Abstract
Tyrosine kinase receptor inhibitors (TKIs) are a relatively new class of targeted anti-cancer agents with vascular endothelial growth factor signalling pathway–inhibiting properties. Hypertension is recognized as one of the most common adverse effects of this anti-angiogenic therapy and is the consequence of reduced production of vasodilatory nitric oxide and reduced prostacyclin production as well as increased production of vasoconstrictive endothelin-1. TKI-induced hypertension is dose dependent and it has been suggested as a marker of treatment effectiveness. In this issue, Saito et al. report the incidence of treatment-related hypertension in patients treated with lenvatinib, a newer TKI, for non-resectable hepatocellular carcinoma. The authors demonstrate that a subset of TKI-treated patients develop fluid retention 3 weeks after treatment initiation as a consequence of lower urinary sodium excretion and thus provides insights into the pathogenesis of blood pressure elevation in the second phase. These findings contribute to a better understanding of TKI-associated hypertension and help in choosing the most appropriate antihypertensive agents in this setting. Active control of hypertension may help more patients benefit from longer TKI therapy, possibly resulting in better cancer outcomes.
Keywords: fluid retention, hypertension, lenvatinib, natriuresis, onco-nephrology, tyrosine kinase inhibitors
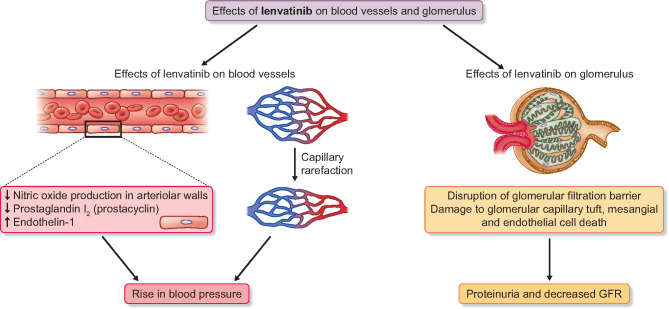
Tyrosine kinase inhibitors (TKIs) are vascular endothelial growth factor (VEGF) signalling pathway inhibitors that are used as targeted anticancer treatment in solid organ cancers, such as renal cell carcinoma, hepatocellular carcinoma, differentiated thyroid carcinoma, medullary thyroid cancer, soft tissue sarcoma, colorectal carcinoma, gastrointestinal stromal tumour, metastatic breast cancer and non-small cell lung carcinoma [1]. They are effective and relatively easy to administer and their use has expanded in recent years. Inhibiting angiogenesis is an effective method of cancer therapy based on the hypothesis that tumour growth and metastases are angiogenesis-dependent processes [2]. Therefore, interrupting pro-angiogenic signalling pathways is a primary goal of newer anti-tumour therapies, with VEGF being a critical target [2]. VEGF exerts its effects through VEGF receptors (VEGFR1, VEGFR2 and VEGFR3). Binding to VEGFR2 activates its intrinsic tyrosine kinase activity and triggers a multitude of downstream signalling cascades [2] related to capillary permeability, production of nitric oxide (NO), endothelial cell proliferation, migration and survival under stress [3, 4] (Figure 1). NO production results in vascular smooth muscle relaxation and, conversely, NO depletion leads to increased vascular tone, increased peripheral resistance and systemic blood pressure (BP) elevation as well as sodium retention and an increase in extracellular fluid volume [2]. VEGFR inhibition also hinders the production of vasodilatory prostacyclin [2] (Figure 1). Activation of the endothelin-1 pathway is another mechanism leading to the constriction of vascular smooth muscle cells [2]. Chronic VEGF depletion causes reduced capillary endothelial cell survival, which can lead to apoptosis and microvascular rarefaction [2, 5] and may increase peripheral resistance. Endothelial injury may also precipitate local thrombosis, leading to a further reduction in microvessel density and vascular perfusion [2] (Figure 1).
FIGURE 1:
Effects of lenvatinib on the blood vessels and glomerulus.
