Abstract
Background
Uromodulin, a tissue-specific tubular glycoprotein, has recently emerged as a promising biomarker for kidney function and tubular integrity. However, the association of serum uromodulin (sUmod) with renal function decline is still unknown in an older general population.
Methods
We analysed the association of sUmod with the estimated glomerular filtration rate (eGFR) and albuminuria in 1075 participants of the population-based Cooperative Health Research in the Region of Augsburg (KORA) F4 study, ages 62–81 years, at baseline and prospectively after a mean follow-up time of 6.5 years (n = 605) using logistic and linear regression models as well as receiver operating characteristics (ROC) analyses.
Results
Cross-sectionally, sUmod was positively associated with eGFR (β = 0.31 ± 0.02 per higher standard deviation sUmod; P < 0.001) and inversely associated with the urinary albumin:creatinine ratio (β = −0.19 ± 0.04; P < 0.001) after adjustment for sex, age, body mass index, arterial hypertension, prediabetes and diabetes. After multivariable adjustment including baseline eGFR, sUmod was not associated with incident chronic kidney disease (CKD), defined as a decrease in eGFR <60 mL/min/1.73 m2 after 6.5 years of follow-up {odds ratio [OR] 1.02 [95% confidence interval (CI) 0.77–1.36] per higher SD sUmod} but was inversely associated with advanced CKD, defined as incident eGFR <45 mL/min/1.73 m2 [OR 0.64 (95% CI 0.42–0.98)]. The ROC showed no added predictive value of sUmod for kidney function decline in the fully adjusted model.
Conclusions
Higher sUmod was inversely associated with progression to advanced kidney disease but does not provide additional predictive value for the development of CKD in elderly participants of the population-based KORA study.
Keywords: albuminuria, eGFR, general community, serum uromodulin
INTRODUCTION
Uromodulin is a glycosylphosphatidylinositol-anchored protein synthesized in tubular cells of the ascending limb of Henle’s loop and released into the urine by proteolytic cleavage [1–3]. In the urinary tract, the renal defensin uromodulin exerts anti-lithogen, anti-infective and immunomodulatory functions [4–11]. Mutations of the uromodulin-coding gene may cause severe kidney damage, such as tubulocystic kidney disease, recurring urinary tract infections, familial juvenile hyperuraemic nephropathy and congenital nephrolithiasis [3, 6, 8, 12–14]. Even small changes in uromodulin concentration or function, e.g. caused by uromodulin loci variants, may trigger or accelerate kidney disease [15–21]. In addition, inflammatory, ischaemic or toxic kidney disease with the loss of epithelia releasing uromodulin might aggravate the kidney function decline [22, 23].
Due to active secretion from the basolateral side of tubular cells into the interstitial space and circulation, uromodulin is also present in the bloodstream [24–26]. Serum uromodulin (sUmod) is a promising kidney tissue biomarker [25–28] that does not directly depend on glomerular filtration but mirrors tubular function and nephron mass [29, 30].
A positive association of sUmod with kidney function has been shown in patients with chronic kidney disease (CKD) [25, 26, 31], after kidney transplantation [25], in patients admitted for coronary angiography [27, 28] and in healthy individuals of different age groups [25, 32]. Longitudinal evaluations are only available in preselected cohorts involving patients with coronary and/or renal disease, in which sUmod is inversely related to kidney function decline [27, 31]. The association of sUmod with renal outcome in older populations from the general community is still unknown. Older people deserve special attention when evaluating kidney function because estimated glomerular filtration rate (eGFR) based on serum creatinine may be unreliable due to variable muscle mass losses, including sarcopenia. Additionally, comorbidities influencing eGFR via hyperfiltration, such as diabetes, obesity and arterial hypertension, accumulate in older age groups. Subsequently, episodes of unforeseen acute kidney failure become more frequent and correct drug dosing may be challenging [33]. Therefore the validation of a complementary kidney tissue marker not directly depending on eGFR is especially crucial for older people. Recently it has been shown that independent of eGFR, sUmod is inversely associated with type 2 diabetes, obesity and metabolic syndrome and its single components, including elevated blood pressure [34–36], providing the rationale to assume that sUmod might be a promising candidate biomarker for kidney function in an elderly general population typically having a high frequency of these risk factors. We here investigated the association of sUmod with kidney function cross-sectionally and longitudinally in elderly participants of the population-based Cooperative Health Research in the Region of Augsburg (KORA) F4/FF4 cohort. The hypothesis was that the results obtained from cohorts including patients with CKD and/or coronary heart disease showing an inverse association of sUmod with kidney function decline are transferable to our older population-based cohort and that sUmod is associated with incident CKD in this study population.
