Abstract
Background
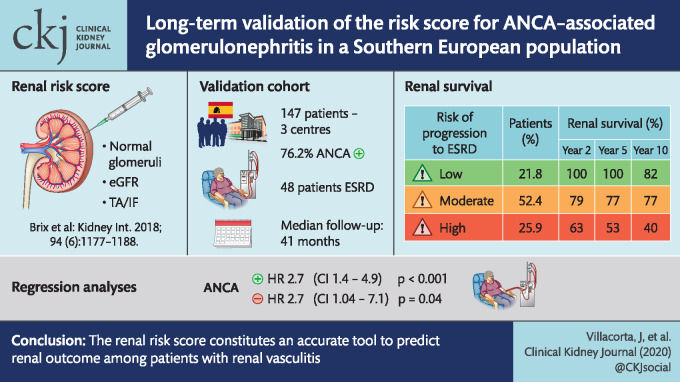
Recently, renal risk score on the basis of three clinicopathologic features to predict end-stage renal disease (ESRD) in antineutrophil cytoplasmic antibody (ANCA)-associated renal vasculitis has been proposed. The aim of this multi-centre study was to validate this renal risk score in a large cohort of southern European patients.
Methods
Data were retrospectively collected from the time of diagnosis by systematic review of medical records from 147 patients with renal vasculitis recruited from three Spanish centres. The renal risk score was calculated in every patient, and renal and global outcomes were analysed according to the risk group assessment.
Results
ANCA serology was positive in 76.2% of patients: 64.6% showed activity against myeloperoxidase (MPO) and 12.2% against proteinase 3 (PR3). The median (interquartile range) follow-up period was 41 months (9.6–104). Forty-eight patients (32.7%) reached ESRD. Patients were classified into the three groups according to the risk of progression to ESRD: 21.8% of patients were classified into low risk, 52.4% were classified into moderate risk and the remaining 25.9% were classified into high risk. The cumulative proportion of renal survival at 2, 5 and 10 years was 100, 100 and 82% in the low-risk group, 79, 77 and 77% in the medium-risk group and 63, 53 and 40% in the high-risk group (P < 0.001). In regression analysis, the risk score was a good predictor for the development of the ESRD among ANCA positive [hazard ratio (HR) = 2.7, 95% confidence interval (CI) 1.4–4.9; P < 0.001] and ANCA negative (HR = 2.7, 95% CI 1.04–7.1, P = 0.04) patients.
Conclusions
The renal risk score constitutes an accurate tool to predict renal outcome among patients with renal vasculitis. This study contributes to validate the risk scoring system in a MPO-predominant population, but also among ANCA-negative vasculitis patients.
Keywords: ANCA, crescentic glomerulonephritis, prognosis, vasculitis
Graphical Abstract
INTRODUCTION
Renal vasculitis is the most common severe manifestation of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) typically presenting with rapidly progressive glomerulonephritis (GN) [1]. Renal impairment at diagnosis predicts poor renal and patient survival [2, 3]. Several studies have also shown the prognostic relevance of renal biopsy in AAV. Histologic factors, such as the percentage of normal glomeruli, the percentage of sclerosed glomeruli and interstitial fibrosis (IF) in the initial renal biopsy, have been proven to be prognostic indicators of renal outcome [4, 5]. An international working group of renal pathologists proposed a classification system for ANCA-associated GN based on glomerulosclerosis, extracapillary proliferation and the percentage of normal glomeruli [6]. This classification comprised four subgroups: focal, crescentic, mixed and sclerotic, and the probability of progressing to end-stage renal disease (ESRD) increased with the ascending sequence of focal, mixed, crescentic and sclerotic GN. Several studies have confirmed the use of the classification system as a predictor of renal outcome, but their differences highlight variation in different population groups [7–10].
