Abstract
Thymus satureioides Coss. (Lamiaceae) is a Moroccan medicinal plant locally known as “Azkouni” or “Zaitra.” It is widely used in traditional medicine to treat various ailments, including hypertension, diabetes, cold, fever, dermatological and circulatory disorders, immune problems, bronchitis, nociception, cooling, pharyngitis, cough, and influenza. The current review aims to critically summarize the literature on ethnopharmacological uses, chemical profile, and pharmacological investigations of T. satureioides in order to provide data support and scientific evidences for further investigations. Electronic databases such as Scopus, PubMed, Web of Science, SciFinder, ScienceDirect, Google Scholar, and Medline were used to gather data on T. satureioides. Chemical characterization of T. satureioides essential oils (EOs) and extracts allowed to identify a total of 139 bioactive compounds, mainly belonging to the terpenoids, phenolic acids, and flavonoids classes. T. satureioides especially its essential oils exhibited numerous biological activities such as antibacterial, antifungal, anti-inflammatory, antioxidant, antidiabetic, anticancer, antiparasitic, and hypolipedemic activities. In light of these findings, further studies to transmute the traditional application of T. satureioides into scientific-based information are strongly required. Additional in vivo pharmacological studies are recommended to validate the results of the in vitro studies. Moreover, comprehensive preclinical and clinical trials on the pharmacological mechanisms of action of this plant and its bioactive compounds on molecular targets should be performed. Finally, more efforts must be focused on toxicological assessments and pharmacokinetic studies, in order to ensure the safety and the efficiency of T. satureioides.
1. Introduction
Thymus satureioides Coss. is a perennial shrub (10–60 cm in height) belonging to the Lamiaceae family and the genus Thymus [1, 2]. T. satureioides is an endemic Moroccan medicinal plant locally known as “Azkuni” or “Zaitra” [3]. This species is widely distributed in the arid and semiarid habitats of the Moroccan High Atlas and Anti-Atlas [1, 4].
In Morocco, T. satureioides has been extensively used in folk medicine against numerous diseases, including arterial hypertension, diabetes, cold, fever [5, 6], dermatological and immune problems, digestive ailments [1, 7, 8], and metabolic disorders [9]. Ethnopharmacological investigations showed that T. satureioides is used for the treatments of bronchitis, skin ailments, nociception, circulatory disorders, urogenital problems, nervous and visual ailments, cooling, pharyngitis, cough, influenza, and as an antispasmodic agent [5, 10–12]. Phytochemical analysis of T. satureioides essential oils and extracts enabled to identify numerous bioactive compounds belonging to several chemical classes, including terpenoids, phenolic acids, flavonoids, steroids, alkaloids, and saponins [13–16].
Several pharmacological reports based on in vitro and in vivo studies have demonstrated that T. satureioides, especially its EOs, exhibit various biological activities such as antibacterial [17, 18], antifungal [19, 20], antioxidant [21, 22], antidiabetic [2], anticancer [23], anti-inflammatory [24], insecticidal [25, 26], and hypolipedemic effects [27]. However, the targeted mechanisms of these pharmacological properties have been poorly investigated.
Although numerous studies reported the ethnomedicinal properties and pharmacological effects of T. satureioides, to the best of our knowledge, no review was published to summarize these reports and suggest the future pharmacological applications of this plant. Therefore, this review was designed to critically summarize all published works on ethnomedicinal uses, phytochemistry, and pharmacological properties of T. satureioides. The current paper aims to provide data support and prospect concerning future research studies on the biological potential of T. satureioides.
2. Research Methodology
All published works about the ethnomedicinal uses, phytochemical composition, and biological activities of T. satureioides were collected, examined, and reported in the present review. An extensive bibliometric survey from different scientific databases such as ScienceDirect, PubMed, Scopus, Web of Science, SpringerLink, Google Scholar, and Medline was used to extract all relevant papers. A total of 79 peer-reviewed papers published in English and French languages were selected to compose this review. The data provided in case reports, editorial/letters, patents, conference papers, and symposiums were excluded because they were considered scientifically unreliable. The search keywords used are “T. satureioides, phytochemical composition of T. satureioides, T. satureioides EOs, biological activities of T. satureioides, the antimicrobial activity of T. satureioides, ethnobotanical study of T. satureioides, and the antioxidant effect of T. satureioides”. ChemDraw Ultra 12.0 Software was used to draw the chemical structures. IUPAC names of the reported chemical compounds were cheeked using PubChem databases (pubchem.ncbi.nlm.nih.gov).
3. Results and Discussion
3.1. Botany, Ecology, and Biogeographic Distribution
T. satureioides is a bushy perennial shrub (10–60 cm in height) with erect branches [1, 2] (Figure 1). Its leaves are opposite, linear, or lanceolate, curled at the edges, grayish on top, and tomentose at the base. The flowers are grouped into ovoid glomerules. The corolla is bilabiate (1/2 cm) with pink or whitish petals [3]. Reproduction of T. satureioides occurs via sexual (seeds) and asexual route through bursts of stump, cuttings, and marcottage [1].
Figure 1.

Thymus satureioides at flowering stage.
T. satureioides is an endemic Moroccan plant, geographically found in the Mediterranean, Thermomediterranean, and Mesomediterranean series, in forest clearings, scrub, matorrals, and low and medium mountains up to 2200 m altitude [3, 27]. This species grows on siliceous limestone substratum and rocky to moderately earthy soils in the High Atlas and Anti-Atlas of Morocco. From a climatic point of view, T. satureioides is located in the arid to subhumid bioclimate, with hot, temperate, and fresh variants [3].
3.2. Ethnomedicinal Use
T. satureioides is one of the medicinal plants commonly used in Moroccan folk medicine to treat many pathological disorders, including diabetes, arterial hypertension, digestives ailments, cold, fever, and respiratory problems [5, 6].
Several ethnobotanical and ethnopharmacological surveys reported these practices and showed that the medicinal use of T. satureioides depends on the plant's part used (Table 1). The aerial parts of T. satureioides were used as a decoction and infusion to treat gastric disorders, chills, cold, fever, and headaches [11], as well as arterial hypertension and diabetes [5, 28]. In addition, Mouhajir et al. [35] showed that the aerial part decoction is used as food disinfectant and against cold and colic.
Table 1.
Ethnomedicinal use of T. satureioides.
| Study area | Parts used | Preparation method | Medicinal use | References |
|---|---|---|---|---|
| Agadir-Ida-Ou Tanane (Morocco) | Aerial parts | Infusion, decoction, cataplasms, and fumigation | Gastrointestinal complaints, influenza, colds, fever, headaches, affections of the annex glands of the digestive tract, respiratory problems, and menstruation pain in women | [5] |
|
| ||||
| Agadir-Ida-Ou-Tanane Province (Southwest Morocco) | Whole plant, flowers, leaves, and stems | Infusion | Respiratory, digestive, skin, circulatory, genital, nervous, visual, and urinary problems | [12] |
|
| ||||
| Beni Mellal (Morocco) | Leaves | Decoction and infusion | Diabetes | [28] |
|
| ||||
| High Atlas mountains (Morocco) | Whole plant | Powder | Gastrointestinal ailments (stomach ache and intestinal trouble) and respiratory disorders such as colds and coughs | [29] |
|
| ||||
| Haouz-Rhamna region (Morocco) | Leaves | Decoction and infusion | Diabetes | [9] |
|
| ||||
| Er-Rich region (High Atlas of Morocco) | Aerial parts | Decoction and infusion | Gastric disorders, chills, cold, fever, headaches, digestive infections, and pain, and it is also used as an antispasmodic agent | [11] |
|
| ||||
| Er-Rich region | Aerial parts | Fumigation | Respiratory diseases, digestive ailments | [11] |
|
| ||||
| Agadir region (Morocco) | Leaves | Infusion | Diabetes | [30] |
|
| ||||
| Chtouka Ait Baha and Tiznit (Morocco) | Leaves | Infusion, maceration, and powder | Diabetes | [31] |
|
| ||||
| Western Middle Atlas region (Morocco) | Leaves and stems | Infusion | Gastrointestinal disorders (bloating, diarrhea) | [32] |
|
| ||||
| Zagora (Morocco) | Leaves | Decoction and powder | Diabetes and used as antinociceptive agent | [33] |
|
| ||||
| Azilal (Morocco) | Aerial parts | Fumigation, infusion | Digestive ailment, colds, and coughs | [34] |
|
| ||||
| Seksaoua region, Western High Atlas (Morocco) | Leaves | Decoction | Cooling, pharyngitis, cough, and influenza | [10] |
|
| ||||
| Morocco | Leaves, aerial part | Decoction, infusion | Coughs and bronchitis | [1] |
|
| ||||
| Beni Mellal region (Morocco) | Whole plant | Infusion | Gastrointestinal ailments | [8] |
|
| ||||
| Berber Peoples of Morocco | Aerial parts | Infusion | Treatment of cold and colic and as food disinfectant | [35] |
|
| ||||
| Marrakech (Morocco) | Aerial parts | Decoction | Digestive ailments | [36] |
|
| ||||
| Errachidia Province (Morocco) | Leaves, flower | Decoction | Arterial hypertension | [37] |
|
| ||||
| Tata Province, Morocco | Aerial part | Decoction | Hypotensive, digestive ailments, diabetes, colds | [6] |
|
| ||||
| Region of Middle Oum Rbia (Morocco) | Whole plant, leaves | Not reported | Dermatological, immune, and digestive and respiratory ailments | [7] |
The whole plant is used to treat dermatological disorders, immune problems, digestive ailments, intestinal troubles, colds, and coughs [7, 8, 29]. The leaves of T. satureioides are mainly known to be used against metabolic disorders, in particular diabetes [9, 12, 31], as well as for the treatments of bloating and diarrhea [32] or against cooling, pharyngitis, cough, and influenza [10].
Other ethnomedicinal studies reported that T. satureioides was also used as an antispasmodic and antinociceptive agent, and for the treatment of bronchitis, skin ailments, circulatory disorders, urogenital problems, nervous and visual ailments, and menstruation pains [1, 5, 11, 12, 33].
3.3. Phytochemistry
Phytochemical screening of T. satureioides EOs and extracts revealed the presence of a total of 139 bioactive compounds, which can be grouped into three main chemical classes, including terpenoids, phenolic acids, and flavonoids (Table 2).
Table 2.
Chemical compounds from T. satureioides.
