Abstract
The common finding of hypokalemic alkalosis in several unrelated disorders may confound the early diagnosis of salt-losing tubulopathy (SLT). Antenatal Bartter syndrome (BS) must be considered in idiopathic early-onset polyhydramnios. Fetal megabladder in BS may allow its distinction from third-trimester polyhydramnios that occurs in congenital chloride diarrhea (CCD). Fetal megacolon occurs in CCD while fecal chloride >90 mEq/L in infants is diagnostic. Failure-to-thrive, polydipsia and polyuria in early childhood are the hallmarks of classic BS. Unlike BS, there is low urinary chloride in hypokalemic alkalosis of intractable emesis and cystic fibrosis. Rarely, renal salt wasting may result from cystinosis, Dent disease, disorders of paracellular claudin-10b and Kir4.1 potassium-channel deficiency. Acquired BS may result from calcimimetic up-regulation of a calcium-sensing receptor or autoantibody inactivation of sodium chloride co-transporters in Sjögren syndrome. A relatively common event of heterozygous gene mutations for Gitelman syndrome increases the likelihood of its random occurrence in certain diseases of adult onset. Finally, diuretic abuse is the most common differential diagnosis of SLT. Unlike the persistent elevation in BS, urinary chloride concentration losses waxes and wanes on day-to-day assessment in patients with diuretic misuse.
Keywords: acquired Bartter, antenatal Bartter, Gitelman, hypochloremic metabolic alkalosis, pseudo-Bartter syndromes
INTRODUCTION
Bartter syndrome (BS) is a rare autosomal recessive (AR) disorder comprising a defect in the thick ascending limb of the loop of Henle (TALH). Its characteristic findings are hypokalemia, metabolic alkalosis, hyperreninemia and hyperplasia of the juxtaglomerular apparatus [1]. Despite urinary salt wasting, secondary hyperaldosteronism results in the maintenance of euvolemia. Its perinatal presentation may include elevated urinary levels of prostaglandin (PG) E2 [2]. A closely related disorder, Gitelman syndrome (GS), is distinguished by a later age of onset, milder clinical manifestations and a lower fatality rate [3–5]. Despite the increased availability of genetic testing, the diagnosis of BS or GS is largely dependent on clinical features, especially in developing countries. Due to the common findings of hypokalemic alkalosis in more prevalent disorders (e.g. emesis), clinicians must be aware of pitfalls that may confound an accurate diagnosis. Whereas severe perinatal BS may be readily diagnosed, childhood and adult forms of the disease are usually more insidious in presentation [3–5]. In the absence of decompensation from a comorbid illness, late-onset disease often maintains near-normal homeostasis by an adaptive increase in oral fluid and salt intake. Accordingly, the objective of this review is to update our knowledge on the salt-losing tubulopathies (SLTs) while highlighting the pathophysiological bases of the major clinical entities that are mistaken for BS or GS.
METHOD
We reviewed relevant literature by conducting a PubMed search using the terms hypokalemic alkalosis, antenatal BS, BS, pseudo-BS, acquired BS, GS and SLT. We retrieved clinical case series, original articles and review article formats. We selected only articles that were published in the English language.
PREVIEW OF HYPOCHLOREMIC METABOLIC ALKALOSIS
Metabolic alkalosis is characterized by a retention of plasma bicarbonate ( >26 mmol/L) that is typically associated with an elevated arterial blood pH >7.45 (alkalemia). It may be initiated either by excessive renal (e.g. use of loop diuretics) or extrarenal loss (e.g. vomiting) of hydrogen ions (H+) [6]. Alkalosis may also result from the extracellular displacement of H+ into the cellular space in response to hypokalemia. Sustaining metabolic alkalosis requires an impaired renal capacity to excrete the bicarbonate load [6]. With the resultant low sodium delivery in the distal convoluted tubule (DCT), there is an increase in the activity of the epithelial sodium channel (ENaC), which in turn causes luminal reabsorption of sodium (Na+) in exchange for H+ and potassium ions (K+) [7]. In contrast to the euvolemia in BS and GS, metabolic alkalosis in conditions mimicking primary aldosterone excess, including Liddle syndrome, licorice ingestion and apparent mineralocorticoid excess, is associated with excessive fluid retention and hypertension [8–13]. Furthermore, the two groups of disorders are differentiated by a secondary elevation of serum aldosterone in BS and GS while there is a suppression of the hormone in the latter [9, 10]. Liddle syndrome is caused by a gain-in-function mutation of ENaC, and licorice contains glycyrrhizinic acid, which is a potent substance that inhibits an enzyme that inactivates cortisol, 11-β-hydroxysteroid dehydrogenase type 2 (HSD11B2) [11, 12]. On the other hand, a syndrome of apparent mineralocorticoid excess results from recessive loss-of-function mutations in the gene for HSD11B2 [9, 13].
RENAL TUBULAR PHYSIOLOGY
TALH
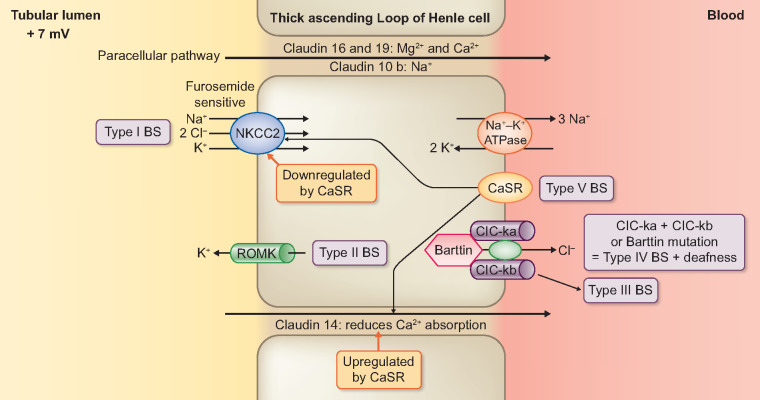
Activity of the apical sodium–potassium dichloride (Na+K+-2Cl−) co-transporter (NKCC2) results in a 25–30% reabsorption of filtered sodium chloride (NaCl) by the TALH (Figure 1) [14]. Poor water permeability of the TALH, due to the absence of aquaporin expression, produces an optimally diluted luminal fluid while generating a medullary osmotic gradient. The recycling of luminal K+ generates a positive luminal potential difference (PD), required for the maintenance of NKCC2 activity [15, 16]. Of the two apical K+ channels, the renal outer medullary K+ channel (ROMK) Kir1.1 mediates 75% of the basal amount of the K+ recycling (Figure 1) [17–19]. The cystic fibrosis (CF) transmembrane regulator protein (CFTR) regulates the ROMK channel [20, 21]. In addition, basolateral Na+/K+-ATPase activity generates the sodium gradient that facilitates the function of the apical NKCC2 [22]. Furthermore, generation of an intracellular negative voltage (−40 to −70 mV) promotes the extrusion of Cl− via the basolateral CLC-Ka and -b channels (Figures 1–3) [22–24]. Barttin, an accessory protein, enhances the function of both channels [24]. Apart from the co-expression with CLC-Kb on TALH and DCT, there is also CLC-Ka on the thin ascending limb [25]. The basolateral K+Cl− cotransporter KCC4 participates in mediating Cl− exit into the blood [26]. The positive luminal PD and preferential permeability of cations over Cl− (aided by claudin-16 and -19) are associated with a net paracellular absorption of Ca2+ and Mg2+ [27–30]. Similarly, claudin-10b exclusively promotes Na+ reabsorption across the paracellular pathway [31]. In contrast, paracellular expression of claudin-14, which is upregulated by the basolateral calcium-sensing receptor (CaSR), produces urinary Ca2+ excretion [30, 32].
FIGURE 1.
Deficits in Electrolyte Transport from the Renal Tubular Lumen across the Thick Ascending Limb of the Loop of Henle Cell Accounting for Types I, II, III, IV and V Bartter Syndrome.