MATERIALS AND METHODS
Study participants
The KORA F4 (2006–08) and FF4 (2013–14) studies are follow-up examinations of the population-based KORA S4 study (1999–2001) in southern Germany. Recruitment and eligibility criteria, study design, standardized sampling methods and data collection (medical history, medication, anthropometric measurements and blood pressure) have been described previously [37–39]. All study participants gave written informed consent. The study was approved by the Ethics Committees of the Bavarian Medical Association in adherence with the Declaration of Helsinki. sUmod was measured in 1119 participants ages 62–81 years of the KORA F4 study with available serum samples (from a total of 1161 participants in this age group). All variables required for the cross-sectional analyses were available in 1075 participants. Of these 1075 participants, 119 died and 336 could not be contacted or declined to take part in the FF4 follow-up examination. Thus the study sample for the FF4 examination comprised 620 participants, of which 15 had to be excluded due to missing covariables (Supplementary data, Figure S1). The mean follow-up period was 6.5 ± 0.3 years.
Clinical diagnosis of diabetes mellitus was based on a validated physician’s diagnosis or the current use of glucose-lowering agents. After overnight fasting of at least 8 h, all participants without clinically diagnosed diabetes underwent a standard 75-g oral glucose tolerance test. Newly diagnosed diabetes, impaired glucose tolerance (IGT), impaired fasting glucose (IFG) and normal glucose tolerance were defined according to the American Diabetes Association diagnostic criteria based on both fasting and post-challenge glucose values (type 2 diabetes: ≥7.0 mmol/L fasting and/or ≥11.1 mmol/L 2-h glucose; IFG: ≥5.6 mmol/L and <7.0 mmol/L fasting glucose; IGT: ≥7.8–<11.1 mmol/L 2-h glucose). Prediabetes was defined as IFG and/or IGT (2-h glucose ≥7.8–<11.1 mmol/L). Participants with a diabetes type other than type 2 diabetes (n = 3) or unclassified glucose tolerance status (n = 22) were excluded. Arterial hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg or known hypertension with the use of antihypertensive drugs.
Laboratory measurements
Blood was kept at room temperature until centrifugation. Plasma and serum samples were assayed immediately or stored at −80°C. Measurements of serum creatinine, cystatin C and glucose were performed as described elsewhere [40]. Urinary creatinine concentration (Jaffe method) was determined on a Cobas Mira chemistry analyser (Greiner, Bahlingen, Germany). Urinary albumin concentration was measured with an immunoturbidimetric test (Tina-quant_Albumin in urine, Boehringer Mannheim, Germany) from a single-spot urine sample that had been stored at −80° C. sUmod was measured as described previously [26] using a commercial enzyme-linked immunosorbent assay kit (Euroimmun, Lübeck, Germany) with a lower detection limit of 2 ng/mL, an intra-assay coefficient of variation of 2.3% and interassay coefficients of variation of 4.4 and 9.5% for sUmod target values of 24.9 and 142.2 ng/mL, respectively. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation based on both serum creatinine and cystatin C [41].
Statistical analyses
The analyses were performed using the statistical environment R, version 3.6.2 (R Foundation, Vienna, Austria). The level of statistical significance was set at 5% (two-sided). Characteristics of the study participants were compared between sUmod quartiles using the Kruskal–Wallis test in the case of approximately normally distributed variables. For skewed distributed variables, analysis of variance tests were performed. Binomial proportions were compared with chi-squared tests. Spearman’s rank test was used to assess the correlation of untransformed sUmod and eGFR and Pearson’s correlation coefficient was used to analyse the correlation of logarithmized sUmod with eGFR. In the regression models, sUmod [log-transformed, continuous per standard deviation [SD]) was used as an exposure variable. eGFR categories, albuminuria and albuminuria categories were employed as outcome variables. The associations of sUmod with eGFR and albuminuria as continuous variables were assessed in linear regression models. The associations of sUmod with categorical variables were investigated using logistic regression models. The results are given as odds ratio [95% confidence interval (CI)] or standardized β coefficient ± standard error (SE), respectively. In multivariable logistic and linear regression analyses, the models were adjusted for the covariates sex, age, body mass index (BMI), arterial hypertension, prediabetes/type 2 diabetes and baseline eGFR (in the longitudinal analyses). The association of sUmod with albuminuria was adjusted for eGFR. The models are indicated in the tables for each analysis. Receiver operating characteristics (ROC) analyses were performed to investigate the predictive value of sUmod for incident CKD using the R package pROC (R Foundation).