From its mechanism of action, it is not unexpected that the most common adverse effect of TKI treatment is BP elevation [6]. Nearly all patients exposed to TKI experience a dose-dependent increase in BP. Increases in BP are expected from the first week of treatment, even from the first day. This highlights the importance of pretreatment evaluation of the patient’s BP and cardiovascular risk. Pre-existing hypertension needs to be well controlled for at least a week before initializing TKI treatment. Monitoring BP weekly during the first cycle of VEGF signalling pathway inhibitor therapy and then at least every 2–3 weeks for the duration of treatment is recommended. Close monitoring will help recognize BP elevations and allows for early treatment. The BP targets in this setting are BPs <140/90 mmHg, or even <130/80 mmHg, in high-risk patients, similar to the targets in patients with diabetes or chronic kidney disease [4]. Importantly, it has been highlighted that even an increase of diastolic BP of 20 mmHg without exceeding the BP goal should urge appropriate antihypertensive treatment, as it seems that the degree of BP change is more crucial than the crossing of a population-based threshold [4]. Besides an increase in BP, the occurrence of severe proteinuria has been linked to the use of TKI and therefore urine protein should be monitored regularly [6, 7]. Evidence suggests that VEGF inhibition selectively reduces nephrin production and causes interruption of the fenestrated endothelium [6, 7].
The early BP increase with VEGF inhibitor treatment, as well as the rapid BP reduction upon drug withdrawal, reflects direct actions of this class of anticancer treatment on the microvasculature [8, 9]. Therefore hypertension, as an on-target class effect of VEGF inhibitors, may serve as a marker of treatment efficacy, indicating the effectiveness of its anti-angiogenic action. Studies have shown that patients developing Grades 2 and 3 hypertension have better outcomes than those who do not develop hypertension [2]. For example, in the phase 3 Study of (E7080) Lenvatinib in Differentiated Cancer of the Thyroid (SELECT) trial, which involved 392 patients with thyroid cancer, treatment-emergent hypertension in the lenvatinib group significantly associated with both progression-free survival and overall survival in the univariate analysis; in the multivariate analysis, only overall survival maintained significance [5, 10]. Similar correlations were seen with other VEGF inhibitors with a variety of tumour types. Moreover, it has been suggested that VEGF polymorphisms may also play a role in the risk for treatment-emergent hypertension [2]. Based on these data, hypertension has been suggested as a biomarker of clinical outcomes for these agents. Interestingly, no correlation was found between treatment-emergent hypertension and proteinuria [5].
Lenvatinib, a newer TKI drug approved since 2015, is indicated as a monotherapy for the treatment of differentiated thyroid cancers resistant to radioactive therapy as well as non-resectable hepatocellular carcinoma. It is an oral multikinase inhibitor of VEGFR1, VEGFR2 and VEGFR3, as well as fibroblast growth factor receptors 1–4, platelet-derived growth factor receptor α, and RET and KIT receptor tyrosine kinases [5]. Lenvatinib is associated with a higher incidence of severe hypertension compared with other TKIs, perhaps indicating a more potent inhibitory effect [6]. In this issue of Clinical Kidney Journal, Saito et al.[1,1] studied the changes in BP during treatment with lenvatinib in patients with non-resectable hepatocellular carcinoma . The study included 25 patients who were followed for 3 weeks after the initiation of lenvatinib. The mean age was 70 ± 9 years; 56% of patients had hypertension before treatment and none had a diagnosis of kidney disease. BP was evaluated before and 1 week after the initiation of lenvatinib treatment using ambulatory BP monitoring. Patients were instructed to continue to perform BP measurements during the third week of treatment and the average BP was recorded. Moreover, changes in kidney function, urinary sodium excretion and proteinuria were evaluated at baseline and at 1 and 3 weeks after the initiation of lenvatinib treatment [11]. Results confirmed BP increases in the early course of lenvatinib treatment, during the first week. The increase in night-time blood pressure was significantly greater than that of daytime BP (Δsystolic BP: 20.4 ± 10.3 versus 12.9 ± 10.5 mmHg at night-time and daytime, respectively; P < 0.05). Forty-eight percent of patients required intensive treatment for hypertension based on the results of the second ambulatory BP monitoring at 1 week of treatment. Interestingly, fluid retention was observed from 3 weeks of treatment onwards, along with a decline in urinary sodium excretion. The mean BP, as well as mean plasma brain natriuretic peptidelevel, were significantly higher in patients experiencing fluid retention compared with those without fluid retention. Of note, 70% of the patients with fluid retention and 20% of those without had Grade III hypertension. Limited proteinuria [median 0.14 g/g creatinine (interquartile range 0.05–0.59)] was detected in 40% of patients in this study. Furthermore, a mild decline in kidney function (eGFR of 66.1 ± 22.0 mL/min/1.73 m2 at 3 weeks compared with 71.3 ± 18.6 mL/min/1.73 m2 at baseline; P < 0.05) was also observed [11]. The authors hypothesize that both proteinuria and kidney function decline are the result of direct cellular injury by TKI and ischaemic injury due to microcirculatory disturbances in glomeruli and kidney interstitium, although this was not specifically investigated in this study. Loop diuretics and intensification of antihypertensive treatment had to be implemented and one patient had to discontinue the lenvatinib treatment because of severe hypertension.
