Abstract
Background
Conservative care (CC) may be a valid alternative to dialysis for certain older patients with advanced chronic kidney disease (CKD). A model that predicts patient prognosis on both treatment pathways could be of value in shared decision-making. Therefore, the aim is to develop a prediction tool that predicts the mortality risk for the same patient for both dialysis and CC from the time of treatment decision.
Methods
CKD Stage 4/5 patients aged ≥70 years, treated at a single centre in the Netherlands, were included between 2004 and 2016. Predictors were collected at treatment decision and selected based on literature and an expert panel. Outcome was 2-year mortality. Basic and extended logistic regression models were developed for both the dialysis and CC groups. These models were internally validated with bootstrapping. Model performance was assessed with discrimination and calibration.
Results
In total, 366 patients were included, of which 126 chose CC. Pre-selected predictors for the basic model were age, estimated glomerular filtration rate, malignancy and cardiovascular disease. Discrimination was moderate, with optimism-corrected C-statistics ranging from 0.675 to 0.750. Calibration plots showed good calibration.
Conclusions
A prediction tool that predicts 2-year mortality was developed to provide older advanced CKD patients with individualized prognosis estimates for both dialysis and CC. Future studies are needed to test whether our findings hold in other CKD populations. Following external validation, this prediction tool could be used to compare a patient’s prognosis on both dialysis and CC, and help to inform treatment decision-making.
Keywords: chronic kidney disease, conservative care, dialysis, end-stage kidney disease, prediction model
INTRODUCTION
End-stage kidney disease (ESKD) is an increasingly large public health burden, with high morbidity and mortality rates [1, 2]. Treatment options for ESKD consist of kidney replacement therapy (KRT) or non-dialytic conservative care (CC). CC consists of on-going treatment and symptom control with medication and diet/lifestyle instructions, and mainly focusses on quality of life. For older patients, dialysis treatment has become the most common treatment in more economically developed countries. However, as the ESKD population ages and focus shifts towards quality of life, a CC approach has emerged as a treatment alternative to dialysis [3]. Studies have shown that in some patient groups dialysis might not have a survival benefit compared with CC, not to mention the treatment burden that comes with dialysis [4, 5]. A recent study showed that the top two health outcome priorities of older chronic kidney disease (CKD) patients are maintaining independence and staying alive [6]. It is therefore important to openly discuss all treatment options, expected outcomes and the patient’s preferences in patients with advanced CKD [5, 7].
In order to foster shared decision-making and personalized treatment, patients should be provided with accurate information on their prognosis for each treatment strategy [5, 8]. There is a large range in survival time of patients on both dialysis and CC. This brings challenges in communicating information on prognosis and selecting patients who would benefit from dialysis or CC [9, 10]. Though multiple prediction models have been developed that predict mortality risk in dialysis patients, no models exist that predict the prognosis on CC [11]. More importantly, no models have been developed that predict mortality for both dialysis and CC at the time of treatment decision. Clinically speaking this is the most relevant time of prediction, as it could provide an individual patient with their hypothetical prognosis on both dialysis and CC. Such a prediction tool could be employed as a decision aid at the time of treatment choice and be of value in patient discussions when considering potential benefits and burdens of both treatment options [8, 12].
The aim of this study is, therefore, to develop and internally validate two prediction models of mortality risk in older CKD patients: one model that predicts mortality in patients who chose dialysis and another model that predicts mortality in patients who chose CC. By calculating individual mortality risks, we hope to facilitate the shared decision process in which benefits and burdens that come with dialysis and CC are considered.
MATERIALS AND METHODS
Study design and population
The Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) statement was followed for the reporting and methods of this study (see Supplementary data) [13]. The study population is a retrospective cohort of patients aged ≥70 years with CKD Stage 4/5, who received nephrology care at a non-academic teaching hospital in the Netherlands [4, 14]. Patients were included when the choice for dialysis or CC was registered in the patient file. As standard care, the physician initiated a shared decision-making process on the preference for dialysis or CC when the estimated glomerular filtration rate (eGFR) dropped to <20 mL/min/1.73 m2. Patients received counselling on different treatment pathways from a multidisciplinary team. Baseline is defined as the time of treatment choice; this means that not all patients in the dialysis group started dialysis during follow-up and some died before initiation. Likewise, in the CC group, patients could still choose to initiate dialysis. Analyses were performed in an intention-to-treat fashion, based on the baseline treatment choice. Inclusion was between 31 October 2004 and 1 May 2016. Exclusion criteria were age <70 years, acute kidney failure or no recorded treatment decision. The study design and population have previously been described in more detail [4, 14]. The study was approved by the local research ethics committee.
Data collection
Baseline data including patient history, clinical parameters and laboratory values were collected from electronic medical records at the time of treatment decision. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Creatinine formula. If laboratory or clinical parameters were not recorded at baseline, the most recent measurement was included within 3 weeks. Definitions of collected predictors are given in Supplementary data.
