Abstract
Background
Evidence indicates that the inverse relationships between phosphate levels and mortality maybe modified by age. Furthermore, malnutrition and inflammation could strengthen the risk associated with phosphate abnormalities. This study aimed to assess the associations between phosphate levels and mortality while accounting for the interactions with age and parameters associated with malnutrition and inflammation in hemodialysis (HD) patients.
Methods
Adult HD patients (n = 245 853) treated in Fresenius Medical Care North America clinics from January 2010 to October 2018 were enrolled. Baseline was defined as Months 4–6 on dialysis, with the subsequent 12 months as the follow-up period. Univariate and multivariate Cox proportional hazard models with spline terms were applied to study the nonlinear relationships between serum phosphate levels and mortality. The interactions of phosphate levels with albumin, creatinine, normalized protein catabolic rate (nPCR) and neutrophil–lymphocyte ratio (NLR) were assessed with smoothing spline analysis of variance Cox proportional hazard models.
Results
Older patients tended to have lower levels of serum phosphate, albumin, creatinine and nPCR. Additionally, both low (<4.0 mg/dL) and high (>5.5 mg/dL) phosphate levels were associated with higher risk of mortality across all age strata. The U-shaped relationships between phosphate levels and outcome persisted even for patients with low or high levels of serum albumin, creatinine, nPCR and NLR, respectively.
Conclusion
The consistent U-shaped relationships between serum phosphate and mortality across age strata and levels of inflammatory and nutritional status should prompt the search for underlying causes and potentially nutritional intervention in clinical practice.
Keywords: hyperphosphatemia, hypophosphatemia, inflammation, malnutrition, protein–energy wasting
INTRODUCTION
The harmful sequelae of hyperphosphatemia (serum phosphate >4.5 mg/dL according to Kidney Disease: Improving Global Outcomes (KDIGO) or >5.5 mg/dL according to Kidney Disease Outcomes Quality Initiative (KDOQI)) [1, 2] in the chronic kidney disease (CKD) population has been well established, especially the relationships with an increased risk of cardiovascular events and mortality [3]. Next to hyperphosphatemia, also low serum phosphate levels (<3.0 according to KDIGO or <3.5 mg/dL according to KDOQI) are related to adverse outcomes in hemodialysis (HD) patients [4–10].
To some extent, the relationship between serum phosphate and outcomes in HD patients may depend on age [5, 11]. Evidence has also indicated that serum phosphate levels tend to decline with age in HD patients [11]. This inverse relationship between age and serum phosphate level seems to be, to some degree, driven by low serum phosphate levels that are mainly observed in elderly patients [5, 6]. However, studies on this subject are scarce. It has also been reported that the prevalence of protein–energy wasting increases with age [12], which may, in combination with the reduced life expectancy, abrogate the more long-term risk of hypophosphatemia [1, 2].
Other factors that may complicate the association between serum phosphate levels and outcomes are phosphate binder use, nutritional and inflammatory indicators and patient characteristics such as gender, race and social economic factors [13–15]. We recently showed that both phosphate levels and conventional nutritional parameters [e.g. serum albumin, creatinine, normalized protein catabolic rate (nPCR), interdialytic weight gain (IDWG)] are inversely associated with mortality, whereas inflammatory parameters such as C-reactive protein and neutrophil–lymphocyte ratio (NLR) are positively associated with death and hospitalization [16–18]. Additionally, Lopes et al. showed an inversed relationship between serum phosphate and nutritional indices [19]. On the other hand, also hyperphosphatemia may be associated with systemic inflammation [20].
A recent study by the Dialysis Outcomes and Practice Patterns Study (DOPPS) showed that the relationship between low phosphate levels and mortality lost statistical significance when adjusted for nutritional parameters [19]. Other studies reported the highest mortality risk in patients with low levels of both phosphate and albumin [13]. However, the direct interaction between phosphate, inflammatory and nutritional parameters and its relationships to outcomes have not been assessed in a large and representative cohort of dialysis patients yet.
So far, studies have generally addressed the relationships between phosphate levels and outcomes by applying serum phosphate as sole predictor. Some studies have shown the added value of analyzing the interaction between two variables and outcome in a continuous fashion via analysis of variance (ANOVA) Cox proportional hazard model [7]. In this study, we explore in a large US HD cohort the relationships between phosphate levels and mortality while accounting for the interactions with age, and nutritional and inflammatory parameters.
MATERIALS AND METHODS
Population and study design
In this retrospective cohort study, all incident HD patients of ≥18 years of age and treated in Fresenius Medical Care North America (FMCNA) clinics between 1 January 2010 and 31 October 2018 were enrolled. Patients were required to be treated in FMCNA clinics within 90 days of their first date on dialysis. The study baseline was defined as Months 4–6 on HD; the follow-up was defined as the subsequent 12 months (i.e. Months 7–18). Clinical and laboratory parameters were averaged during baseline. Demographic and comorbidity data were obtained in Month 4. All-cause mortality was documented during follow-up. Patients were censored when lost to follow-up, transferred to non-FMCNA clinics, kidney transplanted or recovered kidney function. Only patients with at least one baseline serum phosphate value were included. The study was approved by the Western Institutional Review Board (WIRB number ES-18-010).
Two main analyses were performed: first, we assessed the association between serum phosphate levels and all-cause mortality in four age strata (18 ≤ age < 35; 35 ≤ age < 55; 55 ≤ age < 75; age ≥75 years); second, we constructed smoothing spline ANOVA Cox proportional hazard models to assess the association between serum phosphate and mortality while accounting for the interactions with age, serum albumin and creatinine, nPCR and NLR. A smoothing spline ANOVA Cox model assumes that the hazard function is equal to a baseline hazard times the exponential of a multivariate function of covariates. The multivariate function is modeled nonparametrically using tensor product of reproducing kernel Hilbert spaces that leads to a smoothing spline ANOVA model. As in the traditional ANOVA models, this model has main effects and interactions that facilitate model selection and interpretation [21]. Additionally, this methodology allows modeling the joint effects of two or more independent continuous variables without assuming a specific parametric form, thereby avoiding the need to create somewhat arbitrary categories. The results of this analysis are shown as contour plots that are read like a topographic map, which present the 3D features in a 2D way, where the 1D (serum phosphate) levels are represented on Y-axis, the 2D levels (respective biomarkers) are represented on X-axis and the 3D levels (hazard ratios) are represented as ‘altitude’ [22, 23]. An elevation on the ‘altitude’ indicates a higher risk of death at the specific levels of serum phosphate and parameters of interest.
