Abstract
Background
The hepatokine fetuin-A, released by the human liver, promotes pro-inflammatory effects of perivascular fat. The involvement of inflammation in type 2 diabetes mellitus (T2DM) can affect the kidney and contribute to the development of diabetic kidney disease. Therefore we examined the association of urinary fetuin-A protein fragments with renal damage in T2DM patients.
Methods
Urinary peptides of 1491 individuals using proteome data available from the human urine proteome database were analysed. Prediction of proteases involved in urinary peptide generation was performed using the Proteasix tool.
Results
We identified 14 different urinary protein fragments that belong to the region of the connecting peptide (amino acid 301–339) of the total fetuin-A protein. Calpains (CAPN1 and CAPN2), matrix metalloproteinase and pepsin A-3 were identified as potential proteases that were partially confirmed by previous in vitro studies. Combined fetuin-A peptides (mean of amplitudes) were significantly increased in T2DM patients with kidney disease and to a lesser extent with cardiovascular risk. Furthermore, fetuin-A peptide levels displayed a significant negative correlation with baseline estimated glomerular filtration rate (eGFR) values (r = −0.316, P < 0.0001) and with the slope (%) of eGFR per year (r = −0.096, P = 0.023). A multiple regression model including fetuin-A peptide and albuminuria resulted in a significantly improved correlation with eGFR (r = −0.354, P < 0.0001) compared with albuminuria, indicating an added value of this novel biomarker.
Conclusions
The urinary proteome analysis demonstrated the association of fetuin-A peptides with impaired kidney function in T2DM patients. Furthermore, fetuin-A peptides displayed early signs of kidney damage before albuminuria appeared and therefore can be used as markers for kidney disease detection.
Keywords: biomarkers, diabetic kidney disease, fetuin-A, proteomics, urinary peptides
INTRODUCTION
Fetuin-A, also called alpha-2-Heremans–Schmid glycoprotein, is a 46-kDa protein present in the serum and synthesized by hepatocytes (hepatokine). The precursor protein of fetuin-A is composed of three chains, but only the A and B chains, which are encoded by a single messenger RNA transcript, form the active protein [1]. The third chain (connecting peptide) links the A and B chains. Apparently this connecting 40-amino-acid fragment is cleaved by limited proteolysis in a post-translational step that produces A and B chains joined by a disulphide bond. Furthermore, multiple post-translational modifications have been reported [2]. Thus fetuin-A is a secreted partially phosphorylated glycoprotein with complex proteolytic processing that circulates in the blood and extracellular fluids.
Investigations on proteolytic processing might contribute to the understanding of the mechanism to activate fetuin-A. However, specific proteases that are involved in releasing the connecting peptide are more or less unknown. Previously several groups attempted to determine the cleavage sites for the secretion of the connecting peptide. One study revealed that fetuin-A from human plasma is cleaved in its connecting peptide region when digested with different proteases (trypsin, chymotrypsin, elastase plasmin, kallikrein, thrombin and renin) [3]. Amino sequence analysis of the generated fragments demonstrated that only chymotrypsin cleaves the Leu–Leu bond flanking the N-terminal portion of the connecting peptide region. The C-terminus of this fetuin-A peptide, which was generated with chymotrypsin, is at AA position 321. Additionally, matrix metalloproteinases (MMPs) were supposed to be involved in fetuin-A processing, since the bovine homologue was found to be cleaved by MMP3 and MMP7 [4] and interactions between human fetuin-A and MMPs (MMP2, MMP7 and MMP9) have been reported [5]. Furthermore, fetuin-A was identified as a substrate of meprin metalloproteinases (MEP1A and MEP1B) [6]. In a recent study, fetuin-A was identified in vitro as a substrate of transmembrane protease serine 6 (TMPRSS6; matriptase-2) [7]. Arginine and lysine residues located within the connecting peptide of fetuin-A were identified as cleavage sites for TMPRSS6. However, all previous studies investigating the proteolytic cleavage of fetuin-A did not compare the in vitro generated peptides with physiologically occurring peptides in humans.
Fetuin-A plays a role as a physiological inhibitor of insulin receptor tyrosine kinase associated with insulin resistance [8, 9] and a negative acute phase reactant [10]. It also regulates bone remodelling [11] and is a potent systemic calcification inhibitor [12]. The role of fetuin-A in the pathogenesis of type 2 diabetes mellitus (T2DM), obesity, non-alcoholic fatty liver disease (NAFLD) and dyslipidaemia remains unclear and is still under discussion. In a previous study, levels of fetuin-A were increased in NAFLD and the increase of fetuin-A in patients with NAFLD is closely associated with fat accumulation in the liver [13, 14]. Also, Stefan et al. [15, 16] reported a strong relationship between plasma fetuin-A and insulin resistance in patients with NAFLD and pointed to fetuin-A as a predictor of T2DM in humans. A high serum fetuin-A level is a marker of T2DM incidence after adjusting for risk factors [17]. A recent study showed the effect of incretin treatment in patients with T2DM and NAFLD on fetuin-A. In the liraglutide group, fetuin-A was positively correlated with weight, body mass index (BMI) and waist circumference. This highlights that fetuin-A levels are more associated with hepatic fat content rather than glucose levels [18].