DCT
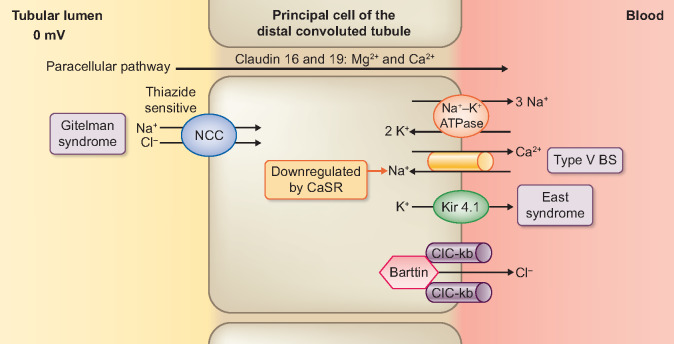
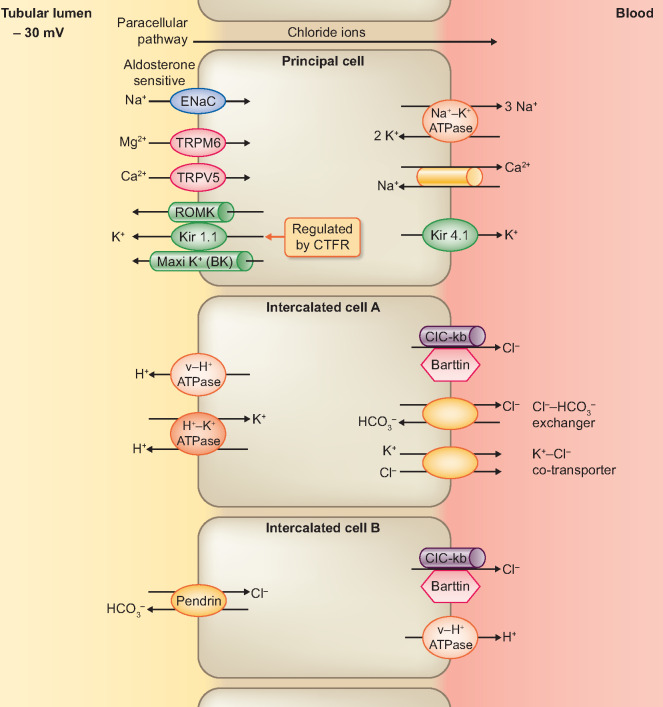
The DCT reabsorbs 6–10% of the sodium content of glomerular filtrate, principally by means of the NaCl cotransporter (NCCT) (Figure 2) [15, 33]. In addition to the NCCT, the expression of an aldosterone-sensitive ENaC on the late DCT causes a progressive change of the lumen PD from 0 to −30 mV [7, 34]. Apart from the generation of a transepithelial voltage of −60 to −90 mV, the basolateral Na+/K+-ATPase pump provides a driving force for the NCCT [35]. In turn, basolateral recycling of the intracellular K+ via the Kir4.1 channel maintains the activity of the Na+/K+-ATPase pump (Figure 2) [36]. Similar to the TALH, a basolateral chloride efflux occurs through CIC-Kb and KCC4 [37, 38]. In addition, the generation of lumen-negative PD by ENaC stimulates the basal amount of K+ recycling through ROMK [35, 39]. With larger tubular fluid flow and a greater demand for K+ secretion, there is an activation of (a large-capacitance) maxi-K+ channel (Figure 3) [18, 19, 40, 41]. Furthermore, active transcellular absorption of both Ca2+ and Mg2+ occurs via transient receptor potential (TRP) channel subfamily V member 5 (TRPV5) and TRP channel subfamily M member 6 (TRPM6), respectively (Figure 3) [42–45]. Finally, types A and B intercalated cells (ICs) are unique cell types that are expressed in DCT and cortical collecting ducts (CCDs) (Figure 3) [46]. Type A ICs have H+-ATPase and H+/K+-ATPase on the apical membrane for the control of metabolic acidosis [46, 47]. A basolateral Cl−/ (AE1) exchanger expels the base generated from the luminal H+ secretion into the blood [46]. In contrast, Type B ICs have the Cl−/ exchanger (Pendrin) on the apical membrane, which is activated by metabolic alkalosis [46].
FIGURE 2.
Deficits in Electrolyte Transport from the Renal Tubular Lumen across the Principal Cell of the Early Distal Convoluted Tubule accounting for Gitelman Syndrome and EAST/ SeSAME Syndrome.
FIGURE 3.
Pattern of Electrolyte Transport Across the Principal Cell, Intercalated Type A Cell and Intercalated Type B Cell of the Late Distal Tubule/ Cortical Collecting Ducts.
PATHOGENESIS AND GENO-PHENOTYPE CORRELATION IN BS AND GS
BS results from several genetic mutations that produce molecular defects in the electrolyte transporters of the TALH and DCT, while inactivation of the thiazide-sensitive NCCT in the DCT causes GS (Table 1, Figures 1 and 2) [1, 2, 4, 5]. To facilitate easier understanding, previous authors have suggested the use of nomenclature that is based on the pharmacological mechanism of equivalent diuretic agents [4, 5].
Table 1.
Clinical, genetic and biochemical features of BS and GS
| Syndromic type | BS I | BS II | BS III | BS IV a | BS IV b | BS V(ADH) | BS V(MAGE) | GS |
|---|---|---|---|---|---|---|---|---|
| Gene mutation | SLC12A1 | KCNJ1 | CLCNKB | BSND | CLCNKA + B | CASR | MAGED2 | SLC12A3 |
| Gene product | NKCC2 | ROMK | CIC-kb | Barttin | CIC-ka + b | CaSR | MAGE-D2 | NCCT |
| Age of onset | Antenatal/newborn | Antenatal/newborn | Infancy | Antenatal/newborn | Antenatal/newborn | Infancy/child | Antenatal/newborn | Child/adults |
| Hyper-PGE2 | ++ | ++ | ± | ++ | ++ | − | + | − |
| Polyuria | ++ | ++ | ± | ++ | ++ | ++ | + | |
| Hypokalemia | + | +a | + | + | + | + | + | ++ |
| Hypochloremia | ± | ± | + | + | + | ± | ± | + |
| Hypomagnesemia | − | − | ± | ± | ± | ++ | ± | ++ |
| Hypercalciuria/nephrocalcinosis | ++/ ++ | ++/ ++ | ± | ± | ± | ++/+ | ± | −/− |
| Hypocalciuria | − | − | − | − | − | − | − | ++ |
| Growth failure | ++ | ++ | ± | ++ | ++ | + | _ | ± |
| Chronic kidney disease | ± | ± | ± | ++ | ++ | ± | _ | − |
| Sensorineural deafness | − | − | − | + | + | − | − | − |
| TALH | ++ | ++ | + | + | + | ±b | ++ | − |
| DCT or CCD | − | + | + | + | + | +b | + | ++ |
| Pharmacological phenotypes | Furosemide | Furosemide/amiloride | Thiazide/furosemide | Thiazide/furosemide | Thiazide/furosemide | Thiazide | Thiazide/furosemide | Thiazide |
Initial hyperkalemia in the newborn. Later there is persistent hypokalemia. bAffects both TALH and DCT but predominantly affects DCT. All disorders manifest hypokalemia, alkalosis and hyperaldosteronism. ++, strong presence; +, presence; ±, variable occurrence or mild events; −/−, strong absence; −, absence; empty box indicates unknown event.
BSDN, Bartter’s syndrome with sensorineural deafness; hyper-PGE2, elevated PGE2 in serum or urine.
Perinatal BS and BS
Although genotype–phenotype correlations are variable in BS, five clinical categories are typically recognized [4].
BS Types I and II
Due to the crucial role of NKCC2 on the apical membrane of TALH (reabsorbs 25% of the filtered NaCl), mutation of SLC12A1 produces type I BS [4, 5, 33, 48]. Required to sustain NKCC2 activity, deficiency of potassium recycling by apical ROMK (KCNJ1 mutation) produces Type II BS (Table 1 and Figure 1) [49]. Consequently, both type I and II produce the most severe manifestations. These may be present initially in the second trimester of pregnancy as polyhydramnios, which may result in premature delivery [1, 2, 50, 51]. Furthermore, defective solute transport across the apical membrane of the macula densa may exacerbate tubular salt wasting both in the fetus and in the newborn [52, 53]. A chloride-defective macula densa produces PGE2, which in turn interacts with the EP4 receptor to release renin from the juxtaglomerular granular cell [54]. The release of angiotensin II produces efferent vasoconstriction, as opposed to nitric oxide–mediated afferent vasodilatation [55]. The resultant increase in glomerular filtration rate (GFR) reduces the fractional sodium reabsorption by the proximal tubule (PT). The subsequent increase in distal sodium delivery, in turn, promotes a secondary aldosteronism [7, 8]. In addition to higher GFR, polyuria results from disruption of the medullary osmotic gradient. Extreme free water loss could lead to an erroneous diagnosis of nephrogenic diabetes insipidus [56]. Impaired NKCC2 reduces the lumen-positive PD that is necessary for paracellular transport of Mg2+ and Ca2+ [27–29, 57]. Hence early-onset severe hypercalciuria may produce medullary nephrocalcinosis [2, 58]. Newborns with type II BS initially present with hyperkalemia due to inhibition of potassium excretion into the lumen of the late DCT [35, 39–41, 58]. As a result of a developmental delay, the compensatory potassium secretion by the luminal maxi K+ channel is absent [59, 60]. Named after the pharmacological target of NKCC2, type I BS is also called a furosemide-type loop disorder. Similarly, type II BS has been designated a furosemide/amiloride phenotype because of the roles of ROMK channels in both TALH and late DCT, coupled with ENaC [4, 5, 61].
