Abstract
Chronic kidney disease (CKD) is a major health problem because of its high prevalence, associated complications and high treatment costs. Several aspects of CKD differ significantly in the Eastern European nephrology community compared with Western Europe because of different geographic, socio-economic, infrastructure, cultural and educational features. The two most frequent aetiologies of CKD, DM and hypertension, and many other predisposing factors, are more frequent in the Eastern region, resulting in more prevalent CKD Stages 3–5. Interventions may minimize the potential drawbacks of the high prevalence of CKD in Eastern Europe, which include several options at various stages of the disease, such as raising public, medical personnel and healthcare authorities awareness; early detection by screening high-risk populations; preventing progression and CKD-related complications by training health professionals and patients; promoting transplantation or home dialysis as the preferred modality; disseminating and implementing guidelines and guided therapy and encouraging/supporting country-specific observational research as well as international collaborative projects. Specific ways to significantly impact CKD-related problems in every region of Europe through education, science and networking are collaboration with non-nephrology European societies who have a common interest in CKD and its associated complications, representation through an advisory role within nephrology via national nephrology societies, contributing to the training of local nephrologists and stimulating patient-oriented research. The latter is mandatory to identify country-specific kidney disease–related priorities. Active involvement of patients in this research via collaboration with the European Kidney Patient Federation or national patient federations is imperative to ensure that projects reflect specific patient needs.
Keywords: chronic renal failure, CKD, dialysis, ESRD, kidney transplantation
CHRONIC KIDNEY DISEASE—A WORLDWIDE PROBLEM
Chronic kidney disease (CKD) is associated with several comorbidities, carrying a huge social and economic burden [1]. CKD affects ∼10% of the world population [2] and ∼850 million people worldwide suffered from CKD in 2018 [3]. Alarmingly, the global all-age prevalence increased by 29.3% between 1990 and 2017 [4], making CKD an ever-growing health burden. Both direct and indirect health effects of CKD are of serious concern, especially as they continue to increase. In 2017, CKD and related cardiovascular disease mortality ranked as the 12th greatest cause of death [4], and it is predicted to become the 5th cause of death worldwide by 2040 [5].
CKD is associated with psychosocial problems, including poorer educational attainment, lower income and reduced well-being and quality of life compared with the healthy population. Consequently these drawbacks perpetuate worse outcomes in CKD because social disadvantage often results in inadequate access to healthcare [6]. Kidney failure, the final stage of CKD, is expensive to treat. The average annual cost of maintenance haemodialysis (HD) globally was Int$22 617 per person in 2016 [7]. The total US Medicare spending on CKD and kidney failure was >$120 billion in 2017, accounting for 7.2% of overall claims paid by Medicare [8]. The mean annual total healthcare costs for different CKD stages or end-stage kidney disease differed significantly among countries in 2019: approximately $40 000 in the USA, $3600 in Italy and $16 000 in the UK [9]. The substantial variation may be linked to differences in healthcare accountancy, clinical practices and/or the cost of care among countries [9].
Several recent landmark publications elegantly describe the overall burden and management of CKD worldwide [1, 7, 10, 11], pointing to significant disparities between countries [12, 13]. Various aspects of CKD differ significantly and are worse in the Eastern European nephrology community than Western Europe because of different geographic, socio-economic, infrastructure, cultural and educational features (Table 1) [2, 12, 14–22].
Table 1.
Reasons for differences in various characteristics of CKD in Eastern European countries compared with Western Europe
| • Unhealthy lifestyle due to rapid urbanization and unplanned infrastructure, which may give rise to increasing rates of non-communicable diseases (especially hypertension and DM). |
| • Less public awareness about CKD. |
| • Deficient recognition and recording of CKD and insufficient statistics on CKD-related morbidity and mortality. |
| • Inadequate management of predisposing diseases (e.g. DM, hypertension, obesity) or causative factors (e.g. herbal remedies) associated with CKD. |
|
• Region- or country-specific problems (e.g. Balkan nephropathy). o Low Tx rates. |
|
• Few nephrologists per million population. o Limited funds for healthcare services. o Inadequate research on CKD. |
The European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) recently commissioned the Nephrology and Public Policy Committee (NPPC) to evaluate the overall burden of CKD across Europe. The NPPC identified eight key topics to stimulate research collaboration and grant applications for screening, diagnosis, prevention and treatment of CKD and advises translating this plan into public policy action [15].
In this document, the NPPC evaluated the extent of current problems and the state of epidemiological and clinical nephrology research related to CKD in the Eastern European nephrology community. It also summarized a potential role that various institutions can have in coping with these problems and how these institutions can contribute [15, 23]. These institutions include the ERA-EDTA, the European Kidney Health Alliance, European Kidney Patients’ Federation and the various national nephrology societies and patient organizations.
UNIQUE FEATURES OF CKD IN EASTERN EUROPE
No consistent definition of Eastern Europe has been agreed upon. The Ural Mountains and River and the Caucasus Mountains form the Eastern geographical border of Europe, but the Western boundaries are controversial [24]. However, the most important difference affecting kidney health between Eastern and Western Europe is not geographical, but economic. According to the World Bank, gross national income (GNI) per capita is significantly lower in Eastern Europe than Western Europe, although Eastern Europe is highly heterogeneous itself (Tables 2 and 3) [25]. For the purpose of this review, we distinguished 12 Eastern European countries with GNI per capita ≤US$12 000 from 11 others with higher GNI based on an arbitrary cut-off of US$1000 of personal income per month. We assumed that several features of lower-GNI Eastern European countries differ not only from Western European countries, but also from higher-income Eastern European countries, including CKD risk factors and CKD care.
Table 2.