Effect of serum phosphate levels on all-cause mortality
To explore nonlinear relationships between serum phosphate levels and outcomes across age groups (all age groups, 18 ≤ age < 35; 35 ≤ age < 55; 55 ≤ age < 75; age ≥75 years), we constructed cubic splines with 4 degrees of freedom. In order to analyze the effect of phosphate levels on outcomes while accounting for the effect of social economic factors, and nutritional and inflammation status, the following three models were constructed: Model 1: unadjusted analysis with serum phosphate as continuous variable and the sole predictor of mortality; Model 2: included in addition to phosphate, age, gender, race, body mass index (BMI), vascular access (VA) type, marital status (MS), diabetes mellitus (DM) and congestive heart failure (CHF); Model 3 (fully adjusted model) comprised Model 2 variables plus serum albumin, creatinine, nPCR and NLR.
Interactions of serum phosphate with age, and nutritional and inflammatory markers
To explore the joint association between baseline serum phosphate levels and age on mortality, we employed ANOVA Cox proportional hazard model with adjustment for gender, race, BMI, VA, MS, DM, CHF, serum albumin, creatinine, nPCR and NLR. The same smoothing ANOVA Cox proportional hazard method was applied to further investigate the joint effect of serum phosphate levels and nutritional (serum albumin and creatinine) and inflammatory markers (nPCR, NLR). These models were adjusted for the aforementioned covariates.
Cross-sections through the contour plots were constructed at discrete levels of age (35, 55, 75 and 80 years, respectively), serum albumin (3.0, 3.5, 3.8 and 4.0 mg/dL, respectively), serum creatinine (4.0, 6.0, 9.0 and 12.0 mg/dL, respectively), nPCR (0.5, 0.8, 1.0 and 1.2 g/kg body weight, respectively) and NLR (2.0, 3.0, 5.0 and 10.0, respectively). This analysis allowed us to visually depict hazard ratio point estimates and their 95% confidence intervals.
Additionally, cubic splines functions with 4 degrees of freedom were performed to assess the relationships between serum phosphate levels and outcomes across serum albumin levels (albumin <3.0; 3.0 ≤ albumin < 3.5; 3.5 ≤ albumin < 3.8; 3.8 ≤ albumin < 4.0; albumin ≥4.0 mg/dL); serum creatinine levels (creatinine <4.0; 4.0 ≤ creatinine < 6.0; 6.0 ≤ creatinine < 9.0; 9.0 ≤ creatinine < 12.0; creatinine ≥12.0 mg/dL); nPCR levels (nPCR <0.5; 0.5 ≤ nPCR < 0.8; 0.8 ≤ nPCR < 1.0; 1.0 ≤ nPCR < 1.2; nPCR ≥1.2 g/kg/day) and NLR levels (NLR <2.0; 2.0 ≤ NLR < 3.0; 3.0 ≤ NLR < 5.0; 5.0 ≤ NLR < 10.0; NLR ≥10.0) in the unadjusted and fully adjusted models.
Predictors of serum phosphate <4.0 mg/dL
Logistic regression models were constructed to identify predictors of baseline serum phosphate ≤4.0 and >4.0 mg/dL, respectively.
Sensitivity analyses
To account for potential indication bias, the above models were applied in patients with or without documented baseline phosphate binder use, respectively. To eliminate a potential interference of hospitalization, we conducted a subset analysis in patients without hospitalizations during the first 6 months on HD. Only patients without missing data were included in the respective adjusted models.
Descriptive statistical analysis
Descriptive statistics were computed in patients stratified by age (four strata) and baseline serum phosphate levels ≤4.0 mg/dL or >4.0 mg/dL. We calculated means and SDs for continuous variables and proportions for categorical variables. ANOVA was used for group comparison of normally distributed variables and the Mann–Whitney U test for non-normally distributed variables. Categorical data were compared by chi-square test. A two-sided P-value <0.05 was considered as statistically significant. All data manipulation analyses were performed with SAS 9.4. Cubic spline functions were performed with R 3.4.4 (survival, splines and ggplot2 packages). ANOVA Cox proportional hazard models were performed with R 3.4.4 mcgv package.
RESULTS
We studied 245 853 HD patients. Baseline characteristics of the entire population and stratified by age are shown in Table 1. Table 2 shows the baseline characteristics in groups with serum phosphate levels <4.0 mg/dL (n = 37 026; 15.13%) and ≥4.0 mg/dL (n = 208 617; 84.87%), respectively.
Table 1.
Baseline characteristics by age strata
| Age group (years) |
P-value | ||||||
|---|---|---|---|---|---|---|---|
| Parameters | All | 18 ≤ Age < 35 | 35 ≤ Age < 55 | 55 ≤ Age < 75 | Age ≥75 | ||
| Number of patients (%) | 245 853 | 13 007 (5.29) | 61 499 (25.01) | 123 088 (50.07) | 48 259 (29.63) | ||
| Demographics | |||||||
| Age, mean (SD) (years) | 62 (14.71) | 29 (4.39) | 47 (5.46) | 65 (5.58) | 81 (4.40) | <0.0001 | |
| Married, % | 41 | 18 | 36 | 45 | 44 | <0.0001 | |
| White, % | 69 | 60 | 61 | 70 | 78 | <0.0001 | |
| Male, % | 58 | 55 | 62 | 57 | 56 | <0.0001 | |
| Catheter as vascular assess, % | 38 | 47 | 40 | 37 | 38 | <0.0001 | |
| BMI | 29 (10.85) | 29 (11.88) | 31 (11.43) | 30 (11.94) | 27 (9.03) | <0.0001 | |
| Height, mean (SD) (cm) | 168 (12.05) | 168 (13.00) | 170 (11.77) | 168 (12.05) | 166 (11.86) | <0.0001 | |
| COPD, % | 8 | 4 | 6 | 9 | 9 | <0.0001 | |
| CVD, % | 61 | 59 | 62 | 62 | 59 | <0.0001 | |
| Infection, % | 13 | 14 | 14 | 13 | 11 | <0.0001 | |
| DM, % | 63 | 31 | 60 | 71 | 57 | <0.0001 | |
| CHF, % | 18 | 9 | 14 | 19 | 22 | <0.0001 | |
| Phosphate binder, % | 48 | 57 | 53 | 48 | 40 | <0.0001 | |
| Calcium base, % | 47 | 45 | 45 | 48 | 52 | <0.0001 | |
| Laboratory data | |||||||
