuminuria in children as young as 8 years old who were born premature [12]. However, it did not differentiate between individuals who were born with LBW and those who had an acute kidney injury event while in the NICU. This makes it difficult to discern if being LBW alone is a relevant risk factor for CKD. Other shortcomings of the medical literature include a small sample size [13], limited racial diversity [14, 15] and incomplete patient birth and medical histories [14].
The question of clinical relevance becomes even more pressing in light of the American Association of Pediatrics’ 2007 recommendation to stop routine screening urinalysis (UA) in all asymptomatic children due to low diagnostic yield and questionable cost-effectiveness [16]. Similarly, the US Preventive Services Task Force also highlighted the lack of sufficient evidence to support CKD screening of asymptomatic adults [17]. Currently, screening patients for CKD is at the discretion of individual physicians.
In our study, we retrospectively compared the renal function of those with a history of LBW to those of NBW at 1–26 years of age. Subjects were free of congenital kidney disease or medical conditions that could lead to renal impairment. In examining the temporal effects of birthweight on kidney disease progression, we hope to provide clinical guidance on screening practices in this particular age group. To our knowledge, this is the largest retrospective cohort study of its kind performed in the USA.
MATERIALS AND METHODS
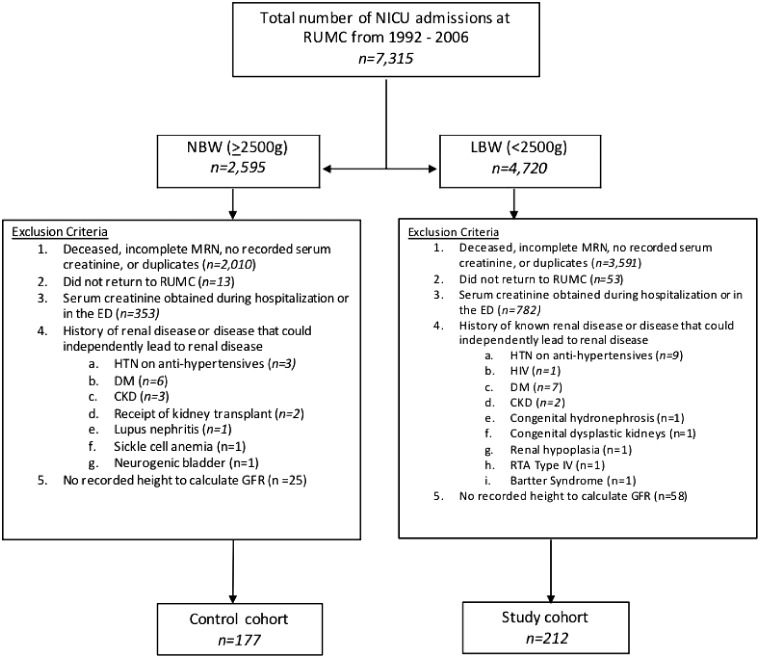
We selected patients from a database of admissions to the NICU at Rush University Medical Center (Chicago, IL, USA) between January 1992 and December 2006. A total of 7315 patients were identified. Among them, 4720 patients were classified as having LBW (<2500 g) and 2595 patients as having NBW (≥2500 g). We excluded patients with incomplete medical record numbers, duplicated charts, missing serum creatinine measurements or those obtained in the inpatient or emergency room setting, missing height measurements, missing follow-up data at our institution or a history of congenital or structural renal disease or medical conditions that may independently lead to renal disease (e.g. human immunodeficiency virus, hypertension and diabetes). After exclusion criteria were applied, 212 patients were included in our study population and 177 patients in our control group (Figure 1). For each patient, the following data were obtained through the electronic medical records: birthweight, gestational age, length of NICU stay, sex, race, medical history, height to calculate estimated GFR, serum creatinine and UA. Serum creatinine was measured using the Jaffé method. GFR was calculated using the original Schwartz equation [18, 19], validated for use in this particular age demographic [20]. The study protocol was approved by the Institutional Review Board at our institution.
FIGURE 1.
Enrollment and exclusion of subjects.
Continuous data are presented as mean ± standard deviation and dichotomous data are reported as a percentage. Differences in continuous and dichotomous variables between groups were analyzed using the Student’s t-test and chi-squared tests, respectively. A P-value < 0.05 was considered statistically significant. All statistical analyses were performed with Excel 2018, version 16.14.1 (Microsoft, Redmond, WA, USA) in consultation with a statistician.