Furthermore, elevated fetuin-A levels impair renal function by inducing pro-inflammatory signalling in vascular and glomerular cells [19, 20]. This may affect the glomerular function and may contribute to kidney disease in T2DM. Therefore we examined the correlation of urinary fetuin-A protein fragments with renal function, measured as estimated glomerular filtration rate (eGFR) in a large cohort of T2DM patients.
MATERIALS AND METHODS
Patients’ proteomics and clinical data
For the urinary proteome analysis, capillary electrophoresis coupled to mass spectrometry (CE-MS) was used and all data were stored in the Human Urinary Proteome Database [21]. In our approach, each peptide is characterized by its molecular mass (Da), CE migration (min) and signal intensity (amplitude). For normalization of analytical and urine dilution variances, amplitudes of the CE-MS raw data were normalized relative to 29 internal standard peptides generally present in at least 90% of all urine samples with small relative standard deviations [22]. The database available at Mosaiques Diagnostics (Hannover, Germany) also included anonymized clinical information of participants enrolled in several studies. All underlying studies complied with the Declaration of Helsinki [23] and received ethical approval from the competent institutional review boards. All participants gave informed written consent. The eGFR was calculated from serum creatinine by the Chronic Kidney Disease Epidemiology Collaboration equation [24]. To harmonize baseline albuminuria across cohorts, we expressed urinary albumin excretion (UAE) in micrograms per minute. This involved a conversion from milligrams per 24 h and a conversion from milligrams per litre, assuming a diuresis of 1500 mL/day. The cut-off threshold for UAE was 20 µg/min, approximately corresponding to 24-h albuminuria of 30 mg [25]. Table 1 summarizes the patients’ data, including eGFR, UAE, age, gender, BMI and cardiovascular risk factors (coronary artery disease, heart failure, dyslipidaemia, hypertension and arteriosclerosis). Diabetic kidney disease (DKD) was defined for patients with eGFR <60 mL/min/1.73 m2 and/or UAE >200 µg/min. The total number of data sets of the individuals in the Human Urinary Proteome Database used for this study was 1491.
Table 1.
Patient’s data
| Characteristics | Patients with DKD | Patients without DKD | Significance |
|---|---|---|---|
| Patients, n | 647 | 844 | |
| Age (years) | 64.43 ± 11.19 | 58.77 ± 8.66 | ** |
| eGFR (mL/min/1.73 m2) | 49.36 ± 22.33 | 75.71 ± 15.01 | ** |
| UAE (µg/min) | 370.38 ± 784.16 | 21.16 ± 34.94 | ** |
| BMI )kg/m2) | 31.11 ± 4.98 | 30.05 ± 5.16 | ** |
| Fetuin-A peptide amplitude | 1799.39 ± 4016.43 | 389.59 ± 487.38 | ** |
| Gender (male), % | 53.06 | 61.10 | ** |
| Cardiovascular risk factors, % | 30.76 | 7.23 | ** |
| Coronary artery disease, % | 6.65 | 1.42 | |
| Dyslipidaemia, % | 15.46 | 5.21 | |
| Heart failure, % | 4.02 | 0.00 | |
| Hypertension, % | 3.71 | 0.47 | |
| Arteriosclerosis, % | 3.71 | 0.47 |
Age, eGFR, UAE, BMI and fetuin-A peptide amplitudes are presented as mean ± SD.
P < 0.01.
Protease prediction
The open-source tool for protease prediction Proteasix (www.proteasix.org) was used for the analysis to link naturally occurring peptides in urine to the proteases potentially involved in their generation [26]. Proteasix uses information about naturally occurring peptides, that is, the corresponding protein UniProt identifier and start/stop amino acid position to predict potential cleaving proteases. Proteasix retrieves information about cleavage sites from protease databases (MEROPS and BRENDA) considering also cleavage site restrictions (from the ENZYME database). A list of predicted proteases is generated as a result of the analysis.
Statistical methods
For statistical analysis, MedCalc software version 12.7.5.0 (SAS Institute, Cary, NC, USA) was used. Correlations were calculated using Pearson’s method and adjustment for confounding factors was performed using multiple regression analysis. A P-value of 0.05 was selected as the significance level.
