Abstract
Background
Within the class of tyrosine kinase inhibitors (TKIs), which are used for the treatment of numerous advanced cancers, lenvatinib is associated with a higher prevalence of hypertension (HT) compared with other TKIs. In this study, we investigated the effect of lenvatinib on blood pressure (BP) and associated factors.
Methods
This single-centre, retrospective observational study included 25 consecutive patients treated with lenvatinib for unresectable hepatocellular carcinoma from April 2018 to December 2018 at the study institution. We assessed changes in BP using ambulatory BP monitoring, urinary sodium excretion, kidney function, use of antihypertensive agents and diuretics, and fluid retention following treatment initiation with lenvatinib.
Results
At 1 week after treatment initiation, the mean BP and the percentage of patients with riser pattern significantly increased compared with those at the baseline. Although there were no significant changes at 1 week, urinary sodium excretion (153.4 ± 51.7 and 112.5 ± 65.0 mEq/day at 1 and 3 weeks, respectively, P < 0.05) and estimated glomerular filtration rate significantly decreased and the number of patients with fluid retention increased at 3 weeks. Furthermore, patients with fluid retention had significantly higher BP or required more intensive BP treatment compared with those without fluid retention.
Conclusions
Lenvatinib might lead to HT without fluid retention soon after the initiation of treatment, subsequently leading to a reduction in urinary sodium excretion, thereby contributing to a rise in BP by fluid retention.
Keywords: ambulatory blood pressure monitoring, blood pressure, lenvatinib, tyrosine kinase inhibitor, urinary sodium excretion
INTRODUCTION
Onconephrology, a recently established subspecialty in Nephrology that has been attracting attention as an important topic in recent years, encompasses topics related to treatments for malignant diseases and nephrological complications including kidney dysfunction, proteinuria, hypertension (HT) and electrolyte and fluid abnormalities, particularly in patients with pre-existing kidney diseases [1, 2]. Among the wide range of anti-cancer drugs, tyrosine kinase inhibitors (TKIs) have been the most frequently used antineoplastic agents in recent years. In addition to their known availability, TKIs are also recognized for the various adverse effects associated with their use [3]. Although TKIs have fewer adverse events compared with other antineoplastic agents, elevated blood pressure (BP) and kidney injury such as proteinuria and impaired kidney function are the most frequent adverse effects of TKIs. One study reported that the prevalence of TKI-induced HT ranged between 17% and 49.6% [4]. While HT is a crucial risk factor for cardiovascular disease, several studies showed that the elevation of BP was significantly associated with better prognosis and reflected the efficacy of vascular endothelial growth factor (VEGF) inhibitors in patients with cancer [5–7]. Therefore, discontinuation or dose reduction during TKI therapy is not recommended.
Lenvatinib (Lenvima®; Eisai, Japan) is an orally administered TKI that targets VEGF receptors 1–3, fibroblast growth factor receptors 1–4, c-KIT receptor, platelet-derived growth factor receptor and rearranged during transfection. Lenvatinib exerts anti-tumour effects by inhibiting angiogenesis [8, 9]. This multi-targeted TKI is approved as first-line treatment of unresectable hepatocellular carcinoma (HCC) and has been widely used in clinical settings. However, some reports suggest that the prevalence of HT is higher with lenvatinib than that with other TKIs [10–14].
In this study, we investigated the effect of lenvatinib on changes in BP and sodium excretion as well as other clinical parameters associated with lenvatinib-mediated increases in BP.
MATERIALS AND METHODS
Study design and population
This was a single-centre, retrospective observational study. Among a total of 40 patients who were admitted to our institute and treated with lenvatinib for unresectable HCC between April and December 2018, 15 patients with insufficient clinical data and/or information were excluded from the present study; thus, 25 patients were included in the study. The salt intake of the study cohort ranged between 7 and 8 g/day, and all patients were treated with oral lenvatinib administered once daily every morning for 3 weeks. The starting lenvatinib dose ranged from 4 to 12 mg/day according to the patient’s body weight. Patients were hospitalized for 2 weeks and then they were followed in an outpatient clinic. The clinical data were collected at baseline and 1 and 3 weeks after the initiation of lenvatinib treatment.
This study was conducted in accordance with the Declaration of Helsinki principles and the study protocol was approved by the appropriate institutional review committee (no. 180341).
