Abstract
Background
Transcellular fluid shifts during dialysis treatment could be related to the frequency and severity of intradialytic hypotension (IDH). We investigated that (i) in addition to ultrafiltration, extracellular fluid (ECF) is further depleted by transcellular fluid shifts and (ii) changes in intracellular fluid (ICF), which have been overlooked so far, or if they were considered, are not understood, might be due to these fluid shifts.
Methods
Thirty-six patients were categorized as haemodynamically stable, asymptomatic IDH or unstable (symptomatic IDH) according to their changes in systolic blood pressure and associated clinical symptoms. Their intradialytic changes in body fluids were studied using bioimpedance spectroscopy measurements and compared among groups.
Results
For IDH-prone patients, data showed a rapid drop in ECF that was more than expected from the ultrafiltration rate (UFR) profile and was associated with a significant increase in ICF (P = 0.001). Study of accumulative loss profiles of ECF revealed a loss in ECF up to 300 ml, more than that predicted from UFR for unstable patients.
Conclusions
The considerable discrepancy between the expected and measured loss in ECF might provide evidence of transcellular fluid shifts possibly induced by changes in plasma osmolarity due to haemodialysis. Moreover, the results suggest a pattern of fluid removal in IDH-prone patients that significantly differs from that in haemodynamically stable patients.
Keywords: bioimpedance spectroscopy, body fluid compartments, extracellular fluid, intradialytic hypotension, transcellular fluid shifts
INTRODUCTION
Despite continuous advances in dialysis technologies, intradialytic hypotension (IDH) remains a frequent complication of haemodialysis (HD). It is defined as a symptomatic drop in systolic blood pressure (SysBP) of >20 mmHg. The main underlying factors behind IDH are volume depletion induced by rapid removal of plasma volume with an ultrafiltration rate (UFR) higher than the plasma-refilling rate, as well as impaired compensatory mechanisms [1]. IDH has been associated with many different clinical events, such as discomfort, reduced treatment efficiency and increased risk of mortality [2].
Transcellular fluid shifts may also take place during HD treatment as a part of the fluid redistribution process and could be associated with dialysis-related complications. These fluid shifts may be induced by the rapid removal of osmotically active substances from the plasma compartment, combined with slow re-equilibration from the intracellular (IC) compartment. Many investigators have suggested that elevated plasma osmolarity may enhance the plasma refilling rate and thus provide better tolerance to variations in BP in some patients [3, 4]. On the other hand, a reduction in osmotic pressure between the extracellular (EC) and the IC compartment may induce a fluid shift in the opposite direction, towards the IC, causing further depletion of the EC volume and, thus, an additional drop in SysBP. Therefore, understanding the dynamics of these fluid shifts is important and tools are required to allow continuous monitoring of these fluid shifts during HD.
Using bioimpedance spectroscopy (BIS) techniques, namely the sum of segmental BIS (sBIS) [5, 6], this pilot study aims to investigate the relationship between the IDH and these fluid shifts. The underlying hypothesis is: (i) IDH might be also induced by transcellular fluid shifts during HD that can be monitored on the basis of estimated changes in both total-body extracellular fluid (ECF) and intracellular fluid (ICF) and (ii) changes in ICF, which have usually not been considered so far for the application of BIS during HD, or if they have, are not understood, are possibly induced by these fluid shifts into the IC compartment. This shall be achieved by analysing the BIS data using different volumetric ratios to explore the relationship between measured reduction in ECF and the applied UFR profile while looking for considerable intergroup discrepancies.
MATERIALS AND METHODS
Subjects
Thirty-six patients with end-stage renal disease undergoing HD (4 h) were studied at the dialysis unit of the Department of Nephrology, RWTH Aachen University Hospital. Dialysate fluid was adjusted to the patient’s requirements with a mean composition of 138 mmol/L sodium, 2.26 ± 0.51 mmol/L potassium, 1.25 mmol/L calcium and 5.5 mmol/L glucose. Bicarbonate (BiBag, Fresenius) was used as a dialysate buffer. The UFR was kept fixed during the treatment at 598 ± 323 mL/h, unless medical intervention was required. Prior to each treatment, the patient’s weight, height and the length and circumference of each body segment (i.e. arm, trunk and leg) were measured. Patients with human immunodeficiency viruses (HIV) or hepatitis virus C infection, pregnancy, pacemakers, amputation of a limb and/or who received a blood transfusion within a week before the measurement were excluded from this study. The study protocol was approved by the local Medical Ethics committee and all patients provided written informed consent [EK No.: EK 081-08 (Ethics Committee at the RWTH Aachen University, Faculty of Medicine)].