Recently, renal risk score on the basis of three clinicopathologic features to predict ESRD has been proposed [11]. It includes parameters such as normal glomeruli, tubular atrophy (TA/IF) and estimated glomerular filtration rate (eGFR) at the time of biopsy. To identify these specific risk factors, histopathologic features of 115 patients in a prospective training cohort and a retrospective validation cohort were analysed. Different models that adjusted for risk factors identified in univariate analysis showed that the percentage of normal glomeruli was an independent predictive factor for ESRD and received the highest weighting for the score, with N1 (10–24% normal glomeruli) and N2 (<10%) receiving 4 and 6 points, respectively. On the other hand, TA/IF >25% (T1) and a baseline eGFR lower of 15 mL/min/1.73 m2 (G1) were weighted at two and three points, respectively. Patients were classified into three different risk groups depending on their sum score: low risk (0–2 points), medium risk (2–7 points) and high risk (8–11 points). The risk score accurately predicted ESRD at 36 months in the training cohort (0, 26 and 68% for low-, medium- and high-risk groups, respectively) and in an independent validation cohort of 90 patients (0, 27 and 78% for low-, medium- and high-risk groups, respectively).
However, both the training cohort and a validation cohort in which the score was analysed were Northern European populations mainly composed of proteinase 3 (PR3)-ANCA patients with granulomatosis with polyangiitis (GPA). These populations significantly differed from vasculitis cohorts in Mediterranean countries, which are characterized by a great predominance of myeloperoxidase (MPO)-ANCA serotype and renal limited forms of vasculitis.
The aim of this multi-centre study was to validate this renal risk score in a large cohort of Southern European patients, analysing the long-term prognostic implications of the clinical and histological features in renal vasculitis.
MATERIALS AND METHODS
Patients
Adult patients diagnosed with renal vasculitis by renal biopsy between 1995 and 2014 from the Nephrology and Pathology Divisions of the Hospital Fundacion Alcorcon (Alcorcon), Hospital Virgen de la Salud of Toledo (Toledo) and Hospital Doce de Octubre (Madrid) were enrolled in this study. Renal biopsy was performed at the time of diagnosis. The diagnosis of renal vasculitis was based on histologic assessment of renal biopsy tissue with haematoxylin and eosin, Masson’s trichrome, periodic acid–Schiff and methenamine silver for light microscopy and staining with antibodies against IgG, IgA, IgM, C1q and C3 for immunofluorescence. Renal vasculitis was defined histologically by the presence of extracapillary proliferation associated with focal glomerular necrosis and/or small vessel vasculitis, in the absence of significant glomerular immune deposits. Pauci-immune was defined when the intensity of glomerular immunoglobulins staining by direct immunofluorescence assay in renal sections was <2+ staining on a scale of 0–4 [12]. Patients with secondary vasculitis or with anti-glomerular basement membrane antibodies were excluded. Secondary vasculitis was considered when associated with cancer, autoimmune disease, infection or drug exposure. The medical records and pathologic data were reviewed and the following information at the time of renal biopsy as well as during follow-up was recorded; patient age, sex, presence of haematuria (five or more red blood cells per high-power field using light microscopy), 24-h urine protein excretion, serum creatinine (sCr) level and eGFR by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine formula [13]. Active urine sediment was defined when haematuria or red blood casts were present. Microscopic polyangiitis (MPA), GPA and renal-limited vasculitis (RLV) were diagnosed according to the American College of Rheumatology and Chapel Hill Consensus Conference (CHCC) criteria [14, 15] (Supplementary data, Table S1). For extra-renal involvement, only manifestations that were both strongly suggestive of vasculitis and included in the Birmingham Vasculitis Activity Score (BVAS) [16] were analysed. The immunosuppressive treatment agents and response rates and relapses in the follow-up were also recorded. Treatment response was defined as the absence of systemic disease activity with improvement or stabilization of renal function in the absence of haematuria. Relapse was defined as the presence of active urine sediment and/or increase in creatinine by >30% attributable to active vasculitis. Informed consent was obtained from each patient. The study was approved by the medical ethics committee at the participating centres.
