Abstract
The number of kidney transplant recipients returning to dialysis after graft failure is steadily increasing over time. Patients with a failed kidney transplant have been shown to have a significant increase in mortality compared with patients with a functioning graft or patients initiating dialysis for the first time. Moreover, the risk for infectious complications, cardiovascular disease and malignancy is greater than in the dialysis population due to the frequent maintenance of low-dose immunosuppression, which is required to reduce the risk of allosensitization, particularly in patients with the prospect of retransplantation from a living donor. The management of these patients present several controversial opinions and clinical guidelines are lacking. This article aims to review the leading evidence on the main issues in the management of patients with failed transplant, including the ideal timing and modality of dialysis reinitiation, the indications for an allograft nephrectomy or the correct management of immunosuppression during graft failure. In summary, retransplantation is a feasible option that should be considered in patients with graft failure and may help to minimize the morbidity and mortality risk associated with dialysis reinitiation.
Keywords: allosensitization, allograft nephrectomy, dialysis, graft failure, immunosuppression, retransplantation
INTRODUCTION
Kidney transplantation represents the best treatment for patients with end-stage kidney disease (ESKD), offering reduced mortality compared with dialysis treatment [1]. Transplantation from a standard-criteria donor is estimated to increase life expectancy by almost 10 years, while it is lower in kidney transplants from marginal donors [2]. In recent years, the occurrence of acute rejection has been significantly reduced [3]; however, no significant impact on long-term outcomes has been reported and a substantial number of patients develop a chronic allograft dysfunction and return to dialysis after a graft failure and frequently relist for transplantation [4]. The number of patients returning to dialysis after kidney transplantation is significantly increasing due to the increased number of kidney transplants performed worldwide, improved management of comorbidities and better survival after kidney transplantation. Although outcomes of people returning to dialysis after graft failure are poor, guidelines for the care of kidney transplant recipients do not include recommendations for safe and adequate management of this transition.
In this review we extensively report the main evidence and recommendations on the management of patients with a failed kidney transplant, focusing on the optimal timing of returning to dialysis treatment, the best dialysis modality, the need for adequate immunosuppression withdrawal and the indications for an allograft nephrectomy and retransplantation.
EPIDEMIOLOGY AND PATIENT OUTCOMES AFTER GRAFT FAILURE
Patients with a failed kidney allograft have steadily increased in recent years, accounting for ~4–5% of the incident dialysis population as reported in US Renal Data System (USRDS) report. Moreover, they represent an important portion of people waitlisted for kidney transplantation (~15%) [5]. A similar trend was also described in the national French Renal Epidemiology and Information Network (REIN) registry [6].
Patient survival after graft failure is still an object of debate since controversial results have been reported in the scientific literature to date. Several studies have shown worse survival in patients returning to dialysis after graft loss than in transplant-naïve patients and in incident patients on dialysis [4, 7–10]. Patient survival after graft loss is reported to be <40% of patients surviving 10 years after dialysis reinitiation. Moreover, overall annual adjusted death after allograft loss (DAGL) rates were >3‐fold higher as compared with mortality before graft loss (9.42% versus 2.81%) [7]. Rao et al. [4] analysed data from the Scientific Registry of Transplant Recipients (SRTR) and showed that mortality among dialysis patients on the waiting list for a first transplant was significantly lower than in patients returning to dialysis after graft failure; the hazard ratios (HRs) were constantly higher across age groups and the risk was greater among patients with diabetes (HR 1.93) compared with non-diabetic patients. In a meta-analysis conducted by Kabani et al. [10], including 40 studies comprising 250 000 transplant recipients with allograft failure, DAGL was highest in the first year following dialysis initiation (12%) and decreased in subsequent years. Furthermore, a study based on the Dialysis Outcomes and Practice Patterns Study (DOPPS) registry showed that patients with failed kidney transplant have reduced quality of life compared with transplant-naïve patients [11]. Several elements should be taken into account when comparing these studies. First, data from the DOPPS and SRTR registries are from the early 2000s and significant improvements in prognosis among the dialysis population have been reported since then. Furthermore, the two populations (incident dialysis patients and patients returning to dialysis after graft loss) are not comparable since differences in comorbidities and age are notable.
More recently, several studies suggest that patients who return to dialysis after kidney transplantation do not have worse survival than incident dialysis patients. An analysis of the REIN registry showed similar survival between transplant recipients <65 years of age with graft failure during 2007–9 and transplant-naïve incident dialysis patients when using a propensity score approach matching for age, gender, diabetes mellitus and year of starting dialysis in order to minimize the differences between the two cohorts [6]; however, a high-risk population (patients >65 years old) was excluded from the analysis. Similarly, Varas et al. [12] conducted a retrospective study on 5216 patients from 65 different sites between 2009 and 2014 and showed similar survival rates after minimizing indication biases with the propensity score matching method. Compared with patients returning to dialysis after graft loss, the incident dialysis patients were older, more commonly male and presented more comorbidities: these differences may have contributed to the worse survival in patients returning on dialysis after graft failure as shown by univariate analysis, while there were no differences in survival in the multivariate analysis with adjustment for these factors [12].
Cardiovascular diseases (CVDs) remained the most important cause of death in patients returning to dialysis after graft failure (~36%), but deaths due to sepsis were more common during this period than during any other interval (17%); minor causes of death in this setting are cerebrovascular disorder and malignancies [8]. The risk for DAGL is related to both immunological and non-immunological factors. Lopez-Gomez et al. [13] showed that patients returning to dialysis after kidney transplant failure suffer from a chronic inflammatory state, which could be associated with increased morbidity and mortality. Moreover, many kidney transplant recipients with chronic kidney disease (CKD) Stages 4 and 5 have CKD-related complications that usually fall below established targets for non-transplant CKD patients (worse blood pressure control, higher serum phosphate, lower bicarbonate and lower haemoglobin). Finally, non-programmed vascular access with the placement of a temporary catheter predicted all-cause mortality among patients returning to dialysis due to more frequent infection complications {HR 5.9 [95% confidence interval (CI) 2.83–12.31]} [14]. In a recent analysis from the USRDS registry of patients returning to dialysis after graft failure, the risk for death was significantly increased in patients without arteriovenous fistula at dialysis initiation and with nutritional issues (albumin <3.5 g/dL and being underweight) [15].