| Serum phosphate, mean (SD) (mg/dL) | 5.28 (1.30) | 6.02 (1.55) | 5.68 (1.38) | 5.21 (1.22) | 4.76 (1.07) | <0.0001 | |
| Serum phosphate ≤4.0, % | 15 | 8 | 9 | 15 | 25 | <0.0001 | |
| Serum albumin, mean (SD) (g/dL) | 3.69 (0.42) | 3.84 (0.49) | 3.74 (0.44) | 3.68 (0.41) | 3.62 (0.39) | <0.0001 | |
| Serum prealbumin, mean (SD) (mg/dL) | 25.89 (8.14) | 29.37 (9.61) | 28.24 (8.44) | 25.41 (7.81) | 23.56 (7.81) | <0.0001 | |
| Serum creatinine, mean (SD) (mg/dL) | 6.98 (2.82) | 9.93 (3.70) | 8.22 (3.10) | 6.60 (2.38) | 5.59 (1.86) | <0.0001 | |
| Serum potassium, mean (SD) (mg/dL) | 4.55 (0.55) | 4.63 (0.59) | 4.60 (0.58) | 4.54 (0.55) | 4.48 (0.51) | <0.0001 | |
| nPCR, mean (SD) (g/kg/day) | 0.93 (4.57) | 1.16 (13.49) | 0.91 (1.34) | 0.93 (4.85) | 0.89 (0.28) | 0.0002 | |
| NLR, mean (SD) | 4.19 (3.47) | 3.61 (3.17) | 3.89 (3.09) | 4.27 (3.60) | 4.52 (3.62) | <0.0001 | |
| Parathyroid hormone, mean (SD) (pg/mL) | 362.59 (316.90) | 509.45 (437.59) | 420.28 (364.98) | 348.44 (292.00) | 285.86 (237.79) | <0.0001 | |
| Serum calcium, mean (SD) (mg/dL) | 8.96 (0.61) | 8.94 (0.70) | 8.91 (0.65) | 8.96 (0.59) | 9.01 (0.56) | <0.0001 | |
| Vitamin D 25, mean (SD) (ng/mL) | 28.67 (15.11) | 23.33 (13.13) | 24.38 (13.70) | 29.18 (14.93) | 34.64 (15.62) | <0.0001 | |
| eKt/V, mean (SD) | 1.55 (4.67) | 1.70 (11.36) | 1.48 (2.91) | 1.56 (5.19) | 1.58 (0.61) | <0.0001 | |
| Treatment data, mean (SD) | |||||||
| Treatment time (min) | 228.86 (23.04) | 226.83 (25.12) | 232.90 (23.60) | 229.97 (22.09) | 228.86 (23.04) | <0.0001 | |
| Postdialysis weight (kg) | 82.50 (22.30) | 81.15 (26.79) | 89.12 (25.96) | 83.43 (21.93) | 82.50 (22.30) | <0.0001 | |
| IDWG (kg) | 2.30 (0.95) | 2.53 (1.06) | 2.63 (1.04) | 2.29 (0.94) | 2.30 (0.95) | <0.0001 | |
| Events | |||||||
| Death, % | 10.06 | 3.57 | 5.32 | 9.98 | 10.06 | <0.0001 | |
| Hospitalized in first 6 months, % | 43.20 | 43.13 | 41.19 | 43.18 | 43.20 | <0.0001 | |
| Hospitalized during baseline, % | 38.25 | 38.78 | 36.84 | 38.15 | 38.25 | <0.0001 | |
| Hospitalizations during baseline, mean (SD) (frequency as count) | 1.76 (1.20) | 2.16 (1.79) | 1.83 (1.32) | 1.71 (1.12) | 1.76 (1.20) | <0.0001 | |
| Hospitalization duration during baseline, mean (SD) (days) | 10.43 (10.24) | 11.74 (12.32) | 10.45 (10.60) | 10.42 (10.12) | 10.43 (10.24) | <0.0001 | |
Table 2.
Baseline characteristics by serum phosphate
| Serum phosphate (mg/dL) |
Δ (95% CI) | ||||
|---|---|---|---|---|---|
| Parameters | All | <4.0 mg/dL | ≥4.0 mg/dL | P-value | |
| Number of patients (%) | 245 853 | 37 206 (15.13%) | 208 647 (84.87%) | ||
| Demographic information | |||||
| Age, mean (SD) (years) | 62 (14.71) | 67 (13.86) | 61 (14.64) | 6 (6.29, 6.61) | <0.0001 |
| Married, % | 41 | 42 | 41 | −1.60 | <0.0001 |
| White, % | 69 | 67 | 69 | −2.0 | <0.0001 |
| Male, % | 58 | 59 | 58 | 1.00 | 0.01 |
| Catheter as vascular assess, % | 38 | 40 | 38 | 2.00 | <0.0001 |
| BMI | 29 (10.85) | 29 (10.13) | 30 (10.95) | −1.76 (−1.88, −1.63) | <0.0001 |
| Height, mean (SD) (cm) | 168 (12.05) | 168 (12.00) | 168 (12.06) | −0.86 (−1.00, −0.72) | <0.0001 |
| Chronic obstructive pulmonary disease, % | 8 | 9 | 8 | 1.00 | <0.0001 |
| CVD, % | 61 | 60 | 62 | −2.00 | <0.0001 |
| Infection, % | 13 | 12 | 13 | −1.00 | 0.50 |
| DM, % | 63 | 61 | 36 | 35.00 | <0.0001 |
| CHF, % | 18 | 20 | 18 | 2.00 | <0.0001 |
| Phosphate binder, % | 48 | 33 | 51 | −18.00 | <0.0001 |
| Calcium base, % | 47 | 55 | 47 | 7.30 | <0.0001 |
| Laboratory information | |||||
| Serum phosphate, mean (SD) (mg/dL) | 5.28 (1.30) | 3.54 (0.40) | 5.56 (1.16) | −2.05 (−2.06, −2.04) | <0.0001 |
| Serum albumin, mean (SD) (g/dL) | 3.69 (0.42) | 3.58 (0.48) | 3.71 (0.71) | −0.14 (−0.14, −0.13) | <0.0001 |
| Serum prealbumin, mean (SD) (mg/dL) | 25.89 (8.14) | 23.28 (7.70) | 26.36 (8.13) | −3.08 (−4.11, −2.04) | <0.0001 |
| Serum creatinine, mean (SD) (mg/dL) | 6.98 (2.82) | 5.23 (2.15) | 7.30 (2.81) | −2.07 (−2.10, −2.04) | <0.0001 |
| Serum potassium, mean (SD) (mg/dL) | 4.55 (0.55) | 4.30 (0.52) | 4.59 (0.54) | −0.29 (−0.30, −0.28) | <0.0001 |
| nPCR, mean (SD) (g/kg/day) | 0.93 (4.57) | 0.78 (0.28) | 0.95 (4.95) | −0.17 (−0.22, −0.11) | <0.0001 |
| NLR, mean (SD) | 4.19 (3.47) | 4.56 (4.73) | 4.12 (3.20) | 0.44 (0.39, 0.48) | <0.0001 |
| Parathyroid hormone, mean (SD) (pg/mL) | 362.59 (316.90) | 252.50 (221.53) | 381.58 (326.86) | −129.02 (−132.6, −125.5) | <0.0001 |
| Serum calcium, mean (SD) (mg/dL) | 8.96 (0.61) | 8.98 (0.57) | 8.95 (0.61) | 0.03 (0.02, 0.04) | <0.0001 |
| Vitamin D 25, mean (SD) (ng/mL) | 28.67 (15.11) | 30.98 (16.11) | 28.28 (14.90) | 2.70 (2.36, 3.05) | <0.0001 |
| eKt/V, mean (SD) | 1.55 (4.67) | 1.57 (0.51) | 1.54 (5.04) | 0.03 (0.00, 0.05) | <0.0001 |
| Treatment information, mean (SD) | |||||
| Treatment time (min) | 228.86 (23.04) | 226.90 (23.03) | 229.20 (23.02) | −2.30 (−2.57, −2.00) | <0.0001 |
| Postdialysis weight (kg) | 82.50 (22.30) | 77.56 (20.99) | 83.37 (23.21) | −5.81 (−6.08, −5.54) | <0.0001 |
| IDWG (kg) | 2.30 (0.95) | 1.98 (0.88) | 2.36 (0.95) | −0.38 (−0.40, −0.37) | <0.0001 |
| Events | |||||
| Death, % | 10.06 | 14.67 | 9.23 | 5.44 | <0.0001 |
| Ever hospitalized during first 6 months on HD, % | 43.20 | 48.60 | 42.24 | 6.36 | <0.0001 |
| Ever hospitalized during baseline, % | 38.25 | 44.04 | 37.21 | 6.83 | <0.0001 |
| Hospitalization frequency during baseline, mean (SD) (count) | 1.76 (1.20) | 1.86 (1.25) | 1.73 (1.19) | 0.13 (0.11, 0.15) | <0.0001 |
| Hospitalization duration during baseline, mean (SD) (days) | 10.43 (10.24) | 12.20 (11.73) | 10.06 (9.85) | 2.14 (1.97, 2.32) | <0.0001 |
CI, confidence interval.
