Abstract
Age-standardized rates of diabetes mellitus (DM)-related complications, such as acute myocardial infarction, stroke or amputations, have decreased in recent years, but this was not associated with a clear reduction of the incidence of advanced chronic kidney disease (CKD) requiring renal replacement therapy. The early detection of diabetic kidney disease (DKD) is a key to reduce complications, morbidity and mortality. Consensus documents and clinical practice guidelines recommend referral of DM patients to nephrology when the estimated glomerular filtration rate falls below 30 mL/min/1.73 m2 or when albuminuria exceeds 300 mg/g urinary creatinine. Conceptually, it strikes as odd that patients with CKD are referred to the specialist caring for the prevention and treatment of CKD only when >70% of the functioning kidney mass has been lost. The increasing global health burden of CKD, driven in large part by DKD, the suboptimal impact of routine care on DKD outcomes as compared with other DM complications, the realization that successful therapy of CKD requires early diagnosis and intervention, the advances in earlier diagnosis of kidney injury and the recent availability of antidiabetic drugs with a renal mechanism of action and lack of hypoglycaemia risk, which additionally are cardio- and nephroprotective, all point towards a paradigm shift in the care for DM patients in which they should be referred earlier to nephrology as part of a coordinated and integrated care approach.
Keywords: diabetes mellitus, diabetic kidney disease, diabetic nephropathy, early referral, multidisciplinary care
Diabetes mellitus (DM) is a key cause of morbidity and mortality, and the disease burden is expected to increase in the next few decades. The International DM Federation estimated in 2017 that ∼425 million adults lived with DM, but 50% remained undiagnosed, and DM caused 4 million deaths [1]. By 2045, DM prevalence will rise to 629 million as Type 2 DM prevalence is increasing in most countries. However, about 80% of DM adults were living in low- and middle-income countries and a majority was aged 40–59 years. DM care cost at least 727 billion dollars in 2017, 12% of total healthcare spending on adults. Additionally, >1 million children were living with Type 1 DM and >21 million live births (1 in 7 births) were from DM mothers [1]. Despite the growing diabetic population, trends in age-standardized rates of DM-related complications, such as acute myocardial infarction, stroke or amputations, have decreased in recent years, but this was not associated to a clear reduction of the incidence of advanced chronic kidney disease (CKD) requiring renal replacement therapy (RRT) [2]. Diabetic kidney disease (DKD) continues to be the leading cause of end-stage renal disease (ESRD) worldwide, being the cause of ESRD in 24–55% of patients [3]. Specifically, in the ERA-EDTA Registry, DM accounts for 23% of incident RRT patients, with an incidence of 43 p.m.p. in males and 22 p.m.p. in females [4, 5]. In this regard, DM displays the largest absolute incidence gap between males and females among major causes of RRT, greatly contributing to the gender gap in RRT incidence [6]. Understanding the reasons underlying this gender gap is a key research priority. Dramatically, despite the high incidence, DM is only the fourth cause of prevalent RRT, illustrating the high mortality of DM patients on RRT and the need to prevent the need for RRT [5]. Moreover, a majority of patients with DKD die before reaching ESRD. Indeed, CKD is one of the fastest-growing global causes of death, predicted to become one of the top global causes of death in a few decades, and DKD is a key contributor to this growth [7, 8]. The early detection of both DM and DKD is a key to reduce complications, morbidity and mortality, as well as the social and economic impact of DM (Figure 1).
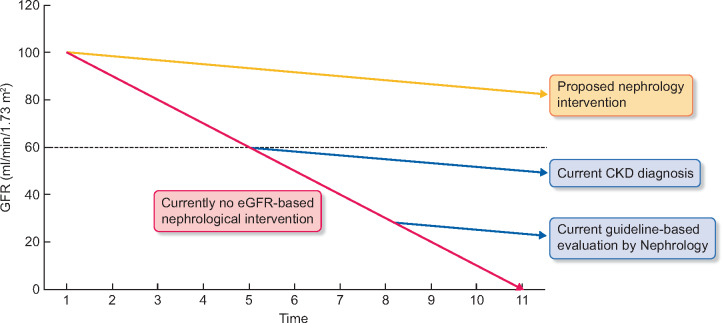
FIGURE 1.
Natural history of DKD (red line) and current eGFR-based cut-off points for the diagnosis of CKD and for the nephrological evaluation according to current guidelines (blue lines). Guidelines also recommend nephrological evaluation when albuminuria exceeds 300 mg/g of urinary creatinine, but such albuminuria may be absent in a significant number of patients with diabetes-associated CKD. Given the dismal outcomes of current clinical practice, we suggest that earlier evaluation by nephrology should be assessed for its impact on outcomes (green lines). We hypothesize that earlier identification of CKD in DKD patients, in association with earlier kidney care intervention, will improve the outcomes of diabetic patients.