RETRANSPLANTATION
At the end of 2017 in the USA, ~12.1% of the adults on the kidney transplant waitlist were waiting for a retransplantation [16], while in the Eurotransplant region, it was ~17.8%. Patients with a failed allograft account for 4–10% of those incident for dialysis therapy and retransplantation offers a significantly lower mortality compared with remaining on dialysis [17, 18]. Changes in the allocation system in the USA in 2014 increased the likelihood of transplant for highly sensitized patients and patients with a previous transplant failure (~13%) [19]. However, retransplanted recipients are more likely to be treated for an acute rejection or hospitalized within 1 year of retransplantation [20]. Moreover, among transplant recipients, transplant-failure patients are at higher risk for death-censored transplant failure and the need for dialysis within 10 years after transplantation [19].
Pre-emptive renal retransplantation is the best option to consider in order to minimize the morbidity associated with dialysis reinitiation and improved clinical outcomes. In a Spanish observational study including 101 patients receiving a second kidney transplantation, pre-emptive recipients showed a low rate of acute rejection and better graft and patient survival at 1 and 5 years, although the difference was not statistically significant [21]. Furthermore, the pre-emptive group showed significantly lower panel reactive antibody (PRA) levels, probably due to better immunosuppression management [21]. A retrospective analysis of USRDS and United Network for Organ Sharing data showed that pre-emptive retransplantation was associated with an increased risk of graft loss compared with non-pre-emptive retransplantation [22].
A more recent analysis from a USRDS cohort of 17 584 recipients of a second kidney transplantation, of which 20% of recipients received a pre-emptive retransplantation, showed that pre-emptive recipients had less acute rejection (12% versus 16%; P < 0.0001) and delayed graft function (DGF) (8% versus 23%; P < 0.0001) [23]; pre-emptive retransplantation was associated with a lower risk of allograft failure from any cause (HR 0.88) and lower death with a functioning graft, but a similar risk of death-censored graft loss (HR 0.98) [23].
In a recent multicentre French cohort study, patients receiving pre-emptive retransplantation showed a lower rate of DGF (2.2% versus 36.6%; P < 0.0001) and cellular and/or humoral rejections, with an improved graft survival [death-censored graft loss HR 0.39 (95% CI 0.18–0.88); P = 0.024] compared with the non-pre-emptive group [24]. Furthermore, an increased waiting time before receiving a retransplantation was associated with the risk of early acute rejection occurring within the first 6 months after transplantation, severe vascular and/or humoral rejection and overall graft failure [25].
Several studies showed that third and subsequent kidney transplantations may be performed safely by experienced surgeons without significant surgical complications influencing long-term graft outcome, which is similar to that with a second transplantation [26, 27]. In a monocentric analysis of a Spanish cohort, third and fourth transplantations showed 1- and 5-year graft survivals of 88% and 76.4% and 71.4% and 42.9%, respectively; the overall rate of operative complications among the series was 25.5% and the most frequent complication was a perirenal haematoma (14.6%) [28].
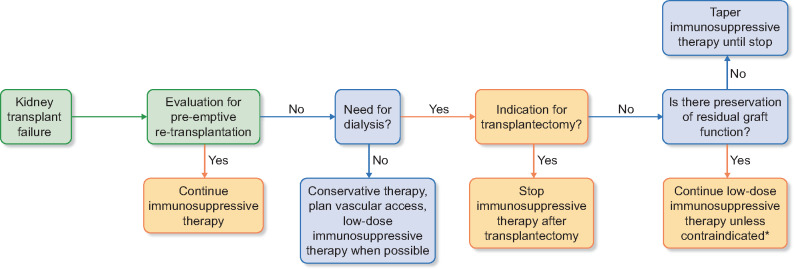
In this scenario, we suggest that the evaluation of the chance for a pre-emptive retransplantation from a deceased or living donor should represent the first step in the management of patients with a failing kidney allograft (Figure 1).
FIGURE 1.
Suggested algorithm for the management of immunosuppressive therapy after kidney transplant failure.
*Contraindications to maintaining immunosuppressive therapy: metabolic (diabetes, hypertension), cardiovascular complications, susceptibility to infections, malignant neoplasia, steroid-associated adverse effects
TIMING AND MODALITIES OF DIALYSIS AFTER GRAFT FAILURE
There are no specific recommendations about the optimal timing for return to dialysis after a failed kidney transplant. The latest studies in the transplant-naïve EKDS population showed comparable mortality rates between patients with early [estimated glomerular filtration rate (eGFR) 10–14 mL/min) or late (eGFR 5–7 mL/min) initiation of dialysis treatment [29]. As reported in Table 1, only a few studies have specifically focused on patients returning to dialysis after graft failure. The larger of these analysed the effect of immunological and non-immunological factors related to mortality in 4741 patients with kidney transplant failure; patients with a higher eGFR at dialysis initiation are at increased risk for all-cause mortality [HR 1.04/mL/min higher (95% CI 1.02–1.06)] [8]. More recently, a review of data from the SRTR (747 failed kidney transplants with dialysis reinitiation with eGFR <15 mL/min/1.73 m2) suggested that earlier initiation of dialysis (eGFR >10 mL/min) leads to worse outcomes: each 1 mL/min/1.73 m2 higher GFR was associated with a 6% higher death risk, particularly in the youngest and healthiest patients [30]. In this scenario, although limited data are available specifically for the transplant population, we suggest that the timing of dialysis initiation should be based on clinical factors and symptoms related to CKD progression rather than eGFR evaluation alone.
Table 1.