Frequency of various aetiologies and risk factors for CKD and prevalence of Stages 3–5 CKD, dialysis and Tx in the Eastern European countries
| Country | GP (millions) | GNI per capita (US$) | DM (%)a | Elevated BP (%)a | Obesity (%)a | Physical inactivity (%)a | Tobacco use (%)a | Salt intake (g/day)a | CKD Stages 3–5 prevalence (crude) (%)a | Prevalence of dialysis (pmp)a | Prevalence of kidney Tx (pmp), deceased donora | Prevalence of kidney Tx (pmp)a, all donorsb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ukraine | 42.4 | 2660 | 9.1 | 32.0 | 26.0 | 21.0 | 27.0 | 11.0 | 6.9 | 180 | 8 | 30 |
| Moldova | 3.5 | 2980 | 9.3 | 33.0 | 20.0 | 12.0 | 24.0 | 10.0 | 5.4 | |||
| Albania | 2.9 | 4860 | 8.3 | 32.0 | 22.0 | – | 29.0 | 9.0 | 3.3 | 442 | 16 | 97 |
| North Macedonia | 2.0 | 5450 | 8.4 | 32.0 | 24.0 | – | – | 10.0 | 3.3 | 763 | 8 | 108 |
| Belarus | 9.5 | 5670 | 9.5 | 32.0 | 27.0 | 15.0 | 26.0 | 11.0 | 6.9 | 265 | 178 | 188 |
| Bosnia and Herzegovina | 3.5 | 5740 | 9.3 | 37.0 | 19.0 | 26.0 | 38.0 | 9.0 | 4.0 | 652 | 31 | 97 |
| Serbia | 7.0 | 6390 | 8.6 | 35.0 | 24.0 | 41.0 | 36.0 | 9.0 | 3.6 | 598 | 49 | 113 |
| Montenegro | 0.6 | 8430 | 8.7 | 34.0 | 25.0 | 46.0 | 9.0 | 3.9 | – | – | ||
| Bulgaria | 7.1 | 8860 | 10.3 | 36.0 | 27.0 | 41.0 | 33.0 | 9.0 | 4.8 | 534 | 71 | 93 |
| Russia | 146.9 | 10 230 | 9.3 | 32.0 | 26.0 | 18.0 | 37.0 | 11.0 | 7.0 | 269 | 42 | 64 |
| Turkey | 80.8 | 10 420 | 13.2 | 30.3 | 32.0 | 31.0 | 28.0 | 14.8 | 5.2 | 767 | 190 | |
| Romania | 19.6 | 11 290 | 8.4 | 35.0 | 25.0 | 38.0 | 28.0 | 10.0 | 4.2 | 1043 | 38 | 98 |
| Average (weighted) | 325.8 | 8904 | 10.2 | 32 | 27.2 | 23.8 | 32.3 | 11.7 | 6.1 | 451 | 42 | 99.6 |
| Croatia | 4.1 | 14 000 | 9.9 | 41.0 | 27.0 | 33.0 | 33.0 | 9.0 | 4.4 | 738 | 501 | 509 |
| Poland | 38.4 | 14 100 | 9.5 | 34.0 | 26.0 | 34.0 | 27.0 | 10.0 | 4.4 | 509 | – | 278 |
| Hungary | 9.8 | 14 780 | 10.0 | 37.0 | 29.0 | 41.0 | 28.0 | 11.0 | 4.2 | – | – | – |
| Latvia | 1.9 | 16 510 | 9.4 | 36.0 | 26.0 | 32.0 | 34.0 | 11.0 | 9.2 | 312 | – | 372 |
| Lithuania | 2.8 | 17 430 | 9.7 | 35.0 | 28.0 | 29.0 | 26.0 | 10.0 | 8.4 | 489 | – | 307 |
| Slovak Republic | 5.4 | 18 260 | 8.6 | 33.0 | 22.0 | 36.0 | 29.0 | 11.0 | 3.6 | 655 | – | |
| Greece | 10.8 | 19 770 | 9.1 | 26.0 | 27.0 | 41.0 | 41.0 | 10.0 | 5.0 | 1077 | 143 | 242 |
| Czech Republic | 10.6 | 20 240 | 9.6 | 34.0 | 29.0 | 33.0 | 32.0 | 10.0 | 3.9 | 682 | 494 | |
| Estonia | 1.3 | 21 140 | 9.3 | 34.0 | 24.0 | 34.0 | 29.0 | 10.0 | 9.6 | 309 | 395 | 411 |
| Slovenia | 2.1 | 24 580 | 9.5 | 38.0 | 23.0 | 35.0 | 20.0 | 11.0 | 3.8 | – | – | – |
| Cyprus | 0.9 | 26 300 | 7.8 | 22.0 | 23.0 | 45.0 | 36.0 | 10.0 | 3.6 | – | – | – |
| Average (weighted) | 88.1 | 16 496 | 9.5 | 33.7 | 26.6 | 35.5 | 29.9 | 10.2 | 4.6 | 629 | NA | 325 |
Nations above and below the thick line indicate low-medium income and high-medium income countries, respectively.
Ratio of each specific parameter in the index population compared with the whole population of that particular country.
The number of transplants from all donors may be greater than the sum of living and deceased donors because of inclusion of transplants with an unknown donor source.
GNI per capita was obtained from IndexMundi [25]. Percentages for DM,elevated BP, obesity, physical inactivity, tobacco use and salt/sodium intake were derived from multiple references [21, 26–28], whereas CKD prevalence was derived from Xie et al. [20] and Suleymanlar et al. [28]. Prevalence of dialysis and Tx were derived from the ERA-EDTA Registry [18].
The phrase ‘elevated blood pressure’ was used interchangeably with the term ‘hypertension’ for the purpose of presenting data in this table.
NA, not available.
Table 3.
Frequency of various aetiologies and risk factors for CKD and prevalence of Stages 3–5 CKD, dialysis and Tx in Western European countries
| Country | GP (millions) | GNI per capita (US$) | DM (%)a | Elevated BP (%)a | Obesity (%)a | Physical inactivity (%)a | Tobacco use (%)a | Salt intake (g/day)a | CKD 3–5 prevalence (crude) (%)a | Prevalence of dialysis (pmp)a | Prevalence of kidney Tx (pmp), deceased donora | Prevalence of kidney Tx (pmp)a, all donorsb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Portugal | 10.3 | 21 990 | 9.2 | 32 | 23 | 46 | 20 | 11 | 3.9 | 1236 | 661 | 729 |
| Spain | 46.6 | 29 340 | 9.4 | 25 | 27 | 30 | 26 | 10 | 6.1 | 593 | 691 | |
| Italy | 60.5 | 33 730 | 8.5 | 30 | 23 | 45 | 22 | 11 | 4.7 | 719 | 214 | 418 |
| France | 66.9 | 41 080 | 8 | 29 | 23 | 32 | 28 | 10 | 3.8 | 716 | 496 | 593 |
| UK | 66 | 41 770 | 7.7 | 20 | 30 | 38 | 21 | 9 | 3.7 | 437 | 347 | 534 |
| Belgium | 11.3 | 45 910 | 6.4 | 24 | 25 | 39 | 26 | 9 | 4 | 744 | 498 | 569 |
| Germany | 83 | 47 090 | 7.4 | 28 | 26 | 46 | 27 | 9 | 5.1 | 1093 | ||
| Finland | 5.5 | 48 280 | 7.7 | 27 | 25 | 19 | 18 | 10 | 3.6 | 361 | 499 | 548 |
| Austria | 8.7 | 49 310 | 6 | 27 | 22 | 33 | 27 | 10 | 4.6 | 518 | 475 | 569 |
| Netherlands | 17.1 | 51 260 | 6.1 | 25 | 23 | 29 | 25 | 8 | 3.3 | 376 | 324 | 662 |
| Sweden | 10.1 | 55 490 | 6.9 | 26 | 22 | 25 | 18 | 9 | 3.5 | 408 | 337 | 579 |
| Denmark | 5.8 | 60 140 | 6.1 | 27 | 21 | 31 | 19 | 8 | 4.1 | 459 | 297 | 499 |
| Ireland | 4.8 | 61 390 | 7.3 | 23 | 27 | 34 | 23 | 9 | 2.8 | |||
| Iceland | 0.3 | 67 960 | 7.1 | 23 | 23 | 14 | 9 | 2.7 | 253 | 184 | 507 | |
| Luxembourg | 0.6 | 70 870 | 6.8 | 26 | 24 | 30 | 23 | 10 | 3.3 | |||
| Norway | 5.3 | 80 610 | 6.6 | 25 | 25 | 34 | 20 | 10 | 2.6 | 297 | 419 | 680 |
| Switzerland | 8.5 | 84 410 | 5.6 | 24 | 21 | 26 | 24 | 9 | 5.3 | 435 | 305 | 502 |
| Average (weighted) | 411.3 | 42 668 | 8 | 26 | 25 | 37 | 24 | 10 | 4 | 702 | 376 | 565 |
Ratio of each specific parameter in the index population compared with the whole population of that particular country.