RESULTS
Demographic and clinical characteristics of patients, including birthweight, gestational age, length of NICU stay, sex, race, height and average age at the time of serum creatinine measurement, are presented in Table 1 (Supplementary data, Table S4). The LBW group had a lower mean birthweight and gestational age than the NBW group (P < 0.0001). The LBW group also had a longer length of stay in the NICU compared with the NBW group (P < 0.0001). There were significantly more blacks who were born with LBW than with NBW (P < 0.05), as expected with the known racial disparity in birthweight in the USA [21]. The average age in which serum creatinine was obtained for the LBW and NBW groups was 14.3 ± 5.1 years (range 1–26) and 14.4 ± 4.9 years (range 1–24), respectively. Mean height did not differ significantly between the two groups.
Table 1.
Demographic characteristics of LBW and NBW subjects and comparison by serum creatinine, GFR and proteinuria
| Characteristics | LBW | n | NBW | n | P-value |
|---|---|---|---|---|---|
| Birthweight (g), mean ± SD | 1453.9 ± 603.9 | 212 | 3307.9 ± 531.3 | 177 | <0.0001 |
| Gestational age (weeks), mean ± SD | 30.5 ± 4.0 | 212 | 38.4 ± 1.9 | 177 | <0.0001 |
| Length of NICU stay (days), mean ± SD | 47.6 ± 43.9 | 212 | 11.9 ± 27.1 | 177 | <0.0001 |
| Males (%) | 54.7 | 116 | 59.3 | 105 | 0.361 |
| Blacks (%) | 55.4 | 113 | 43.9 | 76 | <0.05 |
| Hispanics/Latinos (%) | 31.4 | 64 | 36.4 | 63 | 0.320 |
| Whites (%) | 13.2 | 27 | 19.7 | 34 | 0.0918 |
| Height (cm), mean ± SD | 151.9 ± 21.5 | 212 | 154.2 ± 22.5 | 177 | 0.151 |
| Age at time of serum creatinine assessment (years), mean ± SD | 14.3 ± 5.1 | 212 | 14.4 ± 4.9 | 177 | 0.319 |
| Serum creatinine (mg/dL), mean ± SD | 0.72 ± 0.2 | 212 | 0.72 ± 0.2 | 177 | 0.408 |
| GFR (mL/min/1.73 m2 ), mean ± SD | 127.4 ± 18.7 | 212 | 132.9 ± 23.5 | 177 | <0.01 |
| ≥2+ proteinuria (%) | 4.4 | 7/159 | 1.7 | 2/117 | 0.206 |
GFR was lower in the LBW group [127.4 ± 18.7 mL/min/1.73 m2 (range 85.2–188.2)] versus the NBW group [132.9 ± 23.5 mL/min/1.73 m2 (range 85.7–248.3)], reaching statistical significance (P < 0.01) (Table 1). Proteinuria, defined as ≥2+ proteinuria, was more prevalent in the LBW group (4.4%) than in the NBW group (1.7%), although this did not reach statistical significance.
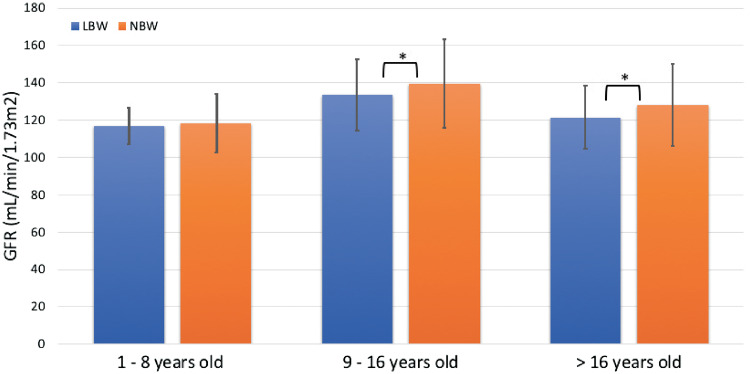
Subjects were further divided into three age groups: 1–8, 9–16 and >16 years. The mean GFR was plotted against the age groups to observe the GFR trend over time (Figure 2). Both LBW and NBW cohorts observed an increase in GFR from the youngest to the middle age group and a decline from the middle to the oldest age group, a trend consistent with other studies [22–25]. GFR in the LBW cohort was consistently lower in all three age groups when compared with the NBW cohort, reaching statistical significance in the middle and oldest age groups (both with P < 0.05).
