Abstract
Background
The recurrence of proteinuria after kidney transplantation (KT) is a characteristic complication of focal segmental glomerulosclerosis (FSGS). It has been suggested that pre-emptive rituximab might prevent recurrences in patients at risk, but there is no agreement about which factors might help to identify such patients.
Methods
We studied 93 kidney transplants with biopsy-proven idiopathic FSGS in order to analyse if preventive rituximab treatment could decrease recurrences in patients at risk.
Results
Fifteen patients (16.1%) presented a recurrence after KT, but when we restricted the analysis to the 34 patients presenting nephrotic syndrome at primary disease onset, the recurrence diagnosis rate increased to 44.1%. All patients with recurrence had complete nephrotic syndrome at the time of diagnosis. After multivariate adjustment, the only significant risk factor for recurrence was the presence of complete nephrotic syndrome at diagnosis. Twelve of the 34 patients at risk for recurrence received rituximab at the time of transplantation. Clinical and analytical characteristics were similar in all patients at risk. The number of recurrences was similar among treated (50%) and non-treated patients (40.9%).
Conclusions
Nephrotic syndrome with hypoalbuminaemia at diagnosis is the most important feature to identify patients at risk of recurrence. Our data suggest that pre-emptive rituximab is not effective to prevent FSGS recurrences.
Keywords: focal segmental glomerulosclerosis, kidney transplantation, recurrence, rituximab
INTRODUCTION
Focal segmental glomerulosclerosis (FSGS) accounts for 20% of the cases of nephrotic syndrome in children and 40% in adults in the USA [1] and a significant proportion of patients evolve towards end-stage renal disease (ESRD). Recurrence of proteinuria after kidney transplantation (KT) is a characteristic complication of the disease that occurs in approximately one-third of the patients [2]. The number of recurrences rises to >70% in patients receiving a new renal allograft after failure of a previous graft because of FSGS recurrence [3] and several studies have shown that FSGS recurrences have an important detrimental influence on graft survival [4, 5].
The following characteristics have been proposed as significant risk factors for FSGS recurrence: childhood onset of the disease [6–8], rapid evolution to ESRD after diagnosis [3, 4, 7], recurrences in previous transplants [4], presence of mesangial hypercellularity in renal biopsy [9, 10], massive proteinuria [4], cyclosporine treatment before transplantation [7, 11] and the lack of response to corticosteroid treatment in patients with previous corticosensitivity [12]. However, there is no general agreement about the importance and significance of these prognostic factors, many of which have been described in single-centre studies with few patients. The same controversy exists about the significance of other suggested risk factors, such as the transplantation of kidneys from African American donors in Caucasian recipients [13] or the use of alemtuzumab or anti-thymocyte globulin as induction therapy at transplantation [14, 15]. The presence of complete nephrotic syndrome with hypoalbuminaemia at the time of FSGS diagnosis has been described as the main predictor of disease recurrence after transplantation [16].
The accurate identification of patients at high risk of recurrence after transplantation would be crucial to provide a pre-emptive treatment when possible. In this regard, some studies have suggested that rituximab administered at the time of transplantation might be effective in preventing recurrences [17, 18], but such efficacy has not been replicated by other groups [19, 20].
This study is the result of a collaboration between four different nephrology departments in Spain and aimed to identify those factors that may predict proteinuria recurrence after KT in a series of biopsy-proven idiopathic FSGS patients, define patients at risk of recurrence and analyse if preventive treatment with rituximab in patients at risk could decrease the rate of recurrences.
MATERIALS AND METHODS
Patients
We performed a multicentre retrospective cohort study including patients with biopsy-proven idiopathic FSGS as the underlying primary renal disease who received a KT between 2000 and 2014.
Patients suspected of having had genetic or secondary forms of FSGS were excluded. That included family history of nephrotic syndrome, human immunodeficiency virus infection, obesity, reflux nephropathy or prior nephrectomy. We identified a total of 93 kidney transplants in 87 patients (6 patients received a second transplant) who were diagnosed with FSGS after renal biopsy of their native kidneys. All patients were Caucasian except six (two African Americans, two Asians, one Arab and one Hispanic). No recipient underwent bilateral native nephrectomy.
Follow-up and data collection
Medical records were reviewed retrospectively. Baseline was defined as the time of diagnostic renal biopsy in native kidneys. Data were collected at baseline and at the time of transplantation. A minimum follow-up time of 12 months after transplantation was required in patients without recurrence of proteinuria. In patients with recurrence, data were collected at Months 1, 3, 6 and 12 and then annually after recurrence. Serum creatinine, estimated glomerular filtration rate (eGFR), 24-h proteinuria and graft loss at the end of follow-up were recorded in all patients. GFR was estimated using the Modification of Diet in Renal Disease formula. Patients were divided into two groups according to the presence or absence of proteinuria recurrence after transplantation. The mean follow-up was 71.7 months (73.5 months for the recurrence group versus 71.4 months for the non-recurrence group).
Definitions
Nephrotic syndrome was defined by the presence of proteinuria >3.5 g/24 h combined with hypoalbuminaemia (serum albumin <3 g/dL).
Recurrence was defined by the reappearance of proteinuria in the absence of acute rejection or urinary tract infection and was confirmed by renal biopsy in all but five cases. In these cases, the clinical course was compatible with a recurrence of FSGS, the presence of donor-specific antibodies (DSAs) was excluded and there was a rapid improvement of proteinuria after the start of plasmapheresis in most of them.
Complete remission was defined as proteinuria ≤0.3 g/24 h along with improvement or stability of renal function (absence of serum creatinine increase >25%); partial remission was defined as a decrease in proteinuria to <3.5 g/24 h (or <50% of the initial proteinuria in cases presenting with proteinuria in the non-nephrotic range) together with improvement or stability of renal function [21]. Relapse was defined as the reappearance of heavy proteinuria once the first recurrence had been treated and remission achieved.