The number of transplants from all donors may be greater than the sum of living and deceased donors because of inclusion of transplants with an unknown donor source.
GNI per capita was obtained from IndexMundi [25]. Percentages for DM, obesity, physical inactivity, tobacco use and salt/sodium intake was derived from the World Health Organization [21], whereas CKD prevalence was derived from Xie et al. [20]. Prevalence of dialysis and Tx were derived from the ERA-EDTA Registry [18].
As the economy is the driving force for social, infrastructure, cultural and educational development, it is also likely one of the main factors defining significant differences in the prevalence, aetiology, predisposing factors and treatment modalities of kidney failure in Eastern Europe (Tables 1 and 2) [2, 12, 14, 16–18, 20–22, 25–27]. All CKD risk factors [i.e. DM (DM), hypertension, obesity, tobacco use, salt intake and physical inactivity] are more prominent in the general population (GP) of Eastern Europe. Accordingly, the prevalence of CKD Stages 3–5 is also significantly higher, especially in lower-income countries (Tables 2 and 3) [18, 20, 21, 25–28].
CKD and its complex array of potential causes and consequences may be neglected as health priorities in these countries. Therefore it is not easy to define simple ‘one-size-fits-all’ solutions. Nevertheless, several strategies for country-specific, patient-oriented research can be proposed while taking into account differences in prevalence, aetiology and economics.
Incidence and prevalence
In general, 80–90% of all CKD cases are in the early [glomerualr filtration rate (GFR) >60 mL/min] stages of the disease [29]. Few studies have analysed the country-specific prevalence of CKD in the Eastern European region. The Chronic REnal Disease in Turkey (CREDIT) study, a Turkish population-based study that included >10 000 subjects, reported prevalence rates for CKD Stages 1–5 of 5.4, 5.2, 4.7, 0.3 and 0.2%, respectively [28]. With age, the percentage of patients in Stages 2 and 3 increased, whereas the percentage of patients with Stage 1 CKD decreased. The overall prevalence of CKD was 15.7% and was higher in females than in males (18.4% versus 12.8%; P < 0.001). In the PolNef study, CKD was reported to affect 18.4% of the GP [30]. In another Polish study (PolSenior), CKD was diagnosed in 29.4% of 4979 randomly selected elderly subjects, but only 3.2% were aware of the disease [31]. In a population-based study on the prevention and awareness of CKD in Albania, among 1.1 million individuals (ages 35–70 years), the prevalence of elevated serum creatinine was 10.3%. Combining estimated GFR (eGFR) and albuminuria, the overall prevalence of CKD in adults (age ≥18 years) was 7.9% (M. Barbullushi, personal communication).
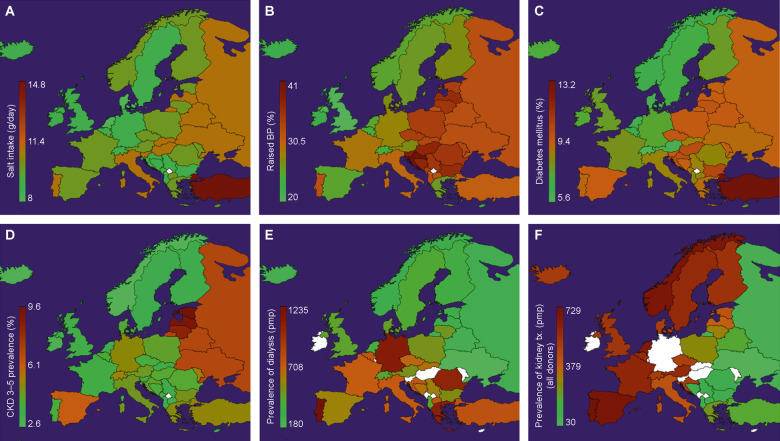
According to data from the World Health Organization, the prevalence of CKD Stages 3–5 is significantly higher in Eastern Europe than in Western Europe (Tables 2 and 3, Figure 1) [18, 20, 21, 26–28]. This likely relates to the higher prevalence of risk factors for CKD in Eastern Europe. Furthermore, the increase in CKD prevalence over time in this region far exceeds that of the USA and Western European countries (Figure 2) [20].
FIGURE 1.
Data reported for various countries in Eastern and Western Europe. (A) Variations in salt consumption. Prevalence of: (B) hypertension (raised blood pressure), (C) DM (raised blood glucose), (D) CKD Stages 3–5, (E) dialysis and (F) Tx. Data were obtained from several references [18, 20, 21, 26–28]. The colour scale from green to brown shows increasing prevalence; white denotes missing data in the ERA-EDTA Registry for those particular countries.
FIGURE 2.
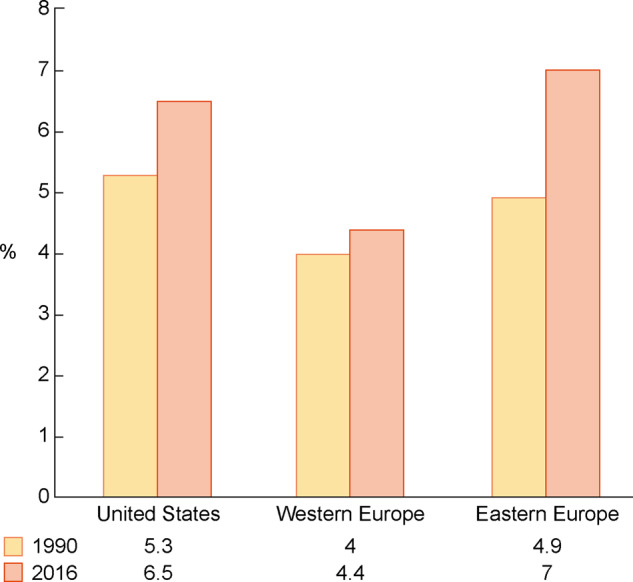
Trends in crude CKD prevalence in various regions of the world from 1990 to 2016. Note that the highest relative increases were noted in Central and Eastern Europe. Adapted from Xie et al. [20].
The frequency of patients requiring kidney replacement therapy (KRT) is well documented in the ERA-EDTA Registry [18] and the International Society of Nephrology (ISN) Global Kidney Health Atlas [7]. A recent report including several countries from Eastern Europe underlines the wide variation in the incidence and prevalence of patients requiring KRT [198 (range 97–654) per million population (pmp) and 934 (range 358–1052) pmp, respectively] [14].
The only European population-based study on the early stages of CKD in children was conducted in Turkey; the prevalence in children ages 5–18 years with an eGFR <75 mL/min/1.73 m2 was 0.94% [32]. Several paediatric publications highlight inequalities within Europe with respect to access to KRT [33, 34]. Importantly, and perhaps consequently, in Eastern Europe, excess mortality is seen in children with kidney failure [35].