Focusing on DKD, the classical presentation includes a progressive increase in albuminuria leading to overt proteinuria. However, in recent years, a non-proteinuric CKD phenotype was described in DM patients, characterized by a progressively decreasing glomerular filtration rate (GFR) in the absence of proteinuria [9]. Thus, the classic tools to identify early kidney involvement (i.e. pathological albuminuria) may not be good enough for the early detection of DKD in these patients. Novel diagnostic tools are required for the early (i.e. before loss of GFR to the point when CKD is diagnosed) diagnosis of DKD in such patients. Urinary proteomics holds promise in this respect [10]. Indeed, in a population rich in diabetics, urinary proteomics allowed better prediction than albuminuria of rapid loss of GFR in individuals with baseline estimated GFR (eGFR) above 60 mL/min/1.73 m2 [11].
In some instances, an atypical presentation (urinary sediment abnormalities, accelerated proteinuria or absence of retinopathy) requires a renal biopsy to rule out non-diabetic renal disease [12]. Still, in renal biopsies performed for atypical presentation, ∼80% of patients have histological diabetic nephropathy, although in 30% diabetic nephropathy was associated with a second nephropathy [13]. Since urinary proteomics may provide information on cause of CKD [14], it would be worth addressing whether in addition to the early identification of rapid progressors, it may provide information on the presence or not of diabetic nephropathy alone or in combination.
Different consensus documents and clinical practice guidelines recommend referral of DM patients to nephrology when eGFR falls below 30 mL/min/1.73 m2 or when albuminuria exceeds 300 mg/g urinary creatinine [12, 15]. The American Diabetes Association (ADA) Standards of Medical Care in Diabetes 2019 statements on nephrology care indicate that (i) DM patients should be referred for evaluation for RRT treatment if they have an eGFR <30 mL/min/1.73 m2 and that (ii) patients should be promptly referred to a physician experienced in the care of kidney disease for uncertainty about the aetiology of kidney disease, difficult management issues and rapidly progressing kidney disease [16]. Other referral criteria include acute decrease in GFR (>25% decrease versus baseline), rapid loss of GFR (eGFR slope faster than −5 mL/min/1.73 m2/year), refractory hypertension, potassium >5.5 or <3.5 mEq/L, renal anaemia (haemoglobin <10.5 g/dL) in patients with CKD and optimal iron availability—that is transferrin saturation index >20% and serum ferritin >100 ng/mL—or alarm signs suggestive of non-diabetic nephropathy [4, 5]. However, there are suggestions that current patterns of referral to nephrology may be too late as they mainly deal with advanced DKD or complications of advanced DKD. Thus, eGFR <45 mL/min/1.73 m2 at the time of referral together with the development of acute kidney injury (AKI) are powerful risk factors for death in DKD patients [17]. Conceptually, it strikes as odd that patients with CKD are referred to the specialist caring for the prevention and treatment of CKD after >70% of the functioning kidney mass has been lost. However, this is currently a key part of the advice of guideline and recommendation bodies.
The past 20 years saw a conceptual change of the CKD definition by expanding from a low GFR to include patients with normal GFR when there is evidence of kidney injury (e.g. albuminuria >30 mg/g), based on the higher all-cause and cardiovascular mortality and risk for AKI and CKD progression of these patients [18]. However, this was not followed by the logical next step: that patients with a kidney disease should be evaluated and eventually followed by a nephrologist earlier than current practice. This is in part due to the insufficient numbers of nephrologists to care for such a large population but also because of lack of clinical trials that have tested nephrological versus general care for CKD and specifically for DKD. There is some discussion on whether global DM care is optimal in nephrology departments, as there are questions over optimal nephrological care for DM patients followed by other specialties. Two Spanish studies, the Mortality and morbidity in renal patients study (MERENA) study of 1129 patients (434 with DM) with CKD G3/G4 [19] and the PECERA study of 900 (364 diabetics) CKD G4/G5 non-dialysis patients [20], identified shortcomings in the integrated care and management of DKD patients. However, we envision early referral to nephrology as part of optimized integrated care of DKD patients.