Assessment of BP
To evaluate the influence of lenvatinib on BP, we performed 24-h ambulatory blood pressure monitoring (ABPM) before and 1 week after the initiation of lenvatinib treatment using a TM-2431 BP monitoring device (A & D Company, Tokyo, Japan). BP was automatically measured every 30 min during daytime and every 60 min during night-time. Daytime and night-time were determined on the basis of a diary of wake-up and sleep times recorded by the patients. In accordance with the guidelines of the Japanese Society of Cardiology, the measurements that did not satisfy all of the following conditions were excluded as measurement errors: (i) 70 mmHg ≤ systolic blood pressure (SBP) ≤ 250 mmHg; (ii) 30 mmHg ≤ diastolic blood pressure (DBP) ≤ 130 mmHg; (iii) 20 mmHg ≤ pulse pressure ≤ 160 mmHg; and (iv) pulse pressure >0.41 × DBP (60–150 mmHg) − 17 mmHg [15]. All patients were instructed to measure BP at home while in a sitting position at least twice every morning and every night after discharge. The data from BP measurements at home were collected at 3 weeks. The average BP for 1 week after discharge was defined as BP at 3 weeks. To evaluate the circadian BP rhythm, the results were classified into the following four types: (i) dipper: nocturnal BP, 10–20% lower than daytime BP; (ii) non-dipper: nocturnal BP, ≤10% lower than daytime BP; (iii) riser: nocturnal BP higher than daytime BP; and (iv) extreme dipper: nocturnal BP, >20% lower than daytime BP [16]. At 3 weeks, the severity of HT was evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0 [17].
Clinical data evaluation
We evaluated changes in kidney function, urinary sodium excretion and proteinuria at baseline and at 1 and 3 weeks after the initiation of lenvatinib treatment. Kidney function was evaluated by serum creatinine level (mg/dL) and estimated glomerular filtration rate (eGFR) (mL/min/1.73 m2). Daily urinary sodium excretion and eGFR were calculated by Kawasaki’s formula and the eGFR formula for Japanese adults, respectively [18–20]. Furthermore, data on prescription for diuretics and leg oedema were collected at baseline and at 1 and 3 weeks in all patients. Fluid retention was defined as the deterioration of leg oedema.
Statistical analysis
All statistical analyses were performed using IBM SPSS statistics software version 25.0 (SPSS, Chicago, IL, USA). Continuous variables were expressed as means ± standard deviation (SD) and medians with interquartile ranges, and differences among time points or groups were analysed by paired t-test or Wilcoxon’s signed-rank test. Categorical variables were expressed as frequencies and percentages and analysed by the Chi-squared test or McNemar’s test. A two-tailed P < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
The clinical characteristics and laboratory data of study participants at baseline are presented in Table 1. The mean age was 70 ± 9 years, and 17 (68.0%) patients were male. Before the initiation of treatment with lenvatinib, 8 (32.0%) and 14 (56.0%) patients had diabetes mellitus and HT, respectively. At baseline, the mean serum creatinine level and eGFR were 0.80 ± 0.21 mg/dL and 71.3 ± 18.6 mL/min/1.73 m2, respectively. None of the cohort patients had a kidney disease diagnosis, and the median proteinuria was 0.05 (0.02–0.11) g/gCre at baseline. The cohort comprised 21 (84.0%) and 4 (16.0%) patients with Child–Pugh Classes A and B cirrhosis, respectively. The aetiologies of HCC included hepatitis B virus and hepatitis C virus infections in 10 (40.0%) and 7 (28.0%) patients, respectively. The remaining patients did not have clear aetiologies. Finally, 14 patients and 1 patient were using antihypertensives and loop diuretics, respectively, at baseline.
Table 1.
Patients characteristics at baseline
| n = 25 | |
|---|---|
| Age, years | 70 ± 9 |
| Male gender, % | 17 (68.0) |
| BMI, kg/m2 | 22.8 ± 3.5 |
| Smoking, % | 15 (60.0) |
| HT, % | 14 (56.0) |
| DM, % | 8 (32.0) |
| HBV infection, % | 10 (40.0) |
| HCV infection, % | 7 (28.0) |
| Child–Pugh A, % | 21 (84.0) |
| Child–Pugh B, % | 4 (16.0) |
| SBP at baseline, mmHg | 125.0 ± 11.8 |
| DBP at baseline, mmHg | 67.1 ± 8.9 |
| Cr, mg/dL | 0.80 ± 0.21 |
| eGFR, mL/min/1.73 m2 | 71.3 ± 18.6 |
| BUN, mg/dL | 16 ± 5 |
| TP, g/dL | 6.9 ± 0.6 |
| Alb, g/dL | 3.4 ± 0.5 |
| HbA1c, % | 6.1 ± 0.8 |
| T-chol, mg/dL | 180 ± 39 |
| Proteinuria, g/gCr | 0.05 (0.02–0.11) |
Values are presented as the mean ± SD or median and interquartile range. BMI, body mass index; DM, diabetes mellitus; HBV, hepatitis B virus; HCV, hepatitis C virus; Cr, creatinine; BUN, blood urea nitrogen; TP, total protein; Alb, albumin; HbA1c, haemoglobin A1c; T-chol, total cholesterol.