List of studies and trials comparing early with late start of dialysis treatment in patients with ESKD
| Author/study | Cohort | Follow-up | Main results |
|---|---|---|---|
| Gill et al. [8] | 4741 with graft failure, returning to dialysis | 15 ± 11 months | Four per cent higher mortality risk after return to dialysis for each 1 mL/min/1.73 m2 higher eGFR at the time of dialysis initiation (HR 1.04; P < 0.01) |
| Molnar et al. [30] | 747 with graft failure, returning to dialysis | 1185 days | In an unadjusted model, each 1 mL/min/1.73 m2 higher eGFR at dialysis reinitiation was associated with a 6% higher risk of death (HR 1.06; P = 0.02); in adjusted models, this finding was not significant (HR 1.02; P = 0.54) |
Regarding the type of renal replacement therapies, whether peritoneal dialysis (PD) or haemodialysis (HD) represents the best treatment option for patients with a failed graft is still a subject of debate. Among transplant-naive patients, those treated with PD enjoy an early survival advantage compared with those treated with HD, but this advantage is not sustained over time [31, 32]. In the past, several comparative studies have been performed in order to compare PD and/or HD in patients returning to dialysis after graft failure (Table 2). Data from series with a limited sample size did not suggest significant differences between the two modalities. Davies et al. [33] compared 28 patients returning to PD and 17 patients returning to HD after graft failure: no significant differences in patient survival between the two groups were observed, but in the adjusted models, the patient survival trend was better for PD patients due to age and comorbidities. Later, De Jonge et al. [34] compared 21 patients starting PD and 39 patients starting HD after graft failure and showed that outcomes did not differ significantly between the two groups, while a tendency towards higher survival and retransplantation rates was described in patients on PD. However, a small study comparing 42 patients returning to PD after graft failure and 43 never-transplanted patients starting PD showed that the outcomes (time to first peritonitis, subsequent infective episodes, transfer to HD and overall survival) were significantly worse in patients with failed transplantation [35].
Table 2.
List of studies and trials comparing outcomes of PD and HD in patients with failed renal transplantation and with transplant-naïve ESKD
| Author/study | Cohort | Type of dialysis | Main results |
|---|---|---|---|
| Davies et al. [33] | 45 patients with renal transplant failure | 28 starting PD treatment and 17 starting HD treatment | No significant difference in the survival of failed transplant patients starting PD as compared with those starting HD (log rank: P = 0.11) |
| De Jonge et al. [34] | 60 patients with renal transplant failure | 21 starting PD treatment and 39 starting HD treatment | Death did not differ significantly between the two groups (P = 0.72). Moreover, there was a tendency towards higher patients’ survival and re-transplantation tended to be more frequent in the PD post-transplant group |
| Perl et al. [36] | 2110 patients with renal transplant failure | 389 starting PD treatment and 1721 starting HD treatment | No difference in overall survival between HD- and PD-treated patients [HR (HD:PD) 1.05 (95% CI 0.85–1.31)], with similar results seen for both early and late survival. |
| Perl et al. [37] | 16 113 patients with renal transplant failure | 1865 starting PD treatment and 14 248 starting HD treatment | Survival in both groups was similar [HR for PD compared with HD 1.09 (95% CI 1.0–1.20)]. Compared with HD, PD is associated with an early survival advantage, inferior late survival and similar overall survival |
| Salazar et al. [38] | 165 patients with renal transplant failure | 16 starting PD treatment and 149 starting HD treatment | Survival prognosis, even adjusted by Charlson comorbidity index, death causes and retransplantation rate had no statistically significant difference |
In a larger database including 2110 patients returning to dialysis (1721 patients on HD versus 389 on PD), survival was not influenced by the initial dialysis modality choice, with similar effects of dialysis modality on both early and late, and patients who underwent pre-emptive transplantation had the greatest survival rate [36]. In a subsequent analysis on 16 113 adults who initiated dialysis after transplant failure from the USRDS, this trend has been confirmed when using a propensity-matched approach [37]. Similar results are reported in a recent retrospective cohort study [38].
In this scenario we suggested that the choice of dialysis modality after graft failure should be based on the clinical characteristics of kidney transplant patients since no clear and definitive evidence is available in the scientific literature. Patients with planned retransplantation (particularly from a living donor) may benefit from PD treatment since it seems to be associated with better outcomes in the early period after graft failure.
MANAGEMENT OF IMMUNOSUPPRESSION
The optimal management of immunosuppression is one of the most challenging decisions following allograft failure [39]. Immunosuppression suspension after graft failure may be immediate, rapid or slow, based on the patient’s characteristics, number and types of immunosuppressive drugs, the presence of residual diuresis and the chance for a retransplant. Furthermore, the timing of graft loss is determinant; when transplant failure occurs early due to a primary non-function (PNF), arterial or venous thrombosis, hyperacute or early refractory acute rejection, immunosuppression cessation (and nephrectomy) is usually indicated to avoid the risk of graft rupture and haemorrhage. Conversely, when graft failure occurs later (after 12–24 months), it is suggested to keep the immunosuppressive therapy, although the maintenance of low-dose immunosuppression presents both benefits and risks, which are listed below [39, 40].
Preservation of residual renal function
Several studies focused on HD patients showed that those with a greater residual renal function showed better renal outcomes compared with those without residual function [41, 42]. Limited data are available on patients with a failed allograft. Davies et al. [33] showed a significant rapid decline in residual renal function in patients starting PD after graft failure compared with transplant-naïve patients, suggesting the importance of maintaining low-dose immunosuppression to preserve residual allograft function. Finally, in a case report described by Elmahi et al. [43], the patient started PD and was maintained on a minimal immunosuppressive regimen with tacrolimus (1 mg/day) and prednisone (5 mg/day): interestingly, the residual renal function remained very well preserved.
Prevention of allosensitization
Sensitization to human leucocyte antigens (HLAs) is a major obstacle for most organ transplants and a risk factor for graft loss; transfusions, graft nephrectomy and immunosuppression withdrawal are the main causes of sensitization and antibody formation [39]. Scornik et al. [44] demonstrated in 104 patients with graft failure that 81% of these patients did not have HLA antibodies at the time of graft loss, but they made HLA antibodies in the follow-up period, whereas none of the patients continuing with immunosuppression did so, suggesting the role of maintaining immunosuppression to prevent sensitization in well-defined circumstances (prompt living donor or pre-emptive transplantation). Furthermore, in a retrospective study comparing early (<3 months) or prolonged (>3 months) immunosuppression withdrawal after graft loss in retransplant candidates, Casey et al. [45] reported a higher rate of non-sensitization at retransplant evaluation (66% versus 30%; P = 0.01) in the prolonged immunosuppression group.