Graft survival was defined as the absence of ESRD. ESRD was defined as an eGFR <15 mL/min/1.73 m2 or a need for chronic dialysis or renal transplantation.
Rituximab as preventive therapy and treatment of recurrences
Pre-emptive treatment
Rituximab, 1 g at induction and 1 g on Day 14 after transplantation, was administered to patients considered at risk for recurrence (those with hypoalbuminaemia and nephrotic proteinuria at baseline).
Treatment of recurrences
Once FSGS recurrence was diagnosed, plasmapheresis was initiated quickly, with exchanges of 1–1, five plasma volumes and 5% albumin replacement. Initially plasmapheresis sessions were performed daily or every other day and then the frequency decreased according to the response. Rituximab (one to four doses of 375 mg/m2) was administered to some patients, usually in combination with plasmapheresis. When rituximab was used in combination with plasmapheresis, it was always infused at least 72 h prior to the next plasmapheresis session.
Aims of the study and outcomes
The aims of the study consisted of the identification of predicting factors for the recurrence of proteinuria after transplantation, evaluation of the efficacy of pre-emptive rituximab administration to prevent recurrences and evaluation of the efficacy of plasmapheresis and other treatments in relapses.
The main outcomes were proteinuria recurrence after transplantation, proteinuria remission, either complete or partial, and graft survival.
Statistical analyses
Data were described as mean ± SD or median and range. Pearson’s chi-square test and Fisher’s exact test were used to compare categorical variables between the two groups and the Student’s t-test was used to compare continuous variables. A multivariate analysis was performed using a binary logistic regression model to relate FSGS recurrence to the total set of independent variables. The forward step method was chosen to develop the model. Survival analysis in the different groups was performed using Kaplan–Meier curves, which were compared by logrank (Mantel–Cox). Statistical significance was accepted at the 95% confidence interval (CI). Statistical analysis was carried out using SPSS for Windows version 20 (IBM, Armonk, NY, USA).
RESULTS
Baseline characteristics of the patients at the time of native kidney biopsy are shown in Table 1. The mean age was 32.9 ± 15.8 years and 69.9% were males. Thirty-four patients (36.5%) showed a complete nephrotic syndrome (nephrotic-range proteinuria and hypoalbuminaemia) at the time of diagnosis. Immunosuppressive treatments (corticosteroids and calcineurin inhibitors) were administered to 32.3% of the patients. The patients’ characteristics at the time of transplantation are shown in Table 2.
Table 1.
Baseline characteristics of the FSGS patients at the time of native kidney biopsy
| Characteristics | All kidney transplants (n = 93) | Recurrence (n = 15) | No recurrence (n = 78) | P-value |
|---|---|---|---|---|
| Age at diagnosis (years) | 32.9 ± 15.8 | 24.5 ± 12.6 | 34.5 ± 16 | 0.037 |
| Gender (males), n/N (%) | 65/93 (69.9) | 11/15 (73.3) | 54/78 (69.2) | 0.751 |
| Serum albumin at diagnosis (g/dL) | 3.2 ± 0.95 | 2.28 ± 0.29 | 3.45 ± 0.91 | 0.000 |
| Proteinuria at diagnosis (g/24 h) | 6.37 ± 4.21 | 9.72 ± 4.18 | 5.61 ± 3.91 | 0.046 |
| Immunosuppressive therapy, n/N (%) | 30/93 (32.3) | 10/15 (66.7) | 20/78 (25.6) | 0.004 |
| Time from diagnosis to ESRD (years) | 8.11 ± 7.64 | 6.17 ± 4.46 | 8.66 ± 8.31 | 0.347 |
| Duration of dialysis (years) | 2.57 ± 2.5 | 1.76 ± 1.46 | 2.74 ± 2.64 | 0.170 |
Values are presented as mean ± SD unless stated otherwise. Bold values highlight variables with statistical significance.
Table 2.
Characteristics of FSGS patients at the time of kidney transplantation
| Characteristics | All kidney transplants (n = 93) | Recurrence (n = 15) | No recurrence (n = 78) | P-value |
|---|---|---|---|---|
| Age at transplantation (years) | 45.9 ± 14.1 | 36.1 ± 10.3 | 47.8 ± 14 | 0.003 |
| Previous transplantation, n/N (%) | 31/93 (33.3) | 2/15 (13.3) | 29/78 (37.2) | 0.082 |
| Recurrence in previous allograft, n/N (%) | 4/93 (4.3) | 1/15 (6.7) | 3/78 (3.8) | 0.005 |
| BMI at transplantation (kg/m2) | 25.9 ± 5.6 | 23.4 ± 5.4 | 26.3 ± 5.6 | 0.175 |
| Donor age (years) | 43.5 ± 16.2 | 32.8 ± 13.2 | 45.6 ± 16 | 0.005 |
| Donor source, n/N (%) | 0.367 | |||
| Deceased | 71/93 (76.3) | 11/15 (73.3) | 60/78 (76.9) | |
| Living related | 6/93 (6.5) | 0/15 (0) | 6/78 (7.7) | |
| Living non-related | 2/93 (2.2) | 1/15 (6.7) | 1/78 (1.3) | |
| Donation after circulatory death | 14/93 (15.1) | 3/15 (20) | 11/78 (14.1) | |
| Number of HLA mismatches | 3.79 ± 1.22 | 3.77 ± 0.92 | 3.80 ± 1.29 | 0.945 |
| Cold ischaemia time (min) | 946.1 ± 432.2 | 939.6 ± 301.6 | 947.4 ± 454 | 0.952 |
| Induction therapy, n/N (%)a | 69/93 (74.2) | 9/15 (60) | 61/78 (78.2) | 0.251 |
| Thymoglobulin | 28/93 (30.1) | 4/15 (26.7) | 24/78 (30.8) | 0.671 |
| Basiliximab | 35/93 (37.6) | 5/15 (33.3) | 30/78 (38.5) | – |
| Others | 7/93 (7.6) | 0/15 (0) | 7/78 (8.9) | – |
| Delayed graft function, n/N (%) | 32/93 (34.4) | 8/15 (53.3) | 24/78 (30.8) | 0.200 |
| Acute rejection, n/N (%) | 22/93 (23.7) | 7/15 (46.7) | 15/78 (19.2) | 0.069 |
Values are presented as mean ± SD unless stated otherwise. Bold values highlight variables with statistical significance.