Predictors and outcome
From the baseline characteristics, a subset of predictors was chosen based on previous literature and clinical expertise of the study group [15, 16]. Aetiological studies in conservatively treated patients, and models predicting mortality in dialysis patients, were considered [5, 11, 15–17]. Three clinical experts (W.J.W.B., W.R.V. and M.v.B.) were asked to rank a list of 13 candidate predictors in order of importance. A basic model with the top 5 predictors and an extended model with the top 10 predictors (with a total of 10 degrees of freedom) were selected based on the expert ranking and consensus meeting (expert ranking and candidate predictors shown in Supplementary data). The goal of this selection method was to prevent overfitting while retaining enough variables for an accurate prediction [18, 19]. The outcome of the prediction model was 2-year mortality, selected based on clinical relevance and optimization of sample size. Follow-up data on death were available until 20 February 2019.
Statistical analysis
Continuous baseline characteristics are presented as mean values with standard deviations (SDs) or median values with interquartile ranges when not normally distributed. Categorical variables are presented as numbers with percentages.
Missing data were assumed to be largely missing at random. To correct for missing data, a 10-fold multiple imputation with fully conditional specification was performed using the R package ‘mice’. All candidate predictors and the outcome were included in the imputation model [20, 21]. The selected predictors were entered into a logistic regression model [22]. The risk models were internally validated and adjusted for overfitting by performing a 250-fold bootstrap analysis in each imputation data set [23]. The prognostic index (PI) can be calculated with the models estimated coefficients (β), individual predictor values (x) and the model constant (a), as PI = a + x1 × β1. With this PI, the mortality risk (P) can be calculated as P = ePI/(1 + ePI).
The predictive performance of the models was assessed by determining the discrimination and calibration. Discrimination is shown by the C-statistic and indicates how well the model can distinguish between people with and without the outcome. Optimism-corrected C-statistics were calculated with bootstrapping and pooled over the 10 imputation data sets with Rubin’s Rules [24]. The calibration is an absolute measure for the accuracy of the predicted probabilities and can be summarized in a calibration slope, intercept, calibration-in-the-large and calibration plot. The optimism-corrected slope and intercept were calculated in the bootstrapped samples. A calibration slope<1 in internal validation implicates overfitting and to correct for this the regression coefficients are shrunk by multiplying them by the slope. The calibration-in-the-large is the observed risk of mortality in the whole population compared with the predicted risk. A calibration plot shows the predicted risk plotted against the observed risk per decile of predicted probability, augmented by a smoothed (loess) regression line [25]. The 45° line indicates perfect agreement between predicted and observed risks. The shrinkage-adjusted coefficients form the final model and were pooled over the imputed data sets by calculating the mean [24]. The shrunken models were then applied for all patients to calculate probability differences between the dialysis and CC models. All analyses were performed in R version 3.5.1.
RESULTS
Baseline characteristics
A total of 366 patients were included. Of these patients, 240 had the intention to start dialysis in the future and 126 had chosen CC. Baseline characteristics stratified by outcome (2-year mortality) are shown in Tables 1 and 2. Most data were complete, with missing data for serum albumin, C-reactive protein (CRP) and body mass index. In the dialysis group, patients who died within 2 years were more often male, had considerably more cardiovascular disease, had slightly more malignancies and diabetes, and had a higher CRP at baseline. In the CC group, patients who died within 2 years were more often male, less often diabetic, had more cardiovascular disease and had a considerably lower eGFR at baseline than patients who survived >2 years. When comparing the dialysis group to the CC group, the largest differences are seen for age and gender; the CC group was 6 years older on average, and had a higher percentage of females.
Table 1.
Baseline characteristics, recorded at treatment decision for the dialysis choice group
| Total | >2 years survival | ≤2 years survival | |
|---|---|---|---|
| n = 240 | n = 162 | n = 78 | |
| Age (years) | 76 (72–79) | 75 (72–78) | 77 (73–81) |
| Male gender, n (%) | 160 (67) | 105 (65) | 55 (71) |
| Primary kidney disease, n (%) | |||
| Vascular disease | 103 (43) | 72 (44) | 31 (40) |
| Diabetes mellitus | 40 (17) | 27 (17) | 13 (17) |
| Other | 39 (16) | 27 (17) | 12 (15) |
| Unknown | 58 (24) | 36 (22) | 22 (28) |
| Comorbidities, n (%) | |||
| Cardiovascular disease | 176 (73) | 105 (65) | 71 (91) |
| Ischaemic heart disease | 109 (45) | 64 (40) | 45 (58) |
| Left ventricular dysfunction | 67 (30) | 29 (18) | 38 (49) |
| Peripheral vascular disease | 111 (46) | 61 (38) | 50 (64) |
| Malignancy | 26 (11) | 16 (10) | 10 (13) |
| Diabetes mellitus | 95 (40) | 62 (38) | 33 (42) |
| Laboratory parameters | |||
| eGFR (mL/min/1.73 m2) | 11.7 (4.0) | 11.7 (3.8) | 11.9 (4.5) |
| Serum albumin (g/L)a | 39.2 (4.6) | 40.0 (3.8) | 37.4 (5.6) |
| CRP (nmol/L)a | 48 (29–95) | 48 (21–57) | 76 (29–167) |
| Clinical parameter | |||
| Body mass index (kg/m²)a | 27.0 (4.5) | 27.2 (4.6) | 26.4 (4.4) |
Normally distributed continuous variables are presented as mean (SD), not normally distributed continuous values are presented as median (IQR). Categorical variables are presented as n (%). Serum albumin can be converted to mmol/L by multiplying by 0.0150. CRP can be converted to mg/L by multiplying by 0.105.