An optimal glycaemic control delays the onset of pathological albuminuria and progression to overt proteinuria (Evidence 1A). Additionally, in some studies, optimal glycaemic control slows CKD progression (Evidence 1B), minimizes vascular damage, especially in the presence of CKD, and reduces comorbidities and mortality (Evidence 1A). However, tight metabolic control is only recommended in DM patients with DKD when hypoglycaemia episodes are minimized (Evidence 1B) [8, 9]. In this regard, there is growing evidence of a nephroprotective effect of some glucagon-like peptide 1 receptor agonists as well as of drugs with a kidney site of action, sodium–glucose 2 transporter (SGLT2) inhibitors [21–23]. These drugs also decrease the risk of cardiovascular events even in non-diabetics [24]. While SGLT2 inhibitors are not currently indicated for patients with more advanced CKD, this may change soon given the cardiovascular and renal benefits [25]. Moreover, they do not increase the risk of hypoglycaemia. The availability of antidiabetic drugs that do not increase the risk of hypoglycaemia may further allow a tighter but still safe glycaemic control in CKD patients. Interestingly, SGLT2 inhibitors may allow deprescription of drugs [24]. This is especially interesting in diabetic patients with CKD who frequently receive multiple medications, thus compromising compliance with antidiabetic drugs and increasing the risk of medication discrepancies resulting in physicians not knowing which drugs patients are actually taking [26, 27].
In conclusion, the increasing global health burden of CKD, driven in large part by DKD, the suboptimal impact of routine care on DKD outcomes as compared with other DM complications, the realization that successful therapy of CKD requires early diagnosis and intervention, the advances in earlier diagnosis of kidney injury, and the recent availability of antidiabetic drugs with a renal mechanism of action and lack of hypoglycaemia risk, which additionally are cardio- and nephroprotective, all point towards a paradigm change in the care for DM patients in which DM patients should be referred earlier to the nephrologist as part of a coordinated and integrated care approach that actively addresses the early stages of renal disease instead of trying to address kidney disease when it is too late to salvage the kidneys as the need for RRT is approaching. We need to practice the ancient aphorism ‘primum non nocere’ and consider the benefits of an early preventive multidisciplinary intervention.
FUNDING
Sources of support: FIS/Fondos FEDER PI18/01386, PI19/00588, PI19/00815, DTS18/00032, ERA-PerMed-JTC2018 (KIDNEY ATTACK AC18/00064 and PERSTIGAN AC18/00071, ISCIII-RETIC REDinREN RD016/0009), Sociedad Española de Nefrología, FRIAT, Comunidad de Madrid en Biomedicina B2017/BMD-3686 CIFRA2-CM.
CONFLICT OF INTEREST STATEMENT
A.O. is a consultant for Sanofi Genzyme, has received speaker fees from Shire, Amicus, Amgen, Otsuka, Mundipharma, Fresenius Medical Care, Kyowa-Kirin, Astra-Zeneca and Menarini, and directs the Mundipharma-Universidad Autónoma de Madrid (UAM) cátedra de Diabetes y Enfermedad Renal. M.J.S. is a consultant for NovoNordisk and has received speaker fees form Janssen, Boehringer, Eli Lilly, AstraZeneca and Esteve. B.F.-F. has served as consultant for Mundipharma and reports speaker fees or travel support from Abbvie, Astrazeneca, Boehringer Ingelheim, Esteve, Menarini, Novartis and Novonordisk. J.L.G. has served as consultant for Boëhringer-Ingelheim, Mundipharma, AstraZeneca and Novonordisk, and has received speaker honoraria from Boëhringer-Ingelheim, Mundipharma, AstraZeneca, Novonordisk, Novartis and Eli Lilly. J.F.N.-G. has served as a consultant and has received speaker fees or travel support from Abbvie, Amgen, AstraZeneca, Boehringer Ingelheim, Esteve, Genzyme, Lilly, Novartis, Servier, Shire and Vifor Fresenius Medical Care, and Renal Pharma. A.M.-C. is a consultant for Boehringer-Ingelheim, Lilly, Esteve and Merck-Sharp-Dhôme, and has received speaker fees from Boëringer-Ingelheim, Lilly, Novo-Nordisk and Esteve.