Thymoglobulin was used in hyperimmunized recipients and in cases of donation after circulatory death, whereas basiliximab was administered to patients with living donors or to elderly recipients (with elderly donors).
FSGS recurrences
Fifteen patients (16.1%) presented an FSGS recurrence after KT. When the analysis was restricted to those patients presenting with nephrotic syndrome at baseline (n = 34), the incidence of recurrences increased to 44.1%. The median time between transplantation and recurrence was 3 months (range 3 days–108 months), although in 7 of the 15 patients the recurrence occurred during the first month after transplantation. Recurrence was confirmed by renal biopsy in 10 of the 15 cases (66.7%). Histopathological findings are shown in Table 3, including Banff scores [22]. In the remaining five cases, the diagnosis was based on clinical features (abrupt onset of massive proteinuria and complete nephrotic syndrome in patients who had had a similar presentation at the onset of biopsy-proven FSGS in the native kidneys and absence of DSAs). The mean proteinuria at the onset of recurrence was 11.8 g/24 h (range 0.9–40.2), mean serum albumin was 3.19 g/dL (range 2.4–4) and mean serum creatinine was 4.3 mg/dL (range 0.85–12).
Table 3.
Histology findings in patients with FSGS recurrence
| Patients with FSGS recurrence | Pre-emptive rituximab | Biopsy | DSA | Treatment of FSGS recurrence | Response to treatment |
|---|---|---|---|---|---|
| Patient 1 | Yes |
|
Negative | Plasmapheresis + rituximab | No |
| Patient 2 | Yes |
|
Negative | Plasmapheresis + rituximab | Yes (partial remission on the day 7 after treatment start) |
| Patient 3 | Yes |
|
Negative | Plasmapheresis + rituximab | No |
| Patient 4 | Yes |
|
Negative | Plasmapheresis + rituximab | Yes (partial remission 1 week after treatment start) |
| Patient 5 | Yes |
|
Negative | Rituximab | Yes |
| Patient 6 | Yes |
|
Not performed | Plasmapheresis + rituximab | Yes (partial remission 2 weeks after treatment start and complete remission after 3 months) |
| Patient 7 | No | Not performed | Negative | No treatment | No |
| Patient 8 | No | Not performed | Not performed | Plasmapheresis + rituximab | Yes (partial remission although plasmapheresis-dependent) |
| Patient 9 | No | Not performed | Negative | Plasmapheresis | Yes (partial remission 3 weeks after treatment start) |
| Patient 10 | No | Not performed | Not performed | Plasmapheresis + rituximab | Yes (partial remission 3 weeks after treatment start although plasmapheresis-dependent) |
| Patient 11 | No |
|
Negative | Plasmapheresis + rituximab | Yes (complete remission 10 days after treatment start) |
| Patient 12 | No | Not performed | Negative | Plasmapheresis + rituximab | No |
| Patient 13 | No |
|
Negative | mTORi withdrawal | Yes |
| Patient 14 | No |
|
Negative | Plasmapheresis + steroids | No |
| Patient 15 | No | Optical microscopy: lesions of segmental hyalinosis with capillary loop collapse and prominence of overlying epithelial cells in 2 glomeruli out of 18. Tubular atrophy involving 15–20% of the area of cortical tubules and mild interstitial fibrosis Banff scorea: i0 t0 v0 g0 ptc0 C4d0 ci1 ct1 cv0 cg0 mm0 ah0 aah0 | Negative | Plasmapheresis + steroids+ intravenous immunoglobulin | Yes (partial remission 4 weeks after treatment start) |
According to the Banff Classification of Kidney Allograft Pathology 2017 Update (22): I, interstitial inflammation; t, tubulitis; v, intimal arteritis; g, glomerulitis; ptc, peritubular capillaritis; Cd4, staining by immunohistochemistry; ci, interstitial fibrosis; ct, tubular atrophy; cv, vascular fibrous intimal thickening; cg, glomerular basement membrane double contours; mm, mesangial matrix expansion; ah, arteriolar hyalinosis; aah, hyaline arteriolar thickening.
Differences between patients with and without FSGS recurrence
As shown in Table 1, at the time of primary FSGS diagnosis, patients who experienced FSGS recurrence after KT were significantly younger and had a significantly higher proteinuria and lower serum albumin than those patients who did not show recurrences. All the patients with recurrences showed a complete nephrotic syndrome at baseline (nephrotic-range proteinuria and hypoalbuminaemia). The number of patients who had received immunosuppressive treatments at baseline was significantly higher among those who later had FSGS recurrence (66.7% versus 25.6%) (Table 1), as well as the number of FSGS recurrences in previous transplants (Table 2).