RESULTS
Release of fetuin-A peptides in urine
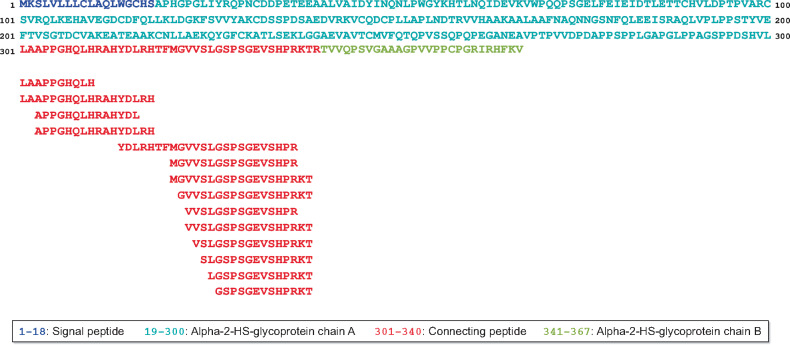
We investigated the association of urinary fetuin-A peptides that were identified previously and stored in the Human Urinary Proteome Database [21]. In total, 14 fetuin-A peptides were identified in urine of the cohort studied. As depicted in Figure 1, these peptides showed a high degree of overlap, covering only the connecting peptide of the fetuin-A protein [from amino acid (AA) 301–339, red sequence]. Interestingly, there was no peptide found containing the C-terminus at AA position 340, the last AA of the connecting peptide in the fetuin-A protein. Most of the peptides demonstrated an inconspicuous cleavage site at their N-terminus and some peptides also at their C-terminus. We identified two fetuin-A peptides with their N-termini at AA position 301, the beginning of the connecting peptide of fetuin-A. In addition, two more peptides comprised an N-terminus at AA position 303. Furthermore, there were seven fetuin-A peptides with their C-termini at AA position 339 (C-terminus of the connecting peptide) and three peptides with a C-terminus at AA position 338, positions indicative of a specific proteolytic cleavage site. Also, there were two peptides with their N-termini at position 322, which could indicate another specific proteolytic cleavage site between phenylalanine and methionine (AA321 and AA322). The further peptides with a stepwise decreasing AA number could be the result of non-specific exopeptidase digests of their N-terminus.
FIGURE 1:
Identified endogenous human urinary fetuin-A peptides and their position in the fetuin-A protein sequence. Urinary fetuin-A peptides were previously identified from a large cohort of 1491 individuals and stored in the Human Urinary Proteome Database [21].
By using bioinformatics approaches, like the open-source tool Proteasix [26], on naturally occurring peptides as cleaved end products, it is possible to track back the enzymes responsible for the generation of these peptides. The majority of human proteases have several protein targets and likewise, one peptide sequence might be cleaved by different proteases. By using Proteasix for the search of fetuin-A as observed substrate, four different proteases were listed: caspase-6, plasmin, neutrophil elastase (ELANE) and TMPRSS6 (http://proteasix.cs.man.ac.uk/GUI_substrate/P02765.txt). However, for most of these proteases, the cleavage sites do not fit exactly with the identified peptides. Only the TMPRSS6 could be a possible protease for the cleavage between AA337 and AA338 of fetuin-A [7]. To further predict proteases possibly involved in the generation of the urinary fetuin-A peptides, in silico protease prediction was performed with Proteasix. The analysis yielded a list of 32 endopeptidases putatively responsible for the generation of the identified fetuin-A peptides in urine (Supplementary data, Table S1). From the 32 predicted proteases, 4 proteases were most frequently selected (predicted protease in ≥50% of total cleavage assignments), namely the calpains CAPN1 and CAPN2, MMP7 and pepsin A-3 (PGA3). Furthermore, when focusing on the potential cleavage sites (AA positions 301 and 339) of the fetuin-A protein for the release of the connecting peptide, CAPN1, CAPN2, MMP7 and PGA3 were predicted. By consideration of the truncated N- and C-terminus of the connecting peptide (AA positions 303 and 337), only the two calpains (CAPN1 and CAPN2) remained as potential proteases for release of the identified urinary fetuin-A peptides. Interestingly, the observed protease TMPRSS6 (see above) was not on the list of the predicted proteases.
Association of fetuin-A peptides with diabetic complications
We used 1491 relevant proteome datasets from patients with T2DM to examine urinary fetuin-A peptides in patients with and without related comorbidities (DKD and cardiovascular risk; see Table 1). Based on the assumption that all the fetuin-A peptides depict the total fetuin-A protein abundance, we calculated the mean amplitude of all peptides in each data set. As shown in Figure 2a, the mean fetuin-A peptide amplitude was significantly (P < 0.0001) increased in T2DM patients with DKD compared with those without kidney disease. Furthermore, we investigated the levels of fetuin-A peptides in T2DM patients suffering from any cardiovascular risk factors (like coronary artery disease, heart failure, arteriosclerosis, dyslipidaemia and hypertension). The total cohort comprised 260 patients with these risk factors, who also had significantly (P = 0.0007) higher fetuin-A levels than diabetes patients without these risk factors (Figure 2b).