For the variables serum albumin, CRP and body mass index, 54 (23%), 67 (28%) and 44 (18%) patients had missing data, respectively.
Table 2.
Baseline characteristics, recorded at treatment decision for the CC group
| Total | >2 years survival | ≤2 years survival | |
|---|---|---|---|
| n = 126 | n = 55 | n = 71 | |
| Age (years) | 82 (79–86) | 82 (78–86) | 83 (80–86) |
| Male gender, n (%) | 68 (54) | 25 (46) | 43 (61) |
| Primary kidney disease, n (%) | |||
| Vascular disease | 65 (52) | 27 (49) | 38 (54) |
| Diabetes mellitus | 16 (13) | 7 (13) | 9 (13) |
| Other | 15 (12) | 10 (18) | 5 (7) |
| Unknown | 30 (24) | 11 (20) | 19 (27) |
| Comorbidities, n (%) | |||
| Cardiovascular disease | 97 (77) | 38 (69) | 59 (83) |
| Ischaemic heart disease | 56 (44) | 21 (38) | 35 (49) |
| Left ventricular dysfunction | 34 (27) | 8 (15) | 26 (37) |
| Peripheral vascular disease | 64 (51) | 23 (42) | 41 (58) |
| Malignancy | 17 (14) | 6 (11) | 11 (16) |
| Diabetes mellitus | 57 (45) | 27 (49) | 30 (42) |
| Laboratory parameters | |||
| eGFR (mL/min/1.73 m2) | 13.4 (4.5) | 15.1 (4.5) | 12.1 (4.1) |
| Serum albumin (g/L)a | 38.8 (3.5) | 39.8 (2.4) | 38.1 (4.0) |
| CRP (nmol/L)a | 48 (29–124) | 48 (19–110) | 52 (31–124) |
| Clinical parameter | |||
| Body mass index (kg/m²)a | 26.2 (4.8) | 27.3 (5.3) | 25.1 (4.0) |
Normally distributed continuous variables are presented as mean (SD), not normally distributed continuous values are presented as median (IQR). Categorical variables are presented as n (%).
For the variables serum albumin, CRP and body mass index, 17 (13%), 33 (26%) and 28 (22%) patients had missing data, respectively.
Follow-up data
All patients were followed for a minimum of 2 years or until death or loss to follow-up. The median time of follow-up was 37 [interquartile range (IQR) 17–58] months in the dialysis group and 17 (IQR 9–34) months in the CC group. In the dialysis group, 78 patients (33%) died within 2 years, and in the CC group 71 patients (56%). In total, seven patients were lost to follow-up and three patients received kidney transplantation; these patients were assumed to survive for 2 years. In the group that chose dialysis, a total of 146 (61%) patients initiated dialysis within the follow-up time; of these patients, 115 initiated haemodialysis and 31 peritoneal dialysis. Dialysis initiation took place after a median of 5 (IQR 2–14) months. In the CC group, two patients eventually initiated haemodialysis. As this information is not yet known at time of prediction, these patients remained in the CC group.
Model development
The following predictors were chosen for the basic models in dialysis and CC patients: age, eGFR, active malignancy, diabetes mellitus and the presence of cardiovascular disease. In the extended models, the following predictors were added: gender, serum albumin and CRP at baseline. The predictor cardiovascular disease was split into the presence of ischaemic heart disease, left ventricular dysfunction and peripheral vascular disease for the extended model. The selected predictors were entered into logistic regression models; a basic model and an extended model were developed to predict mortality on both dialysis and CC.