RESULTS
Study population characteristics
The characteristics of the study population stratified by sUmod quartiles are presented in Table 1. The higher sUmod quartiles showed a more favourable metabolic and cardiovascular risk profile as well as higher eGFR values as compared with the lower sUmod quartiles.
Table 1.
Baseline characteristics of study participants
| Parameter | All participants | sUmod Q1 | sUmod Q2 | sUmod Q3 | sUmod Q4 | P-value |
|---|---|---|---|---|---|---|
| n | 1075 | 269 | 269 | 268 | 269 | – |
| sUmod (ng/mL), mean ± SD | 152.5 (110.0–207.7) | 84.8 (64.9–96.6) | 129.8 (120.7–141.0) | 177.1 (166.3–190.9) | 248.2 (224.9–281.8) | – |
| Sex (female), n (%) | 528 (49) | 108 (40) | 116 (43) | 135 (50) | 169 (63) | <0.001a |
| Age (years), mean ± SD | 70.2 ± 5.5 | 71.8 ± 5.7 | 70.8 ± 5.6 | 69.7 ± 5.3 | 68.8 ± 4.8 | <0.001b |
| BMI (kg/m2), mean ± SD | 28.7 ± 4.5 | 29.7 ± 4.8 | 29.2 ± 4.7 | 28.6 ± 4.0 | 27.4 ± 4.1 | <0.001b |
| Arterial hypertension, n (%) | 669 (62) | 202 (75) | 184 (68) | 152 (57) | 131 (49) | <0.001a |
| Type 2 diabetes, n (%) | 213 (20) | 82 (30) | 59 (22) | 48 (18) | 24 (9) | <0.001a |
| HbA1c (mmol/mol) | 37.7 (35.5–41.0) | 38.8 (36.6–43.2) | 38.8 (36.6–41.0) | 37.7 (35.5–41.0) | 37.7 (34.4–39.9) | <0.001c |
| eGFR (mL/min/1.73 m²), mean ± SD | 76.4 ± 15.7 | 67.9 ± 18.4 | 74.4 ± 14.0 | 80.2 ± 12.8 | 83.2 ± 11.5 | <0.001a |
| Urinary albumin: creatinine ratio (mg/g) | 8.4 (4.7–17.6) | 12.4 (6.4–32.4) | 7.9 (4.4–15.9) | 7.5 (4.5–13.7) | 6.8 (4.3–12.7) | <0.001c |
| Urinary albumin: creatinine ratio ≥30 mg/g, n (%) | 167 (16) | 72 (27) | 42 (16) | 28 (10) | 25 (9) | 0.003a |
Chi-square test.
Kruskal–Wallis test.
Analysis of variance test.
Value presented as median (first quartile–third quartile) unless stated otherwise.
Cross-sectional association of sUmod with eGFR
sUmod correlated with eGFR (Supplementary data, Figure S2). The relationship of untransformed sUmod with eGFR was fairly linear in the lower ranges of both parameters (Supplementary data, Figure S2B). However, at an eGFR >74 mL/min/1.73 m2, the relation of the eGFR and untransformed sUmod was not significant due to the large variation in sUmod values in the higher ranges. Logarithmized sUmod displayed a linear relation with eGFR (Supplementary data, Figure S2C).
sUmod was lower in participants with prevalent kidney disease. The median sUmod was 183.8 ng/mL (first quartile 141.2, third quartile 242.1) in study participants with an eGFR ≥90 mL/min/1.73 m2, 158.6 (115.8–210.3) in participants with an eGFR of 60–89 mL/min/1.73 m2, 112.2 (83.9–132.7) in participants with an eGFR of 45–59 mL/min/1.73 m2 and 75.2 (46.4–99.4) in participants with an eGFR <45 mL/min/1.73 m2. sUmod was also significantly positively associated with the eGFR in the linear regression models (Table 2). In line with these findings, multivariable logistic regression analyses revealed an inverse association of sUmod with a categorized eGFR <90, <60 and <45 mL/min/1.73 m2 in all models (Supplementary data, Table S1).
Table 2.
Association estimates between sUmod and baseline eGFR as continuous variables: β coefficients ± standard error from linear regression models are given per SD sUmod (logarithmized) (n = 1075)
| eGFR | P-value |
|---|---|
| Without adjustment | |
| 0.384 ± 0.023 | <0.001 |
| Adjustment for sex, age, BMI, arterial hypertension and prediabetes/type 2 diabetes | |
| 0.305 ± 0.022 | < 0.001 |
Longitudinal association of sUmod with change in eGFR
Baseline sUmod was not associated with ΔeGFR (change in eGFR from baseline to the follow-up examination) in the crude model (β = −0.02 ± 0.05) or in the model adjusted for sex, age, BMI, hypertension and prediabetes/type 2 diabetes (β = 0.004 ± 0.04).