BS type III, also called the classical variant, is the most common BS phenotype [4, 5]. It is caused by mutations in the CLCNKB gene, which encodes basolateral chloride channel variant b (ClC-kb) (Table 1 and Figures 1–3) [62]. The expression of CIC-kb in both TALH and DCT provides the basis for identifying type III BS as a mixed thiazide–furosemide phenotype [4, 5, 63]. Whereas mutation of CIC-kb in TALH is physiologically compensated by the parallel activity of CIC-ka in the thin ascending limb (which exists only in juxtamedullary nephrons, and it represents 15% of the total nephrons in humans), such is not the case with the loss of ClC-kb in the early DCT [25, 64]. For this reason, the observed variable clinical manifestation of BS is more frequently close to the symptom pattern of GS rather than BS. Thus in a study of a cohort of 115 patients with type III BS, 44.5% presented in childhood as classical variant while 29.5% (with a severe gene mutation) had perinatal BS and 26% of cases were indistinguishable from GS [65, 66]. Compared with types I and II BS, antenatal presentation is less severe and nephrocalcinosis is typically absent in infancy [4, 26, 64].
BS type IV is due to either a double heterozygote mutation of genes that encode basolateral CICs (CLC-NKA and CLC-NKB) or a monogenic mutation of the gene that produces their regulatory protein, Barttin [67, 68]. The absence of CICs on both the TALH and DCT segments accounts for a severe perinatal presentation, characterized by polyhydramnios, extreme prematurity and hypovolemia in the newborn (Table 1 and Figures 1–3) [22–24, 37, 67, 68]. Unlike types I and II BS, hypercalciuria is often self-limiting and medullary nephrocalcinosis is absent. There is a common progression to end-stage kidney disease [69]. Also, a deficiency of chloride transport in the scala media of the inner ear produces concurrent sensorineural deafness [63, 67–69].
BS type V, as produced by autosomal dominant hypocalcemia (ADH), results from inactivation of luminal NKCC2 on TALH and of basolateral sodium–calcium exchanger (NCX1) on the DCT. It represents a gain-of-function mutation of CaSR, the gene responsible for the CaSR (Figures 1 and 2) [5, 70]. A concomitant reduction in paracellular Ca2+ absorption is the consequence of decreased lumen-positive PD and the greater availability of calcium-dependent claudin-14 (Figure 1) [30, 31, 70]. Also, recently classified as type V BS is an X-linked mutation in MAGE-D2 that encodes melanoma-associated antigen D2 (MAGE-D2; essential for fetal expression of NKCC2 and NCCT) [71, 72]. Given the different mechanisms, it may be more appropriate to classify this entity as type VI BS. In one study, perinatal death occurred in about one-third of 13 affected pregnancies, while paradoxically, the surviving infants manifested with self-limiting polyuria and hypokalemic alkalosis [72].
Pseudo-BS
Pseudo-BS presents as hypokalemic metabolic alkalosis, typically in the clinical context of extrarenal salt losses (Table 2). It consists mostly of gastrointestinal disorders such as infantile hypertrophic pyloric stenosis, congenital chloridorrhea and certain eating disorders (EDs). It may also result from excessive cutaneous losses of NaCl in CF. The relationship of these disorders with BS and GS will be discussed in order of their clinical presentation from infancy to adulthood. Although we give prominence to certain topics for their educational values, unlike EDs, most of these disorders are rare.
Table 2.
Comparison of clinical, biochemical and diagnostic features in pseudo-BS variants, BS and GS
| Pseudo-BS versus BS and GS | Idiopathic hypertrophic pyloric stenosis | Congenital chloride diarrhea | CF | Purging behavior | BS | GS |
|---|---|---|---|---|---|---|
| Clinical manifestations | Projectile vomiting, severe dehydration and epigastric mass | Watery diarrhea and severe dehydration | Meconium ileus, mal-absorption, dehydration and pulmonary disease | Vomiting, Russell’s sign, subconjunctival bleeding and sialadenosis | Polyhydramnio, dehydration, FTT and CKD | Asymptomatic, muscle cramp, fatigue and hypokalemic paralysis |
| Gene variants association | Polygenic; BARX1 and EML4-MTA3 | SLC26A3 | CFTR (ΔF508) | None | SLC12A1, CLCNKA, CLCNKB, BSND, KCNJ1, CASR and MAGED2 | SLC12A3 |
| Age of onset | Post-natal 4–6 weeks | Antenatal/early infancy | Early infancy/childhood | Adolescent/young adults | Antenatal/ early infancy/childhood | Late childhood/young adults |
| Polydipsia | None | Yes/no | None | None | Yes | Yes/no |
| Polyuria | None | None | None | None | Yes | Yes/no |
| Hyperaldosteronism | Yes | Yes | Yes | Yes | Yes | Yes |
| Hypokalemia | Yes | Yes | Yes | Yes | Yes | Yes |
| Hypochloremia | Yes | Yes | Yes | Yes | Yes | Yes |
| Hyponatremia | Yes/no | Yes | Yes | Yes | Yes/no | Yes/no |
| Hypercalciuria/nephrocalcinosis | No | No | No | Yes/no | Yes | No |
| Growth failure/weight loss | Yes | Yes | Yes | Yes | Yes | Yes/no |
| Urine chloride | Low | Low | Low | High/low | High | High |
| Diagnostic clues | Abdominal ultrasound | Fecal chloride >90 mmol/L | Sweat chloride/gene variant | Psychosomatic | Electrolyte pattern/gene variant | Electrolyte pattern/gene variant |
FTT, failure to thrive; CKD, chronic kidney disease.
Infantile hypertrophic pyloric stenosis
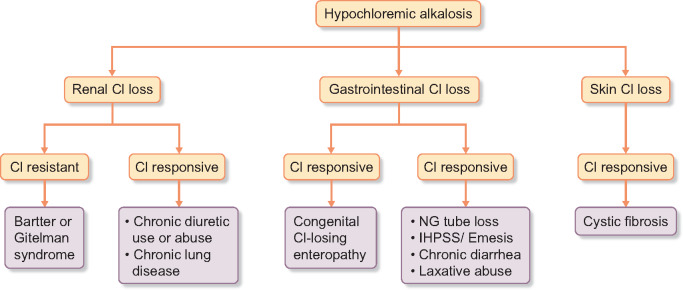
Infantile hypertrophic pyloric stenosis (IHPS) is a potentially life-threatening disorder of young infants. It is far more common than BS. As in perinatal BS, there is a predisposition for (atypical) presentation in premature infants [66, 73]. Affected infants may present with projectile, nonbilious vomiting and an abdominal mass arising from hypertrophy of the pyloric sphincter [74]. Loss of gastric acid results in hypochloremia and secondary hyperaldosteronism produces hypokalemia (Table 2 and Figure 4) [8, 74]. Diagnostic urinary chloride is often <20 mEq/L, while the value is >20 mEq/L in BS (Figure 4) [75]. Mimicking the presentation of antenatal BS is a rare report of an early-onset IHPS in association with prematurity and polyhydramnios [76].
FIGURE 4.
Algorithm for the Differential Diagnosis of Hypochloremic Metabolic Alkalosis.
Congenital chloride diarrhea
Congenital chloride diarrhea (CCD) is a rare AR disorder that is marked by persistent secretory diarrhea in early infancy [77]. The defective gene is SLC26A3, which encodes a chloride/bicarbonate exchanger that occurs on the brush border membranes of ileal and colonic epithelia [77]. Dehydration and hypokalemic hypochloremic alkalosis are common (Table 2 and Figure 4). As in polyuria of BS, an extreme watery stool may soak the diaper of an affected infant [78]. Unlike the second-trimester event (26–30 weeks) in BS, polyhydramnios in CCD presents after 35 weeks of gestational age [78, 79]. Fetal ultrasonography shows megacolon in CCD while megabladder occurs in BS [80, 81]. In contrast to elevated urinary chloride in the fetus (amniotic fluid) or newborn with BS, a finding of fecal chloride >90 mmol/L is diagnostic in CCD [77–79].