Immunosuppression withdrawal with or without transplantectomy has been shown to be an independent predictor of allosensitization. Augustine et al. [46] examined the impact of sensitization in a subgroup of 119 patients with low PRA levels before transplantation and during the follow-up period and showed that the percentage of patients who were highly sensitized increased from 21% at the time of failure to 68% after weaning immunosuppression. Moreover, weaning immunosuppression is a triggered event leading to allograft nephrectomy, since this procedure was required in 41% of patients who were weaned from immunosuppression, while none of the patients who maintained immunosuppression with calcineurin inhibitor treatment did [46]. Similarly, Del Bello et al. [47] analysed 69 patients with a failed kidney transplantation and showed that the production of donor-specific antibodies (DSAs) and non-DSA anti-HLA antibodies may develop in >50% of patients 9 months after allograft nephrectomy. This evidence suggests that allograft nephrectomy did not reduce the risk of allosensitization, while only immunosuppression maintenance could provide a low risk. Finally, the relation between HLA immunogenicity (assessed by differences in donor–recipient HLA amino acid sequence and physicochemical properties) and the development of HLA-specific antibodies after graft failure and relisting for transplantation has been widely described and should be taken into consideration when considering immunosuppression withdrawal [48].
Prevention of graft intolerance syndrome
Immunologic intolerance to a failed renal allograft left in situ is referred to as ‘graft intolerance syndrome’. Most episodes of graft intolerance syndrome appear within the first year of dialysis reinitiation in ~30–50% of patients despite various immunosuppression withdrawal protocols, usually leading to graft nephrectomy [49]. Manifestations of graft intolerance syndrome are similar to symptoms of general infections, including fever, flu-like symptoms, haematuria, local pain and increased graft size or tenderness; usually a persistent inflammatory state and anaemia resistant to erythropoietin are described [50]. Woodside et al. [51] showed that hospitalization with fever within 6 months of graft failure occurred in 44% of patients; only 38% of patients who stopped immunosuppression presented a documented infection, while graft intolerance syndrome was suspected in the remaining patients. In contrast, in patients who maintained immunosuppression after graft failure, hospitalization with fever was related to a documented infection (88% of cases), while graft intolerance syndrome was less frequent [51]. Risk factors for graft nephrectomy because of graft intolerance syndrome include donor age, the number of rejections and shorter graft survival [50, 52].
Potential adverse effects of continuation of immunosuppression
Continuing immunosuppression in patients with graft failure may lead to adverse effects on mortality and morbidity. Smak Gregoor et al. [53] analysed the morbidity and mortality of 197 patients with or without low-dose maintenance immunosuppression after graft failure; the incidence of viral, bacterial and opportunistic infectious complications per patient-year were significantly higher in the immunosuppression continuation group (1.7 versus 0.51, respectively; P < 0.0001). In addition, mortality associated with cardiovascular and infectious complications was higher among patients who continued immunosuppression compared with those whose immunosuppression was discontinued [53].
The timing and modalities of immunosuppression withdrawal are also critical. Kiberd et al. [54] showed an increase in infection-related morbidity in patients whose immunosuppression was tapered over an extended period (mean 14 months) compared with rapid withdrawals of immunosuppression (mean 3 months). Metabolic complications and the use of steroids should be taken into consideration since rapid steroid withdrawal may induce adrenal insufficiency. Clinically this syndrome can manifest with hypotension, malaise, fatigue, fevers, weakness, myalgias, arthralgias, anorexia and weight loss. This condition could be suspected after a failed transplant in dialysis patients with severe fluid overload and frequent hypotensive episodes during dialysis [55]. Conversely, prolonged use of steroids can lead to serious clinical complications such as avascular necrosis, osteoporosis, hyperglycaemia, cataracts, myopathy and increased susceptibility to infections.
Finally, recipients of kidney transplants have a greater risk of developing cancer compared with the general population: the patient’s age, gender and length of exposure to immunosuppressive drugs are the main risk factors for de novo malignancies. The intensity and duration of immunosuppression and the ability of these drugs to promote the replication of various oncogenic viruses and their viral load are important risk factors [56]. Vajdic et al. [57] showed that the incidence of melanoma was lower after resumption of dialysis and reduction of immunosuppression than during transplant function [57], where the relative risk of melanoma peaked in the second year and declined linearly thereafter.
However, the impact of immunosuppression on cancer risk is reversible for some but not all malignancies. Van Leeuwen et al. [58] reported that the incidence of non-Hodgkin’s lymphoma, lip cancer and melanoma was significantly lower after returning to dialysis after graft failure, while similar incidence rates were described for leukaemia, lung cancer and cancers related to ESKD (kidney, urinary tract and thyroid cancers).
Proposed strategies for immunosuppression tapering
There is no single tapering protocol for immunosuppression. According to the British Transplantation Society guideline, anti-proliferative agents (azathioprine and mycophenolate) can be stopped immediately, followed by a gradual taper of calcineurin inhibitors (CNIs) or mammalian target of rapamycin inhibitors, generally weaned over several weeks [59]. No published data define the optimal rate of CNI tapering: one approach is to reduce the dose by 25% per week until withdrawn. Steroids should be the last component to be withdrawn: prednisolone should not be withdrawn faster than 1 mg/month once the dose is <5 mg daily. In the event of clinical manifestations of adrenal insufficiency, it is appropriate to reintroduce steroids at the previous dose and attempt a slower steroid taper [59]. In the case of allograft nephrectomy, all immunosuppressive drugs apart from steroids should be stopped immediately. In the event of severe acute rejection following withdrawal of immunosuppression, it is recommended that steroid therapy should be restarted, followed by transplant nephrectomy when acute inflammation has settled [59].
Finally, steroids at a dose of 5 mg daily should be kept in the case of persistence of residual renal function or with a plan to retransplant, while it should be weaned 1 mg/day/month in all other cases [60]. Figure 1 suggests an algorithm for the correct management of immunosuppressive therapy after graft failure. Future clinical studies focusing on the impact of minimization strategies or immunosuppression withdrawal not only on HLA sensitization but also on the probability of receiving a new transplant or on long-term graft survival are necessary to optimize the management of immunosuppression after graft failure in potential candidates for a second kidney transplant.
ALLOGRAFT NEPHRECTOMY
The rate of surgical allograft nephrectomy after graft failure is highly variable among transplant centres. According to a US report, the cumulative probability of transplantectomy at 1 week, 3 months, 6 months and 1 year after graft failure was 5.3%, 17.6%, 25.0% and 30.9%, respectively [61]; 89.3% of all nephrectomies were performed in the first year after transplant failure [62]. In a single-centre study of only 34 paediatric recipients, children with graft failure within 1 year of transplantation were 4-fold more likely to require transplantectomy than those with graft loss after 1 year (P = 0.04) [63].
