Abstract
Background
Tubular injury plays a critical role in the development of diabetic nephropathy (DN), but current DN therapies do not combat tubular injury. This study was conducted to investigate if tumor necrosis factor (TNF)-α inhibition protects against tubular injury in diabetic rats and to examine the associated mechanisms.
Methods
Kidney biopsy tissues were collected and analyzed from 12 patients with DN and 5 control subjects. Streptozotocin (STZ)-induced diabetic rats were treated with a TNF-α inhibitor for 12 weeks. Renal function, albuminuria, histological injury, renal TNF-α messenger RNA (mRNA) and the NOD- (nucleotide-binding), LRR- (domain-like receptor) and pyrin domain-containing protein 3 (NLRP3) inflammasome were assessed.
Results
Diabetic patients with tubulointerstitial injury (TIN) presented with higher renal tubular expression of TNF-α mRNA and the NLRP3 inflammasome (P < 0.05). TNF-α inhibition reduced albuminuria, glomerular injury and tubular injury in STZ-induced diabetic rats (P < 0.05). Importantly, TNF-α inhibition significantly reduced the NLRP3 inflammasome in tubules (P < 0.05). Moreover, TNF-α inhibition decreased expression of tubular interleukin (IL)-6 and IL-17A mRNA.
Conclusions
TNF-α inhibition protects against TIN by suppressing the NLRP3 inflammasome in DN rats. Future studies may focus on the clinical protective effects of TNF-α inhibition using prospective observation.
Keywords: diabetic nephropathy, inflammasome, NLRP3, TNF-α, tubulointerstitial injury
INTRODUCTION
Diabetic nephropathy (DN) is the most common cause of end-stage renal disease (ESRD) in developed countries, with around one-third of diabetic patients developing nephropathy [1]. A better understanding of DN pathogenic mechanisms is direly needed to identify new therapeutic targets for DN. Although glomerulosclerosis is recognized as a primary feature of DN, tubular injury in DN is closely associated with increased risk of renal function decline [2]. Tubular injury can appear in early stages of DN and may have a crucial role in the early progression of DN [3]. Although the mechanism of tubular injury in diabetes is complex, it is known that key extracellular conditions contribute to damage in proximal tubules including hyperglycemia, proteinuria, hypoxia and inflammation [4, 5]. Tubular injury-related therapies could be an important targeted intervention for DN in the future.
It is well-recognized that NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome activation aggravates DN [6]. Moreover, the NLRP3 inflammasome plays a crucial role in tubular injury and renal fibrosis. NLRP3 deletion protects against renal fibrosis in mice models with 5/6 nephrectomy or unilateral ureteral obstruction [7, 8]. Additionally, high glucose induces NLRP3 inflammasome activation in HK-2 cells [9]. Thus, the NLRP3 inflammasome may be crucial to tubulointerstitial injury (TIN) development in DN.
Tumor necrosis factor (TNF)-α is a pleiotropic cytokine involved in inflammation induction, apoptotic cell death and immune modulation [10]. Moreover, TNF-α is an important transcriptional regulator of NLRP3 inflammasome components [11, 12]. Serum TNF-α and TNF receptors are increased in DN patients and are predictive of renal decline and incidence of ESRD [13–15]. Previous studies have reported that TNF-α inhibition could significantly alleviate diabetic glomerular lesions in diabetic rats [16, 17]. However, the protective effect of TNF-α inhibition on TIN in diabetes has not been investigated. In our preliminary study, diabetic patients with TIN presented with higher messenger RNA (mRNA) expression of TNF-α and NLRP3 inflammasome in renal tubules compared with DN patients without TIN. DN patients with TIN were defined as diabetic glomerular lesions with tubular–interstitial lesions scored more than 1. In the present study, we focused on whether TNF-α inhibition protected against renal TIN in diabetes and examined potential mechanisms. We hypothesized that TNF-α inhibition protects against TIN by suppressing the NLRP3 inflammasome in diabetic rats.