Aetiology
The main aetiologies of CKD in Eastern Europe are similar to the rest of the world: DM, hypertension/vascular disease and glomerulonephritis [12, 14, 18]. Overall, the prevalence of DM, the most frequent cause of CKD, is higher in Eastern Europe than in Western Europe. Weighted averages of 10.2 and 9.5% are noted in low- and medium-income Eastern European countries, respectively, compared with 8% in Western Europe. Specifically, the rates of DM are 10, 10.3 and 13.2% in Hungary, Bulgaria and Turkey, respectively (Tables 2 and 3, Figure 1). Hypertension occurs with a similar pattern; weighted averages are 32 and 33% in low- and medium-income Eastern European countries, respectively, compared with 6% in Western Europe. The prevalence of hypertension has been reported to be 37% in Bosnia and Herzegovina and 41% in Croatia, significantly higher than in Western Europe (Tables 2 and 3, Figure 1). Other factors that predispose to CKD are also more frequent in this region; e.g. tobacco is used by 38, 41 and 46% of the inhabitants of Bosnia and Herzegovina, Greece and Montenegro, respectively, and salt consumption is ≥10 g/day in several countries (Table 2 and Figure 1).
Toxic nephropathies due to unconventional or herbal remedies and environmental toxins causing acute kidney injury (AKI) and CKD are increasing worldwide, especially in low- and middle-income countries (LMICs) [36, 37]. Balkan endemic nephropathy, which is caused by the environmental phytotoxin aristolochic acid, is an important factor in the aetiology of CKD in this region of Europe [38]. However, the overall contribution of this problem to CKD aetiology in Eastern Europe remains unclear.
Healthcare facilities and medical personnel
Treatment of CKD and resultant kidney failure necessitates a high-quality healthcare infrastructure, well-equipped facilities and well-trained medical personnel. All of these requirements closely correlate with national income, thus it is conceivable that there are more shortcomings in this regard in Eastern Europe. Notably, although the prevalence of DM, hypertension and CKD Stages 3–5 is significantly higher in Eastern Europe, the prevalence of dialysis patients and transplant recipients is lower (Tables 2 and 3). These findings suggest that patients in CKD Stages 3–5 die prematurely before experiencing kidney failure or that dialysis and transplantation (Tx) cannot be provided to those who are in need . Possible reasons for this include an inadequate healthcare infrastructure and a shortage of nephrologists in specific regions [7, 14].
Economic concerns/funding
The treatment of kidney failure is costly, therefore financial support from governments is essential to ensure that all patients receive appropriate treatment. This imposes a major burden on the economy. According to the World Bank, only 74% of governments in Eastern and Central Europe provide full support for KRT [7]. Funding of treatment in earlier stages of CKD is even more problematic because of the high number of patients and because the diagnosis may not be made in a timely manner. Although no objective data are available, it is very likely that public funding at earlier stages of CKD is less common or, at least, less comprehensive than reimbursement of KRT.
THE ROADMAP FOR POTENTIAL SOLUTIONS
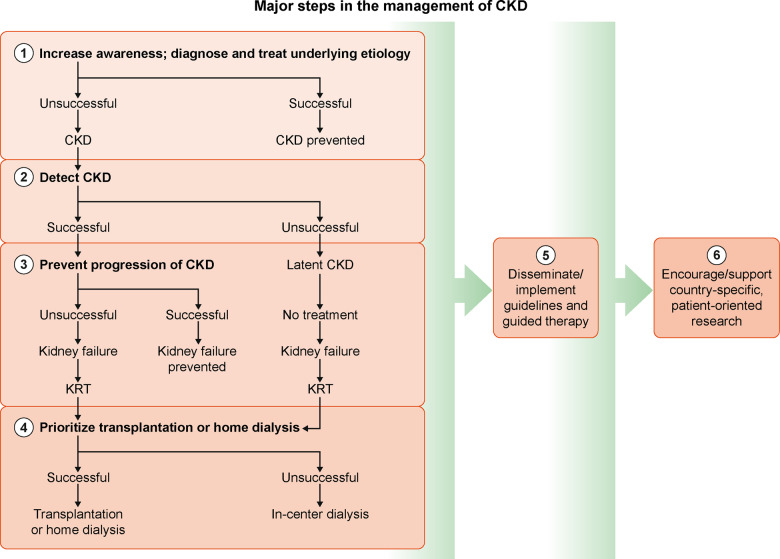
To improve the situation for kidney patients and the nephrology community in Eastern Europe, interventions should be considered at different stages during the course of CKD (Figure 3) [10].
FIGURE 3.
The algorithm outlines the major steps and pragmatic approach for decreasing the incidence and prevalence of CKD and for optimizing management during various stages of the disease. Interventions aim to raise awareness of underlying aetiologies at the beginning and encourage Tx or home dialysis when the stages of kidney failure and KRT dependency are reached.
1. Raise awareness about the burden of CKD and primary diseases/factors leading to CKD
The most cost-effective method of decreasing the burden of CKD is primary prevention (i.e. intervening before CKD occurs or preventing the onset of illness) [1]. This can be accomplished only by raising awareness among the public, medical personnel and authorities of CKD as a major public health problem [22, 39].
The public. Public awareness of CKD is poor, even in developed countries [40]. In a cross-sectional study of >75 000 individuals from 12 LMICs (including Bosnia and Herzegovina and Moldova in Eastern Europe), awareness of CKD was only 6 and 10% in the general and high-risk populations, respectively [41]. In the elderly Polish population (age >65 years), the awareness of CKD was even lower at 3.2% [31].
Potential contributions to detect CKD:
play an advisory role in conducting country-specific population-based surveys and research about the incidence and prevalence of CKD,
collaborate with European and national societies of other specialties to help inform and train their members about early detection of CKD,
organize local epidemiology and registry courses and/or offer fellowships to young nephrologists for training in organizing/optimizing registry systems in their native countries and
support the development of high-quality renal registries (instead of aggregated data) to assess and optimize kidney care.
Proposed research topics:
differences among various regions of a particular country on CKD incidence and prevalence, optimally by use of big data [51];
effects of early versus late referral of CKD patients and
economic burden of CKD as assessed by medical professionals in collaboration with health economists to collaborate with authorities on considering CKD a major public health problem in need of much larger healthcare and research budgets.
Approximately 25–40% of adults with type 2 diabetes and 30% with hypertension develop CKD. These diseases are the primary cause of CKD, yet the majority of patients with these diseases are not aware of this association [13]. Importantly, both type 2 diabetes and hypertension are treatable, and even preventable if predisposing factors (e.g. obesity, salt consumption, tobacco use, sedentary lifestyle and unhealthy nutrition) are addressed promptly. Other aetiologies of CKD are completely avoidable (e.g. toxic nephropathies due to unconventional therapies) [36]. Therefore the target must be to eliminate, or at least reduce, these causative factors by raising public awareness via visual and print media, as well as web-based education and social media [39, 42–44]. Organizing and supporting national campaigns by collaborating with national societies, patient groups, charitable and philanthropic organizations and kidney foundations should also be considered [22].
Healthcare personnel. CKD and its complications should be very familiar to other specialists, not only nephrologists. General practitioners and specialists with a high probability of seeing CKD patients (e.g. internists, cardiologists, diabetologists, endocrinologists, oncologists, urologists and neurologists) should be comfortable identifying and diagnosing CKD [45]. However, this awareness is lacking even in well-developed countries. In a nationwide analysis of >450 000 adults followed by general practitioners in Italy, only 15% had been correctly diagnosed as suffering from CKD because serum creatinine levels in high-risk patients were measured infrequently [46]. Similar observations have been made in other studies [47, 48]. Importantly, late or inaccurate diagnosis may result in delayed referral to the nephrologist, which may then jeopardize the possibility of slowing disease progression and protecting against the complications of CKD. In addition, the risk of delayed or inadvertent initiation of KRT is increased.