EDs/purging behavior
EDs are the main differential diagnoses of BS and GS in adolescents and adults. They may result in life-threatening electrolyte derangements and are associated with a substantial cost of hospitalization [82, 83]. Differences in the electrolyte pattern are observed in the clinical subtypes of EDs. Surreptitious vomiting is the most frequent form. Due to gastric chloride depletion and volume contraction, there is severe hypokalemic alkalosis associated with low urinary chloride (Table 3 and Figure 4) [8, 84, 85]. Laxative abuse, the second most common form, results most frequently from the consumption of enteric stimulants that produce a large volume of watery diarrhea [80, 81, 86]. Unlike metabolic acidosis in diarrhea of short duration, the chronic diarrhea resulting from laxative abuse leads to hypokalemic alkalosis in response to secondary aldosteronism [7, 8, 80, 81, 86]. Although furosemide abuse is the least common purging behavior, it may also occur in the context of surreptitious vomiting, thereby worsening the hypokalemic alkalosis [75, 84]. Due to a common event of salt wasting, differentiation of diuretic abuse and BS and GS may be challenging. Unlike the steady elevation of urine chloride in BS and GS, chloride losses wax and wane on day-to-day urinary assessment among individuals with diuretic misuse [75, 87]. Furthermore, as in the stool analysis for laxatives, urine toxicology may reveal the offending diuretic substance (Table 4) [80]. Due to extrarenal salt loss, surreptitious emesis and laxative abuse are termed pseudo-BS, while the appropriate designation for diuretic abuse is Bartter-like disorder (Tables 2 and 3, Figure 4). There are distinct physical stigmata of purging behavior. Russell’s sign is scarring on the back of the hands that results from repeated scraping against the upper teeth. There may be subconjunctival hemorrhage and swollen parotid glands [81, 86, 88, 89]. Although its presentation in types I and II BS occurs in infancy, there may be adult-onset medullary nephrocalcinosis in chronic diuretic abuse [89]. Unlike the absence of kidney stones, because of persistent polyuria, in BS, laxative abuse increases the likelihood of ammonium urate urolithiasis [90]. Finally, with a report of concurrent hypokalemic alkalosis both in newborn infants (pseudo-BS) and in their mothers, Bartter-like events may complicate chronic diuretic abuse in pregnant women [91, 92].
Table 3.
Comparison of clinical, biochemical and diagnostic features in Bartter-like syndrome and BS and GS
| Bartter-like versus BGS | Drug-induced BS | Cystinosis | Dent disease | Sjögren syndrome | Chronic diuretic abuse | BS | GS |
|---|---|---|---|---|---|---|---|
| Clinical findings | Sepsis, antibiotic and dehydration | Fanconi syndrome, photophobia, rickets and hypothyroidism | Low molecular weight proteinuria, Fanconi syndrome, nephrocalcinosis, hypokalemia, hypercalciuria, and CKD | Rheumatic disease, xerostomia, kerato-conjunctivitis sicca | Vomiting, dehydration, Russell’s sign, sub-conjunctival bleeding and sialadenosis | Polyhydramnios, dehydration, FTT and CKD | Asymptomatic, muscle cramp, fatigue and hypokalemic paralysis |
| Gene variants association | None | CTNS | CLCN5 and OCRL1 | None | None | SLC12A1, CLCNKA, CLCNKB, Barttin, KCNJ1, CASR and MAGED2 | SLC12A3 |
| Age of onset | All age groups | Early infancy/childhood | Childhood | Adults | Adolescents/young adults | Antenatal/early infancy/childhood | Late childhood/young adults |
| Polydipsia | Yes/no | Yes | Yes/no | Yes/no | Yes/no | Yes | Yes/no |
| Polyuria | Yes | Yes | Yes/no | Yes/no | Yes | Yes | Yes/no |
| Hyperaldosteronism | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hypokalemia | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hypochloremia | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hyponatremia | Yes/no | Yes | Yes | Yes/no | Yes | Yes/no | Yes/no |
| Hypercalciuria/nephrocalcinosis | Yes, hyper-calciuria/no, nephrocalcinosis | No | Yes | No | Yes/no | Yes | No |
| Growth failure/weight loss | Yes | Yes | Yes | Yes | Yes | Yes | Yes/no |
| Urine chloride | High | High | High | High | High/low | High | High |
| Diagnostic clues | Acquired BS with aminoglycoside use | Fanconi syndrome/elevated leucocyte cystine | CKD/Lowe syndrome/gene variant | SSA antibody | Psycho-somatic | Electrolyte pattern/gene variant | Electrolyte pattern/gene variant |
FTT, failure to thrive; CKD, chronic kidney disease.
Table 4.
Comparison of clinical, biochemical and diagnostic features in purging behavior variants, BS and GS
| Purging behavior versus BS and GS | Surreptitious vomiting/bulimia | Acute laxative abuse | Chronic laxative abuse | Chronic diuretic abuse | BS | GS |
|---|---|---|---|---|---|---|
| Clinical findings | Vomiting, dehydration, Russell’s sign, subconjunctival bleeding and sialadenosis | Normal physical, diarrhea and dehydration | Other signs of ED, weight loss, dehydration, constipation and edema | Other signs of ED, weight loss, dehydration and edema | Polyhydramnios, dehydration, FTT and CKD | Asymptomatic, muscle cramp, fatigue and hypokalemic paralysis |
| Age of onset | Adolescents/young adults | Adolescents/young adults | Adolescents/young adults | Adolescents/young adults | Antenatal/early infancy/childhood | Late childhood/young adults |
| Hyperaldosteronism | Yes | Yes | Yes | Yes | Yes | Yes |
| Hypokalemia | Yes | Yes | Yes | Yes | Yes | Yes |
| Hypochloremia | Yes | No (acidosis) | Yes | Yes | Yes | Yes |
| Hyponatremia | Yes/no | Yes/no | Yes/no | Yes/no | Yes/no | Yes/no |
| Hypercalciuria/nephrocalcinosis | No | No | No | Yes/no | Yes | No |
| Growth failure/weight loss | Yes | Yes/no | Yes | Yes | Yes | Yes/no |
| Urine chloride | Low | Low | Low | High/low | High | High |
FTT, failure to thrive; CKD, chronic kidney disease.
CF
CF is an AR disorder that results from mutations of the gene that encodes CFTR, a chloride-conducting channel that regulates anion transport and optimizes mucociliary clearance in the airways [93, 94]. Up to 90% of patients with CF have at least one copy of the F508del mutation on chromosome 7 [93]. Depending upon the severity of CFTR dysfunction, a consequence of the specific gene mutation, clinical presentation in affected individuals varies widely [93, 94]. Mimicking BS, children <2 years of age who reside in a hot climate environment may present initially with isolated hypokalemic hypochloremic alkalosis (Table 2 and Figure 4) [95, 96]. Indeed, communities that lack laboratory facilities for sweat testing have used paradoxical findings of metabolic alkalosis in infants with diarrhea as a criterion for the empirical diagnosis of CF [97]. The absence of a regulatory function of CFTR on the renal apical ROMK channel may explain the findings of isolated hypokalemic alkalosis (pseudo-BS) in some patients (Figure 3) [20, 21, 98]. Interestingly, there are reports of the common occurrence of isolated CFTR mutations including T338I, D110E, D110H and 711+1G>T/IVS8-5T in pseudo-BS [98–101].
GS
GS is an AR disorder resulting from mutations of the SLC12A3 gene [102, 103]. As a reflection of the absent NCCT activity in GS, it may also be described as a pure thiazide-like phenotype [4, 5]. Although reasons for the milder clinical expression of GS are incompletely understood, they may include a lower fraction of salt reabsorption by the NCCT in early DCT compared with the NKCC2 in TALH (6% versus 25%) [14, 15, 33]. GS is often asymptomatic in childhood, while fatigue, salt craving, cramps and tetany may be evident in young adults [102, 104, 105]. Occasionally its diagnosis may be a fortuitous event arising from an incidental discovery of subtle laboratory changes [3, 102]. More serious clinical events in older age are hypokalemic rhabdomyolysis, periodic paralysis, seizures and cardiac arrhythmias [103–105]. The signature findings in GS are hypomagnesemia and hypocalciuria. Hypomagnesemia is due to the disruption of transcellular transport of magnesium, which ordinarily occurs through the apical TRPM6 (Figure 3) [106, 107]. On the other hand, hypocalciuria results from a compensatory increase in the PT passive reabsorption of calcium (coupled with sodium) in response to volume depletion in the DCT [107, 108]. Furthermore, absence of the apical NCCT creates an electrochemical gradient that favors a basolateral extrusion of Ca2+ in exchange for an intracellular moving Na+ (Figure 3) [5, 107, 108]. There is also an increase in the abundance of TRPV5, a channel for calcium absorption on the DCT [106, 107].
Bartter- and Gitelman-like disorders
Unlike pseudo-BS, Bartter- and Gitelman-like disorders are due to urinary salt losses and are therefore indistinguishable from BS and GS (Table 3).
Renal tubular claudin-10 gene mutation
Most recently, in four separate publications, there has been a description of an SLT that is easily confused with BS and GS. Therein a total of 22 patients presented with an age range of 4–53 years [109–112]. As a result of the deficiency of claudin-10b (CLDN10 gene) in the tight junction of the TALH, these cases demonstrated impaired paracellular absorption of sodium [31, 109–112]. The resultant increase in sodium delivery at the DCT activates ENaC with a secondary loss of K+ and H+ [7, 8, 31, 112]. Due to a compensatory wider distribution of claudin-16 and -19 at the more distal TALH, there is an increase in paracellular absorption of Ca2+ and Mg2+. Consequently there may be hypermagnesemia and nephrocalcinosis [28, 29, 31]. In addition, a deficit of claudin-10b involving the skin and salivary gland causes anhidrosis and xerostomia in early childhood. Presenting at an older age is hypokalemic alkalosis associated with variable events of hypocalciuria, hypercalciuria, hyposthenuria and loss of GFR [109–112].