Higher baseline sUmod was related to a lower incidence of eGFR <60 mL/min/1.73 m2 after adjustment for sex, age, BMI, hypertension and prediabetes/type 2 diabetes, but this association disappeared after additional adjustment for baseline eGFR. sUmod remained significantly inversely associated with an incident eGFR <45 mL/min/1.73 m2 after multivariable adjustment (Table 3).
Table 3.
ORs (95% CIs) for incident CKD per SD sUmod (logarithmized): results of logistic regression models
| Incident eGFR <60 mL/min/1.73 m² | P-value | Incident eGFR <45 mL/min/1.73 m² | P-value |
|---|---|---|---|
| Yes: n = 126; no: n = 425 | Yes: n = 50; no: n = 544 | ||
| Without adjustment | |||
| 0.73 (0.59–0.91) | 0.005 | 0.44 (0.33–0.61) | <0.001 |
| Adjustment for sex, age, BMI, arterial hypertension and prediabetes/diabetes | |||
| 0.76 (0.59–0.98) | 0.032 | 0.47 (0.33–0.67) | <0.001 |
| Adjustment for sex, age, BMI, arterial hypertension, prediabetes/diabetes and baseline eGFR | |||
| 1.02 (0.77–1.36) | 0.890 | 0.64 (0.42–0.98) | 0.038 |
ROC analysis (Figure 1) showed no added predictive value of sUmod for incident eGFR <60 mL/min/1.73 m2 [area under the curve 0.86 (95% CI 0.82–0.89) for both the base model including baseline eGFR, sex, age, BMI, arterial hypertension and prediabetes/diabetes and the model including sUmod]. For an incident eGFR <45 mL/min/1.73 m2, adding sUmod to the base model increased the area under the curve from 0.88 (95% CI 0.83–0.92) to 0.89 (95% CI 0.84–0.93), which was not significant (P = 0.20).
FIGURE 1.
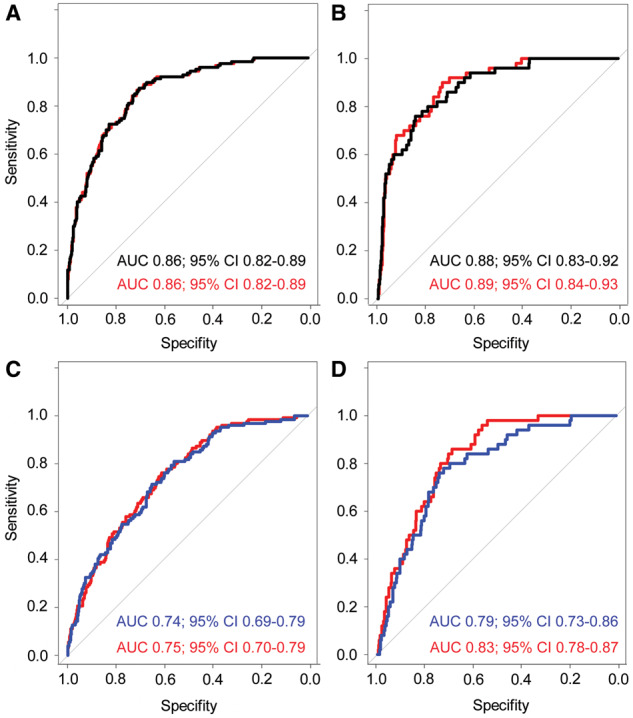
ROC analyses for the predictive value of sUmod for incident eGFR <60 mL/min/1.73 m2 (A and C) and incident eGFR <45 mL/min/1.73 m2 (B and D). Black: base model including sex, age, BMI, arterial hypertension, prediabetes/diabetes and baseline eGFR; blue: base model including sex, age, BMI, arterial hypertension, prediabetes/diabetes and baseline urinary albumin:creatinine ratio and red: respective base model plus sUmod.
Regarding the urinary albumin:creatinine ratio as a known predictor of kidney function, adding sUmod to the ROC model provided no additional predictive value for an incident eGFR <60 mL/min/1.73 m2 [area under the curve 0.75 (95% CI 0.70–0.79)] compared with the base model including the urinary albumin:creatinine ratio, sex, age, BMI, arterial hypertension and prediabetes/diabetes [area under the curve 0.74 (95% CI 0.69–0.79), P = 0.37; Figure 1C]. For an incident eGFR <45 mL/min/1.73 m2, the area under the curve was higher in the model including sUmod [0.83 (95% CI 0.78–0.87)] compared with the base model [0.79 (95% CI 0.73–0.86)]. However, this difference was not statistically significant (P = 0.053; Figure 1D).