MATERIALS AND METHODS
Human renal biopsies
This study was approved by the ethics committee of Shanghai Eighth People’s Hospital. Kidney biopsy tissues were collected from 12 patients with DN and 5 control patients with histologically normal tissue surrounding carcinoma. The control patients did not have diabetes, hypertension or other kidney diseases. Informed consent was obtained from all subjects. Patient demographic data were recorded including age, ethnicity, gender and diabetes duration. Clinical laboratory values were recorded including hemoglobin, glycated hemoglobin, proteinuria and serum creatinine (SCr). TIN was evaluated using a semiquantitative scoring method [18, 19]. TIN was defined as tubular dilation and/or atrophy, tubular interstitial fibrosis or inflammatory cell infiltration. Briefly, TIN was graded from 0 to 3 as follows: 0, normal tubulointerstitium; 1, involving <25% of total area; 2, involving 25–50% of total area; and 3, involving >50% of total area.
According to renal histology appearance, DN patients were divided into two groups: DN group without TIN and DN with TIN group. DN without TIN was defined as diabetic glomerular lesion with few tubulointerstitial lesions scored less than 1. DN combined with TIN was defined as glomerular injury with tubulointerstitial lesions scored more than 1.
Animal model
Animal studies were approved by the Animal Care and Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. All procedures were performed in accordance with the policies for the Care and Use of Laboratory Animals of our institution. Male Sprague–Dawley rats (weighing 250 ± 20 g) were provided by Shanghai Science Academy Animal Center (Shanghai, China). Diabetes was induced by an intraperitoneal (i.p.) injection of streptozotocin (a single dose: streptozotocin (STZ) 65 mg/kg; Sigma-Aldrich, St Louis, MO, USA). Diabetes was confirmed by blood glucose level >16.7 mM at 72 h following STZ injection. Rats were divided into four groups (n = 6 each): normal control group, rats treated with vehicle; STZ-induced diabetic rats treated with same volume of vehicle; STZ-induced diabetic rats with control immunoglobulin G (IgG) (Zhongkang Biotech, Hangzhou, China); and STZ-induced diabetic rats with Humira, a human anti-TNF monoclonal antibody (Abbott, Abbott Park, IL, USA) injected i.p. at 1.0 mg/kg/week. Humira treatment was initiated 7 days after STZ injection and continued weekly for 12 weeks. At the end of the experiments, a 24-h urine collection was conducted using metabolic cages. Blood and renal tissues were collected for analysis. The blood urea nitrogen (BUN), SCr and urine albumin were measured using enzyme-linked immunosorbent assay (ELISA) kits (Abcam, Cambridge, MA, USA). The ratio of kidney weight to body weight was calculated for the kidney weight index.
Histological injury assessment
The paraformaldehyde-fixed kidneys were embedded and then cut into 3 μm sections. Periodic acid–Schiff (PAS) staining was performed to assess histological alterations. As previously described [20], mesangial area was determined by the presence of PAS-positive and nuclei-free areas in the mesangium. Mesangial expansion index, expressed as the fraction of area of mesangial matrix area to glomerular area, was quantitatively measured using the software of ImageJ. The semiquantitative tubular injury scoring methods described above were used to assess TIN.
Measurement of inflammation and oxidative stress markers
Renal malondialdehyde (MDA) and superoxide dismutase (SOD) levels were measured using commercial kits according to the manufacturer’s protocol (Nanjingjianchen, Nanjing, Jiangsu, China). Serum P-selectin and C-reactive protein (CRP) were measured using ELISA assay kits (Shanghai Immune Biotech, China). Renal caspase 1 activity was measured using a commercial colorimetric assay Kit (BioVision, Mountain View, CA, USA) following the manufacturer’s instructions. Renal interleukin (IL)-1β expression was measured using an ELISA kit (Invitrogen, Carlsbad, CA, USA).