FIGURE 2:
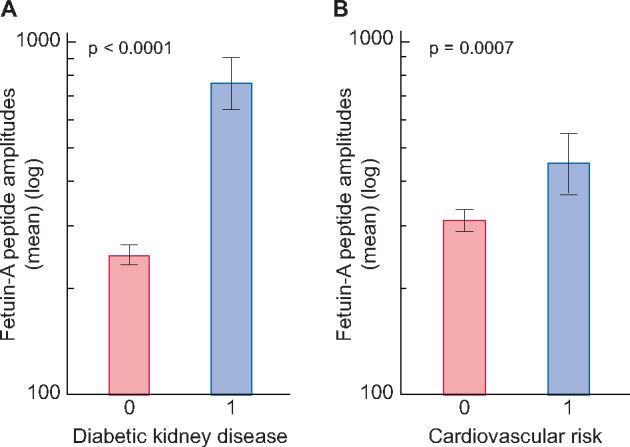
Mean fetuin-A peptide amplitudes (± 95% CI) in urine of T2DM patients with or without (a) DKD (characterized in Table 1) and (b) cardiovascular risk factors, comprising 199 patients with DKD and 61 without DKD (characterized in Table 1).
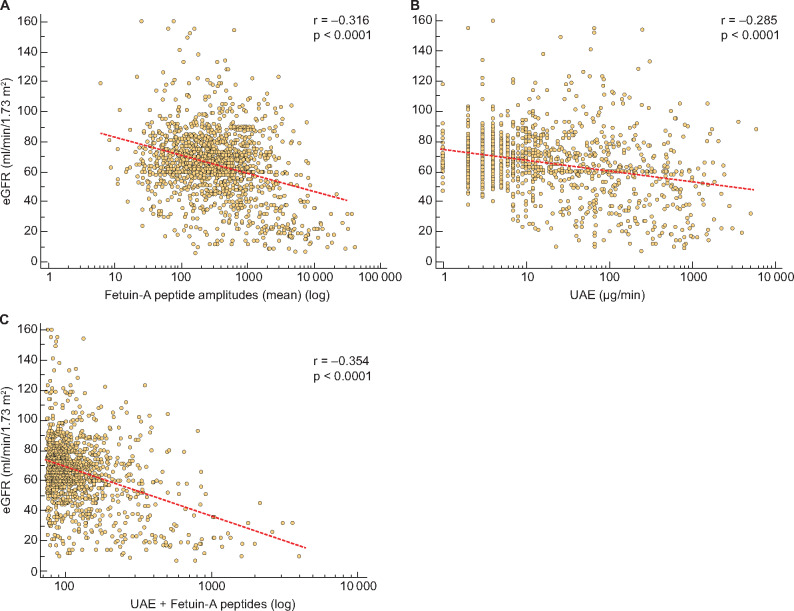
To further investigate the association of fetuin-A with kidney function, we correlated the mean peptide amplitudes with the eGFR of all T2DM patients. As depicted in Figure 3a, a significant negative correlation between fetuin-A peptides and the eGFR was detected (r = −0.316, P < 0.0001). Likewise, the correlation of T2DM patients with kidney disease (n = 647) showed a significant negative correlation (r = −0.256, P < 0.0001). Urinary albumin also showed a significant negative correlation with eGFR, but with a lower correlation factor than with fetuin-A peptides (Figure 3b;r = −0.285, P < 0.0001). To verify that urinary fetuin-A peptides were not depending on co-founding factors, we used partial correlation analysis. After adjustment for urinary albumin, cardiovascular risk factors, gender and age as covariables, the mean amplitude of fetuin-A peptides is still statistically significantly associated with eGFR (r = −0.230, P < 0.0001). To investigate the added value of fetuin-A peptide measurement to clinically used albuminuria, we generated a model with multiple regression analyses with fetuin-A as an independent variable. As depicted in Figure 3c, the generated model resulted in a correlation coefficient (r = −0.354, P < 0.0001) that was significantly higher (P = 0.045) than the correlation with urinary albumin alone.
FIGURE 3:
Scatter diagrams for the correlation of eGFR with (a) mean fetuin-A peptide amplitudes, (b) UAE and (c) regression model (UAE + fetuin-A peptides). Urinary fetuin-A peptides were previously identified and stored in the Human Urinary Proteome Database [21] from 1491 individuals.