FIGURE 2.
Comparison of GFR between LBW and NBW individuals over time. *P < 0.05 for LBW versus NBW.
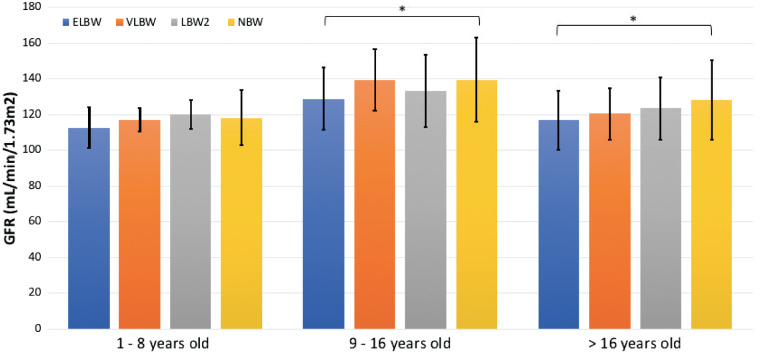
To evaluate whether there was a gradient relationship between birthweight and GFR, the LBW cohort was further stratified into ELBW, very LBW (VLBW) and LBW2 groups, defined as <1000 g at birth, 1000–1499 g at birth and 1500–2499 g at birth, respectively. The mean GFR was again plotted against the three age groups (Figure 3). A similar pattern was observed in that all birthweight cohorts saw an increase in GFR from the youngest to the middle age group and a decline from the middle to the oldest age group. GFR in the ELBW cohort remained consistently lower across all ages when compared with other birthweight groups, reaching statistical significance (P < 0.05) in the middle and oldest cohorts when compared with the NBW population (Table 2). Neither VLBW nor LBW2 cohorts achieved a statistically significant difference compared with the NBW cohort in any age group.
FIGURE 3.
Comparison of GFR between ELBW, VLBW, LBW2 and NBW individuals over time. *P < 0.05 for ELBW versus NBW.
Table 2.
Comparison of ELBW and NBW subjects by age groups
| Age group (years) | ELBW, mean ± SD | n | NBW, mean ± SD | n | P-value |
|---|---|---|---|---|---|
| 1–8 | 112.7 ± 11.5 | 11 | 118.3 ± 15.6 | 22 | 0.155 |
| 9–16 | 128.9 ± 17.5 | 33 | 139.6 ± 23.6 | 94 | <0.05 |
| >16 | 116.8 ± 16.4 | 16 | 128.1 ± 22.2 | 61 | <0.05 |
We observed that LBW males had significantly lower GFRs than NBW males (P < 0.01) (Table 3). The same difference was not observed among females. LBW Hispanic/Latino and white individuals had significantly lower GFRs than their NBW counterparts (both P < 0.05). No significant differences were found between black LBW and NBW participants.
Table 3.
Comparison of LBW and NBW subjects by sex and race
| GFR (mL/min/1.73 m2) by subcategories | LBW, mean ± SD | n | Normal weight, mean ± SD | n | P-value |
|---|---|---|---|---|---|
| Females | 122.3 ± 15.3 | 96 | 123.9 ± 14.9 | 72 | 0.244 |
| Males | 131.7 ± 20.2 | 116 | 139.2 ± 26.2 | 105 | <0.01 |
| Blacks | 124.7 ± 18.3 | 113 | 126.1 ± 15.9 | 76 | 0.289 |
| Females | 119.8 ± 14.5 | 58 | 123.8 ± 14.8 | 34 | 0.112 |
| Males | 129.3 ± 20.3 | 55 | 127.9 ± 16.6 | 42 | 0.371 |
| Hispanics/Latinos | 130.6 ± 18.6 | 64 | 137.3 ± 25.7 | 63 | <0.05 |
| Females | 125.1 ± 15.1 | 25 | 127.3 ± 10.4 | 25 | 0.279 |
| Males | 134.2 ± 19.7 | 39 | 143.9 ± 30.2 | 38 | 0.051 |
| Whites | 128.8 ± 19.1 | 27 | 141.8 ± 27.1 | 34 | <0.05 |
| Females | 123.8 ± 14.4 | 12 | 121.7 ± 17.0 | 10 | 0.379 |
| Males | 132.7 ± 21.3 | 15 | 150.3 ± 26.2 | 24 | <0.05 |
DISCUSSION
In this study we demonstrate LBW is a lone risk factor for GFR decline, as GFR was significantly lower in participants born with LBW than those born with NBW, with a mean difference of 5.5 mL/min/1.73 m2. Our results were consistent with prior studies with a similar focus on the pediatric and young adult population [13, 26]. Previous studies with outcomes that conflicted with ours either had a smaller age range at follow-up [27] or a smaller sample size [28]. Our present study found a significant difference in GFRs between birthweight groups only after participants had reached the adolescent and young adult age group. Thus studies that do not follow participants into this age range may fail to see a difference in renal function. This is likely due to the emergence of renal dysfunction in the late first and second decade of life, in accordance with our data. Additionally, it appears that the ELBW population was the most vulnerable to renal dysfunction. Therefore studies that did not include ELBW infants may fail to see a difference in renal function as well.