Western analysis and quantitative real-time PCR
The glomeruli and tubulointerstitial tissues were isolated as previously described [21] and then stored at −80°C. Western blot was conducted as described previously [22]. The primary antibodies were anti-NLRP3 (Abcam) and anti-β-actin (Proteintech, Chicago, IL, USA). Quantitative real-time polymerase chain reaction (PCR) was conducted as described previously [22]. The specific primers were as follows: human IL-1β: 5ʹ ccccagcccttttgttgag 3ʹ (forward) and 5ʹ ggcgggctttaagtgagtagg 3ʹ (reverse); human NLRP3: 5ʹ agccccgtgagtcccatta 3ʹ (forward) and 5ʹ cgcccagtccaacatcatct 3ʹ (reverse); human TNF-α: 5ʹ ccacggctccaccctctc 3ʹ (forward) and 5ʹ gtcccggatcatgctttcagt 3ʹ (reverse); rat IL-1β: 5ʹ gggcctcaaggggaagaa 3ʹ (forward) and 5ʹ agctgcagggtgggtgtg 3ʹ (reverse); rat IL-6: 5ʹ agcccaccaggaacgaaagt 3ʹ (forward) and 5ʹ caacaacatcagtcccaagaagg 3ʹ (reverse); rat IL-17A: 5ʹ cctgatgctgttgctgctactg 3ʹ (forward) and 5ʹ cctcggcgtttggacaca 3ʹ (reverse); rat IL-18: 5ʹ aacagccaacgaatcccagac 3ʹ (forward) and 5ʹ ggtagacatccttccatccttcac 3ʹ (reverse); rat monocyte chemotactic protein 1: 5ʹ gaatgtgaagttgacccgtaaatct 3ʹ (forward) and 5ʹ taaggcatcacagtccgagtca 3ʹ (reverse); rat P-selectin: 5ʹ tggcgccttggcttctact 3ʹ (forward) and 5ʹagggggcattttccatcatc 3ʹ (reverse); transforming growth factor-β1 (TGF-β1): 5ʹ accgcaacaacgcaatctatg 3ʹ (forward) and 5ʹ cactgcttcccgaatgtctga 3ʹ (reverse); rat TNF-α: 5ʹ aggcgctccccaaaaagat 3ʹ (forward) and 5ʹ caccccgaagttcagtagacaga 3ʹ (reverse) and 18 s rRNA: 5ʹ cggctaccacatccaaggaa 3ʹ (forward) and 5ʹ cctgtattgttatttttcgtcactacct 3ʹ (reverse).
Statistical analysis
Data were expressed as mean ± SEM. One-way ANOVA was performed for parametric data comparison and Kruskal–Wallis test was used for nonparametric data comparison. The Chi-squared test was used for prevalence data comparison. Statistical analysis was calculated by SPSS software (Ver. 19.0) (IBM, Armonk, NY, USA). P < 0.05 was considered statistically significance.
RESULTS
Renal tubular TNF-α and NLRP3 increased in diabetic patients with TIN
There are 12 subjects with DN and 5 control subjects enrolled in this study. According to the renal histology, DN patients were divided into two groups: DN without TIN group (n = 6) and DN with TIN group (n = 6). The ethnicity of all the 17 subjects was Chinese Han. The basic clinical information is presented in Table 1. There were no significant differences in proteinuria between groups (Figure 1A). SCr was slightly elevated in diabetic patients with TIN compared with the DN group (Figure 1B). Although DN combined with TIN group exhibited similar levels of mesangial expansion to the DN group, the tubular scores were significantly higher than that in the DN group (P < 0.05) (Figure 1C and D).
Table 1.
Characterization of patients with DN
| Group | Ethnicity | Gender (male/female) | Age (years) | SBP (mmHg) | DBP (mmHg) | HbA1c (%) |
|---|---|---|---|---|---|---|
| Control | Chinese, Han | 3/2 | 55.8 ± 4.1 | 133.6 ± 2.5 | 79.8 ± 3.6 | 5.6 ± 0.4 |
| DN | Chinese, Han | 4/2 | 58.5 ± 3.3 | 143.3 ± 4.8 | 83.5 ± 5.3 | 7.3 ± 0.5* |
| DN with TIN | Chinese, Han | 4/2 | 60.3 ± 3.5 | 147.8 ± 7.5 | 86.8 ± 2.4 | 7.6 ± 0.4* |
Data were expressed as mean ± SEM. SBP, systolic blood pressure; DBP, diastolic blood pressure.
P < 0.05 versus control group.
FIGURE 1.
Clinical and renal histology characteristics of diabetic TIN patients. (A) Ratio of albuminuria to creatinine; (B) SCr; (C) representative sections for mesangial expansion and semiquantitative analysis; hematoxylin and eosin (HE) staining, original magnification, ×400; and (D) representative sections for tubular injury and semiquantitative analysis; HE staining, original magnification, ×400. Data were expressed as mean ± SEM. *P < 0.05 versus control and #P < 0.05 versus DN.