Patient and donor characteristics related to transplantation are shown in Table 2. Patients with FSGS recurrence received a kidney graft at a younger age and, accordingly, donor age was significantly younger. There were no differences regarding body mass index, other donor characteristics, number of human leucocyte antigen incompatibilities, cold ischaemia time, type of induction therapy, incidence of delayed graft function or maintenance immunosuppressive therapy. Triple therapy based on corticosteroids, tacrolimus and mycophenolate mofetil (MMF) was used in the vast majority of both groups (93.3% in patients with FSGS recurrence, 96.2% in the non-recurrence group). Treatment with mammalian target of rapamycin (mTOR) inhibitors was anecdotal (one patient in each of the two groups).
Factors associated with the risk of FSGS recurrence
As shown in Table 4, a younger age and a lower serum albumin at diagnosis, more frequent immunosuppressive treatments at baseline and a younger age at the time of transplantation were factors significantly associated with the risk of recurrence. By multivariate analysis, the only risk factor for FSGS recurrence was the value of serum albumin at baseline (Table 4).
Table 4.
Predictors of FSGS recurrence after kidney transplantation
| Risk factor | Univariate |
Multivariate |
||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age at diagnosis (years) | 0.96 (0.91–0.99) | 0.043 | – | – |
| Serum albumin at diagnosis (g/dL) | 0.16 (0.03–0.91) | 0.039 | 0.16 (0.03–0.91) | 0.039 |
| Proteinuria at diagnosis (g/24 h) | 1.26 (0.99–1.62) | 0.064 | – | – |
| Immunosuppressive therapy | 5.80 (1.77–19.02) | 0.004 | – | – |
| Recurrence in previous allograft | 0.87 (0.08–9.16) | 0.909 | – | – |
| Age at transplantation (years) | 0.93 (0.89–0.98) | 0.005 | – | – |
Rituximab as prophylaxis for FSGS recurrence
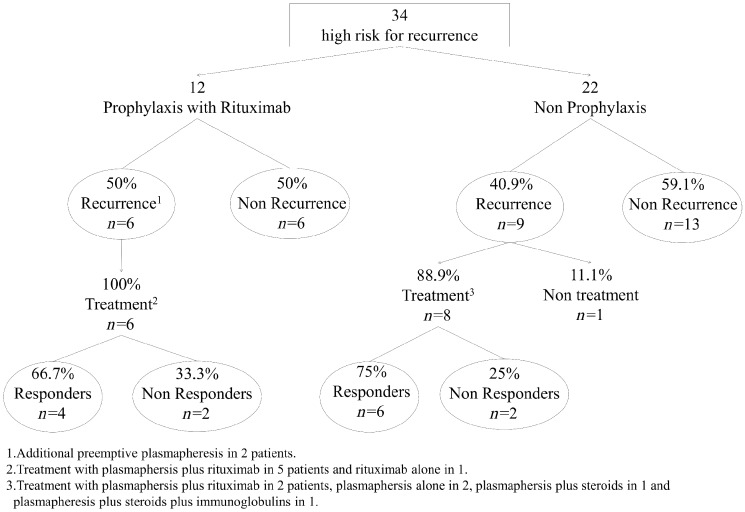
Rituximab (1 g at induction and 1 g 2 weeks after transplantation) was used as a potential prophylactic treatment in 12 of the 34 patients considered at high risk for FSGS recurrence (those with hypoalbuminaemia and nephrotic proteinuria at baseline). Plasma exchange sessions in the days following transplantation were performed, in addition to rituximab, in two of these patients. The clinical and analytical characteristics of these patients, both at baseline and at transplantation, are shown in Table 5. Six patients (50%) presented recurrences and the other six did not (Figure 1). The two patients treated with rituximab and plasmapheresis as prophylactic measures experienced FSGS recurrences.
Table 5.
Comparative characteristics of patients at risk of recurrence (because of the presence of nephrotic syndrome at baseline) treated with pre-emptive rituximab versus non-treated
| Characteristics | Rituximab (n = 12) | Non-rituximab (n = 22) | P-value |
|---|---|---|---|
| At baseline | |||
| Age at diagnosis (years) | 24.5 ± 18.5 | 30 ± 13.7 | 0.358 |
| Gender (males), n/N (%) | 7/12 (58.3) | 14/22 (63.6) | 0.761 |
| Nephrotic syndrome, n/N (%) | 12/12 (100) | 22/22 (100) | 1 |
| Serum albumin at diagnosis (g/dL) | 2.53 ± 0.85 | 2.40 ± 0.51 | 0.745 |
| Proteinuria at diagnosis (g/24 h) | 10.90 ± 4.74 | 6.81 ± 2.98 | 0.102 |
| Immunosuppressive therapy, n/N (%) | 8/12 (66.7) | 14/22 (63.6) | 0.985 |
| Time from diagnosis to ESRD (years) | 5.12 ± 4.44 | 7.58 ± 7.11 | 0.332 |
| Duration of dialysis (years) | 1.91 ± 1.26 | 2.27 ± 2.40 | 0.630 |
| Previous transplantation, n/N (%) | 2/12 (16.7) | 5/22 (22.7) | 0.521 |
| Recurrence in previous allograft, n/N (%) | 2/12 (16.7) | 2/22 (9.1) | 0.359 |
| At transplantation | |||
| Age at transplantation (years) | 35.0 ± 15.2 | 42.4 ± 12.2 | 0.130 |
| BMI at transplantation (kg/m2) | 21.6 ± 4.3 | 25.4 ± 3.5 | 0.050 |
| Residual diuresis >500 mL, n/N (%) | 6/12 (50) | 6/22 (27.3) | 0.350 |
| Donor age (years) | 39.8 ± 17.1 | 38.6 ± 14.8 | 0.831 |
| Donor source, n/N (%) | – | – | 0.239 |
| Deceased | 8/12 (66.7) | 15/22 (68.2) | – |
| Living related | 3/12 (25) | 1/22 (4.5) | – |
| Living non-related | 0/12 (0) | 1/22 (4.5) | – |
| Non-heart-beating donor | 1/12 (8.3) | 5/22 (22.7) | – |
| Number of HLA mismatches | 3.6 ± 1.2 | 3.7 ± 1.3 | 0.886 |
| Cold ischaemia time (min) | 922.3 ± 506.2 | 890.7 ± 405.6 | 0.849 |
| Induction therapy, n/N (%) | 8/12 (66.7) | 14/22 (63.6) | 0.860 |
| Thymoglobulin | 2/12 (16.7) | 9/22 (40.9) | 0.200 |
| Basiliximab | 6/12 (50) | 5/22 (22.7) | – |
| Delayed graft function, n/N (%) | 5/12 (41.7) | 11/22 (50) | 0.426 |
| Acute rejection, n/N (%) | 5/12 (41.7) | 5/22 (22.7) | 0.421 |
Values are presented as mean ± SD unless stated otherwise.