Changes in BP and circadian BP rhythm
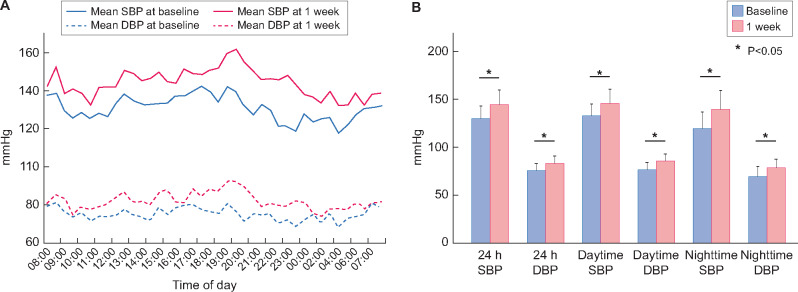
The results of ABPM at baseline and 1 week after the lenvatinib treatment initiation are shown in Figure 1. Both the SBP and DBP values were significantly higher at 1 week compared with those at the baseline throughout the 24 h of monitoring (SBP: 129.7 ± 13.0 and 144.2 ± 15.7 mmHg; DBP: 75.2 ± 7.0 and 83.2 ± 8.0 mmHg at baseline and 1 week), and the increase in night-time BP was significantly greater than that in daytime BP (ΔSBP: 20.4 ± 10.3 versus 12.9 ± 10.5 mmHg at night-time and daytime, respectively, P < 0.05). The analysis of the circadian BP rhythm revealed that the number of patients with the riser pattern increased from 1 (4.0%) to 8 (32.0%) and that the number of those with the dipper pattern decreased from 10 (40.0%) to 5 (20.0%) (Figure 2). After the second ABPM assessment, 12 (48.0%) patients required intensive treatment for HT based on the results. Specifically, calcium channel blockers were prescribed for all patients with HT, and renin−angiotensin−aldosterone system inhibitors were added to the treatment in two (8.0%) patients. As mentioned above, only one (4.0%) patient used loop diuretics until 3 weeks after the initiation of lenvatinib treatment, and seven (28.0%) patients started on loop diuretics after 3 weeks following the lenvatinib treatment initiation.
FIGURE 1:
(A) Variation in SBP and DBP measured with ABPM at baseline and at 1 week after the initiation of treatment with lenvatinib. Mean hourly values of SBP and DBP of all 25 patients were plotted. (B) The 24-h, daytime and night-time BP measurements at baseline and at 1 week after the initiation of treatment with lenvatinib. Blue: baseline, and red: 1 week after the initiation of lenvatinib treatment. BP levels are significantly higher at all time points after treatment initiation compared with those at baseline. The change in mean SBP is significantly greater at night-time than that at daytime (20.4 ± 10.3 versus 12.9 ± 10.5 mmHg, respectively, P < 0.05).
FIGURE 2:
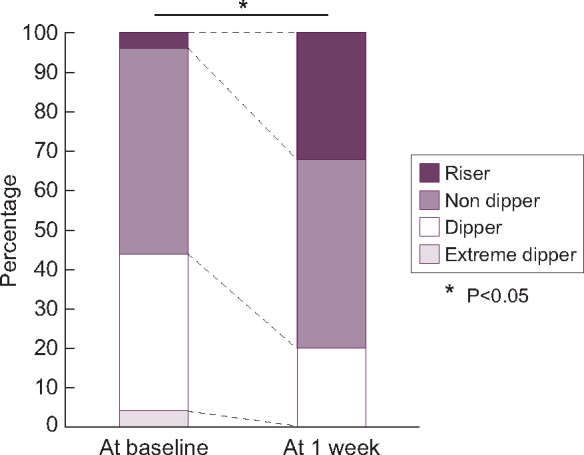
Changes in circadian BP patterns between the baseline and 1 week after the initiation of lenvatinib treatment in patients with and without fluid retention. Black: riser; dark grey: non-dipper; white: dipper; and grey: extreme dipper.