Epilepsy, Ataxia, Sensorineural deafness and Tubulopathy (EAST) or Seizures, Sensorineural deafness, Ataxia, Mental retardation and Electrolyte imbalance (SeSAME) syndrome
EAST syndrome is a rare AR disorder that presents with infantile epilepsy, severe ataxia, sensorineural deafness, mental retardation and renal tubulopathy [113, 114]. Deficient expression of Kir4.1 potassium channel on the DCT, cochlear stria vascularis and glial cells of the brain results from mutations of the KCNJ10 gene [113, 114]. The absence of basolateral potassium recycling suppresses the activity of the apical NCCT by the inhibition of WNK4 and WNK1 [115, 116]. An increase in distal sodium delivery to the late DCT activates secondary aldosteronism [7, 8, 117]. Neurological features often precede the childhood onset of a predominantly Gitelman phenotype, characterized by hypomagnesemia, hypocalciuria and hypokalemic alkalosis [118].
Drug-induced renal salt loss
Aminoglycosides cause self-limited inhibition of NaCl on the TALH and NCCT on the DCT [5, 119]. Multiple cases of aminoglycoside-associated hypokalemic and hypochloremic alkalosis, particularly in premature infants, have been reported in patients receiving gentamicin, netilmicin and amikacin (Table 3) [119–123]. With a mechanism similar to type V BS, these polyvalent cationic molecules enhance the sensitivity of CaSR located on the basal membrane of the renal epithelial cells (Figures 1 and 2) [5, 76].
Hereditary PT defects
Cystinosis
Cystinosis is a rare AR disorder that results from mutations in the cystinosin lysosomal cystine transporter (CTNS) gene [124–126]. The kidney, cornea, bone marrow and thyroid may be involved in an age-dependent manner [124–126]. Typically the severe form of cystinosis presents in late infancy with Fanconi syndrome and growth failure [124–126]. Although the responsible mechanism is unclear, a defect in PT sodium transport associated with secondary aldosteronism in the early stage of disease may produce hypokalemic alkalosis instead of the conventional acidosis (Table 3) [124, 125, 127].
Dent disease
Dent disease is a rare X-linked recessive disorder of the renal PT that manifests as low molecular weight proteinuria, hypercalciuria, nephrocalcinosis and kidney stones [128]. Most frequently it results from the inactivation of the endosomal voltage-gated chloride–hydrogen ion exchanger ClC-5 [130]. Clinical manifestations are variable and may include Fanconi syndrome [81]. The reasons for its presentation as SLT are unknown (Table 3) [128–131].
Sjögren syndrome
In Sjögren syndrome (SS), multiple cases of a GS presentation have been reported (Table 3) [132–136]. These may occur in three clinical contexts: a concurrent manifestation of both disorders in a single patient, autoantibodies in SS producing inactivation of renal tubular cotransporters and autoantibodies in SS promoting the clinical expression of GS in an individual with a heterozygote gene mutation [132, 133]. It is important to differentiate these three entities, given the potential for a response to immunosuppression in the autoimmune variants [134]. The absence of immunohistochemical staining for NCCT on renal tissues and demonstration of its circulating antibody is suggestive of a diagnosis [133, 137]. Finally, genetic analysis is necessary to differentiate autoimmune GS from a genetic mutation of SLC12A3 (GS) in a patient with a diagnosis of SS [133, 138].
CONCLUSION
Because of wide variations and the lack of specificity in clinical presentations, unrelated disorders may mimic SLT. Pseudo-BS occurs in nasogastric fluid losses, intractable emesis, pyloric stenosis, CF and EDs. Unlike BS, urine chloride excretion is low in pseudo-BS. As in BS and GS, renal salt wasting occurs in claudin-10b mutations, EAST syndrome, cystinosis, aminoglycoside toxicity and diuretic abuse. Finally, a relatively common event of heterozygous gene mutations for GS, particularly in the Caucasian population, increases the likelihood of its random occurrence in certain diseases of adult onset.
ACKNOWLEDGEMENTS
We acknowledge and appreciate the editorial assistance of the following individuals: Aviles Diego, MD; Man Oh, MD, PhD; Steven Wadowski, MD; Steven Schwartz, MD, PhD; Juan Kupferman, MD and Mary Mallappallil, MD.
CONFLICT OF INTEREST STATEMENT
The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Simon DB, Lifton RP.. The molecular basis of inherited hypokalemic alkalosis: Bartter’s and Gitelman’s syndromes. Am J Physiol 1996; 271: F961–F966 [DOI] [PubMed] [Google Scholar]
- 2. Seyberth HR. W, Koniger SJ, Rascher W. et al. Role of prostaglandins in hyperprostaglandin E syndrome and in selected renal tubular disorders. Pediatr Nephrol 1987; 1: 491–497 [DOI] [PubMed] [Google Scholar]
- 3. Blanchard A, Bockenhauer D, Bolignano D. et al. Gitelman syndrome: consensus and guidance from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int 2017; 91: 24–33 [DOI] [PubMed] [Google Scholar]
- 4. Seyberth HW, Weber S, Kömhoff M.. Bartter’s and Gitelman’s syndrome. Curr Opin Pediatr 2017; 29: 179–186 [DOI] [PubMed] [Google Scholar]
- 5. Seyberth HW, Schlingmann KP.. Bartter- and Gitelman-like syndromes: salt-losing tubulopathies with loop or DCT defects. Pediatr Nephrol 2011; 26: 1789–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gillion V, Jadoul M, Devuyst O. et al. The patient with metabolic alkalosis. Acta Clin Belg 2019; 74: 34–40 [DOI] [PubMed] [Google Scholar]
- 7. Loffing J, Zecevic M, Féraille E. et al. Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK. Am J Physiol Renal Physiol 2001; 280: F675–F682 [DOI] [PubMed] [Google Scholar]
- 8. Corry DB, Tuck ML.. Secondary aldosteronism. Endocrinol Metab Clin North Am 1995; 24: 511–529 [PubMed] [Google Scholar]
- 9. Mumford E, Unwin RJ, Walsh SB.. Liquorice, Liddle, Bartter or Gitelman—how to differentiate? Nephrol Dial Transplant 2019; 34: 38–39 [DOI] [PubMed] [Google Scholar]
- 10. Nesterov V, Krueger B, Bertog M. et al. In Liddle syndrome, epithelial sodium channel is hyperactive mainly in the early part of the aldosterone-sensitive distal nephron. Hypertension 2016; 67: 1256–1262 [DOI] [PubMed] [Google Scholar]
- 11. Molhuysen JA, Gerbrandy J, de Vries LA. et al. A liquorice extract with deoxycortone-like action. Lancet 1950; 256: 381–386 [DOI] [PubMed] [Google Scholar]
- 12. Gallacher SD, Tsokolas G, Dimitropoulos I.. Liquorice-induced apparent mineralocorticoid excess presenting in the emergency department. Clin Med 2017; 17: 43–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. New MI, Levine LS, Biglieri EG. et al. Evidence for an unidentified steroid in a child with apparent mineralocorticoid hypertension. J Clin Endocrinol Metab 1977; 44: 924–933 [DOI] [PubMed] [Google Scholar]
- 14. Ares GR, Caceres PS, Ortiz PA.. Molecular regulation of NKCC2 in the thick ascending limb. Am J Physiol Renal Physiol 2011; 301: F1143–F1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hebert SC, Mount DB, Gamba G.. Molecular physiology of cation-coupled Cl− cotransport: the SLC12 family. Pflugers Arch 2004; 447: 580–593 [DOI] [PubMed] [Google Scholar]
- 16. Greger R, Schlatter E.. Presence of luminal K+, a prerequisite for active NaCl transport in the cortical thick ascending limb of Henle’s loop of rabbit kidney. Pflugers Arch 1981; 392: 92–94 [DOI] [PubMed] [Google Scholar]
- 17. Wang W. Regulation of the ROMK channel: interaction of the ROMK with associate proteins. Am J Physiol 1999; 277: F826– F831 [DOI] [PubMed] [Google Scholar]
- 18. Hebert SC, Desir G, Giebisch G. et al. Molecular diversity and regulation of renal potassium channels. Physiol Rev 2005; 85: 319–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pluznick JL1, Sansom SC.. BK channels in the kidney: role in K+ secretion and localization of molecular components. Am J Physiol Renal Physiol 2006; 291: F517–F529 [DOI] [PubMed] [Google Scholar]
