Abstract
Background
Diabetic nephropathy (DN) is a major complication of diabetes and the main cause of end-stage renal disease. Extracellular vesicles (EVs) are small cell-derived vesicles that can alter disease progression by microRNA (miRNA) transfer.
Methods
In this study, we aimed to characterize the cellular origin and miRNA content of EVs in plasma samples of type 2 diabetes patients at various stages of DN. Type 2 diabetes patients were classified in three groups: normoalbuminuria, microalbuminuria and macroalbuminuria. The concentration and cellular origin of plasma EVs were measured by flow cytometry. A total of 752 EV miRNAs were profiled in 18 subjects and differentially expressed miRNAs were validated.
Results
Diabetic patients with microalbuminuria and/or macroalbuminuria showed elevated concentrations of total EVs and EVs from endothelial cells, platelets, leucocytes and erythrocytes compared with diabetic controls. miR-99a-5p was upregulated in macroalbuminuric patients compared with normoalbuminuric and microalbuminuric patients. Transfection of miR-99a-5p in cultured human podocytes downregulated mammalian target of rapamycin (mTOR) protein expression and downregulated the podocyte injury marker vimentin.
Conclusions
Type 2 diabetes patients with microalbuminuria and macroalbuminuria display differential EV profiles. miR-99a-5p expression is elevated in EVs from macroalbuminuria and mTOR is its validated mRNA target.
Keywords: diabetic kidney disease, diabetic nephropathy, extracellular vesicles, miRNA, mTOR, podocytes
INTRODUCTION
Diabetic nephropathy (DN) occurs in 20–40% of patients with diabetes and is currently the main cause of chronic kidney disease (CKD) worldwide [1]. DN is considered as a pro-inflammatory and pro-coagulant state, as pro-inflammatory and pro-coagulation processes are main players in DN development [2–8]. These processes contribute to the high risk of DN patients to cardiovascular diseases [9, 10].
Albuminuria is the hallmark for DN diagnosis, which is classified in different stages defined by the American Diabetes Association: normal (<30 mg/24 h), microalbuminuria (30–299 mg/24 h) and clinical proteinuria or macroalbuminuria (≥300 mg/24 h) [11]. The primary injuries in DN take place in the glomeruli, affecting all three layers of the glomerular filtration barrier: the glomerular endothelial cells with the glycocalyx, the glomerular basement membrane and podocytes. The mechanism of diabetes-induced glomerular injury is complex since all glomerular cell types highly interact with each other, and disturbance of one of these interactions can disturb the overall glomerular health. Nevertheless, podocytes are generally considered the weakest link in DN because podocytes lack the ability to regenerate. Thus, podocyte loss cannot be restored, which increases the susceptibility of the remaining podocytes to stress and injury [12].
Extracellular vesicles (EVs) are small particles from the plasma membrane or from multivesicular bodies. They are produced by almost all cell types. EVs are surrounded by a phospholipid bilayer and the composition contains similar molecules to the original cell. EVs are 30–1000 nm in size and contain a plethora of bioactive molecules, including proteins, RNAs and lipids [13]. EVs can deliver these bioactive cargos to other cells and in this way play a role in cell-to-cell communication. The biological function of EVs depends on their characteristics, that is, cellular origin, concentration, composition and function. Under healthy conditions, EVs are involved in maintaining homeostasis, including inflammation, coagulation, angiogenesis and wound healing [13]. EV characteristics may change as a consequence of inflammation and coagulation, but EVs are also active players in the progression of inflammatory- and coagulation-related diseases [14, 15]. Thus, increased circulating EVs can be both a consequence and a cause of disease [16]. Diabetic patients and patients with DN/CKD showed increased levels of total plasma EVs [17–19]. Also, diabetic patients and patients with DN showed increased concentration of circulating EVs derived from platelets, monocytes and endothelial cells [17, 18, 20–23]; however, these EV subpopulations are often studied individually and with different methodological approaches.
MicroRNAs (miRNAs) are small non-coding single-stranded RNAs of approximately 22 nucleotides long. miRNAs regulate gene expression by degrading mRNA or by blocking their ribosomal translation. EVs are the major carriers of miRNAs in the circulation [24] and thereby are an important vehicle to selectively deliver miRNAs to recipient cells. It has been previously described that specific miRNAs are altered in plasma EVs in diabetics [25, 26], as well as in diabetic retinopathy [27]. In the context of DN, miRNA profiles in EVs are frequently studied in urinary samples, but insight into miRNA profiles in plasma EVs in DN patients is lacking. Several studies using in vitro and in vivo animal models indicate, however, that miRNAs play a pivotal role in DN and other kidney diseases [28–30]. Therefore, it is expected that miRNAs in circulating EVs are also involved in the pathophysiology of DN.
Taken together, to our knowledge, no prior studies have shown an overview of a wide panel of plasma EV cellular origins combined with profiles of EV miRNA content in several stages of DN. The aim of our study is to characterize plasma EVs with regard to their cellular origin and miRNA content in type 2 diabetes patients with DN and to evaluate the effect of EV miRNAs in vitro.
MATERIALS AND METHODS
A detailed description of the materials and methods section is provided in the Supplementary Information.