FIGURE 1.
Pre-emptive rituximab in patients at risk for recurrence because of the presence of nephrotic syndrome at baseline.
As shown in Table 5, there were no significant differences in the clinical and analytical characteristics between treated and non-treated patients. The proportion of FSGS recurrences was similar in treated and non-treated cases (50% versus 40.9%, respectively; P = 0.610).
Treatment of recurrences
One of the 15 patients with FSGS recurrence did not receive any specific treatment and restarted dialysis 4 months after recurrence. In the remaining 14 cases, diverse therapeutic measures were used, as shown in Table 6. Plasmapheresis sessions were performed in 12 patients: in combination with rituximab in 8 patients, with increased corticosteroid doses in 1, with increased corticosteroid doses plus immunoglobulins in 1 and as the only therapeutic measure in 2. The number of plasmapheresis was variable; 7 sessions in most, although one patient received up to 30 sessions (mean number 9.9). Plasmapheresis, when used in combination with rituximab, was performed at least 72 h after rituximab infusion.
Table 6.
Treatment and responsiveness of recurrences
| Treatment | Complete remission | Partial remission | No remission |
|---|---|---|---|
| Therapeutic schemes that include plasmapheresis (n = 12) | 3/12 (25) | 5/12 (41.7) | 4/12 (33.3) |
| Plasmapheresis + rituximab (n = 8) | 3/8 (37.5) | 2/8 (25) | 3/8 (37.5) |
| Plasmapheresis alone (n = 2) | 0/2 (0) | 2/2 (100) | 0/2 (0) |
| Plasmapheresis + steroids (n = 1) | 0/1 (0) | 0/1 (0) | 1/1 (100) |
| Plasmapheresis + steroids + intravenous immunoglobulin (n = 1) | 0/1 (0) | 1/1 (100) | 0/1 (0) |
| Rituximab alone (n = 1) | 1/1 (100) | 0/1 (0) | 0/1 (0) |
| mTORi withdrawal (n = 1) | 0/1 (0) | 1/1 (100) | 0/1 (0) |
| Total of treated patients (n = 14) | 4/14 (28.6) | 6/14 (42.8) | 4/14 (28.6) |
| No treatment (n = 1) | 0/1 (0) | 0/1 (0) | 1/1 (100) |
| Total (n = 15) | 4/15 (26.7) | 6/15 (40) | 5/15 (33.3) |
Values are presented as n/N (%).
Rituximab was administered to nine patients with FSGS recurrence, combined with plasmapheresis in eight patients and as the only therapeutic measure in one. Rituximab doses were two fortnightly infusions of 375 mg/m2 in six patients, a single dose of 375 mg/m2 in two patients and four weekly doses of 375 mg/m2 in one patient. Conversion from mTOR inhibitors (mTORis) to MMF was the only therapeutic measure in one patient. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers were administered to 10 of the 15 patients (66.7%).
Proteinuria remission after these different treatments was observed in 10 of 14 patients (71.4%), being complete in 4 and partial in 6 (Table 6). All patients who responded to plasmapheresis achieved remission between 1 and 4 weeks after plasmapheresis was initiated. The addition of rituximab to plasmapheresis did not increase its efficacy (75% of remissions among patients treated with plasmapheresis without rituximab and 62.5% in plasmapheresis plus rituximab patients), although the patient who received rituximab as the only therapeutic measure showed complete remission. A partial remission was observed in the patient who was switched from mTORi to MMF, without the need for other therapeutic strategies.
Impact of FSGS recurrence on graft survival
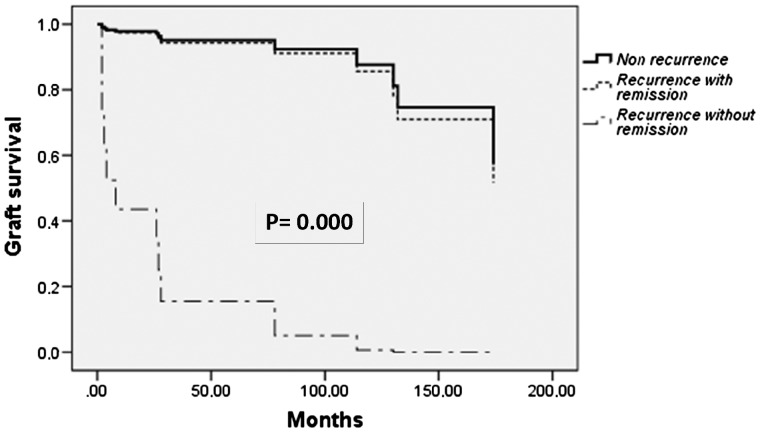
FSGS recurrence had a remarkable impact on graft survival, with 8 of 15 (53%) functioning grafts at the end of follow-up among patients who had suffered recurrences versus 69 of 78 (88.5%) in those who did not [odds ratio (OR) 4.2 (95% CI 1.8–10); P = 0.004]. Within the group of patients with FSGS recurrences, graft survival was significantly greater among patients who showed complete or partial response to treatments (80% of functioning grafts at the end of follow-up as compared with 0% among those who did not respond) (P = 0.015). As shown in Figure 2, graft survival of FSGS recurrent patients achieving proteinuria remission after treatment was similar to that of patients without recurrence, whereas graft survival was very poor among those recurrent patients without treatment response.