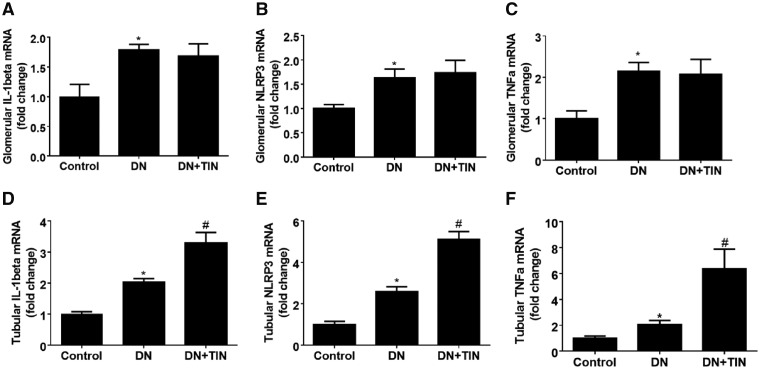
Compared with control subjects, all DN patients had higher renal TNF-α, NLRP3 and IL-1β mRNA expression in both glomeruli and tubules (Figure 2). Importantly, compared with DN patients (without TIN), renal tubular TNF-α, NLRP3 and IL-1β mRNA expressions were significantly increased in the TIN group, while there was no difference in glomeruli (P < 0.05) (Figure 2). Thus, diabetic patients with TIN presented with higher renal tubular expression of TNF-α mRNA and NLRP3 inflammasome.
FIGURE 2.
Renal tubular expression of TNF-α and NLRP3 mRNA increased in diabetic TIN patients. (A) Glomerular IL-1β mRNA; (B) glomerular NLRP3 mRNA; (C) glomerular TNF-α mRNA; (D) tubular IL-1β mRNA; (E) tubular NLRP3 mRNA; and (F) tubular TNF-α mRNA. Data were expressed as mean ± SEM. *P < 0.05 versus control and #P < 0.05 versus DN.
TNF-α inhibition reduces renal injury in DN rats
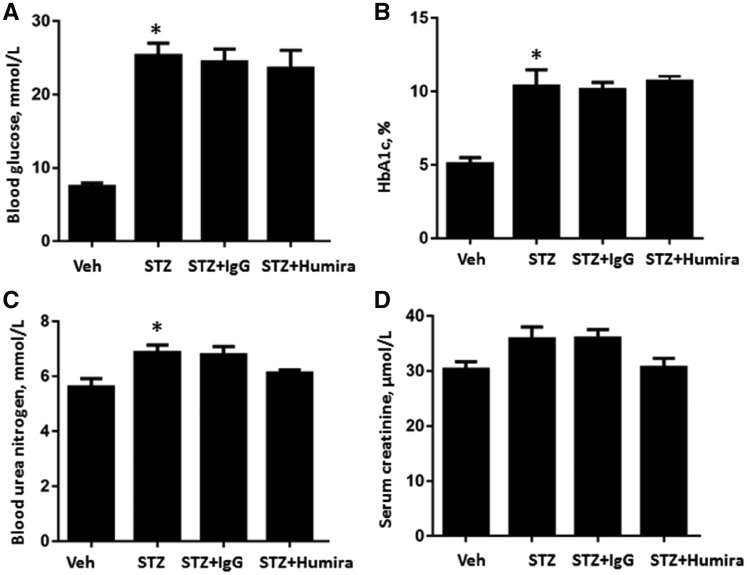
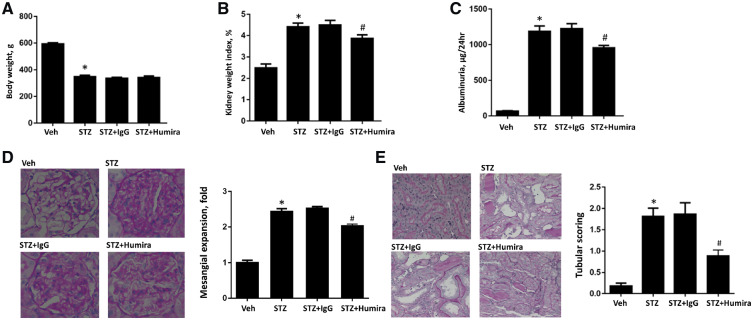
A STZ-induced DN rat model was used to assess the effects of TNF-α inhibition and examine the underlying mechanisms. STZ-induced DN rats had increased blood glucose, HbA1c, SCr, BUN, kidney weight index and albuminuria and reduced body weight at 12 weeks compared with the control group (Figures 3 and 4). TNF-α inhibition had no effect on blood glucose and HbA1c levels (Figure 3A and B). Additionally, there was no significant difference in BUN and SCr between the IgG control antibody-treated DN group and the TNF-α inhibition treatment group (Figure 3C and D). Compared with STZ-induced rats + IgG group, treatment with TNF-α inhibition decreased kidney weight index (P < 0.05) (Figure 4B). Moreover, TNF-α inhibition significantly decreased albuminuria (P < 0.05) (Figure 4C).