Patients
Ninety-three type 2 diabetes patients were randomly selected from a larger, previously described diabetes cohort [31]. After exclusion of current smokers and patients older than 65 years, patients were categorized by urinary albumin excretion levels: normoalbuminuria (<30 mg/day), microalbuminuria (30–300 mg/day) or macroalbuminuria (>300 mg/day). Plasma glucose and lipid profiles, serum and urinary creatinine, plasma high-sensitive C-reactive protein (hsCRP) and HbA1c were determined using standard diagnostic procedures. Estimated glomerular filtration rate by Chronic Kidney Disease Epidemiology Collaboration equation (eGFR)CKD-EPI was calculated according to Levey et al. [32]. This study was approved by the local ethical committee and informed consent was provided by all patients. All investigations have been carried out in accordance with the principles of the Declaration of Helsinki as revised in 2013.
EV measurement by flow cytometry
Blood was drawn and plasma was prepared as previously described [31, 33]. The cellular origin of the EVs was measured by FACS analysis using the A60-Micro flow cytometer (Apogee flow systems). EVs were identified using Calcein Violet 450 AM viability dye (eBioscience) combined with cell-derived surface molecules. Cell-derived surface molecules included: CD61, CD62p, CD34, CD62e, CD235a, CD3, CD14, CD45, CD66b and IgG1. The sample collection, handling and storage, as well as EV measurement methods, have been carried out, where possible, according to the minimal information for studies of EVs 2018 [34].
miRNA profiling, validation and target prediction
EVs RNA was isolated using the exoRNeasy kit (Qiagen) according to the manufacturer’s instructions. EVs miRNA profiling was performed in 18 patients, using the high-throughput miRCURY LNA qPCR assay (Exiqon-Qiagen) as a screening tool. Differentially expressed miRNAs (P < 0.05) were validated in the full patient cohort. Validation was performed using the miRCURY LNA qPCR system (Exiqon-Qiagen). mRNA targets of miR-99a-5p were identified using miRWalk 2.0 [35].
Transfection and stimulation of cultured podocytes
A conditionally immortalized human podocyte cell line (AB8/13; kindly provided by Prof. Moin A. Saleem, University of Bristol, UK) was used to examine the effect of miRNA-99a-5p transfection. Cells were transfected for 24 h with 50 nM of either hsa-miR-99a-5p mimic or negative control mimic (Qiagen) with Lipofectamine 2000 (ThermoFisher) and subsequently stimulated with 30 nM glucose for 24 h. Medium only was used as a non-transfected control. Cells were harvested with Trizol (ThermoFisher) or with radioimmunoprecipitation assay buffer to obtain RNA and protein lysates, respectively. Mammalian target of rapamycin (mTOR) protein expression was corrected for B-actin protein expression. Corrected mTOR expression was normalized for the control condition with medium only.
RESULTS
Cohort characteristics
The demographic and clinical cohort characteristics are shown in Table 1. Age, smoking history, body mass index, diabetes duration, diastolic blood pressure and plasma total cholesterol were similar between groups. The percentage of females was lower in the macroalbuminuria group compared with the normoalbuminuria group. In addition, fasting blood glucose, HbA1c, systolic blood pressure and plasma triglycerides were higher in the macroalbuminuric patients, suggesting poorer glucose and blood pressure regulation compared with normoalbuminuric patients. Compared with normoalbuminuria patients, hsCRP levels were higher in microalbuminuria patients. As expected, in macroalbuminuric patients, the eGFR was lower compared with both normoalbuminuria and microalbuminuria patients. Medication use among the groups is shown in Supplementary data, Table S1.
Table 1.
Comparison of demographic and clinical characteristics
| Demographic | Normoalbuminuria | Microalbuminuria | Macroalbuminuria |
|---|---|---|---|
| n = 32 | n = 28 | n = 33 | |
| Females, % | 50 | 43 | 24a |
| Age, years | 55 (52–58) | 54 (51–56) | 57 (55–59) |
| Smoking history, % | 44 | 39 | 36 |
| Body mass index, kg/m2 | 30 (28–32) | 33 (31–35) | 34 (31–36) |
| Diabetes duration, years | 14 (11–17) | 12 (9–15) | 13 (10–16) |
| Fasting glucose, mmol/L | 8.3 (7.1–9.4) | 8.6 (7.6–9.5) | 9.9 (8.8–11.0)b |
| HbA1c, % | 7.1 (6.7–7.5) | 7.5 (7.1–8.0) | 8.0 (7.6–8.5)c |
| SBP, mmHg | 125 (119–131) | 131 (126–137) | 137 (131–143)b |
| DBP, mmHg | 77 (73–80) | 78 (75–81) | 81 (78–83) |
| Total cholesterol, mmol/L | 4.1 (3.7–4.4) | 4.2 (3.8–4.6) | 4.5 (4.1–4.9) |
| Triglycerides, mmol/L | 1.6 (1.3–1.8) | 1.9 (1.5–2.3) | 2.2 (1.9–2.6)c |
| hsCRP, mg/L | 2.1 (1.4–2.9) | 3.7 (2.5–4.9)b | 4.0 (2.5–5.4) |
| eGFRMDRD, mL/min/1.73 m2 | 88 (78–99) | 89 (81–97) | 73 (65–81)b,d |
| eGFRCKD-EPI, mL/min/1.73 m2 | 89 (81–97) | 90 (83–96) | 74 (66–82)b,d |
| UAE, mg/24 h | 13 (10–16) | 127 (98–155)e | 1023 (767–1279)e,f |
Data are expressed as mean (95% confidence interval).