Only a few and small retrospective studies have reported the principal indications for allograft nephrectomy (Table 3). The main indications for allograft nephrectomy may be related to graft issues (e.g. acute arterial or venous thrombosis, graft infection, malignant neoplasia of the graft, need to create space for a new graft, graft intolerance syndrome) or to immunosuppression (e.g. sepsis, recurrent urinary tract infection, malignant neoplasia outside the graft, adverse effects). Furthermore, the recurrence of primary disease, acute antibody-mediated rejection, refractory acute rejection and polyomavirus infection are often linked to the need for nephrectomy [64]. Although there is no question about the need for allograft nephrectomy in urgent life-threatening situations (e.g. graft haemorrhage, graft thrombosis, graft infection, graft necrosis, etc.), the need for allograft nephrectomies in patients with asymptomatic graft failure should be discussed with the patient and the decision requires careful consideration of potential risks and benefits.
Table 3.
Indications for kidney transplantectomy
| Indications for allograft nephrectomy | |
|---|---|
| Before 12 months from transplantation |
|
|
|
Potential benefits of allograft nephrectomy
Recorded mortality rates range from 0.7 to 14%. The main complications are related to infections and haemorrhage and range from 17 to 60% of surgical procedures, while this percentage is higher in urgent life-threatening procedures [52]. Although nephrectomy is the conventional technique in managing failed kidney allograft, in recent years transvascular embolization has been widely described as a less invasive alternative technique, with minimal complications compared with transplantectomy [65]. In a systematic review and meta-analysis comparing transplant nephrectomy and graft embolization, the mortality rate, as well as procedural complications, was significantly higher in the nephrectomy group [66]. In addition, in patients with residual kidney function, a retained graft may allow more liberal fluid intake and improve anaemia, erythropoietin resistance, hypoalbuminaemia and the chronic inflammatory state [13].
A retrospective study using the USRDS database (10 951 patients returning to dialysis after graft failure, of which 31.5% underwent allograft nephrectomy) demonstrated that nephrectomy was associated with a 32% lower relative risk for all-cause mortality (adjusted HR 0.68) in a mean follow-up period of 2.9 years [67]. Moreover, patients receiving graft nephrectomy were more likely to receive a second transplant during the follow-up period (10.0% versus 4.1%; P = 0.001) and the death rate within 30 days was only 1.5% [67]. Interestingly, in a large retrospective study of 19 107 patients with allograft failure, this procedure was associated with increased mortality among those with early graft loss (graft survival <12 months) [62].
Allograft nephrectomy has been demonstrated to be associated with allosensitization: de novo DSAs were detected as soon as 5 days after transplantectomy, suggesting that the antibodies were performed [47, 68], but the increase in PRAs and DSAs could be explained by pro-inflammatory cytokine production and upregulation of HLA alloantibodies caused by allograft nephrectomy. It has been speculated that a retained allograft may serve as an ‘antibody sponge’ or rapid immunosuppression weaning after the nephrectomy may promote the formation of DSAs.
In a recent single-centre study comparing the allosensitization between three groups of patients with graft failure (Group A: patients receiving both graft nephrectomy and immunosuppressive withdrawal; Group B: patients receiving graft nephrectomy after complete withdrawal of immunosuppression; Group C: patients receiving only withdrawal of immunosuppression), allograft nephrectomy alone was associated with the appearance of Class I HLA antibodies in the serum, while withdrawal of immunosuppression gave rise to Class II HLA antibodies [69]. Graft nephrectomy after withdrawal of immunosuppression led to the de novo appearance of Class I HLA antibodies in the serum but not Class II antibodies. An ongoing French randomized clinical trial is trying to compare the risk of anti-HLA immunization between early and systematic transplantectomy within 6 weeks after return to dialysis versus standard immunosuppressive withdrawal (https://clinicaltrials.gov/ct2/show/NCT01817504).
Allograft nephrectomy and the chance of retransplantation
The rate of allograft nephrectomy before retransplantation ranges from 0.5 to 43% depending on the centre's protocols [22, 70, 71]. Some studies indicate an adverse impact of allograft nephrectomy on various clinical outcomes of a second transplant. In the cyclosporine era, a single-centre study demonstrated that allograft nephrectomy was associated with a significant increase in PRA levels, a higher incidence of DGF and reduced graft survival [72]. In a retrospective single-centre study comparing 121 patients undergoing kidney retransplantation with preliminary allograft nephrectomy to 45 retransplant recipients without the procedure, allograft nephrectomy led to worse graft survival after retransplantation, with an increase in PRA levels, a higher rate of primary non-function (P = 0.05) and acute rejection (P = 0.04); pre-transplant allograft nephrectomy and PRA >70% were independent and significant risk factors associated with graft loss after kidney retransplantation [73]. Recently, in a retrospective analysis of 109 kidney transplant recipients, allograft nephrectomy was an independent risk factor for the development of DSAs and non-DSAs after 12 and 24 months and negatively impacted the chance for retransplantation; maintaining adequate immunosuppression is conversely a protective factor against allosensitization [74].
Conversely, in a retrospective study including patients undergoing retransplantation, there were no significant differences in the rate of acute graft rejection, graft survival and PRA levels between those receiving preliminary nephrectomy prior to retransplantation or not [75]. Using the USRDS database (19 107 patients with graft failure and returning to dialysis), Johnston et al. [62] showed that allograft nephrectomy after early graft loss was associated with a lower risk of second graft failure, whereas transplantectomy for late graft loss (graft survival >1 year) was associated with worse repeat transplant outcome. In a recent Chinese meta-analysis of 1923 patients with failure of the first renal allograft and successively retransplanted, those with allograft nephrectomy showed lower 3- and 5-year graft survival with an increased risk of acute rejection and higher PRA and DGF [76].