Histologic studies
Renal specimens were evaluated using direct immunofluorescence (for immunoglobulins and complement components), light and electron microscopy. For light microscopy, periodic acid–Schiff together with silver methenamine, haematoxylin and eosin, and Masson’s trichrome staining were used. Biopsies were reviewed by two different pathologists. Both pathologists scored the biopsies separately, blinded to patients’ data according to a previously standardized protocol for scoring renal biopsies of patients with AAV [6]. The histological scoring was assessed from haematoxylin and eosin and Masson’s trichrome stains, employing one slide for each staining with at least three sections per slide. Each glomerulus was scored separately on the presence of fibrinoid necrosis, crescents (cellular/fibrous/fibrocellular), glomerulosclerosis (segmental/global), granulomatous reactions, and endocapillary and mesangial cellular proliferation. The standardized definitions of the glomerular lesions were as follows, as described previously by Berden et al. [6]. A minimum of eight glomeruli were considered adequate to classify the specimen. Normal glomerulus was considered when fibrinoid necrosis, sclerotic or proliferative lesions were absent. Cellular crescents were considered when cellular components occupied >10% of the crescent surface, irrespective of whether it was segmental or circumferential or whether it contained other components, such as fibrin, or was accompanied by a periglomerular granulomatous reaction or by breaking of Bowman’s capsule. Crescents were considered fibrous when >90% of the crescent surface wasoccupied by extracellular matrix. Global glomerulosclerosis referred to >80% of sclerotic changes within the glomerular tuft. Interstitial and tubular lesions were scored semi-quantitatively on the basis of the percentage of the tubulointerstitial compartment that was affected: interstitial infiltrates (mild: 0–24%; moderate: 25–49%; and severe ≥50%), IF and TA (mild: 0–24%; moderate 25–49%; and severe: ≥50%). All the biopsies were performed before the initiation of immunosuppressive therapy. Differences in scoring between the two pathologists were resolved by re-reviewing the biopsies and coming to a consensus.
Outcome measures
The primary endpoint of the study was the cumulative percentage of patients who developed ESRD over time censored by death. ESRD was defined as the need for long-term renal replacement therapy (≥6 months), for example dialysis or renal transplantation. Renal survival time for each patient was computed from baseline evaluation at the time of biopsy to the last time of follow-up or the time point of reaching ESRD. According to the renal risk score proposed by Brix et al. [11], each patient received a punctuation depending on: (i) the percentage of normal glomeruli in renal biopsy (N0: ˂10% of normal glomeruli; N1: 10–24% of normal glomeruli; and N2: ≥25% of normal glomeruli); (ii) the degree of TA/IF (T0: ˂25% and T1: ≥25%), and (iii) the eGFR at the onset of the disease (G0 ≥15 mL/min/1.73 m2 and G1 ˂15 mL/min/1.73 m2). Patients were classified into three different risk groups depending on their sum score: low risk (0–2 points), medium risk (3–7 points) and high risk (8–11 points).
Statistical analyses
The Kolmogorov–Smirnov test was used to evaluate the normal distribution of continuous data. Normally distributed variables were expressed as mean [standard deviation (SD)] and compared with the unpaired Student's t-test. Those variables without a normal distribution were expressed as median [interquartile range (IQR)] and compared with the Mann–Whitney U-test. Dichotomous variables were expressed as percentages and compared with the Chi-square statistic or the Fisher’s exact test when appropriate. Survival distributions for the time to event were estimated with the Kaplan–Meier method and the log-rank test. The association of variables with ESRD was assessed with multivariate Cox proportional hazard regression models. Variables previously found to affect renal survival were included in the Cox proportional hazards model. Variables were selected by backward elimination using likelihood ratio tests. Univariate survival comparisons were made using the log-rank test. Hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) were computed. Calculations were performed using SPSS version 17.0 (SPSS, Chicago, IL, USA).
RESULTS
Baseline demographic and clinical features
The main baseline clinical and histologic data of the 147 included patients are shown in Table 1. ANCA serology was positive in 76.2% of patients: 64.6% showed activity against MPO, whereas activity against PR3 was identified in 12.2% of patients. According to the CHCC criteria, 57 (38.8%) of the 147 patients were classified into MPO, 10 (6.8%) patients were identified as GPA and 80 (54.4%) patients as RLV (Supplementary data, Table S2). Median (IQR) sCr at diagnosis was 344.7 mmol/L (194–574) and median estimated eGFR (CKD-EPI) 14.7 mL/min/1.73 m2 (8–27.1). Fifty-two of 147 (35.4%) patients required acute dialysis at the onset. Seventeen (32.6%) of these patients became dialysis-free after therapy. Immunosuppressive treatment consisted of steroids plus cyclophosphamide in 86.3% of the cases and steroids plus other immunosuppressant (rituximab or mycophenolate) in the remaining (Supplementary data, Table S3). Owing to a lack of data or early death, treatment response was not evaluable in 12 patients. In the remaining 135 patients, 94 (69.6%) were responsive to treatment. Only 20 (13.6%) patients experienced a renal relapse of the vasculitis. Fifty-one patients (34.7%) died during the follow-up, infection being the major cause of death, accounting for 37% of the cases, followed by cardiovascular disease (17.4%) (Supplementary data, Table S4). Patient survival at 1, 5 and 10 years was 80.9, 72.1 and 67.3%, respectively.