Healthcare authorities. The creation of CKD-specific policies by healthcare authorities is essential to cope with and reduce the problem. However, only 58% of the countries located in Eastern and Central Europe have CKD-specific healthcare policies, and the application of these programs in practice is even more problematic, as only 29% of LMICs effectively apply such strategies [7].
Potential contributions to raise awareness about the burden of CKD and primary diseases/factors leading to CKD:
Proposed research topics:
collaborate with national nephrology societies and the National Kidney Patients’ Federation to increase awareness of the public, healthcare personnel and authorities toward CKD as a public health priority;
notify European and national societies of other specialties (family practitioners, internists, cardiologists and neurologists) to allow them to educate their members regarding CKD as a public health problem and
stimulate and play an advisory role in country-specific, patient-oriented research.
epidemiological studies on the incidence and prevalence of early stages of CKD and causes of CKD,
longitudinal studies on the course of diseases predisposing to CKD and
surveys on the awareness of public, healthcare personnel and authorities about CKD.
2. Detecting CKD
Although early detection of CKD is a very effective and cost-effective tool for secondary prevention, it is still not as good as primary prevention. CKD is mostly asymptomatic, thus early detection, prevention or halting progression is possible only via screening [22].
Screening of the general population is not cost effective [1, 49]. Elective screening of high-risk groups (e.g. >65 years old, DM or hypertension, a family history of CKD, receiving potentially nephrotoxic drugs and suffering from AKI) has been suggested as a more efficient approach [22]. Thus far, screening strategies vary between countries, but patients with DM (93%) and hypertension (89%) are screened most frequently [7]. Screening practices are influenced by country income and overall is suboptimal in Eastern and Central Europe [7, 30].
Screening consists of two parameters: serum creatinine determination, applying the Chronic Kidney Disease Epidemiology Collaboration formula to estimate the GFR; and measurement of the urine albumin:creatinine ratio (UACR). The Kidney Disease: Improving Global Outcomes guideline defines CKD as ≥3 months of persistent abnormalities in kidney function (e.g. eGFR <60 mL/min/1.73 m2 or UACR ≥30 mg/g) [50]. The most practical approach for screening is a single determination of eGFR and UACR to classify CKD, if abnormal [4]. According to the ISN, the number of patients at high risk of CKD and screened is far from the desired range in the majority of LMICs, including several Eastern European countries [7].
Potential contributions to detect CKD:
play an advisory role in conducting country-specific population-based surveys and research about the incidence and prevalence of CKD,
collaborate with European and national societies of other specialties to help inform and train their members about early detection of CKD,
organize local epidemiology and registry courses and/or offer fellowships to young nephrologists for training in organizing/optimizing registry systems in their native countries and
support the development of high-quality renal registries (instead of aggregated data) to assess and optimize kidney care.
Proposed research topics:
differences among various regions of a particular country on CKD incidence and prevalence, optimally by use of big data [51];
effects of early versus late referral of CKD patients and
economic burden of CKD as assessed by medical professionals in collaboration with health economists to collaborate with authorities on considering CKD a major public health problem in need of much larger healthcare and research budgets.
3. Preventing progression and minimizing complications of CKD
Intervening early in the course of CKD prevents progression to kidney failure and associated complications. It may also provide economic benefits [4]. All stakeholders linked with the disease (patients, physicians and authorities) should have a distinct role in the management protocol.
Patients. Educating patients to modify lifestyle factors is simple and cost-effective. For individuals with CKD, this encompasses smoking cessation [52], decreasing salt intake [53], restricting dietary protein [50], limiting caloric intake in obese subjects [54], following an optimal diet for glycaemic control in diabetic patients [55], adherence to medication [56] and avoiding nephrotoxic herbs and traditional/alternative remedies [57]. Obesity, DM, hypertension, tobacco use and excessive salt intake are more prominent in Eastern Europe. Therefore instruction of and collaboration with people is essential while avoiding a patronizing approach.
Healthcare professionals. Many studies have demonstrated that optimal management of primary kidney diseases and other diseases/factors that may result in slow CKD progression and improve outcomes. Therefore, treating obesity or metabolic syndrome [54, 58], managing DM [59, 60], effectively controlling high blood pressure (BP) [16, 61, 62] and avoiding nephrotoxic drugs [63, 64] should be deemed key therapeutic options by healthcare professionals. Identifying and treating reversible causes of kidney injury early (e.g. hypovolemia, hypotension or urinary tract infection and obstruction) is vital.
CKD is highly prevalent and not all cases can be treated by nephrologists. Other specialists (cardiologists, diabetologists, geriatricians and neurologists) and general practitioners also play a pivotal role in the management of these patients. Therefore these professionals should keep abreast of updates on CKD management [65]. Continuing medical education courses to train all healthcare workers are not practical; therefore, online educational programmes should be considered [22]. This is especially true in LMICs [10], where management of CKD patients is mostly performed by primary care providers. Secondary care referral to a nephrologist should be considered, at the latest, when eGFR falls to 30 mL/min/1.73 m2. The small number of nephrologists and nephrology trainees in Eastern and Central Europe (3.3 pmp; Western Europe 5.8 pmp) cannot be corrected in the near future and is clearly a major drawback to implementing any policy [7].
Healthcare authorities. Authorities should be convinced by the kidney care stakeholders to prioritize CKD as a public health problem, finance educational courses, increase awareness of CKD and organize collaboration among various specialties related to CKD and with scientific societies to achieve these goals. Moreover, consumption of healthy food should be encouraged. Unhealthy nutrition must be limited by economic measures and national campaigns. Introducing measures to reduce the cost of healthy food, tax unhealthy products, restrict unhealthy food advertising and regulate specific food composition (salt, fats, trans-fats and sugar) is mandatory. Notifying the consumer of the health risks of the contents through food labelling has been suggested to be an optimal approach [13].
Healthy environment and healthy kidneys. Reduced environmental pollution is also vital for kidney health because environmental and occupational pollutants frequently cause kidney injury. Long-term exposure to particulate matter, which primarily comprises solid particulates derived from the combustion of coal, gasoline and diesel fuels, may increase the risk of membranous nephropathy, and exposure to heavy metals and industrial and agricultural chemicals predisposes to kidney disease [66]. Central and Eastern Europe have higher levels of pollution, mainly because of continued large-scale use of coal to generate electricity and rapid industrialization at the cost of environmental degradation [67, 68]. According to Greenpeace, the capital of Bulgaria, Sofia, boasted the highest levels of particulate matter ≤2.5 μm in Europe in 2018 [69].
Potential contributions to prevent progression and to minimize the complications of CKD:
collaborate with European and national societies of other specialties (general practitioners, internists, cardiologists and neurologists) to train their members on the management of CKD and collaborate with nephrologists to provide optimum care and
train nephrologists by supporting or contributing to local continuing medical education courses or by any other means (e.g. offering grants to participate in international meetings, organizing e-courses and providing specific evidence-based guidance).
Proposed research topics:
effects of diet and various treatment protocols directly or indirectly related to the progression of CKD. If the initial results of these pilot studies are encouraging, countrywide or transnational long-term projects may be planned considering early experience and lessons learned from the pilot initiatives.