Changes in kidney function, urinary protein, urinary sodium excretion and fluid retention
To analyse the mechanism of underlying lenvatinib-induced HT, we evaluated kidney function, urinary protein excretion and urinary sodium excretion. We also assessed the deterioration of leg oedema at 1 and 3 weeks after the initiation of lenvatinib treatment. We found that there were no significant changes in kidney function, proteinuria and urinary sodium excretion between the baseline and 1 week (Table 2). There was no patient with fluid retention at 1 week. However, significant declines in kidney function and urinary sodium excretion and a significant increase in proteinuria were observed at 3 weeks. Ten patients had the deteriorated leg oedema at 3 weeks and were considered as those with fluid retention.
Table 2.
Changes in kidney parameters and fluid retention
| n = 25 | Baseline | 1 week | 3 weeks |
|---|---|---|---|
| Cr, mg/dL | 0.80 ± 0.21 | 0.82 ± 0.24 | 0.89 ± 0.29a,b |
| eGFR, mL/min/ 1.73 m2 | 71.3 ± 18.6 | 71.0 ± 19.1 | 66.1 ± 22.0a,b |
| Urinary Na excretion, mEq/day | 187.2 ± 62.6 | 153.4 ± 51.7 | 112.5 ± 65.0a,b |
| Proteinuria, g/gCr | 0.05 (0.00–0.11) | 0.10 (0.03–0.20) | 0.14 (0.05–0.59)a,b |
| Patients with the deteriora tion of leg oedema, % | – | 0 (0) | 10 (40.0)b |
Values are presented as the mean ± SD or median and interquartile range.
Baseline versus 3 weeks, P < 0.05
1 week versus 3 weeks, P < 0.05.
Cr, creatinine; Na, sodium.
Severity of HT in patients with and without fluid retention
Three weeks after starting the treatment with lenvatinib, 10 of the 25 patients (40.0%) showed fluid retention. Next, we compared the severity of HT at 3 weeks between the patients with and without fluid retention. The mean BP measured at home at 3 weeks was significantly higher in those with fluid retention than in those without fluid retention (143.6 ± 20.2 mmHg versus 131.3 ± 6.4 mmHg, P < 0.05). Furthermore, mean plasma brain natriuresis peptide (BNP) level at 3 weeks in patients with fluid retention was significantly higher compared with those without fluid retention (137.1 ± 124.1 pg/mL versus 32.4 ± 23.8 pg/mL, P < 0.05), although there was no significant difference at baseline. Even after excluding patients with Child–Pugh Class B cirrhosis, the result was similar (144.6 ± 129.2 versus 30.5 ± 16.9, P < 0.05). Among the patients with fluid retention (n = 10), seven patients needed to start loop diuretics and additional calcium channel blockers were prescribed for three patients. Furthermore, one patient had to discontinue the lenvatinib treatment at 3 weeks because of severe HT. Conversely, none of the patients without fluid retention (n = 15) required loop diuretics or additional antihypertensive treatment at 3 weeks. In addition, the urinary sodium excretion was significantly lower at 3 weeks compared with that at the baseline in both groups (P < 0.05). In the group of patients with fluid retention, urinary sodium excretion was significantly lower at 3 weeks despite comparable urinary sodium excretion between the measurements at baseline and 1 week (Figure 3).
FIGURE 3:
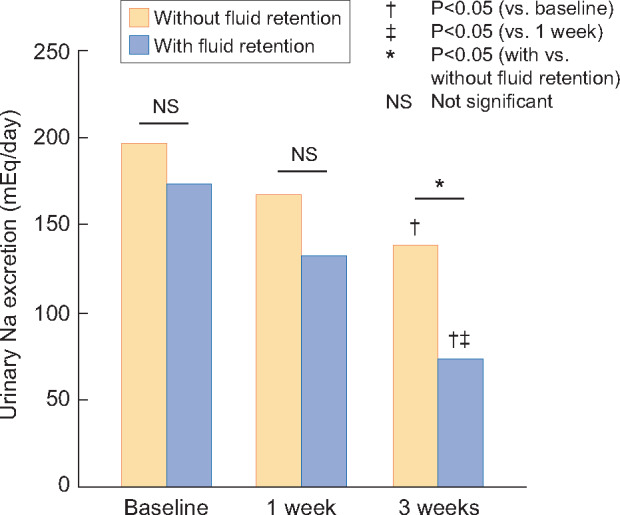
Changes in urinary sodium excretion at baseline and at 1 and 3 weeks after the initiation of lenvatinib treatment in patients with and without fluid retention.