| No. | Compounds | Parts used | Extracts | References |
|---|---|---|---|---|
| 1 | Apigenin | Leaves | Alcohol | [38] |
| 2 | Luteolin | Leaves, Aerial parts | Alcohol, methanol | [14, 38] |
| 3 | Eriodictyol | Leaves | Methanol | [14] |
| 4 | Thymonin | Leaves | Methanol | [14] |
| 5 | Quercetin | Aerial parts | Crude extracts, ethyl acetate, methanol | [39] |
| 6 | Hesperetin | Aerial parts | Crude extracts, ethyl acetate, methanol, aqueous | [13, 27, 39] |
| 7 | Luteolin-3′-O-glucuronide | Leaves | Methanol | [14] |
| 8 | Apigenin-7-O-glucoside | Aerial parts | Dichloromethane | [39] |
| 9 | Hyperoside | Aerial parts | Dichloromethane, ethyl acetate, methanol | [39] |
| 10 | Luteolin-7-O-glucoside | Leaves, Aerial parts | Aqueous, methanol | [13, 14, 24] |
| 11 | Eriodictyol-7-O-glucoside | Leaves | Methanol | [14] |
| 12 | Caffeic acid | Aerial parts, Leaves | Alcohol, ethyl acetate, methanol | [38, 39] |
| 13 | p-Coumaric acid | Leaves | Alcohol | [38] |
| 14 | Ferulic acid | Leaves | Alcohol | [38] |
| 15 | Rosmarinic acid | Aerial parts | Crude extracts | [27, 39] |
| 16 | Chlorogenic acid | Leaves | Alcohol | [38] |
| 17 | Ursolic acid | Leaves | Chloroform | [14] |
| 18 | Oleanolic acids | Leaves | Chloroform | [14] |
| 19 | (E)-Linalool oxide | Aerial parts | EOs | [40] |
| 20 | (E)-p-Menthan-2-one | Aerial parts | EOs | [40] |
| 21 | (E)-Sabinene hydrate | Aerial parts | EOs | [40] |
| 22 | (E)-Verbenol | Aerial parts | EOs | [40] |
| 23 | (E)-β-Ocimene | Flowering top | EOs | [41] |
| 24 | (Z)-Dihydrocarvone | Aerial parts | EOs | [40] |
| 25 | (Z)-Sabinene hydrate | Aerial parts | EOs | [40] |
| 26 | 1,10-di-epi-Cubenol | Whole plant, aerial parts | EOs | [19, 41] |
| 27 | 1,8 Cineole | Aerial parts | EOs | [40] |
| 28 | Thymol methyl ether (2-Isopropyl-5-methylanisole) | Aerial parts | EOs | [40] |
| 29 | 3-Octanol | Whole plant, aerial parts, flowering top | EOs, petroleum ether, ethyl acetate | [19, 23, 41] |
| 30 | 3-Tetradecen-5-yne | Leaves | EOs | [42] |
| 31 | 3-Thujen-2-one | Aerial parts | EOs | [16] |
| 32 | 3-δ-Carene | Aerial parts | EOs | [40] |
| 33 | Alloaromadendrene | Aerial parts, flowering top | EOs | [40, 41] |
| 34 | Alloocimene | Aerial parts | Petroleum ether, EOs | [23, 43] |
| 35 | Aromadendrene | Aerial parts | EOs | [40] |
| 36 | Bicyclogermacrene | Flowering top, aerial parts | EOs | [41, 44] |
| 37 | Borneol | Aerial parts, flowering top | EOs, petroleum ether, ethyl acetate | [23, 41, 45] |
| 38 | Bornyl acetate | Aerial parts, flowering top | EOs, petroleum ether, ethyl acetate | [23, 41, 45] |
| 39 | Bornyl formate | Aerial parts | EOs | [16] |
| 40 | Calamenene | Aerial parts | EOs | [40] |
| 41 | Calarene | Aerial parts | EOs, petroleum ether, ethyl acetate | [23, 43] |
| 42 | Camphene | Aerial parts, whole plant, flowering top | EOs, petroleum ether, ethyl acetate | [19, 41, 46] |
| 43 | Camphene hydrate | Aerial parts | EOs | [44] |
| 44 | Camphenilone | Aerial parts | EOs | [44] |
| 45 | Camphor | Aerial parts, flowering top | EOs | [41, 47] |
| 46 | Carvacrol (5-isopropyl-2-methylphenol) | Aerial parts, flowering top | EOs, petroleum ether, ethyl acetate | [23, 41, 47] |
| 47 | Carvacrol methyl ether | Aerial parts | EOs, petroleum ether, ethyl acetate | [15, 21, 23] |
| 48 | Carvenone | Aerial parts | EOs, petroleum ether, ethyl acetate | [23, 44] |
| 49 | Carveol | Aerial parts | EOs | [40] |
| 50 | Carvone | Aerial parts | EOs | [40] |
| 51 | Caryophyllene oxide | Aerial parts | EOs, petroleum ether, ethyl acetate | [23, 40] |
| 52 | Cedrene oxide | Aerial parts | EOs | [48] |
| 53 | cis-Linalool oxide | Aerial parts, flowering top | EOs | [43, 44] |
| 54 | cis-Ocimene | Aerial parts | EOs | [44] |
| 55 | cis-α-Bisabolene | Leaves | EOs | [42] |
| 56 | Copaene | Aerial parts | EOs | [40] |
| 57 | Crithmene | Aerial parts | EOs | [47] |
| 58 | Dehydro-p-cymene | Aerial parts | EOs | [40] |
| 59 | Dihydrocarvone 1 | Aerial parts | EOs | [40] |
| 60 | Dihydrocarvone 2 | Aerial parts | EOs | [40] |
| 61 | Dodecamethylcyclohexasiloxane | Aerial parts | EOs, petroleum ether | [23, 43] |
| 62 | Eucalyptol | Leaves | EOs | [42] |
| 63 | Eugenol | Aerial parts, whole plant | EOs | [19, 44] |
| 64 | Fenchone | Flowering top | EOs | [16] |
| 65 | Geraniol formate | Aerial parts | Ethyl acetate | [23] |
| 66 | Geranyl linalool | Aerial parts | EOs | [40] |
| 67 | Germacrene-D-4-ol | Flowering top | EOs | [41] |
| 68 | Germacrene | Aerial parts | EOs | [15] |
| 69 | Guaia-3,9-diene | Aerial parts | Petroleum ether, EOs | [23, 43, 48] |
| 70 | Guaiazulene | Aerial parts | EOs | [40] |
| 71 | Hexahydroindan | Aerial parts | EOs, petroleum ether | [23, 43] |
| 72 | Hotrienol | Leaves | EOs | [42] |
| 73 | Isoaromadendrene epoxide | Aerial parts | Petroleum ether, EOs | [23, 40] |
| 74 | Isoborneol | Aerial parts | EOs | [40] |
| 75 | Isobornyl acetate | Aerial parts | EOs, petroleum ether | [23, 43] |
| 76 | Isobornyl formate | Aerial parts | EOs, petroleum ether, ethyl acetate | [23, 44] |
| 77 | Isoledene | Leaves | EOs | [42] |
| 78 | Isothymol methyl ether | Leaves | EOs | [42] |
| 79 | Ledene | Aerial parts | EOs, petroleum ether | [23, 43] |
| 80 | Ledol 6-epi-cubenol | Flowering top | EOs | [41] |
| 81 | Limonene | Aerial parts, flowering top | EOs | [15, 41] |
| 82 | Linalool | Aerial parts, flowering top | EOs | [41, 45] |
| 83 | Linalyl propionate | Aerial parts | Ethyl acetate, petroleum ether | [23] |
| 84 | Thymol methyl ether | Aerial parts, flowering top | EOs | [40, 41] |
| 85 | Myrcene | Aerial parts, flowering top | EOs | [15, 41] |
| 86 | Octan-3-one | Aerial parts | EOs | [45] |
| 87 | Octen-3-ol | Aerial parts | EOs | [40] |
| 88 | p-Cymen-8-ol (2-(4-methylphenyl) propan-2-ol) | Flowering top | EOs | [41] |
| 89 | p-Cymene | Aerial parts, flowering top | EOs, petroleum ether, ethyl acetate | [23, 41, 45] |
| 90 | Pentasiloxane | Aerial parts | Petroleum ether | [23] |
| 91 | Pinocarveol | Aerial parts | EOs | [40] |
| 92 | p-Menth-2-en-1-ol | Aerial parts | EOs | [40] |
| p-Mentha-1.8-diene | Aerial parts | Petroleum ether, ethyl acetate | [23] | |
| 93 | Sabinene | Aerial parts | EOs | [49] |
| 94 | Santolina triene | Aerial parts | EO, petroleum ether | [23, 43] |
| 95 | Spathulenol | Aerial parts | EOs | [40] |
| 96 | tau-Cadinol | Aerial parts, whole plant | EOs | [19, 44] |
| 97 | Terpinen-4-ol | Aerial parts, whole plant | EOs | [19, 44] |
| 98 | Terpinolene | Aerial parts | EOs | [40] |
| 99 | Thuja-2,4(10)-diene | Aerial parts | EOs | [40] |
| 100 | Thujone | Aerial parts, whole plant | EOs | [19, 44] |
| 101 | Thymol | Aerial parts, flowering top | EOs, petroleum ether, ethyl acetate | [23, 41, 42] |
| 102 | Trans-1,2-diphenylcyclobutane | Aerial parts | Petroleum ether | [23] |
| 103 | trans-Pinocarveol | Flowering top | EOs | [41] |
| 104 | trans-Sabinene hydrate | Flowering top | EOs | [41] |
| 105 | Tricyclene | Aerial parts, whole plant, flowering top | EOs | [19, 41, 46] |
| 106 | Valencene | Aerial parts | EOs, petroleum ether | [23, 43] |
| 107 | α-Amorphene | Leaves | EOs | [42] |
| 108 | α-Cadinol | Aerial parts | EOs | [21] |
| 109 | α-Campholenal | Aerial parts | EOs | [44] |
| 110 | α-Campholene aldehyde | Aerial parts | EOs, petroleum ether | [23, 43] |
| 111 | α-Cubebene | Aerial parts | EOs | [40] |
| 112 | α-Curcumene | Aerial parts | EOs | [44] |
| 113 | α-Ferulene | Aerial parts | EOs, petroleum ether | [23, 43] |
| 114 | α-Guajene | Aerial parts | EOs, petroleum ether | [23, 43] |
| 115 | α-Gurjunene | Aerial parts | EOs | [44] |
| 116 | α-Humulene | Aerial parts, flowering top | EOs | [15, 41] |
| α-Muurolene | Aerial parts | Petroleum ether | [23] | |
| 117 | α-Panasisen | Aerial parts | EOs | [40] |
| 118 | α-Pentasiloxane | Aerial parts | EOs | [43] |
| 119 | α-Phellandrene | Aerial parts | EOs, petroleum ether | [23, 43] |
| 120 | α-Pinene | Aerial parts, whole plant | EOs | [19, 46] |
| 121 | α-Terpineol | Aerial parts | EOs | [50] |
| 122 | α-Thujene | Aerial parts, whole plant, flowering top | EOs | [19, 41, 46] |
| 123 | β-Bourbonene | Flowering top | EOs | [41] |
| 124 | β-Caryophyllene | Aerial parts | EOs | [47] |
| 125 | β-Cubebene | Aerial parts | EOs | [40] |
| 126 | β-Gurjunene | Aerial parts | EOs | [40] |
| 127 | β-Ionone | Aerial parts | EOs | [40] |
| 128 | β-Linalool | Aerial parts | Ethyl acetate | [23] |
| 129 | β-Oplopenone | Aerial parts, whole plant | EOs | [19, 44] |
| 130 | β-Patchoulene | Aerial parts | EOs | [40] |
| 131 | β-Phellandrene | Flowering top, aerial parts | EOs | [41, 48] |
| 132 | β-Pinene | Aerial parts, flowering top | EOs, petroleum ether, ethyl acetate | [16, 23, 41] |
| 133 | γ-Cadinene | Aerial parts, flowering top | EOs | [41, 47] |
| 134 | γ-Costol | Aerial parts | EOs | [48] |
| 135 | γ-Methylionone | Aerial parts | EOs | [48] |
| 136 | γ-Muurolene | Aerial parts, flowering top | EOs | [41, 42] |
| 137 | τ-Muurolol | Aerial parts | Petroleum ether | [23] |
| 138 | γ-Terpinene | Aerial parts | EOs, petroleum ether, ethyl acetate | [15, 23, 50] |
| 139 | δ-Cadinene | Aerial parts, flowering top | EOs | [41, 50] |
3.3.1. Phenolic Compounds
Thanks to their phenolic group, the phenolic compounds such as phenolic acids, flavonoids, tocopherols, and tannins are considered as an important group of bioactive compounds that are responsible for a wide range of biological properties such as antimicrobial [51, 52], antioxidant [53] anticancer [54], and litholytic activities [55]. Besides their pharmacological potential, the phenolic compounds, particularly flavonoids, are involved in many physiological processes; they are included in the regulation and protection of vascular plants against several biotic and abiotic stresses [56–58].
There are few studies investigating the chemical composition of T. satureioides extracts. In fact, the phenolic profile of T. satureioides remains not well identified.
Khouya et al. [24] have examined the phenolic composition of the T. satureioides aqueous extracts and reported that they contain high levels of phenolic compounds, which are represented by rosmarinic acid as major phenolic acid and luteolin-7-glycoside and hesperetin as major flavonoids. Another study showed that T. satureioides aqueous extracts were rich in total polyphenols (456.73 ± 6.94 mg caffeic acid equivalent/g of dry plant) and in flavonoid group (172.79 ± 2.12 mg rutin equivalent/g of dry plant) with rosmarinic acid, hesperetin, and luteolin-7-glucoside as major phenolic compounds [13].
In a recent study, Tebaa et al. [59] showed that the aqueous extracts of T. satureioides aerial parts are rich in total polyphenols (285 ± 34.82 μg gallic acid equivalent/mL aqueous extract), in total flavonoids (25.83 ± 4 μg catechin equivalent/mL aqueous extract) and in total tannins (0.032 ± 0.002 μg tannic acid equivalents/mL aqueous extract).
In addition, the chemical composition of the methanol extracts of T. satureioides analyzed by a combination of chromatographic tools (reverse-phase HPLC and 1H NMR analyses) revealed the presence of flavonoids as the main constituents with five essential compounds: luteolin-3′-O-glucuronide, luteolin-7-O-glucoside, eriodictyol-7-O-glucoside, aglycone luteolin, and thymonin [14]. However, other molecules such as ursolic acid and oleanolic acids were identified in the chloroform extract of T. satureioides [14].
The qualitative phytochemical analysis of T. satureioides extracts (hydromethanol, chloroform, ethyl acetate, and butanol extracts) enabled to detect the presence of flavonoids, catechols, gallic tannins, and anthraquinones [60]. Moreover, the quantitative HPLC analysis of crude and organic extracts of T. satureioides aerial parts showed the presence of phenolic acids (caffeic acid and rosmarinic acid) and the flavonoids quercetin and hesperetin in crud and methanolic extracts, whereas rosmarinic acid, hyperoside, quercetin, and hesperetin were detected in ethyl acetate extracts [39].
Interestingly, Kouar et al. [38] have determined the phytochemical profile of alcoholic extract of T. satureioides leaves, using the electrocoagulation and solvent extraction assays, and detected the presence of saponins, sterols, triterpene, tannins, and flavone aglycones. The quantitative analysis showed that T. satureioides alcoholic extract contains high levels of total polyphenols (70.2 ± 0.4 mg of gallic acid equivalents/g extract) and total flavonoids (52.7 ± 0.01 mg of quercetin equivalents/g extract). The high performance liquid chromatography (HPLC) analysis allowed to identify six compounds in this alcoholic extract, including four phenolic acids (12–14, 16) and two flavonoid compounds (1–2) [39].
The phenolic compound content and nature vary depending on the extraction solvent, plant's part used, plant's origin, storage conditions, and analytical method used. Indeed, flavonoids are the main phenolic group detected in T. satureioides extracts with 11 compounds (1–11) (Figure 2). Moreover, five phenolic acids were identified (12–16) (Figure 3).
Figure 2.
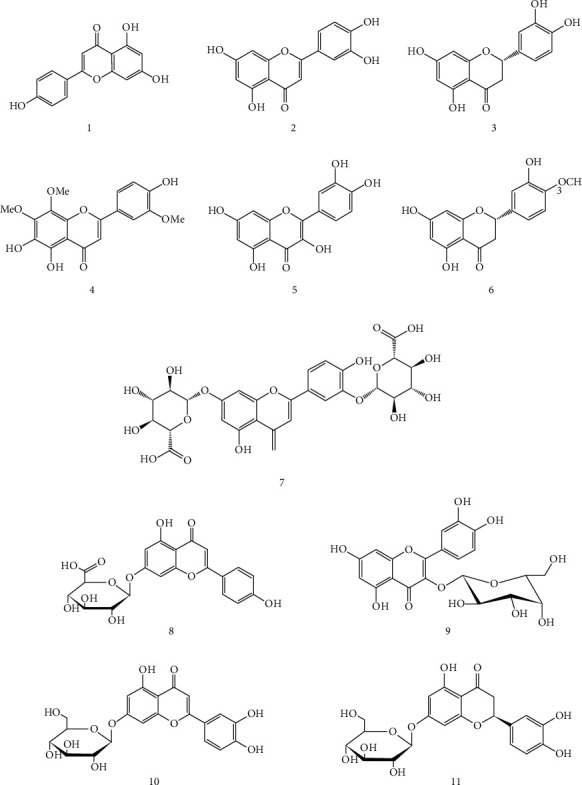
Flavonoid compounds isolated from T. satureioides extracts.