P < 0.05 versus normoalbuminuria with the Fisher’s exact test.
Significant versus normoalbuminuria P < 0.05.
Significant versus normoalbuminuria P < 0.01.
Significant versus microalbuminuria P < 0.05.
Significant versus normoalbuminuria P < 0.0001.
Significant versus microalbuminuria P < 0.0001.
UAE, urinary albumin excretion; eGFRMDRD, estimated glomerular filtration rate determined by Modification of Diet in Renal Disease; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Cellular origin of plasma EVs
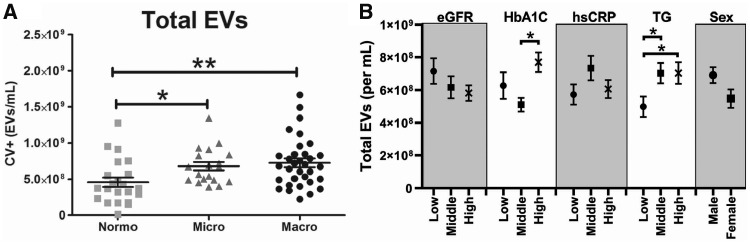
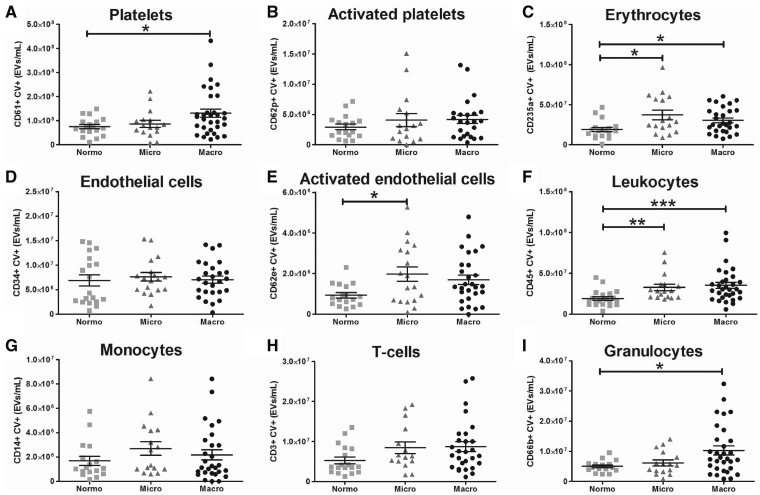
The concentration of total plasma EVs and the concentration of cell-derived EVs are shown in Figures 1 and 2, respectively. The total concentration of plasma EVs was significantly higher in both microalbuminuric and macroalbuminuric patients compared with diabetic controls. EV concentrations were separated by tertile for eGFR, HbA1C, hsCRP, triglycerides and sex (Figure 1B). This figure shows that EV concentrations are the highest in patients within the highest HbA1C and in the middle and high tertile of triglycerides. Platelet (CD61-positive) EVs, but not activated platelet (CD62p-positive) EVs, were higher in macroalbuminuric patients compared with the other two groups. In contrast, endothelial (CD34-positive) cell-derived EVs were similar between groups, whereas activated endothelial cell-derived (CD62e-positive) EVs were higher in the microalbuminuric patients compared with normoalbuminuric patients. Both microalbuminuric and macroalbuminuric patients showed higher concentration of erythrocyte-derived (CD235a-positive) EVs compared with diabetic controls.
FIGURE 1.
Total plasma EVs. (A) Total calcein violet (CV)-positive events in plasma. (B) Total EVs per tertile of eGFR, HbA1C, hsCRP, triglycerides and sex. *P < 0.05, **P < 0.01.
FIGURE 2.
Plasma EV profile. (A–I) Cellular origin of plasma EVs as determined with flow cytometry by double positivity for calcein violet (CV) and a cell-specific marker. *P < 0.05, **P < 0.01, ***P < 0.0001.
Total leucocyte-derived (CD45-positive) EVs were higher in both microalbuminuric and macroalbuminuric patients, likely reflecting the pro-inflammatory state in these patients. In addition, T-cell-derived (CD3-positive) and monocyte-derived (CD14-positive) EVs were similar between groups, and granulocyte-derived (CD66b-positive) EVs were higher in macroalbuminuric patients compared with diabetic controls.