Measurement methodology
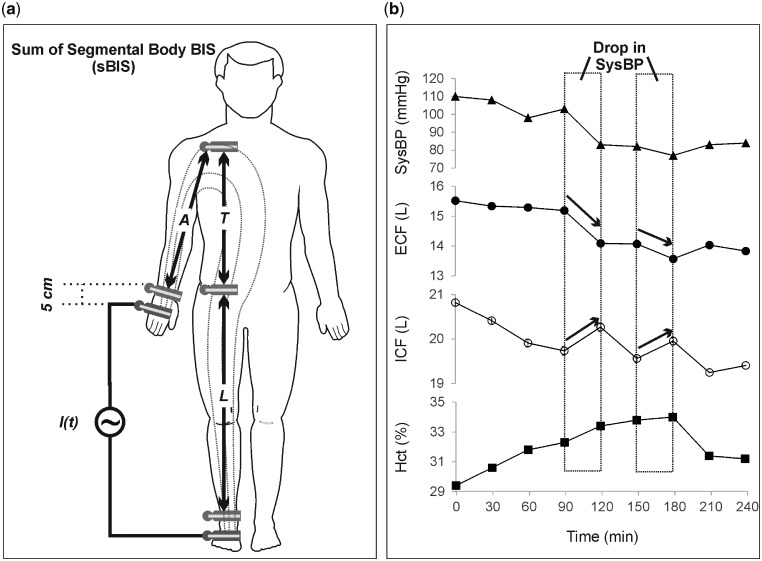
Using a commercial bioimpedance device (Xitron Hydra 4200, Xitron Technologies Inc., San Diego, CA, USA), sBIS measurements were performed with a total of six standard hydrogel–aluminium BIS electrodes. Four electrodes (two for current injection and two for voltage sensing) were located distally at the wrist and ankle, while another two sensing voltage electrodes were located at the top of the shoulder (acromion) and at the hip (anterior superior iliac spine) as shown in Figure 1a. All electrodes were placed on the non-vascular access side of the patient. Impedance data for leg, trunk and arm were recorded sequentially by manually changing the voltage measurements between each pair of the sensing electrodes. The duration of measurement for each segment was about 20 s. Segmental extracellular resistance (Re,s) and intracellular resistance (Ri,s) were extracted by fitting the measured impedance data to the Cole model [7]. Accordingly, segmental fluid volumes of the arm, leg and trunk were then estimated and used to obtain the total body ECF and ICF as described elsewhere [8–10]. Total body extracellular resistance (Re) and intracellular resistance (Ri) were obtained as the sum of all body segmental Re,s and Ri,s, respectively. Fluid overload (FO) was calculated according to Chamney et al. [11]. All measurements were recorded every 30 min in supine position throughout the dialysis treatment from start to finish. An angle of ∼30° was always maintained between the patient’s legs, and also between the patient’s arms and the trunk. Consumption of food and beverages was prohibited during the treatment. Haematocrit (Hct), blood pH, plasma electrolytes and glucose concentrations were obtained using an automated benchtop blood gas analyser (ABL800 Basic, Radiometer Medical ApS, Brønshøj, Denmark). The SysBP and diastolic blood pressures were measured around the upper arm at heart level.
FIGURE 1.
Graph showing (a) the location of the electrodes for sBIS measurements and (b) the changes in ECF and ICF associated with a decrease in SysBP in a representative patient from the unstable group (aged 69 years, pre-dialysis weight 85.5 kg and dry weight 83 kg). A, arm; T, trunk; L, leg segment.
Patient groups and data analysis
For this study, IDH was defined as a continuous drop in SysBP ≥20 mmHg. Accordingly, patients were divided into three groups as follows: haemodynamically stable, asymptomatic IDH or unstable (symptomatic IDH). The clinical symptoms and medical interventions considered relevant to IDH were diaphoresis, nausea, vomiting, oedema, cramps, blurred vision, presyncope, headache, fatigue, fluid infusion, switching off UFR and/or raising of the patient’s legs.