Association of sUmod with albuminuria
sUmod was inversely associated with the urinary albumin:creatinine ratio as a continuous variable and with a urinary albumin:creatinine ratio ≥30 mg/g after multivariable adjustment including eGFR (P < 0.001; Supplementary data, Table S2).
Baseline sUmod was inversely associated with the evolution of a urinary albumin:creatinine ratio ≥30 mg/g in the crude longitudinal analysis (P < 0.001), but the association was no longer significant after multivariable adjustment (Supplementary data, Table S2).
DISCUSSION
We analysed the association of sUmod with kidney function at baseline and with the development of kidney disease during follow-up in a community-based population. sUmod was strongly associated with eGFR in the cross-sectional analysis. These results are in agreement with previous studies assessing different study populations [25–28]. In incipient kidney damage, the uromodulin decrease may precede the eGFR decline, since a progressive nephron loss may initially be compensated for by increased hydraulic pressure and subsequent glomerular hyperfiltration [25, 26]. Hence uromodulin is a promising marker for decreased kidney function already in the early stages and in primarily tubular injury. Since uromodulin is independent of glomerular hyperfiltration, it may be an interesting marker for early kidney disease in diabetes and other states in which eGFR may be disproportionately high due to hyperfiltration [26]. In line with this, we and others have shown that sUmod levels are decreased in type 2 diabetes independent of eGFR [34, 35]. Furthermore, sUmod was associated with proteinuria in the study of Steubl et al. [26] and with albuminuria in our cohort, suggesting that sUmod may, in fact, be a marker for early prevalent diabetic kidney disease.
In our longitudinal analysis, however, baseline sUmod was not associated with a decline of eGFR <60 mL/min/1.73 m2 and did not improve the prediction of kidney function decline in the ROC analysis. These results are in contrast with previous studies assessing the association of uromodulin with renal outcomes. However, these studies used urinary uromodulin and/or were performed in selected cohorts mainly involving participants with pre-existing CKD. The Framingham Heart Study showed an association of higher urinary uromodulin with a reduced risk of kidney disease progression [15]. In the Health ABC Study, urinary uromodulin was inversely associated with incident CKD [42]. In patients with CKD, low sUmod was associated with a higher risk of progression to end-stage renal disease [31] and was a marker of graft failure in recipients of kidney allografts [43]. Finally, sUmod was decreased in patients admitted for coronary angiography who developed CKD during follow-up [27]. This study of Leiherer et al. [27] was smaller and shorter than our study (529 participants at baseline and 340 participants at the follow-up examination after 3.5 years). Furthermore, in the multivariable models, the effect of sUmod on the decline of eGFR was adjusted for the rs13335818 genotype, which strengthened the association of sUmod with incident eGFR <60 mL/min/1.73 m2. Adjusted models without correction for the genotype were not given. However, for the potential use of sUmod as a commonly available biomarker in a broader population, correction for the interaction with genotypes is not feasible.
Our results are in line with the available literature in so far as sUmod was associated with a decline of eGFR <45 mL/min/1.73 m2 in our cohort, indicating that sUmod might be of additional informative value in more advanced kidney disease only. Very recently, Steubl et al. [44] described an inverse association of sUmod with incident end-stage renal disease in 933 participants of the Cardiovascular Health Study with a mean age of 78 years. This study differed from our population in several aspects. Corresponding to the higher age, baseline eGFR (63 versus 76 mL/min/1.73 m2) and sUmod (mean 127.2 versus 162.7 ng/mL) were lower in participants of the Cardiovascular Health Study compared with the KORA population and the albumin:creatinine ratio (13.9 versus 8.4 mg/g) and systolic blood pressure (137 versus 128 mmHg) were higher, indicating an adverse risk profile. Still, despite the higher risk profile and the lower eGFR and sUmod values, sUmod did not predict a decline of kidney function in higher eGFR ranges in this cohort, which is in line with our results.
