Primary hyperoxaluria type I (PHI) is a rare inborn error of glyoxylate metabolism in the liver, leading to endogenous oxalate overproduction inducing hyperoxaluria. Disease hallmarks are severe nephrocalcinosis, recurrent urolithiasis and rapid chronic kidney disease (CKD), with early end-stage renal disease (ESRD) in infantile oxalosis. Liver/kidney transplantation is the only curative option, thus new non-invasive treatments are undoubtedly necessary [1]. Substrate reduction therapies via RNA interference (RNAi) are currently undergoing clinical trials, targeting either glycolate oxidase–RNAi or liver-specific lactate dehydrogenase (LDHA)-RNAi, aimed at reduce endogenous oxalate production and thus urinary oxalate (Uox) excretion.
Stiripentol, an LDHA-targeted oral commercial medication for Dravet syndrome, was recently reported to significantly reduce Uox in one PHI patient with good kidney function after 10 weeks of treatment [2,3]. Here we present our experience of stiripentol compassionate use in two PHI patients with different clinical conditions: CKD (Patient 1) and ESRD (Patient 2).
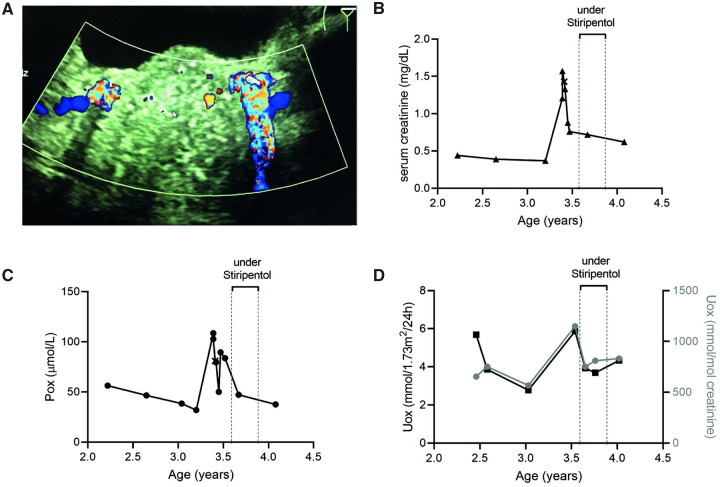
Patient 1 was diagnosed (AGXT: c.33dupC/c.525-1G>A) at the age of 2.5 years. One year later he presented with pyelonephritis, bilateral multiple renal and obstructive prevesical stones, together with increased serum creatinine and plasma oxalate (Pox). Treatment consisted of bilateral nephrostomies, intravenous antibiotics and stone removal. Creatinine, Pox and Uox decreased, but remained elevated (Figure 1). We then administered stiripentol (final dose 50 mg/kg/day) for 10 weeks to avoid further renal deterioration and systemic oxalate deposition. Treatment was safe without any adverse events and the peak of Uox and Pox was occasionally reduced. However, we considered it unsuccessful since Uox remained within its median values and we related Pox reduction to amelioration of kidney function after stone removal. Thus the medication was stopped. Three months later, Uox slightly increased but remained within the range before stiripentol administration. Pox fell below saturation and creatinine decreased further to values similar to those before admission.
FIGURE 1.
Response of Patient 1 (AGXT: c.33dupC/c.525-1G>A) to stiripentol. (A) Ultrasound with twinkling sign of the bilateral prevesical stones and follow-up of (B) serum creatinine (in mg/dL), (C) Pox (µmol/L) and (D) Uox (expressed as the molar oxalate:creatinine ratio in mmol/mol and as oxalate excretion in mmol/1.73 m2/day) since diagnosis and during stiripentol treatment.