In addition to anthropometric characteristics, relevant parameters for group comparison were patient’s blood pressure, UFR and volume, plasma electrolytes and those obtained from BIS measurements. The difference between the groups was tested by one-way analysis of variance, Pearson’s Chi-square test or Student’s t-test, as appropriate. All analyses were carried out using SPSS version 19 (SPSS Inc., Chicago, IL, USA). Regression analysis was applied to assess the correlation between the accumulative ultrafiltration volume (UFV) and accumulative changes in ECF. Intergroup discrepancy was calculated using parameters defined on the basis of BIS and blood analysis according to the equations given below. The predicted (expected) relative change in ECF (represented by ΔECFPredic) as a result of the applied ultrafiltrate profile was calculated using Equation (1), whereas the actual measured change from BIS (expressed as ΔECFMeas) was given by Equation (2).
| (1) |
| (2) |
The osmotic activity of plasma (OsmPL) at a certain time point was estimated as the sum of Na+, K+, Ca2+, glucose and urea concentrations in plasma, according to Equation (3). Here, the factor 0.93 accounts for intermolecular attraction, which may slightly reduce the total osmotic activity as suggested elsewhere [12].
| (3) |
To test for the suitability of Equation (3), laboratory measurements of plasma osmolarity were performed on two randomly selected patients from each group (four measurements per treatment, with an interval of 1 h), and the osmotic gap (i.e. the difference between measured and calculated plasma osmolarity) was noted. Although urea is not an effective osmotic solute for almost all body cells, as it rapidly equilibrates across cell membrane using special urea channels that resemble the well-known aquaporin [13, 14], it was included in Equation (3) since it contributes to plasma osmolarity, especially in dialysis patients. Moreover, relative changes in plasma osmotic activity (ΔOsmPL in percentages) and in Re normalized for the UFV (ΔRe/UFV in percentage per litre) are introduced in Equations (4) and (5), respectively.
| (4) |
| (5) |
Here, τ is 30 min. Finally, two estimated UFV values were calculated either from BIS [UFVestm1, Equation (6)] or from the UFV measured by the dialysis machine minus fluid infusion (FI) [UFVestm2, Equation (7)] and were compared using a Bland–Altman plot.
| (6) |
| (7) |
All results are expressed as mean ± SD unless indicated otherwise. A P-value of ≤0.05 was considered statistically significant.
RESULTS
Table 1 presents the baseline physical and clinical characteristics of the 36 patients and their treatments settings. Apart from the ECF (P = 0.028), there were no significant differences between the groups at baseline with regard to their clinical characteristics or dialysis settings as given in Table 1. About 50% of the patients showed signs of pre-dialysis overhydration mainly in the legs, but with no significant difference in the amount of FO (P = 0.182) among patient groups. Table 2 presents a comparison of 1-h long time episodes with (‘hypotensive’) or without (‘control’) a drop in SysBP of ≥20 mmHg/h, which were selected from unstable patients. Significant increase in Re (P = 0.008), decrease in ECF (P = 0.020) as well as increase in ICF (P = 0.001) were detected after a hypotensive episode. Plasma potassium concentrations (P = 0.013) and Hct level (P = 0.001) were consistent with these changes.
Table 1.