CONCLUSIONS AND RECOMMENDATIONS
Kidney graft loss is an important cause of ESKD. Retransplantation, particularly when pre-emptive, after graft failure presented a similar survival rate compared with the first transplantation and represents an optimal opportunity for a group of patients with kidney graft failure to reduce the complications associated with dialysis reinitiation. Reinitiation of dialysis based on eGFR alone is not justified and could be harmful in some cases: comorbidities, nutritional status and overall wellness of patients returning to dialysis after graft failure should be considered in assessing the optimal timing and modalities of dialysis. Dialysis technique after graft loss does not influence the mortality rate. Based on the actual evidence, PD patients seem to present the greatest survival in the first year (probably due to lower risk of infection and greater preservation of residual renal function), while survival is lower on PD after 2 years (due to PD technique complications and failure). Adequate preparation of patients with failing kidney transplants prior to resuming dialysis is critical to improve outcomes. Future prospective studies are needed to achieve a better understanding of the landscape of patients who return to dialysis after graft failure. Continuation of low-dose immunosuppression is appropriate in pre-dialysis patients and in those with symptomatic rejection to serve as a bridge to allograft nephrectomy. Maintenance low-dose immunosuppression may also be beneficial for the risk of de novo allosensitization that may preclude options for future kidney transplantation, in patients with anticipated living donor re-allograft transplant or those with residual urine output. This is particularly relevant for patients who are likely to require retransplantation within their lifetime. However, considering the risks, in patients with significant comorbid conditions, immunosuppression minimization or withdrawal should be evaluated. Allograft nephrectomy should be considered after an accurate balance of indications and contraindications. Further studies are needed to determine whether allograft nephrectomy after late graft loss may confer a survival advantage over leaving the graft in situ.
AUTHORS’ CONTRIBUTIONS
M.F. and G.C. contributed to the conception and design of the study. P.G., M.G. and V.C. participated in reviewing the literature and writing the paper. M.F. and G.C. supported the final draft editing. A.S., G.S. and L.G. provided critical review and revised the article. All authors gave final approval for the present version to be submitted.
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- 1. Laupacis A, Keown P, Pus N. et al. A study of the quality of life and cost-utility of renal transplantation. Kidney Int 1996; 50: 235–242 [DOI] [PubMed] [Google Scholar]
- 2. Ojo AO, Hanson JA, Meier-Kriesche H. et al. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol 2001; 12: 589–597 [DOI] [PubMed] [Google Scholar]
- 3. Meier-Kriesche H-U, Schold JD, Srinivas TR. et al. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 2004; 4: 378–383 [DOI] [PubMed] [Google Scholar]
- 4. Rao PS, Schaubel DE, Jia X. et al. Survival on dialysis post-kidney transplant failure: results from the Scientific Registry of Transplant Recipients. Am J Kidney Dis 2007; 49: 294–300 [DOI] [PubMed] [Google Scholar]
- 5.United S Renal Data System. USRDS 2011 Annual Data Report. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2015
- 6. Mourad G, Minguet J, Pernin V. et al. Similar patient survival following kidney allograft failure compared with non-transplanted patients. Kidney Int 2014; 86: 191–198 [DOI] [PubMed] [Google Scholar]
- 7. Kaplan B, Meier-Kriesche H-U.. Death after graft loss: an important late study endpoint in kidney transplantation. Am J Transplant 2002; 2: 970–974 [DOI] [PubMed] [Google Scholar]
- 8. Gill JS, Abichandani R, Kausz AT et al... Mortality after kidney transplant failure: the impact of non-immunologic factors. Kidney Int 2002; 62: 1875–1883 [DOI] [PubMed] [Google Scholar]
- 9. Gill JS, Rose C, Pereira BJG et al.. The importance of transitions between dialysis and transplantation in the care of end-stage renal disease patients. Kidney Int 2007; 71: 442–447 [DOI] [PubMed] [Google Scholar]
- 10. Kabani R, Quinn RR, Palmer S. et al. Risk of death following kidney allograft failure: a systematic review and meta-analysis of cohort studies. Nephrol Dial Transplant 2014; 29: 1778–1786 [DOI] [PubMed] [Google Scholar]
- 11. Perl J, Zhang J, Gillespie B. et al. Reduced survival and quality of life following return to dialysis after transplant failure: the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant 2012; 27: 4464–4472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Varas J, Perez-Saez MJ, Ramos R. et al. Returning to haemodialysis after kidney allograft failure: a survival study with propensity score matching. Nephrol Dial Transplant 2019; 34: 667–672 [DOI] [PubMed] [Google Scholar]
- 13. Lopez-Gomez JM, Perez-Flores I, Jofre R. et al. Presence of a failed kidney transplant in patients who are on hemodialysis is associated with chronic inflammatory state and erythropoietin resistance. J Am Soc Nephrol 2004; 15: 2494–2501 [DOI] [PubMed] [Google Scholar]
- 14. Laham G, Pujol GS, Vilches A. et al. Nonprogrammed vascular access is associated with greater mortality in patients who return to hemodialysis with a failing renal graft. Transplantation 2017; 101: 2606–2611 [DOI] [PubMed] [Google Scholar]
- 15. Brar A, Markell M, Stefanov DG. et al. Mortality after renal allograft failure and return to dialysis. Am J Nephrol 2017; 45: 180–186 [DOI] [PubMed] [Google Scholar]
- 16. Hart A, Smith JM, Skeans MA. et al. OPTN/SRTR 2017 Annual Data Report: Kidney. Am J Transplant 2019; 19: 19–123 [DOI] [PubMed] [Google Scholar]
- 17. Marcen R, Teruel JL.. Patient outcomes after kidney allograft loss. Transplant Rev (Orlando) 2008; 22: 62–72 [DOI] [PubMed] [Google Scholar]