Little data exist regarding the antihypertensive drug of choice for TKI-induced hypertension. Treatment should be individualized to each patient’s needs, based on pre-existing comorbidities, drug interactions and adverse effects or intolerances. Angiotensin–renin system inhibitors and calcium channel blockers seem to be reasonable choices [12]. Interestingly, studies in animal models have shown that calcium channel blockers increase microvessel density, while chronic alpha-1 adrenoreceptor blockade can lead to increased capillary density [9]. The effects of angiotensin-converting enzyme inhibition on microvessel rarefaction, however, are more controversial, while available evidence suggests that diuretics and β-blockers have no specific beneficial actions on the microcirculation [9]. Taking into account that QT prolongation is one of the known adverse effects of TKIs, caution is required to avoid electrolyte imbalances as a consequence of diuretics use. The results of this study explain the biphasic pattern of lenvatinib-induced hypertension and are consistent with existing data that suggest an early increase in BP due to imbalances between vasodilators and vasoconstrictors. Vasoconstrictive mechanisms of BP elevations during TKI treatment have been explained above and include NO and prostacyclin-reduced production as well as increased levels on endothelin-1. The study of Saito et al. [11] provides insights into the pathogenesis of BP elevation in the second phase and demonstrates that TKI-treated patients developed fluid retention at 3 weeks as a consequence of lower urinary sodium excretion . These findings are consistent with the notion that hypertension is related to altered natriuresis and fluid overload. Afferent arteriole vasoconstriction and decreased blood flow to the glomeruli as well as inhibition of tubuloglomerular feedback caused by reduced NO function are the proposed mechanisms for the decrease in urinary sodium excretion. Considering the above findings, we can conclude that BP elevation in the later period might be due primarily to the fluid retention caused by a decrease in urinary sodium excretion and diuretics should be the antihypertensive treatment of choice in this setting.
A better understanding of the time and the course of treatment-emergent hypertension will lead to greater awareness, early recognition and appropriate management, allowing patients to benefit from the maximum efficacy of TKI treatment. With proactive BP management, the goal is to avoid discontinuation of TKI because of uncontrolled hypertension and prompt referral to a specialist is indicated in case of difficult-to-control hypertension. At the same time, better pain control by using appropriate medication and avoiding non-steroidal anti-inflammatory drugs will further improve BP management. Other factors contributing to BP elevations might coexist; these include the use of erythropoietin, contraceptive hormones or adrenal steroid hormones as well as excessive alcohol consumption. Active control of hypertension will allow patients to tolerate the highest-dose therapy for the longest period to obtain the maximum benefit from anticancer treatment. On the other hand, uncontrolled hypertension can lead to serious adverse events (like malignant hypertension, heart failure, left ventricular systolic dysfunction and aortic dissection) and therefore can worsen the patient’s survival expectancy and quality of life. Moreover, as the indications of these agents expand to more chronic settings, the need for prevention of cardiovascular disease becomes more apparent. Some patients with short life expectancy and limited treatment choices might be more interested to gain the maximum efficacy from cancer treatment rather than consider hypertensive complications. When commencing anticancer therapy cannot be deferred, TKI treatment can be initiated and maintained, even if BP has not reached the target level yet, as long as it remains under a high-risk level. In cases where hypertension is difficult to manage, a dose reduction or temporary cessation of TKI treatment is warranted. Once hypertension is controlled, TKI treatment can be initiated at the same or lower dose. Of note, the increase in BP is a reversible effect, thus it should be anticipated that once TKI treatment is finished, BP will decrease and antihypertensive medication will have to be reduced or stopped. In humans, data even show regrowth of the capillary network after discontinuation of therapy.