4. Stimulating/prioritizing Tx and home dialysis as KRT modalities
When kidney failure develops, Tx is preferred because of its medical, social and economic advantages over dialysis [1]. However, the prevalence of Tx is low for several reasons. The most important factor is an imbalance between supply and demand of donor organs [18]. According to the 2017 ERA-EDTA Registry, the prevalence of Tx was only 8.3 pmp in Kosovo versus 29.7 pmp in Ukraine [18], but 509 pmp in Croatia (Table 2). The latter is the result of extensive policy measures targeting transplant coordination, donor hospital reimbursement, public awareness, donor legislation and donor quality [70]. Therefore, although challenging, increasing Tx rates is possible in many regions of Europe if appropriate measures are taken. Optimizing the follow-up of transplant recipients may contribute to favourable graft and patient outcomes, although the scarcity of transplant nephrologists in many countries may be a drawback.
If Tx is impossible, and if palliative treatment is not considered, then dialysis remains the only alternative. There is wide variation in the prevalence of dialysis in Europe overall; it is 1077 pmp in Greece versus 180 pmp in Ukraine [18]. Home dialysis, including home HD and peritoneal dialysis (PD), should be preferred over conventional in-centre HD because of better outcomes, quality of life and, in most countries, lower costs [1, 71–73]. These modalities are not frequently utilized in Europe, and even less so in Eastern Europe.
In a recent survey of the distribution of KRT modalities in 10 Eastern and Central European countries, a majority of kidney failure patients (73%) were receiving HD, whereas PD and Tx were used less frequently [14]. If Tx or dialysis is not an option, conservative (palliative) care is an alternative. The number of subjects with kidney failure who do not want or cannot receive dialysis is increasing steadily [74]. Usually these patients are elderly or have multiple or severe comorbidities, although exceptions may occur.
Using the option of end-of-life care, it is essential to respect patient priorities to offer dignity and the best quality of life possible and acceptable to the patient. A number of ethical issues warrant consideration [74, 75]. Reportedly, conservative care is less frequent in Eastern Europe, an issue deserving further investigation. Furthermore, the legal frameworks supporting conservative or palliative care are apparently inadequate [76].
For all treatment modalities, education on diet and adherence to medications may improve prognosis and patient rehabilitation.
Potential contributions to stimulate/prioritize Tx and home dialysis as a modality of KRT:
Proposed research topics:
stimulate multitiered country policies and an approach to promote organ donation, as well as kidney Tx [77];
provide solutions to increase Tx and home dialysis rates and
train local nephrologists on transplant and dialysis patient care.
surveys on the attitudes of the public towards deceased and live donor kidney donation;
surveys on the factors defining the number of transplant recipients and of PD and home self-care and in-centre HD patients who are not transplanted, and also the factors impacting Tx;
short- and long-term outcomes of patients on various KRT modalities;
prevalence of treatment-specific complications (e.g. vascular access complications in HD patients, peritonitis in PD patients; rejections and infections in transplant recipients);
economic and social burden of various KRT modalities and
prevalence and reasons for applying or not applying conservative comprehensive care.
5. Disseminating/implementing guidelines and guided therapy
One of the most effective ways to prevent treatment mistakes is to follow clinical best practice guidelines based on current evidence; thus guided therapy has recently become the standard of care [78]. Simple measures and guided therapy are effective in improving the outcomes of kidney patients; however, guidelines are underused in nephrology [79]. Many barriers to implementing guidelines in LMICs have been suggested, including, but not limited to, language barriers, guideline complexity and lack of familiarity, awareness, agreement, knowledge, motivation, practicalities and skills, all of which can be identified and overcome effectively [80–82].
Potential contributions to disseminating/implementing guidelines and guided therapy:
encourage and support the development of simplified, adapted and patient-involved guidelines in local languages;
identify and help overcome barriers to the implementation of guidelines;
prepare pragmatic educational materials (e.g. slide decks, flow charts, algorithms and patient booklets);
encourage, support and carry out updates to adapted guidelines in parallel with the original publications and
organize educational meetings and interactive workshops on the implementation of guidelines.
Proposed research topics:
surveys about guideline implementation,
studies on the efficacy of guided therapy versus standard treatment protocols and
studies on the economic benefit of guided therapy.
6. Encouraging/supporting country-specific, patient-oriented research
The general and medical infrastructure as well as the priorities of each country may differ significantly between countries. Therefore implementing and supporting country-specific surveillance strategies and conducting patient-oriented clinical studies are vitally important to the detection of major problems and planning health policy [45]. However, a national strategy for non-communicable diseases exists in only half of the countries in Central and Eastern Europe [7] and the quality and quantity of research on this issue is far from adequate.
Potential contributions to encourage/support country-specific, patient-oriented research:
train local nephrologists on research methodology and
assign experts as advisors for patient-oriented research.
CONCLUSIONS
Globally, CKD is an important public health burden with significant medical, social and economic consequences. The extent of this problem is more prominent in LMICs, which includes many Eastern European countries, largely due to a lack of public awareness, suboptimal health infrastructure, higher prevalence of conditions leading to CKD and failure in addressing significant lifestyle factors. Fewer nephrologists, country-specific health problems and restricted funds for healthcare services may further enhance the burden of CKD, and limited country-specific clinical research leads to a lack of specific data on the extent and characteristics of the problem for the Eastern European nephrology community.
Education and organization are suboptimal in Eastern European countries. It is advisable to develop a roadmap for supporting clinical and epidemiological research based on factual data in these countries. The financial support for such activities may be shared between several European institutions to help national societies. The ultimate goal is to coordinate efforts that should lead to improved screening, diagnosis, prevention and treatment of CKD in this part of Europe.
CONFLICT OF INTEREST STATEMENT
Results presented in this article have not been published previously in whole or in part, except in abstract format. The authors have indicated no conflicts of interest.