Older patients tended to have lower levels of phosphate, albumin, creatinine, nPCR, IDWG, post-HD body weight, BMI, parathyroid hormone, phosphate binder use, higher DM prevalence, and mortality and hospitalization rates.
Relation between serum phosphate, phosphate binder use and all-cause mortality
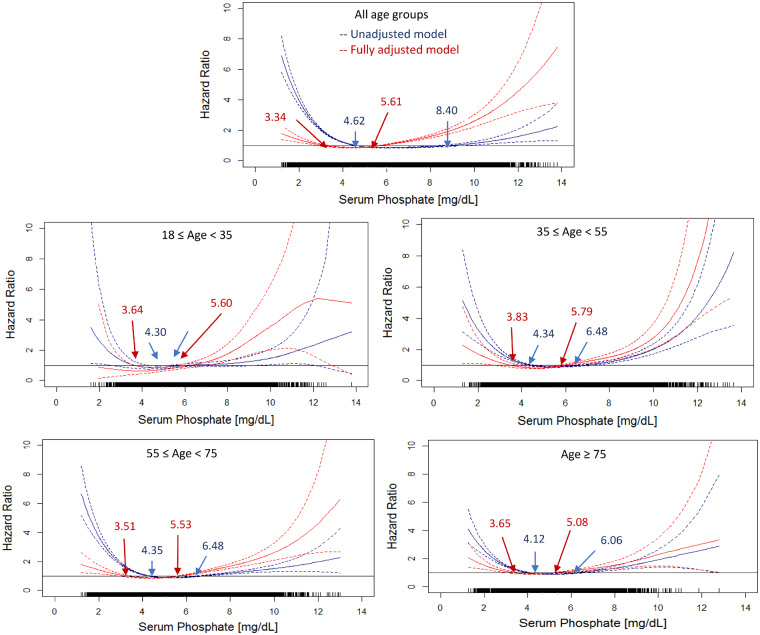
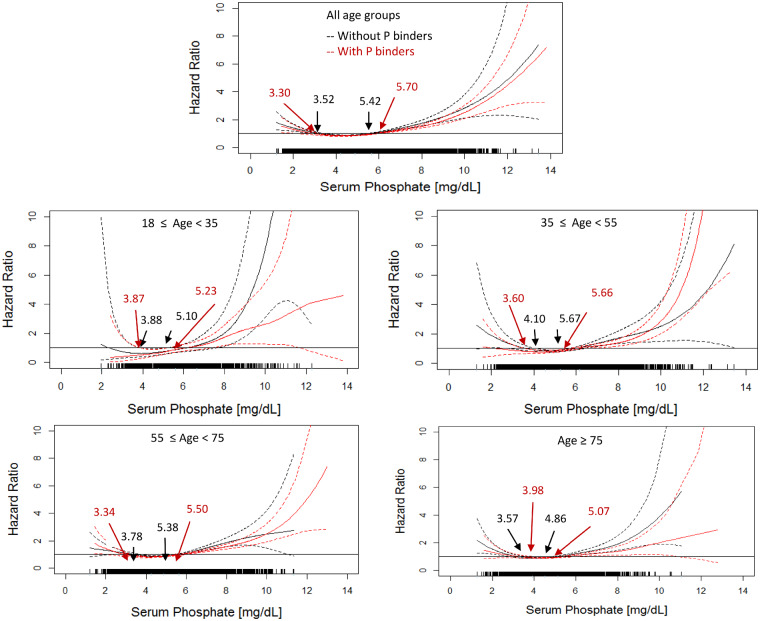
Univariate analyses show a U-shaped relationship between serum phosphate levels and mortality in all age strata (Figure 1) and with or without using phosphate binders during the baseline (Figure 2). The U-shaped relationship persists in fully adjusted models irrespective of age and phosphate binder use (Figure 2). Of note, the inverse associations between hyperphosphatemia and mortality are attenuated in the adjusted analysis (Figures 1 and 3).
FIGURE 1.
Association between all-cause mortality and serum phosphate in unadjusted and adjusted models stratified by different age groups. The blue solid lines show the estimated hazard ratio of the unadjusted model and blue dashed lines show the lower and upper limits of the hazard ratio of the unadjusted model. The red solid lines show the estimated hazard ratio of the fully adjusted model and red dashed lines show the lower and upper limits of the hazard ratio of the adjusted model. Blue arrows show the serum phosphate with the hazard ratio = 1.0 in the unadjusted models. Red arrows show the serum phosphate with the hazard ratio = 1.0 in the adjusted models. The adjusted models were adjusted by gender, race, VA, MS, BMI, DM as comorbidity, CHF as comorbidity, serum albumin, creatinine, nPCR and NLR. To convert serum phosphate to mmol/L, multiply by 0.323.
FIGURE 2.