The lacking association of sUmod with kidney function decline in higher eGFR ranges matches the attenuated cross-sectional correlation of sUmod with eGFR in preserved kidney function. Whereas uromodulin secretion decreases with a substantial reduction of total nephron mass, explaining the strong association with eGFR in the lower ranges, in normal or near-normal kidney function, other factors influencing uromodulin secretion may become more relevant and might explain the loss of a direct association of sUmod with kidney function as measurable by eGFR. For example, sUmod values are higher in women than in men, indicating that, for example, hormonal factors may play a role [34]. Furthermore, genetic variations may influence uromodulin levels in normal kidney function more strongly than the differences in renal reserve and thus influence the association of sUmod with incident kidney disease as demonstrated by Leiherer et al. [27], in whose study sUmod was only associated with incident kidney disease in the group homozygous for the rs13335818 major allele. In addition, reactive increases of uromodulin secreted by each functioning nephron unit may balance the total uromodulin amount in early kidney disease with sufficiently preserved tubular function [45]. The stronger inverse association with more advanced renal disease may derive from an already reduced renal tubular reserve after substantial kidney damage predisposing to progressive kidney function loss.
Study limitations and strengths
Our study included Caucasians ages 62–81 years. The association of sUmod with renal outcomes remains to be investigated in younger populations and other ethnicities. Due to the population-based design, the participants were relatively healthy and at low risk for the development of kidney disease, and only a few participants suffered from CKD Stage 4 or higher. Therefore our study was not suitable to evaluate the association of sUmod with end-stage renal disease. The major strength of our study is the large, well-characterized, community-based cohort representing a typical older European population and the follow-up time of 6.5 years. We measured sUmod with a sensitive and robust enzyme-linked immunosorbent assay. In contrast to uromodulin of urine origin used in most previous studies, which forms various polymers with chancing epitopes and different antigenic sites [46], sUmod is a stable antigen lacking such important pre-analytical disadvantages [26].
CONCLUSIONS
The current study confirms a strong cross-sectional association of sUmod with kidney function in older participants from a large population-based study. However, sUmod did not provide additional predictive value for kidney function decline beyond other known risk factors in our population-based cohort, indicating that the results obtained in cohorts with CKD and/or coronary heart disease are not readily transferable to a general population. Nevertheless, sUmod may be a useful independent biomarker for the identification of individuals at risk for the development of advanced kidney disease.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Victor Herbst, Matthias Block and Wolfgang Schlumberger, Euroimmun, Lübeck for providing the uromodulin assay. We thank Prof. Seymour Rosen, Beth Israel Deaconess Medical Center and Prof. Olivier Devuyst, University of Zürich for valuable discussions.
FUNDING
The KORA study was initiated and financed by the Helmholtz Zentrum München – German Research Center for Environmental Health, which is funded by the German Federal Ministry of Education and Research and by the State of Bavaria. The study was supported by a research grant from the Virtual Diabetes Institute (Helmholtz Zentrum München) and the Clinical Cooperation Group Diabetes, Ludwig-Maximilians-University München and Helmholtz Zentrum München and by the German Diabetes Center, which was supported by the Federal Ministry of Health (Berlin, Germany) and the Ministry of Culture and Science of the State North Rhine Westphalia (Düsseldorf, Germany). Further support was obtained from the Deutsche Diabetes Gesellschaft and the German Research Foundation (RA-45913/3-1).
AUTHORS’ CONTRIBUTIONS
C.M., M.H., A.P., W.K., W.R., B.T., A.L., J.S. and J.S. were involved in the conception and design of the study. C.T., B.T., C.M., M.H., A.P., W.K., W.R., J.S. and J.S. were responsible for data collection. C.T., H.T., A.L., J.S. and J.S. were involved in data analysis, interpretation of results and writing of the manuscript. All authors revised the manuscript critically for important intellectual content and approved the final version.
CONFLICT OF INTEREST STATEMENT
W.K. reports personal fees from AstraZeneca, Novartis, Pfizer, The Medicines Company, DalCor, Kowa, Amgen, Sanofi and Berlin-Chemie and grants and non-financial support from Roche Diagnostics, Beckmann, Singulex and Abbott. J.S. has a patent at the University Charite Berlin pending. The reported disclosures are not related to this article. All other authors declare that they have no conflicts of interest associated with this article. The results presented in this article have not been published previously in whole or part.