Baseline clinical and physical characteristics as mean ± SD with significance (P) of the 36 patients their treatments
| Baseline characteristics | Patients with end-stage renal disease |
|||
|---|---|---|---|---|
| Stable (n = 12) | Asymptomatic (n = 12) | Unstable (n = 12) | P-value | |
| Gender, female (%) | 25.0 | 33.3 | 66.7 | 0.091 |
| Age (years) | 72 ± 16 | 72 ± 10 | 70 ± 16 | 0.957 |
| Height (cm) | 173.3 ± 7.4 | 172.1 ± 8.7 | 167.1 ± 5.1 | 0.094 |
| Weight (kg) | 77.8 ± 10.6 | 78.6 ± 16.0 | 72.6 ± 18.1 | 0.589 |
| BMI (kg/m2) | 26.0 ± 4.1 | 27.2 ± 7.0 | 26.7 ± 6.7 | 0.894 |
| Diabetes (%) | 33.3 | 16.7 | 33.3 | 0.575 |
| Dialysis duration (min) | 232.5 ± 29.0 | 252.5 ± 27.0 | 232.5 ± 26.0 | 0.134 |
| UFR (mL/h) | 593.4 ± 310.9 | 583.0 ± 332.7 | 624.1 ± 265.3 | 0.943 |
| SysBP (mmHg) | 134.5 ± 14.3 | 140.1 ± 4.5 | 127.1 ± 15.7 | 0.252 |
| ECF (L) | 20.8 ± 2.7 | 20.2 ± 5.1 | 16.8 ± 2.9 | 0.028 |
| FO (L) | 4.8 ± 2.6 | 3.9 ± 2.1 | 2.8 ± 2.8 | 0.182 |
| Body temperature (°C) | 36.1 ± 0.3 | 35.9 ± 0.7 | 36.1 ± 0.3 | 0.376 |
| O2 saturation (%) | 95.9 ± 2.0 | 96.6 ± 2.5 | 96.3 ± 2.6 | 0.789 |
| Plasma Na+ (mmol/L) | 137.9 ± 2.2 | 137.3 ± 2.9 | 138.1 ± 3.8 | 0.130 |
| Plasma K+ (mmol/L) | 4.3 ± 0.8 | 4.4 ± 0.9 | 4.7 ± 0.9 | 0.537 |
| Hct (%) | 30.8 ± 6.4 | 31.6 ± 5.1 | 32.3 ± 5.8 | 0.221 |
| Glucose (mg/dL) | 117.7 ± 30.9 | 123.1 ± 27.8 | 119.5 ± 28.2 | 0.899 |
Bold value is statistically significant (P<0.05).
Table 2.
Difference (Δ) in parameters between a pre- and post-hypotensive episode with an SysBP drop of ≥20 mmHg/h or a control episode without a SysBP drop of ≥20 mmHg/h
| Parameters | Control (SysBP drop <20 mmHg/h) | Hypotensive (SysBP drop ≥20 mmHg/h) | P-value |
|---|---|---|---|
| ΔSysBP (mmHg) | −5.8 ± 4.0 | −32.6 ± 11.4 | <0.001 |
| ΔRe (Ω) | 13.4 ± 15.8 | 50.9 ± 41.3 | 0.008 |
| ΔRi (Ω) | 29.5 ± 37.3 | 51.2 ± 83.1 | 0.418 |
| ΔECF (L) | −0.3 ± 0.3 | −0.8 ± 0.7 | 0.020 |
| ΔICF (L) | −0.2 ± 0.3 | 0.2 ± 0.2 | 0.001 |
| ΔNa+ (mmol/L) | 0.5 ± 1.15 | 0.3 ± 0.6 | 0.514 |
| ΔK+ (mmol/L) | −0.2 ± 0.3 | −0.7 ± 0.6 | 0.013 |
| ΔCa2+ (mmol/L) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.503 |
| ΔHct (%) | 0.1 ± 1.1 | 2.0 ± 1.4 | 0.001 |
A total of 12 hypotensive episodes and another 12 control episodes were selected from patients of the unstable group and used for the comparison. All values are expressed as mean ± SD. Bold values are statistically significant (P<0.05).
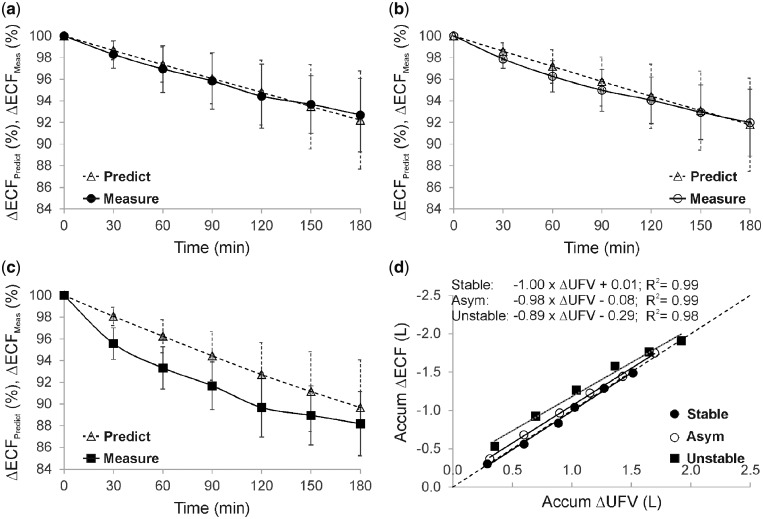
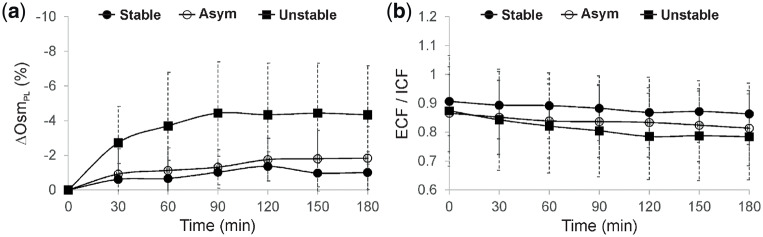
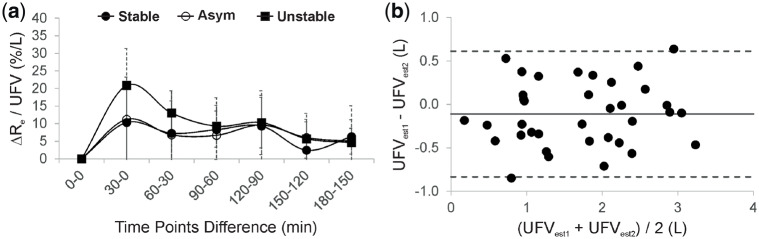