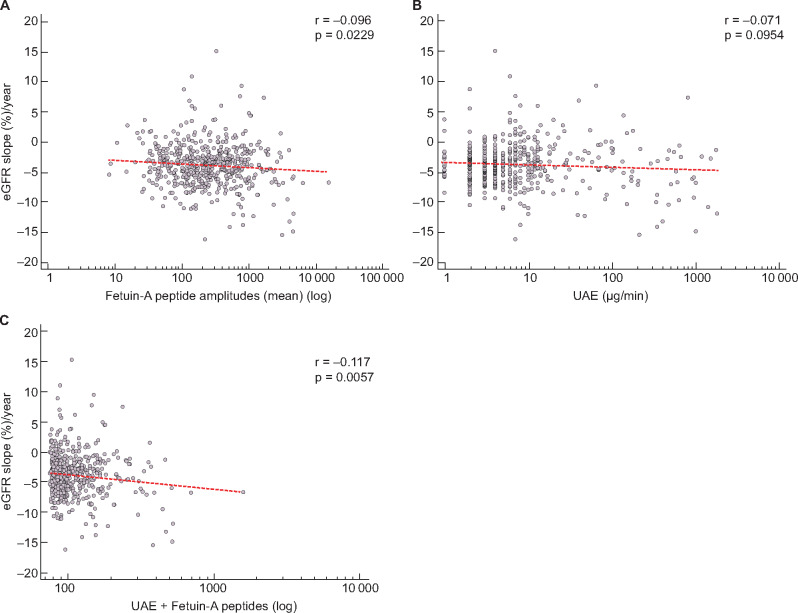
In the next step, we further examined the prognostic value of fetuin-A levels in urine for kidney disease progression. Within the total cohort, follow-up data of eGFR for 559 T2DM patients were available. Based on these follow-up data, we calculated the percentage slope per year of eGFR and correlated these values with the fetuin-A peptides. As depicted in Figure 4a, this resulted in a small but significant negative association (r = −0.096, P = 0.0229). In contrast, urinary albumin was not significantly correlated with eGFR slope (%) per year (Figure 4b;r = −0.071, P = 0.0954). After adjustment for the remaining parameters (age, gender and cardiovascular risk), the mean amplitudes of fetuin-A peptides remained significantly associated with eGFR slope (%) per year (r = −0.086, P = 0.0428). By using the multiple regression model (UAE + fetuin-A), the added value of fetuin-A for albuminuria resulted in a significant correlation (Figure 4c;r = −0.117, P = 0.0057) with eGFR slope (%) per year, in contrast to the non-significant correlation with albuminuria alone.
FIGURE 4:
Scatter diagrams for the correlation of the percentage slope of eGFR per year with (a) mean fetuin-A peptide amplitudes, (b) UAE and (c) regression model (UAE + fetuin-A peptides). Urinary fetuin-A peptides were previously identified and stored in the Human Urinary Proteome Database [21] from 559 individuals with follow-up data for eGFR.
DISCUSSION
In previous studies, higher levels of fetuin-A in blood were associated with an increased risk of T2DM development [27, 28]. The underlying mechanism likely involved insulin resistance caused by the impact of fetuin-A on diverse physiological processes, for example, glucose transporter type 4 translocation and protein kinase B (Akt) activation [27]. In addition, some studies implicated fetuin-A as a potential biomarker for T2DM-related complications, like NAFLD and cardiovascular disease [29]. However, chronic kidney disease (CKD) is also a common comorbidity of T2DM and therefore we supposed that there might also be an association of fetuin-A with DKD. Due to the fact that fetuin-A peptide levels are highly correlated in plasma and urine [30], we used the Human Urinary Proteome Database to investigate urinary fetuin-A protein fragments in T2DM patients to illuminate this hypothesis.
Based on a large patient cohort (n = 1491), we were able to determine a significant difference in the fetuin-A peptide levels in T2DM patients with and without complications like DKD or cardiovascular risk. Furthermore, our data showed that urinary levels of fetuin-A peptides are correlated with kidney function, determined by eGFR, better than albuminuria. Also, based on a subset of patients with follow-up data (n = 560), we were able to demonstrate that fetuin-A peptide levels are negatively correlated with eGFR slope, indicating further development of kidney function. The association of urinary fetuin-A with eGFR decline does not appear to be clinically meaningful but demonstrates that the fetuin-A peptide levels increase earlier than albuminuria appears during DKD progression. Therefore the fetuin-A measurement can provide an added value to urinary albumin measurement in the clinical routine, provided that the conversion of urinary albumin from milligrams per litre, assuming a diuresis of 1500 mL/day, does not decrease the potential of UAE in our study.
The findings of this study could be indirectly confirmed with previous studies because some fetuin-A peptides are also included in the peptide-based classifier CKD273 [31]. This classifier was validated in several studies for the early diagnosis and prediction of CKD progression [32, 33] as well as prediction of the cardiovascular outcome in diabetes patients [34]. In general, these studies showed that using a large number of clinical and proteomics data available at the Human Urinary Proteome Database can be very useful for the evaluation of specific urinary peptide levels under different disease conditions.
The identification of urinary naturally occurring fetuin-A peptides can also provide further insights into the cleavage of the fetuin-A protein. All identified endogenous peptides were derived from the connecting peptide linking the A chain and the B chain of the fetuin-A protein (see Figure 1). This fact verifies the hypothesis that the connecting sequence is cleaved in a post-translational step by proteolysis before fetuin, as A and B chains joined by a disulphide bond, is released into the circulation [1, 35]. Furthermore, the highest AA number of the identified urinary peptides ends at position 339 of the fetuin-A protein and not at position 340. This was also found in other studies [3, 36] and underlined that the endoproteolytic cleavage of the C-terminus and the exoproteolytic removal of arginine at position 340 are closely associated with the site of biosynthesis in the hepatocytes [2] and most probably do not occur in the plasma and urine as well.