REFERENCES
- 1. Cavallone D, Malagolini N, Serafini-Cessi F. et al. Mechanism of release of urinary Tamm-Horsfall glycoprotein from the kidney GPI-anchored counterpart. Biochem Biophys Res Commun 2001; 280: 110–114 [DOI] [PubMed] [Google Scholar]
- 2. Schaeffer C, Santambrogio S, Perucca S. et al. Analysis of uromodulin polymerization provides new insights into the mechanisms regulating ZP domain-mediated protein assembly. Mol Biol Cell 2009; 20: 589–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Devuyst O, Olinger E, Rampoldi L.. Uromodulin: from physiology to rare and complex kidney disorders. Nat Rev Nephrol 2017; 13: 525–544 [DOI] [PubMed] [Google Scholar]
- 4. Pak J, Pu Y, Zhang ZT. et al. Tamm-Horsfall protein binds to type 1 fimbriated Escherichia coli and prevents E. coli from binding to uroplakin Ia and Ib receptors. J Biol Chem 2001; 276: 9924–9930 [DOI] [PubMed] [Google Scholar]
- 5. Raffi HS, Bates JM, Laszik Z. et al. Tamm-Horsfall protein protects against urinary tract infection by proteus mirabilis. J Urol 2009; 181: 2332–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bates JM, Raffi HM, Prasadan K. et al. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection. Kidney Int 2004; 65: 791–797 [DOI] [PubMed] [Google Scholar]
- 7. Serafini-Cessi F, Monti A, Cavallone D.. N-Glycans carried by Tamm-Horsfall glycoprotein have a crucial role in the defense against urinary tract diseases. Glycoconj J 2005; 22: 383–394 [DOI] [PubMed] [Google Scholar]
- 8. Mo L, Huang HY, Zhu XH. et al. N-Glycans carried by Tamm-Horsfall glycoprotein have a crucial role in the defense against urinary tract diseases. Glycoconj J 2005; 22: 383–394 [DOI] [PubMed] [Google Scholar]
- 9. Kreft B, Jabs WJ, Laskay T. et al. Polarized expression of Tamm-Horsfall protein by renal tubular epithelial cells activates human granulocytes. Infect Immun 2002; 70: 2650–2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Säemann MD, Weichhart T, Hörl WH. et al. Tamm-Horsfall protein: a multilayered defence molecule against urinary tract infection. Eur J Clin Invest 2005; 35: 227–235 [DOI] [PubMed] [Google Scholar]
- 11. Darisipudi MN, Thomasova D, Mulay SR. et al. Uromodulin triggers IL-1-dependent innate immunity via the NLRP3 inflammasome. J Am Soc Nephrol 2012; 23: 1783–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hart TC, Gorry MC, Hart PS. et al. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet 2002; 39: 882–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dahan K, Devuyst O, Smaers M. et al. A cluster of mutations in the UMOD gene causes familial juvenile hyperuricemic nephropathy with abnormal expression of uromodulin. J Am Soc Nephrol 2003; 14: 2883–2893 [DOI] [PubMed] [Google Scholar]
- 14. Williams S, Reed AAC, Galvanovskis J. et al. Uromodulin mutations causing familial juvenile hyperuricaemic nephropathy lead to protein maturation defects and retention in the endoplasmic reticulum. Hum Mol Genet 2009; 18: 2963–2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garimella PS, Biggs ML, Katz R. et al. Urinary uromodulin, kidney function, and cardiovascular disease in elderly adults. Kidney Int 2015; 88: 1126–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olden M, Corre T, Hayward C. et al. Common variants in UMOD associate with urinary uromodulin levels: a meta-analysis. J Am Soc Nephrol 2014; 25: 1869–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bleyer AJ, Hart PS, Kmoch S.. Hereditary interstitial kidney disease. Semin Nephrol 2010; 30: 366–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ekici AB, Hackenbeck T, Morinière V. et al. Renal fibrosis is the common feature of autosomal dominant tubulointerstitial kidney diseases caused by mutations in mucin 1 or uromodulin. Kidney Int 2014; 86: 589–599 [DOI] [PubMed] [Google Scholar]
- 19. Reznichenko A, Böger CA, Snieder H. et al. UMOD as a susceptibility gene for end-stage renal disease. BMC Med Genet 2012; 13: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Köttgen A, Hwang SJ, Larson MG. et al. Uromodulin levels associate with a common UMOD variant and risk for incident CKD. J Am Soc Nephrol 2010; 21: 337–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lhotta K. Uromodulin and chronic kidney disease. Kidney Blood Press Res 2010; 33: 393–398 [DOI] [PubMed] [Google Scholar]
- 22. Chakraborty J, Below AA, Solaiman D.. Tamm-Horsfall protein in patients with kidney damage and diabetes. Urol Res 2004; 32: 79–83 [DOI] [PubMed] [Google Scholar]
- 23. Tsai C-Y, Wu T-H, Yu C-L. et al. Increased excretions of β2-microglobulin, IL-6, and IL-8 and decreased excretion of Tamm-Horsfall glycoprotein in urine of patients with active lupus nephritis. Nephron 2000; 85: 207–214 [DOI] [PubMed] [Google Scholar]