Table 1.
Demographic and clinical characteristics of patients with ANCA-associated glomerulonephritis
| Variable | Total, n = 147 |
|---|---|
| Age, mean (SD), years | 60.2 (16) |
| Male, n/N (%) | 85/147 (57.8) |
| ANCA positive, n/N (%) | 112/147 (76.2) |
| MPO, n/N (%) | 95/147 (64.6) |
| PR3, n/N (%) | 18/47 (12.2) |
| Extrarenal involvement, n/N (%) | 65/147 (44.2) |
| BVAS score, mean (SD) | 15.3 (4.4) |
| Dialysis at onset, n/N (%) | 52/147 (35.3) |
| sCr, median (IQR), mmol/L | 344.7 (194–574) |
| eGFR (CKD-EPI), median (IQR), mL/min/1.73 m2 | 14.7 (8–27.1) |
| Urinary protein excretion, median (IQR), g/day | 1.5 (0.7–3) |
| Immunosuppressive therapy, n (%) | 147 (100) |
| Steroids plus CYC, n/N (%) | 127/147 (86.3) |
| Steroids + other agents, n (%) | 20 (13.6) |
| Treatment response, n/N (%) | 94/135 (69.6) |
| Follow-up period, median (IQR), months | 41 (9.6–104) |
| Evolution to ESRD in the follow-up, n/N (%) | 48/147 (32.7) |
| Death, n/N (%) | 51/147 (34.7) |
| Death or ESRD, n (%) | 74/147 (50.3) |
Histologic findings
The main baseline histologic data are shown in Table 2. The mean percentage of glomeruli showing global sclerosis and the mean percentage of glomeruli with extracapillary proliferation were 22 ± 14 and 45.7 ± 26.8%, respectively. Mesangial and endocapillary cellular proliferation were observed in one-third of the biopsies (30.5 and 33.8%, respectively). The mean percentage of glomeruli showing fibrinoid necrosis was 67.5%. Necrotizing lesions of arterioles occurred in 28 of 147 patients (19%). According to Berden’s histopathological classification, of the 147 biopsies studied, 21 (14.3%), 35 (23.8%), 62 (42.2%) and 29 (19.7%) were classified into the sclerotic, mixed, crescentic and focal categories, respectively.
Table 2.
Histological features of patients with ANCA-associated GN
| Variable | Total, n = 147 |
|---|---|
| Number of glomeruli, median (IQR) | 17 (10–24) |
| Percent normal glomeruli, median (IQR) | 20 (8–33) |
| Percent sclerotic glomeruli, median (IQR) | 14 (4–32) |
| Percent crescents, mean (SD) | 45.7 (26.8) |
| Arteries vasculitis, n/N (%) | 28/147 (19) |
| TA, n/N (%) | 96/147 (65.3) |
| Mild, n/N (%) | 76/147 (51.7) |
| Moderate–severe, n/N (%) | 20/147 (13.6) |
| IF, n/N (%) | 112 /147 (76.1) |
| Mild, n/N (%) | 71/147 (48.2) |
| Moderate–severe, n/N (%) | 41/147 (27.8) |
| Histologic subclass, n/N (%) | |
| Focal | 29/147 (19.7) |
| Mixed | 35/147 (23.8) |
| Crescentic | 62/147 (42.2) |
| Sclerotic | 21/147 (14.3) |
Validation of the risk score
The median (IQR) follow-up period was 41 months (9.6–104). Forty-eight patients (32.7%) reached the primary endpoint (ESRD). Variables including sCr at the onset, the percentage of normal glomeruli, the percentage of glomerulosclerosis, the percentage of extracapillary proliferation, the presence of TA and moderate–severe IF were significantly associated with the development of ESRD. When multivariate analysis was performed, only sCr at the onset and the percentage of normal glomeruli at renal biopsy were independent predictors for renal outcome. Table 3 presents multivariable hazard ratios for the prediction of ESRD according to the clinicopathological characteristics of the scoring system.