The study of Nawratil et al. [3] demonstrated that chymotrypsin cleaves the Leu–Leu bond flanking the N-terminal portion of the connecting peptide region and the C-terminus of this generated fetuin-A peptide is at AA position 321 [undefined]. This is in accordance with our findings that there might be a specific proteolytic cleavage site between phenylalanine and methionine (AA321–AA322). However, the cleavage site of the C-terminus from the connecting peptide could not be identified in the previous study. Furthermore, we can confirm a cleavage of the truncated C-terminus between the position at AA337 and AA338 with three of the identified fetuin-A peptides [3]. However, the in silico analysis with Proteasix did not suggest TMPRSS6 for this cleavage site, but calpains (CAPN1 or CAPN2) or plasminogen. Our findings that CAPN1 or CAPN2 are potential proteases for the release of the identified fetuin-A peptides are not shown directly in previous studies. Due to the fact that calpains are intracellular proteinases and fetuin-A is an extracellular protein secreted predominantly by the liver, their interaction under physiologic conditions is questionable. However, in a study by Mellgren et al. [37], this interaction was investigated [undefined]. Furthermore, it also clearly outlined the contribution of calpains to chronic complications of diabetes mellitus, such as DKD [37]. For example, extracellular calpains (triggered by their inhibitor calpastatin) contribute to tubule repair, partly by inducing epithelial cell migration, whereas intracellular calpains triggered by synthetic inhibitors PD150606 and E-64 participate in tubule injury, partly by increasing oxidative stress [38, 39].
Additionally, MMP7 was previously demonstrated to play a role in the release of fetuin-A peptides in urine [4, 5]. These findings can also be confirmed with our study. We found several MMPs potentially responsible for production of the identified urinary fetuin-A peptides. However, MMP7 was the protease involved with the highest frequency (57%) of all cleavages. Elevated serum MMP7 levels have already been previously found in patients with T2DM, diabetic renal disease and diabetic diastolic dysfunction [40, 41] and therefore this protease seems to be one of the best prospects for the cleavage of the connecting peptide and the activation of fetuin-A. Furthermore, MEP1A was previously reported to be a potential protease for fetuin-A [6, 42]. In our in silico analysis we also identified MEP1A. However, the cleavage frequency of MEP1A was only 29% and we found other proteases with higher cleavage frequencies for the identified urinary peptides. Furthermore, we also identified PGA3 as a potential protease for fetuin-A, but this finding was not reported before. Pepsin is synthesized in the gastric mucosa and secreted into the stomach as the zymogen pepsinogen and has its pH optimum at 1.5–2. Therefore it seems unreasonable that this protease could be involved in the digestion of fetuin-A. However, a small amount of pepsinogen was also found in the bloodstream and secreted in the urine; the latter is sometimes referred to as uropepsinogen [43, 44]. Furthermore, a previous study by Senmaru et al. [45] showed that serum pepsinogen ratios are correlated with albuminuria in patients with T2DM. Therefore PGA3 might also be a possible protease for the digestion of fetuin-A, notably with respect to the association with DKD. However, all predicted protease activities have to be confirmed by lab-based generated evidence, particularly if there is no relation to the cleavage of fetuin-A reported.
In conclusion, the urinary proteome analysis demonstrated the association of fetuin-A peptides with impaired kidney function in T2DM patients. Furthermore, fetuin-A peptides displayed progression to kidney disease earlier than albuminuria and can be used as new markers for kidney dysfunction. Furthermore, this study provides more detailed insights into the mechanisms of how fetuin-A can potentially be cleaved to release its connecting peptide.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge the technical personnel at Mosaiques Diagnostics for the proteome analysis of the urine samples and those in charge of providing the urine samples of the different cohorts. The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
FUNDING
The research presented in this article was supported by the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie grant agreement 642937 (RENALTRACT; MSCA-ITN-2014-642937). Furthermore, E.S. received funding from the German Center for Diabetes Research.