miRNA profiles in plasma EVs
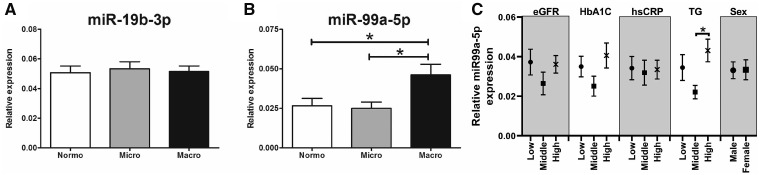
In high-throughput screening analysis, expression levels of 752 miRNAs in plasma EVs were profiled by a quantitative PCR (qPCR) array. Based on the screening analysis in 10 normoalbuminuria and 8 microalbuminuria patients, we selected all miRNAs with P < 0.05 for validation by qPCR in the full cohort of 66 patients. On average, 150 miRNAs per sample were detected. A total of 69 miRNAs were detected in all 18 samples. In this pilot study, none of the miRNAs was found to be differentially expressed between normoalbuminuric and microalbuminuric patients after multiple testing correction (Table 2). Full results of the miRNA profiling are provided in Supplementary data, Table S3. Seven miRNAs were up- or downregulated after individual testing (Table 2); these miRNAs were validated by qPCR in a total of 66 patients, including normoalbuminuric, microalbuminuric or macroalbuminuric patients. miR-136-5p, miR-744-5p, miR-625-3p, miR-205-5p and miR-124-3p were detected in <50% of the patients and therefore these miRNA were not considered relevant in this study. miR-19b-3p and miR-99a-5p were detected in 100 and 95% of the samples, respectively [8% of the samples (n = 2) were undetectable in the normoalbuminuria group and 5% (n = 1) in the microalbuminuria and macroalbuminuria groups]. miR-19b-3p expression was similar between groups, whereas miR-99a-5p was significantly upregulated in EVs from macroalbuminuric patients compared with both normoalbuminuric and macroalbuminuric patients (Figure 3). miR-99a-5p expression was separated per tertile for eGFR, HbA1C, hsCRP, triglycerides and sex (Figure 3C). This figure shows that miR-99a-5p expression was more elevated in the highest tertile for triglycerides compared with the middle and low tertile.
Table 2.
Significantly differentially expressed miRNAs
| miRNA name | Fold change | P-value | q-value |
|---|---|---|---|
| hsa-miR-136-5p | −2.6 | 0.001 | 0.22 |
| hsa-miR-99a-5p | 2.1 | 0.005 | 0.48 |
| hsa-miR-744-5p | −2.1 | 0.016 | 0.90 |
| hsa-miR- 205-5p | 3.0 | 0.039 | 0.90 |
| hsa-miR-625-3p | −3.6 | 0.040 | 0.90 |
| hsa-miR-124-3p | 4.1 | 0.043 | 0.90 |
| hsa-miR-19b-3p | −1.3 | 0.048 | 0.90 |
Seven miRNAs were significantly differentially expressed after individual testing in microalbuminuric patients compared with normoalbuminuric patients.
FIGURE 3.
miRNA expression in plasma EVs. Validation of miR-19b-3p (A) and miR-99a-5p (B) expression in plasma EVs of diabetes patients, determined by qPCR. (C) MiR-99a-5p expression per tertile of eGFR, HbA1C, hsCRP, triglycerides and sex. *P < 0.05. n = 24 for the normoalbuminuria group, n = 20 for the microalbuminuria group and n = 22 for the macroalbuminuria group.
miR-99a-5p target prediction and validation in cultured human podocytes
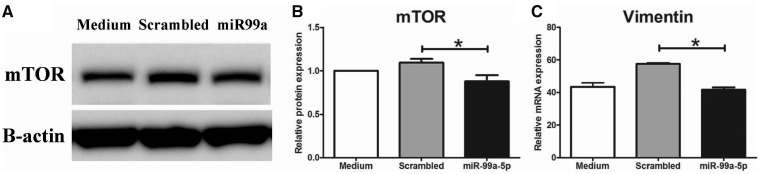
mRNA targets of miR-99a-5p are shown in Supplementary data, Figure S1. mTOR was one of the top hits and was chosen to be validated in vitro as mTOR is known to be involved in the pathogenesis of DN [36]. Transfection of a miR-99a-5p mimic in high glucose-stimulated human podocytes resulted in a downregulation of mTOR protein expression, as shown in Figure 4. Further analysis revealed a downregulation of vimentin after miR-99a-5p transfection compared with the scrambled control, indicating a protective effect to podocyte injury.
FIGURE 4.
miR-99a-5p transfection in human podocytes. (A) Representative western blot of mTOR and B-actin from podocyte lysates cultured in high glucose (30 mM) conditions, transfected with a scrambled miRNA sequence or miR-99a-5p or only medium. (B) Quantification of mTOR protein expression (n = 6). (C) mRNA expression of vimentin (n = 3–4). *P < 0.05.