Association between all-cause mortality and serum phosphate in patients with and without used phosphate binding agents (fully adjusted models). The black solid lines show the estimated hazard ratio of patients who did not use phosphate binding agents during the baseline period and black dashed lines show the lower and upper limits of the hazard ratio. The red solid lines show the estimated hazard ratio of patients that took phosphate binding agents within the baseline and red dashed lines show the lower and upper limits of the hazard ratio. Arrows show the serum phosphate with the hazard ratio = 1.0. The adjusted models were adjusted by gender, race, VA, MS, BMI, DM as comorbidity, CHF as comorbidity, serum albumin, creatinine, nPCR and NLR.
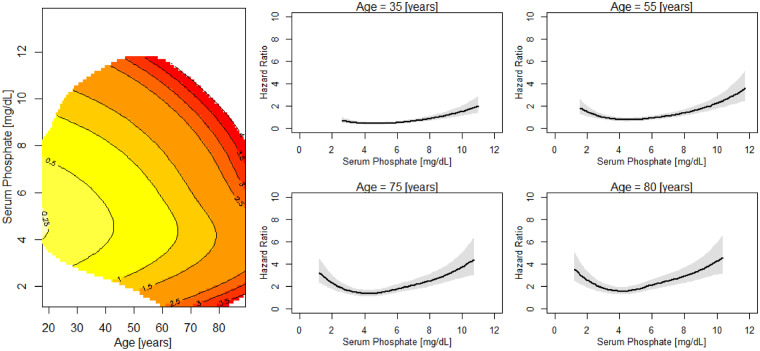
FIGURE 3.
Risk of death across levels of serum phosphate and age. Left: contour plot of the estimated hazard ratio of death in the next year as a joint function of serum phosphate and age for patients with serum phosphate and age fixed at their median values. Estimates of the joint effects are shown in a region with sufficient data decided by posterior SDs. Right: age slices at different serum phosphate ranges. Sections through the contour plot (left) at four age levels. The adjusted model was adjusted by gender, race, VA, MS, BMI, DM as comorbidity, CHF as comorbidity, serum albumin, creatinine, nPCR and NLR.
Predictors of phosphate levels <4.0 mg/dL
Older age, male sex, catheter use as VA, CHF, higher NLR and hospitalization (frequency and duration in days) are predictors of low phosphate levels (Table 3). Low phosphate levels are less likely in patients who are white, and have higher BMI, serum albumin, creatinine, nPCR and IDWG. For these factors, the effect size is similar across age categories.
Table 3.
Predictors of serum phosphate levels <4.0 mg/dL for patients in different age strata
| Predictors | All |
18 ≤ Age < 35 |
35 ≤ Age < 55 |
55 ≤ Age < 75 |
Age ≥75 |
|---|---|---|---|---|---|
|
n = 245 853 |
n = 13 007 |
n = 61 499 |
n = 123 088 |
n = 25 165 |
|
| AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | |
| Age (every 10 years) | 1.40 (1.39–1.41)* | – | – | – | – |
| Married versus nonmarried | 1.06 (1.03–1.08)* | 1.04 (0.88–1.24) | 0.97 (0.91–1.02) | 1.00 (0.96–1.03) | 0.98 (0.94–1.02) |
| White versus nonwhite | 0.91 (0.89–0.93)* | 0.73 (0.64–0.83)* | 0.76 (0.72–0.81)* | 0.81 (0.78–0.84)* | 0.81 (0.77–0.85)* |
| Male versus female | 1.03 (1.01–1.05)* | 0.8 (0.71–0.92)* | 0.88 (0.84–0.94)* | 1.09 (1.05–1.12)* | 1.18 (1.13–1.23)* |
| Catheter as vascular assess | 1.05 (1.03–1.08)* | 1.25 (1.07–1.45)* | 1.02 (0.96–1.09)* | 1.06 (1.03–1.10)* | 1.13 (1.07–1.18)* |
| BMI | 0.97 (0.97–0.98)* | 0.98 (0.97–0.99)* | 0.97 (0.97–0.98)* | 0.98 (0.98–0.99)* | 0.99 (0.99–0.99)* |
| Chronic obstructive pulmonary disease | 1.15 (1.10–1.19)* | 1.21 (0.86–1.7) | 1.07 (0.95–1.21) | 1.09 (1.03–1.15)* | 0.97 (0.91–1.05) |
| CVD | 0.90 (0.88–0.92)* | 0.81 (0.71–0.93)* | 0.91 (0.86–0.96)* | 0.90 (0.87–0.93)* | 0.93 (0.89–0.97)* |
| Infection, % | 1.00 (0.97–1.03) | 0.99 (0.82–1.21) | 1.12 (1.03–1.21)* | 1.01 (0.97–1.06) | 0.93 (0.89–0.97) |
| DM, % | 0.94 (0.92–0.96)* | 0.78 (0.65–0.94)* | 0.86 (0.81–0.91)* | 0.95 (0.92–0.98)* | 0.96 (0.92–1.00) |
| CHF, % | 1.13 (1.10–1.16)* | 0.99 (0.79–1.26) | 0.93 (0.85–1.01) | 1.02 (0.98–1.06) | 1.08 (1.03–1.14)* |
| Phosphate binder, % | 0.48 (0.47–0.49)* | 0.56 (0.49–0.64)* | 0.46 (0.44–0.49)* | 0.52 (0.50–0.53)* | 0.54 (0.51–0.56)* |
| Serum albumin (g/dL) | 0.49 (0.48–0.51)* | 0.86 (0.76–0.98)* | 0.69 (0.65–0.73)* | 0.49 (0.48–0.51)* | 0.44 (0.42–0.46)* |
| Serum prealbumin (mg/dL) | 0.95 (0.94–0.97)* | 0.97 (0.88–1.08) | 0.95 (0.91–0.99)* | 0.95 (0.93–0.97)* | 1.12 (0.88–1.42) |
| Serum creatinine (mg/dL) | 0.67 (0.67–0.68)* | 0.74 (0.72–0.76)* | 0.73 (0.72–0.73)* | 0.68 (0.68–0.69)* | 0.65 (0.64–0.66)* |
| nPCR (g/kg per day) | 0.06 (0.06–0.07)* | 0.09 (0.06–0.14)* | 0.06 (0.05–0.07)* | 0.05 (0.05–0.06)* | 0.07 (0.06–0.08)* |
| NLR | 1.03 (1.03–1.03)* | 1.03 (1.01–1.04)* | 1.02 (1.01–1.03)* | 1.03 (1.02–1.03)* | 1.02 (1.01–1.02)* |
| Parathyroid hormone (pg/mL) | 0.99 (0.99–0.99)* | 0.99 (0.99–0.99)* | 0.99 (0.99–0.99)* | 0.99 (0.99–0.99)* | 0.99 (0.99–0.99)* |
| Vitamin D 25 (ng/mL) | 1.01 (1.01–1.01)* | 1.00 (0.99–1.01) | 1.01 (1.00–1.01)* | 1.01 (1.00–1.01)* | 1.00 (1.00–1.01)* |
| eKt/V | 1.00 (0.99–1.00) | 1.00 (0.99–1.01) | 1.00 (1.00–1.01) | 1.00 (0.99–1.00) | 1.04 (1.00–1.08) |
| IDWG (kg) | 0.61 (0.61–0.62)* | 0.65 (0.60–0.70)* | 0.66 (0.64–0.68)* | 0.68 (0.67–0.69)* | 0.66 (0.64–0.68)* |
| Ever hospitalized during first 6 months | 1.30 (1.28–1.33)* | 1.04 (0.92–1.19) | 1.16 (1.09–1.22)* | 1.32 (1.28–1.36)* | 1.30 (1.25–1.36)* |
| Ever hospitalized during baseline | 1.34 (1.31–1.37)* | 1.04 (0.91–1.19) | 1.18 (1.11–1.24)* | 1.38 (1.34–1.43)* | 1.36 (1.30–1.42)* |
| Hospitalization frequency during baseline | 1.09 (1.07–1.10)* | 1.03 (0.97–1.09) | 1.11 (1.08–1.14)* | 1.15 (1.13–1.17)* | 1.10 (1.05–1.16)* |
| Hospitalization duration during baseline | 1.02 (1.02–1.02)* | 1.01 (1.01–1.02)* | 1.02 (1.02–1.02)* | 1.02 (1.02–1.02)* | 1.02 (1.02–1.03)* |
*P-value < .001. AOR, adjusted odds ratio.