AUTHORS’ CONTRIBUTIONS
P.M. and P.Z. participated in interpretation of the results and were responsible for statistical analyses and the first draft of the manuscript. E.S. and H.M. conceived and planned the experiments, aided in interpreting the results and worked on the manuscript. All authors read, critically revised and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
P.M. and P.Z. are employees of Mosaiques Diagnostics. H.M. is co-founder and a shareholder of Mosaiques Diagnostics. The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Lee CC, Bowman BH, Yang FM.. Human α2-HS-glycoprotein: the A and B chains with a connecting sequence are encoded by a single mRNA transcript. Proc Natl Acad Sci USA 1987; 84: 4403–4407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jahnen-Dechent W, Trindl A, Godovac-Zimmermann J. et al. Posttranslational processing of human α2-HS glycoprotein (human fetuin). Evidence for the production of a phosphorylated single-chain form by hepatoma cells. Eur J Biochem 1994; 226: 59–69 [DOI] [PubMed] [Google Scholar]
- 3. Nawratil P, Lenzen S, Kellermann J. et al. Limited proteolysis of human α2-HS glycoprotein/fetuin. Evidence that a chymotryptic activity can release the connecting peptide. J Biol Chem 1996; 271: 31735–31741 [DOI] [PubMed] [Google Scholar]
- 4. Kubler D, Gosenca D, Wind M. et al. Proteolytic processing by matrix metalloproteinases and phosphorylation by protein kinase CK2 of fetuin-A, the major globulin of fetal calf serum. Biochimie 2007; 89: 410–418 [DOI] [PubMed] [Google Scholar]
- 5. Ochieng J, Green B.. The interactions of alpha 2HS glycoprotein with metalloproteinases. Biochem Mol Biol Int 1996; 40: 13–20 [DOI] [PubMed] [Google Scholar]
- 6. Jefferson T, Uf Dem KU, Bellac C. et al. The substrate degradome of meprin metalloproteases reveals an unexpected proteolytic link between meprin beta and ADAM10. Cell Mol Life Sci 2013; 70: 309–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stirnberg M, Maurer E, Arenz K. et al. Cell surface serine protease matriptase-2 suppresses fetuin-A/AHSG-mediated induction of hepcidin. Biol Chem 2015; 396: 81–93 [DOI] [PubMed] [Google Scholar]
- 8. Ren G, Kim T, Papizan JB. et al. Phosphorylation status of fetuin-A is critical for inhibition of insulin action and is correlated with obesity and insulin resistance. Am J Physiol Endocrinol Metab 2019; 317: E250–E260 [DOI] [PubMed] [Google Scholar]
- 9. Meex RCR, Watt MJ.. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol 2017; 13: 509–520 [DOI] [PubMed] [Google Scholar]
- 10. Mukhopadhyay S, Mondal SA, Kumar M. et al. Proinflammatory and antiinflammatory attributes of fetuin-a: a novel hepatokine modulating cardiovascular and glycemic outcomes in metabolic syndrome. Endocr Pract 2014; 20: 1345–1351 [DOI] [PubMed] [Google Scholar]
- 11. Szweras M, Liu D, Partridge EA. et al. α2-HS glycoprotein/fetuin, a transforming growth factor-β/bone morphogenetic protein antagonist, regulates postnatal bone growth and remodeling. J Biol Chem 2002; 277: 19991–19997 [DOI] [PubMed] [Google Scholar]
- 12. Schafer C, Heiss A, Schwarz A. et al. The serum protein α2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest 2003; 112: 357–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stefan N, Hennige AM, Staiger H. et al. α2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care 2006; 29: 853–857 [DOI] [PubMed] [Google Scholar]
- 14. Reinehr T, Roth CL.. Fetuin-A and its relation to metabolic syndrome and fatty liver disease in obese children before and after weight loss. J Clin Endocrinol Metab 2008; 93: 4479–4485 [DOI] [PubMed] [Google Scholar]
- 15. Stefan N, Schick F, Haring HU.. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med 2014; 371: 2236–2237 [DOI] [PubMed] [Google Scholar]
- 16. Stefan N, Sun Q, Fritsche A. et al. Impact of the adipokine adiponectin and the hepatokine fetuin-A on the development of type 2 diabetes: prospective cohort- and cross-sectional phenotyping studies. PLoS One 2014; 9: e92238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stefan N, Fritsche A, Weikert C. et al. Plasma fetuin-A levels and the risk of type 2 diabetes. Diabetes 2008; 57: 2762–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang LY, Qu XN, Sun ZY. et al. Effect of liraglutide therapy on serum fetuin A in patients with type 2 diabetes and non-alcoholic fatty liver disease. Clin Res Hepatol Gastroenterol 2020; doi: 10.1016/j.clinre.2020.01.007 [DOI] [PubMed] [Google Scholar]
- 19. Siegel-Axel DI, Ullrich S, Stefan N. et al. Fetuin-A influences vascular cell growth and production of proinflammatory and angiogenic proteins by human perivascular fat cells. Diabetologia 2014; 57: 1057–1066 [DOI] [PubMed] [Google Scholar]
- 20. Wagner R, Machann J, Guthoff M. et al. The protective effect of human renal sinus fat on glomerular cells is reversed by the hepatokine fetuin-A. Sci Rep 2017; 7: 2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Latosinska A, Siwy J, Mischak H. et al. Peptidomics and proteomics based on CE-MS as a robust tool in clinical application: the past, the present, and the future. Electrophoresis 2019; 40: 2294–2308 [DOI] [PubMed] [Google Scholar]