Figure 3.
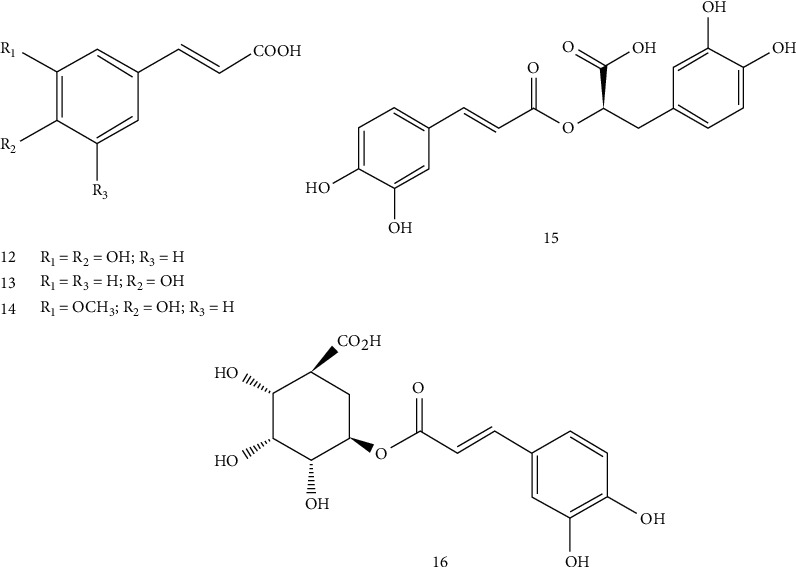
Phenolic acids identified in T. satureioides.
3.3.2. Volatile Compounds
Numerous studies have investigated and characterized the chemical composition of T. satureioides EOs, particularly from the aerial parts.
The chemical analysis showed that T. satureioides EOs are mainly composed of borneol, thymol, carvacrol, camphene, α-pinene, α-terpineol, p-cymene, and linalool (Figure 4).
Figure 4.
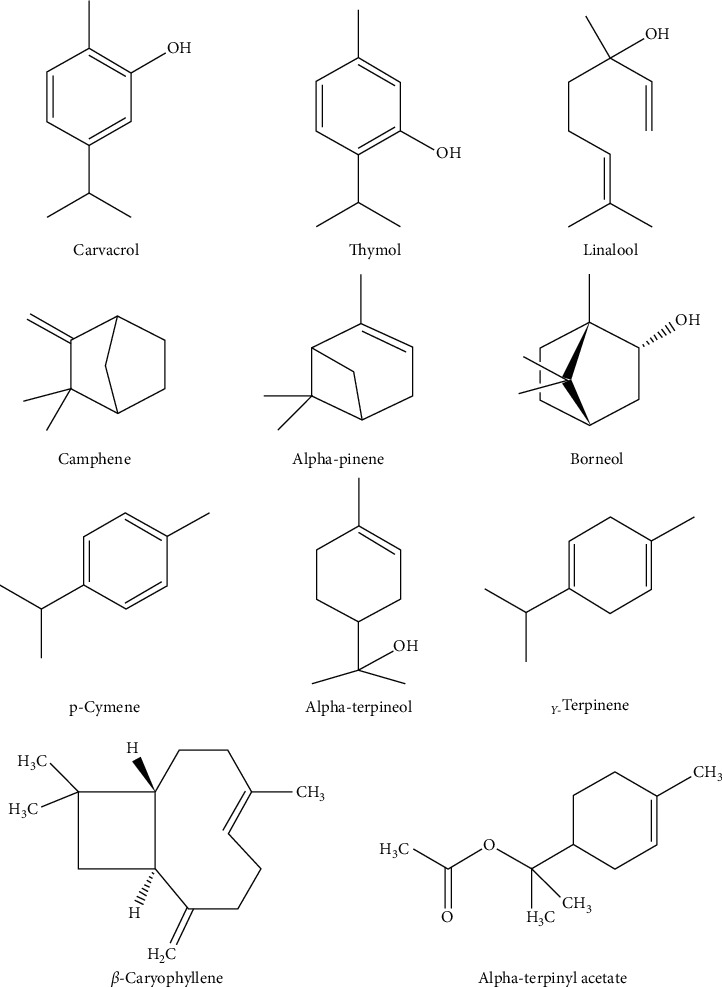
Chemical structure of the main terpenoids identified in T. satureioides.
The percentages and the nature of these volatile compounds vary noticeably depending on several intrinsic and extrinsic factors of the plant, including geographical origin, phenological stage, genotype, plant's part used, and storage and extraction conditions [61, 62].
Sbayou et al. [43] indicated that borneol and thymol are the chief components of T. satureioides EOs with 26.45% and 11.24%, respectively, followed by α-terpinyl acetate (10.99%), β-caryophyllene (8.24%), and camphene (7.16%). The studies carried out on T. satureioides from the High Atlas of Morocco indicated that carvacrol (26.5%) and borneol (20.1%) are the main compounds of its EOs, while thymol was not identified [15, 49].
It is well seen that borneol, carvacrol, and thymol constitute the major proportion of the volatile compounds of T. satureioides. Indeed, in an earlier study, Jaafari et al. [23] described the EOs of T. satureioides harvested in Tiznit region as a “borneol chemotype (59.37%),” those of Marrakech region (Asni-My Brahim) as “carvacrol (35.90%) and borneol (30%) chemotypes,” and the one of Beni Mellal region (Bin El Widane) as “borneol (51.98%) and thymol (26.81%) chemotypes,” thus showing a variation in chemotypes of the T. satureioides EOs according to harvest zones.
A comparative study of T. satureioides leaf and flower EOs, using simultaneous GC-FID and GC-MS tools, showed major differences regarding the main compounds of these two plants' part EOs. Thereby, borneol (a monoterpene alcohol) was the main compound of the flowers EOs with 19.3%, followed by carvacrol (10.0%) and thymol (3.8%), while carvacrol (37%), thymol (13.7%), γ-terpinene (8.4%), and (E)-β-caryophyllene (6.6%) were the main components of the leaves EOs [41].
Another study revealed the presence of 68 volatile compounds representing 93.3% of T. satureioides aerial parts' total EOs using capillary gas chromatography and gas chromatography coupled to mass spectrometry (GC-MS) [40]. These volatile compounds mainly belong to the monoterpenoides class (monoterpene hydrocarbons, oxygenated monoterpenes, and phenolic monoterpenes) such as borneol, carvacrol, thymol, camphene, linalool, and camphor.
3.4. Pharmacological Properties
Numerous pharmacological investigations have shown that T. satureioides essential oils and extracts obtained from different plant parts possess various biological activities, including antibacterial, antioxidant, antifungal, antiparasitic, anticancer, antidiabetic, and anti-inflammatory effects (Figure 5).
Figure 5.
Pharmacological properties of T. satureioides.
3.4.1. Antibacterial Activity
The antibacterial activity of T. satureioides EOs and extracts, against a panel of bacterial strains, including Gram-positive and Gram-negative bacteria, was reported in the literature [16, 63, 64].
Indeed, the EOs obtained from different parts of T. satureioides were evaluated against several pathogenic bacteria known by their drug multiresistance, including Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli, Acinetobacter baumannii, and methicillin-resistant Staphylococcus aureus (MRSA). Table 3 summarizes the published works that investigated the antibacterial activities of T. satureioides. It lists the parts used, the tested extracts, the methods used, the tested strains, and the main results obtained.
Table 3.
Antibacterial activity of T. satureioides.
| Parts used | Extracts | Methods used | Bacteria tested | Key results | References |
|---|---|---|---|---|---|
| Aerial parts | Essential oil (0.93%) | Broth microdilution method | Staphylococcus aureus CCMM B3 | MIC = 4.50 mg/mL | [65] |
| Micrococcus luteus ATCC 10240 | MIC = 4.50 mg/mL | ||||
| Bacillus cereus ATCC 14579 | MIC = 2.50 mg/mL | ||||
| Listeria monocytogenes ATCC 19115 | MIC = 4.50 mg/mL | ||||
| Escherichia coli ATCC 25922 | MIC = 18.00 mg/mL | ||||
| Pseudomonas aeruginosa ATCC 27853 | MIC > 18.4 mg/mL | ||||
| Klebsiella pneumoniae | MIC = 9 mg/mL | ||||
|
| |||||
| Stem | Aqueous extract | Paper disc diffusion assay | Clavibacter michiganensis subsp. michiganensis H195 isolate | Ф = 23.3 ± 2.4 mm | [66] |
|
| |||||
| Leaves | Aqueous extract | Paper disc diffusion assay | Clavibacter michiganensis subsp. michiganensis H195 isolate | Ф = 16.4 ± 0.5 mm | [66] |
|
| |||||
| Aerial part | Essential oil | Agar disc diffusion method | Staphylococcus aureus ATCC 29213 | Ф = 34.67 ± 0.33 mm | [20] |
| Escherichia coli ATCC 25922 | Ф = 20.67 ± 0.27 mm | ||||
| Pseudomonas aeruginosa ATCC 27853 | Ф = 7.00 ± 0.00 mm | ||||
|
| |||||
| Aerial part | Essential oil | Microdilution assay | Escherichia coli ATCC 25922 | MIC = 0.125% | [67] |
| Pseudomonas aeruginosa ATCC 27853 | MIC = 1% | ||||
| Micrococcus luteus ATCC 14452 | MIC = 0.03% | ||||
| Staphylococcus aureus ATCC 29213 | MIC = 0.03% | ||||
| Bacillus subtilis ATCC 6633 | MIC = 0.03% | ||||
| Salmonella typhimurium | MIC = 0.25% | ||||
| Bacillus cereus | MIC = 0.015% | ||||
|
| |||||
| Whole plant | Essential oil (1.78%) | Microdilution assay | Staphylococcus aureus ATCC 25923 | MIC = 2.5 μl/mL | [17] |
| Streptococcus fasciens ATCC 29212) | MIC = 2.5 μl/mL | ||||
| Escherichia coli ATCC 4157 | MIC = 5 μl/mL | ||||
| Pseudomonas aeruginosa ATCC 27853 | MIC = 10 μl/mL | ||||
|
| |||||
| Aerial part | Essential oil | Agar diffusion method Broth microdilution method |
Escherichia coli ATCC 25921 | Ф = 15 ± 0 mm MIC = 0.625 μl/mL MBC = 0.625 μl/mL |
[43] |
| Escherichia coli | Ф = 21 ± 0 mm MIC = 125 μl/mL MBC = 125 μl/mL |
||||
| Pseudomonas aeruginosa ATCC 27853 | Ф = 0 ± 0 mm MIC > 20 μl/mL MBC > 20 μl/mL |
||||
| Pseudomonas aeruginosa | Ф = 0 ± 0 mm MIC > 20 μl/mL MBC > 20 μl/mL |
||||
| Enterobacter cloacae | Ф = 15.5 ± 0.7 mm MIC = 0.625 μl/mL MBC = 0.625 μl/mL |
||||
| Staphylococcus aureus ATCC 29213 | Ф = 23 ± 0 mm MIC = 0.312 μl/mL MBC = 0.312 μl/mL |
||||
| Staphylococcus aureus | Ф = 15 ± 0 mm MIC = 0.625 μl/mL MBC = 0.625 μl/mL |
||||
| Enterococcus faecium | Ф = 16 ± 0 mm MIC = 125 μl/mL MBC = 125 μl/mL |
||||
|
| |||||
| Aerial part | Essential oil | Agar-dilution method | Enterobacter cloacae (clinical strain, nosoco.tech Abdel1) | MIC = 2.9 μg/mL | [68] |
| Escherichia coli CIP 54127 | MIC = 2.9 μg/mL | ||||
| Klebsiella pneumoniae CIP 104216 | MIC = 2.9 μg/mL | ||||
| P. aeruginosa ATCC 15442 | MIC = 11.7 μg/mL | ||||
| Salmonella typhimurium ATCC 133115 | MIC = 2.9 μg/mL | ||||
| Listeria monocytogenes ATCC 35152 | MIC = 5.8 μg/mL | ||||
| Methicillin-resistant Staphylococcus aureus (MRSA) | MIC = 2.9 μg/mL | ||||
| Enterococcus faecalis CIP A185 | MIC = 5.8 μg/mL | ||||
| Streptococcus equinus CIP 56.23 | MIC = 2.9 μg/mL | ||||
| Streptococcus pyogenes CIP 70.3 | MIC = 2.9 μg/mL | ||||
|
| |||||
| Aerial parts | Essential oil | Agar diffusion method Broth microdilution method |
Escherichia coli ATCC25922 | Ф = 12.3 ± 0.6 mm MIC = 1.5% |
[40] |
| Non-O1 Vibrio cholera | Ф = 33.3 ± 2.9 mm MIC = 0.5% |
||||
| Pseudomonas aeruginosa CCMMB11 | Ф = 11.7 ± 1.5 mm MIC = 1.5% |
||||
| Enterobacter cloacae | Ф = 11.7 ± 0.6 mm MIC = 1.5% |
||||
| Klebsiella pneumoniae | Ф = 13.3 ± 0.6 mm MIC = 1.5% |
||||
| Staphylococcus aureus CCMMB3 | Ф = 29.3 ± 2.1 mm MIC = 0.125% |
||||
| Bacillus subtilis ATCC9524 | Ф = 34.3 ± 1.1 mm MIC = 0.003% |
||||
| Bacillus cereus ATCC14579 | Ф = 30 ± 0 mm MIC = 0.003% |
||||
|
| |||||
| Aerial parts | Essential oil (1.86%) | Agar disc diffusion Agar dilution technique |
Staphylococcus aureus CCMM B3 | Ф = 29.67 ± 1.15 mm MIC = 1.78 mg/mL |
[15] |
| Bacillus subtilis ATCC 9524 | Ф = 43.67 ± 1.53 mm MIC = 0.89 mg/mL |
||||
| Bacillus cereus ATCC 14579 | Ф = 43.67 ± 1.53 mm MIC = 0.89 mg/mL |
||||
| Micrococcus luteus ATCC 10240 | Ф = 42.00 ± 1.73 mm MIC = 0.45 mg/mL |
||||
| Escherichia coli ATCC 25922 | Ф = 22.5 ± 1.32 mm MIC = 1.78 mg/mL |
||||
| Escherichia coli CCMM B4 | Ф = 23.00 ± 1.00 mm MIC = 1.78 mg/mL |
||||
| Salmonella sp. CCMM B17 | Ф = 22.33 ± 0.58 mm MIC = 1.78 mg/mL |
||||
| Enterobacter cloacae | Ф = 21.00 ± 1.00 mm MIC = 1.78 mg/mL |
||||
|
| |||||
| Leaves | Essential oil (2.95%(v/w)) | Agar diffusion assay | Bacillus cereus | Ф = 12.5 mm MIC = 80 μg/mL |
[41] |
| Staphylococcus aureus ATCC 5638 | Ф = 8.0 mm MIC = 640 μg/mL |
||||
| Listeria monocytogenes | Ф = 14.5 mm MIC = 40 μg/mL |
||||
| Aeromonas hydrophila | Ф = 11.8 mm MIC = 160 μg/mL |
||||
| Escherichia coli | Ф = 9.0 mm MIC = 320 μg/mL |
||||
| Proteus vulgaris | Ф = 7.4 mm MIC = 640 μg/mL |
||||
| Pseudomonas aeruginosa | ND MIC = 1280 μg/mL |
||||
| Pseudomonas fluorescens | Ф = 7.2 mm MIC = 640 μg/mL |
||||
| Salmonella abony | Ф = 7.8 mm MIC = 640 μg/mL |
||||
|
| |||||
| Inflorescences (flowers) | Essential oil (2.95% (v/)) | Bacillus cereus | Ф = 13.8 mm MIC = 80 μg/mL |
[41] | |
| Staphylococcus aureus ATCC5638 | Ф = 8.4 mm MIC = 320 μg/mL |