Interaction of serum phosphate with age, nutritional and inflammatory markers, and outcome
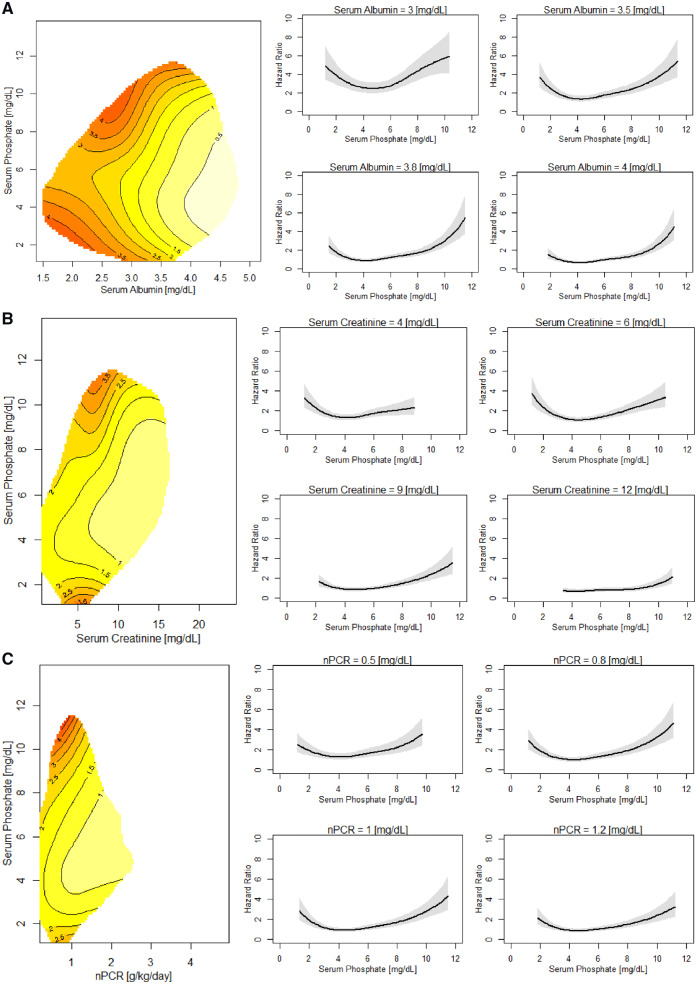
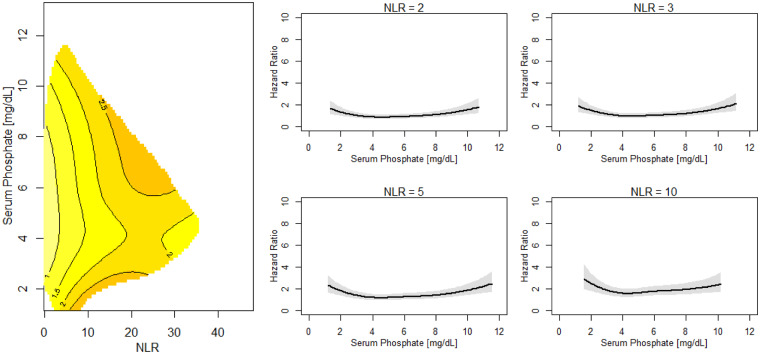
Both high and low serum phosphate levels are related to mortality over a wide range of levels of nutritional and inflammatory markers (Figure 4; Supplementary data, Figures S1A–S3B). In patients with low serum albumin (<3.8 g/dL) and creatinine (<5.5 mg/dL) as well as nPCR (approximately <0.7 g/kg/day), higher phosphate levels are associated with higher mortality. In patients with serum albumin >3.8 g/dL, creatinine >5.5 mg/dL and nPCR >0.7 g/kg/day, lower phosphate levels are associated with increased mortality risk. The adverse effect of higher or lower phosphate, respectively, on mortality can be seen over a wide NLR range (Figure 5; Supplementary data, Figure S4A and B). The observations appear consistently in patients who had at least one hospital admission (not due to VA procedures) during the first 6 months on HD (data not shown).
FIGURE 4.
Risk of death across levels of serum phosphate and (A) serum albumin, (B) serum creatinine and (C) nPCR. (A) Left: contour plot of the estimated hazard ratio of death in the next year as a joint function of serum phosphate and albumin for patients with serum phosphate and albumin fixed at their median values. Right: albumin slices at different serum phosphate ranges. Sections through the contour plot (left) at four albumin levels. The adjusted model was adjusted by gender, race, VA, MS, BMI, DM as comorbidity, CHF as comorbidity, creatinine, nPCR and NLR. (B) Left: contour plot of the estimated hazard ratio of death in the next year as a joint function of serum phosphate and nPCR for patients with serum phosphate and creatinine fixed at their median values. Right: creatinine slices at different serum phosphate ranges. Sections through the contour plot (left) at four creatinine levels. The adjusted model was adjusted for gender, race, VA, MS, BMI, DM as comorbidity, CHF as comorbidity, albumin, nPCR and NLR. (C) Left: contour plot of the estimated hazard ratio of death in the next year as a joint function of serum phosphate and nPCR for patients with serum phosphate and nPCR fixed at their median values. Right: nPCR slices at different serum phosphate ranges. Sections through the contour plot (left) at four nPCR levels. The adjusted model was adjusted by gender, race, VA, MS, BMI, DM as comorbidity, CHF as comorbidity, albumin, creatinine and NLR.