- 22. Jantos-Siwy J, Schiffer E, Brand K. et al. Quantitative urinary proteome analysis for biomarker evaluation in chronic kidney disease. J Proteome Res 2009; 8: 268–281 [DOI] [PubMed] [Google Scholar]
- 23.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–2194 [DOI] [PubMed] [Google Scholar]
- 24. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Basi S, Fesler P, Mimran A. et al. Microalbuminuria in type 2 diabetes and hypertension: a marker, treatment target, or innocent bystander? Diabetes Care 2008; 31(Suppl 2): S194–S201 [DOI] [PubMed] [Google Scholar]
- 26. Klein J, Eales J, Zurbig P. et al. Proteasix: a tool for automated and large-scale prediction of proteases involved in naturally occurring peptide generation. Proteomics 2013; 13: 1077–1082 [DOI] [PubMed] [Google Scholar]
- 27. Bourebaba L, Marycz K.. Pathophysiological implication of fetuin-A glycoprotein in the development of metabolic disorders: a concise review. J Clin Med 2019; 8:2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huth C, Bauer A, Zierer A. et al. Biomarker-defined pathways for incident type 2 diabetes and coronary heart disease-a comparison in the MONICA/KORA study. Cardiovasc Diabetol 2020; 19: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nascimbeni F, Romagnoli D, Ballestri S. et al. Do nonalcoholic fatty liver disease and fetuin-A play different roles in symptomatic coronary artery disease and peripheral arterial disease? Diseases 2018; 6: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Magalhaes P, Pontillo C, Pejchinovski M. et al. Comparison of urine and plasma peptidome indicates selectivity in renal peptide handling. Proteomics Clin Appl 2018; 12: e1700163. [DOI] [PubMed] [Google Scholar]
- 31. Good DM, Zürbig P, Argiles A. et al. Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol Cell Proteomics 2010; 9: 2424–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pontillo C, Jacobs L, Staessen JA. et al. A urinary proteome-based classifier for the early detection of decline in glomerular filtration. Nephrol Dial Transplant 2016; 32: 1510–1516 [DOI] [PubMed] [Google Scholar]
- 33. Pontillo C, Zhang Z, Schanstra J. et al. Prediction of chronic kidney disease stage 3 by CKD273, a urinary proteomic biomarker. Kidney Int Rep 2017; 2: 1066–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Verbeke F, Siwy J, Van BW. et al. The urinary proteomics classifier chronic kidney disease 273 predicts cardiovascular outcome in patients with chronic kidney disease. Nephrol Dial Transplant 2019; doi: 10.1093/ndt/gfz242 [DOI] [PubMed] [Google Scholar]
- 35. Gejyo F, Chang JL, Burgi W. et al. Characterization of the B-chain of human plasma alpha2HS-glycoprotein. The complete amino acid sequence and primary structure of its heteroglycan. J Biol Chem 1983; 258: 4966–4971 [PubMed] [Google Scholar]
- 36. Kellermann J, Haupt H, Auerswald EA. et al. The arrangement of disulfide loops in human alpha2-HS glycoprotein. Similarity to the disulfide bridge structures of cystatins and kininogens. J Biol Chem 1989; 264: 14121–14128 [PubMed] [Google Scholar]
- 37. Wan TT, Li XF, Sun YM. et al. Role of the calpain on the development of diabetes mellitus and its chronic complications. Biomed Pharmacother 2015; 74: 187–190 [DOI] [PubMed] [Google Scholar]
- 38. Breedlove SM, Arnold AP.. Hormonal control of a developing neuromuscular system. I. Complete demasculinization of the male rat spinal nucleus of the bulbocavernosus using the anti-androgen flutamide. J Neurosci 1983; 3: 417–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chatterjee PK, Todorovic Z, Sivarajah A. et al. Inhibitors of calpain activation (PD150606 and E-64) and renal ischemia-reperfusion injury. Biochem Pharmacol 2005; 69: 1121–1131 [DOI] [PubMed] [Google Scholar]
- 40. Ban CR, Twigg SM, Franjic B. et al. Serum MMP-7 is increased in diabetic renal disease and diabetic diastolic dysfunction. Diabetes Res Clin Pract 2010; 87: 335–341 [DOI] [PubMed] [Google Scholar]
- 41. Yang PJ, Ser KH, Lin MT. et al. Diabetes associated markers after bariatric surgery: fetuin-A, but not matrix metalloproteinase-7, is reduced. Obes Surg 2015; 25: 2328–2334 [DOI] [PubMed] [Google Scholar]
- 42. Hedrich J, Lottaz D, Meyer K. et al. Fetuin-A and cystatin C are endogenous inhibitors of human meprin metalloproteases. Biochemistry 2010; 49: 8599–8607 [DOI] [PubMed] [Google Scholar]
- 43. Samloff IM, Townes PL.. Electrophoretic heterogeneity and relationships of pepsinogens in human urine, serum, and gastric mucosa. Gastroenterology 1970; 58: 462–469 [PubMed] [Google Scholar]
- 44. Akutsu T, Saito H, Iwase H. et al. The applicability of ELISA detection of gastric mucosa-expressing proteins for the identification of vomit. Int J Legal Med 2017; 131: 359–364 [DOI] [PubMed] [Google Scholar]
- 46. Senmaru T, Fukui M, Kuroda M. et al. Serum pepsinogen I/II ratio is correlated with albuminuria in patients with type 2 diabetes. Endocr J 2013; 60: 161–166 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.