||||
| Listeria monocytogenes | Ф = 15.2 mm MIC = 40 μg/mL |
||||
| Aeromonas hydrophila | Ф = 14.2 mm; MIC = 80 μg/mL | ||||
| Escherichia coli | Ф = 10.4 mm MIC = 160 μg/mL |
||||
| Proteus vulgaris | Ф = 8.2 mm MIC = 320 μg/mL |
||||
| Pseudomonas aeruginosa | Ф = 6.8 mm MIC = 640 μg/mL |
||||
| Pseudomonas fluorescens | Ф = 8.0 mm MIC = 320 μg/mL |
||||
| Salmonella abony | Ф = 8.6 mm MIC = 320 μg/mL |
||||
|
| |||||
| Aerial part | Ethanolic extract | Agar-well diffusion method Broth microdilution method |
Staphylococcus aureus 25923 | Ф = 6 mm MIC = 6.25 mg/mL MBC = 12.5 mg/mL |
[69] |
| Listeria monocytogenes 4032 | Ф = 8.1 ± 0.31 mm MIC = 6.25 mg/mL MBC = 12.5 mg/mL |
||||
| Bacillus cereus ATCC 14579 | Ф = 13.2 ± 0.23 mm MIC = <0.5 mg/mL MBC = 1 mg/mL |
||||
| Escherichia coli ATCC 25929 | Ф = 6 mm MIC = 25 mg/mL MBC = 50 mg/mL |
||||
| Pseudomonas aeruginosa 195 | Ф = 10 ± 0.043 mm MIC = 25 mg/mL MBC = 50 mg/mL |
||||
| Salmonella enterica | Ф = 6 mm MIC = 12 mg/mL MBC = 25 mg/mL |
||||
|
| |||||
| Aerial part | Essential oil | Disc diffusion assay | Escherichia coli | Ф = 13.66 ± 0.43 mm | [70] |
| Bacillus subtilis | Ф = 26.21 ± 2.08 mm | ||||
| Mycobacterium smegmatis | Ф = 28.34 ± 1.05 mm | ||||
|
| |||||
| Leaves | Essential oil (2.7%) | Disc diffusion assay | Escherichia coli | Ф = 15.5 mm | [71] |
| Staphylococcus aureus | Ф = 30.7 mm | ||||
| Acinetobacter baumannii | Ф = 19 mm | ||||
| Bacillus cereus | Ф = 16 mm | ||||
| Enterobacter cloacae | Ф = 20 mm | ||||
|
| |||||
| Flower | Essential oil (4.1%) | Disc diffusion assay | Escherichia coli | Ф = 20 mm | [71] |
| Staphylococcus aureus | Ф = 45 mm | ||||
| Acinetobacter baumannii | Ф = 35 mm | ||||
| Bacillus cereus | Ф = 38 mm | ||||
| Enterobacter cloacae | Ф = 22 mm | ||||
|
| |||||
| Whole plant | Essential oil | Agar diffusion method Microdilution method |
Staphylococcus aureus | Ф = 16 mm MIC = 1.1% (v/v) |
[18] |
| Bacillus cereus | Ф = 19 mm MIC = 1.1% (v/v) |
||||
| Escherichia coli | Ф = 14.25 mm MIC = 1.25% (v/v) |
||||
|
| |||||
| Flowering plant | Essential oil | Agar dilution method | Escherichia coli O157:H7 | High antibacterial effect against the four pathogenic bacteria (MIC 0.05–0.4% (v/v)) | [72] |
| Listeria monocytogenes 2812 1/2a | |||||
| Salmonella typhimurium SL 1344 | |||||
| Staphylococcus aureus ATCC 29213 | |||||
|
| |||||
| Flowering top | Essential oil | Agar diffusion method | Pseudomonas aeruginosa IH | Ф = 8 mm | [73] |
| Pseudomonas aeruginosa CECT 110T | Ф = 8 mm | ||||
| Pseudomonas aeruginosa CECT 118 | Ф = 8 mm | ||||
| Pseudomonas Fluorescens CECT 378 | Ф = 11 mm | ||||
| Escherichia coli k12 | Ф = 13 mm | ||||
| Staphylococcus aureus MBLA | Ф = 16 mm | ||||
| Staphylococcus aureus CECT 976 | Ф = 15 mm | ||||
| Staphylococcus aureus CECT 794 | Ф = 15 mm | ||||
| Bacillus subtilis DCM 6633 | Ф = 19 mm | ||||
| Bacillus capsulas | Ф = 18 mm | ||||
| Enterococcus faecium CECT 410 | Ф = 13 mm | ||||
| Listeria innocua CECT 4030 | Ф = 21 mm | ||||
| Listeria monocytogenes CECT 4032 | Ф = 19 mm | ||||
|
| |||||
| Aerial part | Essential oil (3.2%) | Microdilution assay | Escherichia coli 1 from patient | MIC = 0.33 mg/mL | [64] |
| Escherichia coli ATCCS | MIC = 43 mg/mL | ||||
| Escherichia coli 2 from patient | MIC = 0.36 mg/mL | ||||
| Escherichia coli 1 from raw sheep milk | MIC = 0.51 mg/mL | ||||
| Escherichia coli 2 from Raw Sheep Milk | MIC = 0.34 mg/mL | ||||
| Escherichia coli 3 from raw sheep milk | MIC = 0.4 mg/mL | ||||
| Enterohemorragic Escherichia coli (EHEC) O157 | MIC = 0.21 mg/mL | ||||
| Enteropathogenic Escherichia coli (EPEC) | MIC = 0.31 mg/mL | ||||
| Enterotoxigenic Escherichia coli (ETEC) | MIC = 0.45 mg/mL | ||||
| Enteroaggregative Escherichia coli (EAggEC) | MIC = 0.5 mg/mL | ||||
| Enteroinvasive Escherichia coli | MIC = 0.7 mg/mL | ||||
|
| |||||
| Leaves | Essential oil 1.35% (v/w) | Disc diffusion method | Microbacterium testaceum | High antibacterial effect (Ф > 20 mm) MIC = 0.025% (v/v) MBC = 0.033% (v/v) |
[63] |
| Serratia marcescens | Important antibacterial activity (15 < Ф < 19 mm) MIC = 0.033% (v/v) MBC = 0.05% (v/v) |
||||
|
| |||||
| Leaves | Aqueous extract 100 mg/mL (w/v) | Disc diffusion method | Microbacterium testaceum | Slight antibacterial activity (Ф < 8 mm) | [63] |
| Serratia marcescens | Slight antibacterial activity (Ф < 8 mm) | ||||
|
| |||||
| Aerial parts | Essential oil | Microdilution method | Mycobacterium aurum A+ | MIC = 0.015% (v/v) MBC = 0.015% (v/v) |
[16] |
| Mycobacterium smegmatis mc2-155 | MIC = 0.062% (v/v) MBC = 0.062% (v/v) |
||||
Ou-Yahia et al. [67] assessed the antibacterial effect of the T. satureioides aerial part EOs against E. coli, Bacillus cereus, P. aeruginosa, Salmonella typhimurium, S. aureus, Micrococcus luteus, and Bacillus subtilis and showed a variable antibacterial activity of the tested EOs. The highest activity was observed against B. cereus and S. aureus with MIC values of 0.015% and 0.03%, respectively, while P. aeruginosa was the most resistant bacterium with a MIC value of 1%.
In another study, testing of the bacteriostatic and bactericidal effects of EOs of T. satureioides leaves and flowering top on E. coli, S. aureus, A. baumannii, B. cereus, and Enterobacter cloacae revealed a significant antibacterial activity (inhibition zone diameters (Ф): 16 mm < Ф < 30.7 mm for leaves EOs; 22 mm < Ф < 45 mm for flowering top EOs) and a bacteriostatic effect against all tested strains except B. cereus [71].
Mekkaoui et al. [70] tested in vitro the antimicrobial effect of EOs of T. satureioides harvested at two different phenological stages (flowering and postflowering) against three pathogenic bacteria responsible for foodborne disease in Morocco (E. coli, B. subtilis, and Mycobacterium smegmatis). They showed that EOs obtained after the flowering stage were more active against the studied strains than those obtained in the flowering stage. In fact, the highest activity was shown against M. smegmatis followed by B. subtilis, while the weakest activity was noticed against E. coli [70]. In the same context, Oussalah et al. [72] evaluated the antibacterial potential of the T. satureioides flower EOs against four pathogenic bacteria including two Gram-positive bacteria: S. aureus and Listeria monocytogenes (2812 1/2a), and two Gram-negative bacteria: E. coli O157:H7 and S. typhimurium (SL 1344), using the broth microdilution method. The results indicated that S. aureus was the most sensitive bacterium with MIC = 0.05% (v/v) followed by E. coli O157:H7 and S. typhimurium (MIC = 0.2% (v/v)), while L. monocytogenes was the least sensitive bacteria to the tested EOs with MIC = 0.4% (v/v).
More interestingly, Amrouche et al. [18] investigated both in vitro and in a food system the antibacterial activity of T. satureioides EOs, extracted from the whole plant, against foodborne bacteria (E. coli, S. aureus, and B. cereus). The paper disc diffusion and broth microdilution methods were used for the in vitro test and the beef minced meat was used as food model. Thereby, the addition of T. satureioides EOs to inoculated beef minced meat decreased the tested strain population after 4 days of storage. Moreover, in vitro investigations indicated that B. cereus was the most sensitive bacteria (Ф = 19 mm and MIC = 1.1%), followed by S. aureus (Ф = 16 mm, MIC = 1.1%) and then E. coli (Ф = 14.25 mm, MIC = 1.25%) [18].
El Abdouni Khayari et al. [65] reported a good antibacterial activity of the T. satureioides aerial part EOs against B. cereus (Ф = 30.00 ± 0.50 mm, MIC = 2.25 mg/mL), followed by M. luteus (Ф = 26.70 ± 0.20 mm, MIC = 4.5 mg/mL), L. monocytogenes (Ф = 19.30 ± 0.60 mm, MIC = 4.5 mg/mL), S. aureus (Ф = 16.30 ± 2.10 mm, MIC = 4.5 mg/mL), K. pneumoniae (Ф = 19.00 ± 1.00 mm, MIC = 9 mg/mL), and E. coli (Ф = 11.70 ± 0.60 mm, MIC = 18 mg/mL), compared to standards: cefixime, gentamicin, and kanamycin, while no effect was observed against P. aeruginosa (ATCC 27853) [65].
Another work demonstrated an antibacterial effect of EOs extracted from wild and cultivated T. satureioides against Gram-positive (S. aureus, M. luteus, B. subtilis, and B. cereus) higher than against Gram-negative strains (E. coli (ATCC 25922), E. coli (CCMM B4), Salmonella sp., and E. cloacae) [15]. The highest activity was found for M. luteus (Ф = 49.17 ± 1.15 mm for wild TS, Ф = 29.67 ± 1.15 mm for cultivated TS), while the weakest activity was noticed against S. aureus (Ф = 30.33 ± 0.58 mm for wild TS, Ф = 47.83 ± 1.32 mm for cultivated TS). Moreover, a considerable activity was also noted against Gram-negative bacteria with inhibition zone diameters ranging from 19.00 ± 0.10 to 23.00 ± 1.00 mm. The microdilution approach revealed a promising antibacterial effect of the tested EOs with MIC values ranging from 0.45 to 1.78 μg/mL [15]. This remarkable antibacterial effect of the T. satureioides EOs is concordant with the results of an earlier investigation, which indicated that T. satureioides E.Os inhibit the growth of a panel of microorganisms (65 Gram-positive and Gram-negative bacterial strains), among which Aeromonas hydrophila, Vibrio cholera, and Stenotrophomonas maltophilia were the most sensitive bacteria with respective MIC values of 0.14 ± 0.4% (v/v), 0.14 ± 0.0% (v/v), and 0.16 ± 0.0% (v/v) [74].