Correlations between clinical parameters and plasma EVs
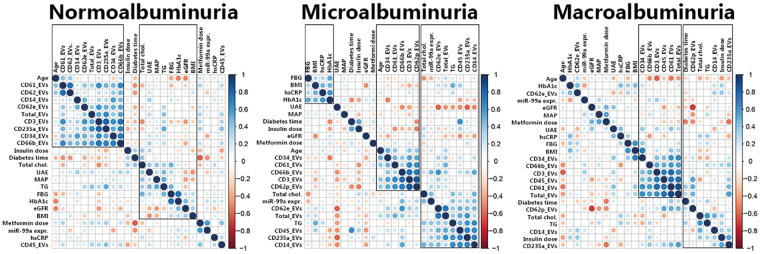
Correlation matrices with hierarchical clustering from the EV profile and clinical parameters are shown in Figure 5. Within the group of microalbuminuric patients, three clusters were formed. In the first cluster, there are positive correlations among fasting blood glucose, BMI, hsCRP and HbA1c, suggesting that in an early stage of DN, glucose levels and systemic inflammation correlate. In the second cluster, age and EVs from endothelial cells, platelets, granulocytes and T-cells cluster together and are positively correlated. Within the third cluster, total cholesterol, triglycerides, EV miR-99a-5p expression and EVs from activated endothelial cells, leucocytes, erythrocytes and monocytes are intercorrelated, indicating that this cluster is related to dyslipidaemia and chronic inflammation.
FIGURE 5.
Correlation matrices. Correlation matrices with unsupervised hierarchical clustering in normoalbuminuria, microalbuminuria and macroalbuminuria patients. Two clusters were recognized in the normoalbuminuria group; in one cluster are the majority of the EV subtypes and age correlated and in the second cluster are most of the metabolic, vascular and renal parameters correlated. In the microalbuminuria group, three clusters were recognized: one cluster with positive correlations between blood glucose, BMI and chronic inflammation; a second cluster with age and some inflammation and coagulation-related EVs; and a third cluster with dyslipidaemia and chronic inflammation parameters, but also miR-99a-5p. In the macroalbuminuria group, two clusters were recognized: one cluster with several inflammation and coagulation related EV subtypes; and a second cluster with diabetes time, dyslipidaemia and platelet-, monocyte- and erythrocyte EVs. FBG, fasting blood glucose; UAE, urinary albumin excretion; MAP, mean arterial pressure; TG, triglycerides; total chol., total cholesterol.
DISCUSSION
This study aimed to characterize plasma EVs by cellular origin and miRNA content in type 2 diabetes patients with DN and to evaluate the consequence of EV miRNAs in vitro. We demonstrated that plasma EV profiles change towards a pro-coagulant and pro-inflammatory profile in DN patients. Also, the expression of EV-incorporated miR-99a-5p was elevated in these patients, which was found to be protective of podocyte injury in vitro.
This study characterized for the first time the plasma profiles of nine EV subtypes, derived from the most prominent coagulation-related and inflammatory cells involved in DN. Other studies often describe only one or a small selection of EV subtypes. Furthermore, this study profiled a large number of EV-incorporated miRNAs in plasma. The characterization of both EV subtypes and miRNA content is unique in plasma samples of DN patients, as the combination of EVs and miRNAs is exclusively studied in urine sample for the discovery of biomarkers [37, 38]. In addition, this study validated mTOR as one of the miR-99a-5p targets in vitro.
Extracellular vesicles
We demonstrated that microalbuminuria, which is the hallmark of Stage 3 (or the incipient stage) of DN, is accompanied by higher levels of total circulating EVs and EVs derived from activated endothelial cells, leucocytes and erythrocytes. A small number of studies that distinguish microalbuminuria from macroalbuminuria found similar EV patterns, but not for all EV subtypes. Endothelial cell-derived EVs were previously found to be higher in microalbuminuric patients [19, 39]; however, our study demonstrates only higher activated endothelial-derived EVs in microalbuminuria, but similar concentration of endothelial-derived EVs between normoalbuminuric and microalbuminuric patients. The study by Yu et al. is one of the few that described a larger panel of EV subtypes in plasma from microalbuminuric patients. They found, corroborating on our findings, elevated concentrations of total EVs, erythrocyte-derived EVs and monocyte-derived EVs [19]. In addition, they found that EVs from platelets, granulocytes and T-lymphocytes were higher in microalbuminuric compared with normoalbuminuric patients. Although we did not find differences in these EVs subtypes, we measured increased concentration of leucocyte-derived EVs as detected by the general leucocyte marker CD45. Taken together, plasma EV profiles are altered in this early stage of DN.
In addition to the incipient stage of DN, overt DN, characterized by macroalbuminuria, was in our study accompanied by higher concentration of total EVs and EVs derived from platelets, erythrocytes, leucocytes and granulocytes. Platelet-, erythrocyte- and leucocyte-derived EVs are often found to be increased in patients with DN [17, 19, 21], but not in all studies [20]. Most other studies also found an altered concentration of endothelial cell- and monocyte-derived EVs in these patients [17, 19, 23], while statistically significant differences in these EV subtypes were absent in our study. Thus, despite some small differences between different studies, overt DN appears to be consistently associated with altered EVs profiles that reflect the pro-inflammatory and pro-coagulation state of these patients.
Of note, it is striking that the abovementioned reports applied different methods to collect, process and identify EVs. The most important difference, in our opinion, is the difference in EV identification. Annexin V is frequently used to identify EVs, because annexin V binds to phosphatidylserine, which is exposed on most, but not all, EVs, especially after a freeze–thaw cycle. The major limitation of annexin V as an EV marker is that annexin V requires addition of calcium in order to bind to phosphatidylserine on EVs. However, adding calcium to plasma may induce plasma clotting, resulting in changing EV concentrations from pro-coagulant EVs. Therefore, annexin V is likely to influence concentrations of total plasma EVs, as well as specific EV subpopulations. For the purpose of accurate and reproducible EV measurements, it is recommended to use one of the other EV markers as described by de Rond et al. [40], but also to follow the guidelines as described by Coumans et al. [41].