FIGURE 3.
TNF-α inhibition had no effect on blood glucose and HbA1c levels in diabetic rats. STZ-induced diabetic rats were treated with TNF-α antibody or normal control IgG and tissue was collected after 12 weeks. (A) Blood glucose; (B) HbA1c; (C) BUN; and (D) SCr. Data were expressed as mean ± standard error. *P < 0.05 versus vehicle control.
FIGURE 4.
TNF-α inhibition alleviated renal injury in diabetic rats. STZ-induced diabetic rats were treated with TNF-α antibody or normal control IgG and tissue were collected after 12 weeks. (A) Body weight; (B) kidney weight index; (C) albuminuria; (D) representative sections for mesangial expansion and semiquantitative analysis; PAS staining, original magnification, ×400; and (E) representative sections for tubular injury and semiquantitative analysis; PAS staining, original magnification, ×400. Data were expressed as mean ± SEM. *P < 0.05 versus vehicle control and #P < 0.05 versus STZ + IgG.
Consistent with the biochemistry data, TNF-α inhibition reduced mesangial expansion, which was a measure of histological glomerular injury in DN rats (P < 0.05) (Figure 4D). Importantly, TNF-α inhibition significantly attenuated tubular injury (P < 0.05) (Figure 4E). Therefore, TNF-α inhibition could alleviate both histologic glomerular injury and tubular injury in DN rats.
TNF-α inhibition alleviated renal inflammation and oxidative stress in DN rats
Inflammatory response and oxidative stress are involved in the pathogenesis of DN. As shown in Figure 5, compared with STZ-induced rats + IgG group, TNF-α inhibition significantly reduced serum CRP (Figure 5A). Meanwhile, TNF-α inhibition decreased TGF-β and P-selectin in both serum and renal cortex (Figure 5B, C and F). TNF-α inhibition decreased renal MDA levels and increased renal SOD levels (Figure 5D and E). Therefore, TNF-α inhibition attenuates kidney injury in diabetic rats in part through suppression of inflammation activation and oxidative stress.
FIGURE 5.
TNF-α inhibition decreased renal inflammation and oxidative stress in diabetic rats. STZ-induced diabetic rats were treated with TNF-α antibody or normal control IgG and tissue were collected after 12 weeks. (A) Serum CRP; (B) serum P-selectin; (C) renal cortical P-selectin mRNA; (D) renal cortical MDA; (E) renal cortical SOD; and (F) renal cortical TGF-β1 mRNA. Data were expressed as mean ± SEM. *P < 0.05 versus vehicle control and #P < 0.05 versus STZ + IgG.