The location of the electrodes for the BIS measurements is illustrated in Figure 1a. A representative example from an unstable is given in Figure 1b to show the changes in ECF, ICF and Hct associated with a decrease in SysBP. Figure 2a–c compares the ‘measured’ changes in ECF (calculated using BIS measurements) with the ‘predicted’ changes (as expected from the UFV curve) among patient groups. Significant differences between predicted and measured loss in ECF were observed between the groups at fixed time points, e.g. at t = 30 min: P < 0.001, and t = 60 min: P = 0.001. Figure 2d shows the relationship between accumulative changes in ECF and accumulative changes in the UFV. The reduction in ECF within unstable patients was higher in comparison with others when normalized to UFV. The relative change in plasma osmotic activity during treatment is shown in Figure 3a); here, plasma osmolarity was calculated according to Equation (2). Osmotic gaps of 4.5 ± 1.6 (stable), 4.4 ± 4.0 (asymptomatic) and 7.9 ± 5.2 (unstable) were found. Figure 3b shows the changes in ECF to ICF ratio for the three patient groups as a function of treatment time. The relative change in ΔRe/UFV in (%/L) is given in Figure 4a. The Bland–Altman plot shows good agreement (bias: −0.11 ± 0.37 L) between changes in the UFV calculated from BIS method (UFVestm1) and those directly recorded from the dialysis machine (UFVestm2) between the time points 0 and 180 min (Figure 4b).
FIGURE 2.
Graph showing (a–c) predicted [Predict from Equation (1) as dotted line] and measured [Measure from Equation (2) as solid line] changes in ECF calculated from the bioimpedance measurements and presented as mean ± SD for the three patient groups, and (d) the accumulative changes (losses) in ECF associated with accumulative UFV as recorded by the dialysis machine during treatment. Data points were taken at the time points 30, 60, 90, 120, 150 and 180 min, respectively, from the start of dialysis. SD lines were omitted from the graph in (d) to maintain visual clarity; max SD was 0.87 for accumulated ΔUFV and 0.93 for accumulated ΔECF.
FIGURE 3.
Trends (mean ± SD) of (a) relative change in plasma osmotic activity (ΔOsmPL) in (%) and (b) the ratio of ECF to ICF during the first 3 h of the treatment for all groups.
FIGURE 4.
Graph showing (a) the relative change in total body extracellular resistance (ΔRe) normalized by UFV , which was removed during the corresponding time interval, for all patient groups and (b) Bland–Altman plot showing the difference (ΔUFV) between UFVestm1 and UFVestm2 plotted against their mean value. The solid line represents the mean value of ΔUFV and the dashed lines are the mean ± 2 SD.
DISCUSSION
Our results showed that the EC in unstable patients is depleted more than expected from applied dialysis settings. If compared with their accumulative UFV during treatment, a substantial fluid up to 300 mL was showed to move from the EC to the IC compartment in these patients. ΔRe/UFV ratios have also reflected similar behaviour, considering that these ratios are not subject to the same inaccuracies arising from estimating body volumes. Furthermore, data confirm that patients at risk of IDH cannot be simply identified from baseline characteristics.