REFERENCES
- 1. Vanholder R, Annemans L, Brown E. et al. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat Rev Nephrol 2017; 13: 393–409 [DOI] [PubMed] [Google Scholar]
- 2. Bello ALA, Lunney M. et al. Global Kidney Health Atlas 2019: A Report by the International Society of Nephrology on the Global Burden of End-stage Kidney Disease and Capacity for Kidney Replacement Therapy and Conservative Care across World Countries and Regions. www.theisn.org/global-atlas (1 September 2020, date last accessed)
- 3. Jager KJ, Kovesdy C, Langham R. et al. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant 2019; 34: 1803–1805 [DOI] [PubMed] [Google Scholar]
- 4. GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020; 395: 709–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foreman KJ, Marquez N, Dolgert A. et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018; 392: 2052–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morton RL, Schlackow I, Mihaylova B. et al. The impact of social disadvantage in moderate-to-severe chronic kidney disease: an equity-focused systematic review. Nephrol Dial Transplant 2016; 31: 46–56 [DOI] [PubMed] [Google Scholar]
- 7.nternational Society of Nephrology. 2019 Global Kidney Health Atlas. https://www.kidneynews.org/careers/resources/2019-global-kidney-health-atlas-released-isn-word-congress (1 September 2020, date last accessed)
- 8. Saran R, Robinson B, Abbott KC. et al. US Renal Data System 2019 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2020; 75: A6–A7 [DOI] [PubMed] [Google Scholar]
- 9. Elshahat S, Cockwell P, Maxwell AP. et al. The impact of chronic kidney disease on developed countries from a health economics perspective: a systematic scoping review. PLoS One 2020; 15: e0230512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levin A, Tonelli M, Bonventre J. et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet 2017; 390: 1888–1917 [DOI] [PubMed] [Google Scholar]
- 11. Zoccali C, Vanholder R, Massy ZA. et al. The systemic nature of CKD. Nat Rev Nephrol 2017; 13: 344–358 [DOI] [PubMed] [Google Scholar]
- 12. Stanifer JW, Muiru A, Jafar TH. et al. Chronic kidney disease in low- and middle-income countries. Nephrol Dial Transplant 2016; 31: 868–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luyckx VA, Cherney DZI, Bello AK.. Preventing CKD in developed countries. Kidney Int Rep 2020; 5: 263–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spasovski G, Rroji M, Vazelov E. et al. Nephrology in the Eastern and Central European region: challenges and opportunities. Kidney Int 2019; 96: 287–290 [DOI] [PubMed] [Google Scholar]
- 15. Massy ZA, Caskey FJ, Finne P. et al. Nephrology and Public Policy Committee propositions to stimulate research collaboration in adults and children in Europe. Nephrol Dial Transplant 2019; 34: 1469–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Non-communicable Disease (NCD) Risk Factor Collaboration. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet 2017; 389: 37–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment. Lancet Diabetes Endocrinol 2014; 2: 634–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ERA-EDTA. ERA-EDTA Registry Annual Report 2017.https://www.era-edta-reg.org/files/annualreports/pdf/AnnRep2017.pdf (1 September 2020, date last accessed)
- 19. Galsworthy M, McKee M.. Europe’s ‘Horizon 2020’ science funding programme: how is it shaping up? J Health Serv Res Policy 2013; 18: 182–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xie Y, Bowe B, Mokdad AH. et al. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int 2018; 94: 567–581 [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Noncommunicable Diseases Country Profiles.https://www.who.int/nmh/countries/en/ (1 September2020, date last accessed)
- 22. Li PK, Garcia-Garcia G, Lui SF. et al. Kidney health for everyone everywhere-from prevention to detection and equitable access to care. Kidney Int 2020; 97: 226–232 [DOI] [PubMed] [Google Scholar]
- 23. Zoccali C, Arici M, Blankestijn PJ. et al. The ERA-EDTA today and tomorrow: a progress document by the ERA-EDTA Council. Nephrol Dial Transplant 2018; 33: 1077–1082 [DOI] [PubMed] [Google Scholar]
- 24.Wikipedia. Eastern Europe. https://en.wikipedia.org/wiki/Eastern_Europe (1 September 2020, date last accessed)
- 25.IndexMundi. GNI Per Capita, Atlas Method (Current US$) - Country Ranking.https://www.indexmundi.com/facts/indicators/NY.GNP.PCAP.CD/rankings (1 September 2020, date last accessed)
- 26. Erdem Y, Akpolat T, Derici U. et al. Dietary sources of high sodium intake in Turkey: SALTURK II. Nutrients 2017; 9: 933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sengul S, Akpolat T, Erdem Y. et al. Changes in hypertension prevalence, awareness, treatment, and control rates in Turkey from 2003 to 2012. J Hypertens 2016; 34: 1208–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suleymanlar G, Utas C, Arinsoy T. et al. A population-based survey of chronic renal disease in Turkey—the CREDIT study. Nephrol Dial Transplant 2011; 26: 1862–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jha V, Garcia-Garcia G, Iseki K. et al. Chronic kidney disease: global dimension and perspectives. Lancet 2013; 382: 260–272 [DOI] [PubMed] [Google Scholar]
- 30. Rutkowski BLA, Oacute L E.. Epidemiology of chronic kidney disease in central and eastern Europe. Blood Purif 2008; 26: 381–385 [DOI] [PubMed] [Google Scholar]
- 31. Chudek J, Wieczorowska-Tobis K, Zejda J. et al. The prevalence of chronic kidney disease and its relation to socioeconomic conditions in an elderly Polish population: results from the national population-based study PolSenior. Nephrol Dial Transplant 2014; 29: 1073–1082 [DOI] [PubMed] [Google Scholar]
- 32. Soylemezoglu O, Duzova A, Yalcinkaya F. et al. Chronic renal disease in children aged 5–18 years: a population-based survey in Turkey, the CREDIT-C study. Nephrol Dial Transplant 2012; 27: iii146–iii151 [DOI] [PubMed] [Google Scholar]
- 33. Chesnaye NC, Schaefer F, Groothoff JW. et al. Disparities in treatment rates of paediatric end-stage renal disease across Europe: insights from the ESPN/ERA-EDTA registry. Nephrol Dial Transplant 2015; 30: 1377–1385 [DOI] [PubMed] [Google Scholar]
- 34. Bonthuis M, Cuperus L, Chesnaye NC. et al. Results in the ESPN/ERA-EDTA registry suggest disparities in access to kidney transplantation but little variation in graft survival of children across Europe. Kidney Int 2020; 98: 464–475 [DOI] [PubMed] [Google Scholar]
- 35. Chesnaye NC, Schaefer F, Bonthuis M. et al. Mortality risk disparities in children receiving chronic renal replacement therapy for the treatment of end-stage renal disease across Europe: an ESPN-ERA/EDTA registry analysis. Lancet 2017; 389: 2128–2137 [DOI] [PubMed] [Google Scholar]
- 36. Jha V. Herbal medicines and chronic kidney disease. Nephrology (Carlton) 2010; 15(Suppl 2): 10–17 [DOI] [PubMed] [Google Scholar]
- 37. Mehta RL, Cerda J, Burdmann EA. et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet 2015; 385: 2616–2643 [DOI] [PubMed] [Google Scholar]
- 38. Jelakovic B, Dika Z, Arlt VM. et al. Balkan endemic nephropathy and the causative role of aristolochic acid. Semin Nephrol 2019; 39: 284–296 [DOI] [PubMed] [Google Scholar]
- 39. Hsiao LL. Raising awareness, screening and prevention of chronic kidney disease: it takes more than a village. Nephrology (Carlton )2018; 23: 107–111 [DOI] [PubMed] [Google Scholar]
- 40. Verhave JC, Troyanov S, Mongeau F. et al. Prevalence, awareness, and management of CKD and cardiovascular risk factors in publicly funded health care. Clin J Am Soc Nephrol 2014; 9: 713–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ene-Iordache B, Perico N, Bikbov B. et al. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob Health 2016; 4: e307–e319 [DOI] [PubMed] [Google Scholar]