Our observation that males accounted for most of the GFR decline is not new. Other studies have demonstrated a similar GFR decline in male LBW infants [29], but not females [30]. However, our study stands in contrast to the findings of a study conducted in the southeastern USA [11]. In examining the association between LBW and ESRD, Lackland et al. [11] found that LBW is associated with an increased risk of ESRD for women but not for men. The opposing findings between our studies with regard to sex differences may reflect our different outcome measurements. Lackland et al. measured ESRD as an endpoint while our study measured initial GFR decline. One hypothesis may be that males tend to exhibit earlier signs of GFR decline but progress to ESRD later in life. Moreover, the study conducted by Lackland et al. comprised a younger cohort with a median age of 34 years. Therefore the same trend in sex differences cannot be extrapolated beyond this age.
In regard to race, our study found that the LBW Hispanic/Latino and white populations had a significantly lower GFR than their NBW counterparts. These findings were not observed in the black population, indicating that LBW only appears to adversely impact Hispanic/Latino and white subjects while having little to no effects on black subjects. Such racial discrepancies may show a relationship to a previous study investigating the association of birthweight with nephron number [31]. Using autopsies from 140 participants 18–65 years of age, Hughson et al. [31] found the correlation between birthweight and nephron number to be stronger among white participants (r = 0.5730, P = 0.0022) than in African Americans (r = 0.2400, P > 0.05). These data suggest that LBW by way of low nephron endowment may play a role in the development of hypertension and eventual renal disease in white subjects but not in African Americans. Although correlation studies suggest that LBW may have contributed to the excess prevalence of ESRD in African Americans [32], our study proposes that there may be unforeseen factors other than LBW accounting for such large racial disparities. Again, our data only include subjects up to 26 years of age; it is unclear if the impact of LBW on different racial groups continues beyond this age.
This study used the Schwartz equation to calculate estimated GFR (eGFR) for adolescents and young adults, as opposed to the adult formulas such as the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. Although there is no current consensus regarding the use of these formulas in this particular age cohort, the decision to use the Schwartz equation was based on evidence provided by two studies [20, 33]. The Schwartz equation demonstrated improved reliability and accuracy in calculating eGFR in adolescents and young adults when compared with the adult equations. Most adult equations have been developed using age as a central component, such as to denote age-related muscle mass decline. Pediatric formulas, on the other hand, were developed using height as a surrogate for muscle mass. Since muscle mass remains constant from young adulthood until middle-age adulthood [34], height-dependent formulas produce less bias than age-dependent formulas. The question of race was not addressed by the aforementioned studies, as the number of black participants was cited as a limitation. In our study, black young adults >16 years of age comprise 75, 62 and 41% of the ELBW, LBW and NBW groups, respectively. One could argue that such racial disparity could lead to a faulty GFR estimate using the Schwartz equation when comparing different weight groups. However, in a separate analysis using the CKD-EPI equation to estimate GFR, race was found to be a statistically nonsignificant factor in our study population. It is hypothesized that the small multiplication coefficient for black subjects in accordance with the CKD-EPI equation is a negligible contribution in the setting of a small sample size.
This study is the largest retrospective cohort study of its kind performed in the USA to longitudinally examine kidney function between various birthweight groups. Limitations of this study include being a single-center study in an urban locale, where differences in socioeconomic status are often more prominent. The current study is unable to control for socioeconomic status, and future studies should explore the interaction of structural violence, LBW and kidney disease. Additionally, since the oldest participant is 26 years old, we currently cannot make conclusions about the progression of renal disease beyond this age. It appears that the beginning of GFR decline occurs around the first to second decade of life, thus future cohort studies should follow participants beyond this age. The sex and racial differences observed in our study should be confirmed in future studies as well. Another limitation includes the use of UA as a method to collect proteinuria data, but false positives can occur in a variety of settings, including but not limited to recent exposure to iodinated radiocontrast agents, alkaline urine and hematuria. An improved approach would be to use a 24-h urine collection or spot albumin or protein:creatinine ratio to quantify proteinuria. Our approach may have overestimated the number of participants having proteinuria.