Recently, Meziani et al. [63] studied the antimicrobial effect of EOs and aqueous and methanolic extract from thyme leaves against Microbacterium testaceum and Serratia marcescens endophytic to date palm by using different approaches including quantitative and qualitative methods. They demonstrated that the EOs were more active against both reported bacteria than aqueous and methanolic extracts (Фs < 15 mm). Thus, these EOs have a great inhibition power against M. testaceum (Ф > 20 mm, MIC = 0.025%, MBC = 0.033%) and a good effect against S. marcescens (15 mm < Ф < 19 mm, MIC = 0.033%, MBC = 0.05%) [63].
3.4.2. Antioxidant Activity
The use of T. satureioides as a food preservative and against several pathologic disorders in Moroccan folk medicine encouraged the research teams to study the antioxidant potential of this plant species. In fact, several works reported the antioxidant activity of T. satureioides EOs as well as its extracts obtained from different plant parts (aerial parts, flowering top, and leaves) using different methods such as 2,2′-azino-bis 3-ethylbenzothiazoline-6-sulphonic acid (ABTS) radical scavenging, ferric reducing power, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, thiobarbituric acid reactive substances (TBARS), 2,2-azobis 2-amidinopropane dihydrochloride (APPH), and β-carotene/linoleic acid bleaching assays [13, 21, 39, 75]. All published works that studied the antioxidant activity of T. satureioides EOs and extracts have been listed and summarized in Table 4.
Table 4.
Antioxidant effects of T. satureioides.
| Parts used | Extracts | Methods used | Findings | Reference |
|---|---|---|---|---|
| Aerial parts | Crude extract | DPPH free radical scavenging activity assay | IC50 = 0.44 ± 0.06 mg/mL | [39] |
| FRAP assay | IC50 = 41.41 ± 4.55 mmol/L | |||
| Ethyl acetate extract | DPPH assay | IC50 = 0.33 ± 0.02 mg/mL | ||
| FRAP assay | IC50 = 82.69 ± 2.29 mmol/L | |||
| Methanolic extract | DPPH assay | IC50 = 0.71 ± 0.09 mg/mL | ||
| FRAP assay | IC50 = 55.99 ± 2.21 mmol/L | |||
| Aqueous extract | DPPH assay | IC50 = 0.85 ± 0.06 mg/mL | ||
| FRAP assay | IC50 = 25.46 ± 2.71 mmol/L | |||
| Dichloromethane extract | DPPH assay | IC50 = 0.48 ± 0.05 mg/mL | ||
| FRAP assay | IC50 = 33.48 ± 0.08 mmol/L | |||
| Aerial parts | Aqueous extract | TBARS method | Significant inhibition of lipid peroxidation product (MDA) | [24] |
| DPPH assay | IC50 = 0.44 ± 0.01 mg/mL | |||
| FRAP assay | IC50 = 40.14 ± 4.55 mmol/g | |||
| Aerial parts | Aqueous extract | ABTS assay | IC50 = 14.65 ± 0.36 μg/mL | [76] |
| Aerial part | Essential oil (1.86%) | DPPH assay | IC50 = 167.00 ± 2.47 μg/mL | [15] |
| Reducing power technique | IC50 = 176.89 ± 1.02 μg/mL | |||
| Aerial parts | Essential oil (2%) | DPPH assay | IC50 = 0.21 ± 1.17 mg/mL | [49] |
| Reducing power assay | IC50 = 0.23 ± 0.67 mg/mL | |||
| β-Carotene/linoleic acid bleaching assay | IC50 = 0.21 ± 1.74 mg/mL | |||
| ABTS assay | IC50 = 0.15 ± 0.36 mg/mL | |||
| Flowering top | Essential oil | Reducing power assay | Absorbance = 0.507 ± 0.019 | [73] |
| DPPH assay | Percent inhibition = 42.99% | |||
| β-Carotene test | Percent inhibition = 74.50% | |||
| Aerial part | Ethyl acetate extract | DPPH assay | IC50 = 109.98 ± 3 μg/mL | [60] |
| Aerial part | Essential oil | Reducing power assay | Percent inhibition = 24.64 ± 0.03% | [47] |
| TEAC assay | TEAC = 159.00 ± 0.01 mMof Trolox/m | |||
| β-Carotene bleaching assay | Percent inhibition = 83.87 ± 0.10% | |||
| DFRS assay | Percent inhibition = 68.55 ± 0.01% | |||
| Aerial part | Essential oil | Ferric reducing capacity | EC50 = 177.13 ± 2.1 mg/mL | [46] |
| DPPH assay | IC50 = 122.53 ± 2.38 μg/mL | |||
| Leaves | Methanolic extract | Free radical scavenging activity (DPPH° test) | SC50 = 14.6 μg | [14] |
| Aerial parts | Essential oil | DPPH assay | IC50 = 0.25 ± 0.03 mg/mL | [43] |
| β-Carotene/linoleic acid assay | Percent inhibition = 81.78 ± 0.37% | |||
| TBARS assay | I50= 300.32 ± 1.50 mg/mL | |||
| Aerial part | Aqueous extract | FRAP assay | IC50 = 50.79 ± 2.02 mmol Trolox/g | [13] |
| Malondialdehyde (MDA) assay | Important antioxidant activity | |||
| DPPH assay | IC50 = 0.480 ± 0.010 mg/mL | |||
| AAPH-induced oxidative erythrocyte hemolysis assay | Neutralization of the free radicals liberated by the AAPH | |||
| Aerial part | Essential oil | Reducing power | IC50 = 85.47 ± 0.95 μg/mL | [21] |
| β-Carotene/linoleic acid assay | IC50 = 64.26 ± 0.70 μg/mL | |||
| DPPH | IC50 = 108.15 ± 1.54 μg/mL | |||
| Aerial part | Essential oil | DPPH assay | IC50 = 0.81 mg/mL | [44] |
| Aerial part | Hot water extract | DPPH assay | IC50 = 15.99 ± 0.47 μg/mL | [77] |
| Reducing power assay | IC50 = 20.33 ± 0.19 μg/mL | |||
| β-Carotene bleaching | IC50 = 14.69 ± 0.69 μg/mL | |||
| Cold water extract | DPPH assay | IC50 = 53.42 ± 1.17 μg/mL | ||
| Reducing power assay | IC50 = 64.32 ± 0.52 μg/mL | |||
| β-Carotene bleaching | IC50 = 50.20 ± 0.33 μg/mL | |||
| Methanol extract | DPPH assay | IC50 = 30.24 ± 0.19 μg/mL | ||
| Reducing power assay | IC50 = 30.48 ± 0.52 μg/mL | |||
| β-Carotene bleaching test | IC50 = 86.38 ± 0.85 μg/mL | |||
| Aerial part | Hexane extract | DPPH assay | IC50 = 275.71 ± 11.26 μg/mL | [22] |
| ABTS assay | IC50 = 127.38 ± 3.83 μg/mL | |||
| FRAP assay | 97.819 ± 0.377 mg equivalent ascorbic acid/g of extract | |||
| Dichloromethane extract | DPPH assay | IC50 = 8.18 ± 0.07 μg/mL | ||
| ABTS assay | IC50 = 80.09 ± 0.65 μg/mL | |||
| FRAP assay | 153.457 ± 0.247 mg equivalent ascorbic acid/g of extract | |||
| Ethyl acetate extract | DPPH assay | IC50 = 23.75 ± 0.67 μg/mL | ||
| ABTS assay | IC50 = 85.16 ± 3.22 μg/mL | |||
| FRAP assay | 123.004 ± 0.377 mg equivalent ascorbic acid/g of extract | |||
| Water-ethanol extract | DPPH assay | IC50 = 3.86 ± 0.07 μg/mL | ||
| ABTS assay | IC50 = 51.27 ± 0.82 μg/mL | |||
| FRAP assay | 233.292 ± 0.377 mg equivalent ascorbic acid/g of extract | |||
| Leaves | Hot aqueous extract | DPPH assay | IC50 = 0.343 ± 0.011 mg/mL | [69] |
| Iron-ferrous chelating power assay | IC50 = 0.4539 ± 0.011 mg/mL | |||
| Cold aqueous extract | DPPH assay | IC50 = 0.652 ± 0.013 mg/mL | ||
| Iron-ferrous chelating power assay | IC50 = 0.6394 ± 0.014 mg/mL | |||
| Ethanolic extract | DPPH assay | IC50 = 0.247 ± 0.011 mg/mL | ||
| Iron-ferrous chelating power assay | IC50 = 0.3341 ± 0.012 mg/mL | |||
| Leaves | Methanolic extract | DPPH assay | Percent inhibition = 92.24% | [75] |
Sbayou et al. [43] reported an important antioxidant activity of the EOs of T. satureioides aerial parts using different methods, namely, DPPH free radical scavenging, TBARS, and β-carotene/linoleic acid assays. In fact, the tested EOs exhibit a strong reduction of DPPH radical (IC50 = 0.25 ± 0.03 mg/mL) compared with ascorbic acid (IC50 = 0.25 ± 0.03 mg/mL) as standard antioxidant. However, the β-carotene/linoleic acid assay showed a moderate antioxidant capacity (I% = 81.78 ± 0.37%) compared to BHT (I% = 98.13 ± 0.94%) as positive control. Taoufik et al. [44] also investigated the antiradical capacity of the T. satureioides aerial part EOs using the DPPH scavenging test and reported an interesting antioxidant effect in a concentration-dependent manner. The IC50DPPH (IC50 = 0.81 mg/mL) value was slightly lower than the antioxidant standards BHT (IC50 = 0.11 mg/mL) and Covi-oxT (IC50 = 0.11 mg/mL).
Another study assessed the antioxidant activity of T. satureioides EOs using several in vitro assays, including ABTS, DPPH, β-carotene/linoleic acid bleaching, and reducing power assays, with quercetin and BHT as antioxidant standards [49]. The antiradical activity indicated that T. satureioides EOs exhibit stronger activity against free radical ABTS (IC50 = 0.15 ± 0.36 μg/mL) and DPPH (IC50 = 0.21 ± 1.17 μg/mL) than the used antioxidant standards. Moreover, β-carotene test and reducing power assays also showed high antioxidant activities with respective IC50 values of 0.21 ± 1.74 μg/mL and 0.23 ± 0.67 μg/mL [49]. In contrast, an investigation carried out by Alaoui-Jamali et al. [46] indicated that the T. satureioides aerial part EOs exhibit a moderate scavenging activity of DPPH radical (IC50 = 122.53 ± 2.38 μg/mL). The ferric (Fe3+) reducing capacity assay showed similar results with an EC50 value equal to 177.13 ± 2.1 μg/mL.
In addition, the antioxidant capacity of T. satureioides extracts was also studied by many researchers. Khouya and his coworkers [39] tested the antioxidant effect of ethyl acetate, methanolic, aqueous, dichloromethane, and crude extracts of T. satureioides aerial parts using DPPH radical scavenging, FRAP, and APPH assays and showed a higher reductive potential of these extracts than the reference compounds (Trolox). In fact, the highest reducing power of ferric metal was shown by the ethyl acetate fraction (IC50 = 82.69 ± 2.29 mmol Trolox/g of dry extract), and the lowest was observed for the aqueous fraction (IC50 = 25.46 ± 2.71 mmol Trolox/g of dry extract). The radical scavenging activity indicated that ethyl acetate fraction exerted the highest antioxidant activity with an IC50 value of 0.33 ± 0.02 mg/mL, followed by crude extract (IC50 = 0.44 ± 0.06 mg/mL), dichloromethane fraction (IC50 = 0.48 ± 0.05 mg/mL), and then methanolic fraction (IC50 = 0.71 ± 0.09 mg/mL). However, the aqueous fraction showed the weakest antiradical capacity (IC50 = 0.85 ± 0.06 mg/mL) [39]. Furthermore, the APPH test indicated that the addition of the tested extracts to suspensions containing erythrocyte and 2,2′-azobis 2-amidinopropane dihydrochloride (APPH) induced an increase in the hemolysis half times [39].
The antioxidant activities of T. satureioides extracts obtained from the aerial part were also examined by Labiad et al. [22] who reported remarkable antioxidant activities for hexane, dichloromethane, ethyl acetate, and hydro-ethanolic extracts, using ABTS radical scavenging, DPPH, and ferric reducing antioxidant power (FRAP) methods, with ascorbic acid as positive control. The hydro-ethanolic extracts exhibited the highest antiradical effect against DPPH and ABTS radicals with IC50 values of 3.86 ± 0.07 μg/mL and 51.27 ± 0.82 μg/mL, respectively, followed by dichloromethane (IC50DPPH = 23.75 ± 0.67 μg/mL, IC50ABTS = 80.09 ± 0.65 μg/mL), ethyl acetate (IC50DPPH = 23.75 ± 0.67 μg/mL, IC50ABTS = 85.16 ± 3.22 μg/mL), and then hexane extracts (IC50DPPH = 275.71 ± 11.26 μg/mL, IC50ABTS = 127.38 ± 3.83 μg/mL). Moreover, the hydro-ethanolic extract also exerted a great FRAP activity (233.292 ± 0.377 mg equivalent Ascorbic acid/g of extract). However, the hexane extract showed the lowest FRAP capacity (97.819 ± 0.377 mg equivalent ascorbic acid/g of extract) [22].