Table 3.
Univariate and multivariate Cox regression analyses with ESRD as the endpoint
| Analysis | Β | P-value | HR (95% CI) |
|---|---|---|---|
| Univariate | |||
| sCr, mg/dL | 0.184 | <0.001 | 1.2 (1.14–1.26) |
| Histologic subclass, n | 0.181 | <0.001 | 1.19 (1.11–1.28) |
| Extracapillary proliferation, % | 0.014 | 0.01 | 1.01 (1–1.02) |
| Sclerotic glomeruli, % | 0.013 | 0.02 | 1.01 (1–1.02) |
| Normal glomeruli, % | −0.34 | 0.001 | −0.96 (–0.94 to –0.98) |
| IF, % | 0.62 | 0.001 | 1.8 (1.2–2.6) |
| TA, % | 0.421 | 0.02 | 1.8 (1.04–2.2) |
| Cox regression analysis | |||
| sCr, mg/dL | 0.151 | <0.001 | 1.16 (1.09–1.23) |
| Normal glomeruli, % | 0.558 | 0.02 | 1.03 (1.01–1.05) |
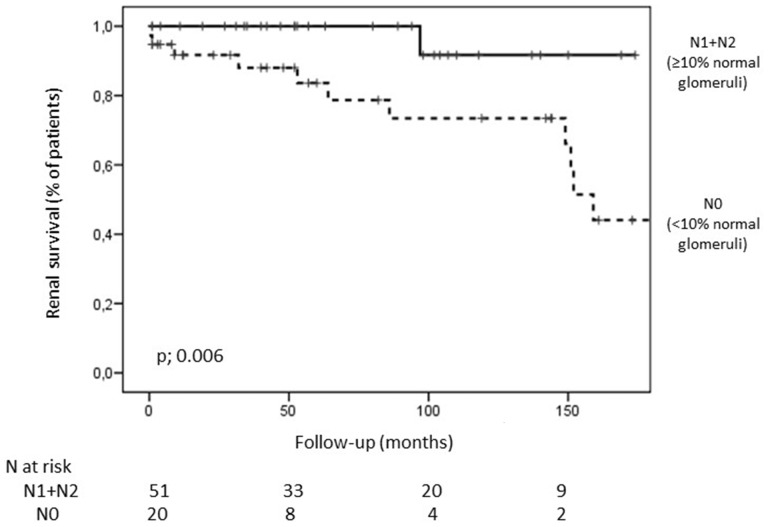
Renal survival was evaluated according to clinicopathologic parameters included in the renal risk score. Only categorization of eGFR at the onset (˂15 or ≥15 mL/min/1.73 m2) and the percentage of normal glomeruli (˂10 or ≥10%), significantly predicted renal outcome among renal vasculitis patients. eGFR categorization (G0 and G1) was a potent predictor for the development of ESRD (HR = 3.09, 95% CI 1.6–5.8; P < 0.001). However, renal outcome among patients presenting with eGFR ≥15 mL/min/1.73 m2 (G1) was significantly poorer between patients with <10% of normal glomeruli (N0) compared with patients presenting with higher percentage of normal glomeruli at renal biopsy (N1 and N2) (Figure 1).
FIGURE 1.
Kaplan–Meier survival curves for the development of ESRD according to the percentage of normal glomeruli at the onset in patients with renal vasculitis presenting with eGFR ≥15 mL/min/1.73 m2.