- 18. Coupel S, Giral-Classe M, Karam G. et al. Ten-year survival of second kidney transplants: impact of immunologic factors and renal function at 12 months. Kidney Int 2003; 64: 674–680 [DOI] [PubMed] [Google Scholar]
- 19. Clark S, Kadatz M, Gill J. et al. Access to kidney transplantation after a failed first kidney transplant and associations with patient and allograft survival: an analysis of national data to inform allocation policy. Clin J Am Soc Nephrol 2019; 14: 1228–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heaphy ELG, Poggio ED, Flechner SM. et al. Risk factors for retransplant kidney recipients: relisting and outcomes from patients’ primary transplant. Am J Transplant 2014; 14: 1356–1367 [DOI] [PubMed] [Google Scholar]
- 21. Florit EA, Bennis S, Rodriguez E. et al. Pre-emptive retransplantation in patients with chronic kidney graft failure. Transplant Proc 2015; 47: 2351–2353 [DOI] [PubMed] [Google Scholar]
- 22. Goldfarb-Rumyantzev AS, Hurdle JF, Baird BC. et al. The role of pre-emptive re-transplant in graft and recipient outcome. Nephrol Dial Transplant 2006; 21: 1355–1364 [DOI] [PubMed] [Google Scholar]
- 23. Johnston O, Rose CL, Gill JS. et al. Risks and benefits of preemptive second kidney transplantation. Transplantation 2013; 95: 705–710 [DOI] [PubMed] [Google Scholar]
- 24. Girerd S, Girerd N, Duarte K. et al. Preemptive second kidney transplantation is associated with better graft survival compared with non-preemptive second transplantation: a multicenter French 2000-2014 cohort study. Transpl Int 2018; 31: 408–423 [DOI] [PubMed] [Google Scholar]
- 25. Wong G, Chua S, Chadban SJ. et al. Waiting time between failure of first graft and second kidney transplant and graft and patient survival. Transplantation 2016; 100: 1767–1775 [DOI] [PubMed] [Google Scholar]
- 26. Herrero E, Portillo JA, Ballestero R. et al. [Experience with third, fourth and fifth kidney transplants and their complications.]. Arch Esp Urol 2017; 70: 815–823 [PubMed] [Google Scholar]
- 27. Loupy A, Anglicheau D, Timsit MO. et al. Impact of surgical procedures and complications on outcomes of third and subsequent kidney transplants. Transplantation 2007; 83: 385–391 [DOI] [PubMed] [Google Scholar]
- 28. Izquierdo L, Peri L, Piqueras M. et al. Third and fourth kidney transplant: still a reasonable option. Transplant Proc 2010; 42: 2498–2502 [DOI] [PubMed] [Google Scholar]
- 29. Cooper BA, Branley P, Bulfone L. et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 2010; 363: 609–619 [DOI] [PubMed] [Google Scholar]
- 30. Molnar MZ, Streja E, Kovesdy CP. et al. Estimated glomerular filtration rate at reinitiation of dialysis and mortality in failed kidney transplant recipients. Nephrol Dial Transplant 2012; 27: 2913–2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McDonald SP, Marshall MR, Johnson DW. et al. Relationship between dialysis modality and mortality. J Am Soc Nephrol2009; 20: 155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yeates K, Zhu N, Vonesh E. et al. Hemodialysis and peritoneal dialysis are associated with similar outcomes for end-stage renal disease treatment in Canada. Nephrol Dial Transplant 2012; 27: 3568–3575 [DOI] [PubMed] [Google Scholar]
- 33. Davies SJ. Peritoneal dialysis in the patient with a failing renal allograft. Perit Dial Int 2001; 21: 280– 284 [PubMed] [Google Scholar]
- 34. de Jonge H, Bammens B, Lemahieu W. et al. Comparison of peritoneal dialysis and haemodialysis after renal transplant failure. Nephrol Dial Transplant 2006; 21: 1669–1674 [DOI] [PubMed] [Google Scholar]
- 35. Sasal J, Naimark D, Klassen J. et al. Late renal transplant failure: an adverse prognostic factor at initiation of peritoneal dialysis. Perit Dial Int 2001; 21: 405–410 [PubMed] [Google Scholar]
- 36. Perl J, Hasan O, Bargman JM. et al. Impact of dialysis modality on survival after kidney transplant failure. Clin J Am Soc Nephrol 2011; 6: 582–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perl J, Dong J, Rose C. et al. Is dialysis modality a factor in the survival of patients initiating dialysis after kidney transplant failure? Perit Dial Int 2013; 33: 618–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Salazar AM, Chávez CO, Álvarez TG. et al. Returning to dialysis after kidney transplant failure. Does the dialysis treatment modality influence the survival prognosis?. Transplantation 2018; 102(7 Suppl 1): S537 . [Google Scholar]
- 39. Pham P-T, Everly M, Faravardeh A. et al. Management of patients with a failed kidney transplant: dialysis reinitiation, immunosuppression weaning, and transplantectomy. World J Nephrol 2015; 4: 148–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pham P-T, Pham P-C.. Immunosuppressive management of dialysis patients with recently failed transplants. Semin Dial 2011; 24: 307–313 [DOI] [PubMed] [Google Scholar]
- 41. Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol 2001; 12: 2158–2162 [DOI] [PubMed] [Google Scholar]
- 42. Shemin D, Bostom AG, Laliberty P et al.. Residual renal function and mortality risk in hemodialysis patients. Am J Kidney Dis 2001; 38: 85–90 [DOI] [PubMed] [Google Scholar]
- 43. Elmahi N, Csongradi E, Kokko K. et al. Residual renal function in peritoneal dialysis with failed allograft and minimum immunosuppression. World J Transplant 2013; 3: 26–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scornik JC, Kriesche H-U.. Human leukocyte antigen sensitization after transplant loss: timing of antibody detection and implications for prevention. Hum Immunol 2011; 72: 398–401 [DOI] [PubMed] [Google Scholar]
- 45. Casey MJ, Wen X, Kayler LK. et al. Prolonged immunosuppression preserves nonsensitization status after kidney transplant failure. Transplantation 2014; 98: 306–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Augustine JJ, Woodside KJ, Padiyar A. et al. Independent of nephrectomy, weaning immunosuppression leads to late sensitization after kidney transplant failure. Transplantation 2012; 94: 738–743 [DOI] [PubMed] [Google Scholar]