In a recent study, Hmidani et al. [76] measured the capacity of aqueous extract of T. satureioides to scavenge the generated radical ABTS+•, using ABTS assay, and showed significant scavenging activity of this extract (IC50ABTS = 14.65 ± 0.36 μg/ml) compared to ascorbic acid as standard (IC50 = 1.96 ± 0.1 μg/ml). These findings support those obtained by Khouya et al. [24], which showed a considerable antioxidant activity of the T. satureioides aerial part aqueous extract. Indeed, the tested aqueous extracts displayed potent scavenging activity against DPPH radical with an IC50 value equal to 0.44 ± 0.01 mg/mL TAE and a higher reducing power of ferric complex (40.14 ± 4.55 mmol Trolox/gTAE) than the Trolox used as positive control (44.33 ± 7.55 mmol Trolox/gTAE). Moreover, the aqueous extracts of T. satureioides exerted a potent protective potential against hemolysis of erythrocytes according to APPH test results [24]. These considerable antioxidant effects of the aqueous extracts were attributed to their high phenolic content [24].
3.4.3. Antifungal Activity
The antifungal activity of T. satureioides, especially its essential oils against several pathogenic fungal, has been reported in the literature [19, 50, 78, 79].
Boukhira et al. [20] evaluated the antifungal activity of T. satureioides EOs obtained from aerial parts against a yeast, Candida albicans (ATCC-10231), and a mould Aspergillus brasiliensis (ATCC-16404), using radial growth inhibition and broth microdilution assays. This study showed that the studied EOs exert effective effect against C. albicans (Ф = 24.67 ± 0.67 mm, MIC = 0.6 μl/mL) and A. brasiliensis (MIC = 1.3 μl/mL).
Asdadi et al. [19] studied the anticandidal activity of T. satureioides EOs (10 μl) against nosocomial fluconazole-resistant strains (Candida dubliniensis, C. albicans, C. glabrata, and C. krusei) using disc diffusion and microdilution methods, with fluconazole (10 μl) and amphotericin B (10 μl) as positive controls. According to this study, C. dubliniensis was the most sensitive strain to the tested EOs (Ф = 85 mm), followed by C. krusei (Ф = 67 mm), while C. albicans and C. glabrata were the least sensitive fungal strains with Ф of 53 mm and 49 mm, respectively. The microdilution assays showed an interesting anticandidal effect with minimal fungicidal concentration (MFC) values ranging between 0.3300 mg/mL and 0.9062 mg/ml [19].
In addition, El Bouzidi et al. [15] assessed the anticandidal activity of T. satureioides EOs obtained from aerial parts against four candida species, including C. albicans, C. glabrata, C. parapsilosis, and C. krusei using disc diffusion and microdilution assays, and fluconazol (40 μl) as reference. The findings of this study showed that all tested strains were more sensitive to the tested EOs (37.67 ± 1.53 mm < Ф < 42.00 ± 1.00 mm) than to the synthetic fungicide (fluconazol) used as a positive control (26.50 ± 0.50 mm < Ф < 29.83 ± 1.15 mm).
More interestingly, Salhi et al. [50] reported the antifungal activity of four chemotypes of T. satureioides EOs from aerial parts, namely, borneol/α-terpineol, borneol/carvacrol/α-terpineol, borneol/carvacrol/thymol, and borneol/camphene/α-terpineol against fungal strains responsible of wood damages (Coniophora puteana BAM Ebw. 15, Gloeophyllum trabeum BAM Ebw.109, Oligoporus placenta FPRL. 280, and Trametes versicolor CTB 863). The studied samples were harvested from four different locations in Southwest Morocco (Oulad Berhil, Amskroud-East, Aoulouz, and Timoulay Aksri), and qualitative and quantitative assays were used for antifungal screening [50]. The results showed that, at a concentration of 1/500 (v/v), all tested chemotypes inhibit the growth of the tested wood-decaying fungi. However, the investigated chemotypes exhibited a variable degree of the antifungal effect. Therefore, the chemotype borneol/carvacrol/thymol was the most active against the tested strains, followed by borneol/camphene/α-terpineol, borneol/α-terpineol, and borneol/carvacrol/α-terpineol. Moreover, the highest antifungal activity was noticed against G. trabeum with MIC ranging between 1/1500 v/v and 1/500 (v/v), followed by C. puteana (1/1250 (v/v) <MIC< 1/500 (v/v)), T. versicolor (MIC = 1/500 (v/v)), and O. placenta (MIC = 1/500 (v/v)) [50].
In the same context, Rahmouni et al. [42] reported fungicide effect of T. satureioides EOs and their major components (thymol, α-terpineol, carvacrol, and borneol) against a phytopathogenic fungus responsible for fusarium wilt on date palm in Morocco, named Fusarium oxysporum f. sp. Albedinis. The results of this study showed that these EOs as well as their major compounds inhibited noticeably the mycelia growth of Fusarium oxysporum f. sp. Albedinis in a concentration-dependent manner. The maximal fungicidal effect of the studied compounds was noticed by thymol with a minimum fungicidal concentration (MFC) value of 03.08 μl/mL, followed by α-terpineol (MFC = 12.20 μl/mL), carvacrol (MFC = 16.96 μl/mL), and borneol (MFC = 22.73 μl/mL) [42]. Furthermore, T. satureioides EO was found to inhibit spore germination of phytopathogenic fungi of citrus, namely, Penicillium digitatum, P. italicum, and Galactomyces citriaurantii at concentrations greater than 500 μl/mL [79].
Recently, El-Bakkal et al. [21] tested the antifungal effect of the EOs of T. satureioides aerial parts against Botrytis cinerea, P. digitatum, and Verticillium dahliae using disc diffusion method and fluconazol (40 μg/disc) as standard antifungal drug. Their results showed a promising antifungal effect, of the studied EOs, against the three tested strains, with inhibition zone diameters ranging from 31.50 ± 1.32 mm to 36.27 ± 1.15 mm compared to fluconazol (25.50 ± 0.50 < Ф < 28.00 ± 0.50).
3.4.4. Anti-Inflammatory Activity
Inflammation is a complex biological process that maintains homeostasis of the organism in response to multiple injuries such as infection, trauma, or immune reaction. It is characterized by pain, heat, redness, and swelling [80].
Inflammation is related to the occurrence of several human pathologies, including heart diseases, Alzheimer's disease, and cancer [81–83]. The mechanisms of the anti-inflammatory response involve various mediators such as phospholipase A2 activation, cytokines, chemokines, reactive oxygen species (ROS) generation, macrophages and mast cells, platelet-activating factor, and nitric oxide (NO) [83].
Khouya et al. [39] have evaluated in vivo the anti-inflammatory activity of T. satureioides crude extracts and fractions (dichloromethane, ethyl acetate, methanol, and aqueous) using croton-oil-induced ear oedema and carrageenan-induced paw oedema in mice and rats. The results of this study showed that topical applications of the dichloromethane and ethyl acetate fractions (1 mg/ear) reduced significantly ear oedema volume of 31.60% and 27.16%, respectively, after 4 h of treatment. The crude extracts exhibited the greatest activity, and its tropical application decreased significantly ear oedema (29.67%) 8 h after treatment. However, the methanol and aqueous fractions did not decrease ear oedema. Moreover, the results of carrageenan oedema assay showed that the ethyl acetate and methanol fractions (60 mg/kg) reduced significantly oedema induced by carrageenan during the first phase (16.40 ± 0.33% and 14.51 ± 1.40%, respectively) [39]. This study confirmed results obtained by the same authors, indicating that aqueous extracts of T. satureioides exhibited a remarkable anti-inflammatory effect in carrageenan-induced rats paw edema and in croton oil-induced mice ear edema [24]. In another previous study, Ismaili et al. [14] investigated the in vivo topical anti-inflammatory effect of methanol and chloroform extracts of T. satureioides leaves, using the croton oil ear test in mice, and showed that chloroform extract induced significant edema inhibition (at a inhibition dose ID50 of 282 μg·cm−2), only three times lower than that of the standard conventional drug indomethacin used as positive control (ID50 = 93 μg·cm−2), while the methanolic extract did not show any topical anti-inflammatory activity.
3.4.5. Antiparasitic Effect
T. satureioides EOs from different plant parts were studied against a number of human, virus, and plant parasites. Indeed, Pavela [84] assessed the toxicity of T. satureioides EOs against the larvae of Culex quinquefasciatus Say (Diptera: Culicidae) and showed its effective larvicidal property with respective lethal concentrations (IC50 and IC90) of 44 μg/ml and 81.5 μg/ml.
Kasrati et al. [49] reported a considerable insecticidal activity of T. satureioides EOs against adults of pest Tribolium castaneum responsible for stored-product deterioration (lethal dose values of LD50 = 0.315 μl/cm2 and LD90 = 0.71 μl/cm2). Moreover, Santana et al. [25] examined the toxicity of T. satureioides EOs against insect pest's larvae of Spodoptera littoralis, insect adults of Myzus persicae and Rhopalosiphum padi, as well as against adults and eggs of root-knot nematodes Meloidogyne javanica. A strong antifeedant effect of T. satureioides EOs was observed against S. littoralis larvae (EC50 = 36.9 ± 22.7 μg/cm2), M. persicae adults (EC50 = 53.53 ± 6.5 μg/cm2), and R. padi (EC50 = 49.0 ± 6.6 μg/cm2). Additionally, an important nematicidal effect was noticed against the tested M. javanica at two different development stages: second-stage juveniles (J2) (LC50 = 0.1 mg/mL and LC90 = 0.2 mg/mL) and eggs (mortality rate of 38.9% after 7 days) [25].
Another study conducted by Avato et al. [26] showed that T. satureioides EOs exhibit a promising nematicidal activity against Meloidogyne incognita juveniles (mortality rate of 10.6 ± 0.7%) and adults of Pratylenchus vulnus (100 ± 0.0%) and Xiphinema index (14.9 ± 0.7%) and that, after 48 h, this effect was dose-dependent.
The acaricidal activity of T. satureioides EOs was also reported in the literature. Ramzi et al. [85] studied the effect of the EOs of T. satureioides aerial parts against adults of Varroa destructor (Acari: Varroidae) and indicated an interesting mortality rate of 50% after 24 h and 80% after 48 h. Additionally, T. satureioides EO was shown to destroy completely the wheat pest Sitophilus oryzae (coleopters) at a concentration of 2.4 × 10−2 μl/cm3 after 24 h [86].
3.4.6. Other Pharmacological Properties
T. satureioides was also reported to exhibit other pharmacological properties such as anticancer, antidiabetic, and hypolipidemic effects.
Jaafari et al. [23] evaluated the in vitro antitumor activity of T. satureioides EOs collected in different regions (High Atlas of Morocco, Bin Elwidane-Beni Mellal, and Tiznit) on P815 mastocytoma cell line using the 3-(4,5-di-methylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. The results showed that all tested EOs exhibit an important cytotoxic effect against P815 cell line with IC50 values from 0.225% (v/v) to 0.24% (v/v). The antiproliferative effect of T. satureioides crude extracts was studied against MCF-7 breast cancer cell line using MTT assay and showed their strong inhibition with a half-inhibitory concentration (IC50) value of 37.5 ± 4.02 μg/mL [24].
Kabbaoui et al. [2] investigated the antidiabetic effect of T. satureioides aqueous extracts obtained from the aerial parts on streptozotocin- (STZ-) induced diabetic rats via the administration of an oral concentration of 500 mg/kg. As a result, T. satureioides aqueous extracts decreased significantly blood glucose levels and improved body weight and glucose tolerance in STZ-diabetic rats.
4. Conclusion and Perspectives
This scientific review reports the ethnomedicinal uses, chemical profile, and pharmacological properties of an endemic Moroccan medicinal plant: T. satureioides. This plant is widely used in Moroccan traditional medicine to treat several diseases such as hypertension, diabetes, skin ailments, and bronchitis.
Indeed, several investigations have demonstrated that T. satureioides exhibits numerous biological activities, including antibacterial, antifungal, antioxidant, anti-inflammatory, anticancer, antidiabetic, and antiparasitic activities. These pharmacological effects have proven the traditional uses of T. satureioides. However, the evidence supporting the traditional practices such as skin disorders, hypertension, influenza, and visual ailments of modern pharmacology is still limited. In this regard, we invite research groups to conduct further studies on the antiviral, antileishmanial, and hypotensive effects of T. satureioides. Furthermore, the pharmacological mechanisms of action, of this plant, on molecular targets need to be explored using current experimental assessments such as network pharmacology, proteomic, and pharmacokinetic. Additionally, an appropriate pharmacological approach should be considered for providing comprehensive pharmacological information for T. satureioides. Moreover, T. satureioides have shown interesting biological effects against some related oxidative stress such as inflammation and cancer. Accordingly, extensive clinical studies should be carried out to determine pharmacodynamic and pharmacokinetic parameters in order to develop drug from T. satureioides.
The phytochemical analysis using different chromatographic tools such as GC-MS and HPLC revealed the presence of a plethora of bioactive compounds mainly belonging to the terpenoids class in the essential oils of T. satureioides. This chemical diversity varied depending on plant's part used, season's harvest, plant's origin, as well as extraction and storage conditions. However, although numerous bioactive compounds have been isolated and identified from T. satureioides essential oils, few pure components have been assessed for their pharmacological effects. Furthermore, few studies have investigated the phenolic content of T. satureioides extracts. Therefore, further efforts should be focused on such area in order to determine in detail the phenolic profile of this species using different extraction solvents and the current spectroscopic tools such as HPLC-DAD, infrared (IR), and 1H NMR technique.
Finally, the acute, subacute, and subchronic toxicity tests are strongly required to verify the innocuity and the safety of this plant.