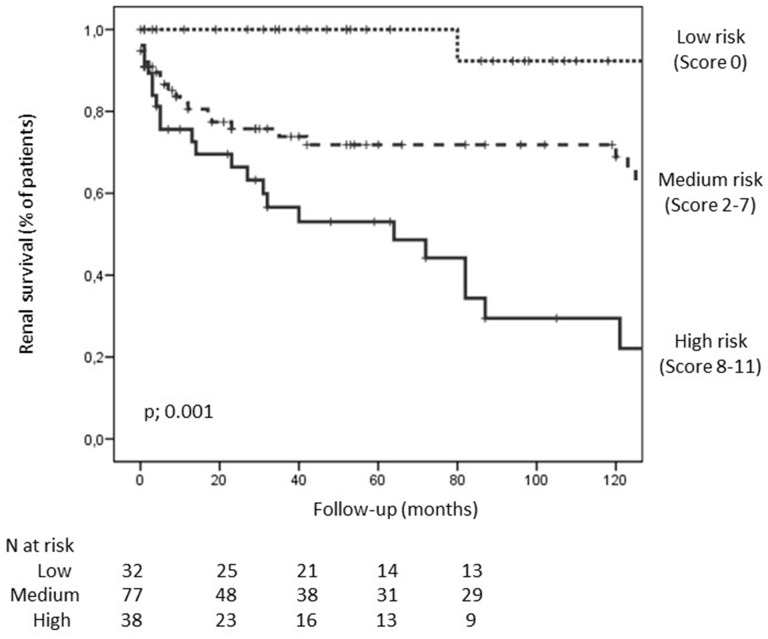
Patients were classified into three groups according to the risk of progression to ESRD: 21.8% of patients were classified into low risk (sum score 0–2), 52.4% were classified into moderate risk (sum score 3–7) and the remaining 25.9% were classified into high risk (sum score 8–11). The cumulative proportion of renal survival at 2, 5 and 10 years was 100, 100 and 82% in the low-risk group, 79, 77 and 77% in the medium-risk group, and 63, 53 and 40% in the high-risk group (P < 0.001) (Figure 2). No differences in renal survival among risk score groups were observed between ANCA-negative and -positive patients. In regression analysis, the risk score was a good predictor for the development of the ESRD among ANCA positive (HR = 2.7, 95% CI 1.4–4.9; P < 0.001) and ANCA-negative (HR = 2.7, 95% CI 1.04–7.1; P = 0.04) patients.
FIGURE 2.
Kaplan–Meier survival curves for the development of ESRD in patients with renal vasculitis according to risk groups (low, medium and high). The survival curves were different between groups (log-rank: P = 0.001).
DISCUSSION
Renal vasculitis is a disease that presents with a rapidly progressive worsening of renal function in the absence of early treatment [17]. Classically, the main prognostic factor described that conditioned renal prognosis in patients with AAV was the degree of renal function impairment at the onset of the disease [2, 18]. Subsequently, it was observed that renal biopsy of patients with vasculitis not only provided a diagnosis, but also prognostic information, so that some histological parameters allowed to discriminate treatment response and renal survival among these patients [19, 20]. Therefore, the histological classification of Berden et al. made it possible to distinguish four histological subclasses (focal, mixed, crescentic and sclerotic), which implied different outcomes [6]. However, this classification excluded interstitial damage and some studies have demonstrated that chronic changes in the interstitium are predictive for renal recovery [20, 21].
Recently, Brix et al. [11] developed and validated a renal risk score to predict the probability of ESRD among 115 AAV patients with renal involvement. In this study, we validate this renal risk score in a large Southern European cohort of patients with renal vasculitis. Of note, our study includes mainly patients with MPO-ANCA serotype and with RLV as the most common form of AAV. Our high incidence of renal limited forms of AAV in our study population is similar to that described in previous studies performed in the same geographical region [22, 23]. This differs from the Brix et al. study, in which half of the population was composed of PR3-ANCA patients. This fact is relevant considering that outcome significantly differs between MPO and PR3 patients, since systemic involvement and relapses are more frequent among PR3 patients [24, 25]. On the other hand, patients with RLV have shown better long-term renal outcome and less frequent relapses compared with patients with GPA and MPA [26, 27]. Despite these demographic and clinical differences, we observed similar cumulative proportion of patients developing ESRD among low- and medium-risk groups of the scoring system. However, 3-year renal survival among patients in the high-risk group was significantly better in our series compared with that reported by Brix et al. (53 versus 32%). We hypothesize that these discrepancies in outcome among patients in high-risk groups could be due to the differences between the study population characteristics, since no differences in the therapeutic approach were observed among patients belonging to the different risk groups.