- 47. Del Bello A, Congy-Jolivet N, Sallusto F. et al. Donor-specific antibodies after ceasing immunosuppressive therapy, with or without an allograft nephrectomy. Clin J Am Soc Nephrol 2012; 7: 1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kosmoliaptsis V, Mallon DH, Chen Y. et al. Alloantibody responses after renal transplant failure can be better predicted by donor-recipient HLA amino acid sequence and physicochemical disparities than conventional HLA matching. Am J Transplant 2016; 16: 2139–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bunthof KLW, Verhoeks CM, van den Brand J. et al. Graft intolerance syndrome requiring graft nephrectomy after late kidney graft failure: can it be predicted? A retrospective cohort study. Transpl Int 2018; 31: 220–229 [DOI] [PubMed] [Google Scholar]
- 50. Bunthof KLW, Hazzan M, Hilbrands LB.. Review: management of patients with kidney allograft failure. Transplant Rev (Orlando) 2018; 32: 178–186 [DOI] [PubMed] [Google Scholar]
- 51. Woodside KJ, Schirm ZW, Noon KA. et al. Fever, infection, and rejection after kidney transplant failure. Transplantation 2014; 97: 648–653 [DOI] [PubMed] [Google Scholar]
- 52. Madore F, Hebert MJ, Leblanc M. et al. Determinants of late allograft nephrectomy. Clin Nephrol 1995; 44: 284–289 [PubMed] [Google Scholar]
- 53. Smak Gregoor PJ, Zietse R, van Saase JL. et al. Immunosuppression should be stopped in patients with renal allograft failure. Clin Transplant 2001; 15: 397–401 [DOI] [PubMed] [Google Scholar]
- 54. Kiberd BA, Belitsky P.. The fate of the failed renal transplant. Transplantation 1995; 59: 645–647 [PubMed] [Google Scholar]
- 55. Verresen L, Vanrenterghem Y, Waer M. et al. Corticosteroid withdrawal syndrome in dialysis patients. Nephrol Dial Transplant 1988; 3: 476–477 [DOI] [PubMed] [Google Scholar]
- 56. Dantal J, Hourmant M, Cantarovich D. et al. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet 1998; 351: 623–628 [DOI] [PubMed] [Google Scholar]
- 57. Vajdic CM, van Leeuwen MT, Webster AC. et al. Cutaneous melanoma is related to immune suppression in kidney transplant recipients. Cancer Epidemiol Biomarkers Prev 2009; 18: 2297–2303 [DOI] [PubMed] [Google Scholar]
- 58. van Leeuwen MT, Webster AC, McCredie MRE. et al. Effect of reduced immunosuppression after kidney transplant failure on risk of cancer: population based retrospective cohort study. BMJ 2010; 340: c570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Andrews PA; Standards Committee of the British Transplantation Society. Summary of the British Transplantation Society guidelines for management of the failing kidney transplant. Transplantation 2014; 98: 1130–1133 [DOI] [PubMed] [Google Scholar]
- 60. Kassakian CT, Ajmal S, Gohh RY. et al. Immunosuppression in the failing and failed transplant kidney: optimizing outcomes. Nephrol Dial Transplant 2016; 31: 1261–1269 [DOI] [PubMed] [Google Scholar]
- 61.United States Renal Data System. 2016 USRDS annual data report: epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease, Bethesda, MD, 2016
- 62. Johnston O, Rose C, Landsberg D. et al. Nephrectomy after transplant failure: current practice and outcomes. Am J Transplant 2007; 7: 1961–1967 [DOI] [PubMed] [Google Scholar]
- 63. Minson S, Munoz M, Vergara I. et al. Nephrectomy for the failed renal allograft in children: predictors and outcomes. Pediatr Nephrol 2013; 28: 1299–1305 [DOI] [PubMed] [Google Scholar]
- 64. Ghyselen L, Naesens M.. Indications, risks and impact of failed allograft nephrectomy. Transplant Rev (Orlando) 2019; 33: 48–54 [DOI] [PubMed] [Google Scholar]
- 65. Gomez-Dos-Santos V, Lorca-Alvaro J, Hevia-Palacios V. et al. The failing kidney transplant allograft. Transplant nephrectomy: current state-of-the-art. Curr Urol Rep 2020; 21: 4. [DOI] [PubMed] [Google Scholar]
- 66. Takase HM, Contti MM, Nga HS. et al. Nephrectomy versus embolization of non-functioning renal graft: a systematic review with a proportional meta-analysis. Ann Transplant 2018; 23: 207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ayus JC, Achinger SG, Lee S. et al. Transplant nephrectomy improves survival following a failed renal allograft. J Am Soc Nephrol2010; 21: 374–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Del Bello A, Congy N, Sallusto F. et al. Anti-human leukocyte antigen immunization after early allograft nephrectomy. Transplantation 2012; 93: 936–941 [DOI] [PubMed] [Google Scholar]
- 69. Lachmann N, Schonemann C, El-Awar N. et al. Dynamics and epitope specificity of anti-human leukocyte antibodies following renal allograft nephrectomy. Nephrol Dial Transplant 2016; 31: 1351–1359 [DOI] [PubMed] [Google Scholar]
- 70. Milongo D, Kamar N, Del Bello A. et al. Allelic and epitopic characterization of intra-kidney allograft anti-HLA antibodies at allograft nephrectomy. Am J Transplant 2017; 17: 420–431 [DOI] [PubMed] [Google Scholar]
- 71. Ahmad N, Ahmed K, Mamode N.. Does nephrectomy of failed allograft influence graft survival after re-transplantation? Nephrol Dial Transplant 2008; 24: 639–642 [DOI] [PubMed] [Google Scholar]
- 72. Sumrani N, Delaney V, Hong JH. et al. The influence of nephrectomy of the primary allograft on retransplant graft outcome in the cyclosporine era. Transplantation 1992; 53: 52–55 [DOI] [PubMed] [Google Scholar]
- 73. Schleicher C, Wolters H, Kebschull L. et al. Impact of failed allograft nephrectomy on initial function and graft survival after kidney retransplantation. Transpl Int 2011; 24: 284–291 [DOI] [PubMed] [Google Scholar]
- 74. Lucisano G, Brookes P, Santos-Nunez E. et al. Allosensitization after transplant failure: the role of graft nephrectomy and immunosuppression – a retrospective study. Transpl Int 2019; 32: 949–959 [DOI] [PubMed] [Google Scholar]
- 75. Surga N, Viart L, Wetzstein M. et al. Impact of renal graft nephrectomy on second kidney transplant survival. Int Urol Nephrol 2013; 45: 87–92 [DOI] [PubMed] [Google Scholar]
- 76. Lin J, Wang R, Xu Y. et al. Impact of renal allograft nephrectomy on graft and patient survival following retransplantation: a systematic review and meta-analysis. Nephrol Dial Transplant 2018; 33: 700–708 [DOI] [PubMed] [Google Scholar]