Model validation and performance
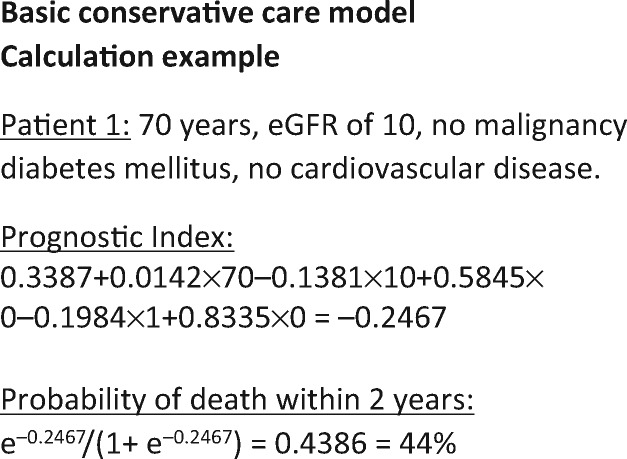
The four developed models were internally validated with bootstrapping. The shrunken regression coefficients of these final models are given in Table 3. Figure 1 gives an example of how to calculate an individual’s mortality probability based on these models. The models’ discrimination is shown in Table 4. The optimism-corrected C-statistics of the basic and extended dialysis models were 0.675 and 0.750, respectively. The basic and extended CC models had an optimism-corrected C-statistic of 0.677 and 0.729. Overall, the C-statistics reflected a moderate discriminatory capacity.
Table 3.
Final multivariate models after internal validation
| Predictors measured at baseline | Shrunken regression coefficients (bootstrap corrected βs) |
|||
|---|---|---|---|---|
| Basic dialysis model | Extended dialysis model | Basic CC model | Extended CC model | |
| Age (per year) | 0.0703 | 0.0737 | 0.0142 | 0.0374 |
| eGFR (per mL/min/1.73 m2) | −0.0049 | 0.0214 | −0.1381 | −0.1049 |
| Malignancy present | 0.1699 | −0.1185 | 0.5845 | 0.4742 |
| Diabetes mellitus present | 0.2792 | 0.0470 | −0.1984 | −0.1753 |
| Cardiovascular disease present | 1.4198 | – | 0.8335 | – |
| Ischaemic heart disease | – | 0.3588 | – | −0.0843 |
| Left ventricular dysfunction | – | 1.1720 | – | 1.2749 |
| Peripheral vascular disease | – | 1.0187 | – | 0.7225 |
| Gender (female) | – | −0.0245 | – | −0.1839 |
| Serum albumin (per g/L) | – | −0.0820 | – | −0.0927 |
| CRP (per nmol/L) | – | 0.0015 | – | 0.0012 |
| Constant | −7.2953 | −4.6099 | 0.3387 | 1.5472 |
FIGURE 1.
Calculation example.
Table 4.
Discrimination of models before and after internal validation
| Apparent C-statistic | Optimism-corrected C-statistic | |
|---|---|---|
| Basic dialysis model | 0.705 | 0.675 |
| Extended dialysis model | 0.790 | 0.750 |
| Basic CC model | 0.719 | 0.677 |
| Extended CC model | 0.797 | 0.729 |
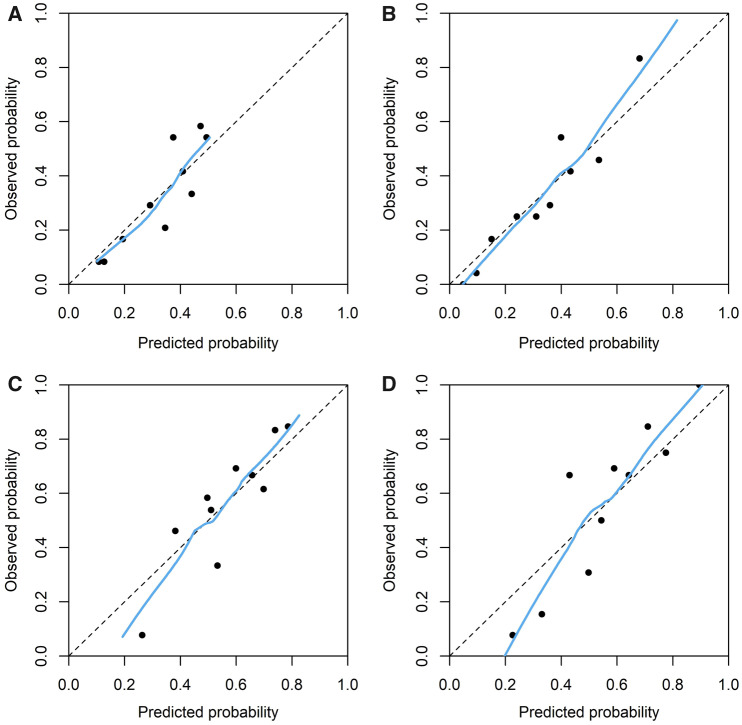
The models’ calibration slopes, intercepts and calibration-in-the-large are shown in Table 5. The internal validation slopes range from 0.665 to 0.844 and the intercepts from −0.112 to 0.084, indicating a considerable level of overfitting in the apparent models before shrinkage. In Figure 2, the calibration plots are shown after correction for this overfitting. Overall, these showed a moderate to good calibration. The CC models showed a large range of predicted risks, indicating the models’ ability to distinguish patients with a low and high absolute risk of mortality.