Abbreviations
- EOs:
Essential oils
- HPLC:
High-performance liquid chromatography
- 1H NMR:
Proton nuclear magnetic resonance
- GC-FID:
Gas chromatograph-flame ionization detection
- GC-MS:
Gas chromatography-mass spectrometry
- MIC:
Minimal inhibitory concentration
- MBC:
Minimum bactericidal concentration
- Ф:
Inhibition zone diameter
- ABTS:
2,2′-Azino-bis 3-ethylbenzothiazoline-6-sulphonic acid
- FRAP:
Ferric reducing antioxidant power
- DPPH:
2,2-Diphenyl-1-picrylhydrazyl
- TBARS:
Thiobarbituric acid reactive substances
- APPH:
2,2-Azobis 2-amidinopropane dihydrochloride
- BHT:
Butylated hydroxytoluene
- MFC:
Minimal fungicidal concentration
- MTT:
3-(4,5-Di-methylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- TAE:
Tannic acid equivalent.
Data Availability
All data analyzed during this investigation are available from the corresponding author.
Conflicts of Interest
The authors declare that they do not have any conflicts of interest.
References
- 1.Bellakhdar J. La Pharmacopée Marocaine, Traditionnelle. Médecine Arabe Ancienne et Savoir Populaire. Casablanca, Morocco: Editions le Fennec; 1997. [Google Scholar]
- 2.Kabbaoui M. E., Chda A., Mejrhit N., et al. Antidiabetic effect of Thymus satureioides aqueous extract in streptozotocin-induced diabetic rats. International Journal of Pharmacy and Pharmaceutical Sciences. 2016;8(9):140–145. doi: 10.22159/ijpps.2016v8i9.12647. [DOI] [Google Scholar]
- 3.Benabid A. Flore et Écosystèmes du Maroc: Evaluation et Préservation de la Biodiversité. Rabat, Morocco: Kalila Wa Dimna Publications; 2000. [Google Scholar]
- 4.Bellakhdar J., Claisse R., Fleurentin J., Younos C. Repertory of standard herbal drugs in the Moroccan pharmacopoea. Journal of Ethnopharmacology. 1991;35(2):123–143. doi: 10.1016/0378-8741(91)90064-k. [DOI] [PubMed] [Google Scholar]
- 5.El Hafian M., Benlandini N., Elyacoubi H., Zidane L., Rochdi A. Étude floristique et ethnobotanique des plantes médicinales utilisées au niveau de la préfecture d’Agadir-Ida-Outanane (Maroc) Journal of Applied Biosciences. 2014;81(1):7198–7213. doi: 10.4314/jab.v81i1.8. [DOI] [Google Scholar]
- 6.Abouri M., El Mousadik A., Msanda F., Boubaker H., Saadi B., Cherifi K. An ethnobotanical survey of medicinal plants used in the Tata province, Morocco. Journal of Medicinal Plant Research. 2012;1(7):99–123. [Google Scholar]
- 7.Ben Akka F., Salhi S., Benkhnigue O., Dahmani J., Douira A., Lahcen Z. Ethnobotanical study of medicinal plants used in the region of middle Oumrbia (Morocco) Plant Archives. 2019;19(2):2005–2017. [Google Scholar]
- 8.El Azzouzi F., Zidane L. La flore médicinale traditionnelle de la région de Béni- Mellal (Maroc) Journal of Applied Biosciences. 2015;91(1):8493–8502. doi: 10.4314/jab.v91i1.8. [DOI] [Google Scholar]
- 9.Benkhnigue O., Akka F. B., Salhi S., Fadli M., Douira A., Zidane L. Catalogue des plantes médicinales utilisées dans le traitement du diabète dans la région d’Al Haouz-Rhamna (Maroc) Journal of Animal and Plant Science. 2014;23(1):3539–3568. [Google Scholar]
- 10.Sbai-Jouilil H., Fadli A., Hafian M., Ayad R., Benharbit O., Zidane L. Floristic and ethnobotanical study of medicinal plants used in the treatment of respiratory diseases in Seksaoua region (western high Moroccan atlas) Annual Research & Review in Biology. 2017;17(6):1–10. doi: 10.9734/arrb/2017/36526. [DOI] [Google Scholar]
- 11.Fadili K., Sekkate C., Alistiqsa F., Haloui Z., Chakir S., Zair T. Ethnobotanical study of medicinal plants from Er-Rich region (Moroccan high atlas) Advances in Environmental Biology. 2017;11(6):27–41. [Google Scholar]
- 12.Ouhaddou H., Boubaker H., Msanda F., El Mousadik A. An ethnobotanical study of medicinal plants of the Agadir Ida Ou Tanane province (southwest Morocco) Journal of Applied Biosciences. 2014;84:7707–7722. [Google Scholar]
- 13.Ramchoun M., Sellam K., Harnafi H., et al. Investigation of antioxidant and antihemolytic properties of Thymus satureioides collected from Tafilalet region, south-east of Morocco. Asian Pacific Journal of Tropical Biomedicine. 2015;5(2):93–100. doi: 10.1016/s2221-1691(15)30151-9. [DOI] [Google Scholar]
- 14.Ismaili H., Milella L., Fkih-Tetouani S., et al. In vivo topical anti-inflammatory and in vitro antioxidant activities of two extracts of Thymus satureioides leaves. Journal of Ethnopharmacology. 2004;91(1):31–36. doi: 10.1016/j.jep.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 15.El Bouzidi L., Jamali C. A., Bekkouche K., et al. Chemical composition, antioxidant and antimicrobial activities of essential oils obtained from wild and cultivated Moroccan Thymus species. Industrial Crops and Products. 2013;43:450–456. doi: 10.1016/j.indcrop.2012.07.063. [DOI] [Google Scholar]
- 16.Chraibi M., Farah A., Lebrazi S., El Amine O., Iraqui Houssaini M., Fikri-Benbrahim K. Antimycobacterial natural products from Moroccan medicinal plants: chemical composition, bacteriostatic and bactericidal profile of Thymus satureioides and Mentha pulegium essential oils. Asian Pacific Journal of Tropical Biomedicine. 2016;6(10):836–840. doi: 10.1016/j.apjtb.2016.08.002. [DOI] [Google Scholar]
- 17.El Hattabi L., Talbaoui A., Amzazi S., et al. Chemical composition and antibacterial activity of three essential oils from south of Morocco (Thymus satureioides, Thymus vulgaris and Chamaelum nobilis) Journal of Materials and Environmental Science. 2016;7(9):3110–3117. [Google Scholar]
- 18.Amrouche T., Djenane D., Dziri F., Danoun K., Djerbal M., Rabinal P. R. Growth inhibition of Staphylococcus aureus, Bacillus cereus, and Escherichia coli assessed in vitro and in food system using thyme and mentha essential oils. Systèmes Agraires et Environnement. 2018;2(2):01–10. [Google Scholar]
- 19.Asdadi A., Alilou H., Akssira M., et al. Chemical composition and anticandidal effect of three thymus species essential oils from southwest of Morocco against the emerging nosocomial fluconazole-resistant strains. Journal of Biology, Agriculture and Healthcare. 2014;4(11):16–26. [Google Scholar]
- 20.Boukhira S., Balouiri M., Bousta F., Stéphane M., Sghir T. M., Bousta D. Antimicrobial activities of essential oil of five plant species from Morocco against some microbial strains. International Journal of Pharmacognosy and Phytochemical Research. 2016;8(11):1901–1906. [Google Scholar]
- 21.El-Bakkal S. E., Zeroual S., Elouazkiti M., et al. Comparison of yield chemical composition and biological activities of essential oils obtained from thymus pallidus and thymus satureioides Coss. grown in wild and cultivated conditions in Morocco. Journal of Essential Oil Bearing Plants. 2020;23(1):1–14. doi: 10.1080/0972060x.2019.1708216. [DOI] [Google Scholar]
- 22.Labiad M. H., Harhar H., Ghanimi A., Tabyaoui M. Phytochemical screening and antioxidant activity of Moroccan Thymus satureioides extracts. Journal of Materials and Environmental Sciences. 2017;8(6):2132–2139. [Google Scholar]
- 23.Jaafari A., Mouse H. A., Rakib E. M., et al. Chemical composition and antitumor activity of different wild varieties of Moroccan thyme. Revista Brasileira de Farmacognosia. 2007;17(4):477–491. doi: 10.1590/s0102-695x2007000400002. [DOI] [Google Scholar]
- 24.Khouya T., Ramchoun M., Hmidani A., et al. Anti-inflammatory, anticoagulant and antioxidant effects of aqueous extracts from Moroccan thyme varieties. Asian Pacific Journal of Tropical Biomedicine. 2015;5(8):636–644. doi: 10.1016/j.apjtb.2015.05.011. [DOI] [Google Scholar]
- 25.Santana O., Andres M. F., Sanz J., Errahmani N., Abdeslam L., Gonzalez-Coloma A. Valorization of essential oils from Moroccan aromatic plants. Natural Product Communications. 2014;9(8):1109–1014. doi: 10.1177/1934578x1400900812. [DOI] [PubMed] [Google Scholar]
- 26.Avato P., Laquale S., Argentieri M. P., Lamiri A., Radicci V., D’Addabbo T. Nematicidal activity of essential oils from aromatic plants of Morocco. Journal of Pest Science. 2017;90(2):711–722. doi: 10.1007/s10340-016-0805-0. [DOI] [Google Scholar]
- 27.Ramchoun M., Harnafi H., Alem C., et al. Hypolipidemic and antioxidant effect of polyphenol-rich extracts from Moroccan thyme varieties. E-SPEN Journal. 2012;7(3):e119–e124. doi: 10.1016/j.clnme.2012.02.005. [DOI] [Google Scholar]
- 28.Fouad Z., Lahcen Z. Antidiabetic medicinal plants in Morocco: ethnobotanical survey of the population of Beni Mellal. Plant Archives. 2020;20(1):337–343. [Google Scholar]
- 29.Montanari B. The Future of Mountain Agriculture. Berlin, Germany: Springer-Verlag; 2013. The future of agriculture in the high atlas mountains of Morocco: the need to integrate traditional ecological knowledge; pp. 51–72. [Google Scholar]
- 30.Ouhaddou H., Alaoui A., Laaribya S., Ayan S. Ethnobotanical survey of medicinal plants used for treating diabetes in Agadir Ida Outanane region, southwestern Morocco. Arabian Journal of Medicinal and Aromatic Plants. 2020;6(2):72–86. [Google Scholar]
- 31.Barkaoui M., Katiri A., Boubaker H., Msanda F. Ethnobotanical survey of medicinal plants used in the traditional treatment of diabetes in Chtouka Ait Baha and Tiznit (western anti-atlas), Morocco. Journal of Ethnopharmacology. 2017;198:338–350. doi: 10.1016/j.jep.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 32.El Azzouzi F., Asserar N., Zaouai F., Benkhnigue O., Hachi M., Zidane L. Ethnomedicinal evaluation of medicinal plants used against gastrointestinal disorders in the western middle atlas region (Morocco) Annual Research & Review in Biology. 2018;28(2):1–11. [Google Scholar]
- 33.Boufous H., Marhoume F., Chait A., Bagri A. Ethnopharmacological survey of medicinal plants with hallucinogenic effect and plants used against pain, inflammatory diseases, diabetes and urinary lithiasis in Zagora “Morocco”. Journal of Intercultural Ethnopharmacology. 2017;6(4):342–350. doi: 10.5455/jice.20170721062527. [DOI] [Google Scholar]
- 34.El Alami A., Farouk L., Chait A. Etude ethnobotanique sur les plantes médicinales spontanées poussant dans le versant nord de l’Atlas d’Azilal (Maroc) Algerian Journal of Natural Products. 2016;4(2):271–282. [Google Scholar]
- 35.Mouhajir F., Hudson J. B., Rejdali M., Towers G. H. N. Multiple antiviral activities of endemic medicinal plants used by Berber peoples of Morocco. Pharmaceutical Biology. 2001;39(5):364–374. doi: 10.1076/phbi.39.5.364.5892. [DOI] [Google Scholar]
- 36.Elkhoudri N., Baali A., Amor H. Maternal morbidity and the use of medicinal herbs in the city of Marrakech, Morocco. Indian Journal of Traditional Knowledge. 2016;15(1):79–85. [Google Scholar]
- 37.Tahraoui A., El-Hilaly J., Israili Z. H., Lyoussi B. Ethnopharmacological survey of plants used in the traditional treatment of hypertension and diabetes in south-eastern Morocco (Errachidia province) Journal of Ethnopharmacology. 2007;110(1):105–117. doi: 10.1016/j.jep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 38.Kouar J., Lamsaddek A., Benchekroun R., et al. Comparison between electrocoagulation and solvent extraction method in the process of the dechlorophyllation of alcoholic extracts from Moroccan medicinal plants Petroselinum crispum, Thymus satureioides and microalgae Spirulina platensis. SN Applied Sciences. 2019;1(1):p. 132. doi: 10.1007/s42452-018-0137-1. [DOI] [Google Scholar]
- 39.Khouya T., Ramchoun M., Hmidani A., et al. Acute toxicity and antiproliferative and procoagulant activities of fractions derived from Thymus satureioides of the Moroccan high atlas. South African Journal of Botany. 2019;121:568–576. doi: 10.1016/j.sajb.2019.01.005. [DOI] [Google Scholar]
- 40.Ghalbane I., Belaqziz R., Ait Said L., Oufdou K., Romane A., El Messoussi S. Chemical composition, antibacterial and antioxidant activities of the essential oils from Thymus satureioides and Thymus pallidus. Natural Product Communications. 2011;6(10):1507–1510. [PubMed] [Google Scholar]
- 41.Jirovetz L., Schmidt E., Höferl M., Wanner J., Abbad A., Gochev V. Comparative study on essential flower and leaf oils of four different wild species of thyme from Morocco. Proceedings of the 4th International Congress on Aromatic and Medicinal Plants; 2012; Sidi Bel-Abbés, Algeria. [Google Scholar]
- 42.Rahmouni A., Saidi R., Khaddor M., Pinto E., Gomes E. D. S. J. C., Maouni A. Chemical composition and antifungal activity of five essential oils and their major components against Fusarium oxysporum f. sp. albedinis of Moroccan palm tree. Euro-Mediterranean Journal for Environmental Integration. 2019;4(1):p. 27. doi: 10.1007/s41207-019-0117-x. [DOI] [Google Scholar]