EV-associated miRNAs
We demonstrated that miR-99a-5p is upregulated in plasma EVs from diabetic patients with macroalbuminuria. In addition, we showed in vitro that miR-99a-5p downregulates mTOR protein expression and thereby we are the first to validate mTOR as a target of miR-99a-5p. mTOR is a key regulator of cellular homeostasis, including cell growth and metabolism with environmental input, and has been found to be involved in various diseases [42]. Pharmacological mTOR inhibition has been shown to ameliorate renal fibrosis, renal inflammation, glomerulosclerosis and glomerular hypertrophy in animal models for DN [43–46]. However, inhibition or genetic deletion of mTOR has also been found to induce podocyte damage and subsequent proteinuria in kidney transplant recipients and in mice [46, 47]. Both dose and exposure time of the mTOR inhibitor seem to play a role [46]. Gödel et al. [47] showed that tight regulation of mTOR activity (both mTOR complexes 1 and 2) is required in podocyte homeostasis. Taken together, mTOR inhibition has beneficial effects on the progression of DN, but more investigation will be required to obtain more insight in the proteinuric effect of (long-term) mTOR inhibition and to find strategies to bypass this effect (e.g. lower dosages or interval treatment). In our study, we found that miR-99a-5p inhibits mTOR expression. miRNA-mediated mTOR inhibition may not be comparable with a therapeutic intervention that inhibits mTOR protein activity. miRNAs are crucial in fine-tuning gene expression, and downregulation of mTOR mRNA expression will affect both mTOR complexes 1 and 2. Thus, miR-99a-5p-mediated downregulation of mTOR in this experiment may have milder effects compared with mTOR inhibition by rapamycin. In order to assess the effect of miR-99a-5p in podocytes, we measured vimentin mRNA expression as a podocyte injury marker as a proof of principle. The miR-99a-5p mimic and its control were transfected via lipofectamine2000, a compound that is slightly toxic to cells [48]. Transfection of the scrambled miRNA control with lipofectamine2000 resulted in a slight, but not statistically significant, upregulation of vimentin, suggesting a harmful effect of the scrambled miRNA, but more likely of lipofectamine2000. Vimentin mRNA expression, however, was downregulated after miR-99a-5p transfection as compared with the scrambled miRNA, but not compared with the medium control. As the medium-only samples were not exposed to lipofectamine2000, the scrambled miRNA control is the right control for the miR-99a-5p transfection. Vimentin upregulation is a marker for epithelial-to-mesenchymal transition (EMT), a key process in DN, thus miR-99a-5p-induced downregulation of vimentin indicates a protective role of this miRNA to podocyte injury. Taken together, our study provided evidence that miR-99a-5p targets mTOR in podocytes in vitro, which seems to suppress podocyte EMT.
Extrapolation of the effect of miR-99a-5p to the in vivo situation is challenging. Increased levels of a possibly protective miRNA in the most severe patient group of DN seem paradoxical. However, the results from our study may indicate that these patients have a late protective mechanism to limit further (renal) injury. An alternative explanation might be that (renal) cells in macroalbuminuric patients increase the packaging and excretion of EV-incorporated miR-99a-5p to the circulation and thereby losing a natural protective mechanism. This principle of selective secretion of molecules via EVs is demonstrated in human breast cancer cells that secrete caspase 3 to promote their own survival [49] and is also demonstrated to be involved in metastasis [50]. Another alternative explanation for the paradoxical effect may be differences in EV uptake between patient groups, which could be either random or selective for miR-99a-5p rich EVs.
Taken together, we demonstrated that the altered plasma EV profile in DN patients reflect the pro-coagulant and pro-inflammatory state of DN. We also demonstrated that miR-99a-5p is enriched in plasma EVs and that this miRNA protects podocytes, likely via mTOR inhibition. Nonetheless, the role of miR-99a-5p enrichment is plasma EVs in the pathogenesis of DN remains to be elucidated. Future research to elaborate this should comprise evaluation of the EV trafficking in these patients. This includes the effect of glomerular leakage and eGFR decline on plasma EV levels, but also assessing the EV production and uptake and the miRNA content of the different EV subtypes. Additionally, it remains unknown whether plasma EVs in macroalbuminuric patients indeed deliver miR-99a-5p to the kidney, and if so, whether it actually suppresses EMT in podocytes in vivo. We did not validate other predicted targets of miR-99a-5p, which could aid in preventing podocyte injury as well (see Supplementary data, Figure S1 for other potential targets), and we did not validate the predicted targets in other renal cells. Further investigation is required to fully understand the role of miR-99a-5p in DN and its potential to become a therapeutic strategy to limit renal complications in the context of diabetes.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
FUNDING
This research was supported by The Netherlands Organisation for Health Research and Development (ZonMw, clinical fellowship grant assigned to J.J.T.H.R., Grant no. 40-00703-97-12480).