Table 5.
Model calibration after internal validation
| Calibration slope | Calibration intercept | Calibration-in-the-large (observed versus expected) | |
|---|---|---|---|
| Basic dialysis model | 0.844 | −0.084 | 32.5% versus 32.6% |
| Extended dialysis model | 0.799 | −0.112 | 32.5% versus 32.6% |
| Basic CC model | 0.796 | 0.052 | 56.3% versus 56.5% |
| Extended CC model | 0.665 | 0.068 | 56.3% versus 56.3% |
FIGURE 2.
Calibration plots of predicted probability (calculated with shrinkage adjusted prediction models). (A) Basic dialysis model, (B) extended dialysis model, (C) basic CC model and (D) extended CC model.
In Table 6, the probabilities calculated by the developed models are shown for three hypothetical patients. To facilitate comparison of the predicted risk for dialysis versus CC, probability differences (mortality probability on CC minus mortality probability on dialysis) were computed. Probability differences were computed in the whole population of 366 patients and ranged from −33% to 59% (meaning a 33% higher risk of mortality on dialysis compared with CC, and a 59% lower risk of mortality on dialysis, respectively). In Figure 3, this large range of probability differences is visualized in a histogram, stratified by actual treatment choice. In total, 35 patients had a probability difference of 0% or smaller, indicating a predicted survival advantage for a CC choice. Of these 35 patients, 26 had also chosen CC in reality. A total of 331 patients had a predicted probability difference >0%, indicating a predicted survival advantage on dialysis. In total, 231 patients of these 331 had also chosen dialysis as treatment. For the 100 patients in this group who chose CC, this prediction was not known at the time of treatment choice and it is unclear to what extent it would have influenced this choice, as many patients chose CC based on an expected lower treatment burden.
Table 6.
Example patients with corresponding mortality probabilities
| Predictors | Patient 1 | Patient 2 | Patient 3 | ||
|---|---|---|---|---|---|
| Extended model | Basic model | Age (per year) | 70 | 80 | 80 |
| eGFR (per mL/min/1.73 m2) | 10 | 15 | 20 | ||
| Malignancy present | No | No | No | ||
| Diabetes mellitus present | Yes | No | Yes | ||
| Cardiovascular disease present | No | Yes | Yes | ||
| Ischaemic heart disease | No | No | Yes | ||
| Left ventricular dysfunction | No | No | No | ||
| Peripheral vascular disease | No | Yes | Yes | ||
| Gender | Male | Female | Male | ||
| Serum albumin (per g/L) | 32 | 35 | 40 | ||
| CRP (nmol/L) | 40 | 29 | 19 | ||
|
| |||||
| Probability of death within 2 years | |||||
| Basic dialysis model | 10% | 42% | 48% | ||
| Basic CC model | 44% | 56% | 34% | ||
| Probability difference (CC minus dialysis) | 34% | 14% | −14% | ||
| Extended dialysis model | 15% | 44% | 47% | ||
| Extended CC model | 51% | 57% | 31% | ||
| Probability difference (CC minus dialysis) | 36% | 13% | −16% | ||
FIGURE 3.
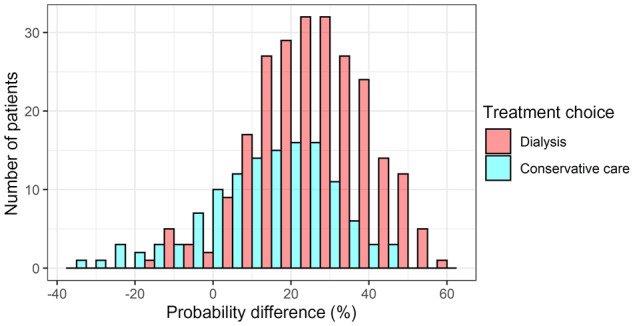
Histogram of probability differences stratified by treatment choice. The difference was calculated by subtracting the mortality probability on dialysis care from the probability on CC. A difference larger than 0% indicates a predicted survival benefit for dialysis choice and smaller than 0% indicates a predicted survival benefit for CC.
DISCUSSION
Four models were developed that predict 2-year mortality for a dialysis and CC treatment pathway in the same patient, and can thereby act as a prediction tool for patients aged ≥70 years with advanced CKD. The performance of the models was moderate to good in terms of discrimination and calibration. However, as this is a small single-centre patient population, further studies are needed to confirm validity of the developed tools in other populations. After external validation, this prediction tool could be used to calculate individually tailored mortality risks, identifying patients who might benefit from dialysis or CC in terms of survival and improving patient discussions surrounding the treatment decision.