At baseline, clinical characteristics of the patients and their treatment settings did not differ significantly among groups. Pre-dialytic ECF levels were, however, generally lower among patients of symptomatic group. Wizemann et al. [15] reported the influence of hydration state on the observed changes in plasma volume during ultrafiltration. They showed that the more compliant interstitium in overhydrated patients might enhance the interstitial hydrostatic pressure during dialysis and thus better preserve the plasma volume. Although this lower ECF value may have contributed to symptomatic IDH in this cohort, it neither contradicted the overall outcome of this study nor solely illustrated the mechanisms of IDH discussed here.
During dialysis, a rapid reduction in EC osmolarity due to ultrafiltrate, without an equivalent drop in IC osmolarity, may build-up an osmotic gradient favouring a fluid shift towards the IC compartment [12, 16]. To test this hypothesis, the first step was to compare the profiles of ECF losses among patient groups. Normally, the UFV is depicted as the sum of losses in both ECF and ICF volume. Figure 1b introduces a representative example from an unstable patient that points in a different direction, in which an episode of severe SysBP drop may have been accompanied by a fluid shift from the EC to the IC compartment. To further investigate this point, hypotensive episodes were compared with control ones selected from unstable patients. If multiple hypotensive episodes occurred for the same patient during the same dialysis session, only the first episode was considered. Subsequent hypotensive episodes were excluded to avoid the possible influence of the applied interventions on the measured parameters. Data analysis revealed that a significant loss in ECF (P = 0.020) was accompanied by an increase in ICF (P = 0.001) after an abrupt drop in SysBP ≥20 mmHg/h, suggesting that a sort of transcellular fluid shift towards IC may be concurrent with IDH. Figure 2a–c compares the expected loss in ECF (as predicted from the UFV profile) to the measured loss in ECF (as estimated from BIS data) among patients groups. For stable patients, the measured loss in ECF followed that predicted from UFV with an equivalent slope throughout HD. IDH-prone patients, however, were characterized by a rapid loss in measured ECF, more than expected from the UFR profile, until the point where medical intervention was required. We considered these large intergroup discrepancies as a sign of a different body fluid redistribution mechanism characterizing unstable patients, especially during the first half of HD. To translate this observation into numeric variables, the accumulative reduction in ECF was investigated in comparison with the collected volume of the ultrafiltrate during HD (Figure 2d). The constant term in the fitted (linear) equations represents the contribution from the IC compartment, while the signs indicate the direction of fluid shift: (−) from EC to IC, and (+) from IC to EC compartments. For unstable patients, ECF is clearly depleted by both UFV and fluid shifts towards the IC, while patients at mid- to no-risk of IDH had a positive shift from the IC compartment. Note that the fitted equations cannot be generalized, as some patients develop a relatively significant increase in ECF at a very low UFV, which was not valid for all patients. Moreover, the amount of transcellular fluid shift is not constant, as it may vary in response to various conditions.
The next step was to study the variation in osmotic activity within the plasma pool for each patient group (Figure 3a). For all patients, plasma osmolarity decreased during dialysis due to the removal of osmotically active solutes by ultrafiltration. However, larger (negative) changes were observed for unstable patients compared with stable ones, taking into account that a relatively similar dialysate fluid was applied to all patients. These rapid osmotic changes are in support of our initial hypothesis. Similar observations have been already reported by others [17–19]. In addition to the induced transcellular fluid shifts, rapid changes in plasma osmolarity may suppress the release of vasopressin, a hormone responsible for increasing the peripheral vascular resistance and arterial blood pressure [20]. It is worth noting that half of the unstable patients in this study were diabetic. This may influence how their body reacts to changes in plasma osmolarity. Previous works [21–24] reported that diabetes mellitus may affect the body fluid distribution and reduces patient tolerance to hypovolaemia. Furthermore, the development of IDH itself or the applied medical interventions may change the fluid distribution status in these patients. This could explain the plateau/increase in plasma osmolarity observed later for the unstable group, during the second half of the treatment.
Finally, additional volumetric ratios, calculated on the basis of BIS, were used to characterize unstable patients. Figure 3b shows the difference in ECF/ICF ratios between the groups. Nearly all patients in this study were characterized by abnormally high ratios at baseline, which indicate either ECF volume overload due to overhydration, or decreased ICF volume due to malnutrition [25]. During treatment, ECF is gradually reduced by the UFR and, thus, the ECF/ICF ratio is also decreased; this was the case for all three groups. However, the faster reduction in unstable group may again support our hypothesis that a substantial fraction of ECF is shifted to IC compartment in unstable patients. Nevertheless, due to the limited accuracy of ICF measurements provided by current BIS methods and an induced ECF/ICF variability due to patient characteristics (e.g. obesity [26]), a more careful interpretation of this particular parameter is required. Also, due to our applied methodology, it was not possible to clearly differentiate between the influence of overhydration and of malnutrition on the ECF/ICF ratios.