Regarding the severity of HT, 20.0% of those without fluid retention and 70.0% of the patients with fluid retention had Grade III HT (Figure 4), reflecting that the patients with fluid retention had more severe HT compared with those without fluid retention. Overall, these results suggested that fluid retention contributed to the elevation of BP at 3 weeks after the initiation of lenvatinib treatment, which was not observed at 1 week.
FIGURE 4:
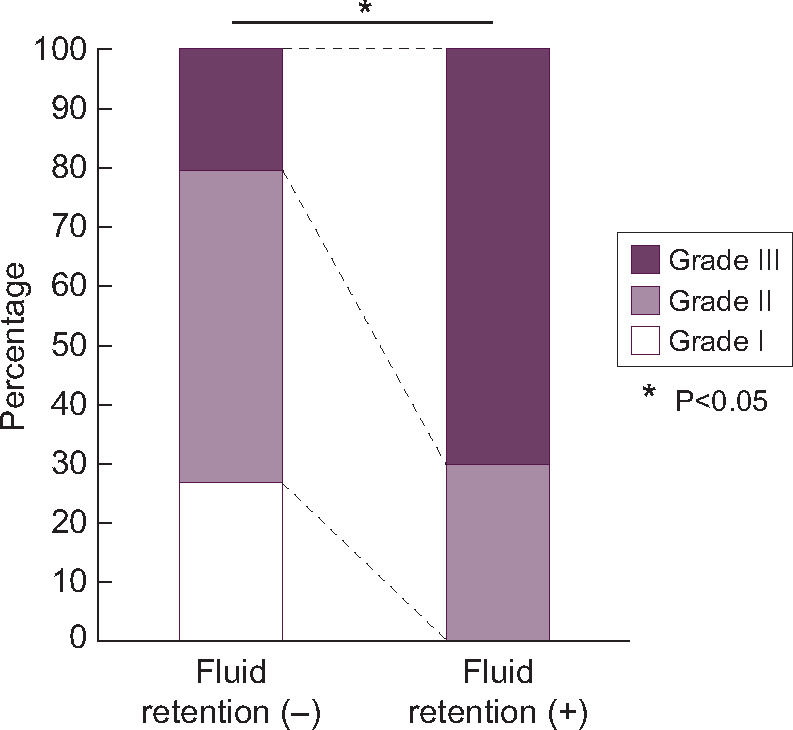
Severity of HT in patients with and without fluid retention. White: grade I, mild HT; light purple: grade II, moderate HT; and dark purple: grade III, severe HT.
DISCUSSION
The present study demonstrated that (i) lenvatinib induced BP elevation and abnormal circadian BP rhythm soon after the initiation of treatment, (ii) changes in kidney function, urinary sodium excretion and oedema status were not observed in the early period after the treatment initiation, (iii) kidney function and urinary sodium excretion declined and the number of patients with leg oedema increased in the later period during the lenvatinib treatment and (iv) urinary sodium excretion was significantly lower in patients with fluid retention compared with those without fluid retention at 3 weeks.
Although the detailed mechanisms of BP elevation induced by lenvatinib remain unclear, the potential mechanisms include the elevation of systemic vascular resistance (SVR) and volume retention. Cardiac output (CO) is regulated by stroke volume and heart rate, and stroke volume depends on blood volume. An increase in CO leads to the elevation of BP, which is the product of SVR and CO. We previously demonstrated that treatment by TKIs increased BP in patients undergoing haemodialysis [21].
In the present study, interestingly, none of the patients exhibited fluid retention or an increase in heart rate in the early period after the initiation of lenvatinib. Therefore, we speculate that the elevation in BP during the early period might be mainly due to an increase in SVR. In general, SVR is modulated by vasodilators and vasoconstrictors. Among the vasodilators, nitric oxide (NO), which plays a key role in BP changes, has been shown to be greatly influenced by TKIs [21–24]. In general, it is known that NO in the kidneys has a function to decrease renal vascular resistance and increases blood flow to glomeruli, leading to reduction in renin secretion and renal perfusion pressure. This means that reduced NO function may inhibit natriuresis and increase circulating volume [25].