To our knowledge, this is the first study to predict mortality on dialysis and CC at the time of treatment decision. Though robust prediction models for mortality on dialysis exist, most predict mortality shortly before or after dialysis initiation [11]. The often-used ‘surprise question’ model was developed on prevalent haemodialysis patients [26]. As of now, the European Renal Best Practice Group recommends using the Renal Epidemiology and Information Network (REIN) score to improve decision-making surrounding KRT choice [27, 28]. The REIN study developed a score that predicts mortality within 3 months after dialysis initiation in older patients [28]. Although these models are relevant for providing dialysis patients with individualized prognosis, they predict after dialysis start, meaning that the included patients survived up to this time point. Therefore, as the authors of the REIN score also point out, the transportability of these models to advanced CKD patients who have yet to decide on a treatment strategy is unsure [28]. Multiple studies have compared survival in patients treated with dialysis and CC from an aetiological perspective and demonstrated a limited survival benefit of dialysis in patients with higher age and more comorbidities [8, 14, 29]. However, no mortality prediction model for patients on CC has previously been developed.
In current treatment decision-making, many older patients feel they lack a choice between dialysis and CC, and in some cases, patients are not informed about treatment alternatives to dialysis at all [30, 31]. Although surveys show that CKD patients want life-expectancy information, whether good or bad, many patients experience a paucity of prognosis information [30, 32–34]. Studies show that nephrologists often have difficulty communicating information on prognosis and disease progression to patients and are rarely trained to do so [32, 35–37]. A prediction tool can inform discussions surrounding these treatment choices and expected prognoses, bringing clinical practice closer to patients’ wants and needs.
The study has a number of limitations. Though it is one of the largest studies including CC patients, the sample size is still quite small for the development of prediction models. A small sample size increases the risk of overfitting, meaning that the models would perform more poorly on new patients. We tried to limit overfitting by pre-selecting predictors instead of using data-driven selection procedures. Also, bootstrapping was performed as internal validation, as this method makes the best use of the available sample size [38]. Secondly, the data were collected in a single centre, which also increases the risk of overfitting. Both these limitations raise doubt on how well the developed models will perform in new patients. Therefore, It is crucial that these models are tested in other patients before use. This external validation should be performed in various centres and other countries to establish the performance in various populations.
Another limitation is the fact that patients in the dialysis and CC groups are not completely interchangeable. The prediction tool’s calculated difference in prognosis may not be entirely due to the treatment choice, but might be due to other differences between the dialysis and CC patient groups. There are many reasons why patients choose dialysis or CC. Some of these reasons may affect prognosis and others may not. We can speculate that personal values and preferences surrounding quality of life and life prolongment would not affect differences in prognosis. However, characteristics such as age and comorbidity can influence both the treatment choice and prognosis. We ensured that such major relevant differences in baseline characteristics were included as predictors and thereby taken into account. Ideally, this study would be performed on randomized controlled trial data as this would ensure that prognosis differences between dialysis and CC are fully caused by the treatment allocation. However, such a trial comparing CC to dialysis has not been completed thus far. Nonetheless, the individualized predictions in this study are more accurate than simply giving the average prognosis in all dialysis or CC patients, which is current practice.
The main strength of the study is that it is the first of its kind, and makes use of rare data in which patients’ treatment decisions are explicitly documented after the patients have taken part in an established process of shared decision-making, and improve the usability of our models. The accurate recording of treatment decision time point is of great importance as this is the clinically relevant time at which to predict and compare prognoses. By using an intention-to-treat method in which patients were categorized into the dialysis or CC group based on their treatment intention, we follow the clinical practice surrounding KRT decision-making. Furthermore, the study was performed in line with the newest methodological recommendations concerning prediction model development [13].
Taking the above-mentioned limitations into account, we would strongly discourage clinical use until the prediction tool has been externally validated. Even then, the developed models are only meant for patients who are eligible for both dialysis and CC, and are ≥70 years old, and we cannot be certain that the difference in predicted prognosis on dialysis and CC is solely due to this treatment choice. This prediction tool should never be used to determine treatment eligibility. It is meant to provide more accurate information on mortality to patients who are considering both treatment options, in order to contribute to an informed decision. Further qualitative studies should be performed to investigate how this prognosis information can best be presented to the patient. However, we do believe that this study is an important first step towards predicting prognosis for these two possible treatment pathways at a time point that is relevant to both patient and physician.
Finally, as previously mentioned, external validation is of great importance before these models are implemented. Testing these models in an external population will ensure accuracy, and allow for updating or recalibration. Not all variables associated with mortality were available in our cohort. Future studies could investigate whether predictors such as frailty, cognition, mobility, dialysis modality or rate of eGFR decline could improve model performance. Future research should also focus on predicting other outcomes such as quality of life, as an expected improved quality of life and more hospital-free days are some of the main benefits of CC over dialysis [14, 39, 40].