- 20. Lu M, Leng Q, Egan ME. et al. CFTR is required for PKA-regulated ATP sensitivity of Kir1.1 potassium channels in mouse kidney. J Clin Invest 2006; 116: 797–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Konstas AA, Koch JP, Tucker SJ. et al. Cystic fibrosis transmembrane conductance regulator-dependent up-regulation of Kir1.1 (ROMK) renal K+ channels by the epithelial sodium channel. J Biol Chem 2002; 277: 25377–25384 [DOI] [PubMed] [Google Scholar]
- 22. Wald H, Scherzer P, Popovtzer MM.. Inhibition of thick ascending limb Na+-K+-ATPase activity in salt-loaded rats by furosemide. Am J Physiol 1989; 256: F549–F555 [DOI] [PubMed] [Google Scholar]
- 23. Greger R, Schlatter E.. Properties of the basolateral membrane of the cortical thick ascending limb of Henle’s loop of rabbit kidney. A model for secondary active chloride transport. Pflugers Arch 1983; 396: 325–334 [DOI] [PubMed] [Google Scholar]
- 24. Waldegger S, Jeck N, Barth P. et al. Barttin increases surface expression and changes current properties of ClC-K channels. Pflügers Arch Eur J Physiol 2002; 444: 411–418 [DOI] [PubMed] [Google Scholar]
- 25. Liu W, Morimoto T, Kondo Y. et al. Analysis of NaCl transport in thin ascending limb of Henle’s loop in CLC-K1 null mice. Am J Physiol Renal Physiol 2002; 282: F451–F457 [DOI] [PubMed] [Google Scholar]
- 26. Mercado A, Song L, Vazquez N. et al. Functional comparison of the K+-Cl− cotransporters KCC1 and KCC4. J Biol Chem 2000; 275: 30326–30334 [DOI] [PubMed] [Google Scholar]
- 27. Hebert SC, Andreoli TE.. Ionic conductance pathways in the mouse medullary thick ascending limb of Henle. The paracellular pathway and electrogenic Cl− absorption. J Gen Physiol 1986; 87: 567–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hou J, Renigunta A, Konrad M. et al. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest 2008; 118: 619–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hou J, Renigunta A, Gomes AS. et al. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci USA 2009; 106: 15350–15355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dimke H, Desai P, Borovac J. et al. Activation of the Ca2+-sensing receptor increases renal claudin-14 expression and urinary Ca2+ excretion. Am J Physiol Renal Physiol 2013; 304: F761–F769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Breiderhoff T, Himmerkus N, Stuiver M. et al. Deletion of claudin-10 (Cldn10) in the thick ascending limb impairs paracellular sodium permeability and leads to hypermagnesemia and nephrocalcinosis. Proc Natl Acad Sci USA 2012; 109: 14241–14246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gong Y, Renigunta V, Himmerkus N. et al. Claudin-14 regulates renal Ca++ transport in response to CaSR signalling via a novel microRNA pathway. EMBO J 2012; 31: 1999–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palmer LG, Schnermann J.. Integrated control of Na transport along the nephron. Clin J Am Soc Nephrol 2015; 10: 676–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wright FS. Increasing magnitude of electrical potential along the renal distal tubule. Am J Physiol 1971; 220: 624–638 [DOI] [PubMed] [Google Scholar]
- 35. Yoshitomi K, Shimizu T, Taniguchi J. et al. Electrophysiological characterization of rabbit distal convoluted tubule cell. Pflugers Arch 1989; 414: 457–463 [DOI] [PubMed] [Google Scholar]
- 36. Schultz SG. Homocellular regulatory mechanisms in sodium-transporting epithelia: avoidance of extinction by “flush-through”. Am J Physiol 1981; 241: F579–F590 [DOI] [PubMed] [Google Scholar]
- 37. Estévez R, Boettger T, Stein V. et al. Barttin is a Cl− channel beta-subunit crucial for renal Cl− reabsorption and inner ear K+ secretion. Nature 2001; 414: 558–561 [DOI] [PubMed] [Google Scholar]
- 38. Velázquez H, Silva T.. Cloning and localization of KCC4 in rabbit kidney: expression in distal convoluted tubule. Am J Physiol Renal Physiol 2003; 285: F49–F58 [DOI] [PubMed] [Google Scholar]
- 39. Schnermann J, Steipe B, Briggs JP.. In situ studies of distal convoluted tubule in rat. II. K secretion. Am J Physiol 1987; 252: F970–F976 [DOI] [PubMed] [Google Scholar]
- 40. Stanton BA, Giebisch GH.. Potassium transport by the renal distal tubule: effects of potassium loading. Am J Physiol 1982; 243: F487–F493 [DOI] [PubMed] [Google Scholar]
- 41. Liu W, Xu S, Woda C. et al. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 2003; 285: F998–F1012 [DOI] [PubMed] [Google Scholar]
- 42. Hoenderop JG, van der Kemp AW, Hartog A. et al. Molecular identification of the apical Ca2+ channel in 1,25-dihydroxyvitamin D3-responsive epithelia. J Biol Chem 1999; 274: 8375–8378 [DOI] [PubMed] [Google Scholar]
- 43. Van Goor MKC, Hoenderop JGJ, van der Wijst J.. TRP channels in calcium homeostasis: from hormonal control to structure-function relationship of TRPV5 and TRPV6. Biochim Biophys Acta Mol Cell Res 2017; 1864: 883–893 [DOI] [PubMed] [Google Scholar]
- 44. Schlingmann KP, Weber S, Peters M. et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet 2002; 31: 166–170 [DOI] [PubMed] [Google Scholar]
- 45. Viering DHHM, de Baaij JHF, Walsh SB. et al. Genetic causes of hypomagnesemia, a clinical overview. Pediatr Nephrol 2017; 32: 1123–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carraro-Lacroix LR, Malnic G.. Acid-base transport by the renal distal nephron. J Nephrol 2010; 23(Suppl 16): S19–27 [PubMed] [Google Scholar]
- 47. Frische S, Chambrey R, Trepiccione F. et al. H+-ATPase B1 subunit localizes to thick ascending limb and distal convoluted tubule of rodent and human kidney. Am J Physiol Renal Physiol 2018; 315: F429–F444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Simon DB, Karet FE, Hamdan JM. et al. Bartter’s syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat Genet 1996; 13: 183–188 [DOI] [PubMed] [Google Scholar]
- 49. Simon DB, Karet FE, Rodriguez-Soriano J. et al. Genetic heterogeneity of Bartter’s syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet 1996; 14: 152–156 [DOI] [PubMed] [Google Scholar]
- 50. Castrop H, Schießl IM.. Physiology and pathophysiology of the renal Na-K-2Cl cotransporter (NKCC2). Am J Physiol Renal Physiol 2014; 307: F991–F1002 [DOI] [PubMed] [Google Scholar]
- 51. Deschenes G, Burguet A, Guyot C. et al. Antenatal form of Bartter’s syndrome. Ann Pediatr 1993; 40: 95–101 [PubMed] [Google Scholar]
- 52. Yang T, Park JM, Arend L. et al. Low chloride stimulation of prostaglandin E2 release and cyclooxygenase-2 expression in a mouse macula densa cell line. J Biol Chem 2000; 275: 37922–37929 [DOI] [PubMed] [Google Scholar]
- 53. Schnermann J, Ploth DW, Hermle M.. Activation of tubulo-glomerular feedback by chloride transport. Pflugers Arch 1976; 362: 229–240 [DOI] [PubMed] [Google Scholar]
- 54. NüSing RM, Treude A, Weissenberger C. et al. Dominant role of prostaglandin E2 EP4 receptor in furosemide-induced salt-losing tubulopathy: a model for hyperprostaglandin E syndrome/antenatal Bartter syndrome. J Am Soc Nephrol 2005; 16: 2354–2362 [DOI] [PubMed] [Google Scholar]
- 55. Schnermann J, Levine DZ.. Paracrine factors in tubuloglomerular feedback: adenosine, ATP, and nitric oxide. Annu Rev Physiol 2003; 65: 501–529 [DOI] [PubMed] [Google Scholar]
- 56. Vergine G, Fabbri E, Pedini A. et al. Bartter syndrome type 1 presenting as nephrogenic diabetes insipidus. Case Rep Pediatr 2018; 2018: 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hou J. Claudins and mineral metabolism. Curr Opin Nephrol Hypertens 2016; 25: 308–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fretzayas A, Gole E, Attilakos A. et al. Expanding the spectrum of genetic mutations in antenatal Bartter syndrome type II. Pediatr Int 2013; 55: 371–373 [DOI] [PubMed] [Google Scholar]