FIGURE 2.
Graft survival according to recurrence with remission, recurrence without remission or non-recurrence.
DISCUSSION
Recurrence of FSGS in KT occurs in approximately one-third of the patients and causes the loss of graft function in a significant number of cases [2, 5]. Therefore the identification of those subjects at risk of recurrence is of vital importance in order to implement preventive therapeutic strategies. Our data show that the presence of complete nephrotic syndrome with hypoalbuminaemia at the time of diagnosis in the native kidneys was the only significant independent risk factor to predict FSGS recurrence. Other risk factors for recurrence described in previous reports, such as younger age at FSGS diagnosis or a rapid evolution to ESRD, failed to confirm their significance in our analysis. The presence of hypoalbuminaemia reached a very high specificity, as all the cases that presented recurrence in our study showed a complete nephrotic syndrome with marked hypoalbuminaemia at the beginning of their disease. Our findings are consistent with the study of Maas et al. [16], who analysed 94 biopsy-proven FSGS patients who had received a KT. As in our study, recurrences were only observed in patients with idiopathic FSGS and nephrotic syndrome at diagnosis, with hypoalbuminaemia being the only independent predictor of recurrence [16].
The importance of the presence of complete nephrotic syndrome with hypoalbuminaemia as a predictor of future recurrences in the allograft is consistent with the pathogenesis invoked for such recurrences. Of the three major a etiological groups of FSGS (idiopathic, secondary and genetic) idiopathic cases are attributed to a hypothetical and not yet identified circulating factor that would alter the normal permeability of the glomerular capillary wall, typically causing massive proteinuria and a complete nephrotic syndrome [23–25]. However, hypoalbuminaemia is a characteristically uncommon finding in secondary FSGS [26–29]. Lastly, genetically based FSGS frequently presents with complete nephrotic syndrome, but recurrences after KT are very uncommon [30, 31].
Treatment with rituximab has proven to be effective in preventing or at least reducing the number of relapses in frequently relapsing or cortico-dependent cases of nephrotic syndrome due to minimal change disease or FSGS in native kidneys [32, 33]. In contrast, rituximab has proven ineffective in cortico-resistant nephrotic syndrome [34]. The mechanisms by which rituximab is effective in frequently relapsing or cortico-dependent nephrotic syndrome are unknown, although some studies suggest that they may be independent of the known effects of the drug on CD20 lymphocytes. It has been suggested that rituximab could regulate the activity of sphingomyelin phosphodiesterase acid-like 3b and acid sphingomyelinase [17], enzymes that are necessary for an adequate preservation of the podocyte cytoskeleton.
The possible beneficial influence of the preventive administration of rituximab to decrease the risk of FSGS recurrence after transplantation is a controversial issue [17–20]. Available information is scarce and difficult to interpret for several reasons: the concomitant use of plasmapheresis as preventive treatment in many patients, the lack of a comparable control group not treated with preventive rituximab, the lack of identification of risk factors for FSGS recurrence or the small number of patients in some studies [17–20]. In contrast, most of our patients received rituximab as the unique preventive measure and were compared with a non-treated group presenting a similar high-risk profile for FSGS recurrence. Our data show that the preventive administration of rituximab is not effective in preventing FSGS recurrence in patients at risk. In our cohort of 34 patients, all of them at risk for recurrence due to the presence of nephrotic syndrome at the time of diagnosis, there was no difference in the rate of recurrences among those who received rituximab (50% of recurrences) and the remaining patients who did not (40.9% of recurrences). Table 7 summarizes the results of studies, including ours, about the use of rituximab and/or plasmapheresis as preventive therapies for FSGS recurrence.
Table 7.
Preventive treatments for FSGS recurrence reported in literature
| Reference | Number of patients (risk factor for recurrence) | Preventive treatment (concomitant therapies) | Recurrence in treated patients (%) | Recurrence in control group |
|---|---|---|---|---|
| Auñón | 12 (hypoalbuminaemia at baseline) |
|
50 |
|
| Alasfar et al. [20] | 37 (≥2 risk factors) |
|
62 |
|
| Fornoni et al. [17] | 27 (young age, rapid progression) | Rituximab, one dose of 375 mg/m2 | 26 |
|
| Gohh et al. [35] | 10 (previous graft lost due to recurrence or rapid progression) | Plasmapheresis | 30 | No control group |
| Park et al. [19] | 9 |
|
22 | 27.7% |
| Audard et al. [18] | 4 (previous graft lost due to recurrence) |
|
0 | No control group |
Plasmapheresis has been the treatment of choice in FSGS recurrence, based on the hypothetical presence of a proteinuric permeabilizing factor that can be removed by this technique [36]. Several series of patients and meta-analysis show a rate of complete or partial remission after plasmapheresis of ∼60–70% [6, 37–39]. Our data are in agreement with these results: 12 of the 15 patients who presented proteinuria recurrence were treated with plasmapheresis and complete or partial remissions were achieved in 66% of them. Interestingly, 8 of these 12 patients also received rituximab infusions in an attempt to increase the effectiveness of plasma exchange, but their remission rates were similar to those of the remaining 4 patients treated with plasmapheresis alone or accompanied by other therapeutic measures such as corticosteroids or intravenous immunoglobulin. Overall, our data suggest that rituximab is not effective in preventing FSGS recurrence. Obviously, prospective studies are needed to definitively resolve these issues.