In conclusion, we have developed and internally validated a prediction tool that provides individualized 2-year mortality risks on CC and dialysis treatment in older patients with advanced CKD. Future studies should test the prediction tool in other patients before use. After external validation, individualized predictions of mortality, combined with expected quality of life and patient preferences, can be of value in shared decision-making when weighing the potential burdens and benefits of dialysis and CC.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
FUNDING
The work on this study by W.R.V. and W.J.W.B. and data collection was funded by unrestricted grants from the St Antonius Research Fund, from Roche to the St Antonius Research Fund and from Zilveren Kruis, Insurance Company. The work on this study by M.v.D. was supported by a grant from the Dutch Kidney Foundation (16OKG12). The funders played no role in study design; collection, analysis and interpretation of data; writing of the report; or in the decision to submit the report for publication.
AUTHORS’ CONTRIBUTIONS
C.L.R., F.W.D., W.J.W.B. and M.v.D. contributed to the research idea and study design; W.R.V. contributed to data acquisition; C.L.R., W.R.V., M.v.B., F.W.D., W.J.W.B. and M.v.D. contributed to data analysis/interpretation; C.L.R. performed the statistical analysis; F.W.D., W.J.W.B. and M.v.D. contributed to supervision. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST STATEMENT
None declared. All authors declare no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work. The results presented in this paper have not been published previously in whole or part, except in abstract form.
Supplementary Material
REFERENCES
- 1. van Walraven C, Manuel DG, Knoll G.. Survival trends in ESRD patients compared with the general population in the United States. Am J Kidney Dis 2014; 63: 491–499 [DOI] [PubMed] [Google Scholar]
- 2. Eckardt KU, Coresh J, Devuyst O. et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet 2013; 382: 158–169 [DOI] [PubMed] [Google Scholar]
- 3. Burns A, Carson R.. Maximum conservative management: a worthwhile treatment for elderly patients with renal failure who choose not to undergo dialysis. J Palliat Med 2007; 10: 1245–1247 [DOI] [PubMed] [Google Scholar]
- 4. Verberne WR, Geers AB, Jellema WT. et al. Comparative survival among older adults with advanced kidney disease managed conservatively versus with dialysis. Clin J Am Soc Nephrol 2016; 11: 633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wongrakpanich S, Susantitaphong P, Isaranuwatchai S. et al. Dialysis therapy and conservative management of advanced chronic kidney disease in the elderly: a systematic review. Nephron 2017; 137: 178–189 [DOI] [PubMed] [Google Scholar]
- 6. Ramer SJ, McCall NN, Robinson-Cohen C. et al. Health outcome priorities of older adults with advanced CKD and concordance with their nephrology providers’ perceptions. J Am Soc Nephrol 2018; 29: 2870–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Couchoud C, Hemmelgarn B, Kotanko P. et al. Supportive care: time to change our prognostic tools and their use in CKD. Clin J Am Soc Nephrol 2016; 11: 1892–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kurella Tamura M, Desai M, Kapphahn KI. et al. Dialysis versus medical management at different ages and levels of kidney function in veterans with advanced CKD. J Am Soc Nephrol 2018; 29: 2169–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jassal SV, Kelman EE, Watson D.. Non-dialysis care: an important component of care for elderly individuals with advanced stages of chronic kidney disease. Nephron Clin Pract 2011; 119: c5–c9 [DOI] [PubMed] [Google Scholar]
- 10. Yoshino M, Kuhlmann MK, Kotanko P. et al. International differences in dialysis mortality reflect background general population atherosclerotic cardiovascular mortality. J Am Soc Nephrol 2006; 17: 3510–3519 [DOI] [PubMed] [Google Scholar]
- 11. Ramspek CL, Voskamp PW, van Ittersum FJ. et al. Prediction models for the mortality risk in chronic dialysis patients: a systematic review and independent external validation study. Clin Epidemiol 2017; 9: 451–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Villain C, Fouque D.. Choosing end-stage kidney disease treatment with elderly patients: are data available? Nephrol Dial Transplant 2019; 34: 1432–1435 [DOI] [PubMed] [Google Scholar]
- 13. Collins GS, Reitsma JB, Altman DG. et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 2015; 350: g7594. [DOI] [PubMed] [Google Scholar]
- 14. Verberne WR, Dijkers J, Kelder JC. et al. Value-based evaluation of dialysis versus conservative care in older patients with advanced chronic kidney disease: a cohort study. BMC Nephrol 2018; 19: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Floege J, Gillespie IA, Kronenberg F. et al. Development and validation of a predictive mortality risk score from a European hemodialysis cohort. Kidney Int 2015; 87: 996–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holme I, Fellstrom BC, Jardin AG. et al. Prognostic model for total mortality in patients with haemodialysis from the Assessments of Survival and Cardiovascular Events (AURORA) study. J Intern Med 2012; 271: 463–471 [DOI] [PubMed] [Google Scholar]