According to Brix et al.'s observations, eGFR at the time of biopsy was the only clinical variable that reached statistical significance to predict outcome in our series. On the other hand, the number of normal glomeruli was the main histological parameter that independently predicted renal survival in both series. Both eGFR and percentage of normal glomeruli were independent predictors of renal outcome, whereas tubular damage did not influence the prognostic model in our series. eGFR categorization (G0 and G1) of the risk score was the main prognostic factor in our series and only the percentage of normal glomeruli at the onset allowed a better prognostic stratification among renal vasculitis patients presenting with more preserved renal function (eGFR ≥15 mL/min/1.73 m2).
Our results also validate the long-term prediction of the renal risk score. Since AAV is a chronic relapsing and remitting autoimmune disease, it seems difficult to assess a long-term prediction of renal outcome among patients who will probably develop a renal relapse during follow-up. However, the particular epidemiology of AAV in our region, with a predominance of renal-limited MPO patients, implied that most of the patients only suffered one renal flare during follow-up. This fact probably contributed to the persistence of significant differences in renal outcome between the risk score groups after several years of follow-up. Therefore, these results cannot be extrapolated to other vasculitis populations presenting higher rate of relapse, such as PR3-ANCA patients.
Finally, this study has the inherent limitations of a retrospective observational investigation and data collection from three centres. However, our analysis has important strengths, mainly the patients’ long-term follow-up, the careful analysis of histological specimens and the use of hard clinical endpoints such as ESRD.
In conclusion, this study confirms that the renal risk score is a good predictor for the development of ESRD among patients with renal vasculitis. Categorization of eGFR at the onset (G0 and G1) constitutes the main prognostic feature of the score, and only the percentage of normal glomeruli at renal biopsy allowed a better prognostic stratification among patients presenting with eGFR ≥15 mL/min/1.73 m2 in our series. Our results contribute to validate the score in a predominant renal limited MPO-ANCA population, but also among ANCA-negative patients.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank all the clinicians and pathologists for their cooperation.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Falk RJ, Jennette JC.. ANCA small-vessel vasculitis. J Am Soc Nephrol 1997; 8: 314–322 [DOI] [PubMed] [Google Scholar]
- 2. Hauer HA, Bajema IM, Van Houwelingen HC. et al. Determinants of outcome in ANCA-associated glomerulonephritis: a prospective clinico-histopathological analysis of 96 patients. Kidney Int 2002; 62: 1732–1742 [DOI] [PubMed] [Google Scholar]
- 3. Ford SL, Polkinghorne KR, Longano A. et al. Histopathologic and clinical predictors of kidney outcomes in ANCA-associated vasculitis. Am J Kidney Dis 2014; 63: 227–235 [DOI] [PubMed] [Google Scholar]
- 4. Bajema IM, Hagen EC, Hansen BE. et al. The renal histopathology in systemic vasculitis. An international survey study of inter- and intra-observer agreement. Nephrol Dial Transplant 1996; 11: 1989–1995 [DOI] [PubMed] [Google Scholar]
- 5. Bajema IM, Hagen EC, Hermans J. et al. ; for the EC/BCR Project for ANCA-Assay Standardisation. Kidney biopsy as a predictor for renal outcome in ANCA-associated necrotizing glomerulonephritis. Kidney Int 1999; 56: 1751–1758 [DOI] [PubMed] [Google Scholar]
- 6. Berden AE, Ferrario F, Hagen C. et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 2010; 21: 1628–1636 [DOI] [PubMed] [Google Scholar]
- 7. Ellis CL, Manno RL, Havill JP. et al. Validation of the new classification of pauci-immune glomerulonephritis in a United States cohort and its correlation with renal outcome. BMC Nephrol 2013; 14: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diaz-Crespo F, Villacorta J, Acevedo M. et al. The predictive value of kidney biopsy in renal vasculitis; a multicenter cohort study. Hum Pathol 2016; 52: 119–127 [DOI] [PubMed] [Google Scholar]