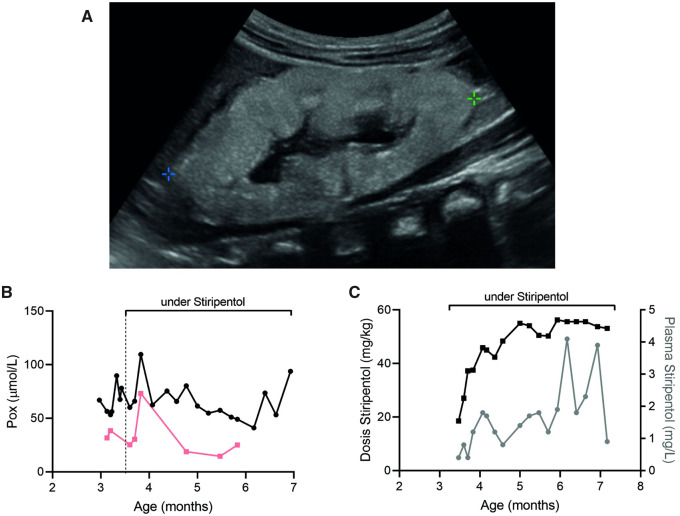
Patient 2 was diagnosed with PHI (AGXT: 508 G>A homozygous) at 3 months of age, with acute anuric kidney failure and infantile oxalosis. The kidneys showed massive nephrocalcinosis and Pox was significantly elevated (Figure 2). Haemodialysis and peritoneal dialysis (3–4 h, 3–4 times per week) plus pyridoxine (B6) treatment (first dose 8 mg, final dosage 17 mg/kg/day) were started immediately while preparing the patient for kidney-after-liver transplantation. Stiripentol treatment (50 mg/kg/day) was started 2 weeks post-diagnosis for 17 weeks, in parallel with dialysis and B6. The treatment was uneventful, without any adverse events. To monitor effectiveness, we measured pre-dialysis Pox regularly after the long free haemodialysis interval (post-weekend). Although stiripentol levels were in therapeutic range, Pox did only decrease as expected with dialysis and pyridoxine (Figure 2). Thus, we stopped stiripentol medication to avoid side effects as described for other indication [4].
FIGURE 2.
Response of Patient 2 (AGXT: 508 G>A homozygous) to stiripentol. (A) Ultrasound at the time of diagnosis, showing hyperechogenic kidney with generalized nephrocalcinosis. Follow-up of (B) Pox levels before (black) and after (pink) haemodialysis, as well as (C) stiripentol dosage (mg/kg) and blood levels (mg/L) in a PHI patient with infantile oxalosis and dialysis treatment.
Stiripentol did not sufficiently reduce oxalate production in either patient, in contrast with the results obtained with LDHA-RNAi (NCT03392896), although medications should not be considered unequivocally equivalent [5]. In Dravet syndrome, stiripentol seems to be effective because of interaction with valproate and clobazam. In the indexed PHI patient [3], non-disclosed additional medications may have modulated the effect of stiripentol in reducing Uox. In PHI, B6 treatment is advised based on genotype. Drug–drug interactions of B6 with stiripentol remains unknown, as well as the effectiveness of combined medication in renal failure. Independently, stiripentol seems useful for occasional prevention of infantile oxalosis when oxalate is extremely elevated. Currently, clinical trials using stiripentol in PHI are ongoing (NCT03819647) [2], which may shed light on its efficacy in a cohort of PHI patients, including B6 and non-B6 responders. However, further pharmacokinetic analyses on stiripentol are definitely needed.
AUTHORS’ CONTRIBUTIONS
B.H. and M.F. obtained the data. B.H. and C.M.-H. analysed the data and wrote the manuscript.
CONFLICT OF INTEREST STATEMENT
B.H. is the Head of Global Medical Affairs at Dicerna Pharmaceuticals. C.M.-H. is a consultant for Dicerna Pharmaceuticals.
REFERENCES
- 1. Weigert A, Martin-Higueras C, Hoppe B.. Novel therapeutic approaches in primary hyperoxaluria. Expert Opin Emerg Drugs 2018; 23: 349–357 [DOI] [PubMed] [Google Scholar]
- 2. Wyatt CM, Drüeke TB.. Stiripentol for the treatment of primary hyperoxaluria and calcium oxalate nephropathy. Kidney Int 2020;97:17–19. [DOI] [PubMed] [Google Scholar]
- 3. Le Dudal M, Huguet L, Perez J. et al. Stiripentol protects against calcium oxalate nephrolithiasis and ethylene glycol poisoning. J Clin Invest 2019; 129: 2571–2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brigo F, Igwe SC, Bragazzi NL.. Stiripentol add-on therapy for focal refractory epilepsy. Cochrane Database Syst Rev 2018; 5: CD009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoppe B, Cochat P, Lemoine S. et al. PHYOX: a safety and tolerability study of DCR-PHXC in primary hyperoxaluria types 1 and 2. Poster TH-PO449. Presented at the American Society of Nephrology Kidney Week 2019, Washington, DC. [Google Scholar]