- 17. Foote C, Kotwal S, Gallagher M. et al. Survival outcomes of supportive care versus dialysis therapies for elderly patients with end-stage kidney disease: a systematic review and meta-analysis. Nephrology (Carlton )2016; 21: 241–253 [DOI] [PubMed] [Google Scholar]
- 18. Vittinghoff E, McCulloch CE.. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 2007; 165: 710–718 [DOI] [PubMed] [Google Scholar]
- 19. Courvoisier DS, Combescure C, Agoritsas T. et al. Performance of logistic regression modeling: beyond the number of events per variable, the role of data structure. J Clin Epidemiol 2011; 64: 993–1000 [DOI] [PubMed] [Google Scholar]
- 20. de Goeij MC, van Diepen M, Jager KJ. et al. Multiple imputation: dealing with missing data. Nephrol Dial Transplant 2013; 28: 2415–2420 [DOI] [PubMed] [Google Scholar]
- 21. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007; 16: 219–242 [DOI] [PubMed] [Google Scholar]
- 22. Royston P, Moons KG, Altman DG. et al. Prognosis and prognostic research: developing a prognostic model. BMJ 2009; 338: b604–b604 [DOI] [PubMed] [Google Scholar]
- 23. Moons KG, Kengne AP, Woodward M. et al. Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart 2012; 98: 683–690 [DOI] [PubMed] [Google Scholar]
- 24. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley, 1987. [Google Scholar]
- 25. Austin PC, Steyerberg EW.. Graphical assessment of internal and external calibration of logistic regression models by using loess smoothers. Statist Med 2014; 33: 517–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cohen LM, Ruthazer R, Moss AH. et al. Predicting six-month mortality for patients who are on maintenance hemodialysis. Clin J Am Soc Nephrol 2010; 5: 72–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Findlay A, Farrington K, Joosten H. et al. Clinical Practice Guideline on management of older patients with chronic kidney disease stage 3b or higher (eGFR<45 mL/min/1.73 m2): a summary document from the European Renal Best Practice Group. Nephrol Dial Transplant 2017; 32: 9–16 [DOI] [PubMed] [Google Scholar]
- 28. Couchoud CG, Beuscart JB, Aldigier JC. et al. Development of a risk stratification algorithm to improve patient-centered care and decision making for incident elderly patients with end-stage renal disease. Kidney Int 2015; 88: 1178–1186 [DOI] [PubMed] [Google Scholar]
- 29. Brown MA, Collett GK, Josland EA. et al. CKD in elderly patients managed without dialysis: survival, symptoms, and quality of life. Clin J Am Soc Nephrol 2015; 10: 260–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ladin K, Lin N, Hahn E. et al. Engagement in decision-making and patient satisfaction: a qualitative study of older patients’ perceptions of dialysis initiation and modality decisions. Nephrol Dial Transplant 2017; 32: 1394–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Llewellyn H, Low J, Smith G. et al. Narratives of continuity among older people with late stage chronic kidney disease who decline dialysis. Soc Sci Med 2014; 114: 49–56 [DOI] [PubMed] [Google Scholar]
- 32. Schell JO, Patel UD, Steinhauser KE. et al. Discussions of the kidney disease trajectory by elderly patients and nephrologists: a qualitative study. Am J Kidney Dis 2012; 59: 495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fine A, Fontaine B, Kraushar MM. et al. Nephrologists should voluntarily divulge survival data to potential dialysis patients: a questionnaire study. Perit Dial Int 2005; 25: 269–273 [PubMed] [Google Scholar]
- 34. Singh P, Germain MJ, Cohen L. et al. The elderly patient on dialysis: geriatric considerations. Nephrol Dial Transplant 2014; 29: 990–996 [DOI] [PubMed] [Google Scholar]
- 35. Davison SN, Jhangri GS, Holley JL. et al. Nephrologists’ reported preparedness for end-of-life decision-making. Clin J Am Soc Nephrol 2006; 1: 1256–1262 [DOI] [PubMed] [Google Scholar]
- 36. Schell JO, Green JA, Tulsky JA. et al. Communication skills training for dialysis decision-making and end-of-life care in nephrology. Clin J Am Soc Nephrol 2013; 8: 675–680 [DOI] [PubMed] [Google Scholar]
- 37. Wachterman MW, Marcantonio ER, Davis RB. et al. Relationship between the prognostic expectations of seriously ill patients undergoing hemodialysis and their nephrologists. JAMA Intern Med 2013; 173: 1206–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Austin PC, Steyerberg EW.. Events per variable (EPV) and the relative performance of different strategies for estimating the out-of-sample validity of logistic regression models. Stat Methods Med Res 2017; 26: 796–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morton RL, Snelling P, Webster AC. et al. Factors influencing patient choice of dialysis versus conservative care to treat end-stage kidney disease. CMAJ 2012; 184: E277–E283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Verberne WR, Das-Gupta Z, Allegretti AS. et al. Development of an international standard set of value-based outcome measures for patients with chronic kidney disease: a report of the International Consortium for Health Outcomes Measurement (ICHOM) CKD Working Group. Am J Kidney Dis 2019; 73: 372–384 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.