REFERENCES
- 1.International Diabetes Federation (IDF). E-library epidemiology and research. https://idf.org (25 October 2019, date last accessed)
- 2. Gregg EW, Williams DE, Geiss L.. Changes in diabetes-related complications in the United States. N Engl J Med 2014; 371: 286–287 [DOI] [PubMed] [Google Scholar]
- 3. Stel VS, Awadhpersad R, Pippias M. et al. International comparison of trends in patients commencing renal replacement therapy by primary renal disease. Nephrology (Carlton) 2019; 24: 1064–1076 [DOI] [PubMed] [Google Scholar]
- 4. Kramer A, Pippias M, Noordzij M. et al. The European Renal Association - European Dialysis and Transplant Association (ERA-EDTA) registry annual report 2016: a summary. Clin Kidney J 2019; 12: 702–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kramer A, Pippias M, Noordzij M. et al. The European Renal Association - European Dialysis and Transplant Association (ERA-EDTA) registry annual report 2015: a summary. Clin Kidney J 2018; 11: 108–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fernández-Prado R, Fernandez-Fernandez B, Ortiz A.. Women and renal replacement therapy in Europe: lower incidence, equal access to transplantation, longer survival than men. Clin Kidney J 2018; 11: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Foreman KJ, Marquez N, Dolgert A. et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018; 392: 2052–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ortiz A, Sánchez-Niño M, Crespo-Barrio M. et al. The Spanish Society of Nephrology (SENEFRO) commentary to the Spain GBD 2016 report: keeping chronic kidney disease out of sight of health authorities will only magnify the problem. Nefrologia 2019; 39: 29–34 [DOI] [PubMed] [Google Scholar]
- 9. Porrini E, Ruggenenti P, Mogensen CE. et al. Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol 2015; 3: 382–391 [DOI] [PubMed] [Google Scholar]
- 10. Pontillo C, Mischak H.. Urinary peptide-based classifier CKD273: towards clinical application in chronic kidney disease. Clin Kidney J 2017; 10: 192–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodríguez-Ortiz ME, Pontillo C, Rodríguez M. et al. Novel urinary biomarkers for improved prediction of progressive eGFR loss in early chronic kidney disease stages and in high risk individuals without chronic kidney disease. Sci Rep 2018; 8: 15940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martínez-Castelao A, Górriz JL, Segura-de la Morena J. et al. Consensus document for the detection and management of chronic kidney disease. Nefrologia 2014; 34: 243–262 [DOI] [PubMed] [Google Scholar]
- 13. Kritmetapak K, Anutrakulchai S, Pongchaiyakul C. et al. Clinical and pathological characteristics of non-diabetic renal disease in type 2 diabetes patients. Clin Kidney J 2018; 11: 342–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Siwy J, Zürbig P, Argiles A. et al. Noninvasive diagnosis of chronic kidney diseases using urinary proteome analysis. Nephrol Dial Transplant 2017; 32: 2079–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gómez-Huelgas R, Martínez-Castelao A, Artola S. et al. ; Grupo de Tabajo para el Documento de Consenso sobre el tratamiento de la diabetes tipo 2 en el paciente con enfermedad renal crónica. Consensus document on treatment of type 2 diabetes in patients with chronic kidney disease. Nefrologia 2014; 34: 34–45 [PubMed] [Google Scholar]
- 16.American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes-2019. Diabetes Care 2019; 42 (Suppl 1): S124–S138 [DOI] [PubMed] [Google Scholar]
- 17. Pinier C, Gatault P, François M. et al. Renal function at the time of nephrology referral but not dialysis initiation as a risk for death in patients with diabetes mellitus. Clin Kidney J 2018; 11: 762–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perez-Gómez MV, Bartsch LA, Castillo-Rodriguez E. et al. Clarifying the concept of chronic kidney disease for non-nephrologists. Clin Kidney J 2019; 12: 258–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martínez-Castelao A, Górriz JL, Portolés JM. et al. Baseline characteristics of patients with chronic kidney disease stage 3 and stage 4 in Spain: the MERENA observational cohort study. BMC Nephrol 2011; 12: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Górriz JL, Pantoja J, Castro C. et al. Prevalencia e impacto de la hiperpotasemia en pacientes con ERC 4-5 no en diálisis. Datos del estudio PECERA. Nefrología 2017; 37 (Suppl 1): 4727575931 [Google Scholar]
- 21. Fernandez-Fernandez B, Fernandez-Prado R, Górriz JL. et al. Canagliflozin and renal events in diabetes with established nephropathy clinical evaluation and study of diabetic nephropathy with atrasentan: what was learned about the treatment of diabetic kidney disease with canagliflozin and atrasentan? Clin Kidney J 2019; 12: 313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Satirapoj B, Korkiatpitak P, Supasyndh O.. Effect of sodium-glucose cotransporter 2 inhibitor on proximal tubular function and injury in patients with type 2 diabetes: a randomized controlled trial. Clin Kidney J 2019; 12: 326–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vergara A, Jacobs-Cachá C, Soler MJ.. Sodium-glucose cotransporter inhibitors: beyond glycaemic control. Clin Kidney J 2019; 12: 322–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Petrie MC, Verma S, Docherty KF. et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA 2020; 323: 1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li J, Fagbote CO, Zhuo M. et al. Sodium-glucose cotransporter 2 inhibitors for diabetic kidney disease: a primer for deprescribing. Clin Kidney J 2019; 12: 620–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schmidt IM, Hübner S, Nadal J. et al. Patterns of medication use and the burden of polypharmacy in patients with chronic kidney disease: the German Chronic Kidney Disease study. Clin Kidney J 2019; 12: 663–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ibrahim J, Hazzan AD1, Mathew AT1. et al. Medication discrepancies in late-stage chronic kidney disease. Clin Kidney J 2018; 11: 507–512 [DOI] [PMC free article] [PubMed] [Google Scholar]