In conclusion, those born with LBW demonstrated a greater GFR decline than those born with NBW, particularly those who were <1000 g, of the male sex and are Hispanic/Latino or white. While GFR in both groups remained within normal limits, we predict that GFR will decline more rapidly in the LBW group, as several studies have already demonstrated the vulnerability of this population for future CKD and ESRD in adulthood. In addition to blood pressure measurements, we recommend a screening UA and renal function panel for those born with ELBW starting at the age of 9 years, as the two oldest cohorts experienced the most pronounced GFR difference when compared with participants born with NBW. Unfortunately, it is during this adolescent time period when many will see their pediatricians less frequently. Every effort should be made to have these patients made aware of their risk for future CKD and the importance of following up and counseling regarding modifiable risk factors for CKD progression, such as smoking, hypertension and obesity [35]. Additionally, the transition from pediatric to adult practitioners may result in a loss of birth and medical histories. Pediatricians and neonatologists should have clear documentation of birthweight, renal function biomarkers, urine studies, blood pressure and height measurements in patient charts. It is worthwhile to note that there was no difference in serum creatinine between the LBW and NBW groups. Health care providers should not feel reassured at the measurement of a normal serum creatinine. Instead, GFR should be calculated using the original Schwartz or ‘bedside Schwartz’ equations, depending on the creatinine measurement methodology used, as a more accurate measurement of kidney function.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Jennifer Poirier for her technical help and invaluable feedback. This article does not contain any studies with human participants or animals performed by any of the authors.
CONFLICT OF INTEREST STATEMENT
None declared. The results of this article have not been published previously in whole or part.
REFERENCES
- 1. Tommiska V, Heinonen K, Ikonen S.. A nationwide short-term follow-up study of extremely low birth weight infants born in Finland in 1996–1997. Pediatrics 2001; 107: e2–e9 [DOI] [PubMed] [Google Scholar]
- 2. Horbar JD, Carpenter JH, Badger GJ. et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics 2012; 129: 1019–1026 [DOI] [PubMed] [Google Scholar]
- 3. Carmody JB, Charlton JR.. Short-term gestation, long-term risk: prematurity and chronic kidney disease. Pediatrics 2013; 131: 1168–1179 [DOI] [PubMed] [Google Scholar]
- 4. Engle WD. Development of fetal and neonatal renal function. Semin Perinatol 1986; 10: 113–124 [PubMed] [Google Scholar]
- 5. Hinchliffe SA, Sargent PH, Howard CV. et al. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest 1991; 64: 777–784 [PubMed] [Google Scholar]
- 6. Rodriguez MM, Gomez AH, Abitbol CL. et al. Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol 2004; 7: 17–25 [DOI] [PubMed] [Google Scholar]
- 7. Sutherland MR, Gubhaju L, Moore L. et al. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J Am Soc Nephrol 2011; 22: 1365–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brenner BM, Garcia DL, Anderson S.. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens 1988; 1: 335–347 [DOI] [PubMed] [Google Scholar]
- 9. White SL, Perkovic V, Cass A. et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis 2009; 54: 248–261 [DOI] [PubMed] [Google Scholar]
- 10. Vikse BE, Irgens LM, Leivestad T. et al. Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol 2008; 19: 151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lackland DT, Bendall HE, Osmond C. et al. Low birth weights contribute to high rates of early-onset chronic renal failure in the southeastern United States. Arch Intern Med 2000; 160: 1472–1476 [DOI] [PubMed] [Google Scholar]
- 12. Salgado CM, Jardim PC, Teles FB. et al. Influence of low birth weight on microalbuminuria and blood pressure of school children. Clin Nephrol 2009; 71: 367–374 [DOI] [PubMed] [Google Scholar]