TNF-α inhibition attenuated renal NLRP3 inflammasome in DN rats
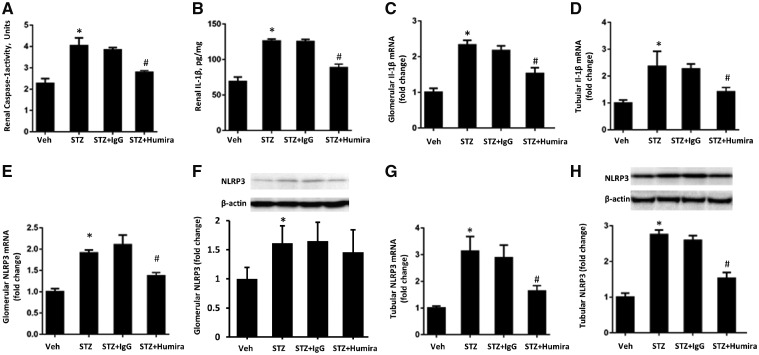
To determine the association between TNF-α pathway and NLRP3 inflammasome activation in TIN, NLRP3 inflammasome in both glomeruli and tubules was separately measured in this study. As shown in Figures 6 and 7, compared with the control group, STZ-induced DN rats had increased renal caspase-1 activity and renal IL-1β expression (P < 0.05) (Figure 6A and B) as well as increased NLRP3, IL-1β and IL-18 mRNA in both glomeruli and tubules (Figures 6C–E G, 7C and F). Compared with the vehicle treatment DN group, TNF-α inhibition significantly reduced NLRP3 mRNA in both glomeruli and tubules (P < 0.05) (Figure 6E and G). However, TNF-α inhibition only decreased NLRP3 protein in tubules, as shown in Figure 6F and H, which suggested that the effects of TNF-α inhibition on NLRP3 might be stronger in renal tubules than glomeruli. Our previous study reported that inhibition of IL-6 and IL-17A could suppress NLRP3 inflammasome activation [22], thus IL-6 and IL-17A mRNA expression in both glomeruli and tubules was further investigated. As expected, TNF-α inhibition decreased IL-6 and IL-17A mRNA expression in both renal glomeruli and tubules (Figure 7A, B, D and E). Therefore, TNF-α inhibition protects against renal TIN in diabetic rats partly through suppression of NLRP3 inflammasome activation by inhibiting IL-6 and IL-17A.
FIGURE 6.
TNF-α inhibition attenuated renal NLRP3 inflammasome in diabetic rats. STZ-induced diabetic rats were treated with TNF-α antibody or normal control IgG and tissue were collected after 12 weeks. (A) Renal caspase-1 activity; (B) renal IL-1β; (C) glomerular IL-1β mRNA; (D) tubular IL-1β mRNA; (E) glomerular NLRP3 mRNA; (F) glomerular NLRP3 protein; (G) tubular NLRP3 mRNA; (H) tubular NLRP3 protein. Data were expressed as mean ± SEM. *P < 0.05 versus vehicle control and #P < 0.05 versus STZ + IgG.
FIGURE 7.
TNF-α inhibition suppressed renal IL-6, IL-17A and IL-18 in diabetic rats. STZ-induced diabetic rats were treated with TNF-α antibody or normal control IgG and tissue were collected after 12 weeks. (A) Glomerular IL-6 mRNA; (B) glomerular IL-17A mRNA; (C) glomerular IL-18 mRNA; (D) tubular IL-6 mRNA; (E) tubular IL-17A mRNA; and (F) tubular IL-18 mRNA. Data were expressed as mean ± SEM. *P < 0.05 versus vehicle control and #P < 0.05 versus STZ + IgG.
DISCUSSION
This study demonstrated that TNF-α inhibition protected against renal TIN in diabetic rats and provided underlying molecular mechanisms for the protective properties of TNF-α inhibition. Diabetic patients with TIN presented with higher renal tubular expression TNF-α and NLRP3 inflammasome. TNF-α inhibition reduced albuminuria, histologic glomerular injury and tubular injury in DN rats. Furthermore, TNF-α inhibition may protect against tubular injury by suppressing the NLRP3 inflammasome and decreasing oxidative stress.
Tubular injury plays a critical role in the development of DN and is closely related with renal prognosis in diabetes. Although renin–angiotensin–aldosterone system (RASS) inhibitors are considered the standard intervention for DN patients, the protective effect of RASS inhibition on tubular injury is unknown. Treatment with the angiotensin-converting enzyme inhibitor enalapril could reduce albuminuria in 16-week-old db/db mice, but does not ameliorate tubular damage [23]. New therapies to diminish tubular injury in DN are urgently needed.