The relative change in Re could be another way to identify patients at risk for IDH, without being subject to possible inaccuracies arising from calculation of ECF and ICF volumes. When normalized for UFV, the influence of the different UFV profiles used on Re is reduced. Figure 4a shows that unstable symptomatic patients have an overall higher reduction in their ΔRe/UFV in comparison with others, regardless of applied UFR settings.
A limitation of the study is the small number of participants. However, our findings were consistent among groups, suggesting that our hypothesis of additional ECF depletion due to transcellular fluid shift may be correct. Precise placement of the electrodes at the boundaries between body segments is very important when applying sBIS to insure reproducibility. This, however, was not always possible, especially in patients with an extreme body mass index (BMI). To gain more insight into the accuracy of the applied method in this study, a Bland–Altman plot was generated from different UFV estimates (Figure 4b). The difference in estimated UFV computed from BIS and that obtained directly from the dialysis machine was −0.11 ± 0.37L, which is within an acceptable range. The clinical characteristics of the patients (e.g. nutritional/health status and response of compensatory mechanisms) should also not be overlooked, as they may alter the pathophysiology of fluid removal during HD. These factors should all be taken into account in future studies.
This pilot study demonstrates the feasibility of applying BIS techniques to monitor transcellular fluid shifts during HD. Although the results did not provide actual quantitative measurements of these fluid shifts, they were able to qualitatively describe the direction of these fluid shifts and correlate them to the changes in both ECF and ICF among patient groups. The results have also indicated that not only Re but also the Ri from the Cole model seems to yield useful information for continuous assessment of body fluid during HD. Nevertheless, due to the small sample size, the outcome of our study is still at the research stage and can be considered a support of our hypothesis. However, larger trials are required before any clinical conclusions can be made.
FUNDING
This work was supported by a grant from the RWTH Exploratory Research Space (ERS) funded by the German Excellence Initiative.
CONFLICT OF INTEREST STATEMENT
The results presented in this article have not been published previously in whole or part, except in abstract format. J.F. and S.L. have received research grants from RWTH Exploratory Research Space (ERS) – German Excellence Initiative.
REFERENCES
- 1. Daugirdas JT. Pathophysiology of dialysis hypotension: an update. Am J Kidney Dis 2001; 38: S11–S17 [DOI] [PubMed] [Google Scholar]
- 2. Shoji T, Tsubakihara Y, Fujii M. et al. Hemodialysis-associated hypotension as an independent risk factor for two-years mortality in hemodialysis patients. Kidney Int 2004; 66: 1212–1220 [DOI] [PubMed] [Google Scholar]
- 3. Depner TA. Assessing adequacy of hemodialysis: urea modeling. Kidney Int 1994; 45: 1522–1535 [DOI] [PubMed] [Google Scholar]
- 4. Kimura G, Van Stone JC, Bauer JH. et al. A simulation study on transcellular fluid shifts induced by hemodialysis. Kidney Int 1983; 24: 542–548 [DOI] [PubMed] [Google Scholar]
- 5. Zhu F, Schneditz D, Wang E. et al. Dynamics of segmental extracellular volumes during changes in body position by bioimpedance analysis. J Appl Physiol (1985) 1998; 85: 497–504 [DOI] [PubMed] [Google Scholar]
- 6. Zhu F, Schneditz D, Levin NW.. Sum of segmental bioimpedance analysis during ultrafiltration and hemodialysis reduces sensitivity to changes in body position. Kidney Int 1999; 56: 692–699 [DOI] [PubMed] [Google Scholar]
- 7. Cole K, Cole R.. Dispersion and absorption in dielectrics. I. alternating-current characteristics. J Chem Phys 1941; 9: 341–351 [Google Scholar]