- 13. Rodriguez-Soriano J et al. Long-term renal follow-up of extremely low birth weight infants. Pediatr Nephrol 2005; 20: 579–584 [DOI] [PubMed] [Google Scholar]
- 14. Ramirez S, Stephen I, Hsu H. et al. Low body weight is a risk factor for proteinuria in multiracial Southeast Asian pediatric population. Am J Kidney Dis 2001; 38: 1045–1054 [DOI] [PubMed] [Google Scholar]
- 15. Hallan S, Euser AM, Irgens LM. et al. Effect of intrauterine growth restriction on kidney function at young adult age: the Nord Trondelag Health (HUNT 2) Study. Am J Kidney Dis 2008; 51: 10–20 [DOI] [PubMed] [Google Scholar]
- 16. Primack W. AAP does not recommend routine urinalysis for asymptomatic youths. AAP News 2010; 31: 16 [Google Scholar]
- 17. Moyer VA. Screening for chronic kidney disease: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 137: 567–570 [DOI] [PubMed] [Google Scholar]
- 18. Schwartz GJ, Brion LP, Spitzer A.. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 1987; 34: 571–590 [DOI] [PubMed] [Google Scholar]
- 19. Schwartz GJ, Gauthier B.. A simple estimate of glomerular filtration rate in adolescent boys. J Pediatr 1985; 106: 522–526 [DOI] [PubMed] [Google Scholar]
- 20. Selistre L, De Souza V, Pierre C. et al. GFR estimation in adolescents and young adults. J Am Soc Nephrol 2012; 23: 989–996 [DOI] [PubMed] [Google Scholar]
- 21. David RJ, Collins JW.. Differing birth weight among infants of U.S.-born blacks, African-born blacks and U.S.-born whites. N Engl J Med 1997; 337: 1209–1214 [DOI] [PubMed] [Google Scholar]
- 22. Schwartz GJ, Work DF.. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 2009; 4: 1832–1843 [DOI] [PubMed] [Google Scholar]
- 23. Brodehl J, Gellissen K, Weber H-P.. Postnatal development of tubular phosphate reabsorption. Clin Nephrol 1892; 17: 163–171 [PubMed] [Google Scholar]
- 24. Gibb DM, Dalton NR, Barratt MT.. Measurement of glomerular filtration rate in children with insulin-dependent diabetes mellitus. Clin Chim Acta 1989; 182: 131–139 [DOI] [PubMed] [Google Scholar]
- 25. Pottel H. Measuring and estimating glomerular filtration rate in children. Pediatr Nephrol 2017; 32: 249–263 [DOI] [PubMed] [Google Scholar]
- 26. Gielen M, Pinto-Sietsma S-J, Zeegers MP. et al. Birth weight and creatinine clearance in young adult twins: influence of genetic, prenatal, and maternal factors. J Am Soc Nephrol 2005; 16: 2471–2476 [DOI] [PubMed] [Google Scholar]
- 27. Rakow A, Johansson S, Legnevall L. et al. Renal volume and function in school-age children born preterm or small for gestational age. Pediatr Nephrol 2008; 23: 1309–1315 [DOI] [PubMed] [Google Scholar]
- 28. Keijzer-Veen MG, Kleinveld HA, Lequin MH. et al. Renal function and size at young adult age after intrauterine growth restriction and very premature birth. Am J Kidney Dis 2007; 50: 542–551 [DOI] [PubMed] [Google Scholar]
- 29. Li SCC, Shlipak M, Bakris G. et al. Low birth weight is associated with chronic kidney disease only in men. Kidney Int 2008; 73: 637–642 [DOI] [PubMed] [Google Scholar]
- 30. Kristner A, Celsi G, Vanpee M. et al. Increased blood pressure but normal renal function in adult women born preterm. Pediatr Nephrol 2000; 15: 215–220 [DOI] [PubMed] [Google Scholar]
- 31. Hughson MD, Douglas-Denton R, Bertram JF. et al. Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int 2006; 69: 671–678 [DOI] [PubMed] [Google Scholar]
- 32. Lackland DT, Egan BM, Fan ZJ. et al. Low birth weight contributes to the excess prevalence of end-stage renal disease in African Americans. J Clin Hypertension 2001; 3: 29–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Selistre L, Rabilloud M, Cochat P. et al. Comparison of the Schwartz and CKD-EPI equations for estimating glomerular filtration rate in children, adolescents and adults: a retrospective cross-sectional study. PLoS Med 2016; 13: e1001979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Levey AS, Inker LA, Coresh J.. GFR estimation: from physiology to public health. Am J Kidney Dis 2014; 63: 820–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chalmers L, Kaskel FJ, Bamgbola O.. The role of obesity and its bioclinical correlates in the progression of chronic kidney disease. Adv Chronic Kidney Dis 2006; 13: 352–364 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.