Although the pathogenesis of tubular injury in DN is unclear, it is known that inflammation is involved in tubular damage [2, 3, 5]. TNF-α is a pleiotropic cytokine that activates TNF receptors, leading to the activation of pro-inflammatory mediators, apoptosis and necroptosis [10]. This cytokine is expressed, synthesized and released by infiltrating and resident cells of the kidney [24]. In the current study, TNF-α inhibition decreased albuminuria and protected from diabetic tubulopathy in STZ-induced diabetic rats. These beneficial effects of TNF-α inhibition might be partly associated with suppression of NLRP3 inflammasome activation by inhibiting IL-6 and IL-17A. This hypothesis is supported by the following findings: first, TNF-α inhibition decreased renal IL-6, IL-17A and NLRP3 inflammasome expression in the present study. Moreover, the NLRP3 inflammasome in both glomeruli and tubules was separately measured; the results demonstrated that TNF-α inhibition significantly reduced NLRP3 inflammasome in tubules. Second, the NLRP3 inflammasome plays a critical role in the pathogenesis and progression of DN, whereas NLRP3 deficiency ameliorates tubular injury in multiple types of renal disease [7, 8]. Third, the presence of IL-17A activates the NLRP3 inflammasome, while neutralization of IL-17A decreases the NLRP3 inflammasome expression [25–27]. Importantly, IL-6 promotes the differentiation of T helper 17 cells, which secrete IL-17, whereas blockade of IL-6 receptor significantly reduced renal NLRP3 inflammasome and IL-17A in diabetic rats [21, 28, 29].
These findings support a crucial role of TNF-α in tubular injury. Several studies should be conducted in the future. TNF-α inhibitors are widely used biochemical drugs in clinical settings, especially in rheumatoid disease, and are considered safe for long-term treatment. TNF-α inhibition may be a viable treatment option for TIN in DN if future clinical studies confirm these protective effects. Moreover, there are different types of TNF-α inhibitors, such as chimeric monoclonal antibody, soluble TNF-α receptor fusion protein and pentoxifylline. These drugs should be tested separately.
There were some limitations in the current study. First, the human renal biopsies sample size was small. Second, streptozotocin-induced type 1 diabetic rats were used in animal experiments. In the future, the effect of TNF-α inhibition on TIN needs been further investigated in type 2 diabetic animal models. In addition, most data were based on mRNA expression level, thus changes on proteomic level were expected and the molecular mechanism should be more deeply investigated in further studies. Third, TNF receptors are an important part in the TNF pathway, thus future study is needed to determine the direct effect of TNF receptors on TIN in DN.
CONCLUSION
This study concludes that TNF-α inhibition can protect against TIN in diabetic rats through suppression of the NLRP3 inflammasome. TNF-α inhibition may be a promising new therapy for the treatment of TIN in DN. Future studies should focus on the clinical protective effect of TNF-α inhibition in prospective clinical trials.
ACKNOWLEDGEMENTS
Thank you to Dr Chun Yang from the Medical College of Wisconsin for technical support.
FUNDING
This work was sponsored by the National Natural Science Foundation of China (81570603 and 81770741) and the Health and Family Planning Commission of Shanghai Xuhui District (SHXH201605).
AUTHORS’ CONTRIBUTIONS
F.W. designed the experiments. D.C., R.L., B.H., J.H., J.Y., T.Z., L.Z., R.W. and F.W. performed the experiments. F.W. and Y.Q. analyzed and interpreted the data. D.C., B.H. and F.W. drafted the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest. The results presented in this paper have not been published previously, in whole or part, except in abstract format.
REFERENCES
- 1. Saran R, Robinson B, Abbott KC. et al. US renal data system 2017 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2018; 71: A7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gilbert RE. Proximal tubulopathy: prime mover and key therapeutic target in diabetic kidney disease. Diabetes 2017; 66: 791–800 [DOI] [PubMed] [Google Scholar]
- 3. Bonventre JV. Can we target tubular damage to prevent renal function decline in diabetes? Semin Nephrol 2012; 32: 452–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin M, Yiu WH, Wu HJ. et al. Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J Am Soc Nephrol 2012; 23: 86–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zeni L, Norden AGW, Cancarini G. et al. A more tubulocentric view of diabetic kidney disease. J Nephrol 2017; 30: 701–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qiu YY, Tang LQ.. Roles of the NLRP3 inflammasome in the pathogenesis of diabetic nephropathy. Pharmacol Res 2016; 114: 251–264 [DOI] [PubMed] [Google Scholar]