The synthesis of NO has been reported to be inhibited by TKIs via the inhibition of VEGF signalling [26]. One clinical study examining patients treated with lenvatinib for 6 days reported the elevation of BP with a reduction in serum NO concentrations [27]. Therefore, the elevation of BP by lenvatinib might be partially caused by a reduction in NO production. Prostacyclin synthesis, which is also decreased by the inhibition of VEGF signalling [28, 29], might also contribute to the lenvatinib-mediated elevation in BP. Conversely, increase in vasoconstrictors is also considered as a crucial mechanism underlying the lenvatinib-induced BP elevation. Clinical and experimental data in humans and rats show that treatment by the TKI sunitinib increased the levels of endothelin-1 [30], which might thus be involved in lenvatinib-induced BP elevation. Thus, we speculate that the BP elevation in the early period after the initiation of lenvatinib treatment might be primarily due to an imbalance between the activity of vasodilators and vasoconstrictors.
In the present study, 10 of the 25 patients exhibited exacerbated leg oedema, and 7 patients were prescribed loop diuretics in the later period following the initiation of lenvatinib treatment. Additionally, reductions in kidney function and urinary sodium excretion were also observed in this period. Moreover, HT was more frequent among patients with fluid retention compared with those without fluid retention. Considering these findings, unlike that observed in the early period, the BP elevation in the later period might be primarily influenced by fluid retention due to a decrease in urinary sodium excretion. Decreased blood flow to the glomeruli is one mechanism that can contribute to a decrease in urinary sodium excretion. TKIs are reported to contract afferent arterioles via the inhibition of NO production and the increase in vasoconstrictive factors [31, 32]. Although this might lead to not only a reduction in intraglomerular blood flow but also impaired kidney function, a decrease in urinary sodium excretion was observed even in patients without an obvious deterioration of kidney function in the present study. Besides this potential mechanism, TKIs could reduce urinary sodium excretion by hindering tubuloglomerular feedback through the inhibition of NO release from the macula densa and the weakening of the effects of NO on the function of various transporters in tubular cells [33–35]. Furthermore, despite the intake of a low-sodium diet during hospitalization, most of the patients in the present study had an excessive intake of sodium after discharge from the hospital. These mechanisms of fluid retention and BP elevation might be at play in patients treated with lenvatinib in the present study.
Previous studies reported that various kidney lesions observed in patients treated with TKIs [4, 36–38], including glomerulonephritis, tubulointerstitial nephritis and thrombotic microangiopathy (TMA). The underlying pathophysiological mechanisms can be classified into direct cellular injury by TKIs and ischaemic injury due to microcirculatory disturbances. Proteinuria, a common adverse event observed with TKIs, is considered to reflect the presence of direct glomerular injury comprising endothelial and podocyte injury via the inhibition of VEGF signalling [39, 40]. Clinical trials using lenvatinib have reported that the prevalence of patients with proteinuria ranged from 20% to 30% [11, 13]. In the present study, 10 patients (40.0%) exhibited slight proteinuria. Although not so much as the presence of proteinuria, TKIs can also lead to a reduction in kidney function [41]. In the present study, we found that the kidney function was decreased after the lenvatinib treatment initiation based on the observed changes in eGFR from baseline to 3 weeks. Although the underlying mechanisms have not been elucidated, TMA due to the inhibition of VEGF signalling and decreased NO synthesis is considered to be involved [42, 43]. Microcirculatory disturbances in glomeruli and kidney interstitium also appear to contribute to the deterioration of kidney function.
The present study has several limitations that should be acknowledged. First, the sample size was relatively small. Secondly, the levels of several factors related to changes in BP, such as endothelin-1, NO, prostacyclin, renin activity, aldosterone and epinephrine, were not determined. Future studies should include these parameters to determine the detailed mechanism underlying lenvatinib-mediated changes in BP.
In the present study, SBP and DBP were significantly elevated after the initiation of treatment with lenvatinib for unresectable HCC. Our results suggest that the elevation in BP might exhibit a biphasic pattern. Therefore, we speculate that the elevation in BP in the early period might be due to a change in SVR, whereas that in the later period might be due to sodium retention. Further studies are warranted to elucidate the detailed mechanisms underlying the changes in BP due to lenvatinib.
ACKNOWLEDGEMENTS
The authors would like to thank Kohei Okamoto and Mao Shimizu for their contributions of data collection. We also thank Dr Yoshihiko Yano for entrusting us with the management of treatment-associated HT and allowing us to share the patients' clinical data.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no other conflict of interest.