FIGURE 5.
Risk of death across levels of serum phosphate and NLR. Left: contour plot of the estimated hazard ratio of death in the next year as a joint function of serum phosphate and NLR for patients with serum phosphate and NLR fixed at their median values. Right: NLR slices at different serum phosphate ranges. Sections through the contour plot (left) at four NLR levels. The adjusted model was adjusted by gender, race, VA, MS, BMI, DM as comorbidity, CHF as comorbidity, serum albumin, creatinine and nPCR.
DISCUSSION
The main finding of our research into a large, diverse and representative US HD population is a U-shaped association between phosphate levels and all-cause mortality that persists across age strata and after multivariate adjustments for markers reporting nutrition and inflammation, and is independent of phosphate binder use.
Our study corroborates the adverse relationship between hyperphosphatemia and mortality in HD patients [24]. However, only a few studies have focused on this relationship as a function of age. Lertdumrongluk et al. [5] have shown a relationship between hyperphosphatemia and mortality in patients >75 years old. We corroborate this finding and extended our research into octogenarians, in which we describe an equally clear relationship between high phosphate and mortality. Therefore, there does not appear to be an age threshold above which the association between hyperphosphatemia and mortality vanishes. In contrast, the curves describing the association between low and high serum phosphate levels and increased mortality appeared even to be shifted to the left in the very elderly.
Next to hyperphosphatemia, our study also shows an increased mortality risk with phosphate levels <3.4–4.6 mg/dL (depends on age strata), the threshold by which hypophosphatemia is usually defined in the dialysis literature and guidelines [1, 2, 24]. The relationship between low phosphate levels and mortality was recently confirmed in a meta-analysis [24]. Our results appear to be in contrast to the study by Lertdumrongluk et al., who observed a relationship between low phosphate levels and mortality only in patients >65 years [5]. However, in their study, this relationship was present in the unadjusted analysis but lost statistical significance after adjusting for nutritional, inflammatory and social economic factors. In this study, even after extensive adjustment for demographic, economic, nutritional and inflammatory parameters, the relationship between low phosphate levels and mortality persisted across wide ranges of age. Also in the study of Lee et al., a relationship between phosphate levels <3.5 mg/dL and increased all-cause and infection-related mortalities were observed in the adjusted analysis, although results were not separated by age [6].
It is likely that hyperphosphatemia is directly causative of adverse outcomes because of its association with vascular calcifications [25, 26] and premature aging. Low phosphate levels are an indicator of inflammation, malnutrition or underlying disease, although in the presence of very low levels, also cardiopulmonary and leukocyte function may be impaired [6, 8]. Indeed, in our study, serum albumin, creatinine and nPCR were lower in patients with serum phosphate levels <4.0 mg/dL, whereas NLR as a marker of inflammation was higher. In a study in 3552 Portuguese dialysis patients, lean tissue index was lower in the group with serum phosphate <3.5 mg/dL, although also age was significantly higher in this group [10].
We additionally explored the interaction between phosphate levels with several inflammatory and nutritional parameters. Our study differs from previous ones by a separate continuous analysis between phosphate and a range of nutritional and inflammatory markers, including, serum creatinine, albumin and NLR, while adjusting for the others using smoothing spline ANOVA Cox proportional hazard model. Serum creatinine can be viewed as a marker influenced by muscle mass [27]. Previous studies have shown the relationship between these markers and outcome in HD patients [7, 28]. nPCR calculated from urea kinetic modeling reflects daily steady dietary protein intake. Various publications had illustrated the negative associations of lower nPCR and evaluated risk of death in HD patients [29–31]. Over a wide range of these parameters, the U-shaped curve between phosphate and mortality was observed. Most notably, the relationship between hyperphosphatemia and outcome persisted in patients with biochemical evidence of inflammation and malnutrition, whereas the risk associated with low phosphate levels also remained present in patients with normal serum albumin levels. We suggest that in this case, low phosphate levels may be a sign of impending malnutrition. Our group has previously indicated that various nutritional markers decline ∼6 months before hospitalizations. Our subset analysis in patients with at least one admission of hospitalization during the first 6 months on HD indicated that the adverse effect of low and high phosphate persisted, and in all levels of nutritional and inflammatory status [18]. Therefore, low phosphate levels, which persist after modification of phosphate binder therapy and hospitalizations, may prompt a call to action in and search for modifiable risk factors such as low protein intake. Low phosphate levels were also associated with increased mortality in patients using phosphate binders. As the use of phosphate binders in HD patients is generally associated with improved outcomes [19], we suggest that the relationship between low phosphate levels and mortality, in this case, is primarily a reflection of underlying nutritional abnormalities.
The strengths of this manuscript are the availability of a very large database and the use of innovative statistical techniques that study the interaction between two risk factors in relation to outcome in a continuous way. The drawbacks are its observational nature and the absence of data on residual renal function (RRF), which may when present be associated with lower phosphate levels. However, as RRF is generally protective in relation to mortality, the presence of RRF in a model is more likely to attenuate the relationship between low serum phosphate levels and adverse outcomes. Moreover, there is a lack of detailed information on dietary intake and nutritional intervention, which potentially could add additional insights to this study.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the FMCNA Team for putting together the data from patients treated in North America.
AUTHORS’ CONTRIBUTIONS
J.P.K., P.K., F.W.M., and X.Y. proposed the research idea and wrote the paper. J.P.K., F.M.v.d.S. and P.K. supervised the medical insights. X.Y., L.A.U. and Y.W. developed the study plan and performed the statistical analysis. J.G.R. helped revised the manuscript. All the other coauthors provided critical comments in developing the analysis plan and writing the manuscript, and approved the final version of the manuscript. The authors thank Herman Rosen, MD, FASN, Weil Cornell Medical College and Icahn School of Medicine at Mount Sinai, New York, NY, for reviewing the manuscript.
CONFLICT OF INTEREST STATEMENT
The author(s) received no specific funding for this work. F.M.v.d.S., J.P.K. and Y.W. declare no conflicts of interest. X.Y., J.G.R. and P.K. are employees of Renal Research Institute, a wholly owned subsidiary of Fresenius Medical Care (FMC). L.A.U. and F.W.M. are employees of Fresenius Medical Care Global Medical Office. L.A.U., P.K. and F.W.M. have stock in FMC. F.W.M. has directorships in American National Bank & Trust, Mid-Atlantic Renal Coalition and Sound Physicians. F.W.M. is the chairman of Pacific Care Renal Foundation 501(c)(3) nonprofit. P.K. received author honoraria from UpToDate. The results presented in this paper have not been published previously in whole or part, except had been selected as free communication at the ERA-EDTA Congress 2019 in Budapest, Hungary.