- 43.Sbayou H., Boumaza A., Hilali A., Amghar S. Chemical composition and antibacterial and antioxidant activities of Thymus satureioides Coss. essential oil. International Journal of Pharmacy and Pharmaceutical Sciences. 2016;8(10):183–187. doi: 10.22159/ijpps.2016v8i10.13742. [DOI] [Google Scholar]
- 44.Taoufik F., Anejjar A., Asdadi A., et al. Synergic effect between Argania spinosa cosmetic oil and Thymus satureioides essential oil for the protection of the carbon steel against the corrosion in sulfuric acid medium. Journal of Materials and Environmental Science. 2017;8:582–593. [Google Scholar]
- 45.Zerrifi S. E. A., Kasrati A., Redouane E. M., et al. Essential oils from Moroccan plants as promising ecofriendly tools to control toxic cyanobacteria blooms. Industrial Crops and Products. 2020;143 doi: 10.1016/j.indcrop.2019.111922.111922 [DOI] [Google Scholar]
- 46.Alaoui-Jamali C., Kasrati A., Leach D., Abbad A. Étude comparative de l’activité insecticide des huiles essentielles des espèces de thyms originaires du Sud-Ouest marocain. Phytothérapie. 2018;16(5):268–274. doi: 10.3166/s10298-016-1051-6. [DOI] [Google Scholar]
- 47.Wang H.-F., Yih K.-H., Yang C.-H., Huang K.-F. Anti-oxidant activity and major chemical component analyses of twenty-six commercially available essential oils. Journal of Food and Drug Analysis. 2017;25(4):881–889. doi: 10.1016/j.jfda.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lemrhari A., Zouhair R., El Kahkahi R., Elidrissi M., Amchrouk A., Elhourri M. Chemical composition and differentiation of essential oils of Morocco’s different varieties of thyme. Global Journal of Pure and Applied Chemistry Research. 2016;4(1):30–35. [Google Scholar]
- 49.Kasrati A., Alaoui Jamali C., Fadli M., et al. Antioxidative activity and synergistic effect of Thymus saturejoides Coss. essential oils with cefixime against selected food-borne bacteria. Industrial Crops and Products. 2014;61:338–344. doi: 10.1016/j.indcrop.2014.07.024. [DOI] [Google Scholar]
- 50.Salhi N., Fidah A., Rahouti M., Ismaili M. R., Ramzi H., Kabouchi B. Chemical composition and fungicidal effects of four chemotypes of Thymus satureioides Coss. essential oils originated from south-west of Morocco. Journal of Materials and Environmental Sciences. 2018;9(2):514–519. [Google Scholar]
- 51.Et-Touys A., Fellah H., Mniouil M., et al. Screening of antioxidant, antibacterial and antileishmanial activities of Salvia officinalis L. extracts from Morocco. British Microbiology Research Journal. 2016;16(5):1–10. doi: 10.9734/bmrj/2016/28307. [DOI] [Google Scholar]
- 52.Bouyahya A., Bakri Y., Khay E. O., et al. Antibacterial, antioxidant and antitumor properties of Moroccan medicinal plants: a review. Asian Pacific Journal of Tropical Disease. 2017a;7(1):57–64. [Google Scholar]
- 53.Bouyahya A., Moussaoui N., Abrini J., Bakri Y., Dakka N. Determination of phenolic contents, antioxidant and antibacterial activities of strawberry tree (Arbutus unedo L.) leaf extracts. British Biotechnology Journal. 2016;14(3):1–10. doi: 10.9734/bbj/2016/26488. [DOI] [Google Scholar]
- 54.Aneb M., Talbaoui A., Bouyahya A., et al. In vitro cytotoxic effects and antibacterial activity of Moroccan medicinal plants Aristolochia longa and Lavandula multifida. European Journal of Medicinal Plants. 2016;16(2):1–13. doi: 10.9734/ejmp/2016/28534. [DOI] [Google Scholar]
- 55.Bouyahya A., Guaouguaou F. E., Guaouguaou F.-E., Nadia Dakka N., Bakri Y. Pharmacological activities and medicinal properties of endemic Moroccan medicinal plant Origanum compactum (Benth) and their main compounds. Asian Pacific Journal of Tropical Disease. 2017b;7(10):628–640. doi: 10.12980/apjtd.7.2017d7-31. [DOI] [Google Scholar]
- 56.Božović M., Ragno R. Calamintha nepeta (L.) Savi and its main essential oil constituent pulegone: biological activities and chemistry. Molecules. 2017;22(2):p. 290. doi: 10.3390/molecules22020290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DellaGreca M., Previtera L., Purcaro R., Zarrelli A. Cinnamic ester derivatives from Oxalis pes-caprae (Bermuda buttercup) Journal of Natural Products. 2007;70(10):1664–1667. doi: 10.1021/np0702786. [DOI] [PubMed] [Google Scholar]
- 58.DellaGreca M., Fiorentino A., Monaco P., Previtera L., Zarrelli A. A new dimeric 9,10-dihydrophenanthrenoid from the rhizome of Juncus acutus. Tetrahedron Letters. 2002;43(14):2573–2575. doi: 10.1016/s0040-4039(02)00308-8. [DOI] [Google Scholar]
- 59.Tebaa L., Douma M., Tazart Z., Manaut N., Mouhri K. H., Loudiki M. Assessment of the potentially algicidal effects of Thymus satureioides Coss. and Artemisia herba alba L. against Microcystis aeruginosa. Applied Ecology and Environmental Research. 2018;16(1):903–912. doi: 10.15666/aeer/1601_903912. [DOI] [Google Scholar]
- 60.Fadili K., Amalich S., N’dedianhoua S. K., et al. Polyphenols content and antioxidant activity of two species from Moroccan high atlas: Rosmarinus officinalis and Thymus satureioides. International Journal of Innovation and Scientific Research. 2015;4(17):24–33. [Google Scholar]
- 61.Bouyahya A., Dakka N., Talbaoui A., et al. Correlation between phenological changes, chemical composition and biological activities of the essential oil from Moroccan endemic Oregano (Origanum compactum Benth) Industrial Crops and Products. 2017;108:729–737. doi: 10.1016/j.indcrop.2017.07.033. [DOI] [Google Scholar]
- 62.Bouyahya A., Zengin G., Belmehdi O., et al. Origanum compactum Benth., from traditional use to biotechnological applications. Journal of Food Biochemistry. 2020;44(8) doi: 10.1111/jfbc.13251.e13251 [DOI] [PubMed] [Google Scholar]
- 63.Meziani R., Mazri M. A., Essarioui A., et al. Towards a new approach of controlling endophytic bacteria associated with date palm explants using essential oils, aqueous and methanolic extracts from medicinal and aromatic plants. Plant Cell, Tissue and Organ Culture (PCTOC) 2019;137(2):285–295. doi: 10.1007/s11240-019-01570-1. [DOI] [Google Scholar]
- 64.Bouazza F., Hassikou R., Satrani B., Ghanmi M., ENnadir J., Khedid K. In vitro antibacterial activity of Thymus satureioides, Mentha pulegium, and Origanum vulgare essential oils against Escherichia coli isolated from raw sheep milk. International Journal of Agriculture Innovations and Research. 2014;3(1):210–217. [Google Scholar]
- 65.El Abdouni Khayari M., Jamali C. A., Kasrati A., et al. Antibacterial activity of essential oils of some Moroccan aromatic herbs against selected food-related bacteria. Journal of Essential Oil Bearing Plants. 2016;19(5):1075–1085. doi: 10.1080/0972060x.2015.1004123. [DOI] [Google Scholar]
- 66.Talibi I., Amkraz N., Askarne L., et al. Antibacterial activity of Moroccan plants extracts against Clavibacter michiganensis subsp. michiganensis, the causal agent of tomatoes’ bacterial canker. Journal of Medicinal Plants Research. 201;5(17):4332–4338. [Google Scholar]
- 67.Ou-yahia D., Chraibi M., Farah A., Fikri-Benbrahim K. Antimicrobial and antioxidant activities of Moroccan Thymus satureioides essential oil. Journal of Materials and Environmental Sciences. 2017;8(2):1948–1952. [Google Scholar]
- 68.Asbahani A. E., Addi E. H. A., Miladi K., et al. Preparation of medical cotton textile activated by Thymus leptobotrys essential oil colloidal particles: evaluation of antifungal properties. Journal of Colloid Science and Biotechnology. 2014;3(3):253–261. doi: 10.1166/jcsb.2014.1096. [DOI] [Google Scholar]
- 69.Ramdan B., Amakran A., Bakrim N., Vannier B., Greche H., Nhiri M. Anti-glycation and radical scavenging activities of hydro-alcohol and aqueous extracts of nine species from Lamiaceae family. Journal of Medicinal Plants Studies. 2017;5(1):331–345. [Google Scholar]
- 70.Mekkaoui S., Farah A., Elkhanchoufi A., Elkhetabi A., Moutaouafiq S., Mimouni S. Effect of harvest period on yield, chemical composition and antibacterial activity of the essential oils of Thymus satureioides. International Journal of Pharmacognosy and Phytochemical Research. 2017;9(1):17–21. [Google Scholar]
- 71.Boukhraz A., Elhartiti H., Barrahi M., et al. Evaluation of the bacteriostatic and bactericidal activity of essential oil of Thymus satureioides. International Journal of Research Studies in Science, Engineering and Technology. 2016;3(3):24–28. [Google Scholar]
- 72.Oussalah M., Caillet S., Saucier L., Lacroix M. Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E. coli O157:H7, Salmonella typhimurium, Staphylococcus aureus and Listeria monocytogenes. Food Control. 2007;18(5):414–420. doi: 10.1016/j.foodcont.2005.11.009. [DOI] [Google Scholar]
- 73.Bouhdid S., Idaomar M., Zhiri A., Baudoux D., Skali N. S., Abrini J. Thymus essential oils: chemical composition and in vitro antioxidant and antibacterial activities. Proceedings of the 2nd International Congress of Biochemistry; 2006; Agadir, Morocco. [Google Scholar]
- 74.Mayaud L., Carricajo A., Zhiri A., Aubert G. Comparison of bacteriostatic and bactericidal activity of 13 essential oils against strains with varying sensitivity to antibiotics. Letters in Applied Microbiology. 2008;47(3):167–173. doi: 10.1111/j.1472-765x.2008.02406.x. [DOI] [PubMed] [Google Scholar]
- 75.El Guiche R., Tahrouch S., Amri O., El Mehrach K., Hatimie A. Antioxidant activity and total phenolic and flavonoid contents of 30 medicinal and aromatic plants located in the south of Morocco. International Journal of New Technology and Research. 2015;1(3):7–11. [Google Scholar]
- 76.Hmidani A., Bouhlali E. D. T., Khouya T., et al. Antioxidant, anti-inflammatory and anticoagulant activities of three Thymus species grown in southeastern Morocco. Future Journal of Pharmaceutical Sciences. 2019;5(1):p. 4. doi: 10.1186/s43094-019-0005-x. [DOI] [Google Scholar]
- 77.Kasrati A., Alaoui Jamali C., Abbad A. Antioxidant properties of various extract from selected wild Moroccan aromatic and medicinal species. Trends in Phytochemical Research. 2017;1(4):175–182. [Google Scholar]
- 78.Feyaerts A., Mathé L., Luyten W., Van Dijck P. Comparison between the vapor-phase-mediated anti-Candida activity of conventional and organic essential oils. Natural Volatiles and Essential Oils. 2018;5(1):1–6. [Google Scholar]
- 79.Boubaker H., Karim H., El Hamdaoui A., et al. Chemical characterization and antifungal activities of four Thymus species essential oils against postharvest fungal pathogens of citrus. Industrial Crops and Products. 2016;86:95–101. doi: 10.1016/j.indcrop.2016.03.036. [DOI] [Google Scholar]
- 80.Ferrero M. L., Nielsen O. H., Anderson P. S., Girardin S. E. Chronic inflammation: importance of NOD2 and NALP3 in interleukin‐1 beta generation. Clinical and Experimental Immunology. 2007;147:227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Balkwill F., Charles K. A., Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7(3):211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 82.Woodward M., Rumley A., Welsh P., MacMahon S., Lowe G. A comparison of the associations between seven hemostatic or inflammatory variables and coronary heart disease. Journal of Thrombosis and Haemostasis. 2007;5(9):1795–1800. doi: 10.1111/j.1538-7836.2007.02677.x. [DOI] [PubMed] [Google Scholar]
- 83.Mueller C., Laule-Kilian K., Christ A., Brunner-La Rocca H. P., Perruchoud A. P. Inflammation and long-term mortality in acute congestive heart failure. American Heart Journal. 2006;151(4):845–850. doi: 10.1016/j.ahj.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 84.Pavela R. Larvicidal property of essential oils against Culex quinquefasciatus Say (Diptera: Culicidae) Industrial Crops and Products. 2009;30(2):311–315. doi: 10.1016/j.indcrop.2009.06.005. [DOI] [Google Scholar]
- 85.Ramzi H., Ismaili M. R., Aberchane M., Zaanoun S. Chemical characterization and acaricidal activity of Thymus satureioides C. & B. and Origanum elongatum E. & M. (Lamiaceae) essential oils against Varroa destructor Anderson & Trueman (Acari: Varroidae) Industrial Crops and Products. 2017;108:201–207. doi: 10.1016/j.indcrop.2017.06.031. [DOI] [Google Scholar]
- 86.Ainane A., Khammour F., M’hamad E., et al. Composition chimique et activité anti insecticide des huiles essentielles de Thymus du Maroc: Thymus capitatus, Thymus bleicherianus et Thymus satureioides. Proceedings Biosune. 2018;1:96–100. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed during this investigation are available from the corresponding author.