REFERENCES
- 1. Salahudeen AK, Bonventre JV.. Onconephrology: the latest frontier in the war against kidney disease. J Am Soc Nephrol 2013; 24: 26–30 [DOI] [PubMed] [Google Scholar]
- 2. Abudayyeh AA, Lahoti A, Salahudeen AK.. Onconephrology: the need and the emergence of a subspecialty in nephrology. Kidney Int 2014; 85: 1002–1004 [DOI] [PubMed] [Google Scholar]
- 3. Rimassa L, Danesi R, Pressiani T. et al. Management of adverse events associated with tyrosine kinase inhibitors: improving outcomes for patients with hepatocellular carcinoma. Cancer Treat Rev 2019; 77: 20–28 [DOI] [PubMed] [Google Scholar]
- 4. Semeniuk-Wojtaś A, Lubas A, Stec R. et al. Influence of tyrosine kinase inhibitors on hypertension and nephrotoxicity in metastatic renal cell cancer patients. Int J Mol Sci 2016; 17: 2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rini BI, Cohen DP, Lu DR. et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst 2011; 103: 763–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scartozzi M, Galizia E, Chiorrini S. et al. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol 2009; 20: 227–230 [DOI] [PubMed] [Google Scholar]
- 7. Wirth LJ, Tahara M, Robinson B. et al. Treatment-emergent hypertension and efficacy in the phase 3 study of (E7080) lenvatinib in differentiated cancer of the thyroid (SELECT). Cancer 2018; 124: 2365–2372 [DOI] [PubMed] [Google Scholar]
- 8. Lorusso L, Pieruzzi L, Biagini A. et al. Lenvatinib and other tyrosine kinase inhibitors for the treatment of radioiodine refractory, advanced, and progressive thyroid cancer. Onco Targets Ther 2016; 9: 6467–6477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stjepanovic N, Capdevila J.. Multikinase inhibitors in the treatment of thyroid cancer: specific role of lenvatinib. Biologics 2014; 8: 129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yu ST, Ge JN, Luo JY. et al. Treatment-related adverse effects with TKIs in patients with advanced or radioiodine refractory differentiated thyroid carcinoma: a systematic review and meta-analysis. Cancer Manag Res 2019; 11: 1525–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schlumberger M, Tahara M, Wirth LJ. et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015; 372: 621–630 [DOI] [PubMed] [Google Scholar]
- 12. Kiyota N, Schlumberger M, Muro K. et al. Subgroup analysis of Japanese patients in a phase 3 study of lenvatinib in radioiodine-refractory differentiated thyroid cancer. Cancer Sci 2015; 106: 1714–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kudo M, Finn RS, Qin S. et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018; 391: 1163–1173 [DOI] [PubMed] [Google Scholar]
- 14. Ikeda K, Kudo M, Kawazoe S. et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol 2017; 52: 512–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.JCS Joint Working Group. Guidelines for the clinical use of 24 hour ambulatory blood pressure monitoring (ABPM) (JCS2010): – digest version –. Circ J 2012; 76: 508–519 [DOI] [PubMed] [Google Scholar]
- 16. Shimamoto K, Ando K, Fujita T. et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH2014). Hypertens Res 2014; 37: 253–390 [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute. Protocol Development: Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events (CTCAE) v5.0 https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50 (4 January 2019, date last accessed)
- 18. Kawasaki T, Itoh K, Uezono K. et al. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin Exp Pharmacol Physiol 1993; 20: 7–14 [DOI] [PubMed] [Google Scholar]
- 19. Kawamura M, Kusano Y, Takahashi T. et al. Effectiveness of a spot urine method in evaluating daily salt intake in hypertensive patients taking oral antihypertensive drugs. Hypertens Res 2006; 29: 397–402 [DOI] [PubMed] [Google Scholar]
- 20. Matsuo S, Imai E, Horio M. et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992 [DOI] [PubMed] [Google Scholar]
- 21. Nakai K, Fujii H, Kono K. et al. Hypertension induced by tyrosine-kinase inhibitors for the treatment of renal cell carcinoma in hemodialysis patients: a single-center experience and review of the literature. Ther Apher Dial 2017; 21: 320–325 [DOI] [PubMed] [Google Scholar]
- 22. Kappers MH, Smedts FM, Horn T. et al. The vascular endothelial growth factor receptor inhibitor sunitinib causes a preeclampsia-like syndrome with activation of the endothelin system. Hypertension 2011; 58: 295–302 [DOI] [PubMed] [Google Scholar]