REFERENCES
- 1. National Kidney Foundation. KDOQI US commentary on the 2017 KDIGO clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Am J Kidney Dis 2017; 70: 737–751 [DOI] [PubMed] [Google Scholar]
- 2. Isakova T, Nickolas TL, Denburg M. et al. KDOQI US Commentary on the 2017 KDIGO Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Am J Kidney Dis 2017; 70: 737–751 [DOI] [PubMed] [Google Scholar]
- 3. Block GA, Hulbert-Shearon TE, Levin NW. et al. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 1998; 31: 607–617 [DOI] [PubMed] [Google Scholar]
- 4. Fernandez-Martin JL, Dusso A, Martinez-Camblor P. et al. Serum phosphate optimal timing and range associated with patients survival in haemodialysis: the COSMOS study. Nephrol Dial Transplant 2019; 34: 673–681 [DOI] [PubMed] [Google Scholar]
- 5. Lertdumrongluk P, Rhee CM, Park J. et al. Association of serum phosphorus concentration with mortality in elderly and nonelderly hemodialysis patients. J Ren Nutr 2013; 23: 411–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee JE, Lim JH, Jang HM. et al. Low serum phosphate as an independent predictor of increased infection-related mortality in dialysis patients: a prospective multicenter cohort study. PLoS One 2017; 12: e0185853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rymarz A, Gibińska J, Zajbt M. et al. Low lean tissue mass can be a predictor of one-year survival in hemodialysis patients. Ren Fail 2018; 40: 231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amanzadeh J, Reilly RF Jr.. Hypophosphatemia: an evidence-based approach to its clinical consequences and management. Nat Rev Nephrol 2006; 2: 136–148 [DOI] [PubMed] [Google Scholar]
- 9. Tentori F, Blayney MJ, Albert JM. et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2008; 52: 519–530 [DOI] [PubMed] [Google Scholar]
- 10. Garagarza C, Valente A, Caetano C. et al. Hypophosphatemia: nutritional status, body composition, and mortality in hemodialysis patients. Int Urol Nephrol 2017; 49: 1243–1250 [DOI] [PubMed] [Google Scholar]
- 11. Lorenzo V, Martin M, Rufino M. et al. Protein intake, control of serum phosphorus, and relatively low levels of parathyroid hormone in elderly hemodialysis patients. Am J Kidney Dis 2001; 37: 1260–1266 [DOI] [PubMed] [Google Scholar]
- 12. Kim JC, Kalantar-Zadeh K, Kopple JD.. Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J Am Soc Nephrol 2013; 24: 337–351 [DOI] [PubMed] [Google Scholar]
- 13. Zitt E, Lamina C, Sturm G. et al. Interaction of time-varying albumin and phosphorus on mortality in incident dialysis patients. Clin J Am Soc Nephrol 2011; 6: 2650–2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galassi A, Cupisti A, Santoro A. et al. Phosphate balance in ESRD: diet, dialysis and binders against the low evident masked pool. J Nephrol 2015; 28: 415–429 [DOI] [PubMed] [Google Scholar]
- 15. Kumar VA, Tilluckdharry N, Xue H. et al. Serum phosphorus levels, race, and socioeconomic status in incident hemodialysis patients. J Ren Nutr 2016; 26: 10–17 [DOI] [PubMed] [Google Scholar]
- 16. Ye X, Dekker MJE, Maddux FW. et al. Dynamics of nutritional competence in the last year before death in a large cohort of US hemodialysis patients. J Ren Nutr 2017; 27: 412–420 [DOI] [PubMed] [Google Scholar]
- 17. Usvyat LA, Barth C, Bayh I. et al. Interdialytic weight gain, systolic blood pressure, serum albumin, and C-reactive protein levels change in chronic dialysis patients prior to death. Kidney Int 2013; 84: 149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thijssen S, Wong MM, Usvyat LA. et al. Nutritional competence and resilience among hemodialysis patients in the setting of dialysis initiation and hospitalization. Clin J Am Soc Nephrol 2015; 10: 1593–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lopes AA, Tong L, Thumma J. et al. Phosphate binder use and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS): evaluation of possible confounding by nutritional status. Am J Kidney Dis 2012; 60: 90–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Navarro-Gonzalez JF, Mora-Fernandez C, Muros M. et al. Mineral metabolism and inflammation in chronic kidney disease patients: a cross-sectional study. Clin J Am Soc Nephrol 2009; 4: 1646–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gu C. Smoothing Spline ANOVA Models. New York, NY: Springer, 2013. [Google Scholar]
- 22. Wang Y. Smoothing Splines: Methods and Applications. Chapman & Hall, 2011 [Google Scholar]
- 23. Dekker M, Konings C, Canaud B. et al. Pre-dialysis fluid status, pre-dialysis systolic blood pressure and outcome in prevalent haemodialysis patients: results of an international cohort study on behalf of the MONDO initiative. Nephrol Dial Transplant 2018; 33: 2027–2034 [DOI] [PubMed] [Google Scholar]
- 24. Hou Y, Li X, Sun L. et al. Phosphorus and mortality risk in end-stage renal disease: a meta-analysis. Clin Chim Acta 2017; 474: 108–113 [DOI] [PubMed] [Google Scholar]
- 25. Goodman WG, Goldin J, Kuizon BD. et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 2000; 342: 1478–1483 [DOI] [PubMed] [Google Scholar]
- 26. Kooman JP, Kotanko P, Schols AM. et al. Chronic kidney disease and premature ageing. Nat Rev Nephrol 2014; 10: 732–742 [DOI] [PubMed] [Google Scholar]
- 27. Patel SS, Molnar MZ, Tayek JA. et al. Serum creatinine as a marker of muscle mass in chronic kidney disease: results of a cross-sectional study and review of literature. J Cachexia Sarcopenia Muscle 2013; 4: 19–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pecoits-Filho R, Lindholm B, Stenvinkel P.. The malnutrition, inflammation, and atherosclerosis (MIA) syndrome – the heart of the matter. Nephrol Dial Transplant 2002; 17 (Suppl 11): 28–31 [DOI] [PubMed] [Google Scholar]
- 29. Shinaberger CS, Kilpatrick RD, Regidor DL. et al. Longitudinal associations between dietary protein intake and survival in hemodialysis patients. Am J Kidney Dis 2006; 48: 37–49 [DOI] [PubMed] [Google Scholar]
- 30. Eriguchi R, Obi Y, Streja E. et al. Longitudinal associations among renal urea clearance-corrected normalized protein catabolic rate, serum albumin, and mortality in patients on hemodialysis. Clin J Am Soc Nephrol 2017; 12: 1109–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hakim RM, Levin N.. Malnutrition in hemodialysis patients. Am J Kidney Dis 1993; 21: 125–137 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.