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
REFERENCES
- 1. Gross JL, de Azevedo MJ, Silveiro SP. et al. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 2005; 28: 164–176 [DOI] [PubMed] [Google Scholar]
- 2. Lopes de Faria JB, Silva KC, Lopes de Faria JM.. The contribution of hypertension to diabetic nephropathy and retinopathy: the role of inflammation and oxidative stress. Hypertens Res 2011; 34: 413–422 [DOI] [PubMed] [Google Scholar]
- 3. Stegenga ME, van der Crabben SN, Levi M. et al. Hyperglycemia stimulates coagulation, whereas hyperinsulinemia impairs fibrinolysis in healthy humans. Diabetes 2006; 55: 1807–1812 [DOI] [PubMed] [Google Scholar]
- 4. Duran-Salgado MB, Rubio-Guerra AF.. Diabetic nephropathy and inflammation. World J Diab 2014; 5: 393–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grassi G, Mancia G, Nilsson PM.. Specific blood pressure targets for patients with diabetic nephropathy? Diabetes Care 2016; 39 (Suppl 2): S228–S233 [DOI] [PubMed] [Google Scholar]
- 6. Group AC, Patel A, MacMahon S. et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572 [DOI] [PubMed] [Google Scholar]
- 7. Lattenist L, Ochodnicky P, Ahdi M. et al. Renal endothelial protein C receptor expression and shedding during diabetic nephropathy. J Thromb Haemost 2016; 14: 1171–1182 [DOI] [PubMed] [Google Scholar]
- 8. Roelofs JJ, Vogt L.. Diabetic Nephropathy. Cham, Switzerland: Springer, 2019, 270–91 [Google Scholar]
- 9. Almdal T, Scharling H, Jensen JS. et al. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med 2004; 164: 1422–1426 [DOI] [PubMed] [Google Scholar]
- 10. Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diab 2008; 26: 77–82 [Google Scholar]
- 11. Molitch ME, DeFronzo RA, Franz MJ. et al. Nephropathy in diabetes. Diab Care 2004; 27 (Suppl 1): S79–S83 [DOI] [PubMed] [Google Scholar]
- 12. Lin JS, Susztak K.. Podocytes: the weakest link in diabetic kidney disease? Curr Diab Rep 2016; 16: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van der Pol E, Boing AN, Harrison P. et al. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev 2012; 64: 676–705 [DOI] [PubMed] [Google Scholar]
- 14. Boilard E, Nigrovic PA, Larabee K. et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 2010; 327: 580–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diehl P, Aleker M, Helbing T. et al. Increased platelet, leukocyte and endothelial microparticles predict enhanced coagulation and vascular inflammation in pulmonary hypertension. J Thromb Thrombolysis 2011; 31: 173–179 [DOI] [PubMed] [Google Scholar]
- 16. Zaldivia MTK, McFadyen JD, Lim B. et al. Platelet-derived microvesicles in cardiovascular diseases. Front Cardiovasc Med 2017; 4: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Almquist T, Mobarrez F, Jacobson SH. et al. Effects of lipid-lowering treatment on circulating microparticles in patients with diabetes mellitus and chronic kidney disease. Nephrol Dial Transplant 2016; 31: 944–952 [DOI] [PubMed] [Google Scholar]
- 18. Lu GY, Xu RJ, Zhang SH. et al. Alteration of circulatory platelet microparticles and endothelial microparticles in patients with chronic kidney disease. Int J Clin Exp Med 2015; 8: 16704–16708 [PMC free article] [PubMed] [Google Scholar]
- 19. Yu M, Xie R, Zhang Y. et al. Phosphatidylserine on microparticles and associated cells contributes to the hypercoagulable state in diabetic kidney disease. Nephrol Dial Transplant 2018; 33: 2115–2127 [DOI] [PubMed] [Google Scholar]
- 20. Rodrigues KF, Pietrani NT, Fernandes AP. et al. Circulating microparticles levels are increased in patients with diabetic kidney disease: a case-control research. Clin Chim Acta 2018; 479: 48–55 [DOI] [PubMed] [Google Scholar]
- 21. Omoto S, Nomura S, Shouzu A. et al. Significance of platelet-derived microparticles and activated platelets in diabetic nephropathy. Nephron 1999; 81: 271–277 [DOI] [PubMed] [Google Scholar]
- 22. Nomura S, Shouzu A, Omoto S. et al. Activated platelet and oxidized LDL induce endothelial membrane vesiculation: clinical significance of endothelial cell-derived microparticles in patients with type 2 diabetes. Clin Appl Thromb Hemost 2004; 10: 205–215 [DOI] [PubMed] [Google Scholar]
- 23. Omoto S, Nomura S, Shouzu A. et al. Detection of monocyte-derived microparticles in patients with Type II diabetes mellitus. Diabetologia 2002; 45: 550–555 [DOI] [PubMed] [Google Scholar]
- 24. Diehl P, Fricke A, Sander L. et al. Microparticles: major transport vehicles for distinct microRNAs in circulation. Cardiovasc Res 2012; 93: 633–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jansen F, Wang H, Przybilla D. et al. Vascular endothelial microparticles-incorporated microRNAs are altered in patients with diabetes mellitus. Cardiovasc Diabetol 2016; 15: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Katayama M, Wiklander OPB, Fritz T. et al. Circulating exosomal miR-20b-5p is elevated in Type 2 diabetes and could impair insulin action in human skeletal muscle. Diabetes 2019; 68: 515–526 [DOI] [PubMed] [Google Scholar]