Our study has all the limitations inherent in retrospective analysis, so our results should be considered as preliminary. The number of patients at risk for FSGS recurrence was relatively small, which limits the statistical power to detect associations. Given the multicentric and retrospective character of the study, there was no previously agreed upon protocol for the prevention and treatment of FSGS recurrences. Another limitation could be the lack of biopsy to confirm FSGS recurrence in 5 of 15 patients. However, in patients without biopsy, the clinical presentation with complete nephrotic syndrome, the absence of DSA and the rapid response to plasmapheresis in most of them made other diagnoses, such as antibody-mediated rejection, unlikely. On the other hand, it has several strengths, including the relatively large series of FSGS patients (n = 93), who were well characterized clinically and histologically, in whom those factors predicting recurrences were investigated and identified.
Finally, our data illustrate well the importance of achieving a remission in FSGS recurrence after transplantation. Graft survival was very poor in those recurrent patients in whom remission was not achieved, whereas the prognosis of patients with remission was similar to that of patients who did not experience recurrence. On the other hand, there was a non-significant trend for a higher rate of acute rejection in the FSGS recurrent group (41.7% versus 22.7%; P = 0.421) and it could contribute to the poorer graft survival. Previous studies have also shown a higher rate of acute rejection in patients with FSGS recurrence [40], although the pathogenic mechanisms underlying this association are unknown.
In conclusion, our study suggests that low levels of serum albumin at the time of FSGS diagnosis is the main risk factor in predicting FSGS recurrence after KT. Pre-emptive treatment with rituximab seems to be ineffective in preventing recurrence in patients at risk, but further prospective studies are needed to confirm these results.
FUNDING
Work in this report was funded by the Instituto de Salud Carlos III; REDinREN (RD012/0021) and Fondo de Investigaciones Sanitarias (ISCIII/FEDER) (13/02502, ICI14/00350 and 16/01685) to M.P. M.J.P.-S. has support from a Rio Hortega-ISCIII contract 2016–17 and a grant from the Spanish Society of Nephrology. J.P. is supported by grants PI13/00598 and PI16/00619 (Spanish Ministry of Health ISCIII FIS-FEDER) and RD16/0009/0013 (ISCIII FEDER RedinRen).
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. D’Agati VD, Kaskel FJ, Falk RJ.. Focal segmental glomerulosclerosis. N Engl J Med 2011; 365: 2398–2411 [DOI] [PubMed] [Google Scholar]
- 2. Ivanyi B. A primer on recurrent and de novo glomerulonephritis in renal allografts. Nat Clin Pract Nephrol 2008; 4: 446–457 [DOI] [PubMed] [Google Scholar]
- 3. Tejani A, Stablein DH.. Recurrence of focal segmental glomerulosclerosis posttransplantation: a special report of the North American Pediatric Renal Transplant Cooperative Study. J Am Soc Nephrol 1992; 2: S258–S263 [DOI] [PubMed] [Google Scholar]
- 4. Dall’Amico R, Ghiggeri G, Carraro M.. Prediction and treatment of recurrent focal segmental glomerulosclerosis after renal transplantation in children. Am J Kidney Dis 1999; 34: 1048–1055 [DOI] [PubMed] [Google Scholar]
- 5. Briganti EM, Russ GR, McNeil JJ. et al. Risk of renal allograft loss from recurrent glomerulonephritis. N Engl J Med 2002; 347: 103–109 [DOI] [PubMed] [Google Scholar]
- 6. Lee SE, Min SI, Kim YS. et al. Recurrence of idiopathic focal segmental glomerulosclerosis after kidney transplantation: experience of a Korean tertiary center. Pediatr Transplant 2014; 18: 369–376 [DOI] [PubMed] [Google Scholar]
- 7. Sener A, Bella AJ, Nguan C. et al. Focal segmental glomerular sclerosis in renal transplant recipients: predicting early disease recurrence may prolong allograft function. Clin Transplant 2009; 23: 96–100 [DOI] [PubMed] [Google Scholar]
- 8. Hickson LJ, Gera M, Amer H. et al. Kidney transplantation for primary focal segmental glomerulosclerosis: outcomes and response to therapy for recurrence. Transplantation 2009; 87: 1232–1239 [DOI] [PubMed] [Google Scholar]
- 9. Couser W. Recurrent glomerulonephritis in the renal allograft: an update of selected areas. Exp Clin Transplant 2005; 3: 283–288 [PubMed] [Google Scholar]
- 10. Weber S, Tönshoff B.. Recurrence of focal-segmental glomerulosclerosis in children after renal transplantation: clinical and genetic aspects. Transplantation 2005; 80: S128–S134 [DOI] [PubMed] [Google Scholar]
- 11. Pardon A, Audard V, Caillard S. et al. Risk factors and outcome of focal and segmental glomerulosclerosis recurrence in adult renal transplant recipients. Nephrol Dial Transplant 2006; 21: 1053–1059 [DOI] [PubMed] [Google Scholar]
- 12. Ding WY, Koziell A, McCarthy HJ. et al. Initial steroid sensitivity in children with steroid-resistant nephrotic syndrome predicts post-transplant recurrence. J Am Soc Nephrol 2014; 25: 1342–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abbott KC, Sawyers ES, Oliver JD 3rd. et al. Graft loss due to recurrent focal segmental glomerulosclerosis in renal transplant recipients in the United States. Am J Kidney Dis 2001; 37: 366–373 [DOI] [PubMed] [Google Scholar]
- 14. Raafat R, Travis LB, Kalia A. et al. Role of transplant induction therapy on recurrence rate of focal segmental glomerulosclerosis. Pediatr Nephrol 2000; 14: 189–194 [DOI] [PubMed] [Google Scholar]