- 42. Gheewala PA, Peterson GM, Zaidi STR. et al. Public knowledge of chronic kidney disease evaluated using a validated questionnaire: a cross-sectional study. BMC Public Health 2018; 18: 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chin HJ, Ahn JM, Na KY. et al. The effect of the World Kidney Day campaign on the awareness of chronic kidney disease and the status of risk factors for cardiovascular disease and renal progression. Nephrol Dial Transplant 2010; 25: 413–419 [DOI] [PubMed] [Google Scholar]
- 44. Schatell D. Web-based kidney education: supporting patient self-management. Semin Dial 2013; 26: 154–158 [DOI] [PubMed] [Google Scholar]
- 45.International Society of Nephrology. 12 Recommendations to Global Kidney Health https://www.theisn.org/images/12_recommendations_to_global_Kidney_Health.pdf (1 September 2020, date last accessed)
- 46. Minutolo R, De Nicola L, Mazzaglia G. et al. Detection and awareness of moderate to advanced CKD by primary care practitioners: a cross-sectional study from Italy. Am J Kidney Dis 2008; 52: 444–453 [DOI] [PubMed] [Google Scholar]
- 47. Ravera M, Noberasco G, Weiss U. et al. CKD awareness and blood pressure control in the primary care hypertensive population. Am J Kidney Dis 2011; 57: 71–77 [DOI] [PubMed] [Google Scholar]
- 48. Stevens LA, Fares G, Fleming J. et al. Low rates of testing and diagnostic codes usage in a commercial clinical laboratory: evidence for lack of physician awareness of chronic kidney disease. J Am Soc Nephrol 2005; 16: 2439–2448 [DOI] [PubMed] [Google Scholar]
- 49. Qaseem A, Humphrey LL, Fitterman N. et al. Screening, monitoring, and treatment of stage 1 to 3 chronic kidney disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2013; 159: 770–747 [DOI] [PubMed] [Google Scholar]
- 50.Kidney Disease: Improving Global Outcomes CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [DOI] [PubMed] [Google Scholar]
- 51. Saez-Rodriguez J, Rinschen MM, Floege J. et al. Big science and big data in nephrology. Kidney Int 2019; 95: 1326–1337 [DOI] [PubMed] [Google Scholar]
- 52. Orth SR, Hallan SI.. Smoking: a risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients—absence of evidence or evidence of absence? Clin J Am Soc Nephrol 2008; 3: 226–236 [DOI] [PubMed] [Google Scholar]
- 53. Hosohata K. Biomarkers for chronic kidney disease associated with high salt intake. Int J Mol Sci 2017; 18: 2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kovesdy CP, Furth SL, Zoccali C. et al. Obesity and kidney disease: hidden consequences of the epidemic. Kidney Int 2017; 91: 260–262 [DOI] [PubMed] [Google Scholar]
- 55. Hahr AJ, Molitch ME.. Management of diabetes mellitus in patients with chronic kidney disease. Clin Diabetes Endocrinol 2015; 1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mechta Nielsen T, Frojk Juhl M, Feldt-Rasmussen B. et al. Adherence to medication in patients with chronic kidney disease: a systematic review of qualitative research. Clin Kidney J 2018; 11: 513–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Luyckx VA. Nephrotoxicity of alternative medicine practice. Adv Chronic Kidney Dis 2012; 19: 129–141 [DOI] [PubMed] [Google Scholar]
- 58. Hsu CY, McCulloch CE, Iribarren C. et al. Body mass index and risk for end-stage renal disease. Ann Intern Med 2006; 144: 21–28 [DOI] [PubMed] [Google Scholar]
- 59. Wanner C, Inzucchi SE, Lachin JM. et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375: 323–334 [DOI] [PubMed] [Google Scholar]
- 60. Thomas MC, Brownlee M, Susztak K. et al. Diabetic kidney disease. Nat Rev Dis Primers 2015; 1: 15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xie X, Liu Y, Perkovic V. et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis 2016; 67: 728–741 [DOI] [PubMed] [Google Scholar]
- 62. Ruggenenti P, Perticucci E, Cravedi P. et al. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19: 1213–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pannu N, Nadim MK.. An overview of drug-induced acute kidney injury. Crit Care Med 2008; 36(Suppl 4): S216–S223 [DOI] [PubMed] [Google Scholar]
- 64. Lazarus B, Coresh J, Grams ME.. Adverse effects of proton pump inhibitors in chronic kidney disease–reply. JAMA Intern Med 2016; 176: 869–870 [DOI] [PubMed] [Google Scholar]
- 65. Jafar TH, Allen JC, Jehan I. et al. Health education and general practitioner training in hypertension management: long-term effects on kidney function. Clin J Am Soc Nephrol 2016; 11: 1044–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xu X, Nie S, Ding H. et al. Environmental pollution and kidney diseases. Nat Rev Nephrol 2018; 14: 313–324 [DOI] [PubMed] [Google Scholar]
- 67. Little RE. Public health in central and eastern Europe and the role of environmental pollution. Annu Rev Public Health 1998; 19: 153–172 [DOI] [PubMed] [Google Scholar]
- 68. Fitzgerald EF, Schell LM, Marshall EG. et al. Environmental pollution and child health in central and Eastern Europe. Environ Health Perspect 1998; 106: 307–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Agence France-Presse. Air Pollution Hotspots in Europe.https://www.ednh.news/air-pollution-hotspots-in-europe/(1 September 2020, date last accessed)
- 70. Zivcic-Cosic S, Busic M, Zupan Z. et al. Development of the Croatian model of organ donation and transplantation. Croat Med J 2013; 54: 65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kerr PG, Jaw J.. Home hemodialysis: what is old is new again. Contrib Nephrol 2017; 190: 146–155 [DOI] [PubMed] [Google Scholar]
- 72. Klarenbach S, Manns B.. Economic evaluation of dialysis therapies. Semin Nephrol 2009; 29: 524–532 [DOI] [PubMed] [Google Scholar]
- 73. van der Tol A, Stel VS, Jager KJ. et al. A call for harmonization of European kidney care: dialysis reimbursement and distribution of kidney replacement therapies. Nephrol Dial Transplant 2020; 35: 979–986 [DOI] [PubMed] [Google Scholar]
- 74. Russon L, Mooney A.. Palliative and end-of-life care in advanced renal failure. Clin Med 2010; 10: 279–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rak A, Raina R, Suh TT. et al. Palliative care for patients with end-stage renal disease: approach to treatment that aims to improve quality of life and relieve suffering for patients (and families) with chronic illnesses. Clin Kidney J 2017; 10: 68–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. van Biesen W, van de Luijtgaarden MW, Brown EA. et al. Nephrologists’ perceptions regarding dialysis withdrawal and palliative care in Europe: lessons from a European Renal Best Practice survey. Nephrol Dial Transplant 2015; 30: 1951–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. European Kidney Health Alliance. Joint Statement. Thematic Network on Improving Organ Donation and Transplantation in the EU.. http://ekha.eu/wp-content/uploads/FINAL_Joint-Statement-of-the-Thematic-Network-on-Organ-Donation-and-Transplantation.pdf (1 September 2020, date last accessed)
- 78. Kredo T, Bernhardsson S, Machingaidze S. et al. Guide to clinical practice guidelines: the current state of play. Int J Qual Health Care 2016; 28: 122–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kamath CC, Dobler CC, Lampman MA. et al. Implementation strategies for interventions to improve the management of chronic kidney disease (CKD) by primary care clinicians: protocol for a systematic review. BMJ Open 2019; 9: e027206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jha V, Arici M, Collins AJ. et al. Understanding kidney care needs and implementation strategies in low- and middle-income countries: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) controversies conference. Kidney Int 2016; 90: 1164–1174 [DOI] [PubMed] [Google Scholar]
- 81. van der Veer SN, Tomson CR, Jager KJ. et al. Bridging the gap between what is known and what we do in renal medicine: improving implementability of the European Renal Best Practice guidelines. Nephrol Dial Transplant 2014; 29: 951–957 [DOI] [PubMed] [Google Scholar]
- 82.National Institute for Health and Clinical Excellence. How to Change Practice: Understand, Identify and Overcome Barriers to Change.https://www.nice.org.uk/media/default/about/what-we-do/into-practice/support-for-service-improvement-and-audit/how-to-change-practice-barriers-to-change.pdf (1 September 2020, date last accessed)