- 23. Mayer EL, Dallabrida SM, Rupnick MA. et al. Contrary effects of the receptor tyrosine kinase inhibitor vandetanib on constitutive and flow-stimulated nitric oxide elaboration in humans. Hypertension 2011; 58: 85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kandula P, Agarwal R.. Proteinuria and hypertension with tyrosine kinase inhibitors. Kidney Int 2011; 80: 1271–1277 [DOI] [PubMed] [Google Scholar]
- 25. Schnackenberg C, Patel AR, Kirchner KA. et al. Nitric oxide, the kidney and hypertension. Clin Exp Pharmacol Physiol 1997; 24: 600–606 [DOI] [PubMed] [Google Scholar]
- 26. Kappers MH, van Esch JH, Sleijfer S. et al. Cardiovascular and renal toxicity during angiogenesis inhibition: clinical and mechanistic aspects. J Hypertens 2009; 27: 2297–2309 [DOI] [PubMed] [Google Scholar]
- 27. Sueta D, Suyama K, Sueta A. et al. Lenvatinib, an oral multi-kinase inhibitor, -associated hypertension: potential role of vascular endothelial dysfunction. Atherosclerosis 2017; 260: 116–120 [DOI] [PubMed] [Google Scholar]
- 28. Robinson ES, Khankin EV, Karumanchi SA. et al. Hypertension induced by vascular endothelial growth factor signaling pathway inhibition: mechanisms and potential use as a biomarker. Semin Nephrol 2010; 30: 591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. He H, Venema VJ, Gu X. et al. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J Biol Chem 1999; 274: 25130–25135 [DOI] [PubMed] [Google Scholar]
- 30. Kappers MH, van Esch JH, Sluiter W. et al. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension 2010; 56: 675–681 [DOI] [PubMed] [Google Scholar]
- 31. Ito S, Arima S, Ren YL. et al. Endothelium-derived relaxing factor/nitric oxide modulates angiotensin II action in the isolated microperfused rabbit afferent but not efferent arteriole. J Clin Invest 1993; 91: 2012–2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Imig JD, Roman RJ.. Nitric oxide modulates vascular tone in preglomerular arterioles. Hypertension 1992; 19: 770–774 [DOI] [PubMed] [Google Scholar]
- 33. Ren YL, Garvin JL, Carretero OA.. Role of macula densa nitric oxide and cGMP in the regulation of tubuloglomerular feedback. Kidney Int 2000; 58: 2053–2060 [DOI] [PubMed] [Google Scholar]
- 34. Thorup C, Persson AE.. Inhibition of locally produced nitric oxide resets tubuloglomerular feedback mechanism. Am J Physiol 1994; 267: F606–F611 [DOI] [PubMed] [Google Scholar]
- 35. Garvin JL, Herrera M, Ortiz PA.. Regulation of renal NaCl transport by nitric oxide, endothelin, and ATP: clinical implications. Annu Rev Physiol 2011; 73: 359–376 [DOI] [PubMed] [Google Scholar]
- 36. Furuto Y, Hashimoto H, Namikawa A. et al. Focal segmental glomerulosclerosis lesion associated with inhibition of tyrosine kinases by lenvatinib: a case report. BMC Nephrol 2018; 19: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baek SH, Kim H, Lee J. et al. Renal adverse effects of sunitinib and its clinical significance: a single-center experience in Korea. Korean J Intern Med 2014; 29: 40–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Izzedine H, Escudier B, Lhomme C. et al. Kidney diseases associated with anti-vascular endothelial growth factor (VEGF): an 8-year observational study at a single center. Medicine (Baltimore) 2014; 93: 333–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tesařová P, Tesař V.. Proteinuria and hypertension in patients treated with inhibitors of the VEGF signalling pathway – incidence, mechanisms and management. Folia Biol (Praha) 2013; 59: 15–25 [PubMed] [Google Scholar]
- 40. Hayman SR, Leung N, Grande JP. et al. VEGF inhibition, hypertension, and renal toxicity. Curr Oncol Rep 2012; 14: 285–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Paschke L, Lincke T, Mühlberg KS. et al. Anti VEGF-TKI treatment and new renal adverse events not reported in phase III trials. Eur Thyroid J 2018; 7: 308–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Usui J, Glezerman IG, Salvatore SP. et al. Clinicopathological spectrum of kidney diseases in cancer patients treated with vascular endothelial growth factor inhibitors: a report of 5 cases and review of literature. Hum Pathol 2014; 45: 1918–1927 [DOI] [PubMed] [Google Scholar]
- 43. Eremina V, Jefferson JA, Kowalewska J. et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 2008; 358: 1129–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]