- 15. Hubsch H, Montané B, Abitbol C. et al. Recurrent focal glomerulosclerosis in pediatric renal allografts: the Miami experience. Pediatr Nephrol 2005; 20: 210–216 [DOI] [PubMed] [Google Scholar]
- 16. Maas RJ, Deegens JK, van den Brand JA. et al. A retrospective study of focal segmental glomerulosclerosis: clinical criteria can identify patients at high risk for recurrent disease after first renal transplantation. BMC Nephrol 2013; 14: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fornoni A, Sageshima J, Wei C. et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med 2011; 3: 85ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Audard V, Kamar N, Sahali D. et al. Rituximab therapy prevents focal and segmental glomerulosclerosis recurrence after a second renal transplantation. Transpl Int 2012; 25: e62–e66 [DOI] [PubMed] [Google Scholar]
- 19. Park HS, Hong Y, Sun IO. et al. Effects of pretransplant plasmapheresis and rituximab on recurrence of focal segmental glomerulosclerosis in adult renal transplant recipients. Korean J Intern Med 2014; 29: 482–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alasfar S, Matar D, Montgomery RA. et al. Rituximab and therapeutic plasma exchange in recurrent focal segmental glomerulosclerosis postkidney transplantation. Transplantation 2018; 102: e115–e120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kidney Disease: Improving Global Outcomes Glomerulonephritis Work Group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2012; 2: 139–274 [Google Scholar]
- 22. Roufosse C, Simmonds N, Clahsen-van Groningen M. et al. A 2018 reference guide to the Banff classification of renal allograft pathology. Transplantation 2018; 102: 1795–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lagrue G, Branellec A, Blanc C. et al. A vascular permeability factor in lymphocyte culture supernatants from patients with nephrotic syndrome. II. Pharmacologic and physiochemical properties. Biomedicine 1975; 23: 73–75 [PubMed] [Google Scholar]
- 24. Zimmerman SW. Increased urinary protein excretion in the rat produced by serum from a patient with recurrent focal glomerular sclerosis after renal transplantation. Clin Nephrol 1984; 22: 32–38 [PubMed] [Google Scholar]
- 25. Sharma M, Sharma R, Reddy SF. et al. Proteinuria after injection of human focal segmental glomerulosclerosis factor. Transplantation 2002; 73: 366–372 [DOI] [PubMed] [Google Scholar]
- 26. Praga M, Morales E, Herrero JC. et al. Absence of hypoalbuminemia despite massive proteinuria in focal segmental glomerulosclerosis secondary to hyperfiltration. Am J Kidney Dis 1999; 33: 52–58 [DOI] [PubMed] [Google Scholar]
- 27. Praga M, Hernández E, Morales E. et al. Clinical features and long-term outcome of obesity-associated focal segmental glomerulosclerosis. Nephrol Dial Transplant 2001; 16: 1790–1798 [DOI] [PubMed] [Google Scholar]
- 28. Kambham N, Markowitz GS, Valeri AM. et al. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 2001; 59: 1498–1509 [DOI] [PubMed] [Google Scholar]
- 29. D’Agati VD, Chagnac A, de Vries AP. et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol 2016; 12: 453–471 [DOI] [PubMed] [Google Scholar]
- 30. Felldin M, Nordén G, Svalander C. et al. Focal segmental glomerulosclerosis in a kidney transplant population: hereditary and sporadic forms. Transplant Int 1998; 11: 16–21 [DOI] [PubMed] [Google Scholar]
- 31. Jungraithmayr TC, Hofer K, Cochat P. et al. Screening for NPHS2 mutations may help predict FSGS recurrence after transplantation. J Am Soc Nephrol 2011; 22: 579–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ruggenenti P, Ruggiero B, Cravedi P. et al. Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol 2014; 25: 850–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iijima K, Sako M, Nozu K. et al. Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2014; 384: 1273–1281 [DOI] [PubMed] [Google Scholar]
- 34. Fernandez-Fresnedo G, Segarra A, González E. et al. Rituximab treatment of adult patients with steroid-resistant focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 2009; 4: 1317–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gohh RY, Yango AF, Morrissey PE. et al. Preemptive plasmapheresis and recurrence of FSGS in high-risk renal transplant recipient. Am J Transplant 2005; 5: 2907–2912 [DOI] [PubMed] [Google Scholar]
- 36. Zimmerman SW. Plasmapheresis and dipyridamole for recurrent focal glomerular sclerosis. Nephron 1985; 40: 241–245 [DOI] [PubMed] [Google Scholar]
- 37. Ponticelli C. Recurrence of focal segmental glomerular sclerosis (FSGS) after renal transplantation. Nephrol Dial Transplant 2010; 25: 25–31 [DOI] [PubMed] [Google Scholar]
- 38. Schachter ME, Monahan M, Radhakrishnan J. et al. Recurrent focal segmental glomerulosclerosis in the renal allograft: single center experience in the era of modern immunosuppression. Clin Nephrol 2010; 74: 173–181 [DOI] [PubMed] [Google Scholar]
- 39. Moroni G, Gallelli B, Quaglini S. et al. Long-term outcome of renal transplantation in adults with focal segmental glomerulosclerosis. Transpl Int 2010; 23: 208–216 [DOI] [PubMed] [Google Scholar]
- 40. Kim EM, Striegel J, Kim Y. et al. Recurrence of steroid-resistant nephrotic syndrome in kidney transplants is associated with increased acute renal failure and acute rejection. Kidney Int 1994; 45: 1440–1445 [DOI] [PubMed] [Google Scholar]