- 24. El-Achkar TM, McCracken R, Liu Y. et al. Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. Am J Physiol Physiol 2013; 304: F1066–F1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scherberich JE, Gruber R, Nockher WA. et al. Serum uromodulin—a marker of kidney function and renal parenchymal integrity. Nephrol Dial Transplant 2018; 33: 284–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steubl D, Block M, Herbst V. et al. Plasma uromodulin correlates with kidney function and identifies early stages in chronic kidney disease patients. Medicine (Baltimore) 2016; 95: e3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leiherer A, Muendlein A, Saely CH. et al. The value of uromodulin as a new serum marker to predict decline in renal function. J Hypertens 2018; 36: 110–118 [DOI] [PubMed] [Google Scholar]
- 28. Delgado GE, Kleber ME, Scharnagl H. et al. S erum uromodulin and mortality risk in patients undergoing coronary angiography. J Am Soc Nephrol 2017; 28: 2201–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pivin E, Ponte B, de Seigneux S. et al. Uromodulin and nephron mass. Clin J Am Soc Nephrol 2018; 13: 1556–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garimella PS, Sarnak MJ.. Uromodulin in kidney health and disease. Curr Opin Nephrol Hypertens 2017; 26: 136–142 [DOI] [PubMed] [Google Scholar]
- 31. Lv L, Wang J, Gao B. et al. Serum uromodulin and progression of kidney disease in patients with chronic kidney disease. J Transl Med 2018; 16: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Risch L, Lhotta K, Meier D. et al. The serum uromodulin level is associated with kidney function. Clin Chem Lab Med 2014; 52: 1755–1761 [DOI] [PubMed] [Google Scholar]
- 33. Helldén A, Bergman U, Odar-Cederlöf I. The importance of correct estimation of renal function for drug treatment in hospitalized elderly patients, especially women: a prospective observational study. Clin Nephrol 2019; 91: 254–264 [DOI] [PubMed] [Google Scholar]
- 34. Then C, Then H, Meisinger C. et al. Serum uromodulin is associated with but does not predict type 2 diabetes in elderly KORA F4/FF4 study participants. J Clin Endocrinol Metab 2019; pii: jc.2018-02557 [DOI] [PubMed] [Google Scholar]
- 35. Leiherer A, Muendlein A, Saely CH. et al. Serum uromodulin is associated with impaired glucose metabolism. Medicine (Baltimore) 2017; 96: e5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Then C, Then H, Lechner A. et al. Serum uromodulin is inversely associated with the metabolic syndrome in the KORA F4 study. Endocr Connect 2019; pii: EC-19-0352.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Holle R, Happich M, Löwel H. et al. KORA—a research platform for population based health research. Gesundheitswesen 2005; 67(Suppl 1): S19–S25 [DOI] [PubMed] [Google Scholar]
- 38. Rathmann W, Strassburger K, Heier M. et al. Incidence of Type 2 diabetes in the elderly German population and the effect of clinical and lifestyle risk factors: KORA S4/F4 cohort study. Diabet Med 2009; 26: 1212–1219 [DOI] [PubMed] [Google Scholar]
- 39. Meisinger C, Rückert IM, Rathmann W. et al. Retinol-binding protein 4 is associated with prediabetes in adults from the general population: the Cooperative Health Research in the region of Augsburg (KORA) F4 study. Diabet Care 2011; 34: 1648–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Seissler J, Feghelm N, Then C. et al. Vasoregulatory peptides pro-endothelin-1 and pro-adrenomedullin are associated with metabolic syndrome in the population-based KORA F4 study. Eur J Endocrinol 2012; 167: 847–853 [DOI] [PubMed] [Google Scholar]
- 41. Inker LA, Schmid CH, Tighiouart H. et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367: 20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Garimella P, Katz R, Ix J. et al. Association of urinary uromodulin with kidney function decline and mortality: the health ABC study. Clin Nephrol 2017; 87: 278–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bostom A, Steubl D, Garimella PS. et al. Serum uromodulin: a biomarker of long-term kidney allograft failure. Am J Nephrol 2018; 47: 275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Steubl D, Buzkova P, Garimella PS. et al. Association of serum uromodulin with ESKD and kidney function decline in the elderly: the cardiovascular health study. Am J Kidney Dis 2019; 74: 501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thornley C, Dawnay A, Cattell WR.. Human Tamm-Horsfall glycoprotein: urinary and plasma levels in normal subjects and patients with renal disease determined by a fully validated radioimmunoassay. Clin Sci 1985; 68: 529–535 [DOI] [PubMed] [Google Scholar]
- 46. Youhanna S, Weber J, Beaujean V. et al. Determination of uromodulin in human urine: influence of storage and processing. Nephrol Dial Transplant 2014; 29: 136–145 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