In conclusion, TKIs are effective and relatively easy to administer. The treatment goal should be to maximize anti-tumour effects while minimizing therapy-associated toxicities. Understanding the mechanisms of hypertension will lead to effective management of this complication. The findings of Saito et al. [11] are important to further elucidate the detailed mechanisms underlying the changes in BP due to lenvatinib and TKI treatment in general . Although this is an observational retrospective study with a small sample size, these findings could help improve awareness and vigilance, aiming for early management of hypertension and help deciding on the preferred class of antihypertensive agents. Further studies are required to establish the optimal antihypertensive treatment in this setting and determine the possible use of BP, as well as albuminuria, as biomarkers for anti-tumour treatment efficacy. An unanswered question at this time is whether the dose of lenvatinib should be increased in patients who do not develop hypertension. Awareness of potential adverse events will lead to early recognition and appropriate management, thus improving patient outcomes.
CONFLICT OF INTEREST STATEMENT
B.S. is a senior clinical investigator of the Research Foundation Flanders (1842919N).
REFERENCES
- 1. Pottier C, Fresnais M, Gilon M. et al. Tyrosine kinase inhibitors in cancer: breakthrough and challenges of targeted therapy. Cancers (Basel) 2020; 12: 731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robinson ES, Khankin EV, Karumanchi SA. et al. Hypertension induced by vascular endothelial growth factor signaling pathway inhibition: mechanisms and potential use as a biomarker. Semin Nephrol 2010; 30: 591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lenihan DJ, Kowey PR.. Overview and management of cardiac adverse events associated with tyrosine kinase inhibitors. Oncologist 2013; 18: 900–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maitland ML, Bakris GL, Black HR. et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst 2010; 102: 596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wirth LJ, Tahara M, Robinson B. et al. Treatment-emergent hypertension and efficacy in the phase 3 study of (E7080) lenvatinib in differentiated cancer of the thyroid (SELECT). Cancer 2018; 124: 2365–2372 [DOI] [PubMed] [Google Scholar]
- 6. Pandey AK, Singhi EK, Arroyo JP. et al. Mechanisms of VEGF inhibitor-associated hypertension and vascular disease. Hypertension 2018; 71: e1–e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kandula P, Agarwal R.. Proteinuria and hypertension with tyrosine kinase inhibitors. Kidney Int 2011; 80: 1271–1277 [DOI] [PubMed] [Google Scholar]
- 8. Touyz RM, Lang NN, Herrmann J. et al. Recent advances in hypertension and cardiovascular toxicities with vascular endothelial growth factor inhibition. Hypertension 2017; 70: 220–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levy BI, Ambrosio G, Pries AR. et al. Microcirculation in hypertension: a new target for treatment? Circulation 2001; 104: 735–740 [DOI] [PubMed] [Google Scholar]
- 10. Schlumberger M, Tahara M, Wirth LJ. et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015; 372: 621–630 [DOI] [PubMed] [Google Scholar]
- 11. Saito K, Fujii H, Kono K. et al. Changes in blood pressure during the treatment with the tyrosine kinase inhibitor lenvatinib. Clin Kidney J 2021; 14: 325--331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rimassa L, Danesi R, Pressiani T. et al. Management of adverse events associated with tyrosine kinase inhibitors: improving outcomes for patients with hepatocellular carcinoma. Cancer Treat Rev 2019; 77: 20–28 [DOI] [PubMed] [Google Scholar]