- 8. De Lorenzo A, Andreoli A, Matthie J. et al. Predicting body cell mass with bioimpedance by using theoretical methods: a technological review. J Appl Physiol 1997; 82: 1542–1558 [DOI] [PubMed] [Google Scholar]
- 9. Fenech M, Jaffrin MY.. Extracellular and intracellular volume variations during postural change measured by segmental and wrist-ankle bioimpedance spectroscopy. IEEE Trans Biomed Eng 2004; 51: 166–175 [DOI] [PubMed] [Google Scholar]
- 10. Matthie JR. Second generation mixture theory equation for estimating intracellular water using bioimpedance spectroscopy. J Appl Physiol 2005; 99: 780–781 [DOI] [PubMed] [Google Scholar]
- 11. Chamney PW, Wabel P, Moissl UM. et al. A whole-body model to distinguish excess fluid from hydration of major body tissues. Am J Clin Nutr 2007; 85: 80–89 [DOI] [PubMed] [Google Scholar]
- 12. Ursino M, Colí L, Brighenti C. et al. Prediction of solute kinetics, acid-base status, and blood volume changes during profiled hemodialysis. Ann Biomed Eng 2000; 28: 204–216 [DOI] [PubMed] [Google Scholar]
- 13. Brahm J. Urea permeability of human red cells. J Gen Physiol 1983; 82: 1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao D, Sonawane ND, Levin MH. et al. Comparative transport efficiencies of urea analogues through urea transporter UT-B. Biochim Biophys Acta 2007; 1768: 1815–1821 [DOI] [PubMed] [Google Scholar]
- 15. Wizemann V, Leibinger A, Mueller K. et al. Influence of hydration state on plasma volume changes during ultrafiltration. Artif Organs 1995; 19: 416–419 [DOI] [PubMed] [Google Scholar]
- 16. Fernandez de Canete J, Del Saz Huang P.. First-principles modeling of fluid and solute exchange in the human during normal and hemodialysis conditions. Comput Biol Med 2010; 40: 740–750 [DOI] [PubMed] [Google Scholar]
- 17. Mann H, Stiller S.. Urea, sodium, and water changes in profiling dialysis. Nephrol Dial Transplant 1996; 11 (Suppl 8): 10–15 [DOI] [PubMed] [Google Scholar]
- 18. Henrich WL, Woodard TD, Blachley JD. et al. Role of osmolality in blood pressure stability after dialysis and ultrafiltration. Kidney Int 1980; 18: 480–488 [DOI] [PubMed] [Google Scholar]
- 19. Rodrigo F, Shideman J, McHugh R. et al. Osmolality changes during hemodialysis. Natural history, clinical correlations, and influence of dialysate glucose and intravenous mannitol. Ann Intern Med 1977; 86: 554–561 [DOI] [PubMed] [Google Scholar]
- 20. Van der Zee S, Thompson A, Zimmerman R. et al. Vasopressin administration facilitates fluid removal during hemodialysis. Kidney Int 2007; 71: 318–324 [DOI] [PubMed] [Google Scholar]
- 21. Basu A, Jensen MD, McCann F. et al. Effects of pioglitazone versus glipizide on body fat distribution, body water content, and hemodynamics in type 2 diabetes. Diabetes Care 2006; 29: 510–514 [DOI] [PubMed] [Google Scholar]
- 22. Brunet P, Saingra Y, Leonetti F. et al. Tolerance of haemodialysis: a randomized cross-over trial of 5-h versus 4-h treatment time. Nephrol Dial Transplant 1996; 11 (Suppl 8): 46–51 [DOI] [PubMed] [Google Scholar]
- 23. Low PA, Benrud-Larson LM, Sletten DM. et al. Autonomic symptoms and diabetic neuropathy: a population-based study. Diabetes Care 2004; 27: 2942–2947 [DOI] [PubMed] [Google Scholar]
- 24. Shideman JR, Buselmeier TJ, Kjellstrand CM.. Hemodialysis in diabetics: complications in insulin-dependent patients accepted for renal transplantation. Arch Intern Med 1976; 136: 1126–1130 [DOI] [PubMed] [Google Scholar]
- 25. Lin YP, Yu WC, Hsu TL. et al. The extracellular fluid-to-intracellular fluid volume ratio is associated with large-artery structure and function in hemodialysis patients. Am J Kidney Dis 2003; 42: 990–999 [DOI] [PubMed] [Google Scholar]
- 26. Mingrone G, Bertuzzi A, Capristo E. et al. Unreliable use of standard muscle hydration value in obesity. Am J Physiol Endocrinol Metab 2001; 280: E365–E371 [DOI] [PubMed] [Google Scholar]