- 27. Mazzeo A, Beltramo E, Lopatina T. et al. Molecular and functional characterization of circulating extracellular vesicles from diabetic patients with and without retinopathy and healthy subjects. Exp Eye Res 2018; 176: 69–77 [DOI] [PubMed] [Google Scholar]
- 28. Trionfini P, Benigni A, Remuzzi G.. MicroRNAs in kidney physiology and disease. Nat Rev Nephrol 2015; 11: 23–33 [DOI] [PubMed] [Google Scholar]
- 29. Wei Q, Mi QS, Dong Z.. The regulation and function of microRNAs in kidney diseases. IUBMB Life 2013; 65: 602–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ichii O, Horino T.. MicroRNAs associated with the development of kidney diseases in humans and animals. J Toxicol Pathol 2018; 31: 23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ahdi M, Gerdes VE, Graaff R. et al. Skin autofluorescence and complications of diabetes: does ethnic background or skin color matter? Diabetes Technol Ther 2015; 17: 88–95 [DOI] [PubMed] [Google Scholar]
- 32. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ochodnicky P, Lattenist L, Ahdi M. et al. Increased circulating and urinary levels of soluble TAM receptors in diabetic nephropathy. Am J Pathol 2017; 187: 1971–1983 [DOI] [PubMed] [Google Scholar]
- 34. Thery C, Witwer KW, Aikawa E. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018; 7: 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dweep H, Gretz N.. miRWalk2.0: a comprehensive atlas of microRNA-target interactions. Nat Methods 2015; 12: 697. [DOI] [PubMed] [Google Scholar]
- 36. Viana SD, Reis F, Alves R.. Therapeutic use of mTOR inhibitors in renal diseases: advances, drawbacks, and challenges. Oxid Med Cell Longev 2018; 2018: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jia Y, Guan M, Zheng Z. et al. miRNAs in urine extracellular vesicles as predictors of early-stage diabetic nephropathy. J Diabetes Res 2016; 2016: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barutta F, Tricarico M, Corbelli A. et al. Urinary exosomal microRNAs in incipient diabetic nephropathy. PLoS One 2013; 8: e73798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang PH, Huang SS, Chen YH. et al. Increased circulating CD31+/annexin V+ apoptotic microparticles and decreased circulating endothelial progenitor cell levels in hypertensive patients with microalbuminuria. J Hypertens 2010; 28: 1655–1665 [DOI] [PubMed] [Google Scholar]
- 40. de Rond L, van der Pol E, Hau CM. et al. Comparison of generic fluorescent markers for detection of extracellular vesicles by flow cytometry. Clin Chem 2018; 64: 680–689 [DOI] [PubMed] [Google Scholar]
- 41. Coumans FAW, Brisson AR, Buzas EI. et al. Methodological guidelines to study extracellular vesicles. Circ Res 2017; 120: 1632–1648 [DOI] [PubMed] [Google Scholar]
- 42. Saxton RA, Sabatini DM.. mTOR signaling in growth, metabolism, and disease. Cell 2017; 169: 361–371 [DOI] [PubMed] [Google Scholar]
- 43. Lloberas N, Cruzado JM, Franquesa M. et al. Mammalian target of rapamycin pathway blockade slows progression of diabetic kidney disease in rats. J Am Soc Nephrol 2006; 17: 1395–1404 [DOI] [PubMed] [Google Scholar]
- 44. Wittmann S, Daniel C, Stief A. et al. Long-term treatment of sirolimus but not cyclosporine ameliorates diabetic nephropathy in the rat. Transplantation 2009; 87: 1290–1299 [DOI] [PubMed] [Google Scholar]
- 45. Yang Y, Wang J, Qin L. et al. Rapamycin prevents early steps of the development of diabetic nephropathy in rats. Am J Nephrol 2007; 27: 495–502 [DOI] [PubMed] [Google Scholar]
- 46. Ma MKM, Yung S, Chan TM.. mTOR inhibition and kidney diseases. Transplantation 2018; 102: S32–S40 [DOI] [PubMed] [Google Scholar]
- 47. Godel M, Hartleben B, Herbach N. et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest 2011; 121: 2197–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang T, Larcher LM, Ma L. et al. Systematic screening of commonly used commercial transfection reagents towards efficient transfection of single-stranded oligonucleotides. Molecules 2018; 23: 2564–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Boing AN, Stap J, Hau CM. et al. Active caspase-3 is removed from cells by release of caspase-3-enriched vesicles. Biochim Biophys Acta 2013; 1833: 1844–1852 [DOI] [PubMed] [Google Scholar]
- 50. Dickman CT, Lawson J, Jabalee J. et al. Selective extracellular vesicle exclusion of miR-142-3p by oral cancer cells promotes both internal and extracellular malignant phenotypes. Oncotarget 2017; 8: 15252–15266 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.