- 7. Gong W, Mao S, Yu J. et al. NLRP3 deletion protects against renal fibrosis and attenuates mitochondrial abnormality in mouse with 5/6 nephrectomy. Am J Physiol Renal Physiol 2016; 310: F1081–F1088 [DOI] [PubMed] [Google Scholar]
- 8. Vilaysane A, Chun J, Seamone ME. et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol 2010; 21: 1732–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen K, Zhang J, Zhang W. et al. ATP-P2X4 signaling mediates NLRP3 inflammasome activation: a novel pathway of diabetic nephropathy. Int J Biochem Cell Biol 2013; 45: 932–943 [DOI] [PubMed] [Google Scholar]
- 10. Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol 1992; 10: 411–452 [DOI] [PubMed] [Google Scholar]
- 11. McGeough MD, Wree A, Inzaugarat ME. et al. TNF regulates transcription of NLRP3 inflammasome components and inflammatory molecules in cryopyrinopathies. J Clin Invest 2017; 127: 4488–4497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Amaral FA, Bastos LF, Oliveira TH. et al. Transmembrane TNF-alpha is sufficient for articular inflammation and hypernociception in a mouse model of gout. Eur J Immunol 2016; 46: 204–211 [DOI] [PubMed] [Google Scholar]
- 13. Gohda T, Niewczas MA, Ficociello LH. et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol 2012; 23: 516–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Niewczas MA, Gohda T, Skupien J. et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol 2012; 23: 507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coca SG, Nadkarni GN, Huang Y. et al. Plasma biomarkers and kidney function decline in early and established diabetic kidney disease. J Am Soc Nephrol 2017; 28: 2786–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Omote K, Gohda T, Murakoshi M. et al. Role of the TNF pathway in the progression of diabetic nephropathy in KK-A(y) mice. Am J Physiol Renal Physiol 2014; 306: F1335–F1347 [DOI] [PubMed] [Google Scholar]
- 17. Awad AS, You H, Gao T. et al. Macrophage-derived tumor necrosis factor-alpha mediates diabetic renal injury. Kidney Int 2015; 88: 722–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Radford MG Jr, Donadio JV Jr, Bergstralh EJ. et al. Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol 1997; 8: 199–207. [DOI] [PubMed] [Google Scholar]
- 19. Angelotti ML, Ronconi E, Ballerini L. et al. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells 2012; 30: 1714–1725 [DOI] [PubMed] [Google Scholar]
- 20. Lin Y, Sun Z.. Thyroid hormone ameliorates diabetic nephropathy in a mouse model of type II diabetes. J Endocrinol 2011; 209: 185–191 [DOI] [PubMed] [Google Scholar]
- 21. Richardson JC, Waterson P, Simmons NL.. Isolation and culture of renal cortical tubules from neonate rabbit kidneys. Q J Exp Physiol 1982; 67: 287–301 [DOI] [PubMed] [Google Scholar]
- 22. Wu R, Liu X, Yin J. et al. IL-6 receptor blockade ameliorates diabetic nephropathy via inhibiting inflammasome in mice. Metabolism 2018; 83: 18–24 [DOI] [PubMed] [Google Scholar]
- 23. Marquardt A, Al-Dabet MM, Ghosh S. et al. Farnesoid X receptor agonism protects against diabetic tubulopathy: potential add-on therapy for diabetic nephropathy. J Am Soc Nephrol 2017; 28: 3182–3189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Al-Lamki RS, Mayadas TN.. TNF receptors: signaling pathways and contribution to renal dysfunction. Kidney Int 2015; 87: 281–296 [DOI] [PubMed] [Google Scholar]
- 25. Kim SR, Kim HJ, Kim DI. et al. Blockade of interplay between IL-17A and endoplasmic reticulum stress attenuates LPS-induced lung injury. Theranostics 2015; 5: 1343–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yan J, Li Y, Yang H. et al. Interleukin-17A participates in podocyte injury by inducing IL-1beta secretion through ROS-NLRP3 inflammasome-caspase-1 pathway. Scand J Immunol 2018; 87: e12645. [DOI] [PubMed] [Google Scholar]
- 27. Zhang S, Yu N, Zhang R. et al. Interleukin-17A induces IL-1beta secretion from RPE cells via the NLRP3 inflammasome. Invest Ophthalmol Vis Sci 2016; 57: 312–319 [DOI] [PubMed] [Google Scholar]
- 28. Kimura A, Kishimoto T.. IL-6: regulator of Treg/Th17 balance. Eur J Immunol 2010; 40: 1830–1835 [DOI] [PubMed] [Google Scholar]
- 29. Serada S, Fujimoto M, Mihara M. et al. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 2008; 105: 9041–9046 [DOI] [PMC free article] [PubMed] [Google Scholar]