- 9. Iwakiri G, Fujimoto S, Kitagawa K. et al. Validation of a newly proposed histopathological classification in Japanese patients with anti-neutrophil cytoplasmic antibody-associated glomerulonephritis. BMC Nephrol 2013; 14: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quintana LF, Perez NS, De Sousa E. et al. ANCA serotype and histopathological classification for the prediction of renal outcome in ANCA-associated glomerulonephritis. Nephrol Dial Transplant 2014; 29: 1764–1176 [DOI] [PubMed] [Google Scholar]
- 11. Brix SR, Noriega M, Tennstedt P. et al. Development and validation of a renal risk score in ANCA-associated glomerulonephritis. Kidney Int 2018; 94: 1177–1188 [DOI] [PubMed] [Google Scholar]
- 12. Harris AA, Falk RJ, Jennette JC.. Crescentic glomerulonephritis with a paucity of glomerular immunoglobulin localization. Am J Kidney Dis 1998; 32: 179–184 [DOI] [PubMed] [Google Scholar]
- 13. Levey AS, Stevens LA, Schmid CH. et al. ; for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leavitt RY, Fauci AS, Bloch DA. et al. The American College of Rheumatology 1990 criteria for the classification of Wegener’s granulomatosis. Arthritis Rheum 2010; 33: 1101–1107 [DOI] [PubMed] [Google Scholar]
- 15. Jennette JC, Falk RJ, Bacon PA. et al. 2012 revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum 2013; 65: 1–11 [DOI] [PubMed] [Google Scholar]
- 16. Luqmani RA, Bacon PA, Moots RJ. et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM 1994; 87: 671–678 [PubMed] [Google Scholar]
- 17. Jennette JC, Falk RJ.. Small-vessel vasculitis. N Engl J Med 1997; 337: 1512–1523 [DOI] [PubMed] [Google Scholar]
- 18. Hedger N, Stevens J, Drey N. et al. Incidence and outcome of pauci-immune rapidly progressive glomerulonephritis in Wessex, UK: A 10-year retrospective study. Nephrol Dial Transplant 2000; 15: 1593–1599 [DOI] [PubMed] [Google Scholar]
- 19. Tanna A, Guarino L, Tam F. et al. Long-term outcome of anti-neutrophil cytoplasm antibody-associated glomerulonephritis: evaluation of the international histological classification and other prognostic factors. Nephrol Dial Transplant 2015; 30: 1185–1192 [DOI] [PubMed] [Google Scholar]
- 20. Berden AE, Jones RB, Erasmus DD. et al. ; on behalf of the European Vasculitis Society. Tubular lesions predict renal outcome in antineutrophil cytoplasmic antibody-associated glomerulonephritis after rituximab therapy. J Am Soc Nephrol 2012; 23: 313–321 [DOI] [PubMed] [Google Scholar]
- 21. de Lind van Wijngaarden R, Hauer H, Wolterbeek R. et al. Clinical and histologic determinants of renal outcome in ANCA-associated vasculitis: a prospective analysis of 100 patients with severe renal involvement. J Am Soc Nephrol 2006; 17: 2264–2274 [DOI] [PubMed] [Google Scholar]
- 22. Gonzalez-Gay MA, Garcia-Porrua C, Guerrero J. et al. The epidemiology of the primary systemic vasculitides in northwest Spain: implications of the Chapel Hill Consensus Conference definitions. Arthritis Rheum 2003; 49: 388–393 [DOI] [PubMed] [Google Scholar]
- 23. Fujimoto S, Watts RA, Kobayashi S. et al. Comparison of the epidemiology of anti-neutrophil cytoplasmic antibody-associated vasculitis between Japan and the UK. Rheumatology 2011; 50: 1916–1920 [DOI] [PubMed] [Google Scholar]
- 24. Franssen CF, Stegeman CA, Kallenberg CG. et al. Antiproteinase 3- and antimyeloperoxidase associated vasculitis. Kidney Int 2000; 57: 2195–2206 [DOI] [PubMed] [Google Scholar]
- 25. Murosaki T, Sato T, Akiyama Y. et al. Difference in relapse-rate and clinical phenotype by autoantibody-subtype in Japanese patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Mod Rheumatol 2017; 27: 95–101 [DOI] [PubMed] [Google Scholar]
- 26. Göçeroğlu A, Berden AE, Fiocco M. et al. ; on behalf of the European Vasculitis Society (EUVAS). ANCA-associated glomerulonephritis: risk factors for renal relapse. PLoS One 2016; 11: e0165402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Furuta S, Chaudhry AN, Arimura Y. et al. Comparison of the phenotype and outcome of granulomatosis with polyangiitis between UK and Japanese cohorts. J Rheumatol 2017; 44: 216–222 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.