- 59. Suzuki Y, Yasuoka Y, Shimohama T. et al. Expression of the K channel Kir7.1 in the developing rat kidney: role in K excretion. Kidney Int 2003; 63: 969–975 [DOI] [PubMed] [Google Scholar]
- 60. Woda CB, Miyawaki N, Ramalakshmi S. et al. Ontogeny of flow-stimulated potassium secretion in rabbit cortical collecting duct: functional and molecular aspects. Am J Physiol Renal Physiol 2003; 285: F629– F639 [DOI] [PubMed] [Google Scholar]
- 61. Walsh PR, Tse Y, Ashton E. et al. Clinical and diagnostic features of Bartter and Gitelman syndromes. Clin Kidney J 2018; 11: 302–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Simon DB, Bindra RS, Mansfield TA. et al. Mutations in the chloride channel gene, CLCNKB, cause Bartter’s syndrome type III. Nat Genet 1997; 17: 171–178 [DOI] [PubMed] [Google Scholar]
- 63. Hebert SC. Bartter syndrome. Curr Opin Nephrol Hypertens 2003; 12: 527–532 [DOI] [PubMed] [Google Scholar]
- 64. Konrad M, Vollmer M, Lemmink HH. et al. Mutations in the chloride channel gene CLCNKB as a cause of classic Bartter syndrome. J Am Soc Nephrol 2000; 11: 1449–1459 [DOI] [PubMed] [Google Scholar]
- 65. Seys E, Andrini O, Keck M. et al. Clinical and genetic spectrum of Bartter syndrome type 3. J Am Soc Nephrol 2017; 28: 2540–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hsu P, Klimek J, Nanan R.. Infantile hypertrophic pyloric stenosis: does size really matter? J Paediatr Child Health 2014; 50: 827–828 [DOI] [PubMed] [Google Scholar]
- 67. Schlingmann KP, Konrad M, Jeck N. et al. Salt wasting and deafness resulting from mutations in two chloride channels. N Engl J Med 2004; 350: 1314–1319 [DOI] [PubMed] [Google Scholar]
- 68. Birkenhäger R, Otto E, Schürmann MJ. et al. Mutation of BSND causes Bartter syndrome with sensorineural deafness and kidney failure. Nat Genet 2001; 29: 310–314 [DOI] [PubMed] [Google Scholar]
- 69. Jeck N, Reinalter SC, Henne T. et al. Hypokalemic salt-losing tubulopathy with chronic renal failure and sensorineural deafness. Pediatrics 2001; 108: E5–E5 [DOI] [PubMed] [Google Scholar]
- 70. Bonny O, Edwards A.. Calcium reabsorption in the distal tubule: regulation by sodium, pH, and flow. Am J Physiol Renal Physiol 2013; 304: F585–F600 [DOI] [PubMed] [Google Scholar]
- 71. Legrand A, Treard C, Roncelin I. et al. Prevalence of novel MAGED2 mutations in antenatal Bartter syndrome. Clin J Am Soc Nephrol 2018; 13: 242–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Laghmani K, Beck BB, Yang SS. et al. Polyhydramnios, transient antenatal Bartter’s syndrome, and MAGED2 mutations. N Engl J Med 2016; 374: 1853–1863 [DOI] [PubMed] [Google Scholar]
- 73. Stark CM, Rogers PL, Eberly MD. et al. Association of prematurity with the development of infantile hypertrophic pyloric stenosis. Pediatr Res 2015; 78: 218–222 [DOI] [PubMed] [Google Scholar]
- 74. Peters B, Oomen MW, Bakx R. et al. Advances in infantile hypertrophic pyloric stenosis. Expert Rev Gastroenterol Hepatol 2014; 8: 533–541 [DOI] [PubMed] [Google Scholar]
- 75. Carmody JB. Focus on diagnosis: urine electrolytes. Pediatr Rev 2011; 32: 65–68 [DOI] [PubMed] [Google Scholar]
- 76. Houben C, Kiely E.. Congenital hypertrophic pyloric stenosis with associated polyhydramnios in a premature infant. Eur J Pediatr Surg 1997; 7: 184–185 [DOI] [PubMed] [Google Scholar]
- 77. Konishi K, Mizuochi T, Yanagi T. et al. Clinical features, molecular genetics, and long-term outcome in congenital chloride diarrhea: a nationwide study in Japan. J Pediatr 2019; 214: 151–157.e6. [DOI] [PubMed] [Google Scholar]
- 78. Gujrati K, Rahman AJ, Gulsher. Congenital chloride losing diarrhoea. J Pak Med Assoc 2014; 64: 339–341 [PubMed] [Google Scholar]
- 79. Rachid ML, Dreux S, Czerkiewicz I. et al. Fetal urine biochemistry in antenatal Bartter syndrome: a case report. Clin Case Rep 2016; 4: 876–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mehler PS, Rylander M.. Bulimia Nervosa – medical complications. J Eat Disord 2015; 3: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Weinstein HD, Halabis JA.. Subconjunctival hemorrhage in bulimia. J Am Optom Assoc 1986; 57: 366–367 [PubMed] [Google Scholar]
- 82. Forney KJ, Buchman-Schmitt JM, Keel PK. et al. The medical complications associated with purging. Int J Eat Disord 2016; 49: 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Patel RS, Olten B, Patel P. et al. Hospitalization outcomes and comorbidities of bulimia nervosa: a nationwide inpatient study. Cureus 2018; 10: e2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bahia A, Mascolo M, Gaudiani JL. et al. Pseudo Bartter syndrome in eating disorders. Int J Eat Disord 2012; 45: 150–153 [DOI] [PubMed] [Google Scholar]
- 85. Mitchell JE, Pyle RL, Eckert ED. et al. Electrolyte and other physiological abnormalities in patients with bulimia. Psychol Med 1983; 13: 273–278 [DOI] [PubMed] [Google Scholar]
- 86. Williams JF, Friedman IM, Steiner H.. Hand lesions characteristic of bulimia. Arch Pediatr Adolesc Med 1986; 140: 28–29 [DOI] [PubMed] [Google Scholar]
- 87. Jones BF, Trevillian PR.. Variability of urinary chloride—a clue to diuretic abuse. Nephron 1992; 61: 472–472 [DOI] [PubMed] [Google Scholar]
- 88. Park KK, Tung RC, de Luzuriaga AR.. Painful parotid hypertrophy with bulimia: a report of medical management. J Drugs Dermatol 2009; 8: 577–579 [PubMed] [Google Scholar]
- 89. Kim YG, Kim B, Kim MK. et al. Medullary nephrocalcinosis associated with long-term furosemide abuse in adults. Nephrol Dial Transplant 2001; 16: 2303–2309 [DOI] [PubMed] [Google Scholar]
- 90. Dick WH, Lingeman JE, Preminger GM. et al. Laxative abuse as a cause for ammonium-urate renal calculi. J Urol 1990; 143: 244–247 [DOI] [PubMed] [Google Scholar]
- 91. Higuchi R, Sugimoto T, Hiramatsu C. et al. Neonatal pseudo-Bartter syndrome due to maternal eating disorder. J Perinatol 2008; 28: 646–648 [DOI] [PubMed] [Google Scholar]
- 92. Mathot M, Maton P, Henrion E. et al. Pseudo-Bartter syndrome in a pregnant mother and her fetus. Pediatr Nephrol 2006; 21: 1037–1040 [DOI] [PubMed] [Google Scholar]
- 93. Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med 2006; 173: 475–482 [DOI] [PubMed] [Google Scholar]
- 94. Elborn JS. Cystic fibrosis. Lancet 2016; 388: 2519–2531 [DOI] [PubMed] [Google Scholar]
- 95. Qiu L, Yang F, He Y. et al. Clinical characterization and diagnosis of cystic fibrosis through exome sequencing in Chinese infants with Bartter-syndrome-like hypokalemia alkalosis. Front Med 2018; 12: 550–558 [DOI] [PubMed] [Google Scholar]
- 96. Kintu B, Brightwell A.. Episodic seasonal pseudo-Bartter syndrome in cystic fibrosis. Paediatr Respir Rev 2014; 15: 19–21 [DOI] [PubMed] [Google Scholar]
- 97. Kabra SK, Kabra M, Shastri S. et al. Diagnosing and managing cystic fibrosis in the developing world. Paediatr Respir Rev 2006; 7(Suppl 1): S147–S150 [DOI] [PubMed] [Google Scholar]
- 98. Tinsa F, Hadj Fredj S, Bel Hadj I. et al. Pseudo-Bartter syndrome as the sole manifestation of cystic fibrosis in a child with 711+G>T/IVS8-5T mutation: a new face of an old disease. Ann Biol Clin 2017; 75: 466–473 [DOI] [PubMed] [Google Scholar]
- 99. Weller F, Wiebicke W, Tummler B.. Turkish infant with hypoelectrolytemia and metabolic alkalosis as the sole manifestations of a mild form of cystic fibrosis (mutation D110H). Klin Padiatr 2000; 212: 41–43 [DOI] [PubMed] [Google Scholar]
- 100. Padoan R, Bassotti A, Seia M. et al. A novel missense mutation (D110E) in exon 4 of CFTR (ABCC7) in a CF infant presenting with hypochloraemic metabolic alkalosis. Hum Mutat 2000; 15: 485–485 [DOI] [PubMed] [Google Scholar]
- 101. Leoni GB, Pitzalis S, Podda R. et al. A specific cystic fibrosis mutation (T338I) associated with the phenotype of isolated hypotonic dehydration. J Pediatr 1995; 127: 281–283 [DOI] [PubMed] [Google Scholar]
- 102. Gitelman HJ, Graham JB, Welt LG.. A new familial disorder characterized by hypokalemia and hypomagnesemia. Trans Assoc Am Physicians 1966; 79: 221–235 [PubMed] [Google Scholar]
- 103. Fujimura J, Nozu K, Yamamura T. et al. Clinical and genetic characteristics in patients with gitelman syndrome. Kidney Int Rep 2019; 4: 119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Jeck N, Schlingmann KP, Reinalter SC. et al. Salt handling in the distal nephron: lessons learned from inherited human disorders. Am J Physiol Regul Integr Comp Physiol 2005; 288: R782–R795 [DOI] [PubMed] [Google Scholar]
- 105. Cruz DN, Shaer AJ, Bia MJ. et al. Gitelman’s syndrome revisited: an evaluation of symptoms and health-related quality of life. Kidney Int 2001; 59: 710–717 [DOI] [PubMed] [Google Scholar]
- 106. Schnoz C, Carrel M, Loffing J.. Loss of sodium chloride co-transporter impairs the outgrowth of the renal distal convoluted tubule during renal development. Nephrol Dial Transplant 2019; 5: 411–432 [DOI] [PubMed] [Google Scholar]
- 107. Loffing J, Vallon V, Loffing-Cueni D. et al. Altered renal distal tubule structure and renal Na+ and Ca2+ handling in a mouse model for Gitelman's syndrome. J Am Soc Nephrol 2004; 15: 2276–2288 [DOI] [PubMed] [Google Scholar]
- 108. Nijenhuis T, Vallon V, van der Kemp AW. et al. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest 2005; 115: 1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bongers EMHF, Shelton LM, Milatz S. et al. A novel hypokalemic-Alkalotic salt-losing tubulopathy in patients with CLDN10 mutations. J Am Soc Nephrol 2017; 28: 3118–3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hadj-Rabia S, Brideau G, Al-Sarraj Y. et al. Multiplex epithelium dysfunction due to CLDN10 mutation: the HELIX syndrome. Genet Med 2018; 20: 190–201 [DOI] [PubMed] [Google Scholar]
- 111. Klar J, Piontek J, Milatz S. et al. Altered paracellular cation permeability due to a rare CLDN10B variant causes anhidrosis and kidney damage. PLoS Genet 2017; 13: e1006897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Meyers N, Nelson-Williams C, Malaga-Dieguez L. et al. Hypokalemia associated with a claudin 10 mutation: a case report. Am J Kidney Dis 2019; 73: 425–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Freudenthal B, Kulaveerasingam D, Lingappa L. et al. KCNJ10 mutations disrupt function in patients with EAST syndrome. Nephron Physiol 2011; 119: 40–48 [DOI] [PubMed] [Google Scholar]
- 114. Bockenhauer D, Feather S, Stanescu HC. et al. Epilepsy, ataxia, sensorineural deafness. N Engl J Med 2009; 360: 1960–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhang C, Wang L, Zhang J. et al. KCNJ10 determines the expression of the apical Na-Cl cotransporter (NCC) in the early distal convoluted tubule (DCT1). Proc Natl Acad Sci USA 2014; 111: 11864–11869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wang MX, Cuevas CA, Su XT. et al. Potassium intake modulates the thiazide-sensitive sodium-chloride cotransporter (NCC) activity via the Kir4.1 potassium channel. Kidney Int 2018; 93: 893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wang WH. Basolateral Kir4.1 activity in the distal convoluted tubule regulates K secretion by determining NaCl cotransporter activity. Curr Opin Nephrol Hypertens 2016; 25: 429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Scholl UI, Dave HB, Lu M. et al. SeSAME/EAST syndrome—phenotypic variability and delayed activity of the distal convoluted tubule. Pediatr Nephrol 2012; 27: 2081–2090 [DOI] [PubMed] [Google Scholar]
- 119. Zietse R, Zoutendijk R, Hoorn EJ.. Fluid, electrolyte and acid–base disorders associated with antibiotic therapy. Nat Rev Nephrol 2009; 5: 193–202 [DOI] [PubMed] [Google Scholar]
- 120. Chou CL, Chau T, Lin SH. et al. Acquired Bartter-like syndrome associated with gentamicin administration. Am J Med Sci 2005; 329: 144–149 [DOI] [PubMed] [Google Scholar]
- 121. Singh J, Patel M, Gupta K. et al. Acquired Bartter syndrome following gentamicin therapy. Indian J Nephrol 2016; 26: 461–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chrispal A, Boorugu H, Prabhakar A. et al. Amikacin-induced type 5 Bartter-like syndrome with severe hypocalcemia. J Postgrad Med 2009; 55: 208–210 [DOI] [PubMed] [Google Scholar]
- 123. Landau D, Kher KK.. Gentamicin-induced Bartter-like syndrome. Pediatr Nephrol 1997; 11: 737–740 [DOI] [PubMed] [Google Scholar]
- 124. Whyte MP, Shaheb S, Schnaper HW.. Cystinosis presenting with features suggesting Bartter syndrome. Clin Pediatr (Phila) 1985; 24: 447–451 [DOI] [PubMed] [Google Scholar]
- 125. Çaltik A, Akyüz SG, Erdogan Ö. et al. Rare presentation of cystinosis mimicking bartters syndrome: reports of two patients and review of the literature. Ren Fail 2010; 32: 277–280 [DOI] [PubMed] [Google Scholar]
- 126. Özkan B, Çayır A, Koşan C. et al. Cystinosis presenting with findings of Bartter syndrome. Case report. J Clin Res Pediatr Endocrinol 2011; 3: 101–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Yildiz B, Durmuş-Aydoğdu S, Kural N. et al. A patient with cystinosis presenting transient features of Bartter syndrome. Turk J Pediatr 2006; 48: 260–262 [PubMed] [Google Scholar]
- 128. Bogdanović R, Draaken M, Toromanović A. et al. A novel CLCN5 mutation in a boy with Bartter-like syndrome and partial growth hormone deficiency. Pediatr Nephrol 2010; 25: 2363–2368 [DOI] [PubMed] [Google Scholar]
- 129. Fisher SE, Vanbakel I, Lloyd SE. et al. Cloning and characterization of CLCN5, the human kidney chloride channel gene implicated in dent disease (an X-linked hereditary nephrolithiasis). Genomics 1995; 29: 598–606 [DOI] [PubMed] [Google Scholar]
- 130. Okamoto T, Tajima T, Hirayama T. et al. A patient with dent disease and features of Bartter syndrome caused by a novel mutation of CLCN5. Eur J Pediatr 2012; 171: 401–404 [DOI] [PubMed] [Google Scholar]
- 131. Besbas N, Ozaltin F, Jeck N. et al. CLCN5 mutation (R347X) associated with hypokalaemic metabolic alkalosis in a Turkish child: an unusual presentation of Dents disease. Nephrol Dial Transplant 2005; 20: 1476–1479 [DOI] [PubMed] [Google Scholar]
- 132. Kusuda T, Hosoya T, Mori T. et al. Acquired Gitelman syndrome in an anti-SSA antibody-positive patient with a SLC12A3 heterozygous mutation. Intern Med 2016; 55: 3201–3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Gu X, Su Z, Chen M. et al. Acquired Gitelman syndrome in a primary Sjögren syndrome patient with a SLC12A3 heterozygous mutation: a case report and literature review. Nephrol 2017; 22: 652–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Casatta L, Ferraccioli GF, Bartoli E.. Hypokalaemic alkalosis, acquired Gitelman’s and Bartter’s syndrome in chronic sialoadenitis. Br J Rheumatol 1997; 36: 1125–1128 [DOI] [PubMed] [Google Scholar]
- 135. Francois H, Mariette X.. Renal involvement in primary Sjogren syndrome. Nat Rev Nephrol 2016; 12: 82–93 [DOI] [PubMed] [Google Scholar]
- 136. Bastani B, Haragsim L, Gluck S. et al. Lack of H-ATPase in distal nephron causing hypokalaemic distal RTA in a patient with Sjogren’s syndrome. Nephrol Dial Transplant 1995; 10: 908–909 [PubMed] [Google Scholar]
- 137. Kim YK, Song HC, Kim WY. et al. Acquired Gitelman syndrome in a patient with primary Sjögren syndrome. Am J Kidney Dis 2008; 52: 1163–1167 [DOI] [PubMed] [Google Scholar]
- 138. Mishima E, Mori T, Sohara E. et al. Inherited, not acquired, Gitelman syndrome in a patient with Sjogren’s syndrome: importance of genetic testing to distinguish the two forms. CEN Case Rep 2017; 6: 180–184 [DOI] [PMC free article] [PubMed] [Google Scholar]